- 1Vorarlberg Institute for Vascular Investigation and Treatment, Molecular Biology Laboratory, Dornbirn, Austria
- 2Department of Haematology and Oncology, Academic Teaching Hospital Feldkirch, Feldkirch, Austria
- 3Private University of the Principality of Liechtenstein, Triesen, Liechtenstein
- 4Onkologie Ravensburg, Ravensburg, Germany
- 5Medical Central Laboratories, Feldkirch, Austria
- 6Department of Internal Medicine, Academic Teaching Hospital Bregenz, Bregenz, Austria
- 7Praxis und Tagesklinik Prof. Dr. Oettle Helmut Prof. Mayer Frank, Friedrichshafen, Germany
Cell surface syndecans and glypicans play important roles in the development and prognosis of colorectal cancer (CRC). Their soluble forms from proteoglycan shedding can be detected in blood and have been proposed as new prognostic biomarkers in several cancer entities. However, studies on circulating syndecan-1 (SDC1) and glypican-4 (GPC4) in CRC are limited. We, therefore, evaluated the impact of plasma SDC1 and GPC4 on the prognosis of metastatic (m)CRC patients. The present study included 93 patients with mCRC. The endpoints were progression-free survival (PFS) and overall survival (OS) at 12 months. SDC1 and GPC4 levels were measured in plasma using enzyme-linked immunosorbent assays. Plasma levels of SDC1 and GPC4 were significantly correlated. Significant correlations of these two markers were also found with carcinoembryonic antigen (CEA). Kaplan-Meier curve analyses indicated that PFS and OS probabilities significantly decreased with increasing levels of SDC1 and GPC4, respectively. Multivariable Cox regression analyses showed that both markers were significantly associated with PFS and OS independently from clinicopathological characteristics including CEA. Respective adjusted hazard ratios (HR) together with corresponding 95% confidence intervals for one standard deviation change of SDC1 were 1.32 [1.02-1.84] for PFS and 1.48 [1.01-2.15] for OS. Adjusted HRs [95% confidence intervals] of GPC4 were 1.42 [1.07-1.89] for PFS and 2.40 [1.51-3.81] for OS. Results from area under the receiver operating characteristic curve analyses suggest that GPC4 and SDC1 add additional prognostic values to CEA for OS. In conclusion, we showed significant associations of circulating SDC1 and GPC4 with poor survival of mCRC patients.
Introduction
Colorectal cancer (CRC) is the third most prevalent cancer diagnosis in the world (1). At least half of the patients with diagnosed CRC will develop metastatic CRC (mCRC) (2). Despite recent advances in systemic treatment options and surgical management strategies (3, 4) prognosis of mCRC remains grim, with a relative 5‐year survival rate of only 14% (5).
Several studies found a central and significant contributory role of members of the heparan sulphate proteoglycan (HSPG) family in the development and prognosis of cancer, including CRC (6, 7). HSPGs are mainly present in the cell membrane and extracellular matrix, where they may interact with cytokines, chemokines, and growth factors playing active roles in cell adhesion, migration, proliferation, and signaling pathways. Syndecans, which have a transmembrane domain in their core proteins, and glycosylphosphatidylinositol-anchored glypicans are the two main subfamilies of membrane HSPGs (8).
Syndecan-1 (SDC1, alias CD138) is the major cell-surface proteoglycan in endothelial cells (9, 10) and is involved in the remodeling and angiogenesis of CRC tissue (11). Loss of SDC1 expression in CRC cells has been associated with metastatic potential and shorter survival in several (11–13) but not all studies (14). SDC1 can be released from the cell surface into the bloodstream after proteoglycan shedding, mainly induced by matrix metalloproteinases and other proteins (15). Activated shedding of membrane SDC1 and other HSPGs is mainly observed under various pathogenic conditions, including cancer (6, 15). However, only a few studies have investigated the association of circulating SDC1 with the prognosis of CRC linking serum SDC1 with chemotherapy resistance and survival in mCRC patients (16, 17).
Glypican-4 (GPC4) is another cell-surface proteoglycan involved in regulating several oncogenic pathways including Wnt, bone morphogenic protein, fibroblast growth factor 2, and hepatocyte growth factor (18–20). However, studies on GPC4 in cancer are rather limited. GPC4 expression has been associated with oxaliplatin resistance in ovarian carcinoma cells (21) and 5-fluorouracil resistance in pancreatic cancer cells (22). Shedded GPC4 can be detected in blood also and has been linked with insulin resistance (23) as well as with several metabolic disorders (23–30). However, the association between circulating GPC4 and the prognosis of patients with mCRC or other types of cancer is unknown so far.
In the present study, we therefore prospectively analyzed the impact of circulating SDC1 and GPC4 on the survival of mCRC patients and evaluated their prognostic values as new biomarkers for mCRC.
Materials and methods
Study subjects
From October 2017 through June 2020, patients with mCRC were assessed for eligibility at the ‘Oncology Study Center Ravensburg’ (Ravensburg, Germany) at the ‘Department of Hematology, Oncology, Gastroenterology and Infectiology’ of the Academic Teaching Hospital Feldkirch (Feldkirch, Austria), and at a private medical practice in Friedrichshafen, Germany (‘Gemeinschaftspraxis Oettle und Mayer’). Eligible patients were aged ≥18 years and diagnosed with mCRC starting first line or second line of therapy. All patients had to give written informed consent for participation in the present study. Baseline clinicopathological parameters were obtained from medical records, including age, sex, tumor histopathology, RAS mutation status, metastatic status, serum carcinoembryonic antigen (CEA) levels, and scheduled medical treatments. Patients were followed up until disease progression, death or end of the observational period on June 30th, 2021. The endpoints were progression-free survival (PFS; defined as the time from baseline to disease progression or death from any cause) and overall survival (OS) at 12 months. Only patients with available clinical and laboratory data and complete follow-up were included in the present study. The study protocol was approved by the Ethics Commission of the State Chamber of Medicine of Baden-Württemberg (Germany) and by the Ethics Committee of the State of Vorarlberg (Austria) and is following the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Laboratory measurements
At baseline, blood samples were collected, and plasma samples were stored at -80°C until used for the analysis of SDC1 and GPC4, respectively. Circulating SDC1 and GPC4 levels were determined via commercially available enzyme-linked immunosorbent assays (ELISA; Thermo Fisher Scientific, Waltham, MA USA; article number: EHSDC1 and Cloude-Clone; Houston, Texas; article number: SEA998Hu, respectively) following the manuals of the manufacturers.
Statistical analyses
Differences in baseline characteristics according to tertiles of SDC1 and GPC4 were tested for statistical significance with Chi-squared tests for trend for categorical and Jonckheere Terpstra tests for continuous variables, respectively. Continuous variables are given as median and interquartile range (defined as the range from the 25th to the 75th percentile). Normal distribution was assessed using Kolmogorov-Smirnov indicating that SDC1 and GPC4 values were not normally distributed (each p-value <0.001). Non-normal distribution of plasma SDC1 and GPC4, respectively, was visually verified using a quantile-quantile plot as displayed in Supplementary Figure 1. Correlation analyses were performed by calculating non-parametric Spearman rank correlation coefficients. Differences between SDC1 and GPC4 levels and categorical variables were tested for statistical significance using the Mann–Whitney–U test. Survival curves were generated using the Kaplan-Meier method and compared using log-rank-Mantel-Cox tests. Hazard ratios (HRs) and 95% confidence intervals (CIs) of the HRs were derived from univariable and multivariable Cox proportional hazards models. Z-transformation was applied before logistic regression analysis. In addition, area under the receiver operating characteristic curve (ROC-AUC) analyses were performed and the statistical significance of the difference between AUCs was tested with the method of DeLong (31). To examine the prognostic values of SDC1 and GPC4 as prognostic biomarkers for mCRC, binary logistic regression models were fitted with PFS and OS, respectively, as the dependent variable. A basic model comprising serum CEA, which represents one of the most extensively used prognostic biomarkers in CRC (32), as the independent variable was compared with further models including SDC1 and/or GPC4 as additional markers. Statistical analyses were performed with SPSS 28.0.0 (SPSS, Inc., Chicago, IL) and GraphPad Prism 8 (GraphPad Software, San Diego, CA).
Results
Patients’ characteristics
Stored plasma samples for SDC1 and GPC4 analysis were available from 93 patients with complete follow-up data, which were included in the present study. The clinicopathological characteristics of the study cohort are shown in Table 1. Patients’ characteristics stratified by tertiles of SDC1 and GPC4 are given in Supplementary Tables 1 and 2, respectively. Age, sex, primary tumor localization, number of metastatic sites, and kind of scheduled therapy did not differ significantly between tertiles of SDC1 or GPC4. GPC4 levels increased with increasing SDC1 tertiles and vice versa (each P-value < 0.001). Spearman’s correlation analysis using SDC1 and GPC4 as continuous variables confirmed the highly significant correlation between these two parameters (rho = 0.521; P < 0.001). In addition, CEA levels were significantly associated with tertiles of SDC1 and GPC4 (P < 0.001 and P = 0.005, respectively). Respective Spearman’s correlation coefficient values were rho = 0.484; P < 0.001 and rho = 0.351; P < 0.001 for soluble SDC1 and GPC4, respectively, using continuous variables. Scatter plots visualizing the correlations between SDC1, GPC4, and CEA are displayed in Supplementary Figure 2.
Prospective study
Circulating SDC1 and GPC4 values were significantly increased in patients showing a progression of the disease (52.7%; n=49) and in patients who died (21.5%; n=20) during the 12-month follow-up period (Figure 1). Kaplan-Meier estimates representing the probabilities of PFS and OS stratified by tertiles of SDC1 and GPC4, respectively, are displayed in Figure 2. PFS probability significantly decreased from the 1st tertile of plasma SDC1 over the 2nd SDC1 tertile to the 3rd tertile of SDC1. The same was true for OS. Similarly, increasing GPC4 tertiles were significantly associated with decreased probabilities of PFS and OS. The significant associations of SDC1 and GPC4 with survival were confirmed by univariable and multivariable Cox regression analyses using these markers as continuous variables. Respective hazard ratios together with corresponding confidence intervals are shown in Figure 3. Plasma SDC1 and GPC4 were associated with PFS and OS independently from clinicopathological characteristics including CEA. In particular, the association between circulating GPC4 and OS remained highly significant in each regression model. Multivariable Cox regression analysis including both SDC1 and GPC4 in the same regression model further showed that the association with OS remained significant for GPC4 but not for SDC1 (HR = 2.15 [1.43-3.22]; P < 0.001 and HR = 0.96 [0.65-1.43]; P = 0.847, respectively). The same was true for PFS (HR = 1.47 [1.01–1.97]; P = 0.010 and HR = 1.08 [0.83–1.41]; P = 0.553, respectively).
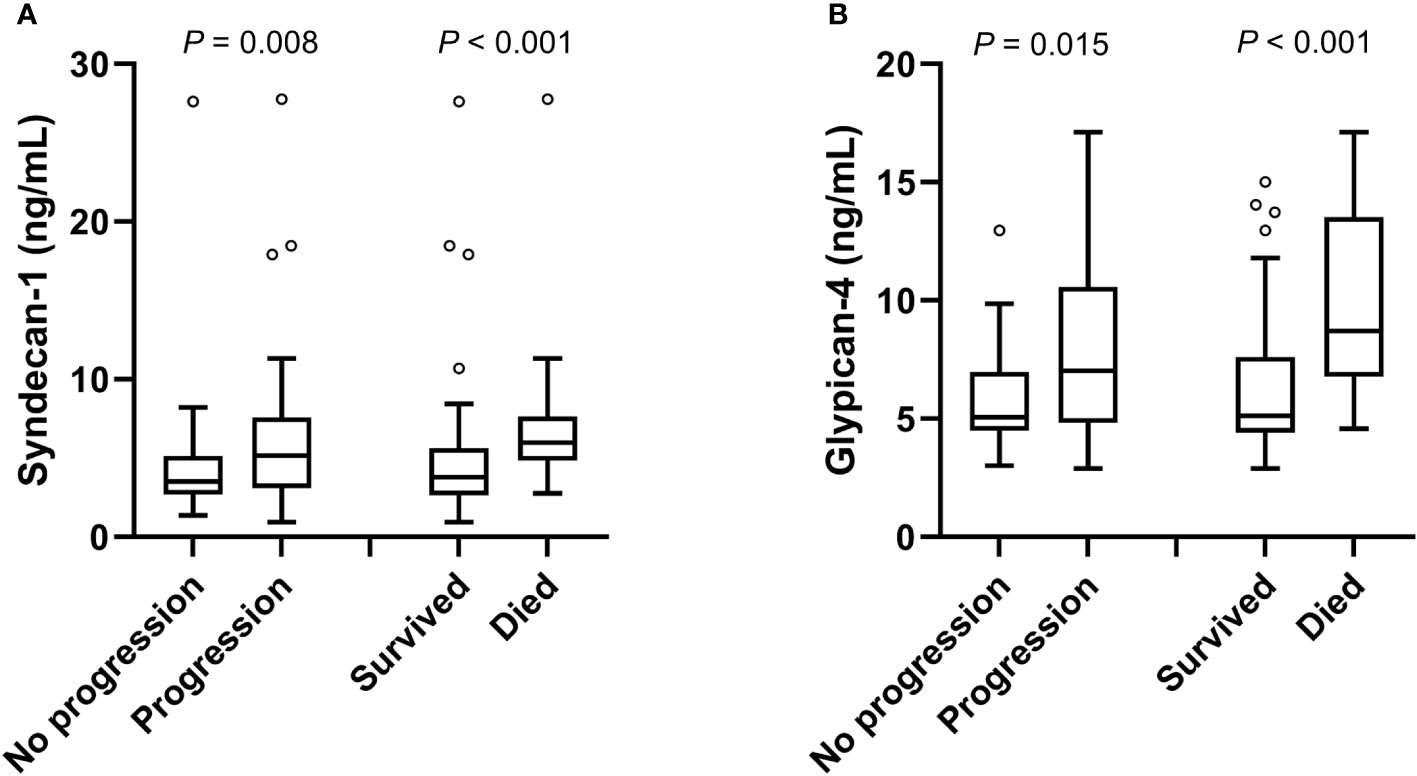
Figure 1 Plasma levels of SDC1 and GPC4 with respect to disease progression and mortality. Plasma levels of (A) SDC1 and (B) GPC4 with respect to disease progression and mortality are shown as box plots using the Tukey method for plotting the whiskers and outliers. P-values were calculated using the Mann-Whitney U test.
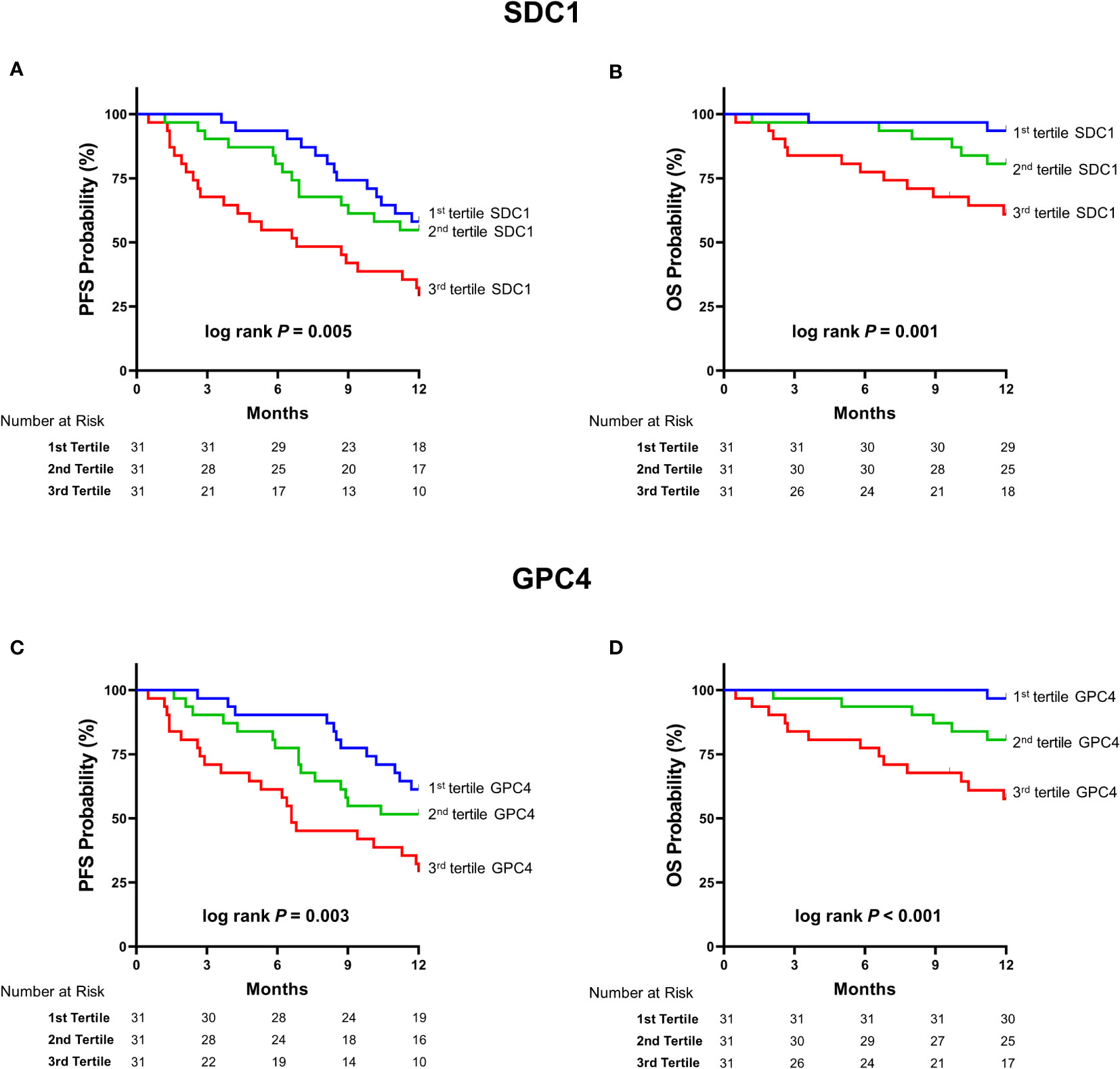
Figure 2 Kaplan Meier estimates of survival according to tertiles of SDC1 and GPC4. Kaplan–Meier curves of (A) PFS stratified by tertiles of SDC1 (B) OS stratified by tertiles of SDC1 (C) PFS stratified by tertiles of GPC4 (D) OS stratified by tertiles of OS. P-values were obtained by Log-Rank-Mantel-Cox-tests. SDC1, syndecan-1; GPC4, glypican-4; PFS, progression-free survival; OS, overall survival.
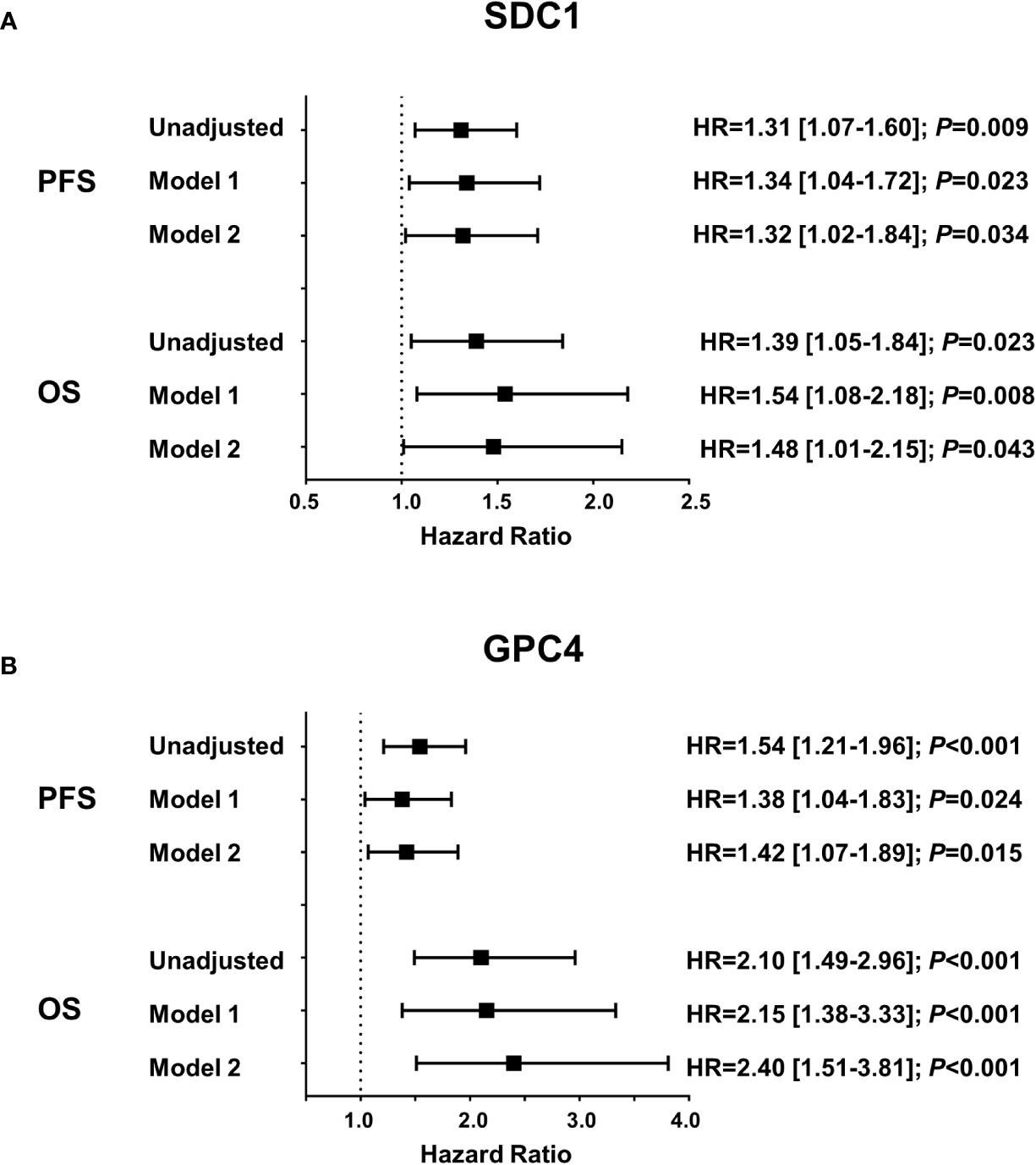
Figure 3 Association of plasma SDC1 and GPC4 with progression free survival and overall survival. Results were obtained from Cox proportional hazards regression analyses and are presented as hazard ratios (HR) and 95% confidence intervals [CI] for one standard deviation change of (A) SDC1 and (B) GPC4. Model 1 includes age, sex, number of metastatic sites, and scheduled therapy; model 2 additionally includes CEA. PFS, progression-free survival; OS, overall survival; SDC1, syndecan-1; GPC4, glypican-4; CEA, carcinoembryonic antigen.
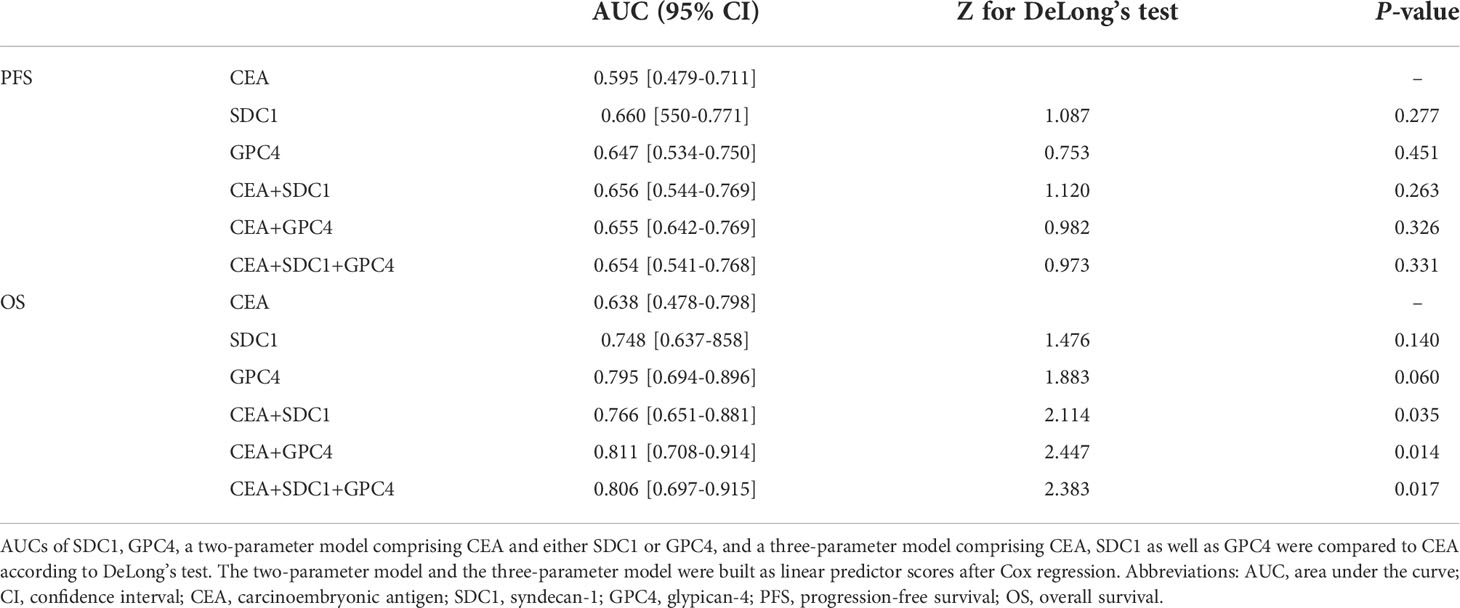
Area under the receiver operating characteristic curve (ROC-AUC) analyses were used to compare the performance of SDC1 and GPC4 as biomarkers for PFS or OS with CEA. Table 2 shows the AUCs and the results from DeLong’s test indicating a significant additional prognostic value of SDC1 and/or GPC4 to a model comprising CEA as the independent variable for the 12-month OS. Highest AUC for OS was observed for the two-parameter model comprising GPC4 and CEA. That said, no additional prognostic value of SDC1 and/or GPC4 to CEA was observed for PFS.
Discussion
In the present study, we showed that high plasma levels of SDC1 and GPC4 independently predicted outcomes in patients with mCRC. Studies on GPC4 in cancer are rather limited and a significant association between plasma GPC4 and the outcome of CRC patients has not been described so far. Furthermore, our study is in line with two previous studies demonstrating that circulating SDC1 is significantly linked with the prognosis of CRC (16, 17). In this regard, Wang et al. showed that CRC patients with high SDC1 serum levels showed a poorer disease-free survival (17) and Jary et al. identified soluble SDC1 as an independent prognostic risk factor of OS in mCRC patients (16). In addition, significant associations between circulating SDC1 and the outcome of patients with other tumor entities including lung cancer (33, 34), prostate cancer (35), bladder cancer (36), and multiple myeloma (37) have been reported in the literature.
It has been suggested that shed SDC1 is associated with chemotherapy resistance, probably linking soluble SDC1 with poor survival of cancer patients. Wang et al. demonstrated that CRC patients with high pre-operative SDC1 levels were less responsive to 5-fluorouracil, oxaliplatin, irinotecan, cisplatin, or paclitaxel chemotherapy (17). As a possible mechanism of chemotherapy resistance in CRC, the authors proposed that shed SDC1 may interact with soluble heparin-binding EGF-like growth factor enhancing the activation of EGFR and downstream signaling, which in turn decreases the chemotherapeutical sensitivity of colon cancer cells (38). It has been further shown that the shed of SDC1 is associated with chemotherapy resistance in castration-resistance prostate cancer (39). In addition, in vitro studies showed that shed SDC1 may bind VEGF stimulating endothelial invasion and tumor angiogenesis in myeloma cells (40). In fact, activated SDC1 shedding has been associated with increased metastasis in various cancers, indicating that soluble SDC1 represents a major facilitator for malignant cellular invasion (41). Consequently, targeting SDC1 and preventing SDC1 shedding has been proposed as promising approaches to prevent tumor progression; respective putative therapeutic strategies have been summarized by Guo et al. (41). However, it remains unclear if SDC1 shedding probably involved in paracrine communication within the tumor microenvironment may also lead to quantifiable concentrations of the free SDC1 ectodomain in blood.
Our study further demonstrated that plasma levels of SDC1 and GPC4 were significantly correlated in mCRC patients. Interestingly, multivariable Cox regression analyses as well as ROC-AUC analyses indicated that plasma GPC4 even outperformed SDC1 as a prognostic biomarker of mCRC. The close correlation between these two biomarkers is not surprising because simultaneous shedding of different HSPGs is found under inflammatory and other pathological conditions (42). Consequently, common pathophysiological mechanisms may have led to measurable blood levels of SDC1 and GPC4 predicting the course of mCRC patients. However, concerning the limited knowledge of shed GPC4 on cancer prognosis, the molecule should not be neglected and deserves further investigations.
Serum SDC1 has been proposed not only as a prognostic marker in cancer but also in cardiovascular disease, kidney disease, diabetes, and sepsis [as reviewed by Leppeda et al. (43)]. Circulating GPC4 has been previously associated with several disorders linked to insulin resistance including elevated systolic blood pressure (27), non-alcoholic fatty liver disease (26), and kidney disease (30, 44). Recently, we showed that circulating GPC4 is associated with increased overall mortality risk in coronary angiography patients (45) as well as in patients with peripheral artery disease (46). Notably, cardiovascular diseases, hypertension, and diabetes are common comorbidities in CRC patients (47) and may have a large impact on both blood levels of shed proteoglycans and survival. Therefore, circulating SDC1 and GPC4 might be markers of general organ dysfunction rather than specific tumor markers. In CRC, CEA represents the most extensively used biomarker to monitor disease progression and to estimate CRC patients’ prognosis (32). Nevertheless, there is a growing consensus that using alternative biomarkers may further improve the assessment of mCRC prognosis (48–50). For this reason, the determination of the tumor marker CEA together with markers of organ dysfunction such as SDC1 or GPC4 may appear advantageous in estimating the prognosis of CRC since they can provide complementary information. In fact, our study’s ROC-AUC analyses suggest that circulating SDC1 and/or GPC4 add additional prognostic value to CEA concerning OS.
Our study has several limitations. By design, our study population was composed of consecutively recruited mCRC patients; our results, therefore, are not necessarily applicable to other cancer stages or cancer types. Due to the consecutive study design, included patients were not uniformly treated and the limited sample size does not allow a detailed subgroup analysis based on individual therapy regimes. However, our study population covers a large proportion of mCRC patients seen in clinical practice providing real-world data linking the impact of circulating HSPGs with the outcome of mCRC. The observational design of our study does not allow definite conclusions regarding causal relationships between investigated biomarkers and study outcomes. The molecular backgrounds behind our findings are needed to be addressed in further studies.
In conclusion, we showed significant associations of circulating SDC1 and GPC4 with poor survival of mCRC patients. Future research appears worthwhile to further evaluate the value of circulating proteoglycans as predictors of colorectal cancer.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Ethics Commission of the State Chamber of Medicine of Baden-Württemberg (Germany) and the Ethic Committee of the State of Vorarlberg (Austria). The patients/participants provided their written informed consent to participate in this study.
Author contributions
AM designed the study, obtained funding, interpretated data, and wrote the manuscript. LS recruited patients, collected and interpretated data, and revised the manuscript for important intellectual content. ThD recruited patients, collected and interpretated data, and critically revised the manuscript for important intellectual content. CH performed laboratory analyses, interpretated data, and critically revised the manuscript for important intellectual content. AL performed statistical analyses and critically revised the manuscript. KG performed laboratory analyses and revised the manuscript. HD critically revised the manuscript for important intellectual content. TW recruited patients, collected and interpretated data, and critically revised the manuscript for important intellectual content. PR recruited patients and collected data. FM recruited patients, collected and interpretated data, and revised the manuscript for important intellectual content. CN collected data and revised the manuscript. ToD designed the study, collected and interpretated data, and revised the manuscript for important intellectual content. All authors contributed to the article and approved the submitted version.
Funding
The present study was partly financed by the European Union’s European Regional Development Fund through the INTERREG V program “Alpenrhein-Bodensee-Hochrhein”; Project Number: ABH055. The sponsors had no involvement in the study design, in the collection, analysis, and interpretation of data or the writing of the manuscript or in the decision to submit the manuscript for publication.
Acknowledgments
The authors sincerely thank all patients participating in this research study and all involved nurses and physicians for supporting patient recruitment and sample collection.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1045995/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Ivey GD, Johnston FM, Azad NS, Christenson ES, Lafaro KJ, Shubert CR. Current surgical management strategies for colorectal cancer liver metastases. Cancers (Basel) (2022) 14:1063. doi: 10.3390/CANCERS14041063
3. Riedesser JE, Ebert MP, Betge J. Precision medicine for metastatic colorectal cancer in clinical practice. Ther Adv Med Oncol (2022) 14:17588359211072703. doi: 10.1177/17588359211072703
4. Cherri S, Libertini M, Zaniboni A. New drugs for the treatment of metastatic colorectal cancer. World J Gastroint Oncol (2021) 13:1551–60. doi: 10.4251/WJGO.V13.I11.1551
5. Wang J, Li S, Liu Y, Zhang C, Li H, Lai B. Metastatic patterns and survival outcomes in patients with stage IV colon cancer: A population-based analysis. Cancer Med (2020) 9:361. doi: 10.1002/CAM4.2673
6. Vicente CM, da Silva DA, Sartorio PV, Silva TD, Saad SS, Nader HB, et al. Heparan sulfate proteoglycans in human colorectal cancer. Anal Cell Pathol (2018) 2018:8389595. doi: 10.1155/2018/8389595
7. Marques C, Reis CA, Vivès RR, Magalhães A. Heparan sulfate biosynthesis and sulfation profiles as modulators of cancer signalling and progression. Front Oncol (2021) 11:778752. doi: 10.3389/FONC.2021.778752
8. Bernfield M, Götte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, et al. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem (1999) 68:729–77. doi: 10.1146/ANNUREV.BIOCHEM.68.1.729
9. Kumar-Singh A, Parniewska MM, Giotopoulou N, Javadi J, Sun W, Szatmári T, et al. Nuclear syndecan-1 regulates epithelial-mesenchymal plasticity in tumor cells. Biol (Basel) (2021) 10:521. doi: 10.3390/BIOLOGY10060521/S1
10. Szatmári T, Ötvös R, Hjerpe A, Dobra K. Syndecan-1 in cancer: Implications for cell signaling, differentiation, and prognostication. Dis Markers (2015) 2015:796052. doi: 10.1155/2015/796052
11. Mitselou A, Skoufi U, Tsimogiannis KE, Briasoulis E, Vougiouklakis T, Arvanitis D, et al. Association of syndecan-1 with angiogenesis-related markers, extracellular matrix components, and clinicopathological features in colorectal carcinoma. Anticancer Res (2012) 32:3977–85.
12. Al-Maghrabi J. Loss of expression of syndecan-1 is associated with tumor recurrence, metastatic potential, and poor survival in patients with colorectal carcinoma. Pakistan J Med Sci (2021) 37:1–7. doi: 10.12669/PJMS.37.1.2592
13. Li K, Li L, Wu X, Yu J, Ma H, Zhang R, et al. Loss of SDC1 expression is associated with poor prognosis of colorectal cancer patients in northern China. Dis Markers (2019) 2019:3768708. doi: 10.1155/2019/3768708
14. Wei HT, Guo EN, Dong BG, Chen LS. Prognostic and clinical significance of syndecan-1 in colorectal cancer: a meta-analysis. BMC Gastroenterol (2015) 15:152. doi: 10.1186/S12876-015-0383-2
15. Piperigkou Z, Mohr B, Karamanos N, Götte M. Shed proteoglycans in tumor stroma. Cell Tissue Res (2016) 365:643–55. doi: 10.1007/S00441-016-2452-4
16. Jary M, Lecomte T, Bouché O, Kim S, Dobi E, Queiroz L, et al. Prognostic value of baseline seric syndecan-1 in initially unresectable metastatic colorectal cancer patients: a simple biological score. Int J Cancer (2016) 139:2325–35. doi: 10.1002/IJC.30367
17. Wang X, Zuo D, Chen Y, Li W, Liu R, He Y, et al. Shed syndecan-1 is involved in chemotherapy resistance via the EGFR pathway in colorectal cancer. Br J Cancer (2014) 111:1965–76. doi: 10.1038/BJC.2014.493
18. Strate I, Tessadori F, Bakkers J. Glypican4 promotes cardiac specification and differentiation by attenuating canonical wnt and bmp signaling. Dev (2015) 142:1767–76. doi: 10.1242/dev.113894
19. Ford-Perriss M, Turner K, Guimond S, Apedaile A, Haubeck HD, Turnbull J, et al. Localisation of specific heparan sulfate proteoglycans during the proliferative phase of brain development. Dev Dyn (2003) 227:170–84. doi: 10.1002/dvdy.10298
20. Karihaloo A, Kale S, Rosenblum ND, Cantley LG. Hepatocyte growth factor-mediated renal epithelial branching morphogenesis is regulated by glypican-4 expression. Mol Cell Biol (2004) 24:8745–52. doi: 10.1128/mcb.24.19.8745-8752.2004
21. Varma RR, Hector SM, Clark K, Greco WR, Hawthorn L, Pendyala L. Gene expression profiling of a clonal isolate of oxaliplatin resistant ovarian carcinoma cell line A2780/C10. Oncol Rep (2005) 14:925–32. doi: 10.3892/or.14.4.925
22. Cao J, Ma J, Sun L, Li J, Qin T, Zhou C, et al. Targeting glypican-4 overcomes 5-FU resistance and attenuates stem cell–like properties via suppression of wnt/β-catenin pathway in pancreatic cancer cells. J Cell Biochem (2018) 119:9498–512. doi: 10.1002/jcb.27266
23. Ussar S, Bezy O, Blüher M, Kahn CR. Glypican-4 enhances insulin signaling via interaction with the insulin receptor and serves as a novel adipokine. Diabetes (2012) 61:2289–98. doi: 10.2337/db11-1395
24. Huang K, Park S. Heparan sulfated glypican-4 is released from astrocytes predominantly by proteolytic shedding. BioRxiv (2021) 8:ENEURO.0069-21.2021. doi: 10.1101/2021.02.17.431702
25. Traister A, Shi W, Filmus J. Mammalian notum induces the release of glypicans and other GPI-anchored proteins from the cell surface. Biochem J (2008) 410:503–11. doi: 10.1042/BJ20070511
26. Yoo HJ, Hwang SY, Cho GJ, Hong HC, Choi HY, Hwang TG, et al. Association of glypican-4 with body fat distribution, insulin resistance, and nonalcoholic fatty liver disease. J Clin Endocrinol Metab (2013) 98:2897–901. doi: 10.1210/jc.2012-4297
27. Zhu HJ, Pan H, Cui Y, Wang XQ, Wang LJ, Li NS, et al. The changes of serum glypican4 in obese patients with different glucose metabolism status. J Clin Endocrinol Metab (2014) 99:E2697–701. doi: 10.1210/jc.2014-2018
28. Li K, Xu X, Hu W, Li M, Yang M, Wang Y, et al. Glypican-4 is increased in human subjects with impaired glucose tolerance and decreased in patients with newly diagnosed type 2 diabetes. Acta Diabetol (2014) 51:981–90. doi: 10.1007/s00592-014-0652-5
29. Zhang K, Zhu H, Wang L, Yang H, Pan H, Gong F. Serum glypican4 and glycosylphosphatidylinositol-specific phospholipase d levels are associated with adipose tissue insulin resistance in obese subjects with different glucose metabolism status. J Endocrinol Invest (2021) 44:781–90. doi: 10.1007/s40618-020-01372-9
30. Cha JJ, Min HS, Kim K, Lee MJ, Lee MH, Kim JE, et al. Long-term study of the association of adipokines and glucose variability with diabetic complications. Korean J Intern Med (2018) 33:367–82. doi: 10.3904/kjim.2016.114
31. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics (1988) 44:837. doi: 10.2307/2531595
32. Campos-da-Paz M, Dórea JG, Galdino AS, Lacava ZGM, Santos Md.FMA. Carcinoembryonic antigen (CEA) and hepatic metastasis in colorectal cancer: Update on biomarker for clinical and biotechnological approaches. Recent Pat Biotechnol (2018) 12:269–79. doi: 10.2174/1872208312666180731104244
33. Joensuu H, Anttonen A, Eriksson M, Mäkitaro R, Alfthan H, Kinnula V, et al. Soluble syndecan-1 and serum basic fibroblast growth factor are new prognostic factors in lung cancer. Cancer Res (2002) 62:5210–7.
34. Anttonen A, Leppä S, Ruotsalainen T, Alfthan H, Mattson K, Joensuu H. Pretreatment serum syndecan-1 levels and outcome in small cell lung cancer patients treated with platinum-based chemotherapy. Lung Cancer (2003) 41:171–7. doi: 10.1016/S0169-5002(03)00196-X
35. Szarvas T, Reis H, vom Dorp F, Tschirdewahn S, Niedworok C, Nyirady P, et al. Soluble syndecan-1 (SDC1) serum level as an independent pre-operative predictor of cancer-specific survival in prostate cancer. Prostate (2016) 76:977–85. doi: 10.1002/pros.23186
36. Olah C, Tschirdewahn S, Hoffmann MJ, Krafft U, Hadaschik B, Nyirady P, et al. Soluble syndecan-1 levels are associated with survival in platinum-treated bladder cancer patients. Diagnostics (2020) 10:864. doi: 10.3390/DIAGNOSTICS10110864
37. Seidel C, Sundan A, Hjorth M, Turesson I, Dahl IMS, Abildgaard N, et al. Serum syndecan-1: a new independent prognostic marker in multiple myeloma. Blood (2000) 95:388–92. doi: 10.1182/BLOOD.V95.2.388
38. Chen SJ, Luan J, Zhang HS, Ruan CP, Xu XY, Li QQ, et al. EGFR-mediated G1/S transition contributes to the multidrug resistance in breast cancer cells. Mol Biol Rep (2012) 39:5465–71. doi: 10.1007/S11033-011-1347-4
39. Szarvas T, Sevcenco S, Módos O, Keresztes D, Nyirády P, Kubik A, et al. Circulating syndecan-1 is associated with chemotherapy-resistance in castration-resistant prostate cancer. Urol Oncol (2018) 36:312.e9–312.e15. doi: 10.1016/J.UROLONC.2018.03.010
40. Purushothaman A, Uyama T, Kobayashi F, Yamada S, Sugahara K, Rapraeger AC, et al. Heparanase-enhanced shedding of syndecan-1 by myeloma cells promotes endothelial invasion and angiogenesis. Blood (2010) 115:2449–57. doi: 10.1182/BLOOD-2009-07-234757
41. Guo S, Wu XY, Lei T, Zhong R, Wang YR, Zhang L, et al. The role and therapeutic value of syndecan-1 in cancer metastasis and drug resistance. Front Cell Dev Biol (2021) 9:784983. doi: 10.3389/FCELL.2021.784983
42. Fisher J, Linder A, Bentzer P. Elevated plasma glypicans are associated with organ failure in patients with infection. Intensive Care Med Exp (2019) 7:2. doi: 10.1186/s40635-018-0216-z
43. Lepedda AJ, Nieddu G, Piperigkou Z, Kyriakopoulou K, Karamanos N, Formato M. Circulating heparan sulfate proteoglycans as biomarkers in health and disease. Semin Thromb Hemost (2021) 47:295–307. doi: 10.1055/s-0041-1725063
44. Muendlein A, Brandtner EM, Leiherer A, Geiger K, Heinzle C, Gaenger S, et al. Evaluation of the association of serum glypican-4 with prevalent and future kidney function. Sci Rep (2022) 12:10168. doi: 10.1038/s41598-022-14306-7
45. Muendlein A, Brandtner EM, Leiherer A, Geiger K, Heinzle C, Gaenger S, et al. Serum glypican-4 is a marker of future vascular risk and mortality in coronary angiography patients. Atherosclerosis (2022) 345:33–8. doi: 10.1016/J.ATHEROSCLEROSIS.2022.02.015
46. Muendlein A, Heinzle C, Leiherer A, Geiger K, Brandtner EM, Gaenger S, et al. Serum glypican-4 is associated with the 10-year clinical outcome of patients with peripheral artery disease. Int J Cardiol (2022) 369:54–9. doi: 10.1016/J.IJCARD.2022.08.018
47. Van Leersum NJ, Janssen-Heijnen MLG, Wouters MWJM, Rutten HJT, Coebergh JW, Tollenaar RAEM, et al. Increasing prevalence of comorbidity in patients with colorectal cancer in the south of the Netherlands 1995-2010. Int J Cancer (2013) 132:2157–63. doi: 10.1002/IJC.27871
48. Yu Z, Chen Z, Wu J, Li Z, Wu Y. Prognostic value of pretreatment serum carbohydrate antigen 19-9 level in patients with colorectal cancer: A meta-analysis. PloS One (2017) 12:.e0188139. doi: 10.1371/JOURNAL.PONE.0188139
49. Wang C, Zhou X, Ma L, Zhuang Y, Wei Y, Zhang L, et al. Galectin-3 may serve as a marker for poor prognosis in colorectal cancer: A meta-analysis. Pathol Res Pract (2019) 215:152612. doi: 10.1016/J.PRP.2019.152612
Keywords: colorectal cancer, proteoglycans, shedding, syndecan-1 (SDC1), glypican-4, biomarker, prognosis
Citation: Muendlein A, Severgnini L, Decker T, Heinzle C, Leiherer A, Geiger K, Drexel H, Winder T, Reimann P, Mayer F, Nonnenbroich C and Dechow T (2022) Circulating syndecan-1 and glypican-4 predict 12-month survival in metastatic colorectal cancer patients. Front. Oncol. 12:1045995. doi: 10.3389/fonc.2022.1045995
Received: 16 September 2022; Accepted: 10 October 2022;
Published: 24 October 2022.
Edited by:
Mauro Sergio Pavao, Federal University of Rio de Janeiro, BrazilReviewed by:
Demitrios Vynios, University of Patras, GreeceJenny Karousou, University of Insubria, Italy
Copyright © 2022 Muendlein, Severgnini, Decker, Heinzle, Leiherer, Geiger, Drexel, Winder, Reimann, Mayer, Nonnenbroich and Dechow. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Axel Muendlein, YXhlbC5tdWVuZGxlaW5Adml2aXQuYXQ=
 Axel Muendlein
Axel Muendlein Luciano Severgnini2,3
Luciano Severgnini2,3 Christine Heinzle
Christine Heinzle Andreas Leiherer
Andreas Leiherer