
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol., 14 November 2022
Sec. Radiation Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1045752
This article is part of the Research TopicCase Reports in Radiation Oncology : 2022View all 17 articles
Background: Pancreatic neuroendocrine tumor (NET) is rare, and the majority presents late in their clinical course. Here, we present a huge locally advanced pancreatic NET having Hi-Art helical Tomotherapy that resulted in a 68% reduction in target volume during adaptive image-guided radiotherapy (IGRT).
Case summary: A 63-year-old man without any history of systemic disease developed voiding difficulty for several months. Associated symptoms included poor appetite, nausea, distended abdomen, and body weight loss. Further magnetic resonance imaging showed a large multilobulated tumor in the left upper abdomen. Tumor biopsy revealed well-differentiated, grade 2, neuroendocrine tumor. Complete resection was unattainable. Therefore, Lanreotide was prescribed initially. However, tumor progression up to the greatest diameter of 18 cm was noted on computed tomography 5 months later. Thus, he stopped Lanreotide and commenced on concurrent chemoradiotherapy (CCRT). With a total dose of 70 Gy in 35 fractions, we generated two adaptive treatment plans during the whole course. Laparoscopic subtotal pancreatectomy with spleen preservation was performed after neoadjuvant CCRT. It has been more than 3 years after IGRT, and he remains cancer free and reports no side effects during regular follow-ups.
Conclusion: Tomotherapy caused tumor size reduction and hence facilitated surgical possibility for this originally unresectable pancreatic NET. Neoadjuvant IGRT incorporated with adaptive treatment planning enhanced delivery accuracy. In this case of pancreatic NET resistant to Lanreotide, inter-fractional tumor regression from 1910 to 605 cc (68%) was documented.
Pancreatic neuroendocrine tumor (NET) is a rare type of neuroendocrine neoplasm (NEN) that arises from endocrine cell in pancreatic tissue, accounting for only 3% of all pancreatic tumors (1). The majority of pancreatic NETs are non-functional without defined clinical syndrome or abnormal hormone profiles, and their presentation is often delayed until significant mass effect or distant metastasis (2). In this situation, curative surgical resection is often intricate. However, the role of surgical resection in the treatment of pancreatic NET is imperative. Hill et al. have investigated the impact of resection on overall survival. Resection of pancreatic NET was related to significantly improved survival in contrast with those patients who were recommended for surgery but did not undergo resection (114 vs. 35 month; p<0.01) (2).
Surgery was, however, not recommended in cases of giant size and small probability of complete resection. Combining different treatment modalities prior to definitive surgical intervention was hence applied. Radiotherapy used as a main treatment of primary pancreatic NET is novel and not often reported, although it has long been regarded as an approved treatment option in palliative symptom relief (3). Here, we present a case of locally advanced unresectable pancreatic NET who underwent neoadjuvant concurrent chemoradiation (CCRT) via Tomotherapy and subsequent surgical resection successfully. To the best of our knowledge, this is the first reported pancreatic NET who had 68% regression of target volume during IGRT of 70 Gy.
A 63-year-old man without any history of systemic disease developed voiding difficulty for several months prior to his presence in the hospital. Associated symptoms included poor appetite, nausea, distended abdomen, and body weight loss. Further abdominal magnetic resonance imaging (MRI) showed a large multilobulated tumor with the size of 16.1 × 14.9 × 14.5 cm in the left upper abdomen (Figure 1A). During physical examination, a very big abdominal mass was palpated in the left upper quadrant with firm texture. The mass was fixed with regular border. His laboratory data, such as alpha-fetoprotein and carcinoembryonic antigen, were within normal limits. He received tumor biopsy in which it revealed tissue fragment infiltrated by tumor cells bearing relatively uniform round nuclei and high nucleus–cytoplasm ratio arranged in sheet or rosette-like patterns. Immunohistochemical staining showed positive for chromogranin A, synaptophysin, and somatostatin receptor 2A (SSTR2A) (Figures 2A–C). Moreover, the mitotic activity was about 3 per 10 high-power fields. The Ki-67 labeling index was about 4%. Well-differentiated, grade 2, neuroendocrine tumor was diagnosed.
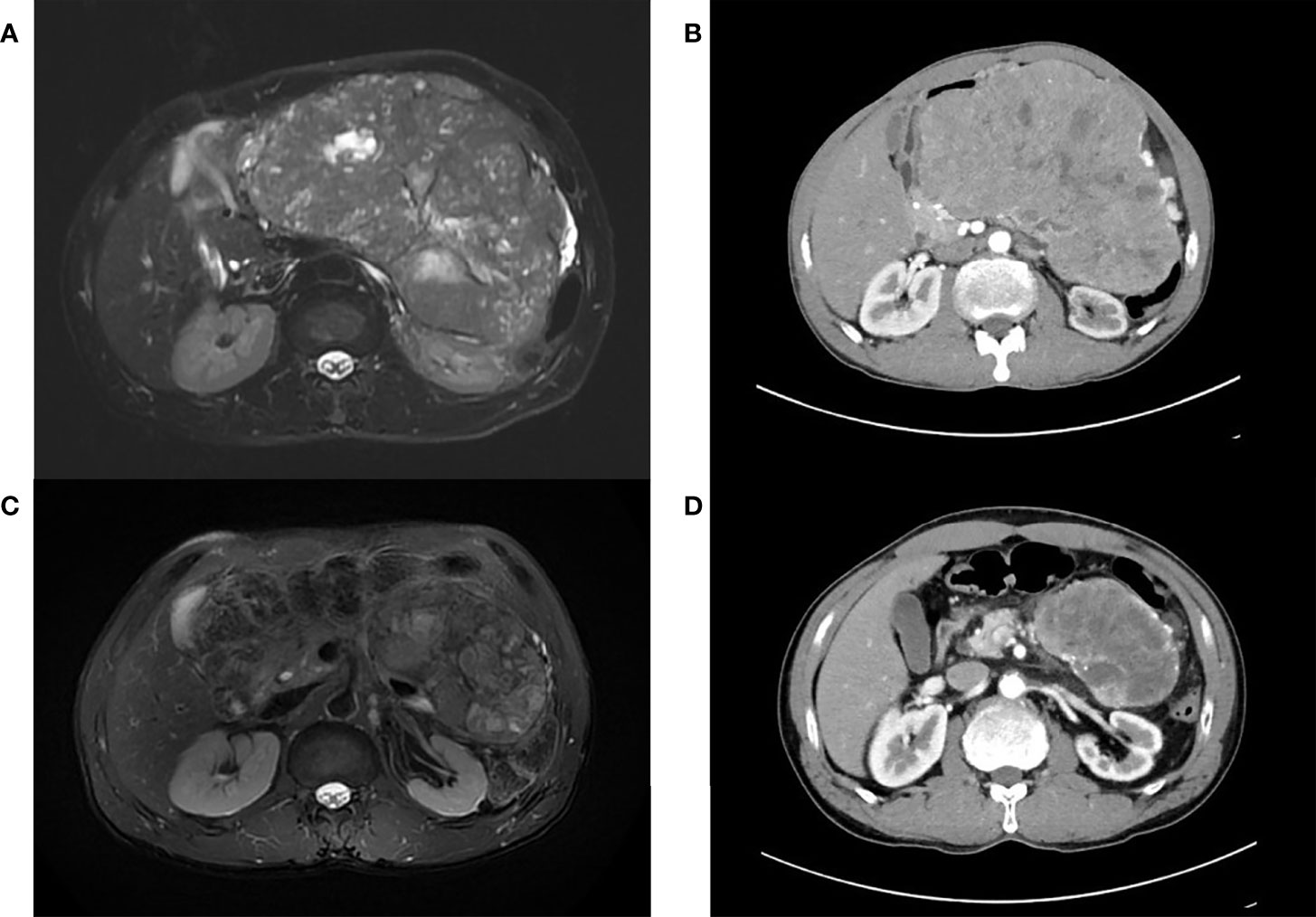
Figure 1 (A) Pretreatment magnetic resonance imaging (MRI) image depicting a multilobulated tumor with the size of 16.1 cm × 14.9 cm × 14.5 cm in the left upper abdomen. (B) Tumor progression up to the greatest diameter of 18 cm in the axial view of computerized tomography (CT) scans after treatment of Lanreotide. (C) Prominent tumor shrinkage after concurrent chemoradiation. (D) At least 50% reduction of tumor axial perpendicular diameters in preoperative CT.
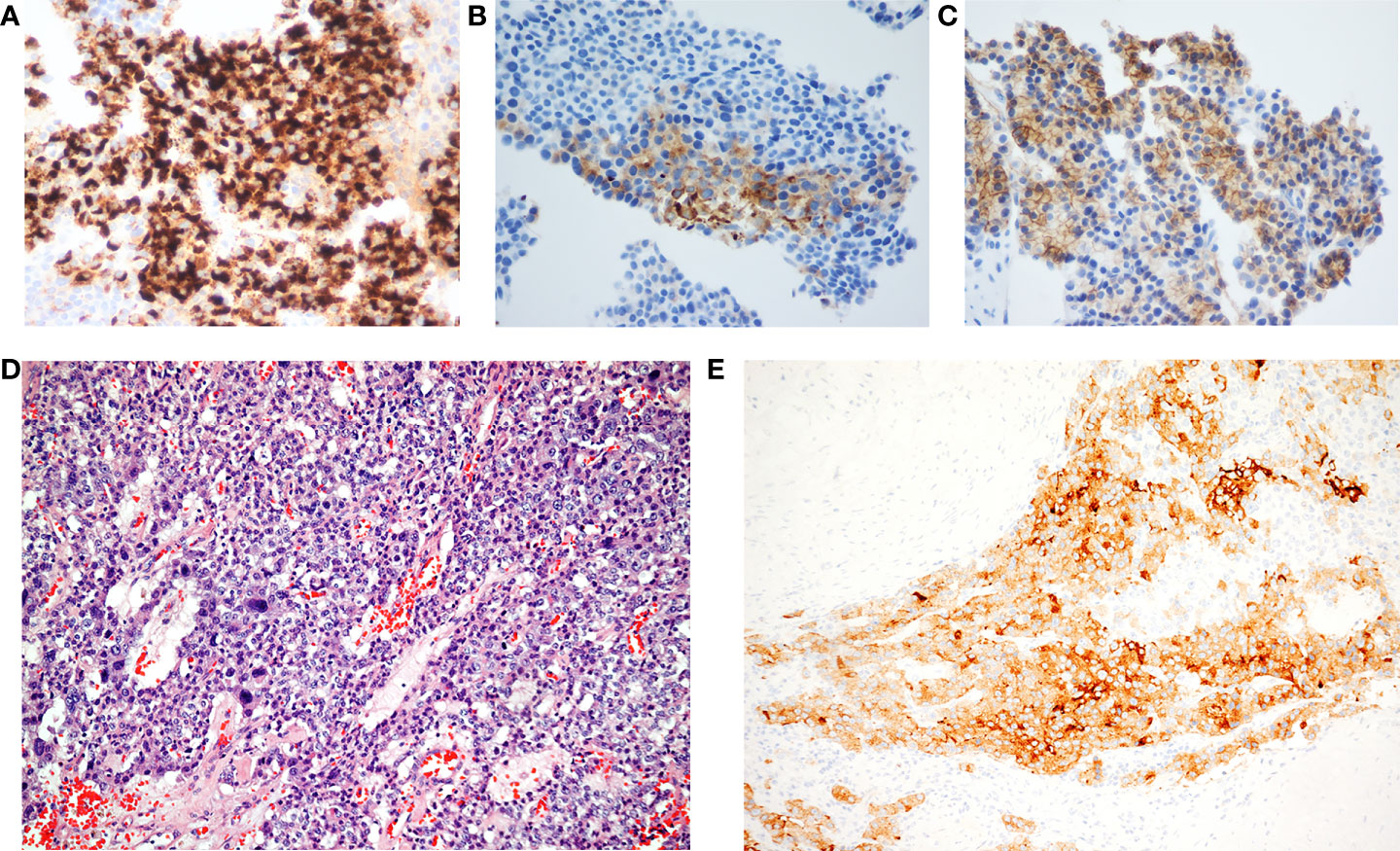
Figure 2 (A) Chromogranin, (B) synaptophysin, (C) somatostatin receptor 2A, and (D) monomorphous round to cuboidal cells arranging in solid and trabecular patterns. These cells have rich cytoplasm, and salt and pepper nuclei (hematoxylin–eosin stain; original magnification, 100×). (E) The immunohistochemical study reveals immunoreactivity of synaptophysin (original magnification, 100×).
There was no metastasis or regional lymph node involvement under initial MRI and further contrast-enhanced computerized tomography (CT). Because the tumor size was too large to differentiate the primary affected organ and the border of the tumor was implicated with surrounding organ, complete resection was not achievable. Therefore, Lanreotide was given initially. However, marked enlargement up to the greatest diameter of 18 cm was noted on CT scan 5 months later (Figure 1B). Coinciding with this, he complained about bulging abdomen interfering with digestion. In the second-line therapeutic regimens, there are multiple anti-tumor therapy including Everolimus- or Sunitinib-based targeted therapy, chemotherapy, and even peptide receptor radionuclide therapy (PRRT). Among these therapies, PRRT was not achievable in our hospital, and reimbursement for Temozolomide for pancreatic NET was not included in the National Health Insurance of our country. Given the bulky and progressive disease status, a second-line therapeutic regimen with cytotoxic chemotherapy was taken into consideration. After offering multidisciplinary treatment options in the full discussion with the patient and his families, CCRT was chosen for strengthening local control. Thus, he began to receive capecitabine + oxaliplatin (XELOX) concurrently with image-guided radiotherapy (IGRT).
We utilized the Hi-Art helical Tomotherapy, version 2.2.4.1 (TomoTherapy, Inc., Madison, WI). The planned total dose was 70 Gy in 35 fractions. The dose statistics was provided in the supplementary material regarding doses of the various organs at risk at each of the three plans, e.g., kidneys, liver, stomach, and spinal cord. After 14 fractions, we performed adaptive treatment planning to better suit regressed tumor. The target volume has shrunk from 1,910 to 1,057 cc (Figures 3A, B). Again, after 10 more fractions, IGRT revealed further shrinkage. Another new adaptive plan was administered, since the target volume has shrunk from 1,057 to 605 cc (Figures 3B, C). Corresponding with radiological response, his urinary and abdominal symptoms improved. The tumor volumes of the enhanced CT before and after radiotherapy have been calculated by the radiation oncologist utilizing a segmentation tool program. It was 2,199.35 cc before radiotherapy and 315.54 cc after radiotherapy. On the third month of CCRT, MRI showed a continuously decreased tumor dimension (Figure 1C). The axial perpendicular diameters of the tumor reduced at least 50% in preoperative CT after CCRT (Figure 1D).
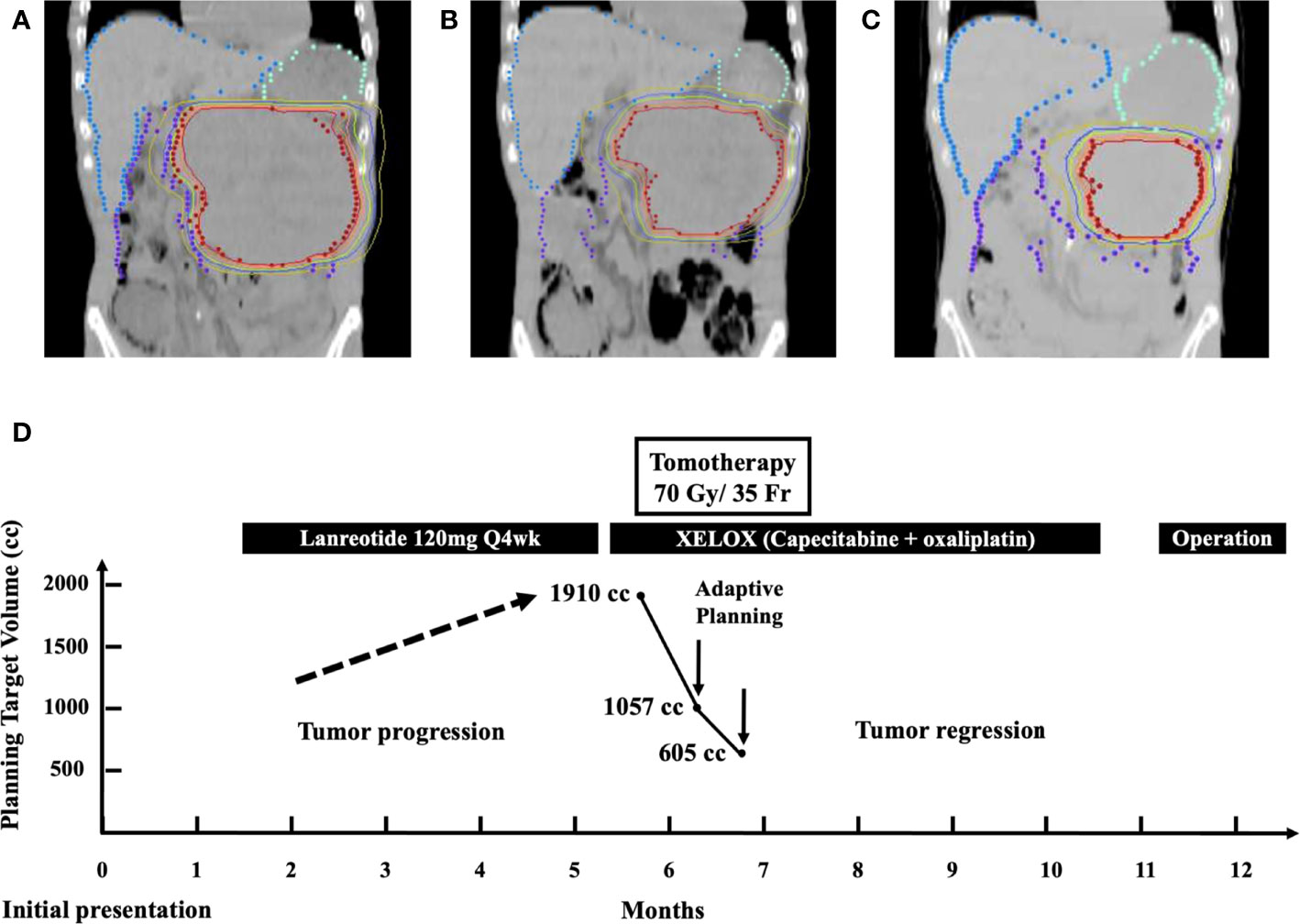
Figure 3 (A) Isodose curves depicting a target volume of 1,910 cc in the initial treatment plan of Tomotherapy. (B) Target volume reduced from 1,910 to 1,057 cc after 14 fractions. (C) Target volume reduced from 1,057 to 605 cc after 10 fractions. (D) Multidisciplinary treatment course of the patient from the day of initial presentation until definitive surgery: adaptive Tomotherapy plans were administrated twice at the timing of substantial target volume reduction from 1910 to 1,057 cc and from 1,057 to 605 cc, respectively.
His baseline performance status before CCRT was Eastern Cooperative Oncology Group (ECOG) grade 1 with only mild urinary frequency but no other complaint. There was also no acute radiation-induced nausea, diarrhea, or abdominal cramping. The radiotherapy-related toxicity was evaluated by the Common Terminology Criteria for Adverse Events (CTCAE) v4.0. Radiotherapy was well-tolerated without acute toxicities >2. In addition, his kidney function, as measured by creatinine clearance, remains mostly the same throughout the radiotherapy. He developed grade 1 radiation-induced dermatitis with mild erythema and later worsened because of a weekend trip swimming in the ocean. Grade 2 dermatitis subsided after Tomotherapy. Apart from avoiding disease progression, CCRT under current regimens resulted in tremendous tumor volume shrinkage. To achieve the best prognosis for the patient, curative surgical resection of tumor was indicated. Laparoscopic subtotal pancreatectomy with spleen preservation was performed, and the surgeons did not report any unusual difficulty. Pathology confirmed a pancreatic NET with the size of 12 × 10 × 6 cm and weighed 315.9 g with American Joint Committee on Cancer (AJCC) stage II, ypT3 (Figure 2). The surgical margin was 15 mm and uninvolved by tumor, which was confined to the pancreas.
The patient recovered well without post-operative complication. There was neither diabetes mellitus, postoperative ileus, nor surgery-related infection after subtotal pancreatectomy. Then, he was under regular surveillance in the outpatient department with abdominal CT every 3 months in the first year post-resection and every 12 months after the first year post-resection. It has been more than 46 months since his diagnosis of pancreatic NET, and he has no recurrence or distant metastasis during regular follow-ups. The overview of the clinical course of this patient is illustrated in Figure 3D.
Surgical resection is the only treatment that can cure pancreatic NETs, and it is recommended to remove all localized and limited metastatic disease (4). However, for our patient who presented with a sizeable tumor burden causing high surgical risk and impossibility of complete tumor resection, other modality like somatostatin analogues was applied. Within 5 months of commencing Lanreotide, this locally advanced tumor kept progressing. We shifted to CCRT for tumor growth control and symptom alleviation. Radiotherapy acted as a bridge forward to curative surgical resection. This patient was able to receive curative operation when the tumor became smaller to less than one-third of the original size after CCRT.
Although radiotherapy is not considered a curative method as much as surgery, Iwata et al. demonstrated that radiotherapy was effective for local control in pancreatic NET. This retrospective study included 11 patients with pancreatic NET who received radiotherapy with maximum dose of 60 Gy in 30 fractions and ended up having 100% disease control rate (1). In addition, symptomatic relief owing to reduction in the physical pressure from large tumor burden was obvious after radiotherapy. However, Iwata et al. disclosed that median progression-free survival (PFS) and median overall survival for patients with pancreatic NET were 5.5 months (95% confidence interval [CI], 3.7–28.2 months) and 35.9 months (95% CI, 9.04 months—not reached), respectively (1). On the contrary, our patient had been alive without disease recurrence for 45 months. In our case, radiotherapy was delivered up to 70 Gy in 35 fractions via Tomotherapy with image-guided and adaptive planning ability. Nine of 11 patients in the study of Iwata et al. utilized three-dimensional conformal radiation therapy, which delivered 50–54 Gy in 25–30 fractions (1). Moreover, the fact that 8 of 11 patients were in metastasis status and only one patient underwent post-RT surgical resection was the major cause of the different prognosis between the study of Iwata et al. and our case (1).
Tomotherapy allows radiation oncologists to visualize inter-fractional radiation responses. Kupelian et al. illustrated the benefit of IGRT in head and neck tumors by comparing the severity of inter-fractional setup errors. Even if imaging guidance was performed every other day, about 10% of all fractions still had a setup error over 5 mm (5). In addition to setup errors and organ mobilization, the dimension of targeted tumor was likely to change after each fraction, which may cause daily deviation. Different anatomic sites also have various setup uncertainties. Studies have shown that inter-fractional displacement in the lung is the largest followed by that in the abdomen (6). The mean 3D displacement (average of lateral, longitudinal, vertical, and rotational direction) of inter-fractional variation in the abdomen was 4.4 mm (6). The advantage of applying IGRT to avoid daily variation before abdominal irradiation is evident.
The recent advancements in radiotherapy technologies have made the delivery of the highly conformal dose to the target volume possible. The organ at risk that needed to be considered first was the kidneys. With the aim of maximal renal parenchymal sparing, radiotherapy was delivered. We provide the dose statistics in the supplementary material regarding the doses of the various organs at risk at each of the three plans, e.g., kidneys, liver, stomach, and spinal cord. With daily IGRT via Tomotherapy, we have carefully prescribed 70 Gy in 35 fractions, exceeding the doses of previous reports for pancreatic NET while causing no chronic-radiation-induced side effect (7). Bresciani et al. reported minimal toxicities from 66 Gy in 30 fractions delivered via Tomotherapy for para-aortic lymphadenopathy in the upper abdomen (8). The majority of patients received 45–50.4 Gy in 1.8 Gy per fraction as their abdominal radiation course. Radiation-induced diarrhea or emesis is commonly seen during abdominal–pelvic radiotherapy. Wang et al. has calculated that mean dose to the small bowel is associated with radiation-induced emesis. They suggested to limit the constraint of the small bowel mean dose to <63% of the prescribed dose (median, 28.35 Gy) (9). In the present case, the mean dose of the small bowel was 26.56, 24.97, and 18.39 Gy, respectively, in all three plans.
Somatostain analogues (SSAs) have been used in advanced or grade 1 or 2 (Ki-67 <10%) enteropancreatic, somatostatin receptor-positive NET (10), and the National Comprehensive Cancer Network (NCCN) guidelines have regarded it as an appropriate drug for symptom control and prevention of tumor progression (3). However, the huge tumor remained enlarged and resistant to Lanreotide in our case. The clinically predictive factors for tumor resistance in SSA can be determined by baseline tumor growth rate, and the patients can then be stratified by disease status and documented progression status to individualized treatment protocol (11). In pathological or molecular aspect, the NCCN guideline recommended using SSA on patients with positive SSTR2A expression (3). Recent research also showed the significant correlation between SSTR2A expression and the clinical efficacy of Lanreotide (12). Increased Ki-67 index and poorly differentiated NET also had unsatisfying SSA treatment outcome (13). In addition, genetic difference had been found between poorly differentiated neuroendocrine carcinoma (NEC) and well-differentiated NET. Poorly differentiated NEC, which includes small- and large- cell NEC, has frequent loss of immunolabeling patterns in p53 and Rb (14). On the other hand, loss of nuclear death domain-associated protein (DAXX) and alpha-thalassemia X-linked intellectual disability syndrome (ATRX) immunolabeling was observed in 5 of 11 (45%) well-differentiated pancreatic NET (14). There is also more clinical value of the finding of ATRX and DAXX gene mutations. In one recent meta-analysis, altered ATRX and DAXX gene had significant correlation with the prognosis of pancreatic NET (15). Disease- and relapse-free survival significantly decreased in patients who had ATRX and DAXX mutations (15). However, the present case did not undergo genetic testing as part of the assessment. More advanced investigation on molecular characteristics of pancreatic NET can help us predict the prognosis and set individualized clinical practice.
Our patient had a huge locally advanced abdominal tumor cured without chronic treatment-related complication. We searched on PubMed and Medline databases for articles written in English from 2016 to 2022 with keywords such as “Abdominal mass,” “Neuroendocrine tumor,” and “locally advanced.” Table 1 shows the clinicopathological characteristics of eight recent cases. The definitive treatment for NET is surgical resection, and the resectability is associated with size and location. The cases whose primary site was in the liver or duodenum or colon received surgery as primary treatment even if the tumor size was up to 20 cm × 16 cm × 11 cm (18–20, 22). Tumors with the pancreas as the primary site and with an initial tumor diameter of 4/3.8 cm were able to be resected (16), and yet, some researchers preferred chemotherapy and radiotherapy in tumors with the greatest diameter up to 9.8 cm (17). In the case of a 9.8-cm pancreatic NET, systemic therapy followed by CCRT with 54 Gy in 30 fractions was applied and reached partial response (17). Similar to our case, CCRT was used. With unprecedented 70 Gy, a 68% reduction in target volume followed by a successful conversion to resectable status was presented in our case. Quite the opposite, colorectal NET was rare and often diagnosed very late, and bowel perforation was noted in the case of Alshammari et al. (19). Namikawa et al. presented a case of gastric NET that developed hepatic metastasis (diameter up to 25 cm). Spontaneous rupture of hepatic metastasis was noted 8 months after initial treatment with everolimus plus somatostatin (21).
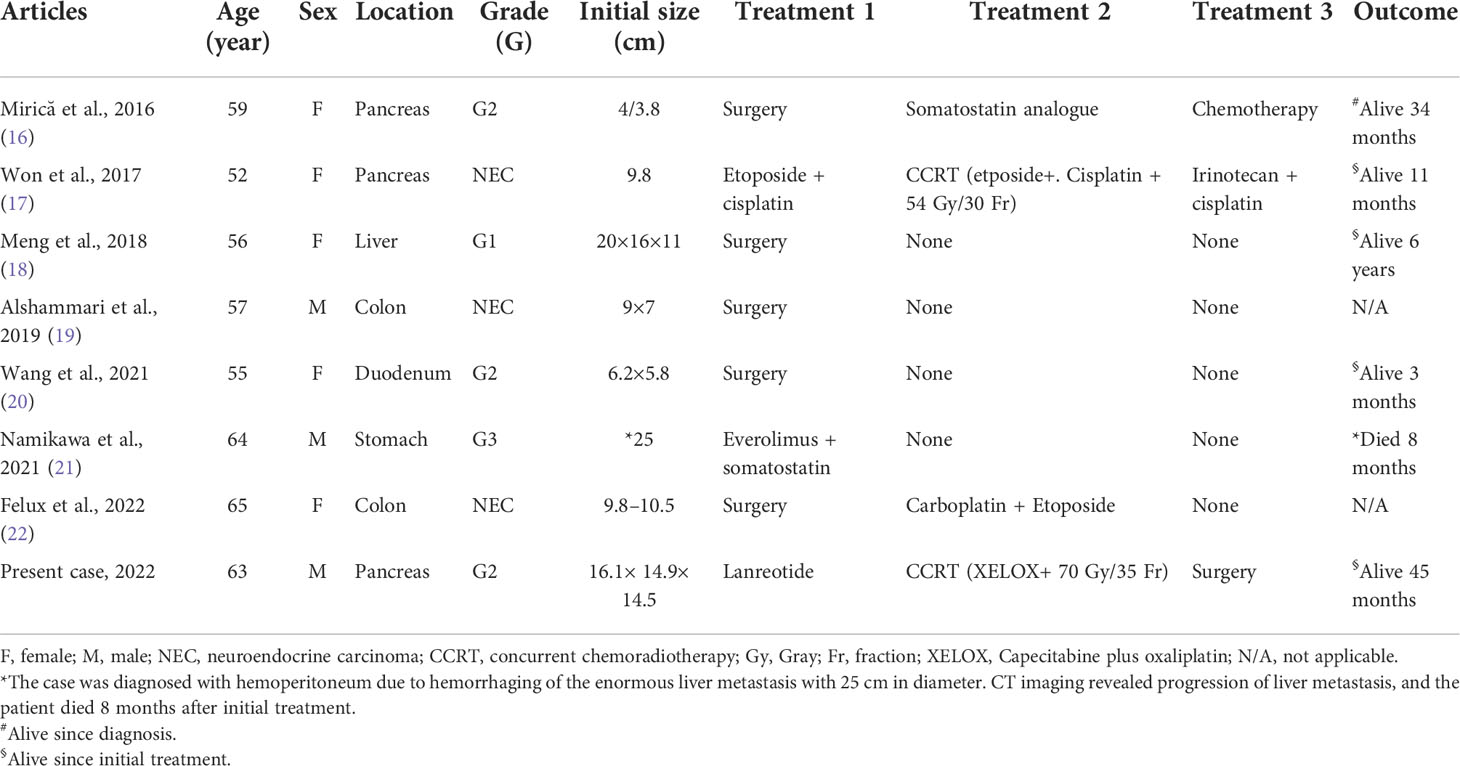
Table 1 Clinicopathological characteristics of reported cases of locally advanced abdominal neuroendocrine tumor.
Further prospective studies with larger patient numbers are required to establish the role of IGRT in huge NET (7). However, it is often not expected to see one with such considerable size in the present case. It is imperative to take into consideration various treatment options for the best benefits of each individual patient. As in our case, IGRT showed its value in optimizing the therapeutic ratio by maximizing target dose safely. Most of all, the conversion of surgical suitability has extended his disease-free survival.
IGRT via Tomotherapy has eased the patients’ symptoms from such 18-cm pancreatic NET in this case. Apart from complete relief of abdominal and pelvic discomfort, CCRT caused a 68% target volume reduction (1,910 to 605 cc) and facilitated further surgical resectability. To the best of our knowledge, this is the first reported case using Tomotherapy to deliver 70 Gy to a pancreatic NET with such favorable outcome. Adaptive planning helps to modify doses according to volumetric changes.
I was at first disheartened with the diagnosis of an inoperable tumor. When I came to the Department of Radiotherapy, I was dismayed and yet impressed by the coordination of simulation scanning and resource intensive re-planning due to markedly shrinkage of the tumor. Following more and more fractions of Tomotherapy, I felt vigorous because of a flatter belly coinciding with the diminished tumor volume. I remember swimming at the beach during the radiation treatment course, and the belly skin became painful, which was later relieved by topical medication from Dr. Lee. She advised me to be heedful of skin care. Owing to the strikingly smaller size after radiotherapy, I was able to take on surgery followed by an uneventful postoperative recovery. It has been almost 4 years, and I am energetic with my cancer-free life.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
K-YT and JL wrote the first draft of the manuscript and made the table. K-YT and Y-SH contributed to image review and figure legends. K-YT generated the timeline figure. H-HL treated the patient, conceived the paper layout, and revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1045752/full#supplementary-material
1. Iwata T, Ueno H, Itami J, Ito Y, Inaba K, Morizane C, et al. Efficacy of radiotherapy for primary tumor in patients with unresectable pancreatic neuroendocrine tumors. Jpn J Clin Oncol (2017) 47(9):826–31. doi: 10.1093/jjco/hyx081
2. Hill JS, McPhee JT, McDade TP, Zhou Z, Sullivan ME, Whalen GF, et al. Pancreatic neuroendocrine tumors: The impact of surgical resection on survival. Cancer (2009) 115(4):741–51. doi: 10.1002/cncr.24065
3. Shah MH, Goldner WS, Benson AB, Bergsland E, Blaszkowsky LS, Brock P, et al. Neuroendocrine and adrenal tumors, version 2. 2021 Nccn Clin Pract Guidelines Oncol J Natl Compr Canc Netw (2021) 19(7):839–68. doi: 10.6004/jnccn.2021.0032
4. Akirov A, Larouche V, Alshehri S, Asa SL, Ezzat S. Treatment options for pancreatic neuroendocrine tumors. Cancers (Basel) (2019) 11(6):828. doi: 10.3390/cancers11060828
5. Kupelian P, Langen K. Helical tomotherapy: Image-guided and adaptive radiotherapy. Front Radiat Ther Oncol (2011) 43:165–80. doi: 10.1159/000322420
6. Zhou J, Uhl B, Dewit K, Young M, Taylor B, Fei DY, et al. Analysis of daily setup variation with tomotherapy megavoltage computed tomography. Med Dosim (2010) 35(1):31–7. doi: 10.1016/j.meddos.2009.01.005
7. Chan DL, Thompson R, Lam M, Pavlakis N, Hallet J, Law C, et al. External beam radiotherapy in the treatment of gastroenteropancreatic neuroendocrine tumours: A systematic review. Clin Oncol (R Coll Radiol) (2018) 30(7):400–8. doi: 10.1016/j.clon.2018.03.006
8. Bresciani S, Garibaldi E, Cattari G, Maggio A, Di Dia A, Delmastro E, et al. Dose to organs at risk in the upper abdomen in patients treated with extended fields by helical tomotherapy: A dosimetric and clinical preliminary study. Radiat Oncol (2013) 8:247. doi: 10.1186/1748-717x-8-247
9. Wang YM, Chen YF, Lee PY, Ho MW, Huang EY. Radiation-induced emesis (Rie) in extended-field radiotherapy for gynecological malignancies: Dosimetric and non-dosimetric factors. Curr Oncol (2021) 28(5):3602–9. doi: 10.3390/curroncol28050308
10. Caplin ME, Pavel M, Ćwikła JB, Phan AT, Raderer M, Sedláčková E, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med (2014) 371(3):224–33. doi: 10.1056/NEJMoa1316158
11. Carmona-Bayonas A, Jiménez-Fonseca P, Lamarca Á, Barriuso J, Castaño Á, Benavent M, et al. Prediction of progression-free survival in patients with advanced, well-differentiated, neuroendocrine tumors being treated with a somatostatin analog: The getne-trasgu study. J Clin Oncol (2019) 37(28):2571–80. doi: 10.1200/jco.19.00980
12. Kasajima A, Papotti M, Ito W, Brizzi MP, La Salvia A, Rapa I, et al. High interlaboratory and interobserver agreement of somatostatin receptor immunohistochemical determination and correlation with response to somatostatin analogs. Hum Pathol (2018) 72:144–52. doi: 10.1016/j.humpath.2017.11.008
13. Lee L, Ramos-Alvarez I, Jensen RT. Predictive factors for resistant disease with Medical/Radiologic/Liver-directed anti-tumor treatments in patients with advanced pancreatic neuroendocrine neoplasms: Recent advances and controversies. Cancers (Basel) (2022) 14(5):1250. doi: 10.3390/cancers14051250
14. Yachida S, Vakiani E, White CM, Zhong Y, Saunders T, Morgan R, et al. Small cell and Large cell neuroendocrine carcinomas of the pancreas are genetically similar and distinct from well-differentiated pancreatic neuroendocrine tumors. Am J Surg Pathol (2012) 36(2):173–84. doi: 10.1097/PAS.0b013e3182417d36
15. Wang F, Xu X, Ye Z, Qin Y, Yu X, Ji S. Prognostic significance of altered Atrx/Daxx gene in pancreatic neuroendocrine tumors: A meta-analysis. Front Endocrinol (Lausanne) (2021) 12:691557. doi: 10.3389/fendo.2021.691557
16. Mirică A, Bădărău IA, Mirică R, Păun S, Păun DL. A rare case of metastasized non-functional pancreatic neuroendocrine tumor with a good long-term survival. J Med Life (2016) 9(4):369–72. doi: 10.22336/jml.2016.0409
17. Won YG, Seo KJ, Hyeon J, Shin OR, Chang E, Sun S, et al. Gastroenteropancreatic-origin neuroendocrine carcinomas: Three case reports with favorable responses following localized radiotherapy and a review of literature. Med (Baltimore) (2017) 96(49):e9009. doi: 10.1097/md.0000000000009009
18. Meng XF, Pan YW, Wang ZB, Duan WD. Primary hepatic neuroendocrine tumor case with a preoperative course of 26 years: A case report and literature review. World J Gastroenterol (2018) 24(24):2640–6. doi: 10.3748/wjg.v24.i24.2640
19. Alshammari TF, Hakami RA, Alali MN, AlShammari S, Zayed MA, AlSohaibani MO, et al. A perforated colonic neuroendocrine tumor with liver metastasis: A case report and literature review. Am J Case Rep (2019) 20:920–5. doi: 10.12659/ajcr.916288
20. Wang X, Wu Y, Cao X, Zhang X, Cheng Y, Kong L. Duodenal neuroendocrine tumor: A rare case report. Med (Baltimore) (2021) 100(6):e24635. doi: 10.1097/md.0000000000024635
21. Namikawa T, Yokota K, Yamaguchi S, Fukudome I, Munekage M, Uemura S, et al. Spontaneous intra-abdominal hemorrhage of a well-differentiated, grade 3 gastric neuroendocrine tumor during drug-based treatment. Clin J Gastroenterol (2021) 14(4):1244–9. doi: 10.1007/s12328-021-01433-3
Keywords: Tomotherapy, pancreas, abdomen, unresectable neuroendocrine tumor (NET), neuroendocrine neoplasm (NEN), image-guided radiotherapy (IGRT), adaptive planning, case report
Citation: Tu K-Y, Huang Y-S, Lau J and Lee H-H (2022) Adaptive Tomotherapy for locally advanced unresectable pancreatic neuroendocrine tumor: Case report and literature review. Front. Oncol. 12:1045752. doi: 10.3389/fonc.2022.1045752
Received: 16 September 2022; Accepted: 25 October 2022;
Published: 14 November 2022.
Edited by:
Mattia Falchetto Osti, Sapienza University of Rome, ItalyReviewed by:
Vincent Vinh-Hung, Centre Hospitalier de la Polynésie Française (CHPF), French PolynesiaCopyright © 2022 Tu, Huang, Lau and Lee. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hsin-Hua Lee, ZHIuaGgubGVlQGdtYWlsLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.