
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 17 November 2022
Sec. Gastrointestinal Cancers: Hepato Pancreatic Biliary Cancers
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1039086
 Jiayan Wu1†
Jiayan Wu1† Jiandong Yu1†
Jiandong Yu1† Zhiping Chen1†
Zhiping Chen1† Hongquan Zhu2†
Hongquan Zhu2† Chengrui Zhong1
Chengrui Zhong1 Yongling Liang1
Yongling Liang1 Ziyan Mai1
Ziyan Mai1 Zejin Lin1
Zejin Lin1 Yunle Wan1*
Yunle Wan1* Guolin Li1*
Guolin Li1*Objectives: Gastric cancer with liver metastasis (GCLM) is highly aggressive and has a poor prognosis. This study aims to evaluate the survival benefit of primary tumor resection (PTR) for gastric cancer with liver metastasis.
Methods: Data on patients with GCLM was extracted from the Surveillance, Epidemiology, and End Results (SEER) database from 2010 to 2015. A 1:1 propensity score matching (PSM) analysis was performed to minimize the heterogeneity between the PTR and no-PTR groups. The Kaplan–Meier method and Cox regression analysis were used to assess the impact of primary tumor resection (PTR) on overall survival (OS) and cause-specific survival (CSS).
Results: A total of 3,001 patients with GCLM were included, with 328 patients treated with primary tumor resection (PTR), whereas the other 2,673 patients were not. Patients with PTR had a significantly higher OS and CSS rate than those without PTR in unmatched and PSM cohorts. In an unmatched cohort, the median OS was 12.0 months (95% CI, 10 months to 14 months) for those who underwent PTR and 4 months (95% CI, 4 months to 5 months) for those without PTR; the median CSS for those who underwent PTR was 12.0 months (95% CI, 10 months to14 months) and 4 months (95% CI, 4 months to 5 months) for those without PTR, respectively. After PMS, the median OS was 12.0 months (95% CI, 10 months to 17 months) for those who underwent PTR and 7 months (95% CI, 5 months to 10 months) for those without PTR, respectively; the median CSS for those who underwent PTR was 12.0 months (95% CI, 11 months to 17 months) and 7 months (95% CI, 5 months to 8 months) for those without PTR, respectively. In addition, multivariate Cox analysis in the PSM cohort showed that PTR, age, degree of tumor differentiation, and chemotherapy were independent prognostic factors for OS and CSS in GCLM. Specifically, PTR was a significant protective factor for OS (HR: 0.427; 95% CI, 0.325 to 0.561, P <0.001) and CSS (HR: 0.419; 95% CI, 0.313 to 0.561, P <0.001).
Conclusion: Primary tumor resection improves the survival of gastric cancer patients with liver metastasis.
Gastric cancer is the fifth most common malignant tumor, and it is the fourth-leading cause of cancer-related deaths in the world. In 2020, over one million (1,089,103) new cases were reported, with an estimated 769,000 deaths worldwide (1). Besides, because of a lack of early symptoms, over 80% of gastric cancer patients were at an advanced stage at the time of diagnosis. Some of them had distant metastases. The metastases of gastric cancer include three routes: hematogenous, lymphatic, and peritoneal dissemination (2, 3); and the liver is the most common metastatic organ. For gastric cancer patients with liver metastasis (GCLM), guidelines regard palliative gastrectomy as a choice when surgery is unavoidable (4) and chemotherapy can be considered to be adopted (5, 6). The AIO-FLOT3 trial (7) showed that patients who were treated with gastrectomy and chemotherapy had better OS (22.9 vs. 10.7 months) than those treated with chemotherapy alone. However, the phase III study REGATTA trial (8) reported that the overall survival (OS) and progression-free survival (PFS) of patients who were treated with palliative surgery plus chemotherapy had no significant difference with those who were treated with chemotherapy only. Consequently, whether palliative gastrectomy improves the prognosis of GCLM remains unclear. The necessity of primary tumor resection for GCLM patients is a debatable issue.
Therefore, the aim of the study was to investigate the survival influence of primary tumor resection in GCLM patients based on the Surveillance, Epidemiology, and End Results (SEER) database.
This study was approved by the Ethics Committee of the Sixth Affiliated Hospital of Sun Yat-sen University, and this study does not need informed patient consent because the data was collected from the SEER database.
The Surveillance, Epidemiology, and End Results (SEER) program, sponsored by the National Carcinoma Institute of the United States of America and established in 1973, reported a variety of cancer cases with patient characteristics and patient survival from 19 regional areas of the USA, representing approximately 34.6% of the US population. We used the SEER*Stat software (version 8.4.0) with the ID number 10355-Nov2021 to obtain patient characteristics and survival information. This software was used to identify gastric cancer with liver metastasis (GCLM) (Figure 1). Firstly, GCLM patients were retrieved based on the Site and Morphology value ({Site and Morphology. Primary Site-labeled} = ‘C16.0,’ ‘C16.1,’ ‘C16.2,’ ‘C16.3,’ ‘C16.4,’ ‘C16.5,’ ‘C16.6,’ ‘16.7,’ ‘C16.8,’ ‘C16.9’). Secondly, gastric cancer with liver metastasis between 2010 and 2015 with a microscopic diagnosis was chosen. The inclusion criteria for our study were as follows: (1) being diagnosed with gastric cancer only; (2) confirmed liver metastases; (3) confirmed with histologically documented cancer; and (4) patients who had a primary tumor resection. The exclusion criteria in this study were as follows: (1) patients with more than one primary tumor or unknown cancer; (2) combined with other organ metastases; and (3) unknown primary tumor resection. Thirdly, all the variables collected in this study were as follows: (1) year of diagnosis (2010/2011/2012/2013/2014/2015); (2) age at diagnosis (>/<65y); (3) sex (Male/Female); (4) race (Black/White/Other); (5) marital status (Divorced/Married/Single/Other); (6) income (<60,000/>60,000); (7) residence (Metropolitan/Rural/Urban); (8) reporting source (Hospital/no-Hospital); (9) PRCDA (No/Yes); (10) original record (Hispanic/Non-Hispanic); (11) tumor differentiation (Grade I/Grade II/Grade III/Grade IV/Unknown); (12) tumor T-stage (T0/T1/T2/T3/T4/TX); (13) tumor N-stage (N0/N1/N2/N3/NX); (14) pathological type (adenocarcinoma/Gastrointestinal stromal/Intestinal type/Signet ring cell/Other); (15) tumor location (Body/Cardia/Fundus/Gastric antrum/Greater/Lesser/Pylorus/Other); (16) tumor size (<1 cm/>1 cm/Unknown); (17) chemotherapy (No/Yes); (18) radiotherapy (No/Yes); (19) primary tumor resection (No/Yes); and (20) survival rate.
The aim of the study was to research the effect of primary tumor resection in patients with gastric cancer with liver metastasis, and this research was a retrospective study. Consequently, the potential covariates between primary tumor resection and the non-surgery cohort were not balanced, which might distort the fundamental relationship of primary tumor resection with OS and CSS. Therefore, propensity score matching (PSM) was further performed to reduce this influence. And according to the nearest neighbor matching method with a setting caliper value of 0.1 (9), patients were propensity matched 1:1 into PTR and no-PTR groups. All the covariates used for matching in this study were as follows: age, year of diagnosis, sex, race, marital status, income, residence, reporting source, PRCDA, original record, tumor differentiation, tumor T-stage, tumor N-stage, pathological type, tumor location, tumor size, chemotherapy, and radiotherapy.
The Kaplan‐Meier method was used to estimate OS and CSS before and after PSM, and the log-rank tests were conducted to compare survival differences in the PTR and no-PTR groups. The Cox proportional hazards regression method was performed to identify prognostic factors for OS and CSS. In the univariate Cox model, variables with a P-value <0.05 were further incorporated into the multivariate Cox analysis to identify the independent prognostic factors of OS and CSS. All statistical analyses in this study were performed with R software (version 4.1.3; https://www.r-project.org/), and a two-tailed P <0.05 was considered statistically significant.
In this study, a total of 5,313 patients diagnosed with microscopically confirmed gastric cancer and liver metastasis between 2010 and 2015 were extracted from the SEER database. Based on the inclusion and exclusion criteria in our study, 3,001 patients were enrolled for further analysis (Figure 1). Of those, 328 (12.3%) patients underwent PTR, whereas 2,673 (87.7%) did not. The baseline characteristics of the overall population are shown in Table 1. A 1:1 PSM analysis was performed to balance the available covariates such as sex, race, degree of tumor differentiation, tumor T-stage, tumor N-stage, pathological type, tumor location, and tumor size between the PTR and no-PTR groups, a 1:1 PSM analysis was performed. The baseline characteristics that were well-balanced in the matched cohort after PSM are also summarized in Table 1.
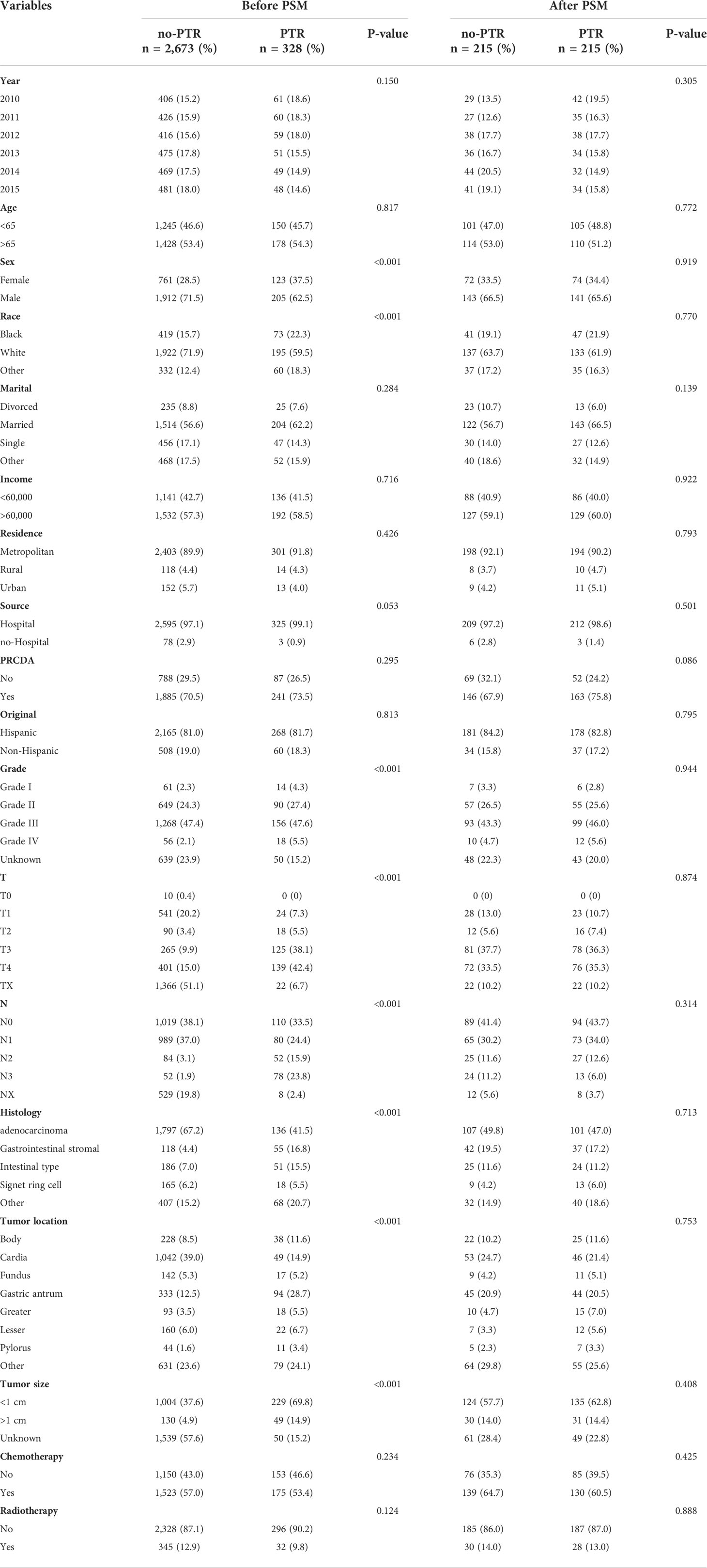
Table 1 Baseline characteristics of the selected patients before and after propensity score matching.
A Kaplan‐Meier analysis was performed to calculate the OS and CSS of the overall population cohort before PSM. The results showed that patients with PTR had a significantly higher OS and CSS rate than those without PTR (log-rank p <0.001, Figure 2A; log-rank p <0.001, Figure 2B). The median OS was 12.0 months (95% CI, 10 months to 14 months) for those who underwent PTR and 4 months (95% CI, 4 months to 5 months) for those without PTR, respectively. The median CSS for those who underwent PTR was 12.0 months (95% CI, 10 months to 14 months) and 4 months (95% CI, 4 months to 5 months) for those without PTR, respectively.
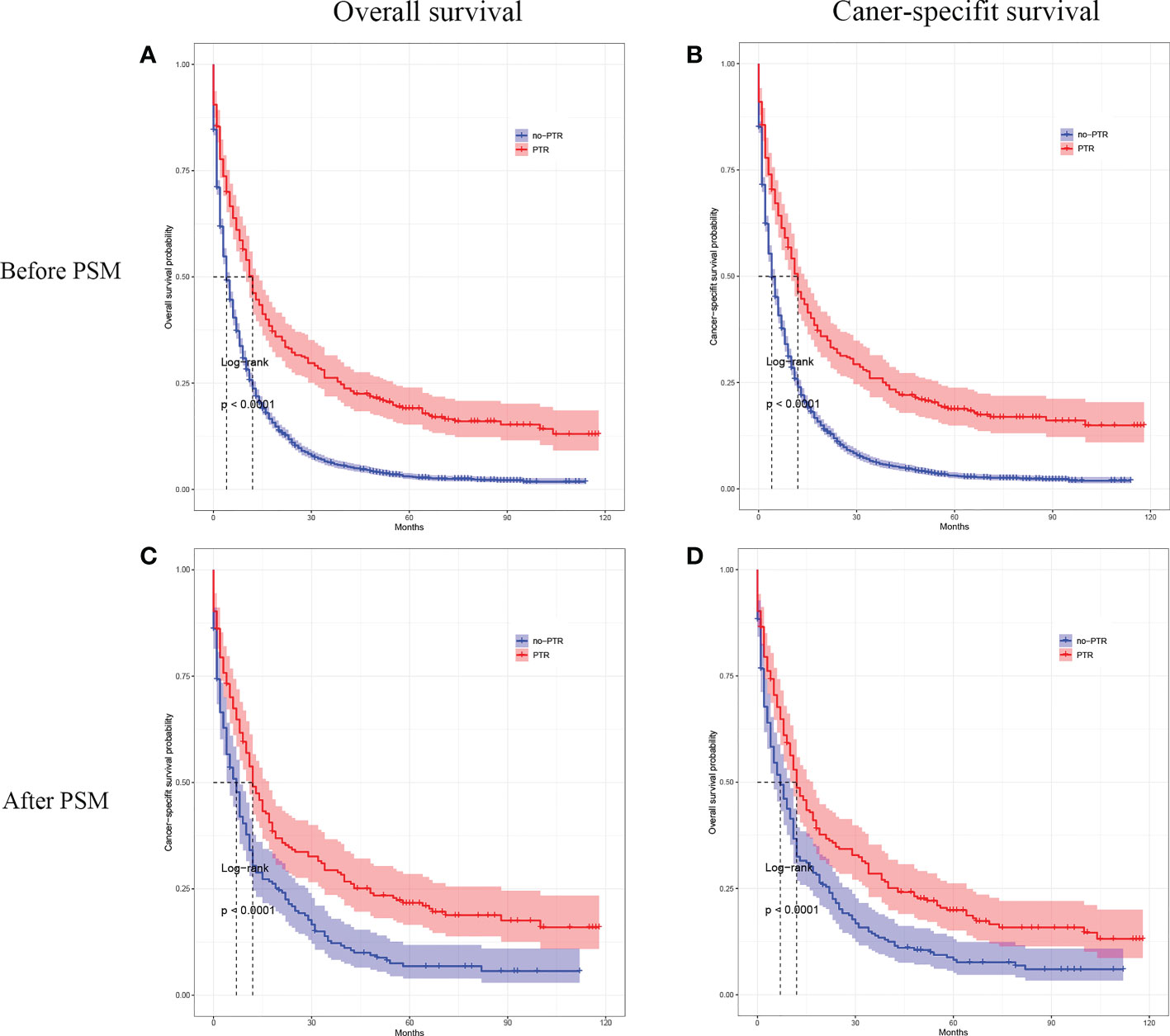
Figure 2 Kaplan–Meier curves for overall survival and cancer-specific survival of patients with primary tumor resection versus without primary tumor resection before and after the propensity score matching.
In the matched cohort, patients with PTR also had a significantly higher OS and CSS rate than those without PTR (log-rank p <0.001, Figure 2C; log-rank p <0.001, Figure 2D). After PMS, the median OS was 12.0 months (95% CI, 10 months to 17 months) for those who underwent PTR and 7 months (95% CI, 5 months to 10 months) for those without PTR, respectively. The median CSS for those who underwent PTR was 12.0 months (95% CI, 11 months to 17 months) and 7 months (95% CI, 5 months to 8 months) for those without PTR, respectively. Furthermore, the Cox proportional hazards model was performed to confirm the prognostic significance of PTR in patients with gastric cancer and liver metastasis (Table 2). In the univariable Cox analysis for the matched cohort, PTR, age, degree of tumor differentiation, tumor N-stage, pathological type, tumor size, and chemotherapy were significantly associated with OS, and these variables were all included in the multivariate Cox analysis. Multivariate Cox analysis revealed that PTR, age, degree of tumor differentiation, and chemotherapy were independent prognostic factors for OS of the patients with gastric cancer and liver metastasis. Specifically, PTR was a significant protective factor for OS (HR: 0.427; 95% CI, 0.325 to 0.561, P <0.001). As for mortality risk, it was higher in patients with age >65 y, poorly differentiated or undifferentiated and no chemotherapy than in patients with age <65 y, well-differentiated or moderately differentiated and chemotherapy, respectively (Table 2).
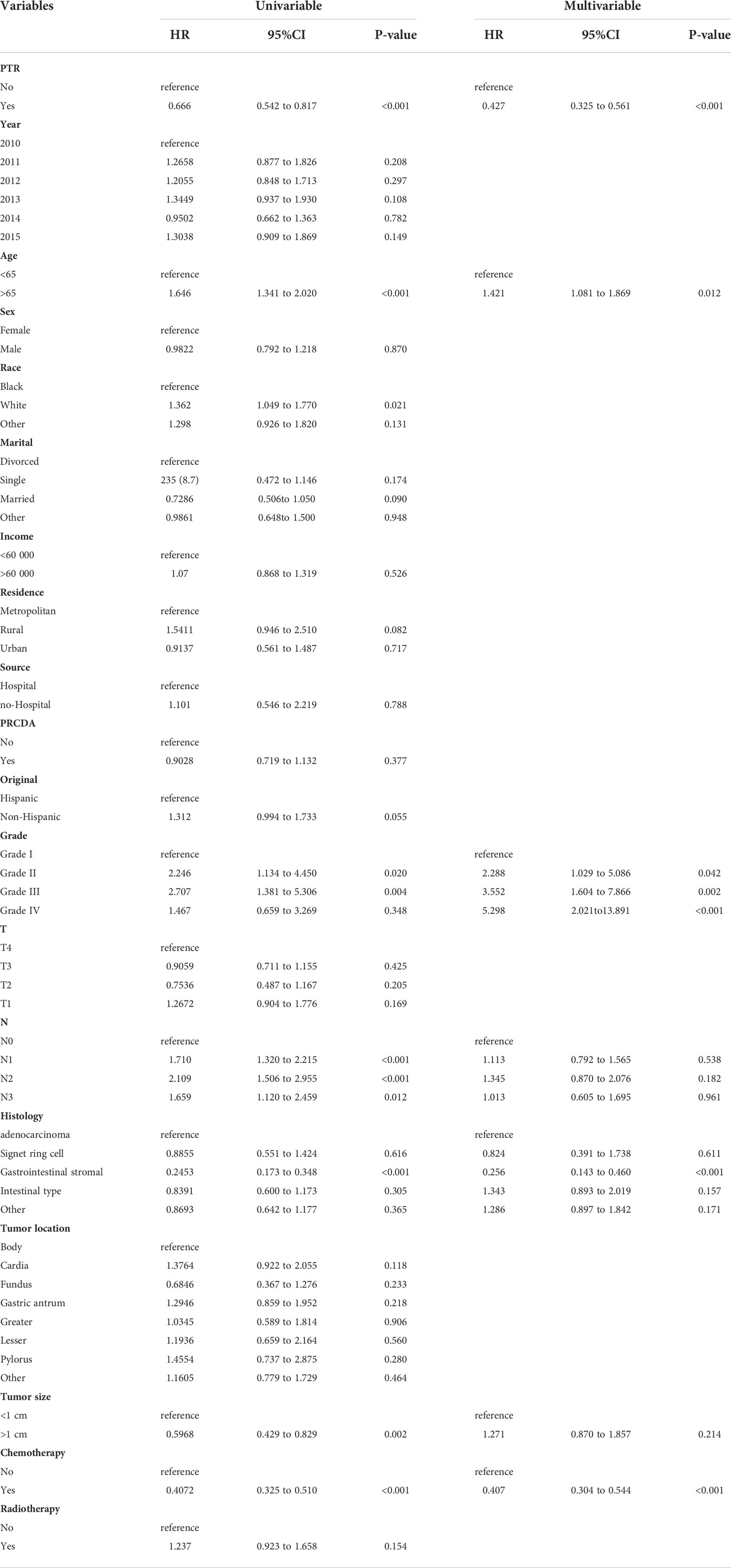
Table 2 Univariate and multivariate analysis of prognostic factors for OS in the propensity score matched cohort.
Cox analysis showed that PTR, age, tumor differentiation, and chemotherapy were independent prognostic factors for OS in patients with gastric cancer and liver metastasis. Therefore, we used subgroup analysis to explore the effect of PTR on overall survival. In the age subgroup with a median age of <65, the overall survival and cancer-specific survival of the PTR group were longer than the no-PRT group (log-rank P <0.001, Figure 3A and log-rank P <0.001, Figure 3B). Similarly, in the age subgroup with a median age of >65, the overall survival and cancer-specific survival of the PTR group were longer than the no-PRT group (log-rank P <0.001, Figure 3C and log-rank P <0.001, Figure 3D). Based on subgroup analysis of tumor differentiation, overall survival and cancer-specific survival rate of the PTR group were significantly higher than those of the no-PTR group (log-rank P <0.001, Figure 4A and log-rank P <0.001, Figure 4B) in patients with well or moderately differentiated tumors (Grades I–II). Also, in the patients with poorly differentiated or undifferentiated tumors (Grades III–IV), the overall survival and cancer-specific survival rates of the PTR group were significantly higher than those of the no-PTR group (log-rank P <0.001, Figure 4C and log-rank P <0.001, Figure 4D). In the chemotherapy subgroup, the overall survival and cancer-specific survival of the PTR group were longer than the no-PRT group in the patients with chemotherapy (log-rank P <0.001, Figure 5A and log-rank P <0.001, Figure 5B). Likewise, in the subgroup without chemotherapy, the overall survival and cancer-specific survival of the PTR group were also longer than the no-PRT group (log-rank P <0.001, Figure 5C and log-rank P <0.001, Figure 5D).
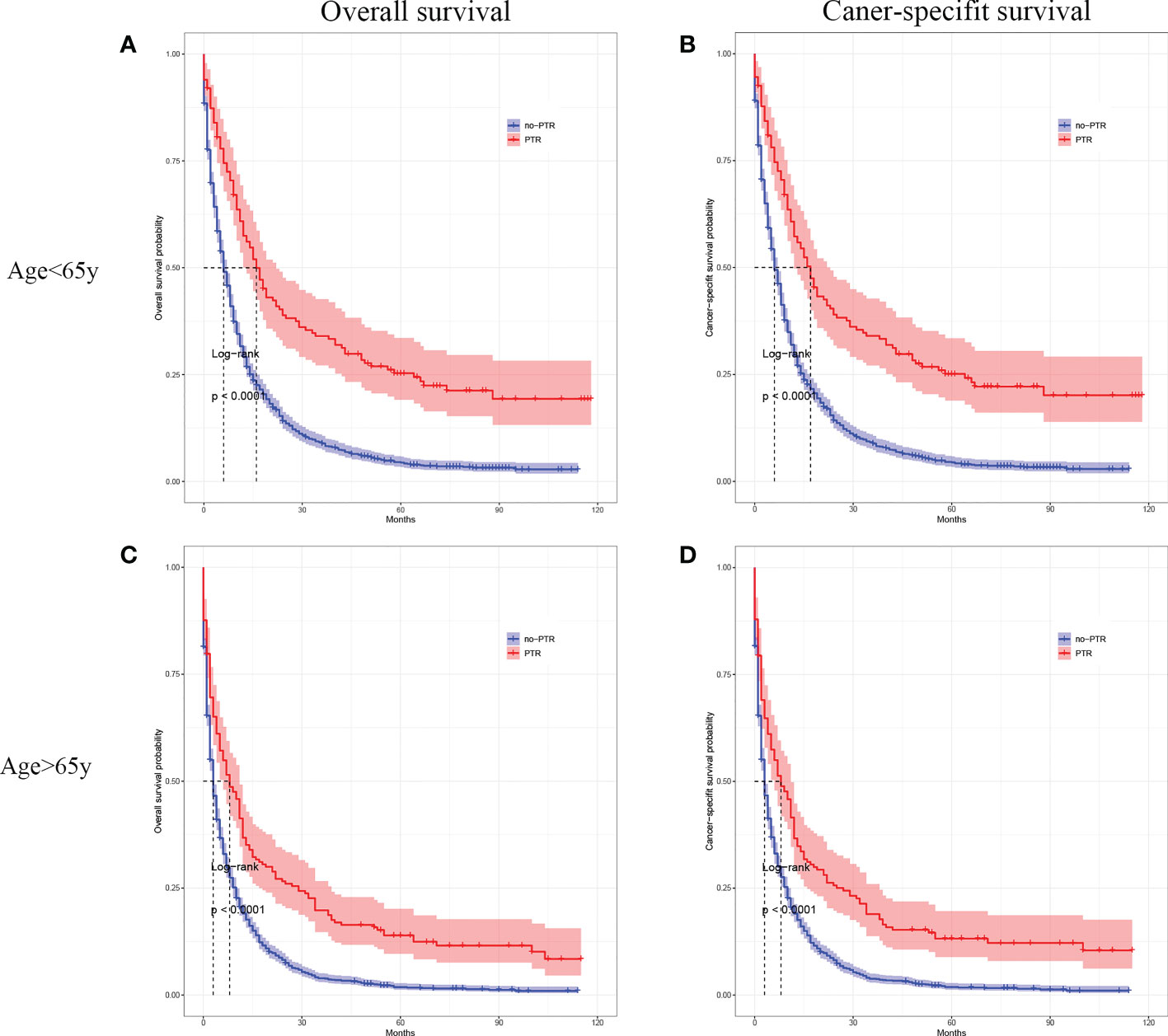
Figure 3 Kaplan–Meier curves for overall survival and carcinoma-specific survival of patients with primary tumor resection versus without primary tumor resection stratified by age in the unmatched cohort.
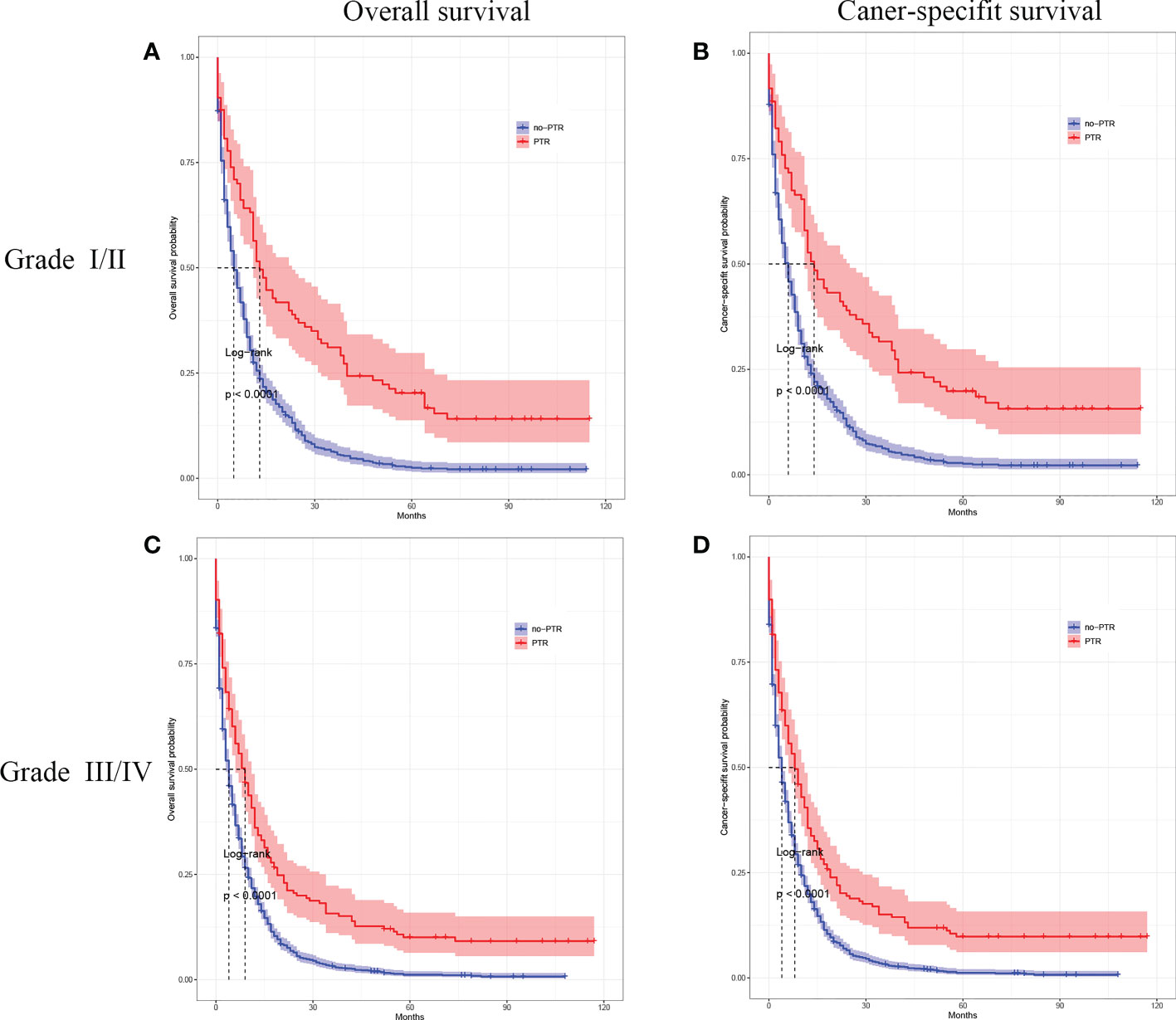
Figure 4 Kaplan–Meier curves for overall survival and carcinoma-specific survival of patients with primary tumor resection versus without primary tumor resection stratified by tumor differentiation in the unmatched cohort.
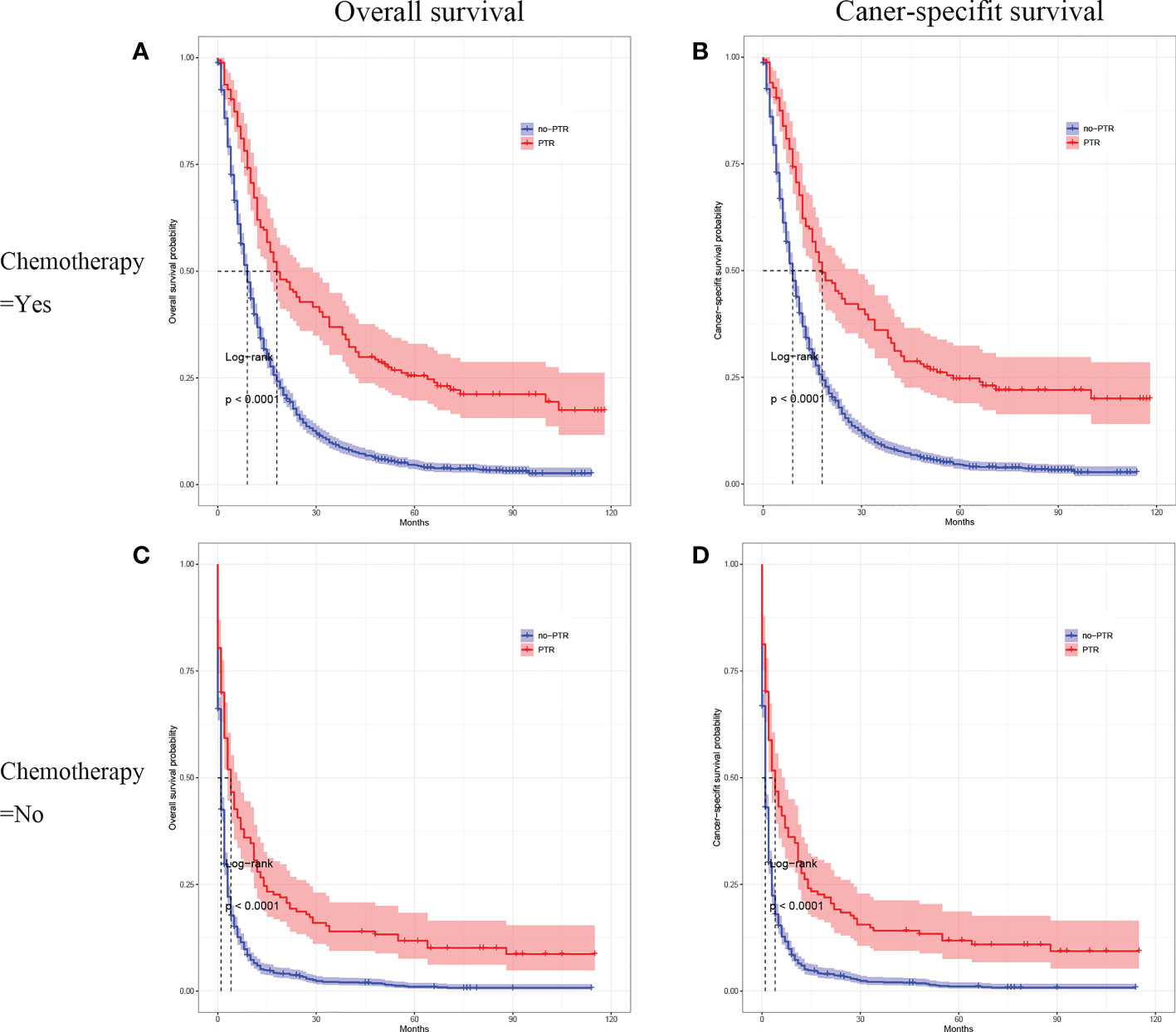
Figure 5 Kaplan–Meier curves for overall survival and carcinoma-specific survival of patients with primary tumor resection versus without primary tumor resection stratified by chemotherapy in the unmatched cohort.
Gastric cancer with liver metastasis (GCLM), the deadliest disease, is considered an advanced gastric cancer with a poor prognosis (10). GCLM includes two types: synchronous metastases and metachronous metastases. Synchronous metastases are defined as metastases that appear before surgical resection or after gastrectomy within 6 months, whereas metachronous metastases occur after gastrectomy over 180 days (11–13). At the initial time of diagnosis, about 4%–14% of gastric cancer patients presented with liver metastases due to its non-specific symptoms (14). However, approximately 37% of gastric cancer patients developed metachronous liver metastases after gastrectomy (15). GCLM was considered an incurable disease and the first-line treatment for it was systematic chemotherapy according to EMSO’s or NCCN’s guidelines (16, 17). The role of surgical resection is still controversial and debated because of limited data to support routine surgery resection (18). A gastrectomy is considered to be performed when severe gastrointestinal symptoms such as obstruction or refractory hemorrhage occur. However, more and more surgeons investigated the role of surgery resection in patients with liver metastases inspired by survival benefits of surgery of colorectal cancer patients with liver metastases (13, 19, 20). Some retrospective studies showed that surgery, including liver resection or primary tumor resection in GCLM, significantly improved survival (11, 21–23). But owing to the single center and small case data, the benefit of primary tumor resection for gastric patients with liver metastases needs to be further confirmed.
In our study, all 328 patients from multiple centers underwent gastric primary tumor resection, including partial, sub-total, or total gastrectomy, which confirmed that primary tumor resection, as an independent protective factor, improves overall and cancer-specific survival of gastric cancer patients with liver metastasis. Firstly, using the data collected from the SEER database, this study showed big improvements in survival outcomes of PTR to GCLM patients in the overall patient cohort. Secondly, after PSM with the aim of balancing the potential covariates between the primary tumor resection and non-surgery cohort, the better OS and CSS of GCLM patients in the PTR group than in the no-PTR group, and our research confirmed that PTR, age, degree of tumor differentiation, and chemotherapy were independent prognostic factors for OS of the patients with gastric cancer and liver metastasis. Specifically, PTR was a significant protective factor for OS (HR: 0.427; 95% CI, 0.325 to 0.561, P <0.001). Thirdly, subgroup analysis showed that GCLM patients with age <65 y, well-differentiated or moderately differentiated, and chemotherapy have better overall survival in the PTR group than the no-PRT group.
However, a recent clinical trial showed contradictory and inconsistent results of gastrectomy in GCLM patients. The AIO-FLOT3 trial showed that patients who were treated with gastrectomy and chemotherapy had better OS (22.9 vs. 10.7 months) than those treated with chemotherapy alone, while the REGATTA trial reported that the overall survival (OS) and progression-free survival (PFS) of patients who were treated with palliative surgery plus chemotherapy had no significant difference compared to those who were treated with chemotherapy alone. But in other metastatic diseases such as ovarian and renal tumors, primary tumor resection can result in better survival, as reported in clinical trials (24–26). Why primary tumor resection can prolong the survival of metastatic tumor patients remains uncertain. Recently, a possible mechanism was reported that recovering the immune system by primary tumor resection improved patient survival (27, 28). Also, another possible mechanism was reported that the high circulating tumor cells (CTCs) in the blood resulted from primary tumor causes such as liver or lung metastases (29, 30). So, primary tumor resection might prolong the survival of metastatic tumor patients by reducing circulating tumor cells (27). Therefore, we consider that primary tumor resection improves the survival of gastric cancer patients with liver metastasis possibly by recovering the immune system and reducing circulating tumor cells.
Some opponents argue that resection of the primary tumor in metastasis tumor patients might delay the start of systemic therapy and increase postoperative complications, thus affecting survival time (31). Besides, medical costs and the quality of postoperative life should be considered before surgery (32). Consequently, a multidisciplinary team should evaluate whether gastric cancer patients with liver metastasis are suitable for excising the primary tumor.
There are several limitations to this study. Firstly, this study based on the SEER database has the natural limitation of incomplete information. This database didn’t record patients’ performance status and comorbidities; preoperative or postoperative complications; the details of liver metastases and their treatment; or chemotherapy regimens. Those factors affect the prognosis of patients with GCLM, but we cannot acquire that relevant information from the SEER database. Secondly, due to its retrospective nature, selection bias in our study was unavoidable. So we used PSM analysis to balance covariates between the PTR and no-PTR groups to reduce other confounding biases. However, there might be other unobserved confounders not included in the propensity score matching. Thirdly, it is not reported whether the clinical symptoms of the primary gastric tumor might affect the selection of PTR surgery. All in all, only a well-designed randomized control trial can avoid those biases and verify our findings.
In conclusion, this propensity score-matched, population-based study showed that primary tumor resection yields an association with favorable overall and cancer-specific survival in gastric cancer patients with liver metastasis. A well-designed prospective randomized controlled trial should be performed to confirm this initial conclusion.
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Conception and design: YW and GL. Administrative support: YW. Provision of study materials or patients: JW, HZ, and CZ. Collection and assembly of data: JY, YL, ZM, and ZL. Data analysis: JW. Manuscript writing: All authors. All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
This study was supported by the National Natural Science Foundation of China (No. 8180102403 ; No. 30671987) and National Key Clinical Discipline.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71:209–49. doi: 10.3322/caac.21660
2. Hirotsu Y, Hada M, Amemiya K, Oyama T, Mochizuki H, Omata M. Multi-regional sequencing reveals clonal and polyclonal seeding from primary tumor to metastases in advanced gastric cancer. J Gastroenterol (2020) 55:553–64. doi: 10.1007/s00535-019-01659-6
3. Cortes-Guiral D, Hubner M, Alyami M, Bhatt A, Ceelen W, Glehen O, et al. Primary and metastatic peritoneal surface malignancies. Nat Rev Dis Primers (2021) 7:91. doi: 10.1038/s41572-021-00326-6
4. Eom SS, Choi W, Eom BW, Park SH, Kim SJ, Kim YI, et al. A comprehensive and comparative review of global gastric cancer treatment guidelines. J Gastric Cancer (2022) 22:3–23. doi: 10.5230/jgc.2022.22.e10
5. Li M, Yang B. Long-term outcomes after different treatments for gastric cancer with synchronous liver metastasis: A PRISMA systematic review and network meta-analysis. Med (Baltimore) (2022) 101:e29533. doi: 10.1097/MD.0000000000029533
6. Nakayama N, Ishido K, Chin K, Nishimura K, Azuma M, Matsusaka S, et al. A phase I study of s-1 in combination with nab-paclitaxel in patients with unresectable or recurrent gastric cancer. Gastric Cancer (2017) 20:350–7. doi: 10.1007/s10120-016-0614-4
7. Al-Batran SE, Homann N, Pauligk C, Illerhaus G, Martens UM, Stoehlmacher J, et al. Effect of neoadjuvant chemotherapy followed by surgical resection on survival in patients with limited metastatic gastric or gastroesophageal junction cancer: The AIO-FLOT3 trial. JAMA Oncol (2017) 3:1237–44. doi: 10.1001/jamaoncol.2017.0515
8. Fujitani K, Yang HK, Mizusawa J, Kim YW, Terashima M, Han SU, et al. Gastrectomy plus chemotherapy versus chemotherapy alone for advanced gastric cancer with a single non-curable factor (REGATTA): A phase 3, randomised controlled trial. Lancet Oncol (2016) 17:309–18. doi: 10.1016/S1470-2045(15)00553-7
9. Chen X, Hu W, Huang C, Liang W, Zhang J, Wu D, et al. Survival outcome of palliative primary tumor resection for colorectal cancer patients with synchronous liver and/or lung metastases: A retrospective cohort study in the SEER database by propensity score matching analysis. Int J Surg (2020) 80:135–52. doi: 10.1016/j.ijsu.2020.06.024
10. Luo Z, Rong Z, Huang C. Surgery strategies for gastric cancer with liver metastasis. Front Oncol (2019) 9:1353. doi: 10.3389/fonc.2019.01353
11. Thelen A, Jonas S, Benckert C, Lopez-Hanninen E, Neumann U, Rudolph B, et al. Liver resection for metastatic gastric cancer. Eur J Surg Oncol (2008) 34:1328–34. doi: 10.1016/j.ejso.2008.01.022
12. Li SC, Lee CH, Hung CL, Wu JC, Chen JH. Surgical resection of metachronous hepatic metastases from gastric cancer improves long-term survival: A population-based study. PloS One (2017) 12:e182255. doi: 10.1371/journal.pone.0182255
13. Markar SR, Mikhail S, Malietzis G, Athanasiou T, Mariette C, Sasako M, et al. Influence of surgical resection of hepatic metastases from gastric adenocarcinoma on long-term survival: Systematic review and pooled analysis. Ann Surg (2016) 263:1092–101. doi: 10.1097/SLA.0000000000001542
14. Kim JJ. Epidemiology of gastroesophageal junction adenocarcinoma in korea. J Gastric Cancer (2018) 18:328–38. doi: 10.5230/jgc.2018.18.e38
15. Cui JK, Liu M, Shang XK. Hepatectomy for liver metastasis of gastric cancer: A meta-analysis. Surg Innov (2019) 26:692–7. doi: 10.1177/1553350619856491
16. Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Cooke D, Corvera C, et al. Gastric cancer, version 2.2022, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2022) 20:167–92. doi: 10.6004/jnccn.2022.0008
17. Lordick F, Carneiro F, Cascinu S, Fleitas T, Haustermans K, Piessen G, et al. Gastric cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol (2022) 33:1005–20. doi: 10.1016/j.annonc.2022.07.004
18. Kataoka K, Kinoshita T, Moehler M, Mauer M, Shitara K, Wagner AD, et al. Current management of liver metastases from gastric cancer: What is common practice? new challenge of EORTC and JCOG. Gastric Cancer (2017) 20:904–12. doi: 10.1007/s10120-017-0696-7
19. Quan D, Gallinger S, Nhan C, Auer RA, Biagi JJ, Fletcher GG, et al. The role of liver resection for colorectal cancer metastases in an era of multimodality treatment: A systematic review. Surgery (2012) 151:860–70. doi: 10.1016/j.surg.2011.12.018
20. Hur H, Ko YT, Min BS, Kim KS, Choi JS, Sohn SK, et al. Comparative study of resection and radiofrequency ablation in the treatment of solitary colorectal liver metastases. Am J Surg (2009) 197:728–36. doi: 10.1016/j.amjsurg.2008.04.013
21. Lee JW, Choi MH, Lee YJ, Ali B, Yoo HM, Song KY, et al. Radiofrequency ablation for liver metastases in patients with gastric cancer as an alternative to hepatic resection. BMC Cancer (2017) 17:185. doi: 10.1186/s12885-017-3156-1
22. Turanli S. The value of resection of primary tumor in gastric cancer patients with liver metastasis. Indian J Surg (2010) 72:200–5. doi: 10.1007/s12262-010-0053-0
23. Granieri S, Altomare M, Bruno F, Paleino S, Bonomi A, Germini A, et al. Surgical treatment of gastric cancer liver metastases: Systematic review and meta-analysis of long-term outcomes and prognostic factors. Crit Rev Oncol Hematol (2021) 163:103313. doi: 10.1016/j.critrevonc.2021.103313
24. Flanigan RC, Salmon SE, Blumenstein BA, Bearman SI, Roy V, McGrath PC, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med (2001) 345:1655–9. doi: 10.1056/NEJMoa003013
25. Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: A randomised trial. Lancet (2001) 358:966–70. doi: 10.1016/s0140-6736(01)06103-7
26. van der Burg ME, Vergote I. The role of interval debulking surgery in ovarian cancer. Curr Oncol Rep (2003) 5:473–81. doi: 10.1007/s11912-003-0008-8
27. van Rooijen KL, Shi Q, Goey K, Meyers J, Heinemann V, Diaz-Rubio E, et al. Prognostic value of primary tumour resection in synchronous metastatic colorectal cancer: Individual patient data analysis of first-line randomised trials from the ARCAD database. Eur J Cancer (2018) 91:99–106. doi: 10.1016/j.ejca.2017.12.014
28. Turner N, Tran B, Tran PV, Sinnathamby M, Wong HL, Jones I, et al. Primary tumor resection in patients with metastatic colorectal cancer is associated with reversal of systemic inflammation and improved survival. Clin Colorectal Cancer (2015) 14:185–91. doi: 10.1016/j.clcc.2015.02.004
29. Budd GT, Cristofanilli M, Ellis MJ, Stopeck A, Borden E, Miller MC, et al. Circulating tumor cells versus imaging–predicting overall survival in metastatic breast cancer. Clin Cancer Res (2006) 12:6403–9. doi: 10.1158/1078-0432.CCR-05-1769
30. Wong NS, Kahn HJ, Zhang L, Oldfield S, Yang LY, Marks A, et al. Prognostic significance of circulating tumour cells enumerated after filtration enrichment in early and metastatic breast cancer patients. Breast Cancer Res Treat (2006) 99:63–9. doi: 10.1007/s10549-006-9181-4
31. Chen JN, Shoucair S, Wang Z, Habib JR, Zhao FQ, Yu J, et al. Primary tumor resection for rectal cancer with unresectable liver metastases: A chance to cut is a chance for improved survival. Front Oncol (2021) 11:628715. doi: 10.3389/fonc.2021.628715
Keywords: gastric cancer, liver metastasis, primary tumor resection, prognosis, SEER
Citation: Wu J, Yu J, Chen Z, Zhu H, Zhong C, Liang Y, Mai Z, Lin Z, Wan Y and Li G (2022) Survival benefit of primary tumor resection for gastric cancer with liver metastasis: A propensity score-matched, population-based study. Front. Oncol. 12:1039086. doi: 10.3389/fonc.2022.1039086
Received: 07 September 2022; Accepted: 27 October 2022;
Published: 17 November 2022.
Edited by:
Christian Cotsoglou, ASST Brianza, ItalyReviewed by:
Sissi Paleino, ASST Vimercate, ItalyCopyright © 2022 Wu, Yu, Chen, Zhu, Zhong, Liang, Mai, Lin, Wan and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guolin Li, bGlnbGluQG1haWwuc3lzdS5lZHUuY24=; Yunle Wan, d2FueXVubGVAbWFpbC5zeXN1LmVkdS5jbg==
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.