
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 23 December 2022
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1040452
This article is part of the Research Topic Case Reports in Thoracic Oncology: 2022 View all 42 articles
It is unclear whether pleural/pericardial drainage and pleurodesis/pericardiodesis should be performed before or after initiating chemotherapy in patients with chemotherapy-sensitive small-cell lung cancer. A 76-year-old woman presented to the emergency department with progressive dyspnea on exertion for a week. Chest computed tomography showed a mass shadow anterior to the left upper lobe, bilateral pleural effusions, and a circumferential pericardial effusion surrounding the heart. We diagnosed extensive-stage small-cell lung cancer based on the clinical course and pathological findings. We first performed pleurodesis and pericardial drainage and successfully initiated immune checkpoint inhibitor combined chemotherapy, with improved performance status. This case highlights the importance of aggressive drainage and pleurodesis/pericardiodesis, and suggests that drainage and pleurodesis/pericardiodesis should be considered before systemic chemotherapy in patients with concurrent pericardial or pleural effusions, even in patients with small-cell lung cancer that is sensitive to chemotherapy.
Small-cell lung cancer (SCLC) is rapidly progressive and is highly sensitive to chemotherapy compared to non-small-cell lung cancer. SCLC with concurrent pleural and pericardial effusions at the time of the initial presentation is rare. A retrospective observational study found that of 765 patients with SCLC, 63 had pleural effusions, 17 had pericardial effusions, and 16 had both (1). Recently, the initiation of chemotherapy with immune checkpoint inhibitors has been reported to improve the clinical outcomes of patients with SCLC (2). Although early initiation of chemotherapy with immune checkpoint inhibitors (ICIs) is desirable to improve the prognosis and symptoms of patients with SCLC, there is concern that patients with SCLC and pericardial or pleural effusions may not be able to initiate chemotherapy safely due to poor performance status (PS). Moreover, it is unclear whether pleural/pericardial drainage and pleurodesis/pericardiodesis should be performed before or after initiating chemotherapy should in patients with chemotherapy-sensitive SCLC. Here, we report a case of successful initiation of chemotherapy combined with ICI therapy in a patient with SCLC and pleural and pericardial effusions, after performing pleurodesis and pericardial drainage.
A 76-year-old woman presented to the emergency department with progressive dyspnea on exertion for a week. Her medical history included hypertension, dyslipidemia, and hyperuricemia. Her vital signs at the visit were: body temperature 36.1°C, pulse rate 68 beats/min, blood pressure 130/78 mmHg, respiratory rate 18 breaths/min, and oxygen saturation 92% breathing ambient air, and her consciousness was clear. Physical examination revealed decreased breath sounds in the lower left side of the chest and decreased cardiac sounds. Blood tests revealed white blood cell count 7,700/μL, aspartate aminotransferase 52 IU/L, alanine aminotransferase 55 IU/L, C-reactive protein 0.97 mg/dL, pro-gastrin-releasing peptide 71.9 pg/mL, and neuron specific enolase 254 ng/mL. Chest computed tomography (CT) showed a mass shadow anterior to the left upper lobe, bilateral pleural effusions (Figure 1A), and pericardial effusion circumferentially around the entire heart (Figure 1B). We performed pericardial drainage on day 2 because of concern about the possibility of the development of cardiac tamponade and for diagnosis. Pericardial fluid was exudative, but cytology of the fluid was negative for malignant cells. On day 4, the patient required oxygen therapy because of an increased left pleural effusion, and we performed pleural drainage. Pleural effusion was exudative, and cytology of the fluid was positive for small cell lung cancer. On day 8, we performed a CT-guided lung biopsy. Hematoxylin and eosin staining of the biopsy specimen revealed dense sheet-like growth of bare nucleated atypical cells with increased chromatin (Figure 2A), and immunostaining was positive for insulinoma-associated protein 1 (Figure 2B) and chromogranin A (Figure 2C) and negative for thyroid transcription factor-1, p40, and leukocyte common antigen (not shown). We diagnosed extensive-stage small cell lung cancer (ES-SCLC) based on the clinical course and pathological findings. The pleural fluid was drained over 5,000ml and we confirmed lung re-expansion on chest X-ray. Later, we performed talc pleurodesis to control the pleural effusion (Figures 1C, D) and improve the PS, which was 2 at the initial visit, and subsequently improved to 0. We did not perform pericardiodesis due to concern about the risk of adverse events. Instead, we administered a combination regimen of carboplatin, etoposide, and atezolizumab as first-line chemotherapy. After 4 cycles of first-line chemotherapy, we evaluated the patient to have progressive disease, so we administered amrubicin as second-line chemotherapy. However, her general condition worsened after 2 cycles, and considering her wishes, she was treated with best supportive care alone after that. The patient died approximately 9 months after diagnosis. The timeline of all relevant interventions from the initial visit to the introduction of treatment is shown in Figure 3.
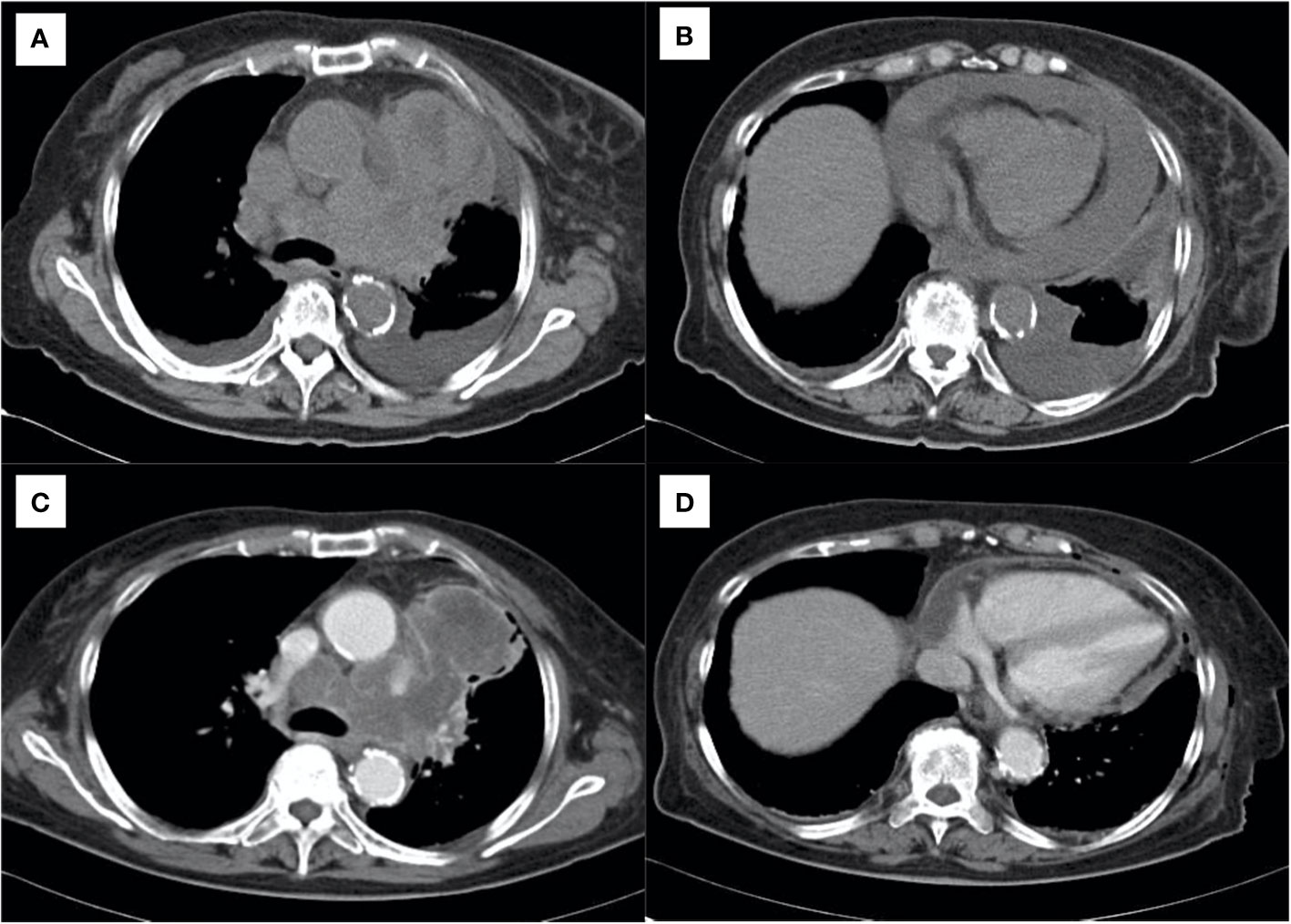
Figure 1 Chest computed tomography showing (A) primary lung cancer and bilateral pleural effusions and (B) pericardial effusion. (C, D) After drainage of the effusions and pleurodesis, showing control of the effusions before starting systemic chemotherapy.
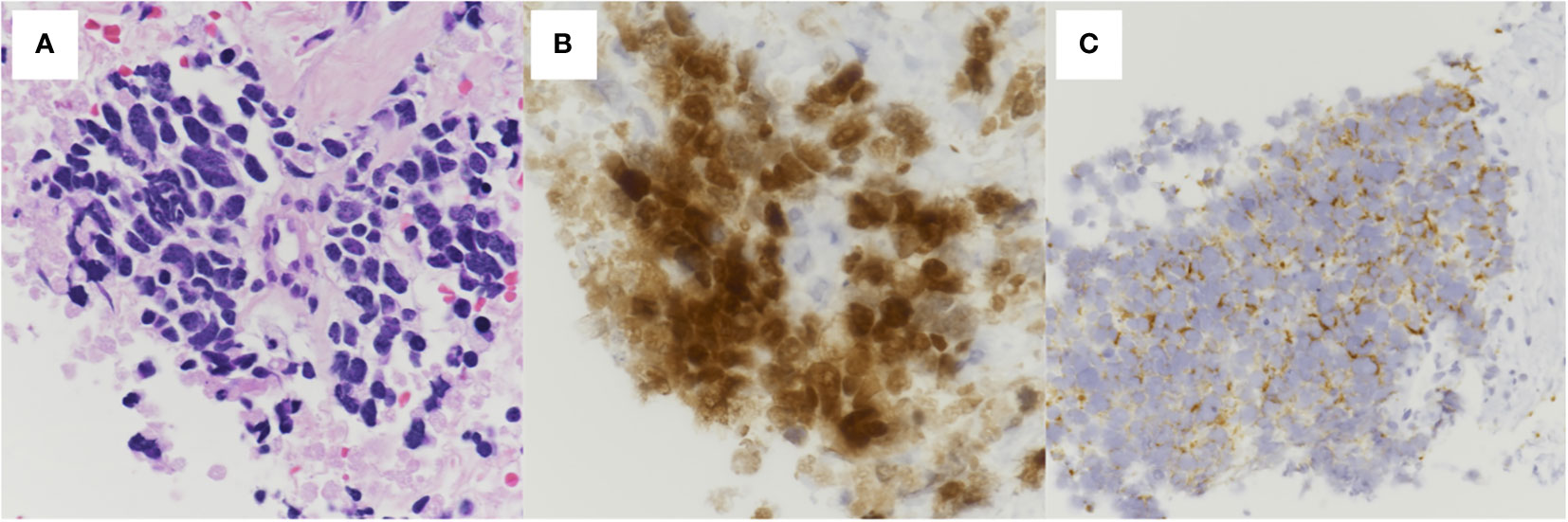
Figure 2 Histopathological findings of the pulmonary tumor. (A) Hematoxylin and eosin staining showing dense sheet-like growth of bare nucleated atypical cells with increased chromatin (400× magnification). Immunostaining showing positive (B) insulinoma-associated protein 1 (400× magnification) and (C) chromogranin A (200× magnification).
Based on the TNM classification, the 5-year survival rate for patients with SCLC with T4 lesions and pleural effusions is 3.6%, with a median overall survival of 7 months (3). Patients with ES-SCLC with pleural effusion on the same side as the primary tumor have a significantly lower overall survival rate than ES-SCLC without pleural effusion (20.9 months vs. 11.8 months, p < 0.001) (4). In patients with SCLC, the presence of malignant pleural effusions is an independent prognostic factor for SCLC, and reduce the median overall survival time by approximately 4 months, and are associated with lower 1-year and 2-year survival rates (17% and 6%, respectively) (5). The median overall survival of patients with SCLC and pericardial effusions is 14.2 months, which is approximately 7 months shorter than that of SCLC patients without pericardial effusions (1). In addition, metastasis of SCLC to the pericardium generally occurs at a relatively late stage in the disease (6). Therefore, the early initiation of chemotherapy is desirable in patients with SCLC patients and pleural effusions, pericardial effusions, or both.
In the IMpower133 trial, which tested the efficacy of a regimen combining atezolizumab, carboplatin, and etoposide compared to placebo, combination chemotherapy increased the overall survival by 2 months (12.3 months vs. 10.3 months, p < 0.01), with no increase in serious adverse events compared to standard therapy (2). In addition, ICIs, including pembrolizumab and durvalumab, combined with cytotoxic anticancer agents improve progression-free survival and overall survival (7, 8). Thus, early initiation of chemotherapy with ICIs, especially in patients with SCLC and pleural or pericardial effusions, who generally have a poor prognosis (9), may contribute to improving the clinical outcome.
A prospective observational study with malignant pleural effusion control as an outcome, conducted in cancer patients with pharmacologically sensitive and non-pharmacologically sensitive tumors of whom 13% had SCLC, found that factor that had the greatest effect on controlling malignant pleural effusions was not whether the patients received systemic chemotherapy, but whether they underwent pleurodesis (10). Another prospective observational study of 509 patients with lung cancer and malignant pleural effusions found that compared to chemotherapy alone, early treatment of malignant pleural effusions reduced the need for future re-intervention (23.5% vs. 53.8%, p < 0.01) (11). In patients with non-small cell lung cancer, intrapericardial chemotherapy alone and a combination of intrapericardial chemotherapy and systemic chemotherapy improve control of cancer-related pericarditis (12–14). These results suggest that in patients with SCLC and pleural and pericardial effusions, drainage and pleurodesis/pericardiodesis prior to chemotherapy might lead to better fluid control and improved symptom control and prognosis.
In this case, we were concerned about the increased risk of adverse events with combined pleurodesis and pericardiodesis; therefore, we performed pleurodesis and pericardial drainage, without pericardiodesis. As a result, chemotherapy could be safely introduced with improved PS. However, there are several limitations in this case. First, the clinical course of chemotherapy preceded by pericardial or pleural drainage is uncertain. Randomized controlled trials of non-small cell lung cancer with pleural effusion suggested that a pleural effusion control rate of 86.9-92.9% may be achieved with chemotherapy alone without pleurodesis (15, 16). Local approaches, such as drainage to the pericardial and thoracic space, are unlikely to result in serious outcomes (12, 17) but may delay the administration of chemotherapy in about 2% of patients (18). Second, the patient’s clinical response was not adequate. The patient had to stop chemotherapy during the second round of chemotherapy due to poor performance status, resulting in death 9 months after diagnosis. In other words, the clinical course could have been different if chemotherapy had been administered prior to the procedure. Third, the impact of pleurodesis on PET/CT, which may be performed to determine the efficacy of chemotherapy, needs to be considered (19). In this patient, PET/CT was not performed due to the inaccessibility and financial burden of PET/CT. We needed to plan our treatment strategy with these matters in mind carefully.
This case highlights the importance of considering aggressive drainage and pleurodesis/pericardiodesis before systemic chemotherapy in patients with SCLC and concurrent pericardial or pleural effusions, even in patients with SCLC that is sensitive to chemotherapy.
I went to the hospital because I was short of breath, and had fluid drained from around my heart and lungs. This treatment was successful, and I was able to receive chemotherapy before the cancer worsened. Fortunately, I did not experience any major side effects of chemotherapy, and I am continuing to live my daily life.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
AK, YF, MS, and MI collected and interpreted the clinical data. AK and YF drafted the manuscript. MS, MI, and HS reviewed and revised the manuscript. All authors read and approved the final version of the manuscript.
We would like to thank Editage (www.editage.jp) for English language editing.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Niho S, Kubota K, Yoh K, Goto K, Ohmatsu H, Nihei K, et al. Clinical outcome of small cell lung cancer with pericardial effusion but without distant metastasis. J Thorac Oncol (2011) 6:796–800. doi: 10.1097/JTO.0b013e318208ec77
2. Horn L, Mansfield AS, Szczęsna A, Havel L, Krzakowski M, Hochmair MJ, et al. First-line atezolizumab plus chemotherapy in extensive-stage small-cell lung cancer. N Engl J Med (2018) 379:2220–9. doi: 10.1056/NEJMoa1809064
3. Ignatius Ou SH, Zell JA. The applicability of the proposed IASLC staging revisions to small cell lung cancer (SCLC) with comparison to the current UICC 6th TNM edition. J Thorac Oncol (2009) 4:300–10. doi: 10.1097/JTO.0b013e318194a355
4. Niho S, Kubota K, Yoh K, Goto K, Ohmatsu H, Nihei K, et al. Clinical outcome of chemoradiation therapy in patients with limited-disease small cell lung cancer with ipsilateral pleural effusion. J Thorac Oncol (2008) 3:723–7. doi: 10.1097/JTO.0b013e31817c606a
5. Shojaee S, Singh I, Solsky I, Nana-Sinkam P. Malignant pleural effusion at presentation in patients with small-cell lung cancer. Respiration (2019) 98:198–202. doi: 10.1159/000499372
6. Megyesfalvi Z, Tallosy B, Pipek O, Fillinger J, Lang C, Klikovits T, et al. The landscape of small cell lung cancer metastases: Organ specificity and timing. Thorac Cancer (2021) 12:914–23. doi: 10.1111/1759-7714.13854
7. Rudin CM, Awad MM, Navarro A, Gottfried M, Peters S, Csőszi T, et al. Pembrolizumab or placebo plus etoposide and platinum as first-line therapy for extensive-stage small-cell lung cancer: Randomized, double-blind, phase III KEYNOTE-604 study. J Clin Oncol (2020) 38:2369–79. doi: 10.1200/JCO.20.00793
8. Goldman JW, Dvorkin M, Chen Y, Reinmuth N, Hotta K, Trukhin D, et al. Durvalumab, with or without tremelimumab, plus platinum-etoposide versus platinum-etoposide alone in first-line treatment of extensive-stage small-cell lung cancer (CASPIAN): Updated results from a randomised, controlled, open-label, phase 3 trial. Lancet Oncol (2021) 22:51–65. doi: 10.1016/S1470-2045(20)30539-8
9. Nakazawa K, Kurishima K, Tamura T, Kagohashi K, Ishikawa H, Satoh H, et al. Specific organ metastases and survival in small cell lung cancer. Oncol Lett (2012) 4:617–20. doi: 10.3892/ol.2012.792
10. Holling N, Patole S, Medford ARL, Maskell NA, Bibby AC. Is systemic anticancer therapy associated with higher rates of malignant pleural effusion control in people with pharmacologically sensitive tumors?: A retrospective analysis of prospectively collected data. Chest (2021) 160:1915–24. doi: 10.1016/j.chest.2021.05.027
11. Chiang KY, Ho JC, Chong P, Tam TC, Lam DC, Ip MS, et al. Role of early definitive management for newly diagnosed malignant pleural effusion related to lung cancer. Respirology (2020) 25:1167–73. doi: 10.1111/resp.13812
12. Moriya T, Takiguchi Y, Tabeta H, Watanabe R, Kimura H, Nagao K, et al. Controlling malignant pericardial effusion by intrapericardial carboplatin administration in patients with primary non-small-cell lung cancer. Br J Cancer (2000) 83:858–62. doi: 10.1054/bjoc.2000.1397
13. Maruyama R, Yokoyama H, Seto T, Nagashima S, Kashiwabara K, Araki J, et al. Catheter drainage followed by the instillation of bleomycin to manage malignant pericardial effusion in non-small cell lung cancer: A multi-institutional phase II trial. J Thorac Oncol (2007) 2:65–8. doi: 10.1097/JTO.0b013e31802c8260
14. Lestuzzi C, Bearz A, Lafaras C, Gralec R, Cervesato E, Tomkowski W, et al. Neoplastic pericardial disease in lung cancer: Impact on outcomes of different treatment strategies. A multicenter study. Lung Cancer (2011) 72:340–7. doi: 10.1016/j.lungcan.2010.10.013
15. Usui K, Sugawara S, Nishitsuji M, Fujita Y, Inoue A, Mouri A, et al. A phase II study of bevacizumab with carboplatin-pemetrexed in non-squamous non-small cell lung carcinoma patients with malignant pleural effusions: North East Japan study group trial NEJ013A. Lung Cancer (2016) 99:131–6. doi: 10.1016/j.lungcan.2016.07.003
16. Tamiya M, Tamiya A, Yamadori T, Nakao K, Asami K, Yasue T, et al. Phase2 study of bevacizumab with carboplatin-paclitaxel for non-small cell lung cancer with malignant pleural effusion. Med Oncol (2013) 30:676. doi: 10.1007/s12032-013-0676-7
17. Saka H, Oki M, Kitagawa C, Kogure Y, Kojima Y, Saito A, et al. Sterilized talc pleurodesis for malignant pleural effusions: A phase II study for investigational new drug application in Japan. Jpn J Clin Oncol (2018) 48:376–81. doi: 10.1093/jjco/hyy020
18. Singh N, Aggarwal AN, Behera D, Jindal SK. Intercycle delays during chemotherapy of non-small cell lung cancer in a health care resource-constrained setting and their effect on overall survival. J Thorac Oncol (2010) 5:236–9. doi: 10.1097/JTO.0b013e3181c3f5f7
Keywords: pericardial effusion, pleural effusion, pleurodesis, small-cell lung cancer, immune checkpoint inhibitor
Citation: Kashima A, Fukuda Y, Shimamura M, Ijichi M and Sagara H (2022) Successful treatment of extensive-stage small cell lung cancer with concurrent pleural and pericardial effusions: Case report. Front. Oncol. 12:1040452. doi: 10.3389/fonc.2022.1040452
Received: 09 September 2022; Accepted: 05 December 2022;
Published: 23 December 2022.
Edited by:
Kohei Fujita, National Hospital Organization Kyoto Medical Center, JapanReviewed by:
Ahmed El Bastawisy, Cairo University, EgyptCopyright © 2022 Kashima, Fukuda, Shimamura, Ijichi and Sagara. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yosuke Fukuda, eS5mLjA0MjNAbWVkLnNob3dhLXUuYWMuanA=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.