- 1Department of Community Health Sciences, College of Applied Medical Sciences, King Saud University, Riyadh, Saudi Arabia
- 2Leeds Institute of Health Sciences, School of Medicine, University of Leeds, Leeds, United Kingdom
Background: Monitoring cancer trends in a population is essential for tracking the disease’s burden, allocating resources, and informing public health policies. This review describes variations in commonly employed methods to estimate colorectal cancer (CRC) incidence trends.
Methods: We performed a systematic literature search in four databases to identify population-based studies reporting CRC incidence trends, published between January 2010 and May 2020. We extracted and described data on methods to estimate trends and assess model validity, and the software used.
Results: This review included 145 articles based on studies conducted in five continents. The majority (93%) presented visual summaries of trends combined with absolute, relative, or annual change estimates. Fourteen (10%) articles exclusively calculated the relative change in incidence over a given time interval, presented as the percentage of change in rates. Joinpoint regression analysis was the most commonly used method for assessing incidence trends (n= 65, 45%), providing estimates of the annual percentage change (APC) in rates. Nineteen (13%) studies performed Poisson regression and 18 (12%) linear regression analysis. Age-period-cohort modeling- a type of generalized linear models- was conducted in 18 (12%) studies. Thirty-nine (37%) of the studies modeling incidence trends (n=104, 72%) indicated the method used to evaluate model fitness. The joinpoint program (52%) was the statistical software most commonly used.
Conclusion: This review identified variation in the calculation of CRC incidence trends and inadequate reporting of model fit statistics. Our findings highlight the need for increasing clarity and transparency in reporting methods to facilitate interpretation, reproduction, and comparison with findings from previous studies.
Introduction
Quantifying and monitoring cancer incidence in a population are essential for tracking the disease burden and resource planning. Observing changes in cancer rates over time can enhance our understanding of its historical evolution, the potential social and environmental risk factors leading to cancer, and the impact of implementing interventions and policies. To produce reliable findings on population-level incidence trends, investigators usually rely on population-based cancer registries for providing valid cancer data.
Recent years have witnessed an extensive focus on studying the epidemiology of colorectal cancer (CRC). CRC is a major global health problem, and its incidence rate has increased over the past decades. According to the GLOBOCAN 2020 estimates of cancer incidence, CRC is the third most common cancer and the second leading cause of cancer-related deaths worldwide (1). In CRC, survival outcomes are associated with the clinical stage at diagnosis (2); thus, it is one of the few cancers where screening is considered a critical preventive measure (3). Many population-based reports and epidemiological studies have investigated trends in CRC incidence over time and what societal, environmental, or political changes have been related to these transitions in incidence. Time trend analysis of CRC by age group has also been critical in developing and evaluating secondary prevention efforts such as screening programs (4, 5).
Different methods have been utilized to assess CRC incidence trends. Visual summaries in the form of graphs and descriptive tables are widely used, most often complementing the use of advanced statistical methods. A well-known, established approach for quantifying trends is the estimated annual percentage change (APC), representing the yearly average change in incidence rate. The APC is usually estimated by computing the regression model’s slope fitted to the log-transformed incidence rates (6). Different statistical models have been used to estimate the APC, such as linear, Poisson, and joinpoint regression. Some modeling strategies account for age, calendar period, and birth cohort effects on incidence trend estimates (7). The derived inferences from these modeling techniques are imperative for directing resource allocation and public health policies. Yet, the integrity of these inferences largely depends on the modeling procedure’s validity. Thus, previous methodological studies have underscored the importance of assessing model validity, including evaluating candidate models, selecting the final model, and assessing model assumptions or performance (8, 9).
When choosing the statistical software to conduct the trend analysis, it is essential to note that different software uses different methods and permits different outputs to be reported. Also, not all software requires the same technical skills; some need coding experience, while others are considered user-friendly in terms of learning the tool and implementing the analysis. Therefore, researchers should be aware of the most commonly used tools to assess trends and what output is usually reported.
To our knowledge, no previous study has examined and summarized the methods used to assess incidence trends in the literature and the extent of reporting model validity assessment. This review was set up to answer the following questions: 1- What are the various statistical methods reported in the literature for assessing CRC incidence trends, and what type of parameters are reported? 2- What model validity measures are reported in studies using statistical modeling? 3- What software is employed to conduct the analysis?
The current study was conducted in parallel with a comprehensive review describing incidence rate measures and evaluating the quality of reporting incidence methods (10).
Methods
The reporting of this systematic review followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (11).
Study identification
In May 2020, we searched Embase, Medline, Web of Science, and the Cochrane Library for studies reporting temporal trends in CRC incidence, published since 2010. In consultation with an information specialist, we developed a search strategy that included keywords and a combination of subject headings, including “colorectal cancer,” “incidence,” “trends,” and “registry” (the complete search strategy is provided in Supplementary File 1. We also checked the reference lists of identified studies to detect potentially missed articles.
Study selection
We included studies that fulfilled all of the following criteria: 1) population-based retrospective studies using registry data to measure and report the incidence trends of colorectal cancer, 2) written in English, and 3) a full text is published. We excluded studies conducted in selected population groups (i.e., CRC incidence trends amongst patients with specific diseases), measured the incidence of multiple cancer types, or only reported trend estimates calculated in previous research. Furthermore, studies published as commentaries, case studies, clinical trials, case-control studies, reviews, conference proceedings, abstracts, or posters were excluded from this review.
Selection process
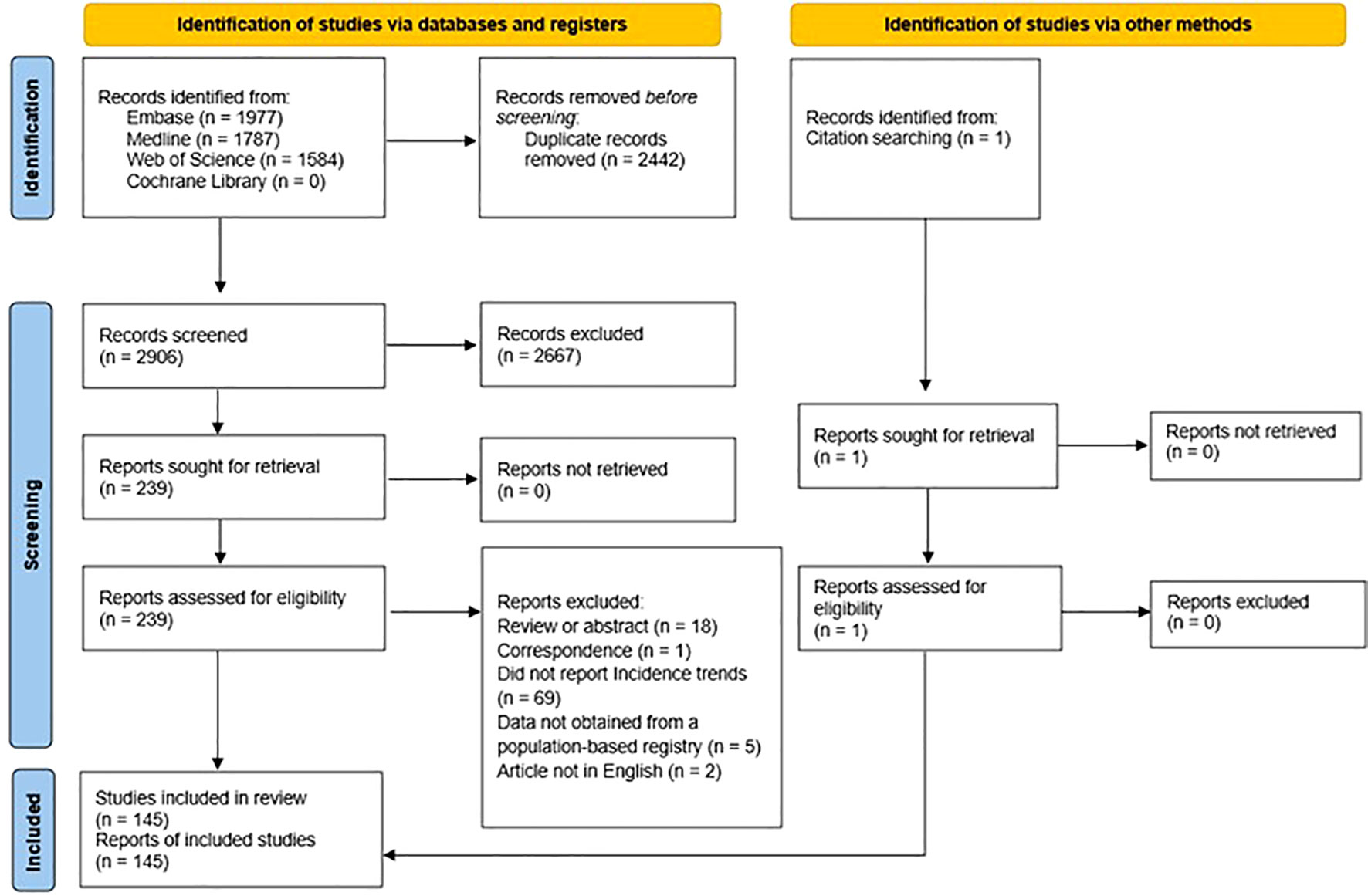
Figure 1 summarizes the selection process. After importing all potential abstracts into the screening web app “Rayyan” (12), two independent reviewers screened all titles and abstracts against the inclusion-exclusion criteria to exclude clearly irrelevant articles. Reviewers resolved disagreements through discussion, and in cases where a consensus decision was not reached by screening the title and abstract, the reviewers examined the full text. We used Cohen’s κ statistic to calculate the inter-reviewer agreement rate for title/abstract screening. After the screening process, we further assessed all articles selected for full-text review. If no consensus was reached, we consulted a third reviewer.
Data extraction and synthesis
One reviewer independently extracted the data using a standardized form pilot-tested in ten studies. The form included details on the author and publication year, country, main study outcomes, observation period, methods for calculating incidence trends, model fit statistics (if applicable), and the software used. To ensure the robustness of the extraction process, a second reviewer cross-checked a random sample of 25% (n=36). Discrepancies were resolved by consensus agreement.
Quality assessment
We assessed the quality of all included studies using a prespecified checklist adapted for this review and based on the Joanna Briggs Institute Critical Appraisal tool for prevalence studies (13) and the Appraisal tool for Cross-Sectional Studies (14). We chose relevant criteria from each tool to create a 10-item checklist for this study (Supplementary Table 2.1). Items were assigned a score of 1 if “demonstrated in the study” or 0 if “not demonstrated or unclear”. We calculated and presented an overall score for each study, with higher scores indicating studies of higher quality. This overall quality score should be interpreted cautiously, as each quality indicator’s scores are often subjectively justified (15).
Data analysis
The general characteristics of included studies and the methods utilized to assess incidence trends were described using descriptive statistics, reported as frequencies and percentages.
Methods used to measure incidence trends
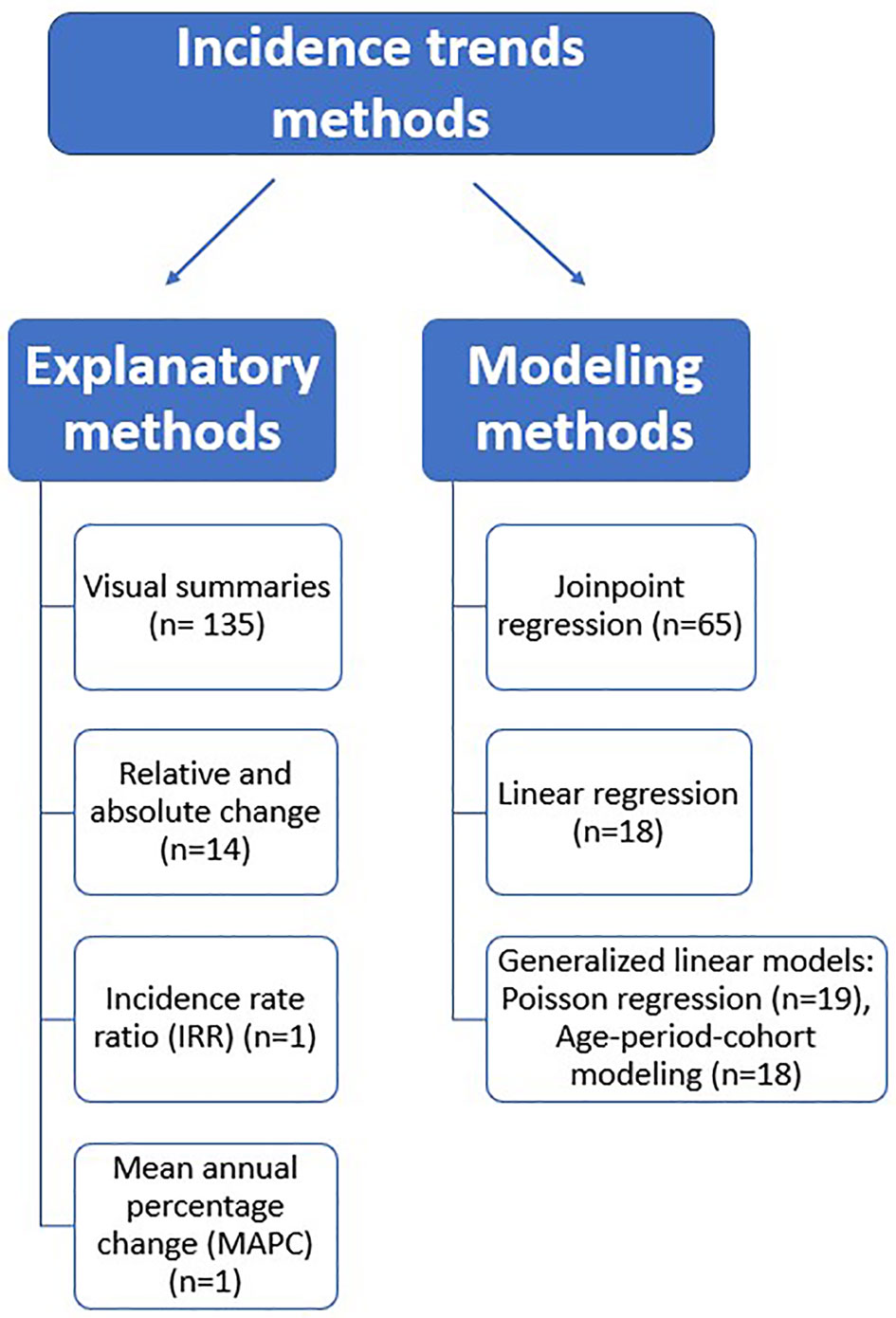
Based on the findings of this review, we classified the reported methods into explanatory and modeling methods (see Figure 2).
Explanatory methods
Our definition of explanatory methods included visual summaries (graphical and tabular presentation of trends) and simple arithmetic calculations employed to estimate incidence trends.
Modeling methods
The reviewed modeling methods used regression analysis to fit a relationship between a dependent variable (incidence rate) and an independent variable (time). This review’s most commonly reported statistical modeling methods were subdivided into the following groups: joinpoint regression, linear regression, and generalized linear models such as Poisson regression and age-period-cohort models. The choice of the modeling method will depend on the researcher’s aim. When the interest is only to examine the annual percentage change in rates, linear or Poisson regression would be an appropriate option. However, joinpoint regression would be a suitable choice when the research aims to identify points in time where rates change in direction (join points) and magnitude. Thus, joinpoint analysis is especially useful when evaluating trends over a long time period when diagnostic techniques have changed, or relevant prevention or screening interventions have been implemented. Additionally, studies focusing on disentangling the simultaneous and independent effect of age (biological processes of aging), birth cohorts (exposures/experiences that vary from one generation to the next), and period (external factors that affect all age groups similarly at a specific calendar time) on cancer incidence should use age-period cohort modeling methods. Supplementary File 2 provides a summarized description of each of the aforementioned statistical techniques.
Results
The combined search initially yielded 5,348 articles, and after removing duplicates, we identified and screened 2,906 titles. Of these, 2,667 studies were excluded at the title/abstract level, and another 94 were excluded after full-text assessment. The remaining 145 articles were included in the review. The inter-reviewer agreement for the title/abstract screening had a Cohen’s κ value of 94% (Supplementary Table 1). The PRISMA flow diagram (Figure 1) shows details of excluded reports.
Characteristics of included studies
The eligible articles comprised studies from North America, including the United States of America (n=58, 40%) and Canada (n=3, 2%), Europe (n=34, 23%), Asia (n=33, 23%), Oceania (n=6, 4%), Africa (n=3, 2%), and eight (6%) multi-country study. In addition to incidence, the two other study outcomes most commonly reported were mortality (n=35, 24%) and survival (n=30, 21%). Overall, 21 (14%) studies covered a study period of fewer than ten years, and the remaining covered ten years or more of observation. The characteristics and details of the studies (16–160) are provided in Supplementary Tables 3, 4. Detailed quality score information for all included studies is presented in Supplementary Table 2.2.
Summaries of incidence trends methods
Explanatory methods
The majority of included studies (93%) presented trends using visual summaries combined with other statistical methods. Yet, 23 (16%) studies analyzed trends through only graphical and tabular presentations of incidence rates. Garcia et al. (88) and Murphy et al. (134) reported the absolute change in incidence, representing the arithmetic difference between any two rates at different time points. Fourteen articles assessed trends by calculating the relative change in incidence over a given time interval, presented as the percentage of change in rates. None of these studies reported confidence interval estimates, and only two indicated the significance of trends (75, 140). Cheng et al. (39) and Garcia et al. (82) provided details on the calculation employed for deriving the relative change; the following formula was reported:
In one study, Nooyi et al. (87) measured trends by calculating the mean annual percentage change using the formulae:
Furthermore, Fedewa et al. (85) used incidence rate ratios to illustrate changing patterns in incidence by comparing more recent years versus earlier ones.
Statistical modeling methods
Joinpoint regression
Joinpoint regression analysis (n= 65, 45%) was the most commonly used method for assessing incidence trends by fitting a series of joined straight lines to estimate annual percentage change (APC) in incidence rates. More than half (n= 37, 57%) of the studies using this method calculated and reported only the APC. Of these, 15 (40%) reported one APC for the entire observation period, whereas 22 (59%) reported several APCs for different time segments during the study period. Eleven (17%) studies only reported the calculation of the average annual percentage change (AAPC), and 16 (25%) studies reported both the APC and AAPC. Only four studies explicitly stated the difference in calculation between the APC and AAPC. Further details on the estimated trend parameters and the number of joinpoints in the regression model are provided in Supplementary Table 5. The joinpoint trend analysis software developed by the National Cancer Institute (NCI) (161) was the only tool reported for this type of regression modeling. Examining the reporting of parameter setting in the joinpoint software revealed an overall inadequacy, with the model selection method being the most reported information (52%). Details on parameter setting reporting for the joinpoint program are provided in Supplementary Tables 4 and 5.
Linear regression modeling
Of all included studies, 18 (12%) used linear regression analysis to estimate linear trends. The majority of them (89%) reported the percentage of change in rates. Chittleborough et al. (41) reported trends as the difference per decade, and Baniasadi et al. (26) reported only the model formulae with no estimates for trends. Only nine studies indicated the method used to fit the linear regression model. The least-squares estimation method was the only reported one, and eight studies further indicated that the weighted-least squares technique was employed. None of these studies justified chosen model estimation procedure. Five studies performed a log transformation of the linear model (23, 25, 59, 105, 111). Different software was used for linear regression analysis, with SPSS (162) being the most commonly used (n=4, 22%).
Generalized linear models
Overall, 19 (13%) employed a Poisson regression model to quantify changes in incidence rates. Sixteen of these studies reported measures such as the incidence rate ratio or percentage of change to illustrate incidence trends. Abdifard et al. (17) and Abdifard et al. (16) indicated incidence trends by merely presenting the slope of the regression line. In one study, Dehghani et al. (46) explained only the significance of the incidence trend with no reporting of any other parameter indicating the pattern of rates over time. Five studies reported the use of Poisson regression to conduct age-period-cohort analysis. Only two studies (28, 63) reported consideration for dispersion, and only one (63) indicated the use of negative binomial distribution to correct overdispersion. The most commonly reported software for conducting Poisson regression was Stata (163) (n=6, 31%), followed by SAS (164) (n=5, 26%).
Age-period-cohort modeling- another type of generalized linear models - was employed to measure incidence trends in 18 (12%) studies. The model’s parameters used to estimate trends varied across these studies. The period/cohort rate ratio (ratio of rates in a specific period/cohort relative to reference period/cohort) was the most reported measure presented in 14 studies. Although reference values are usually arbitrarily chosen, nine studies in this review used the middle calendar period and birth cohort as reference categories. Chambers et al. (34) and Wessler et al. (149) took the earliest periods and cohorts as the reference, while Siegel et al. (114) chose the cohort with the lowest incidence rates. Estimations for the Local drift (age-specific net annual percentage change) and Net drift (age-adjusted annual percentage change) were indicated in seven and six studies, respectively. Supplementary Table 4 provides details of all reported parameters. The most common software for this type of modeling was the publicly available age-period-cohort analysis web tool (n=8, 44%), developed by the NCI (165).
Other methods
Models that were reported only once in the reviewed literature included: time-series analysis (144), interrupted time-series analysis (83), bayesian analysis of spatio-temporal conditional autoregressive models (142), and the LOESS method (66). Six studies reported the APC without explaining the used model to derive this estimate (31, 35, 39, 121, 145, 159).
Association between number of years covered and the statistical method chosen
We further examined the studies to identify if the number of years studied had influenced the statistical method chosen (i.e., studies covering fewer years tend to use specific methods). We found no evidence to support any connection.
Model validity measures
Thirty-nine (37%) of the studies modeling incidence trends (n=104, 72%) explicitly indicated the method used to evaluate model fitness. Of all studies that utilized joinpoint regression analysis, 34 reported the method employed to select the final model. The permutation test (166), the only technique used, was either explicitly indicated in the text or cited in the reference list. Of these 34 studies, only five clearly reported the number of joinpoints in the best-fitting model.
Additionally, six studies (21, 34, 40, 62, 114, 149) indicated other approaches to assess model fitness. Of these, one study (21) failed to report the assessment’s result, while the remaining indicated a good model fit. Detailed information on the employed model fit statistics is provided in Supplementary Table 4.
Software
Most studies (n=111, 77%) reported the software used for incidence trend analysis. The most common (52%) was the joinpoint program (161). Other reported software included SPSS (13%) (162), STATA (12%) (163), and SAS (11%) (164) (see Supplementary Table 4).
Discussion
To our knowledge, this is the first study to examine variations in the methods employed in calculating incidence trends of CRC. The 145 articles retrieved provided valuable information on the most commonly reported methods and parameters for measuring trends.
Methods used to measure incidence trends
Explanatory methods
Some studies in this review relied solely on explanatory methods to investigate trends. The exclusive use of visual summaries (graphical and tabular presentation of incidence) may lead to an erroneous and subjective interpretation of findings. We also noted that many studies that used only visual summaries covered an observation period of ten years or more. Yet, it was unclear why other methods were not used in conjunction with visual summaries.
Fourteen studies in this review reported trends as the relative change or percentage of change (PC) in rates between different periods. A positive PC corresponds to an increase in incidence rates, while a negative PC corresponds to a decreasing trend. Relative changes in incidence can be misleading because an absolute small difference can result in a significant percentage change. Therefore, it is important to provide readers with estimates of absolute and relative differences with confidence intervals to interpret incidence trends accurately.
Statistical modeling methods
This review identified different statistical modeling techniques for characterizing CRC incidence trends. The most commonly reported method was the joinpoint regression analysis using the NCI’s joinpoint program (161). Several APCs for varying periods could be generated depending on the number of joinpoints included in the model and the final selected model. Reporting the APC for each joinpoint segment provides a detailed description of how disease risk changes over time. Yet, to facilitate comparisons of incidence trends for various groups, it is essential to develop a summary measure of incidence trends that accounts for varying trends over sub-time intervals. Hence, in 2009, Clegg et al. (167) proposed the average annual percentage change (AAPC) as a summary measure of trends, computed as a weighted average of the slope coefficients of the joinpoint regression line, with weights corresponding to the length of each subinterval. The APC and AAPC have different interpretations, and thus, it is emphasized that both should be reported, if possible, to provide a comprehensive analysis of trends. The calculation of the AAPC has been incorporated into the joinpoint trend analysis Software (161).
In this review, 15 studies reported only one APC over the entire study period. These studies did not clarify why only a single APC was estimated, whether segmented analysis was not possible -due to the number of data points included- or has yielded insignificant findings, or if this measure reflects the AAPC. Eleven articles reported the AAPC without indicating if the final selected model provided APCs for different time segments, which would have provided an enhanced description of trends. Most studies failed to explain the interpretation of the AAPC, what it represents, and the calculation differences between the APC and AAPC. Providing the reader with a clear description of these parameters and their meaning is vital for understanding trends, reproducing findings, and making potential comparisons in future studies. Furthermore, this review highlighted inadequate reporting on the parameters set in the joinpoint program. Such details are essential for replicating the analysis or justifying when the researcher’s findings differ from previously published trends estimates using the same data source.
Poisson regression was the second most reported method in this review. Our results indicated underreporting of the verification of model assumptions concerning dispersion. Authors should inform readers if any model assumptions did not hold and how it was handled in the analysis.
When exploring cancer burden, disentangling the effects of age, cohort, and period is vital for a comprehensive analysis and understanding of incidence trends. Due to issues related to data availability and concerns about the statistical interpretability of age, period, and cohort analysis, researchers’ uptake and interest in this type of assessment were limited. To facilitate the conduction of this analysis, Rosenberg et al. (165) developed a freely available and easy-to-use web tool that provides researchers with a panel of estimable functions for age-period-cohort analysis. This tool was the most used in this review. Furthermore, we noted that nine studies used age-period-cohort modeling and joinpoint regression to analyze their data. This analysis approach of combining methods is imperative for strengthening the analysis, revealing emerging cancer trends, and enhancing our understanding of cancer etiology and natural history.
Among all studies that assessed and reported CRC incidence trends via statistical modeling, less than half reported model fit statistics in this review. Most of them focused on documenting the method used without further explanation of the model fit analysis. Examining model fitness is one aspect of assessing the statistical model’s validity; it is defined as “a measure of the discrepancy between the observed empirical distribution of the observations in the data set and the ‘best-fitting’ probability distribution computed from the estimated probability model” (8). Model fit statistics might include graphical assessment such as residual plots or quantitative evaluation such as log-likelihood tests and goodness-of-fit measures (8, 9). Despite the used methods, authors should provide details on model fit statistics in the manuscript or as supplemental or web-based data. Ensuring transparency by providing sufficient information on the modeling building procedure will support an accurate interpretation of the research findings and facilitate future analysis replication.
To our knowledge, this study is the first to review the methods employed to estimate incidence trends across different populations and settings. In cancer studies, the quality and reliability of the cancer registry data are essential for evaluating cancer trends. It was not within the scope of the current review to examine data quality reporting; yet, in a previous publication, we assessed reporting quality in CRC incidence studies and noted a substantial deficit in reporting registry-data quality control procedures and findings (10).
This review was limited to studies assessing the incidence of CRC using registry data. Thus, we might have missed other trend analysis methods used to analyze different data sources and diseases in the last decade. Despite this, our results inform well about a variety of commonly used incidence trends methods and thus support future researchers in choosing potential methods and parameters that will enhance the comparability of their research. Although we searched multiple databases and included studies from different countries, we included only English articles in this review. Thus, we might have missed relevant papers in other languages.
Conclusion
This review described the most commonly reported methods for measuring CRC incidence trends over the past decade. Visual summaries are always a good starting point for observing trends, preferably followed by modeling. Joinpoint regression was the most reported method, identifying points in time where incidence rates change. We also noted an increased uptake of age-period-cohort modeling to disentangle the effect of age, period, and birth cohort on incidence trends. Our findings highlighted the need for increased clarity and transparency in reporting incidence trends methods to facilitate interpretation and comparison of results with previous studies and help identify and address limitations of the analysis.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
NA, MP-R, RW, and FS conceived the idea for this review and the research questions. NA developed and conducted the search, screened the studies, performed data extraction and analysis, and wrote the first draft. AA participated in the screening process. SA participated in data extraction. RW, CB, and FS provided overall supervision and critically appraised the results. All authors contributed to the article and approved the submitted version.
Funding
This study is part of Norah Alsadhan’s PhD study, funded by King Saud University, Riyadh, Kingdom of Saudi Arabia.
Acknowledgments
The authors wish to acknowledge Natalie King (Information Specialist, University of Leeds) for her help with the development of the search strategy for this systematic review.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1049486/full#supplementary-material
Abbreviations
APC, Annual percentage change; AAPC, Average annual percentage change; CRC, Colorectal cancer; NCI, National Cancer Institute; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; PC, Percentage of change; SAS, Statistical Analysis System; SPSS Statistical Package for The Social Sciences.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Gorin SS. Multilevel approaches to reducing diagnostic and treatment delay in colorectal cancer. Ann Fam Med (2019) 17(5):386–9. doi: 10.1370/afm.2454
3. Schreuders EH, Ruco A, Rabeneck L, Schoen RE, Sung JJ, Young GP, et al. Colorectal cancer screening: a global overview of existing programmes. Gut (2015) 64(10):1637–49. doi: 10.1136/gutjnl-2014-309086
4. Wolf AMD, Fontham ETH, Church TR, Flowers CR, Guerra CE, LaMonte SJ, et al. Colorectal cancer screening for average-risk adults: 2018 guideline update from the American cancer society. CA: A Cancer J Clin (2018) 68(4):250–81. doi: 10.3322/caac.21457
5. Bénard F, Barkun AN, Martel M, von Renteln D. Systematic review of colorectal cancer screening guidelines for average-risk adults: Summarizing the current global recommendations. World J gastroenterology (2018) 24(1):124–38. doi: 10.3748/wjg.v24.i1.124
6. Fay MP, Tiwari RC, Feuer EJ, Zou Z. Estimating average annual percent change for disease rates without assuming constant change. Biometrics (2006) 62(3):847–54. doi: 10.1111/j.1541-0420.2006.00528.x
7. Rosenberg PS, Anderson WF. Age-period-cohort models in cancer surveillance research: ready for prime time? Cancer Epidemiol Biomarkers Prev (2011) 20(7):1263–8. doi: 10.1158/1055-9965.EPI-11-0421
8. Henley SS, Golden RM, Kashner TM. Statistical modeling methods: challenges and strategies. Biostatistics Epidemiol (2020) 4(1):105–39. doi: 10.1080/24709360.2019.1618653
9. Eddy DM, Hollingworth W, Caro JJ, Tsevat J, McDonald KM, Wong JB. Model transparency and validation: a report of the ISPOR-SMDM modeling good research practices task force-7. Med Decis Making (2012) 32(5):733–43. doi: 10.1177/0272989X12454579
10. Alsadhan N, Almaiman A, Pujades-Rodriguez M, Brennan C, Shuweihdi F, Alhurishi SA, et al. A systematic review of methods to estimate colorectal cancer incidence using population-based cancer registries. BMC Med Res Methodology (2022) 22(1):144. doi: 10.1186/s12874-022-01632-7
11. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ (2021) 372:n71. doi: 10.1136/bmj.n71
12. Ouzzani M, Hammady H, Fedorowicz Z, Elmagarmid A. Rayyan–a web and mobile app for systematic reviews. Systematic Rev (2016) 5(1):210. doi: 10.1186/s13643-016-0384-4
13. Munn Z, Moola S, Riitano D, Lisy K. The development of a critical appraisal tool for use in systematic reviews addressing questions of prevalence. Int J Health Policy Manage (2014) 3(3):123–8. doi: 10.15171/ijhpm.2014.71
14. Downes MJ, Brennan ML, Williams HC, Dean RS. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS). BMJ Open (2016) 6(12):e011458. doi: 10.1136/bmjopen-2016-011458
15. Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Cochrane handbook for systematic reviews of interventions version 5.2.0 (updated June 2017). Cochrane (2017). Available at: www.training.cochrane.org/handbook.
16. Abdifard E, Amini S, Bab S, Masroor N, Khachian A, Heidari M. Incidence trends of colorectal cancer in Iran during 2000-2009: A population-based study. Med J Islam Repub Iran (2016) 30:382.
17. Abdifard E, Ghaderi S, Hosseini S, Heidari M. Incidence trends of colorectal cancer in the West of Iran during 2000-2005. Asian Pac J Cancer Prev (2013) 14(3):1807–11. doi: 10.7314/apjcp.2013.14.3.1807
18. Abreu MH, Matos E, Castro Pocas F, Rocha R, Pinto J, Lopes C. Staging and survival of rectal cancer in Vila Nova de gaia, Portugal. Eur J Gastroenterol Hepatol (2010) 22(2):151–6. doi: 10.1097/MEG.0b013e3283307c5c
19. Al Dahhan SA, Al Lami FH. Epidemiology of colorectal cancer in Iraq, 2002-2014. Gulf J Oncolog (2018) 1(26):23–6.
20. Araghi M, Fidler MM, Arnold M, Jemal A, Bray F, Soerjomataram I. The future burden of colorectal cancer among US blacks and whites. J Natl Cancer Inst (2018) 110(7):791–3. doi: 10.1093/jnci/djx287
21. Araghi M, Soerjomataram I, Bardot A, Ferlay J, Cabasag CJ, Morrison DS, et al. Changes in colorectal cancer incidence in seven high-income countries: a population-based study. Lancet Gastroenterol Hepatology (2019) 4(7):511–8. doi: 10.1016/S2468-1253(19)30147-5
22. Ashktorab H, Vilmenay K, Brim H, Laiyemo AO, Kibreab A, Nouraie M. Colorectal cancer in young African americans: Is it time to revisit guidelines and prevention? Dig Dis Sci (2016) 61(10):3026–30. doi: 10.1007/s10620-016-4207-1
23. Austin H, Henley SJ, King J, Richardson LC, Eheman C. Changes in colorectal cancer incidence rates in young and older adults in the united states: what does it tell us about screening. Cancer Causes Control (2014) 25(2):191–201. doi: 10.1007/s10552-013-0321-y
24. Aziz H, Pandit V, DiGiovanni RM, Ohlson E, Gruessner AC, Jandova J, et al. Increased incidence of early onset colorectal cancer in Arizona: A comprehensive 15-year analysis of the Arizona cancer registry. J Gastrointest Dig Syst (2015) 5(5):345. doi: 10.4172/2161-069X.1000345
25. Bailey CE, Hu CY, You YN, Bednarski BK, Rodriguez-Bigas MA, Skibber JM, et al. Increasing disparities in the age-related incidences of colon and rectal cancers in the united states, 1975-2010. JAMA Surg (2015) 150(1):17–22. doi: 10.1001/jamasurg.2014.1756
26. Baniasadi N, Moghtader E, Khajehkazemi R, Mohebbi E. Increasing trend in colorectal cancer incidence in the southeast of Iran 2003-2013: A population based cancer registry study. Asian Pac J Cancer Prev (2015) 16(13):5257–60. doi: 10.7314/apjcp.2015.16.13.5257
27. Bhurgri Y, Khan T, Kayani N, Ahmad R, Usman A, Bhurgri A, et al. Incidence and current trends of colorectal malignancies in an unscreened, low risk Pakistan population. Asian Pac J Cancer Prev (2011) 12(3):703–8.
28. Boyce S, Nassar N, Lee CY, Suen MK, Al Zahrani S, Gladman MA. Young-onset colorectal cancer in new south Wales: a population-based study. Med J Aust (2016) 205(10):465–70. doi: 10.5694/mja16.00237
29. Braendegaard Winther S, Baatrup G, Pfeiffer P, Qvortrup C. Academy of geriatric cancer r. trends in colorectal cancer in the elderly in Denmark, 1980-2012. Acta Oncol (2016) 55(Suppl 1):29–39. doi: 10.3109/0284186X.2015.1114674
30. Brenner H, Schrotz-King P, Holleczek B, Katalinic A, Hoffmeister M. Declining bowel cancer incidence and mortality in Germany. Dtsch Arztebl Int (2016) 113(7):101–6. doi: 10.3238/arztebl.2016.0101
31. Brouwer NPM, Bos A, Lemmens V, Tanis PJ, Hugen N, Nagtegaal ID, et al. An overview of 25 years of incidence, treatment and outcome of colorectal cancer patients. Int J Cancer (2018) 143(11):2758–66. doi: 10.1002/ijc.31785
32. Caldarella A, Crocetti E, Messerini L, Paci E. Trends in colorectal incidence by anatomic subsite from 1985 to 2005: a population-based study. Int J Colorectal Dis (2013) 28(5):637–41. doi: 10.1007/s00384-013-1672-2
33. Carroll R, Zhao S. Trends in colorectal cancer incidence and survival in Iowa SEER data: The timing of it all. Clin Colorectal Cancer (2019) 18(2):e261–74. doi: 10.1016/j.clcc.2018.12.001
34. Chambers AC, Dixon SW, White P, Williams AC, Thomas MG, Messenger DE. Demographic trends in the incidence of young-onset colorectal cancer: a population-based study. Br J Surg (2020) 107(5):595–605. doi: 10.1002/bjs.11486
35. Chatterjee S, Chattopadhyay A, Levine PH. Between-ward disparities in colorectal cancer incidence and screening in Washington DC. J Epidemiol Glob Health (2015) 5(4 Suppl 1):S1–9. doi: 10.1016/j.jegh.2015.08.001
36. Alsanea N, Abduljabbar AS, Alhomoud S, Ashari LH, Hibbert D, Bazarbashi S. Colorectal cancer in Saudi Arabia: incidence, survival, demographics and implications for national policies. Ann Saudi Med (2015) 35(3):196–202. doi: 10.5144/0256-4947.2015.196
37. Chauvenet M, Cottet V, Lepage C, Jooste V, Faivre J, Bouvier AM. Trends in colorectal cancer incidence: a period and birth-cohort analysis in a well-defined French population. BMC Cancer (2011) 11:282. doi: 10.1186/1471-2407-11-282
38. Chen TA, Kang HY, Chang HC, Lin WC, Chao TM, Horng JT. Gender differences in colorectal cancer during the past 20 years in Taiwan. Int J Colorectal Dis (2012) 27(3):345–53. doi: 10.1007/s00384-011-1318-1
39. Cheng L, Eng C, Nieman LZ, Kapadia AS, Du XL. Trends in colorectal cancer incidence by anatomic site and disease stage in the united states from 1976 to 2005. Am J Clin Oncol (2011) 34(6):573–80. doi: 10.1097/COC.0b013e3181fe41ed
40. Chernyavskiy P, Kennerley VM, Jemal A, Little MP, Rosenberg PS. Heterogeneity of colon and rectum cancer incidence across 612 SEER counties, 2000-2014. Int J Cancer (2019) 144(8):1786–95. doi: 10.1002/ijc.31776
41. Chittleborough TJ, Gutlic I, Pearson JF, Watson A, Bhatti LA, Buchwald P, et al. Increasing incidence of young-onset colorectal carcinoma a 3-country population analysis. Dis Colon Rectum (2020) 63(7):903–10. doi: 10.1097/DCR.0000000000001631
42. Chong VH, Telisinghe PU, Bickle I, Abdullah MS, Lim E, Chong CF. Increasing incidence of colorectal cancer, starting at a younger age for rectal compared to colon cancer in Brunei darussalam. Asian Pac J Cancer Prev (2015) 16(12):5063–7. doi: 10.7314/apjcp.2015.16.12.5063
43. Clarke N, McDevitt J, Kearney PM, Sharp L. Increasing late stage colorectal cancer and rectal cancer mortality demonstrates the need for screening: a population based study in Ireland, 1994-2010. BMC Gastroenterol (2014) 14:92. doi: 10.1186/1471-230X-14-92
44. Crocetti E, Buzzoni C, Zappa M. Colorectal cancer incidence rates have decreased in central Italy. Eur J Cancer Prev (2010) 19(6):424–5. doi: 10.1097/CEJ.0b013e32833b48b6
45. Crosbie AB, Roche LM, Johnson LM, Pawlish KS, Paddock LE, Stroup AM. Trends in colorectal cancer incidence among younger adults-disparities by age, sex, race, ethnicity, and subsite. Cancer Med (2018) 7(8):4077–86. doi: 10.1002/cam4.1621
46. Dehghani SL, Rezaianzadeh A, Safe M, Tabatabaee H. Trends of incidence of colorectal cancer in Iran. 2003–2010: Rev Latinoamericana Hiperten; (2019) 14(4):456–61. doi: 170263002017
47. Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, et al. Annual report to the nation on the status of cancer, 1975-2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer (2010) 116(3):544–73. doi: 10.1002/cncr.24760
48. Ellis L, Abrahao R, McKinley M, Yang J, Somsouk M, Marchand LL, et al. Colorectal cancer incidence trends by age, stage, and Racial/Ethnic group in California, 1990-2014. Cancer Epidemiol Biomarkers Prev (2018) 27(9):1011–8. doi: 10.1158/1055-9965.EPI-18-0030
49. Eser S, Chang J, Charalambous H, Silverman B, Demetriou A, Yakut C, et al. Incidence patterns of colorectal cancers in four countries of the middle East cancer consortium (Cyprus, Jordan, Israel, and izmir, Turkey) compared with those in the united states surveillance, epidemiology, and end results program. Turk J Gastroenterol (2018) 29(1):36–44. doi: 10.5152/tjg.2018.17263
50. Exarchakou A, Donaldson LJ, Girardi F, Coleman MP. Colorectal cancer incidence among young adults in England: Trends by anatomical sub-site and deprivation. PloS One (2019) 14(12):e0225547. doi: 10.1371/journal.pone.0225547
51. Feletto E, Yu XQ, Lew JB, St John DJB, Jenkins MA, Macrae FA, et al. Trends in colon and rectal cancer incidence in Australia from 1982 to 2014: Analysis of data on over 375,000 cases. Cancer Epidemiol Biomarkers Prev (2019) 28(1):83–90. doi: 10.1158/1055-9965.EPI-18-0523
52. Hasanpour-Heidari S, Fazel A, Semnani S, Khandoozi SR, Amiriani T, Sedaghat S, et al. Temporal and geographical variations in colorectal cancer incidence in northern Iran 2004-2013. Cancer Epidemiol (2019) 59:143–7. doi: 10.1016/j.canep.2019.02.003
53. Lemmens V, van Steenbergen L, Janssen-Heijnen M, Martijn H, Rutten H, Coebergh JW. Trends in colorectal cancer in the south of the Netherlands 1975-2007: rectal cancer survival levels with colon cancer survival. Acta Oncol (2010) 49(6):784–96. doi: 10.3109/02841861003733713
54. May FP, Glenn BA, Crespi CM, Ponce N, Spiegel BMR, Bastani R. Decreasing black-white disparities in colorectal cancer incidence and stage at presentation in the united states. Cancer Epidemiol Biomarkers Prev (2017) 26(5):762–8. doi: 10.1002/cncr.31076
55. Klugarova J, Klugar M, Muzik J, Jarkovsky J, Licenik R, Burilova P, et al. Use of epidemiological analyses in development of colorectal cancer clinical practice guidelines in the Czech republic. Int J Evid Based Healthc (2019) 17(Suppl 1):S57–61. doi: 10.1097/XEB.0000000000000187
56. Koblinski J, Jandova J, Nfonsam V. Disparities in incidence of early- and late-onset colorectal cancer between hispanics and whites: A 10-year SEER database study. Am J Surg (2018) 215(4):581–5. doi: 10.1016/j.amjsurg.2017.03.035
57. Martinsen RP, Morris CR, Pinheiro PS, Parikh-Patel A, Kizer KW. Colorectal cancer trends in California and the need for greater screening of Hispanic men. Am J Prev Med (2016) 51(6):e155–63. doi: 10.1016/j.amepre.2016.05.019
58. Giddings BH, Kwong SL, Parikh-Patel A, Bates JH, Snipes KP. Going against the tide: increasing incidence of colorectal cancer among koreans, filipinos, and south asians in California, 1988-2007. Cancer Causes Control (2012) 23(5):691–702. doi: 10.1007/s10552-012-9937-6
59. Missaoui N, Jaidaine L, Abdelkader AB, Trabelsi A, Mokni M, Hmissa S. Colorectal cancer in central Tunisia: increasing incidence trends over a 15-year period. Asian Pac J Cancer Prev (2011) 12(4):1073–6.
60. Kelly JJ, Alberts SR, Sacco F, Lanier AP. Colorectal cancer in alaska native people, 2005-2009. Gastrointest Cancer Res (2012) 5(5):149–54.
61. Loomans-Kropp HA, Umar A. Increasing incidence of colorectal cancer in young adults. J Cancer Epidemiol (2019) 2019:9841295. doi: 10.1155/2019/9841295
62. Gandhi J, Davidson C, Hall C, Pearson J, Eglinton T, Wakeman C, et al. Population-based study demonstrating an increase in colorectal cancer in young patients. Br J Surg (2017) 104(8):1063–8. doi: 10.1002/bjs.10518
63. Lopez-Abente G, Ardanaz E, Torrella-Ramos A, Mateos A, Delgado-Sanz C, Chirlaque MD, et al. Changes in colorectal cancer incidence and mortality trends in Spain. Ann Oncol (2010) 21(Suppl 3):iii76–82. doi: 10.1093/annonc/mdq091
64. McClements PL, Madurasinghe V, Thomson CS, Fraser CG, Carey FA, Steele RJ, et al. Impact of the UK colorectal cancer screening pilot studies on incidence, stage distribution and mortality trends. Cancer Epidemiol (2012) 36(4):e232–42. doi: 10.1016/j.canep.2012.02.006
65. Ladabaum U, Clarke CA, Press DJ, Mannalithara A, Myer PA, Cheng I, et al. Colorectal cancer incidence in Asian populations in California: effect of nativity and neighborhood-level factors. Am J Gastroenterol (2014) 109(4):579–88. doi: 10.1038/ajg.2013.488
66. Fowler B, Samadder NJ, Kepka D, Ding Q, Pappas L, Kirchhoff AC. Improvements in colorectal cancer incidence not experienced by nonmetropolitan women: A population-based study from Utah. J Rural Health (2018) 34(2):155–61. doi: 10.1111/jrh.12242
67. Meester RGS, Mannalithara A, Lansdorp-Vogelaar I, Ladabaum U. Trends in incidence and stage at diagnosis of colorectal cancer in adults aged 40 through 49 years, 1975-2015. Jama (2019) 321(19):1933–4. doi: 10.1001/jama.2019.3076
68. Li Z, Yang L, Du C, Fang X, Wang N, Gu J. Characteristics and comparison of colorectal cancer incidence in Beijing with other regions in the world. Oncotarget (2017) 8(15):24593–603. doi: 10.18632/oncotarget.15598
69. Jayarajah U, Udayanga V, Fernando A, Samarasekera DN, Seneviratne S. The incidence and patterns of colorectal cancers in Sri Lanka from 2001 to 2010: Analysis of national cancer registry data. Eur J Cancer Care (Engl) (2020) 29(4):e13247. doi: 10.1111/ecc.13247
70. Katsidzira L, Chokunonga E, Gangaidzo IT, Rusakaniko S, Borok M, Matsena-Zingoni Z, et al. The incidence and histo-pathological characteristics of colorectal cancer in a population based cancer registry in Zimbabwe. Cancer Epidemiol (2016) 44:96–100. doi: 10.1016/j.canep.2016.08.001
71. Lee Y-C, Hsu C-Y, Chen SL-S, Yen AM-F, Chiu SY-H, Fann JC-Y, et al. Effects of screening and universal healthcare on long-term colorectal cancer mortality. Int J Epidemiol (2019) 48(2):538–48. doi: 10.1093/ije/dyy182
72. Merrill RM, Anderson AE. Risk-adjusted colon and rectal cancer incidence rates in the united states. Dis Colon Rectum (2011) 54(10):1301–6. doi: 10.5114/aoms.2011.24138
73. Klimczak A, Kempinska-Miroslawska B, Mik M, Dziki L, Dziki A. Incidence of colorectal cancer in Poland in 1999-2008. Arch Med Sci (2011) 7(4):673–8. doi: 10.5114/aoms.2011.24138
74. Khiari H, Ben Ayoub HW, Ben Khadhra H, Hsairi M. Colorectal cancer incidence trend and projections in Tunisia (1994 - 2024). Asian Pac J Cancer Prev (2017) 18(10):2733–9. doi: 10.22034/APJCP.2017.18.10.2733
75. Jandova J, Ohlson E, Torres BSM, DiGiovanni R, Pandit V, Elquza E, et al. Racial disparities and socioeconomic status in the incidence of colorectal cancer in Arizona. Am J Surg (2016) 212(3):485–92. doi: 10.1016/j.amjsurg.2015.08.024
76. Li K, Lin GZ, Li Y, Dong H, Xu H, Song SF, et al. Spatio-temporal analysis of the incidence of colorectal cancer in guangzhou, 2010-2014. Chin J Cancer (2017) 36(1):60. doi: 10.1186/s40880-017-0231-6
77. Meza R, Jeon J, Renehan AG, Luebeck EG. Colorectal cancer incidence trends in the united states and united kingdom: evidence of right- to left-sided biological gradients with implications for screening. Cancer Res (2010) 70(13):5419–29. doi: 10.1158/0008-5472.CAN-09-4417
78. Jafri NS, Gould M, El-Serag HB, Duan Z, Davila JA. Incidence and survival of colorectal cancer among hispanics in the united states: a population-based study. Dig Dis Sci (2013) 58(7):2052–60. doi: 10.1007/s10620-012-2454-3
79. McDevitt J, Comber H, Walsh PM. Colorectal cancer incidence and survival by sub-site and stage of diagnosis: a population-based study at the advent of national screening. Ir J Med Sci (2017) 186(1):113–21. doi: 10.1007/s11845-016-1513-8
80. Khachfe HH, Salhab HA, Fares MY, Khachfe HM. Probing the colorectal cancer incidence in Lebanon: an 11-year epidemiological study. J Gastrointest Cancer (2020) 51(3):805–12. doi: 10.1007/s12029-019-00284-z
81. Meyer JE, Narang T, Schnoll-Sussman FH, Pochapin MB, Christos PJ, Sherr DL. Increasing incidence of rectal cancer in patients aged younger than 40 years: an analysis of the surveillance, epidemiology, and end results database. Cancer (2010) 116(18):4354–9. doi: 10.1002/cncr.25432
82. Garcia S, Pruitt SL, Singal AG, Murphy CC. Colorectal cancer incidence among hispanics and non-Hispanic whites in the united states. Cancer Causes Control (2018) 29(11):1039–46. doi: 10.1007/s10552-018-1077-1
83. Brenner DR, Ruan Y, Shaw E, De P, Heitman SJ, Hilsden RJ. Increasing colorectal cancer incidence trends among younger adults in Canada. Prev Med (2017) 105:345–9. doi: 10.1016/j.ypmed.2017.10.007
84. Brenner DR, Heer E, Sutherland RL, Ruan Y, Tinmouth J, Heitman SJ, et al. National trends in colorectal cancer incidence among older and younger adults in Canada. JAMA Netw Open (2019) 2(7):e198090. doi: 10.1001/jamanetworkopen.2019.8090
85. Fedewa SA, Siegel RL, Jemal A. Are temporal trends in colonoscopy among young adults concordant with colorectal cancer incidence? J Med Screen (2019) 26(4):179–85. doi: 10.1177/0969141319859608
86. Melnitchouk N, Shabat G, Lu P, Lyu H, Scully R, Leung K, et al. Colorectal cancer in Ukraine: Regional disparities and national trends in incidence, management, and mortality. J Glob Oncol (2018) 4:1–8. doi: 10.1200/JGO.18.00145
87. Nooyi SC, Murthy NS, Shivananjaiah S, Sreekantaiah P, Mathew A. Trends in rectal cancer incidence–Indian scenario. Asian Pac J Cancer Prev (2011) 12(8):2001–6.
88. Siegel RL, Torre LA, Soerjomataram I, Hayes RB, Bray F, Weber TK, et al. Global patterns and trends in colorectal cancer incidence in young adults. Gut (2019) 68(12):2179–85. doi: 10.1136/gutjnl-2019-319511
89. Al-Zalabani A. Preventability of colorectal cancer in Saudi Arabia: Fraction of cases attributable to modifiable risk factors in 2015-2040. Int J Environ Res Public Health (2020) 17(1):320. doi: 10.3390/ijerph17010320
90. Augustus GJ, Roe DJ, Jacobs ET, Lance P, Ellis NA. Is increased colorectal screening effective in preventing distant disease? PloS One (2018) 13(7):e0200462. doi: 10.1371/journal.pone.0200462
91. Davis DM, Marcet JE, Frattini JC, Prather AD, Mateka JJ, Nfonsam VN. Is it time to lower the recommended screening age for colorectal cancer? J Am Coll Surg (2011) 213(3):352–61. doi: 10.1016/j.jamcollsurg.2011.04.033
92. Domati F, Maffei S, Kaleci S, Di Gregorio C, Pedroni M, Roncucci L, et al. Incidence, clinical features and possible etiology of early onset (</=40 years) colorectal neoplasms. Intern Emerg Med (2014) 9(6):623–31. doi: 10.1007/s11739-013-0981-3
93. Koblinski J, Jandova J, Pandit V, Omesiete P, Nfonsam V. Disparities in colon and rectal cancer queried individually between hispanics and whites. J Gastrointest Oncol (2019) 10(4):632–40. doi: 10.21037/jgo.2019.02.08
94. Vuik FE, Nieuwenburg SA, Bardou M, Lansdorp-Vogelaar I, Dinis-Ribeiro M, Bento MJ, et al. Increasing incidence of colorectal cancer in young adults in Europe over the last 25 years. Gut (2019) 68(10):1820–6. doi: 10.1136/gutjnl-2018-317592
95. Shafqat H, Ali S, Salhab M, Olszewski AJ. Survival of patients with neuroendocrine carcinoma of the colon and rectum: a population-based analysis. Dis Colon Rectum (2015) 58(3):294–303. doi: 10.1097/DCR.0000000000000298
96. Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA Cancer J Clin (2017) 67(3):177–93. doi: 10.3322/caac.21395
97. Savijarvi S, Seppa K, Malila N, Pitkaniemi J, Heikkinen S. Trends of colorectal cancer incidence by education and socioeconomic status in Finland. Acta Oncol (2019) 58(11):1557–63. doi: 10.1080/0284186X.2019.1652340
98. Rahman R, Schmaltz C, Jackson CS, Simoes EJ, Jackson-Thompson J, Ibdah JA. Increased risk for colorectal cancer under age 50 in racial and ethnic minorities living in the united states. Cancer Med (2015) 4(12):1863–70. doi: 10.1002/cam4.560
99. Nfonsam VN, Vijayasekaran A, Pandit V, Vera E, Aziz H, Nzuonkwelle S, et al. Patients diagnosed with colorectal cancer in rural areas in Arizona typically present with higher stage disease. J Gastrointest Dig Syst (2015) 5(5):346. doi: 10.4172/2161-069X.1000346
100. Van Beck KC, Jasek J, Roods K, Brown JJ, Farley SM, List JM. Colorectal cancer incidence and mortality rates among new York city adults ages 20-54 years during 1976-2015. JNCI Cancer Spectr (2018) 2(4):pky048. doi: 10.1093/jncics/pky048
101. Wong MCS, Huang J, Lok V, Wang J, Fung F, Ding H, et al. Differences in incidence and mortality trends of colorectal cancer worldwide based on sex, age, and anatomic location. Clin Gastroenterol Hepatol (2021) 19(5):955–966.e61. doi: 10.1016/j.cgh.2020.02.026
102. Mosli MH, Al-Ahwal MS. Colorectal cancer in the kingdom of Saudi Arabia: need for screening. Asian Pac J Cancer Prev (2012) 13(8):3809–13. doi: 10.7314/apjcp.2012.13.8.3809
103. Mosli MH, Al-Ahwal MS. Does the increasing trend of colorectal cancer incidence in jeddah reflect a rise in the kingdom of Saudi Arabia? Asian Pac J Cancer Prev (2012) 13(12):6285–8. doi: 10.7314/apjcp.2012.13.12.6285
104. Russo AG, Andreano A, Sartore-Bianchi A, Mauri G, Decarli A, Siena S. Increased incidence of colon cancer among individuals younger than 50 years: A 17 years analysis from the cancer registry of the municipality of Milan, Italy. Cancer Epidemiol (2019) 60:134–40. doi: 10.1016/j.canep.2019.03.015
105. Sheneman DW, Finch JL, Messersmith WA, Leong S, Goodman KA, Davis SL, et al. The impact of young adult colorectal cancer: incidence and trends in Colorado. Colorectal Cancer (2017) 6(2):49–56. doi: 10.2217/crc-2017-0008
106. Oliphant R, Brewster DH, Morrison DS. The changing association between socioeconomic circumstances and the incidence of colorectal cancer: a population-based study. Br J Cancer (2011) 104(11):1791–6. doi: 10.1038/bjc.2011.149
107. Perdue DG, Haverkamp D, Perkins C, Daley CM, Provost E. Geographic variation in colorectal cancer incidence and mortality, age of onset, and stage at diagnosis among American Indian and Alaska native people, 1990-2009. Am J Public Health (2014) 104(Suppl 3):S404–14. doi: 10.2105/AJPH.2013.301654
108. Murphy CC, Sandler RS, Sanoff HK, Yang YC, Lund JL, Baron JA. Decrease in incidence of colorectal cancer among individuals 50 years or older after recommendations for population-based screening. Clin Gastroenterol Hepatol (2017) 15(6):903–9.e6. doi: 10.1016/j.cgh.2016.08.037
109. Shah AB, Sarfati D, Blakely T, Atkinson J, Dennett ER. Trends in colorectal cancer incidence rates in new Zealand, 1981-2004. ANZ J Surg (2012) 82(4):258–64. doi: 10.1111/j.1445-2197.2011.05995.x
110. Siegel RL, Miller KD, Goding Sauer A, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA Cancer J Clin (2020) 70(3):145–64. doi: 10.3322/caac.21601
111. Shin A, Kim KZ, Jung KW, Park S, Won YJ, Kim J, et al. Increasing trend of colorectal cancer incidence in Korea, 1999-2009. Cancer Res Treat (2012) 44(4):219–26. doi: 10.4143/crt.2012.44.4.219
112. Patel P, De P. Trends in colorectal cancer incidence and related lifestyle risk factors in 15-49-year-olds in Canada, 1969-2010. Cancer Epidemiol (2016) 42:90–100. doi: 10.1016/j.canep.2016.03.009
113. Vardanjani HM, Haghdoost A, Bagheri-Lankarani K, Hadipour M. Estimation and projection of prevalence of colorectal cancer in Iran, 2015-2020. Adv BioMed Res (2018) 7:20. doi: 10.4103/abr.abr_178_16
114. Siegel RL, Fedewa SA, Anderson WF, Miller KD, Ma J, Rosenberg PS, et al. Colorectal cancer incidence patterns in the united states, 1974-2013. J Natl Cancer Inst (2017) 109(8):djw322. doi: 10.1093/jnci/djw322
115. Pescatore P, Scheiden R, Abeywickrama KH, Braun M, Capesius C. Evolution of colorectal cancer epidemiology in a setting of opportunistic screening. a 20 year national survey in Luxembourg. Acta Gastroenterol Belg (2013) 76(1):25–33.
116. Murphy CC, Singal AG, Baron JA, Sandler RS. Decrease in incidence of young-onset colorectal cancer before recent increase. Gastroenterology (2018) 155(6):1716–9.e4. doi: 10.1053/j.gastro.2018.07.045
117. Siegel RL, Ward EM, Jemal A. Trends in colorectal cancer incidence rates in the united states by tumor location and stage, 1992-2008. Cancer Epidemiol Biomarkers Prev (2012) 21(3):411–6. doi: 10.1158/1055-9965.EPI-11-1020
118. Siegel RL, Medhanie GA, Fedewa SA, Jemal A. State variation in early-onset colorectal cancer in the united states, 1995-2015. J Natl Cancer Inst (2019) 111(10):1104–6. doi: 10.1093/jnci/djz098
119. Sung JJY, Chiu HM, Jung KW, Jun JK, Sekiguchi M, Matsuda T, et al. Increasing trend in young-onset colorectal cancer in Asia: More cancers in men and more rectal cancers. Am J Gastroenterol (2019) 114(2):322–9. doi: 10.14309/ajg.0000000000000133
120. Rafiemanesh H, Pakzad R, Abedi M, Kor Y, Moludi J, Towhidi F, et al. Colorectal cancer in Iran: Epidemiology and morphology trends. EXCLI J (2016) 15:738–44. doi: 10.17179/excli2016-346
121. Sierra MS, Forman D. Burden of colorectal cancer in central and south America. Cancer Epidemiol (2016) 44(Suppl 1):S74–81. doi: 10.1016/j.canep.2016.03.010
122. Zhu J, Tan Z, Hollis-Hansen K, Zhang Y, Yu C, Li Y. Epidemiological trends in colorectal cancer in China: An ecological study. Dig Dis Sci (2017) 62(1):235–43. doi: 10.1007/s10620-016-4362-4
123. Palmieri G, Paliogiannis P, Scognamillo F, Budroni M, Cesaraccio R, Pulighe F, et al. Colorectal cancer epidemiology in an area with a spontaneous screening program. Acta Med Mediterranea (2013) 29:231.
124. Reggiani-Bonetti L, Di Gregorio C, Pedroni M, Domati F, Barresi V, Marcheselli L, et al. Incidence trend of malignant polyps through the data of a specialized colorectal cancer registry: clinical features and effect of screening. Scand J Gastroenterol (2013) 48(11):1294–301. doi: 10.3109/00365521.2013.838301
125. Nowicki A, Dahms S. Incidence, morbidity and 5-year survival of colorectal cancer patients in the kujawsko-pomorskie voivodship in 2005-2011, based on data from the national health fund. Pol Przegl Chir (2018) 90(4):1–8. doi: 10.5604/01.3001.0011.8175
126. Phipps AI, Scoggins J, Rossing MA, Li CI, Newcomb PA. Temporal trends in incidence and mortality rates for colorectal cancer by tumor location: 1975-2007. Am J Public Health (2012) 102(9):1791–7. doi: 10.2105/AJPH.2011.300393
127. Oppelt KA, Luttmann S, Kraywinkel K, Haug U. Incidence of advanced colorectal cancer in Germany: comparing claims data and cancer registry data. BMC Med Res Methodol (2019) 19(1):142. doi: 10.1186/s12874-019-0784-y
128. Murphy CC, Wallace K, Sandler RS, Baron JA. Racial disparities in incidence of young-onset colorectal cancer and patient survival. Gastroenterology (2019) 156(4):958–65. doi: 10.1053/j.gastro.2018.11.060
129. Innos K, Reima H, Baburin A, Paapsi K, Aareleid T, Soplepmann J. Subsite- and stage-specific colorectal cancer trends in Estonia prior to implementation of screening. Cancer Epidemiol (2018) 52:112–9. doi: 10.1016/j.canep.2017.12.016
130. Siegel R, Desantis C, Jemal A. Colorectal cancer statistics, 2014. CA Cancer J Clin (2014) 64(2):104–17. doi: 10.3322/caac.21220
131. Sia CS, Paul E, Wale RJ, Lynch AC, Heriot AG, Warrier SK. No increase in colorectal cancer in patients under 50 years of age: a Victorian experience from the last decade. Colorectal Dis (2014) 16(9):690–5. doi: 10.1111/codi.12648
132. Rejali M, Daneshi S, Hadipour M, Tavazohi H, Vardanjani HM. Temporal trends of incidence of colorectal cancer in isfahan, Iran, 2000-2011. Int J Prev Med (2018) 9:22. doi: 10.4103/2008-7802.225931
133. Sarakarn P, Suwanrungruang K, Vatanasapt P, Wiangnon S, Promthet S, Jenwitheesuk K, et al. Joinpoint analysis trends in the incidence of colorectal cancer in khon kaen, Thailand (1989 - 2012). Asian Pac J Cancer Prev (2017) 18(4):1039–43. doi: 10.22034/APJCP.2017.18.4.1039
134. Keum N, Giovannucci EL. Folic acid fortification and colorectal cancer risk. Am J Prev Med (2014) 46(3 Suppl 1):S65–72. doi: 10.1016/j.amepre.2013.10.025
135. Singh KE, Taylor TH, Pan CG, Stamos MJ, Zell JA. Colorectal cancer incidence among young adults in California. J Adolesc Young Adult Oncol (2014) 3(4):176–84. doi: 10.1089/jayao.2014.0006
136. Stock C, Pulte D, Haug U, Brenner H. Subsite-specific colorectal cancer risk in the colorectal endoscopy era. Gastrointest Endosc (2012) 75(3):621–30. doi: 10.1016/j.gie.2011.10.025
137. Sun M, Wang Y, Sundquist J, Sundquist K, Ji J. Temporal trends of sex disparity in incidence and survival of colorectal cancer: Variations by anatomical site and age at diagnosis. Clin Epidemiol (2020) 12:73–81. doi: 10.2147/CLEP.S240006
138. Tawadros PS, Paquette IM, Hanly AM, Mellgren AF, Rothenberger DA, Madoff RD. Adenocarcinoma of the rectum in patients under age 40 is increasing: impact of signet-ring cell histology. Dis Colon Rectum (2015) 58(5):474–8. doi: 10.1097/DCR.0000000000000318
139. Thirunavukarasu P, Sathaiah M, Singla S, Sukumar S, Karunamurthy A, Pragatheeshwar KD, et al. Medullary carcinoma of the large intestine: a population based analysis. Int J Oncol (2010) 37(4):901–7. doi: 10.3892/ijo_00000741
140. Thuraisingam R, Jandova J, Pandit V, Michailidou M, Nfonsam VN. Assessing the national trends in colon cancer among native americans: A 12 year SEER database study. Am J Surg (2017) 214(2):228–31. doi: 10.1016/j.amjsurg.2016.11.033
141. Troeung L, Sodhi-Berry N, Martini A, Malacova E, Ee H, O’Leary P, et al. Increasing incidence of colorectal cancer in adolescents and young adults aged 15-39 years in Western Australia 1982-2007: Examination of colonoscopy history. Front Public Health (2017) 5:179. doi: 10.3389/fpubh.2017.00179
142. Ugarte MD, Etxeberria J, Goicoa T, Ardanaz E. Gender-specific spatio-temporal patterns of colorectal cancer incidence in navarre, Spain (1990-2005). Cancer Epidemiol (2012) 36(3):254–62. doi: 10.1016/j.canep.2011.10.004
143. Ullah MF, Fleming CA, Mealy K. Changing trends in age and stage of colorectal cancer presentation in Ireland - from the nineties to noughties and beyond. Surgeon (2018) 16(6):350–4. doi: 10.1016/j.surge.2018.03.006
144. Wan Ibrahim NR, Chan HK, Soelar SA, Azmi AN, Mohd Said R, Abu Hassan MR. Incidence, clinico-demographic profiles and survival rates of colorectal cancer in northern Malaysia: Comparing patients above and below 50 years of age. Asian Pac J Cancer Prev (2020) 21(4):1057–61. doi: 10.31557/APJCP.2020.21.4.1057
145. Wang DY, Thrift AP, Zarrin-Khameh N, Wichmann A, Armstrong GN, Thompson PA, et al. Rising incidence of colorectal cancer among young hispanics in Texas. J Clin Gastroenterol (2017) 51(1):34–42. doi: 10.1097/MCG.0000000000000563
146. Wang H, Mejia de Grubb MC, Gonzalez SJ, Sidani M, Ma J, Zoorob RJ. Temporal trends in colorectal cancer incidence among Asian American populations in the united states, 1994-2013. Family Med Community Health (2017) 5(1):56–64. doi: 10.15212/FMCH.2017.0103
147. Wang W, Chen W, Lin J, Shen Q, Zhou X, Lin C. Incidence and characteristics of young-onset colorectal cancer in the united states: An analysis of SEER data collected from 1988 to 2013. Clin Res Hepatol Gastroenterol (2019) 43(2):208–15. doi: 10.1016/j.clinre.2018.09.003
148. Wen D, Zou W, Wen X, Yang Y, Chen Y, He Y, et al. Urban-rural disparity in colorectal cancer incidence and increasing trend in relation to socioeconomic development and urbanization in China. J Int Med Res (2018) 46(10):4181–96. doi: 10.1177/0300060518791090
149. Wessler JD, Pashayan N, Greenberg DC, Duffy SW. Age-period-cohort analysis of colorectal cancer in East Anglia, 1971-2005. Cancer Epidemiol (2010) 34(3):232–7. doi: 10.1016/j.canep.2010.03.012
150. Wu H, Zhou P, Zhang W, Jiang Y, Liu XL, Zhang L, et al. Time trends of incidence and mortality in colorectal cancer in changning district, shanghai, 1975-2013. J Dig Dis (2018) 19(9):540–9. doi: 10.1111/1751-2980.12667
151. Yee YK, Gu Q, Hung I, Tan VP, Chan P, Hsu A, et al. Trend of colorectal cancer in Hong Kong: 1983-2006. J Gastroenterol Hepatol (2010) 25(5):923–7. doi: 10.1111/j.1440-1746.2009.06130.x
152. Yeo H, Betel D, Abelson JS, Zheng XE, Yantiss R, Shah MA. Early-onset colorectal cancer is distinct from traditional colorectal cancer. Clin Colorectal Cancer (2017) 16(4):293–9.e6. doi: 10.1016/j.clcc.2017.06.002
153. Yoon M, Kim N, Nam B, Joo J, Ki M. Changing trends in colorectal cancer in the republic of Korea: contrast with Japan. Epidemiol Health (2015) 37:e2015038. doi: 10.4178/epih/e2015038
154. Zhabagin K, Igissinov N, Manambayeva Z, Adylkhanov T, Sandybayev M, Nurgazin M, et al. Temporal epidemiological assessment of colorectal cancer incidence and mortality in East Kazakhstan, 2004-2013. Asian Pac J Cancer Prev (2015) 16(15):6413–6. doi: 10.7314/apjcp.2015.16.15.6413
155. Zhang B, Xie SH, Yu IT. Differential incidence trends of colon and rectal cancers in Hong Kong: an age-period-cohort analysis. Cancer Commun (Lond) (2018) 38(1):42. doi: 10.1186/s40880-018-0311-2
156. Zhou Q, Li K, Lin GZ, Shen JC, Dong H, Gu YT, et al. Incidence trends and age distribution of colorectal cancer by subsite in guangzhou, 2000-2011. Chin J Cancer (2015) 34(8):358–64. doi: 10.1186/s40880-015-0026-6
157. Zhu C, Bassig BA, Zaridze D, Boyle P, Dai M, Li Q, et al. A birth cohort analysis of the incidence of ascending and descending colon cancer in the united states, 1973-2008. Cancer Causes Control (2013) 24(6):1147–56. doi: 10.1007/s10552-013-0193-1
158. Zorzi M, Dal Maso L, Francisci S, Buzzoni C, Rugge M, Guzzinati S, et al. Trends of colorectal cancer incidence and mortality rates from 2003 to 2014 in Italy. Tumori (2019) 105(5):417–26. doi: 10.1177/0300891619838336
159. Zorzi M, Mangone L, Sassatelli R, Baracco S, Budroni M, Castaing M, et al. Incidence trends of colorectal cancer in the early 2000s in italy. figures from the IMPATTO study on colorectal cancer screening. Epidemiologia e prevenzione (2015) 39:115–25.
160. Ohri A, Robinson A, Liu B, Bhuket T, Wong R. Updated assessment of colorectal cancer incidence in the U.S. by age, sex, and Race/Ethnicity. Dig Dis Sci (2020) 65(6):1838–49. doi: 10.1007/s10620-019-05913-y
161. Joinpoint Regression Program, Version 4.8.0.1. Statistical methodology and applications branch, surveillance research program, national cancer institute. (2020). Available at: https://surveillance.cancer.gov/joinpoint/.
165. Rosenberg PS, Check DP, Anderson WF. A web tool for age-period-cohort analysis of cancer incidence and mortality rates. Cancer Epidemiol Biomarkers Prev (2014) 23(11):2296–302. doi: 10.1158/1055-9965.EPI-14-0300
166. Kim HJ, Fay MP, Feuer EJ, Midthune DN. Permutation tests for joinpoint regression with applications to cancer rates. Stat Med (2000) 19(3):335–51. doi: 10.1002/(sici)1097-0258(20000215)19:3<335::aid-sim336>3.0.co;2-z
Keywords: incidence, trends, colorectal cancer, methods, population-based study, systematic review
Citation: Alsadhan N, Almaiman A, Pujades-Rodriguez M, Brennan C, Shuweihdi F, Alhurishi SA and West RM (2022) Statistical methods for measuring trends in colorectal cancer incidence in registries: A systematic review. Front. Oncol. 12:1049486. doi: 10.3389/fonc.2022.1049486
Received: 20 September 2022; Accepted: 08 November 2022;
Published: 30 November 2022.
Edited by:
Maria Paula Curado, A.C.Camargo Cancer Center, BrazilReviewed by:
Yujie Zhang, Xi’an Medical University, ChinaYibing Ruan, Alberta Health Services, Canada
Copyright © 2022 Alsadhan, Almaiman, Pujades-Rodriguez, Brennan, Shuweihdi, Alhurishi and West. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Norah Alsadhan, bmFsc2FkaGFuQGtzdS5lZHUuc2E=
 Norah Alsadhan
Norah Alsadhan Alaa Almaiman1
Alaa Almaiman1 Sultana A. Alhurishi
Sultana A. Alhurishi Robert M. West
Robert M. West