
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 20 October 2022
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1042869
 Kang-Jun Zhang1,2
Kang-Jun Zhang1,2 Tai-Wei Ye1
Tai-Wei Ye1 Wen-Feng Lu3
Wen-Feng Lu3 Fei-Qi Xu1,2
Fei-Qi Xu1,2 Ya-Ming Xie1
Ya-Ming Xie1 Dong-Dong Wang1
Dong-Dong Wang1 Zun-Qiang Xiao1
Zun-Qiang Xiao1 Si-Yu Liu4
Si-Yu Liu4 Wei-Feng Yao1
Wei-Feng Yao1 Jian Cheng1
Jian Cheng1 Guo-Liang Shen1
Guo-Liang Shen1 Jun-Wei Liu1
Jun-Wei Liu1 Cheng-Wu Zhang1
Cheng-Wu Zhang1 Dong-Sheng Huang1,5,6*
Dong-Sheng Huang1,5,6* Lei Liang1,5*
Lei Liang1,5*Background & aims: The long-term prognosis of patients with metabolic syndrome (MS) and hepatitis B virus-related hepatocellular carcinoma (HBV-HCC) after radical hepatectomy remains unclear. The purpose of this study was to elucidate the effect of MS on long-term survival for patients with HBV-related HCC after hepatectomy.
Methods: Patients with HBV-HCC after hepatectomy were included. Patients were stratified into MS-HBV-HCC and HBV-HCC groups. Clinical features and surgical outcomes were compared between the two groups, and COX regression analysis was used to determine independent risk factors associated with overall survival (OS) and recurrence-free survival (RFS).
Result: 389 patients (MS-HBV-HCC group: n=50, HBV-HCC group: n=339) were enrolled for further analysis. Baseline characteristics showed that patients with MS-HBV-HCC were associated with a high rate of elderly patients, ASA score, and co-morbid illness, but a lower rate of anatomy hepatectomy. There were no significant differences in perioperative complications. After excluding patients who relapsed or died within 90 days after surgery, multivariate Cox regression analysis showed MS was an independent risk factor of OS (HR 1.68, 95% CI 1.05-2.70, P = 0.032) and RFS (HR 1.78, 95% CI 1.24-2.57, P = 0.002).
Conclusion: MS is an independent risk factor for poor OS and RFS in HBV-infected HCC patients after radical hepatectomy. This suggests that we need to strengthen postoperative follow-up of the relevant population and encourage patients to develop a healthy lifestyle.
Primary liver cancer remains the sixth most common cancer worldwide and is one of the top three causes of cancer-related deaths, with hepatocellular carcinoma (HCC) accounting for 75-85% (1, 2). Hepatitis B virus (HBV) infection is the most important risk factor for HCC development in the Asia-Pacific region, especially in China. Current guidelines have recommended routine antiviral therapy for HCC patients with HBV infection after surgery (3), but the recurrence rate is still high. Therefore, it is necessary to actively explore the risk factors affecting long-term survival after surgery.
Metabolic syndrome (MS) is a clinical syndrome of obesity, dyslipidemia (low high-density lipoproteinemia and/or high triglyceridemia), hyperglycemia (diabetes or impaired glucose regulation), and hypertension (4, 5). Previous studies have shown that hepatectomy for MS-HCC has a higher but acceptable operative risk, but the long-term survival outcome remains controversial (6–11). Reasons for the controversy include a small sample size or an uneven baseline of enrolled patients. Studies focusing on one factor in MS may also lead to biased results because many factors are related and should be analyzed comprehensively (12–14). Furthermore, patients with early recurrence and death, especially within 90 days, mainly due to surgical and tumor factors (15), should be excluded. Most HCC patients in the Asia-Pacific region have a background of chronic HBV infection, and the long-term prognosis of HBV-HCC patients with MS is still unclear.
The aim of this study is to elucidate the effect of MS on long-term survival after radical hepatectomy in HBV-infected HCC patients, excluding patients who relapsed or died within 90 days after surgery.
Data from a Chinese large single-center database of patients who underwent curative-intent liver resection for HBV-infected HCC between January 2011 and January 2018 at Zhejiang Provincial People’s Hospital. MS is diagnosed when at least three of the following conditions are met: abdominal obesity (waist circumference ≥ 90cm in men; ≥ 80 cm for women); dyslipidemia (triglyceride ≥ 1.70 mmol/L or high-density lipoprotein cholesterol < 1.04 mmol/L); diagnosed with diabetes or glucose intolerance (fasting blood glucose ≥ 6.1 mmol/L); treated for hypertension or hypertension (blood pressure ≥ 130/≥ 85 mmHg); abdominal obesity with a body mass index (BMI) ≥ 25.0 kg/m2 (16). Patients younger than 18 years of age, with recurrent HCC, concomitant tumors, incomplete clinical data, concomitant HCV or no concomitant HBV infection were excluded. All patients underwent R0 resection, in which all microscopic and gross tumors were removed.
Selection criteria for hepatectomy for HCC were constant during the study period, such as previously reported tumor location and number, liver function reserve, and future residual liver volume (17, 18). This study was approved by the Institutional Evaluation Committee of Zhejiang Provincial People’s Hospital according to the Helsinki Declaration (No. QT2022238).
There were seven patient-related variables: age, sex, smoking history, Performance status (PS), American Society of Anesthesiologists (ASA) score, co-morbid illness, and preoperative anti-HBV therapy. There were seven liver-related and laboratory variables: preoperative HBV-DNA levels, alanine aminotransferase (ALT), aspartate aminotransferase (AST), alpha-fetoprotein (AFP), cirrhosis, portal hypertension, and Child-Pugh grade. There were six tumor-related and pathological variables: maximum tumor diameter, tumor number, satellite nodules, vascular invasion (macro- or micro-), tumor differentiation (good/moderate or poor), and tumor encapsulation (incomplete or complete). There were five surgery-related variables: intraoperative blood loss, intraoperative blood transfusion, the scope of liver resection (minor or major), type of liver resection (anatomic or non-anatomic) (19), and resection margin.
After hepatectomy, patients were followed up according to a standardized recurrence surveillance protocol. The date of tumor recurrence, date of death and cause of death, and date of last follow-up were recorded. Short-term outcomes included postoperative hospital stay, postoperative morbidity, severe morbidity (20), postoperative 90-day mortality, and postoperative 90-day death or recurrence.
SPSS 25.0 software was used for statistical analysis of the data. Continuous variables were represented by mean or median (range), and categorical variables were represented by numbers (n, %). Continuous variables were compared using Student’s T test or Mann-Whitney U test and comparisons between categorical variables were performed using either χ2 test or Fisher’s exact test, as appropriate. OS and RFS were calculated using the Kaplan-Meier method, and multivariate COX regression was used to determine whether MS was an independent risk factor for worse OS and RFS. P < 0.05 was considered statistically significant.
Of the 559 patients treated with hepatectomy for HCC at our center, 170 patients did not meet the inclusion criteria and were excluded. 389 HBV-infected HCC patients were collected for further analysis. Among them, 339 (87.1%) patients without MS and 50 (12.9%) patients with MS. Table 1 shows that the proportion of patients with MS complicated with anatomic liver resection was lower (32.0% vs. 49.3%, P = 0.022), but the proportion of patients with age ≥ 60 years, ASA score > 2 and co-morbid illness was higher (both P < 0.05). There were no significant differences in postoperative hospital stay, postoperative 30-day morbidity, serious complications, postoperative 90-day mortality, and postoperative 90-day death or recurrence between the two groups (all P > 0.05).
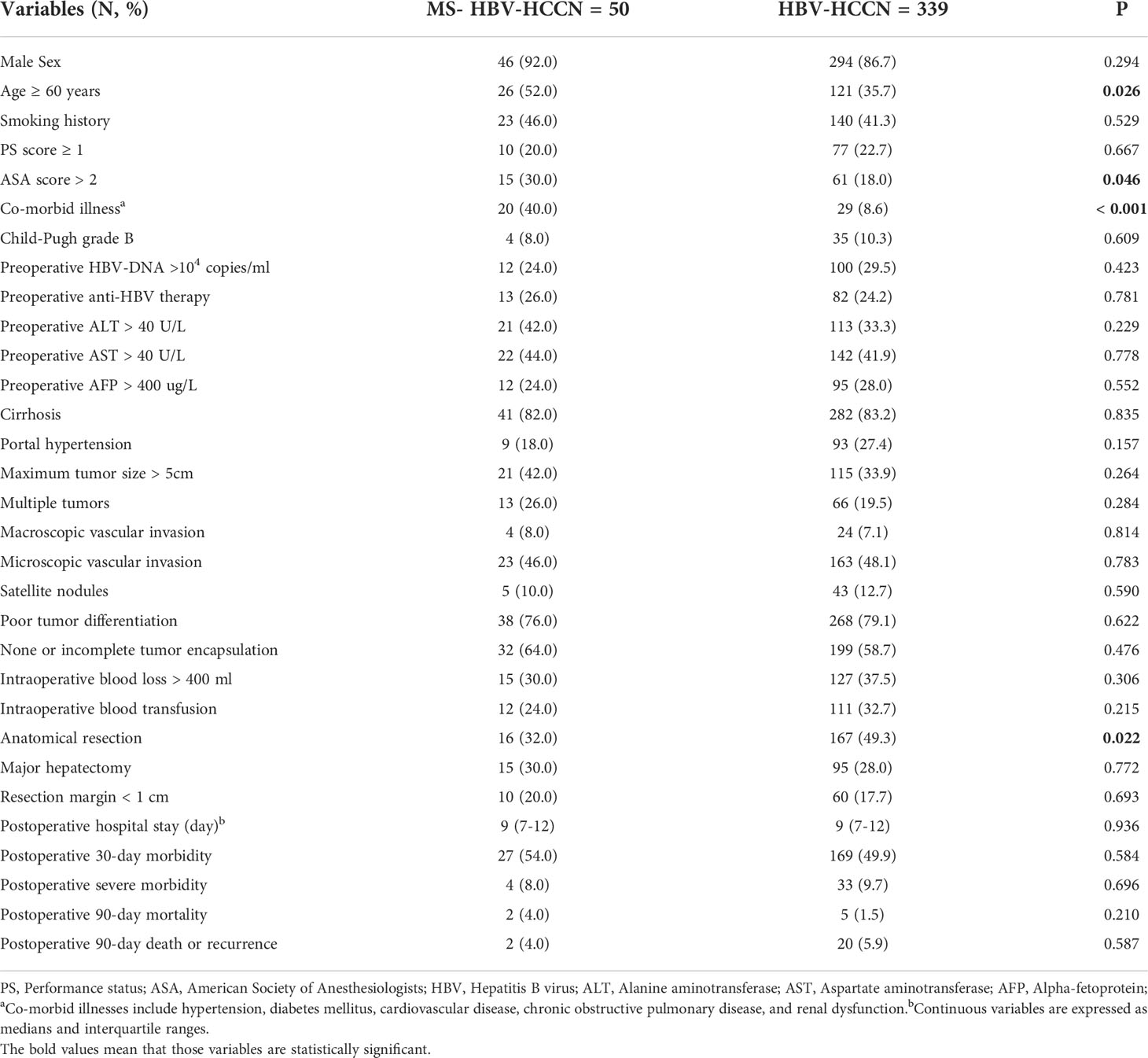
Table 1 Comparison of clinical and pathological characteristics and short-term outcomes between the study groups in the whole study population.
Patients who died or relapsed within 90 days were excluded from our study cohort to reduce the impact of tumor and surgery (n = 22). At a median follow-up of 40.2 months, 123 (33%) and 196 (53%) patients died and HCC recurred, respectively. Table 2 shows that the 1-, 3- and 5-year OS of patients with or without MS after radical hepatectomy for HCC were 91.7%, 54.2% and 12.5%, and 95.0%, 63.0% and 27.3%, respectively. RFS of 1-, 3- and 5-year were 68.8%, 22.9% and 2.1%, and 80.9%, 46.1% and 17.2%, respectively. Patients with MS had poor 5-year OS, 3-year RFS, and 5-year RFS (all P < 0.05). In Figure 1, Kaplan-Meier method was used to compare OS and RFS curves of the two groups. By log-rank test, OS and RFS of HCC patients with MS were worse than those without MS (hazard ratio (HR) 1.69, 95% confidence interval (CI) 1.06 – 2.71, P = 0.027; HR 1.92, 95% CI 1.34 – 2.75, P < 0.001, respectively).
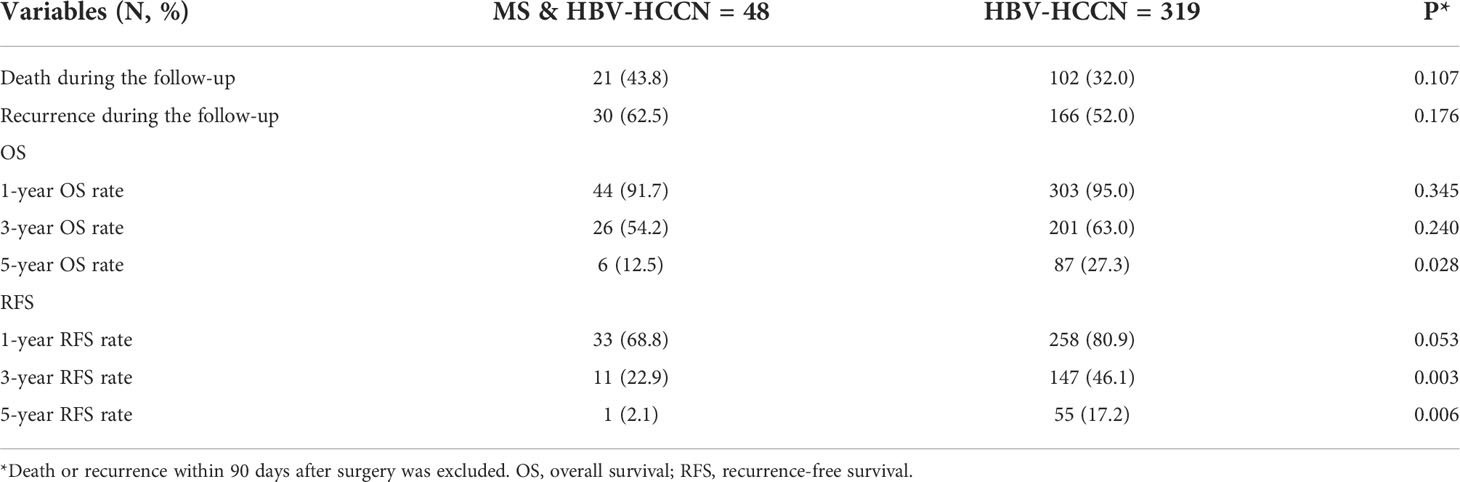
Table 2 Comparisons of long-term outcomes between HBV-infected patients with and without metabolic syndrome.
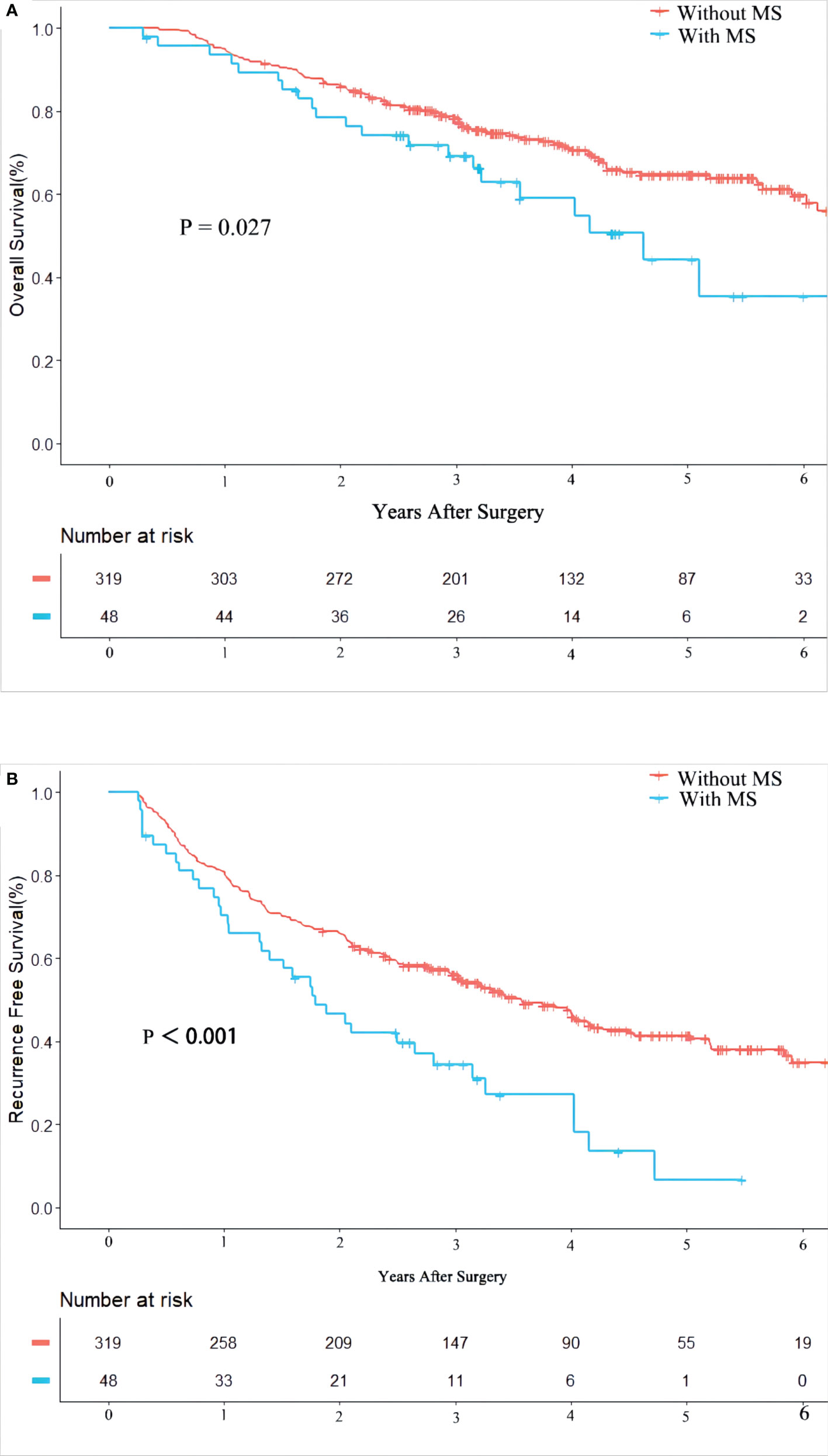
Figure 1 Kaplan-Meier Curves of overall survival (A) and recurrence free survival (B) between HBV-infected patients with and without metabolic syndrome.
Table 3 and Table 4 showed the univariate and multivariate COX-regression analysis results of OS and RFS in patients with HBV-infected HCC after hepatectomy. The results showed that MS was an independent risk factor for worse OS and RFS (HR 1.68, 95% CI 1.05 – 2.70, P = 0.032; HR 1.78, 95% CI 1.24 – 2.57, P = 0.002). Furthermore, the results also showed PS score ≥ 1, preoperative HBV-DNA level > 104 copies/ml, macroscopic vascular invasion, microscopic vascular invasion, satellite nodules, incomplete tumor encapsulation, and resection margin < 1cm were independent risk factors associated with poorer OS. Meanwhile, preoperative HBV-DNA level > 104 copies/ml, tumor size, multiple tumors, macroscopic vascular invasion, microscopic vascular invasion, satellite nodules, incomplete tumor encapsulation, and resection margin < 1cm were independent risk factors associated with worse RFS (all P < 0.05).
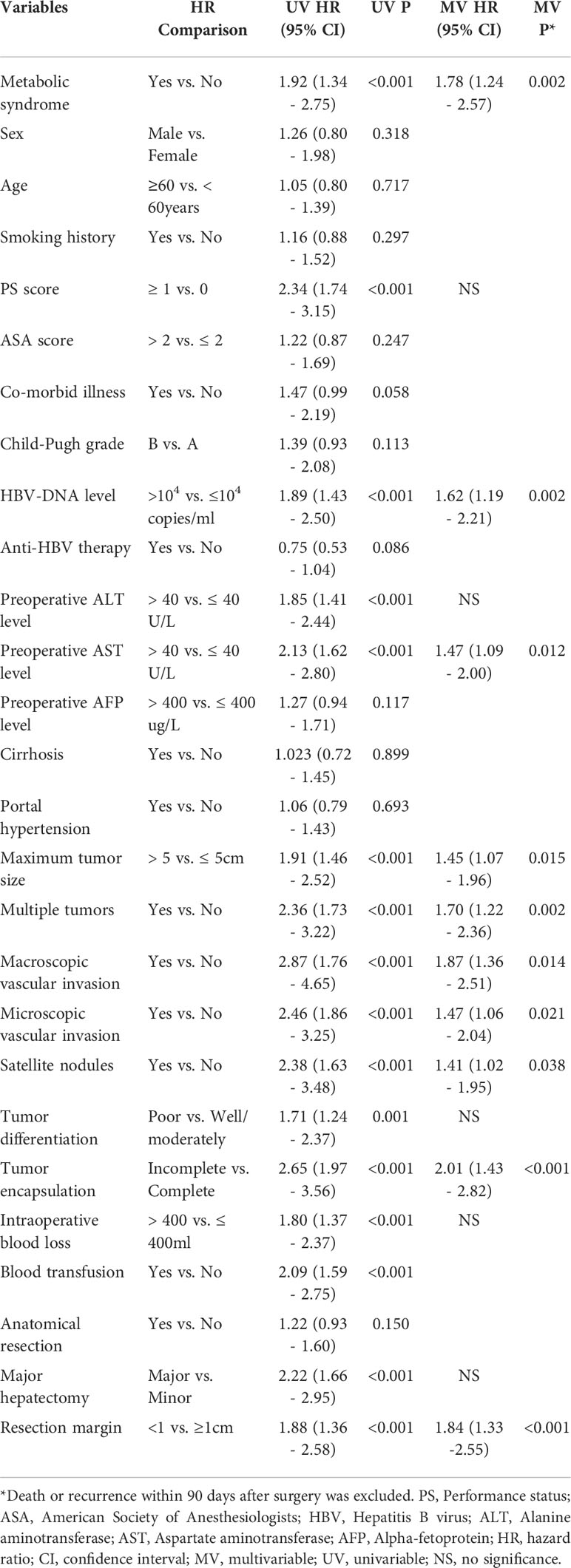
Table 4 Univariable and multivariable Cox regression analyses on risk factors of recurrence-free survival.
In this study, a total of 389 patients with HCC complicated with HBV infection were enrolled to evaluate the effect of MS on long-term survival after radical hepatectomy. According the excluding criterion, 389 patients were finally included for further analysis. HBV-infected HCC patients with MS were mostly overweight elderly males with solitary large tumors (92.0% male, 52.0% ≥ 60 years old, 74.0% single tumor and 42.0% > 50 mm). In the multivariate COX regression analysis, MS was an independent risk factor affecting OS (HR 1.68, 95% CI 1.05–2.70, P = 0.032) and RFS (HR 1.78, 95% CI 1.24–2.57, P = 0.002). In other words, MS increased nearly 1.7 folds risk of tumor recurrence and death.
At present, hepatectomy is still the preferred method for radical treatment of HCC. However, the postoperative recurrence rate was still high, with an overall recurrence rate of more than 70% within 5 years (17, 21, 22). At the same time, studies have shown that the causes of patients’ early recurrence and death, especially within 90 days, are mainly surgical and tumor factors (15). Although the development of neoadjuvant and adjuvant therapies has improved the prognosis of liver cancer, there is no definite treatment plan. Therefore, actively exploring the risk factors of long-term recurrence and death after hepatectomy is an important clinical topic to be solved urgently.
MS is associated with a variety of tumors and has a long-term impact on patient survival. Because NAFLD is often associated with insulin resistance, central obesity, dyslipidemia, hypertension, and hyperglycemia, it is often considered a hepatic manifestation of the MS (23–26). A growing body of evidence suggests that the relationship between NAFLD and MS is bidirectional, exacerbating each other’s conditions (27). NAFLD progresses to non-alcoholic steatohepatitis (NASH) due to hepatic steatosis and chronic substrate overload leading to lipotoxicity (28). Hepatic lipid accumulation leads to cellular metabolic changes and accumulation of potentially toxic metabolites leading to the occurrence of liver tumors (29–31).
Similar to the characteristics of metabolism-related HCC previously reported (6, 7, 11, 32), HBV-infected HCC patients with MS were more likely to be overweight elderly men with a single large tumor (92.0% male, 52.0% ≥ 60 years old, 74.0% single tumor and 42.0% > 50 mm). Comorbidity, older age, and high-risk lifestyle behaviors [such as sedentary behavior or unbalanced diet (33)] associated with MS further hinder tumor management.
MS-HCC can occur in non-cirrhotic liver parenchyma in previous studies (2, 6, 11), but there was no difference in cirrhosis between the two groups in this study (82.0% vs. 83.2%, P=0.835). This is related to the co-infection with HBV in this study population, and studies have shown that MS has a detrimental effect on the course of viral hepatitis, especially by accelerating the progression of liver fibrosis (34).
Previous studies have reported that hepatectomy for patients with MS is safe, feasible and beneficial to prognosis, although the probability of postoperative complications is higher (6, 8, 10, 35, 36). Similarly, the incidence of postoperative complications was also high among HBV-infected HCC patients with MS in our cohort, although there was no difference between the two groups (52.1% vs. 49.8%, P= 0.772). This may be due to the high proportion of patients with cirrhosis in both groups, even up to 80%, which is similar to the characteristics of HBV-HCC (7). A high proportion of cirrhosis is closely related to a high incidence of complications (37, 38).
The long-term prognosis of HBV-HCC patients with MS after radical hepatectomy remains unclear. In 2018, Tian Y et al. compared hepatectomy in MS-HCC patients with HBV-HCC patients. Patients were stratified according to the AJCC Cancer Staging Manual, Seventh Edition (2010) to compare the long-term outcomes of MS-HCC, MS-HBV-HCC, and HBV-HCC patients. It was found that compared with the other two groups, MS-HCC patients in AJCC stage I had higher RFS and OS, and there was no significant difference in RFS and OS in MS-HCC, MS-HBV-HCC and HBV-HCC patients in AJCC stage II, III and IV (7). In 2020, Tian Y et al. in the study of BCLC stage 0 or A HCC patients, compared with patients without MS, the long-term survival of most HBV-related HCC patients with hepatectomy in the presence of MS was comparable (10). In addition, a multi-center retrospective study by Viganò et al. found that the prognosis of the MS-HCC cohort was better than that of the HCV-HCC cohort after a propensity matching analysis (6). However, factors such as obesity and diabetes are often associated with a worse prognosis (12–14). The components of MS are increasingly linked to a variety of cancers, including increased disease risk and worsening outcomes. In this cohort, death and recurrence within 90 days after surgery were excluded, effectively controlling the direct impact of the tumor itself and surgery. We found that 5-year OS and RFS were significantly worse in HBV-HCC patients with MS (12.5% vs. 27.3%, P = 0.028; 2.1% vs. 17.2%, P = 0.006 respectively). By multivariate Cox regression analysis, MS was determined to be an independent risk factor for OS and RFS. In addition, preoperative HBV-DNA level > 104 copies/ml, microscopic vascular invasion, tumor encapsulation (none or incomplete), resection margin < 1cm were also independent risk factors for worse OS and RFS in HBV-infected HCC patients (all P < 0.05).
The diagnosis of MS is cumbersome and often goes unnoticed. The gold standard for diagnosing NAFLD is pathological examination of liver tissue. Once cirrhosis has progressed, diagnosis becomes more difficult due to the loss of fat (39). At present, there is no specific diagnostic method, so the prevention of MS and the treatment after the occurrence of HCC are particularly critical. Abdominal ultrasound monitoring is recommended every 4-6 months in all patients with cirrhosis (40). Of course, if technology develops in the future, more sensitive and cheaper serum tests should be used for surveillance.
There are currently no approved drugs to treat MS/NAFLD, and diet, lifestyle changes, and exercise remain the mainstay of treatment (41). There is no doubt that hepatitis B virus infection must also receive regular antiviral treatment. In elderly patients with MS, cardiopulmonary function tests must be fully evaluated before surgery. Attention must be paid to perioperative management of these patients, and timely and effective treatment of postoperative complications is needed. Regular follow-up tests after surgery must also be emphasized.
There are some defects in this study. First of all, this study is retrospective and there are some unavoidable biases. Secondly, the cases in this study were all infected with HBV, so whether it can be applied to patients infected with HCV needs further study.
In conclusion, this study demonstrates that MS is an independent risk factor for worse OS and RFS in HBV-infected patients after radical hepatectomy for HCC. This suggests that we need to strengthen postoperative follow-up of the relevant population and encourage patients to develop a healthy lifestyle.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
K-JZ, T-WY, W-FL, and F-QX contributed equally to this work. LL and D-SH had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: K-JZ, LL, and D-SH. Acquisition, analysis, or interpretation of data: T-WY, W-FL, F-QX, Y-MX, D-DW, Z-QX, S-YL, W-FY, JC, and G-LS. Drafting of the manuscript: K-JZ and T-WY. Critical revision of the manuscript for important intellectual content: J-WL and C-WZ. Statistical analysis: T-WY, D-DW and Y-MX. Obtained funding: LL. Administrative, technical, or material support: C-WZ, D-SH, and LL. Study supervision: D-SH, LL. All authors contributed to the article and approved the submitted version.
This work was supported by Zhejiang Provincial People’s Hospital (No. ZRY2020A004), and the Health Commission of Zhejiang Province (No.2022KY532).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer CL declared a shared affiliation with the author W-FL to the handling editor at the time of review.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
MS, metabolic syndrome; HBV, hepatitis virus B; HCC, hepatocellular carcinoma; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; ASA, American Society of Anesthesiologists; OS, overall survival; RFS, recurrence-free survival; PS, performance status; ALT, alanine aminotransferase; AST, aspartate transaminase; AFP, alpha-fetoprotein; HR, hazard ratio; CI, confidence interval; MV, multivariable; UV, univariable.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Llovet JM, Kelley RK, Villanueva A, Singal AG, Pikarsky E, Roayaie S, et al. Hepatocellular carcinoma. Nat Rev Dis Primers (2021) 7:6. doi: 10.1038/s41572-020-00240-3
3. Omata M, Cheng AL, Kokudo N, Kudo M, Lee JM, Jia J, et al. Asia-Pacific clinical practice guidelines on the management of hepatocellular carcinoma: a 2017 update. Hepatol Int (2017) 11(4):317–70. doi: 10.1007/s12072-017-9799-9
4. Lemieux I, Després JP. Metabolic syndrome: Past, present and future. Nutrients (2020) 12(11):3501. doi: 10.3390/nu12113501
5. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, et al. Diagnosis and management of the metabolic syndrome: an American heart Association/National heart, lung, and blood institute scientific statement. Circulation (2005) 112:2735–52. doi: 10.1161/CIRCULATIONAHA.105.169404
6. Viganò L, Conci S, Cescon M, et al. Liver resection for hepatocellular carcinoma in patients with metabolic syndrome: A multicenter matched analysis with HCV-related HCC. J Hepatol (2015) 63:93–101. doi: 10.1016/j.jhep.2015.01.024
7. Tian Y, Lyu H, He Y, Xia Y, Li J, Shen F. Comparison of hepatectomy for patients with metabolic syndrome-related HCC and HBV-related HCC. J Gastrointest Surg (2018) 22(4):615–23. doi: 10.1007/s11605-017-3629-1
8. Koh YX, Tan HJ, Liew YX, Syn N, Teo JY, Lee SY, et al. Liver resection for nonalcoholic fatty liver disease-associated hepatocellular carcinoma. J Am Coll Surg (2019) 229:467–78.e1. doi: 10.1016/j.jamcollsurg.2019.07.012
9. Yang T, Hu LY, Li ZL, Liu K, Wu H, Xing H, et al. Liver resection for hepatocellular carcinoma in non-alcoholic fatty liver disease: A multicenter propensity matching analysis with HBV-HCC. J Gastrointest Surg (2020) 24:320–9. doi: 10.1007/s11605-018-04071-2
10. Tian Y, Li T, Qi S, Alhourani H, Luo B, Chenqin J, et al. The impact of metabolic syndrome (MetS) on surgical outcome for patients with mostly HBV-related hepatocellular carcinoma (HCC) underwent hepatectomy. J Surg Oncol (2020). doi: 10.1002/jso.26055
11. Conci S, Cipriani F, Donadon M, Marchitelli I, Ardito F, Famularo S, et al. Hepatectomy for metabolic associated fatty liver disease (MAFLD) related HCC: Propensity case-matched analysis with viral- and alcohol-related HCC. Eur J Surg Oncol (2022) 48:103–12. doi: 10.1016/j.ejso.2021.07.015
12. Kobayashi A, Kaido T, Hamaguchi Y, Okumura S, Shirai H, Yao S, et al. Impact of sarcopenic obesity on outcomes in patients undergoing hepatectomy for hepatocellular carcinoma. Ann Surg (2019) 269:924–31. doi: 10.1097/SLA.0000000000002555
13. Hashimoto M, Tashiro H, Kobayashi T, Kuroda S, Hamaoka M, Ohdan H. Influence of higher BMI for hepatitis b- and c-related hepatocellular carcinomas. Langenbecks Arch Surg (2017) 402(5):745–55. doi: 10.1007/s00423-017-1589-2
14. Shen GL, Lu Y, Liang L, Lu WF, Diao YK, Xiao ZQ, et al. Impact of diabetes mellitus on the long-term prognosis of patients with hepatocellular carcinoma after hepatectomy. Expert Rev Gastroenterol Hepatol (2022) 16:473–8. doi: 10.1080/17474124.2022.2063837
15. Llovet JM, Schwartz M, Mazzaferro V. Resection and liver transplantation for hepatocellular carcinoma. Semin Liver Dis (2005) 25(2):181–200. doi: 10.1055/s-2005-871198
16. Fan JG, Kim SU, Wong VW. New trends on obesity and NAFLD in Asia. J Hepatol (2017) 67(4):862–73. doi: 10.1016/j.jhep.2017.06.003
17. Xu XF, Xing H, Han J, Li ZL, Lau WY, Zhou YH, et al. Risk factors, patterns, and outcomes of late recurrence after liver resection for hepatocellular carcinoma: A multicenter study from China. JAMA Surg (2019) 154:209–17. doi: 10.1001/jamasurg.2018.4334
18. Yang T, Lu JH, Lau WY, Zhang TY, Zhang H, Shen YN, et al. Perioperative blood transfusion does not influence recurrence-free and overall survivals after curative resection for hepatocellular carcinoma: A propensity score matching analysis. J Hepatol (2016) 64:583–93. doi: 10.1016/j.jhep.2015.10.012
19. Strasberg SM, Phillips C. Use and dissemination of the brisbane 2000 nomenclature of liver anatomy and resections. Ann Surg (2013) 257(3):377–82. doi: 10.1097/SLA.0b013e31825a01f6
20. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg (2004) 240(2):205–13. doi: 10.1097/01.sla.0000133083.54934.ae
21. Vibert E, Schwartz M, Olthoff KM. Advances in resection and transplantation for hepatocellular carcinoma. J Hepatol (2020) 72(2):262–76. doi: 10.1016/j.jhep.2019.11.017
22. Tabrizian P, Jibara G, Shrager B, Schwartz M, Roayaie S. Recurrence of hepatocellular cancer after resection: patterns, treatments, and prognosis. Ann Surg (2015) 261(5):947–55. doi: 10.1097/SLA.0000000000000710
23. Cauchy F, Belghiti J. A clinical perspective of the link between metabolic syndrome and hepatocellular carcinoma. J Hepatocell Carcinoma (2015) 2:19–27. doi: 10.2147/JHC.S44521
24. Yki-Järvinen H. Non-alcoholic fatty liver disease as a cause and a consequence of metabolic syndrome. Lancet Diabetes Endocrinol (2014) 2(11):901–10. doi: 10.1016/S2213-8587(14)70032-4
25. Nderitu P, Bosco C, Garmo H, Holmberg L, Malmström H, Hammar N, et al. The association between individual metabolic syndrome components, primary liver cancer and cirrhosis: A study in the Swedish AMORIS cohort. Int J Cancer (2017) 141:1148–60. doi: 10.1002/ijc.30818
26. Huang DQ, El-Serag HB, Loomba R. Global epidemiology of NAFLD-related HCC: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol (2021) 18(4):223–38. doi: 10.1038/s41575-020-00381-6
27. Wainwright P, Byrne CD. Bidirectional relationships and disconnects between NAFLD and features of the metabolic syndrome. Int J Mol Sci (2016) 17(3):367. doi: 10.3390/ijms17030367
28. Sunny NE, Bril F, Cusi K. Mitochondrial adaptation in nonalcoholic fatty liver disease: Novel mechanisms and treatment strategies. Trends Endocrinol Metab (2017) 28(4):250–60. doi: 10.1016/j.tem.2016.11.006
29. Hardy T, Oakley F, Anstee QM, Day CP. Nonalcoholic fatty liver disease: Pathogenesis and disease spectrum. Annu Rev Pathol (2016) 11:451–96. doi: 10.1146/annurev-pathol-012615-044224
30. Nakagawa H, Hayata Y, Kawamura S, Yamada T, Fujiwara N, Koike K. Lipid metabolic reprogramming in hepatocellular carcinoma. Cancers (Basel) (2018) 10(11): 447. doi: 10.3390/cancers10110447
31. Anstee QM, Reeves HL, Kotsiliti E, Govaere O, Heikenwalder M. From NASH to HCC: current concepts and future challenges. Nat Rev Gastroenterol Hepatol (2019) 16(7):411–28. doi: 10.1038/s41575-019-0145-7
32. Mohamad B, Shah V, Onyshchenko M, Elshamy M, Aucejo F, Lopez R, et al. Characterization of hepatocellular carcinoma (HCC) in non-alcoholic fatty liver disease (NAFLD) patients without cirrhosis. Hepatol Int (2016) 10:632–9. doi: 10.1007/s12072-015-9679-0
33. Bovolini A, Garcia J, Andrade MA, Duarte JA. Metabolic syndrome pathophysiology and predisposing factors. Int J Sports Med (2021) 42(3):199–214. doi: 10.1055/a-1263-0898
34. Wang CC, Cheng PN, Kao JH. Systematic review: chronic viral hepatitis and metabolic derangement. Aliment Pharmacol Ther (2020) 51(2):216–30. doi: 10.1111/apt.15575
35. Cauchy F, Zalinski S, Dokmak S, Fuks D, Farges O, Castera L, et al. Surgical treatment of hepatocellular carcinoma associated with the metabolic syndrome. Br J Surg (2013) 100:113–21. doi: 10.1002/bjs.8963
36. Bhayani NH, Hyder O, Frederick W, Schulick RD, Wolgang CL, Hirose K, et al. Effect of metabolic syndrome on perioperative outcomes after liver surgery: A national surgical quality improvement program (NSQIP) analysis. Surgery (2012) 152(2):218–26. doi: 10.1016/j.surg.2012.05.037
37. Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet (2021) 398(10308):1359–76. doi: 10.1016/S0140-6736(21)01374-X
38. Tsochatzis EA, Bosch J, Burroughs AK. Liver cirrhosis. Lancet (2014) 383(9930):1749–61. doi: 10.1016/S0140-6736(14)60121-5
39. Siegel AB, Zhu AX. Metabolic syndrome and hepatocellular carcinoma: two growing epidemics with a potential link. Cancer (2009) 115(24):5651–61. doi: 10.1002/cncr.24687
40. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. Eur J Cancer (2012) 48:599–641. doi: 10.1016/j.ejca.2011.12.021
Keywords: metabolic syndrome, hepatitis virus B, hepatocellular carcinoma, hepatectomy, survival
Citation: Zhang K-J, Ye T-W, Lu W-F, Xu F-Q, Xie Y-M, Wang D-D, Xiao Z-Q, Liu S-Y, Yao W-F, Cheng J, Shen G-L, Liu J-W, Zhang C-W, Huang D-S and Liang L (2022) Impact of metabolic syndrome on the long-term prognosis of patients with hepatitis B virus-related hepatocellular carcinoma after hepatectomy. Front. Oncol. 12:1042869. doi: 10.3389/fonc.2022.1042869
Received: 13 September 2022; Accepted: 07 October 2022;
Published: 20 October 2022.
Edited by:
Jiang Chen, Zhejiang University, ChinaReviewed by:
Hao Xing, Second Military Medical University, ChinaCopyright © 2022 Zhang, Ye, Lu, Xu, Xie, Wang, Xiao, Liu, Yao, Cheng, Shen, Liu, Zhang, Huang and Liang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Lei Liang, bGlhbmdsMTk5MkBob3RtYWlsLmNvbQ==; Dong-Sheng Huang, aHVhbmdkc2h6OTlAaG90bWFpbC5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.