
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 06 January 2023
Sec. Breast Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1043185
This article is part of the Research Topic Reviews in Breast Cancer View all 20 articles
Background: Early diagnosis of axillary lymph node metastasis is very important for the recurrence and prognosis of breast cancer. Currently, Lymph node biopsy is one of the important methods to detect lymph node metastasis in breast cancer, however, its invasiveness might bring complications to patients. Therefore, this study investigated the diagnostic performance of multiple ultrasound diagnostic methods for axillary lymph node metastasis of breast cancer.
Materials and methods: In this study, we searched PubMed, Web of Science, CNKI and Wan Fang databases, conducted Bayesian network meta-analysis (NMA) on the studies that met the inclusion criteria, and evaluated the consistency of five different ultrasound imaging techniques in axillary lymph node metastasis of breast cancer. Funnel graph was used to evaluate whether it had publication bias. The diagnostic performance of each ultrasound imaging method was ranked using SUCRA
Results: A total of 22 papers were included, US+CEUS showed the highest SUCRA values in terms of sensitivity (SEN) (0.874), specificity (SPE) (0.911), positive predictive value (PPV) (0.972), negative predictive value (NPV) (0.872) and accuracy (ACC) (0.990).
Conclusion: In axillary lymph node metastasis of breast cancer, the US+CEUS combined diagnostic method showed the highest SUCRA value among the five ultrasound diagnostic methods. This study provides a theoretical basis for preoperative noninvasive evaluation of axillary lymph node metastases in breast cancer patients and clinical treatment decisions.
Systematic review registration: https://www.crd.york.ac.uk/PROSPERO/, identifier CRD42022351977.
Breast Cancer is the most common malignancy tumor in women worldwide, and its incidence is much higher than other cancers (1), it ranks first in incidence and second in mortality among female malignant tumors (2). The occurrence of axillary lymph nodes metastasis is a key factor affecting the recurrence and prognosis of breast cancer. In order to avoid the spread of cancer cells through lymph nodes, axillary lymph node dissection is often performed in breast cancer patients. Although this method can effectively inhibit the recurrence of breast cancer and improve the prognosis, it may cause a series of complications, such as lymph node edema, Cellulitis, etc. Currently, the axillary staging and treatment of early breast cancer has changed from complete axillary lymph node dissection to sentinel lymph node biopsy (SLNB), which has a higher accuracy rate and a lower rate of postoperative complications (3). However, as an invasive procedure, SLNB may still lead to postoperative complications such as subcutaneous effusion, nerve injury, and restriction of shoulder joint movement, and the incidence of SLNB is 7.1% (4). Therefore, an accurate assessment of the extent of axillary lymph node involvement by non-invasive methods before surgery can minimize the incidence of postoperative complications caused by invasive methods. In non-invasive diagnosis, the sensitivity (SEN) of axillary lymph node palpation is only 33% to 68% (5), computer tomography (CT), positron emission tomography (PET) and other diagnosis methods (6) have the disadvantages of high price, radiation, etc., and do not show the obvious correlation in the evaluation of axillary lymph node metastasis in breast cancer (7, 8).
As one of the main detection methods of non-invasive imaging, ultrasound (US) has the advantages of no radiation, economy, convenience, and real-time imaging, and has become a common imaging method for the diagnosis of axillary lymph node metastasis in breast cancer. However, some studies have shown that 2D ultrasound has low SEN and specificity(SPE) in detecting benign and malignant lymph nodes due to its poor imaging of deep axillary lymph nodes and inability to show typical morphological changes (9). Ultrasound elastography (UE), contrast-enhanced ultrasound (CEUS), and other techniques may allow better differentiation between benign and malignant masses (10, 11). Studies have shown that elastography has high diagnostic performance in distinguishing benign from metastatic LNs, however, Park et al (12) showed that elastography did not have a significant advantage in evaluating metastatic lymph nodes. Tsai et al (13) found that US+UE showed higher SEN and SPE than US and UE alone. With the continuous progress of ultrasound technology, CEUS is widely used in clinical practice, and has higher SEN and SPE for lymph node metastasis, so that accuracy of diagnosing axillary lymph node metastasis in breast cancer is better improved.
The diagnostic performance of ultrasound diagnostic techniques for breast Cancer axillary lymph nodes is still controversial, and the results obtained by different clinical trials are also different. Therefore, we comprehensively analyze the diagnostic performance of US, UE, CEUS, US+UE, and US+CEUS.
This study conducted an NMA of the diagnostic performance of US, UE, CEUS, US+UE, and US+CEUS using two or more published studies of ultrasound imaging methods, comparison of different ultrasound imaging techniques for detection of SEN, SPE, positive predictive value (PPV), negative predictive value (NPV), accuracy (ACC) in axillary lymph node metastases. Helping clinicians find more accurate methods for diagnosing axillary lymph node metastases in breast cancer thereby improving patient outcomes.
We searched for relevant studies published in Chinese National Knowledge Infrastructure, PubMed, Web of Science, and Wan Fang before July 2022. Using “lymph node”, “Lymphatic Metastasis”, Elasticity imaging Techniques”, “Ultrasonography”, “Breast” cancer”, “ Contrast Ultrasound “ and other keywords were searched (Table 1). The included references were also screened to ensure that all included references met the inclusion and exclusion criteria.
The relevant inclusion criteria are as follows: 1) Population: patients with pathologically proven breast cancer with axillary lymph node metastasis; 2) Diagnosis method: including two or more ultrasound imaging methods; 3) Study result should include calculable indicators such as true-positive (TP), false-positive (FP), true-negative (TN), false-negative (FN) of describe the diagnostic performance of the study; 4) Type of study: diagnostic trial.
The relevant exclusion criteria include the following aspects: (1) The study population is non-human studies or studies with axillary lymph node metastases of breast cancer without pathological confirmation; (2) the diagnostic performance indicators in the studies are incomplete; (3) Editorials, reviews, case reports, meeting minutes, guidelines, etc.
The titles and abstracts of retrieved articles were read by two authors, respectively. studies that do not meet the inclusion criteria will be excluded according to the inclusion and exclusion criteria established in this study.
Data extraction was performed on the originally included studies and was independently extracted by two investigators. The extracted data included: 1) The first author; 2) Research publication time; 3) Country of the first author; 4) The mean of the patient’s age; 5) Diagnostic method; 6) Sample size; 7) The results of the study were TP, FP, TN, FN.
This meta-analysis has been registered on the PROSPERO website with registration number CRD42022336701.We divided the different ultrasound diagnostic methods in the included study into five groups, namely US, UE, CEUS, US+UE, and US+CEUS, and used NMA to analyze the diagnostic performance of the five groups in the diagnosis of axillary lymph node metastasis in breast cancer. According to the PRISMA NMA list, Stata’s(version-15.1) -Markov chain Monte Carlo model was used. The NMA was aggregated and analyzed in a Bayes-based framework, and the five groups of data were compared directly and indirectly. The diagnostic performance of each diagnostic method was judged by analyzing its SEN, SPE, PPV, NPV, and ACC indicators, and using the P value or I2 to evaluate heterogeneity. P value <0.05 or I2>90% indicates that the heterogeneity was large.
We also use the nodal method to evaluate the inconsistency in NMA, using the surface under the cumulative ranking curve (SUCRA) to calculate the probability of each imaging mode. The value of SUCRA is between 0 and 1(0≤SUCRA ≤ 1), when SUCRA is 1, it indicates that the intervention is absolutely effective, and when SUCRA is 0, it indicates that the intervention is absolutely ineffective. According to the value of SUCRA, the pros and cons of the diagnostic methods can be sorted, so as to screen out the most effective diagnostic methods.
This study used funnel plots to detect possible publication bias, and the results showed that the distribution of funnel plots was roughly symmetric, suggesting that there was no publication bias or other bias in the study (Figure 1).
This study found 8072 studies from the database based on keywords, of which 1999 articles were extracted from PubMed, 3502 articles were extracted from Web of Science,1214 articles were extracted from Wan Fang, and 1357 articles were extracted from CNKI. A total of 8050 studies that did not meet the inclusion criteria were excluded from this study, and 22 studies were finally included (3, 10, 12–31) (Table 2). We included published studies using two or more ultrasound imaging methods, and analyzed and evaluated the extracted diagnostic indicators.
A total of 7776 patients (range, 42-313) were included in 22 studies (3, 10, 12–31), all of whom were pathologically confirmed to have lymph node metastases of breast cancer. Among these studies, there were 2 retrospective studies and 20 randomized controlled studies. There were many studies on the US, UE, and US+UE in the included literature, among which 18 studies compared US vs UE,11 studies compared US vs US+UE, 11 studies compared UE vs US+UE, 5 studies compared US vs CEUS. Five studies compared US vs US+CEUS (Table 3). The quality assessment of the literature was based on QUADAS-2 scale to evaluate 22 studies from four aspects: Patient Selection, Reference Standard, Index Test, and Flow Timing. The results show that the overall quality of the included studies was relatively satisfactory (Figure 2). Among the 22 articles, 5 had an unclear risk of bias in the Index Test, which may be due to the differences in the operators performing the tests and their experience levels.
The Network evidence diagram was shown in Figure 3. In this study, the consistency of direct comparison and indirect comparison of the included studies was analyzed, and the results showed that all studies were P > 0.05, indicating that the studies had good consistency.
NMA showed that US+CEUS [MD=0.15, 95%CI (0.02, 0.28)] was superior to the control group (CEUS) in diagnosing SEN in axillary lymph node metastasis of breast cancer (Table 4A). US+CEUS ranked first in SEN for axillary lymph node metastasis of breast cancer in different methods(SUCRA: 87.4% as shown in Table 5) (Figure 4).
NMA showed that US+CEUS [MD=0.16, 95% CI (0.01, 0.31)] was superior to the control group (UE) in diagnosing of SPE in axillary lymph node metastasis in breast cancer. US+CEUS [MD=0.21, 95%CI (0.07, 0.35)] and CEUS [MD=0.17, 95%CI (0.03, 0.31)] were superior to the control group (US) in diagnosing of SPE in axillary lymph node metastasis in breast cancer (Table 4B). US+CEUS ranked first in SPE for axillary lymph node metastasis of breast cancer in different methods(SUCRA: 90.8% as shown in Table 5) (Figure 5).
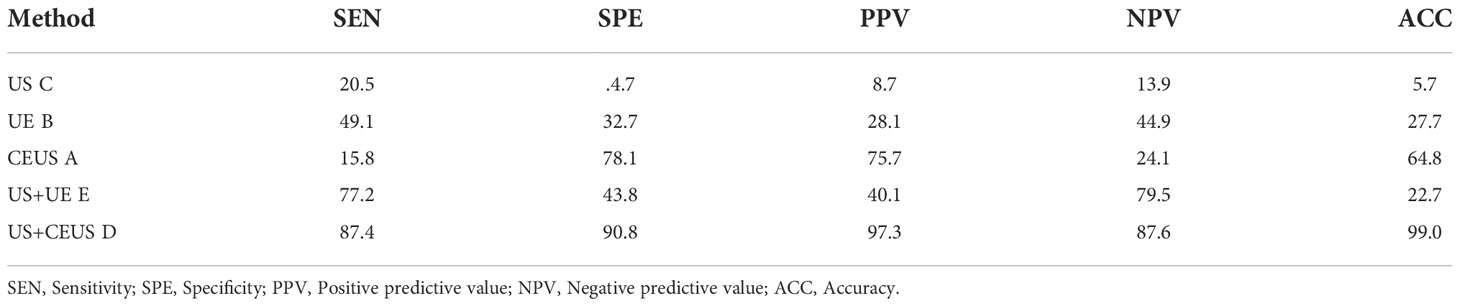
Table 5 SUCRA values of preoperative detection of axillary lymph node metastases in breast cancer patients by 5 different ultrasonic diagnostic methods.
NMA showed that US+CEUS [MD=0.18, 95%CI (0.05, 0.31)] was superior to the control group (US+UE) in diagnosing of PPV in axillary lymph node metastasis of breast cancer. US+CEUS [MD=0.20, 95%CI (0.08, 0.34)] was better than control group (UE) in diagnosing of PPV in axillary lymph node metastasis of breast cancer. US+CEUS [MD=0.22, 95%CI (0.11, 0.33)] and CEUS [MD=0.15, 95%CI (0.04, 0.26)] were superior to the control group (US) in the diagnosing of PPV in axillary lymph node metastasis of breast cancer (Table 4C). US+CEUS ranked first in PPV for axillary lymph node metastasis of breast cancer in different methods (SUCRA: 97.3% as shown in Table 5) (Figure 6).
NMA showed that US+CEUS [MD=0.10, 95%CI (0.01, 0.19)] and US+UE [MD=0.08, 95%CI (0.02, 0.14)] were superior to the control group (US+UE) in diagnosing of NPV in axillary lymph node metastasis of breast cancer (Table 4D). US+CEUS ranked first in NPV for axillary lymph node metastasis of breast cancer in different methods (SUCRA:87.6% as shown in Table 5) (Figure 7).
NMA showed that US+CEUS was superior to the control group in diagnosing of ACC in axillary lymph node metastasis of breast cancer (US+UE, UE, US) (Table 4E). US+CEUS ranked first in ACC for axillary lymph node metastasis of breast cancer in different methods(SUCRA:99.0% as shown in Table 5) (Figure 8).
Early identification of axillary lymph node metastasis in breast cancer is crucial for the prognosis and treatment of breast cancer patients, and SLNB is a necessary means to detect whether breast cancer has lymph node metastasis (32). However, SLNB usually carries a risk of acute or long-term complications including nerve damage, lymphedema, and wound infection etc (33). Therefore, accurate prediction of axillary lymph node metastasis in breast cancer by non-invasive diagnosis is an urgent problem to be solved. This study evaluated the diagnostic performance of US, UE, CEUS, US+UE, and US+CEUS of axillary lymph node metastasis in breast cancer patients with in detail from five aspects: SEN, SPE, PPV, NPV, and ACC. This is the first systematic review and NMA of non-invasive imaging modalities of ultrasound diagnostic methods in patients with pathologically confirmed breast cancer with axillary lymph node metastases. A total of 22 articles were included in this study, with a total of 7776 patients (range, 42-313), The combined ultrasound method was significantly better than the single ultrasound method in the diagnosis of axillary lymph node metastasis in breast cancer. Compared with other diagnostic methods, US+CEUS showed obvious advantages in predicting axillary lymph node metastasis in breast cancer in all aspects. The SUCRA values showed that CEUS had higher SEN and higher accuracy than US and UE alone in a single diagnostic method. Our analysis showed that US+CEUS could be an effective non-invasive diagnostic method for clinical diagnosis of axillary lymph node metastasis in breast cancer.
The US is considered to be a routine non-invasive diagnostic method for diagnosing axillary lymph node metastasis in breast cancer. The status of axillary lymph node s is usually assessed by blood flow, size, and shape. However, US diagnosis usually relies on the doctor’s own experience and skills, and there may be a higher misdiagnosis rate, and its SEN and SPE are quite different (27). The SPE and SEN of this diagnostic method in this study were 70% and 86%, respectively, similar to the results of Qing.Z et al. (28). UE is widely used in the diagnosis of superficial organs and lymph node metastases. Wang J et al. (24) believed that traditional two-dimensional ultrasound technology is not ideal for the differential diagnosis of breast cancer axillary lymph node metastases, while UE can accurately reflect tissue stiffness. Thus, the types of breast cancer axillary lymph node metastasis can be identified semi-quantitatively. We analyzed the 12 included articles and found that the SEN and SPE of UE for breast cancer axillary lymph node metastasis were 83% and86%, respectively, which were consistent with the results of Choi J.J et al (15).
The morphology of lymph nodes and blood flow distribution are studied using conventional ultrasound, although it is difficult to identify small infiltrative foci that do not result in morphological changes in lymph nodes; Doppler ultrasound is unable to detect anterior lymph nodes because of its low signal-to-noise ratio, inability to see microvessels, and difficulty displaying tissue perfusion. The examination of abdominopelvic and superficial organ lesions as well as the detection of SLN in breast cancer have all benefited from the widespread use of CEUS, a novel technology for the dynamic assessment of tissue perfusion utilizing ultrasonic contrast agent (UCA). It has been commonly used for the diagnosis of benign and malignant breast cancer and the assessment of axillary lymph node metastasis. Ultrasonography under enhanced conditions can reveal some of the new and immature tissues around the tumor, and the boundary and internal blood flow of the primary breast cancer are more clearly shown compared to conventional ultrasound. It is mainly by injecting a contrast agent into the patient’s body to enhance the outline of the axillary lymph node according to the concentration of the contrast agent in the patient’s axillary lymph nodes. compared with normal lymph nodes, metastatic lymph nodes showed longer duration of enhancement as well as higher imaging intensities. CEUS has been shown to be more accurate than other ultrasound methods in previous studies, and our study showed the same results with a SEN and SPE of 82% and 88%, respectively.
Most of the current clinical prediction models of axillary lymph node metastasis of breast cancer are based on clinicopathological characteristics such as age, tumor size, and histological grade. However, these clinicopathological features are usually acquired intraoperatively or postoperatively, and the diagnostic performance of single diagnostic imaging is not ideal. Therefore, we analyzed combined diagnostic methods, such as US+CEUS, and US+UE. Compared with previous studies, the combined diagnostic method was significantly higher than the single diagnostic method in terms of diagnostic performance, especially the US+CEUS combined diagnostic method showed satisfactory predictive results in terms of SEN and SPE, the mean reason is that conventional ultrasound must first locate lymph nodes in order to distinguish between benign and malignant ones; however, some lymph nodes are challenging to distinguish from nearby tissues and are frequently missed. However, some lymph nodes are hard to spot in the tissues around them and are frequently missed. By using enhanced microbubbles to detect these occult lymph nodes, CEUS can aid in their detection. It can also correct some lymph nodes that conventional ultrasonography incorrectly labeled as benign due to minor metastases. Traditional ultrasonography misdiagnoses lymph nodes as benign because of minor metastases. The combined diagnosis of the two can offer a thorough assessment of the lymph nodes’ size, shape, internal structure, and lymphatic drainage, and evaluation of the internal anatomy, lymphatic drainage, size, morphology, and diagnostic value of axillary lymph nodes. There were still some limitations in the study. First, this study needs to include kinds of literature containing two or more diagnostic methods. However, it is found that the number of such articles is limited through search, resulting in an uneven number of studies on each diagnostic method. Second, some of the results of this study may have an impact on the results of the study due to differences in the number of patients between studies. Third, due to the differences in the experience level of the radiologist in the diagnosis of diseases, there are potential differences in the studies. In view of the above deficiencies, it is suggested that readers should reasonably refer to and select the diagnostic method of this study according to clinical practice and actual results.
In conclusion, the analysis of this study showed that single US, UE, and CEUS have limited diagnostic performance in diagnosing axillary lymph nodes metastases in breast cancer. Compared with single ultrasound imaging, US + CEUS have highest diagnostic performance of axillary lymph nodes metastasis in breast cancer in the combined diagnosis, which can provide a reliable basis for breast cancer axillary lymph nodes metastasis, However, due to the lack of literature, more prospective studies are still needed to confirm this conclusion.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Study concept and design: S-RW, Q-LL. Acquisition of data: Q-LL, MC, S-RW, TZ. Analysis and interpretation of data: S-RW, TZ. Drafting of the manuscript: TZ, P-SZ. Critical revision of the manuscript for important intellectual content: JL. Approval of the final manuscript: JL, S-RW. Study supervision: JL. All authors contributed to the article and approved the submitted version.
This work was supported by the Non-profit Central Research Institute Fund of Chinese Academy of Medical Sciences(2020-PT330-003); Open Research Fund of NHC Key Laboratory of Prevention and Treatment of Central Asia High Incidence Diseases; The First Affiliated Hospital of Shihezi University School of Medicine Youth Fund Project (QN202107); The First Affiliated Hospital of Shihezi University School of Medicine Youth Fund Project (QN202126); Supported by the National Natural Science Foundation of China (82060318).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer MX declared a shared parent affiliation with the author X-WC to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
3. Chang JM, Leung JWT, Moy L, Ha SM, Moon WK. Axillary nodal evaluation in breast cancer: State of the art. Radiology (2020) 295(3):500–15. doi: 10.1148/radiol.2020192534
4. Balasubramanian I, Fleming CA, Corrigan MA, Redmond HP, Kerin MJ, Lowery AJ. Meta-analysis of the diagnostic accuracy of ultrasound-guided fine-needle aspiration and core needle biopsy in diagnosing axillary lymph node metastasis. Br J Surg (2018) 105(10):1244–53. doi: 10.1002/bjs.10920
5. Valente SA, Levine GM, Silverstein MJ, Rayhanabad JA, Weng-Grumley JG, Ji L, et al. Accuracy of predicting axillary lymph node positivity by physical examination, mammography, ultrasonography, and magnetic resonance imaging. Ann Surg Oncol (2012) 19(6):1825–30. doi: 10.1245/s10434-011-2200-7
6. Suvannarerg V, Chitchumnong P, Apiwat W, Lertdamrongdej L, Tretipwanit N, Pisarnturakit P, et al. Diagnostic performance of qualitative and quantitative shear wave elastography in differentiating malignant from benign breast masses, and association with the histological prognostic factors. Quant Imaging Med Surg (2019) 9(3):386–98. doi: 10.21037/qims.2019.03.04
7. Qiu SQ, Zeng HC, Zhang F, Chen C, Huang WH, Pleijhuis RG, et al. A nomogram to predict the probability of axillary lymph node metastasis in early breast cancer patients with positive axillary ultrasound. Sci Rep (2016) 6:21196. doi: 10.1038/srep21196
8. Choi YJ, Ko EY, Han BK, Shin JH, Kang SS, Hahn SY. High-resolution ultrasonographic features of axillary lymph node metastasis in patients with breast cancer. Breast (2009) 18(2):119–22. doi: 10.1016/j.breast.2009.02.004
9. Alvarez S, Añorbe E, Alcorta P, López F, Alonso I, Cortés J. Role of sonography in the diagnosis of axillary lymph node metastases in breast cancer: A systematic review. AJR Am J Roentgenol (2006) 186(5):1342–8. doi: 10.2214/AJR.05.0936
10. Wojcinski S, Dupont J, Schmidt W, Cassel M, Hillemanns P. Real-time ultrasound elastography in 180 axillary lymph nodes: elasticity distribution in healthy lymph nodes and prediction of breast cancer metastases. BMC Med Imaging (2012) 12:35. doi: 10.1186/1471-2342-12-35
11. Sadigh G, Carlos RC, Neal CH, Dwamena BA. Ultrasonographic differentiation of malignant from benign breast lesions: A meta-analytic comparison of elasticity and BIRADS scoring. Breast Cancer Res Treat (2012) 133(1):23–35. doi: 10.1007/s10549-011-1857-8
12. Park YM, Fornage BD, Benveniste AP, Fox PS, Bassett RL Jr., Yang WT. Strain elastography of abnormal axillary nodes in breast cancer patients does not improve diagnostic accuracy compared with conventional ultrasound alone. AJR Am J Roentgenol (2014) 203(6):1371–8. doi: 10.2214/AJR.13.12349
13. Tsai WC, Lin CK, Wei HK, Yu BL, Hung CF, Cheng SH, et al. Sonographic elastography improves the sensitivity and specificity of axilla sampling in breast cancer: a prospective study. Ultrasound Med Biol (2013) 39(6):941–9. doi: 10.1016/j.ultrasmedbio.2012.12.013
14. Zhao QL, Xia XN, Zhang Y, He JJ, Sheng W, Ruan LT, et al. Elastosonography and two-dimensional ultrasonography in diagnosis of axillary lymph node metastasis in breast cancer. Clin Radiol (2018) 73(3):312–8. doi: 10.1016/j.crad.2017.09.013
15. Choi JJ, Kang BJ, Kim SH, Lee JH, Jeong SH, Yim HW, et al. Role of sonographic elastography in the differential diagnosis of axillary lymph nodes in breast cancer. J Ultrasound Med (2011) 30(4):429–36. doi: 10.7863/jum.2011.30.4.429
16. Zhou J, Zhang Q, Zhang Q, Yan L, Gao Q. Evaluation of the property of axillary lymph nodes and analysis of lymph node metastasis factors in breast cancer by ultrasound elastography. Comput Math Methods Med 2022 (2022), 8066289. doi: 10.1155/2022/8066289
17. Xu Y, Bai X, Chen Y, Jiang L, Hu B, Hu B, et al. Application of real-time elastography ultrasound in the diagnosis of axillary lymph node metastasis in breast cancer patients. Sci Rep (2018) 8(1):10234. doi: 10.1038/s41598-018-28474-y
18. Zhao Q, Sun JW, Zhou H, Du LY, Wang XL, Tao L, et al. Pre-operative conventional ultrasound and sonoelastography evaluation for predicting axillary lymph node metastasis in patients with malignant breast lesions. Ultrasound Med Biol (2018) 44(12):2587–95. doi: 10.1016/j.ultrasmedbio.2018.07.017
19. Luo S, Yao G, Hong Z, Zhang S, Wang W, Zhang J, et al. Qualitative classification of shear wave elastography for differential diagnosis between benign and metastatic axillary lymph nodes in breast cancer. Front Oncol (2019) 9:533. doi: 10.3389/fonc.2019.00533
20. Meng L, Ailian L, Tianxiang L, Fenging F, Dan G, Xuan S, et al. The value of acoustic palpation tissue and quantitative techniques and conventional ultrasound in qualitative diagnosis axillary lymph nodes in breast cancer. Chin J Clin (2019) 13(04):281–5.
21. Luo C, Lu L, Zhang W, Li X, Zhou P, Ran Z. The value of shear wave elastography in the diagnosis of breast cancer axillary lymph node metastasis and its correlation with molecular classification of breast masses. Front Oncol (2022) 12:846568. doi: 10.3389/fonc.2022.846568
22. Pulappadi VP, Paul S, Hari S, Dhamija E, Manchanda S, Kataria K, et al. Role of shear wave elastography as an adjunct to axillary ultrasonography in predicting nodal metastasis in breast cancer patients with suspicious nodes. Br J Radiol (2022) 95(1134):20220055. doi: 10.1259/bjr.20220055
23. Ng WL, Omar N, Ab Mumin N, Ramli Hamid MT, Vijayananthan A, Rahmat K. Diagnostic accuracy of shear wave elastography as an adjunct tool in detecting axillary lymph nodes metastasis. Acad Radiol (2022) 29 Suppl 1:S69–s78. doi: 10.1016/j.acra.2021.03.018
24. Wang J, Ben Z, Gao S, Lyu S, Wei X. The role of ultrasound elastography and virtual touch tissue imaging in the personalized prediction of lymph node metastasis of breast cancer. Gland Surg (2021) 10(4):1460–9. doi: 10.21037/gs-21-199
25. Seo M, Sohn YM. Differentiation of benign and metastatic axillary lymph nodes in breast cancer: Additive value of shear wave elastography to b-mode ultrasound. Clin Imaging (2018) 50:258–63. doi: 10.1016/j.clinimag.2018.04.013
26. Youk JH, Son EJ, Kim JA, Gweon HM. Pre-operative evaluation of axillary lymph node status in patients with suspected breast cancer using shear wave elastography. Ultrasound Med Biol (2017) 43(8):1581–6. doi: 10.1016/j.ultrasmedbio.2017.03.016
27. Du LW, Liu HL, Gong HY, Ling LJ, Wang S, Li CY, et al. Adding contrast-enhanced ultrasound markers to conventional axillary ultrasound improves specificity for predicting axillary lymph node metastasis in patients with breast cancer. Br J Radiol (2021) 94(1118):20200874. doi: 10.1259/bjr.20200874
28. Zhang Q, Agyekum EA, Zhu L, Yan L, Zhang L, Wang X, et al. Clinical value of three combined ultrasonography modalities in predicting the risk of metastasis to axillary lymph nodes in breast invasive ductal carcinoma. Front Oncol (2021) 11:715097. doi: 10.3389/fonc.2021.715097
29. Liwen D, Haiyan G, Jing D, Hui W, Chunbei Y, Cuiying L. The diagnostic value of conventional ultrasonography combined with contrast-enhanced ultrasonography in evaluating axillany lymph nodes in breast cancer patients. Cancer Imaging (2020) 29(04):397–405.
30. Shaofen W, Junging L. The diagnostic value of conventional ultrasonography combined with contrast-enhanced ultrasonography in metastatic sentinel lymph nodes of breast cancer. Clinical Medicine Research and Practice (2021) 6(35):114–6.
31. Xiaodan Z, Xinjia W. Application analysis of ultrasonography combined with contrast-enhanced ultrasonography in the diagnosis of metastatic sentinel lymph nodes in breast cancer. Electronic J Clin Med Literature (2019) 6(13):165.
32. Garcia-Etienne CA, Ferrari A, Della Valle A, Lucioni M, Ferraris E, Di Giulio G, et al. Management of the axilla in patients with breast cancer and positive sentinel lymph node biopsy: An evidence-based update in a European breast center. Eur J Surg Oncol (2020) 46(1):15–23. doi: 10.1016/j.ejso.2019.08.013
Keywords: ultrasound, ultrasound elastography, contrast-enhanced ultrasound, breast cancer, lymph nodes metastasis, network meta-analysis
Citation: Li J, Wang S-R, Li Q-L, Zhu T, Zhu P-S, Chen M and Cui X-W (2023) Diagnostic value of multiple ultrasound diagnostic techniques for axillary lymph node metastases in breast cancer: A systematic analysis and network meta-analysis. Front. Oncol. 12:1043185. doi: 10.3389/fonc.2022.1043185
Received: 13 September 2022; Accepted: 25 November 2022;
Published: 06 January 2023.
Edited by:
Maria Rosaria De Miglio, University of Sassari, ItalyReviewed by:
Mingxing Xie, Huazhong University of Science and Technology, ChinaCopyright © 2023 Li, Wang, Li, Zhu, Zhu, Chen and Cui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jun Li, MTI4NzQyNDc5OEBxcS5jb20=; Xin-Wu Cui, Y3VpeGlud3VAbGl2ZS5jbg==
†These authors have contributed equally to this article
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.