
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 09 November 2022
Sec. Gynecological Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1036710
This article is part of the Research Topic New Molecular Approaches to Improve Gynecological Cancer Management View all 11 articles
Background: Clinically, few patients with locally advanced cervical cancer (LACC) are insensitive to neoadjuvant chemotherapy (NACT). Recent studies have reported that circulating microRNAs (miRNAs) may be involved in the response to NACT. The aim of this study was to discover the potential miRNAs that can predict the response to NACT in LACC.
Methods: Pair-matched blood samples of 39 LACC patients before and after receiving NACT were collected. Seven paired samples were used for microRNA microarray analysis. Targeted miRNAs were selected by bioinformatics analysis and were validated by quantitative reverse transcription–polymerase chain reaction (qRT-PCR). All 39 patients were assigned into either the responders group or the non-responders group after NACT. The predictive performance of selected microRNA was evaluated by sensitivity, specificity, accuracy, and the area under the receiver operating characteristic (ROC) curve.
Results: A total of 17 miRNAs downregulated before NACT and upregulated after NACT were selected according to microarray analysis in our previous study, and miR-326 and miR-376a-3p were selected for further exploration. According to the responses and the evaluation criteria, 25 patients reached partial response (PR) and 14 patients remained stable. Further qRT-PCR analysis showed that miR-326 significantly downregulated before NACT and upregulated after NACT in 12 responders (p = 0.02). The expression of miR-376a-3p showed no statistical difference before and after NACT in these 12 responders. Then, miR-326 provided an AUC-ROC of 0.75 (p = 0.04) in the discrimination between the responders and non-responders groups. The cutoff value of ROC for miR-326 to predict the response of NACT was <0.023, the sensitivity was 88.89%, and the specificity was 50%.
Conclusions: The expression of miR-326 significantly upregulated after NACT in responders. miR-326 may be a biomarker for predicting the response to NACT in LACC patients. The results may optimize individualized treatments for LACC patients.
Cervical cancer ranks second in cancer incidence and death, following breast cancer, in women, with an estimated 570,000 cases and 311,000 deaths in 2018 worldwide (1). The majority of the cases are diagnosed as squamous cell carcinoma (SCC). Human papillomavirus (HPV) infection is the necessary but not sufficient cause of cervical cancer (2). Other important factors are young age at first coitus, multiple sexual partners, high parity, the presence of foreskin in the male partner, cigarette smoke, diet, and so on (3, 4). The risk factors associated with the development of HPV have been summarized in Table 1. After Friedlander et al. firstly reported in 1983 that cervical cancer was responsive to cisplatin-based combination chemotherapy (5), neoadjuvant chemotherapy (NACT) followed by radical hysterectomy or radiotherapy has been investigated. NACT can inhibit tumor micrometastases, improve the radiosensitivity of the tumor, and effectively shrink the tumor volume. The ovarian and vaginal functions could be reserved in patients who underwent NACT followed by radical surgery (6). However, the response of NACT varies due to individual differences and the complexity of cancer. Previous studies reported that around 30% of SCC patients were non-responsive to the chemotherapy (7). Identifying responders to NACT could improve their prognosis and optimize individualized treatment. For the non-responders, doctors could stop them from wasting precious time receiving ineffective treatments and suggest other effective treatments.
At present, the standard approach for the evaluation of response to NACT is MRI. However, the use of MRI increases the risk of false-positive results and is not a precise way for evaluating the NACT response. Diffusion-weighted MRI can measure the movement of water molecules in tissue, and this molecular imaging can identify the changes during the process of NACT (8). However, it cannot be popularized in developing countries due to the fact that it is costly. Therefore, it is important to identify effective serum biomarkers predicting the NACT response.
MiRNAs are small non-coding RNAs of 18–25 nucleotides that regulate gene expression at the posttranscriptional levels and are involved in the development of multiple malignant tumors (9). Recent studies have discovered that miRNAs may play a key role in predicting response to NACT in cancers (10, 11). The main idea of this study was to find out the potential miRNAs that can predict the response to NACT in LACC patients.
This study aimed to discover certain circulating miRNAs that could serve as markers for response to NACT in LACC patients. First, the serum samples of seven SCC patients before and after one or two cycles of NACT were collected for microRNA microarray analysis. miRNAs were selected after bioinformatics analysis and literature review. Second, the serum samples of 32 SCC patients before NACT and after one to two cycles of NACT were collected. Third, according to the response evaluation criteria for solid tumors (RECST, version 1.1), SCC patients with complete or partial response were assigned into the chemo-sensitive group (responders) and those with stable or progressive disease into the chemo-insensitive group (non-responders). Fourth, candidate miRNAs were validated both in the responders and non-responders using qRT-PCR analysis. Finally, 18 patients were randomly assigned into the testing group, and the remaining 21 patients were assigned into the validation group. Overall, the study was designed (Figure 1).
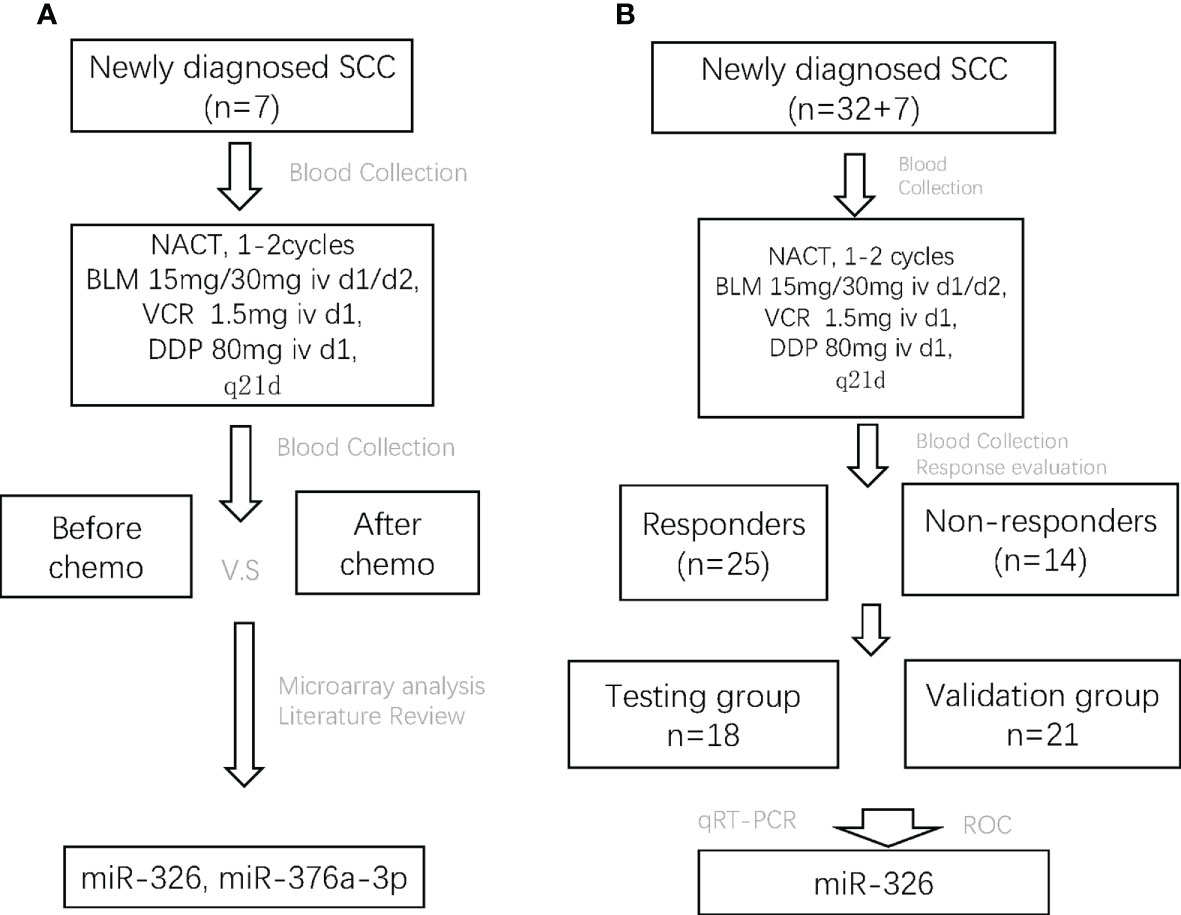
Figure 1 The flowchart of the whole study design. SCC, squamous cell carcinoma; NACT, neoadjuvant chemotherapy; BLM, bleomycin; VCR, vincristine; DDP, cisplatin; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
All patients were recruited from the West China Second University Hospital (Chengdu, P.R. of China) between July 2014 and June 2017. This study was approved by the Ethics Committee of West China Second University Hospital. Informed consent was obtained from all participants. All methods were performed in accordance with the relevant guidelines and regulations. All of the participants were genetically unrelated, were ethnic Han Chinese, and donated 5 ml of peripheral blood in every collection. The inclusion criteria were as follows: (1) newly diagnosed with SCC, stages IB–IIB, according to the 2009 FIGO classification; (2) had no prior hysterectomy, pelvic radiotherapy, or systemic chemotherapy; (3) had no other metabolic diseases, such as diabetes mellitus; and (4) had no any inflammations. The basic clinical information of patients is shown in Table 2. The SCC diagnosis and staging were assessed by two experienced pathologists. Once the samples were collected, tubes were kept upright at room temperature for 30 min and then stored in a 4°C refrigerator. After centrifugation at 4000 rpm, 4°C for 10 min, the serum was extracted and distributed into aliquots of 1 ml per 1.5 ml tube. Then the serum tubes were stored in a -80°C freezer.
Eligible patients received two cycles of NACT in the form of a regimen consisting of bleomycin, vincristine, and cisplatin. Once every 21 days, the patients received bleomycin at 15/30 mg intravenously (iv) on the first day and second day with vincristine at 1.5 mg iv and cisplatin at 80 mg iv on the first day. The doses and schedules of the drug administration were modified according to a drug toxicity evaluation before each course. Radical hysterectomy plus pelvic lymphadenectomy were performed after two cycles of chemotherapy.
The response evaluation was based on the response evaluation criteria for solid tumors (RECST, version 1.1) (12) and was classified into four categories—CR: complete response, all the lesions disappeared (any pathological lymph nodes must have a reduction in short axis to <10mm); PR: partial response, at least a 30% decrease in the sum of diameters of lesions; PD: progressive disease, at least a 20% increase in the sum of diameters of lesions; and SD: stable disease, neither a shrinkage that qualified as the PR nor a sufficient increase that qualified as the progressive disease (PD). In our study, responders (chemo-sensitivity) were defined as CR plus PR; non-responders (chemo-insensitivity) were SD plus PD.
RNA was extracted from the serum using a miRNeasy Serum/Plasma Kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions. RNA was quantified and its purity evaluated by the absorption ratio at 260/280 nm using NanoDrop 2000 (Thermo Fisher Scientific, Massachusetts, USA). The ratio of 260/280 varied from 1.8 to 2.1. Then, cDNA synthesis was performed using a miScript II RT Kit (Qiagen, Hilden, Germany). The expression levels of 192 human mature miRNAs were examined using the miRCURY LNA™ Universal RT microRNA PCR system, Serum/Plasma Focus microRNA PCR Panel (Exiqon, Vedbaek, Denmark). Microarrays were scanned using the ABI PRISM7900 system (Applied Biosystems, Foster, USA), and fold changes in the miRNA expression between the two groups were calculated using the 2-ΔCt method. Raw data were normalized by quantile normalization and the robust multichip average algorithm. All miRNA level files were imported into GeneSpring 11.0 software (Agilent Technologies, Santa Clara, CA, USA).
The relative expression of two miRNAs was validated by qRT-PCR assay. RNA was extracted from the serum using a miRNeasy Serum/Plasma Kit (QIAGEN, Inc.) according to the manufacturer’s instructions. The reverse-transcription reactions were carried out by a miScript II RT Kit (QIAGEN, Inc.). qRT-PCR was performed using a miScript SYBR Green PCR Kit (QIAGEN, Inc.). Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used for the internal control of miRNA. Each sample was run in three independent experiments in triplicate. The reactions were amplified at 95°C for 15 min followed by 40 cycles at 94°C for 15 s and 55°C for 30 s.
Quantitative variables were expressed as mean ± SD, and categorical variables were the number of events (%). GraphPad Prism (Version 7, GraphPad Software Inc.) was applied for data analysis with all data assessed for a normal distribution and equal variance. Statistical comparisons between two groups were performed by Student’s t-test or the chi-square test. The difference in miRNA levels between groups was evaluated using the Mann–Whitney unpaired test, and, for before/after comparison within one group, the Wilcoxon matched-pairs signed rank test was used. Receiver operating characteristic (ROC) curves were constructed to evaluate the predictive performance of the potential biomarkers. All P values were bilateral, with P < 0.05 being statistically significant.
Between July 2014 and June 2017, a total of 39 available SCC patients who met the inclusion criteria were enrolled in this study. Twenty-five (64%) of them were evaluated as PR and were assigned into the responders group, whereas the remaining 14 (36%) patients remained stable and were assigned into the non-responders group. Their demographic and clinical characteristics are shown in Table 2. Each characteristic was comparable in both groups.
Pair-matched blood samples of seven SCC patients before and after receiving NACT were collected to perform microRNA microarray analysis. Seventeen significantly differentially expressed microRNAs were selected according to bioinformatics analysis in our previous study. These 17 microRNAs were significantly downregulated before NACT and upregulated after NACT (Figure 2). Combined with the literature review (13, 14), miR-326 and miR-376a-3p were selected for further explorations.
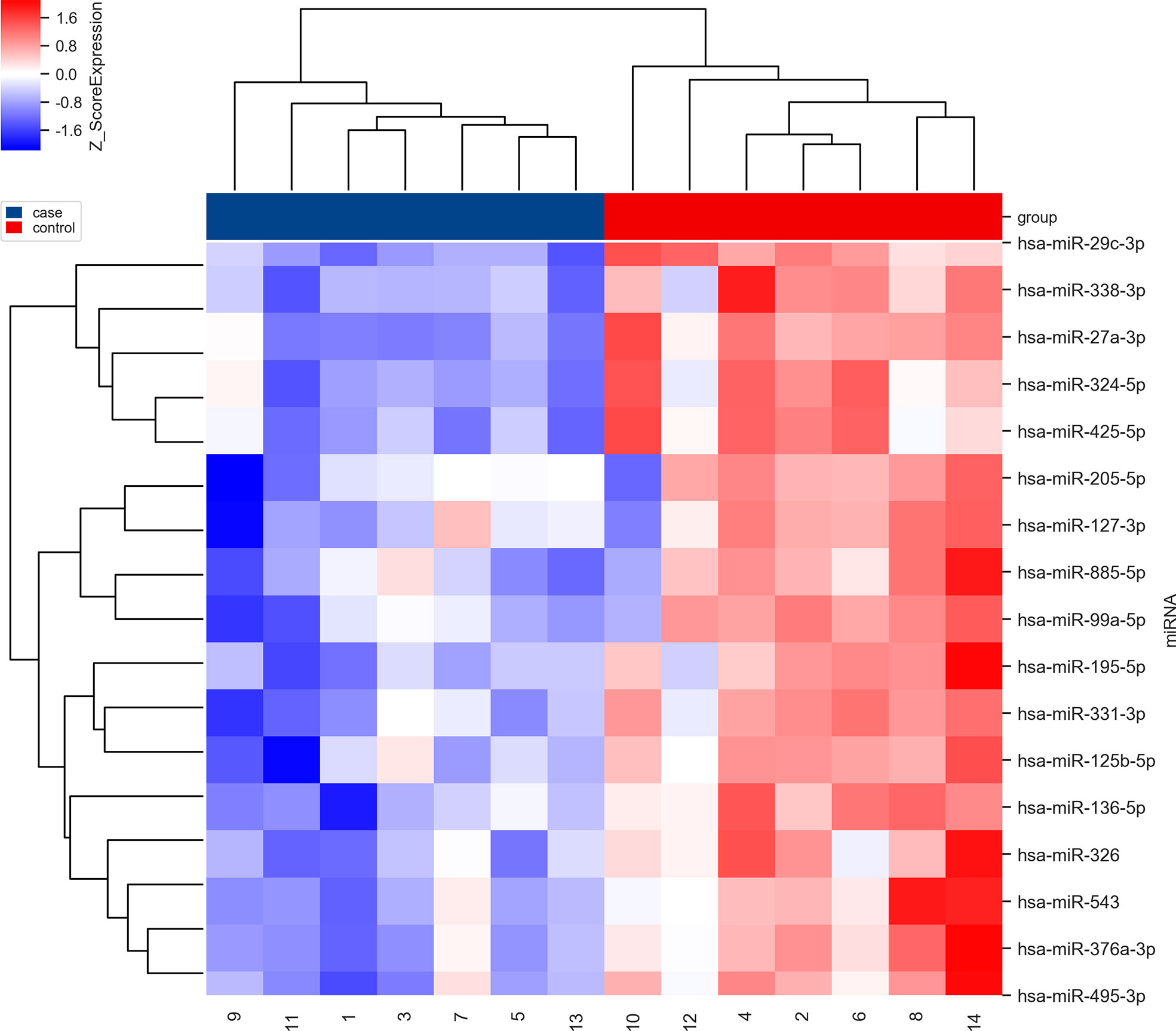
Figure 2 The clustering map of dysregulated microRNAs before (in blue) and after (in red) NACT in the first seven SCC patients. The blue ones mean downregulated and the red ones mean upregulated.
The pair-matched blood samples of 18 SCC patients before and after receiving NACT were used to explore the expression change of miR-326 by using qRT-PCR. According to the new response evaluation criteria in solid tumors (RECST, version 1.1), 12 patients achieved PR and were classified into the responders group, and the remaining six patients achieved SD and were classified into the non-responders group. After the qRT-PCR analysis, miR-326 was significantly downregulated before NACT and upregulated after NACT in 12 responders (p = 0.02, Figure 3A), whereas the expression before and after NACT had no statistical difference in the non-responders (Figure 3B). The expressions of miR-376a-3p before and after NACT had no statistical difference neither in the responders group nor the non-responders group (Figures 3C, D).
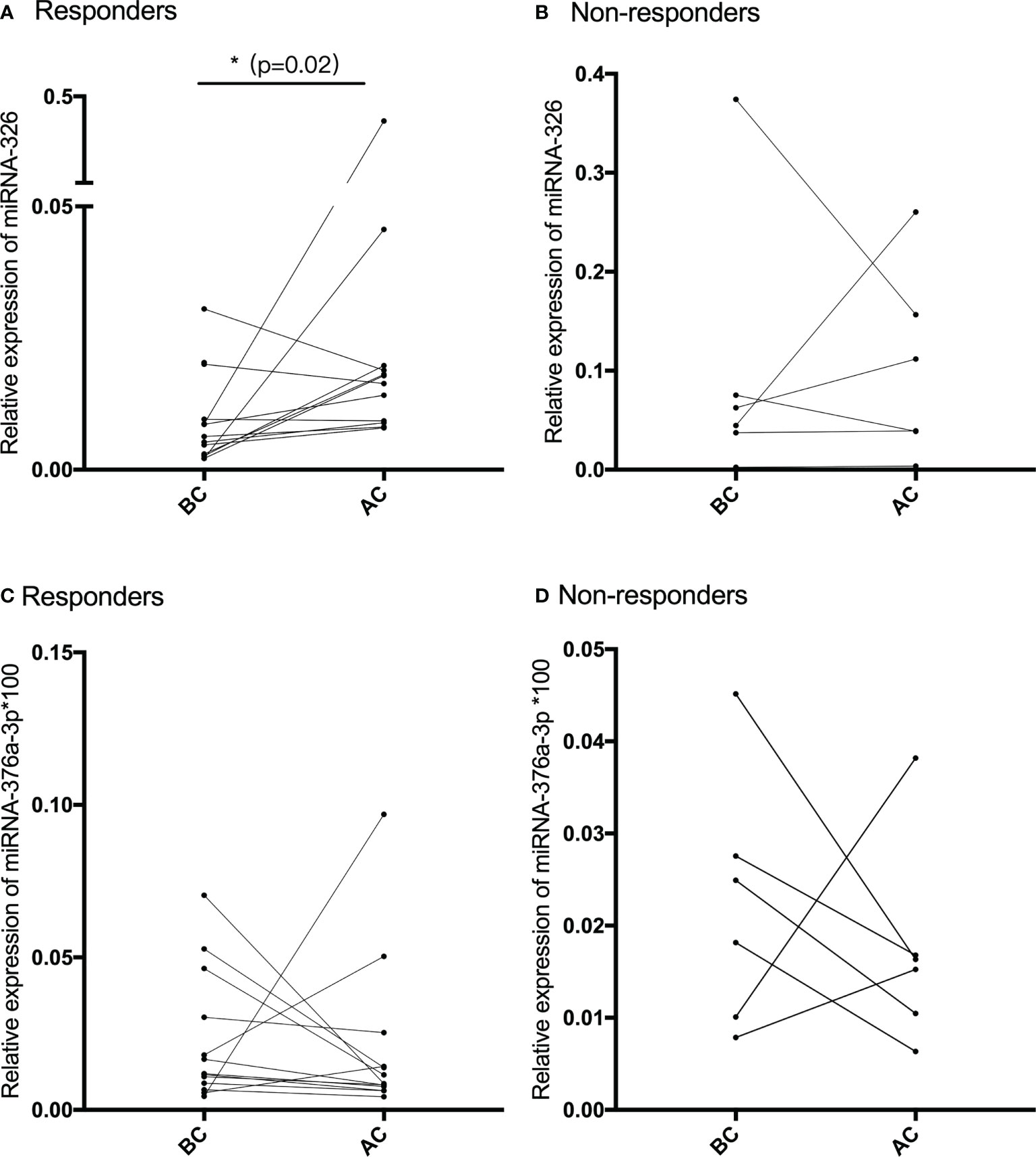
Figure 3 (A, B) The relative expression of miR-326 before and after NACT in chemotherapy-sensitive patients (responders) and chemotherapy-resistant patients (non-responders). (C, D) The relative expression of miR-376a-3p before and after NACT in chemotherapy-sensitive patients (responders) and chemotherapy-resistant patients (non-responders).
miR-326 was selected to be the candidate biomarker for predicting the NACT response and was validated by 21 SCC patients in a further analysis. Among them, 13 patients achieved PR, and the remaining eight patients were in the SD group. The predictive performance was evaluated by sensitivity, specificity, accuracy, and the area under the ROC. miR-326 provided an AUC-ROC of 0.75 (p = 0.04) in the discrimination between the PR and SD groups (Figure 4A). The cutoff value of ROC for miR-326 to predict the response of NACT was <0.023, the sensitivity was 88.89%, and the specificity was 50%. Before NACT, the relative expression of miR-326 in the responders was much lower than that in the non-responders (p = 0.02) (Figure 4B).
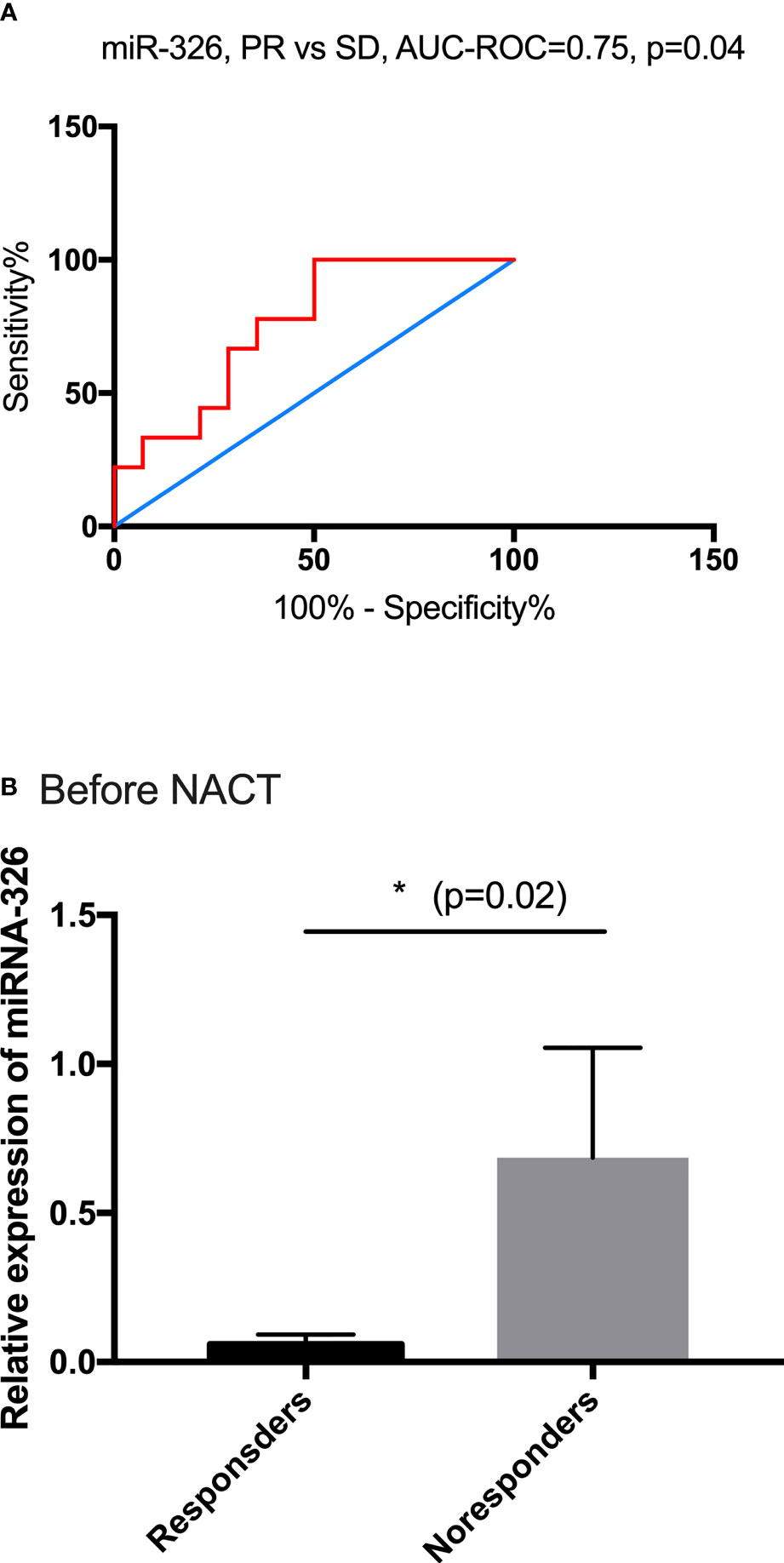
Figure 4 The predictive performance of miR-326. (A) The ROC curve for PR and SD based on the relative expression of miR-326, AUC-ROC = 0.75. (B) Before NACT, the baseline expressions of miR-326 in responders were much lower than those in non-responders (p = 0.02).
In a meta-analysis, NACT followed by radical surgery showed a highly significant reduction in the risk of death compared with radiotherapy alone (hazard ratio [HR] = 0.65, 95% CI [0.53, 0.8], P = 0.0004), with an absolute improvement of 14% in survival at 5 years, increasing from 50% to 64% (15). However, many patients cannot receive follow-up surgery because of drug toxicity or insensitivity to NACT resulting to delaying the time to receive chemoradiotherapy. Therefore, it is meaningful to find some predictive biomarkers to predict the response to NACT in newly diagnosed LACC patients.
MicroRNAs are stable and detectable in the serum, and changes in the levels of specific microRNAs could allow the detection of clinical conditions (16, 17). Feng et al. reported that miR-15a, miR-16-1, miR-29c, miR-34a, and miR-155 could be novel non-invasive biomarkers for the diagnosis of diffuse large B-cell lymphoma (DLBCL) with AUC-ROCs of 0.7722, 0.7002, 0.6672, 0.8538, and 0.7157, respectively (18).
Searching for effective prevention and management of cancers has always been at the top of medical concerns list. MicroRNAs (miRNAs), less than 22 nt small non-coding RNAs, play important roles by regulating mRNAs in cancer-related processes (9). There are still some small non-coding RNAs linked to other gynecologic cancers, which have been shown to contribute positively to the prognosis prediction of oncology patients. Piergentili et al. summarized ncRNAs with good predictive values such as miR-101, miR-152, and miR-205 in the treatment of endometrial cancer and recognized their great potential to improve risk stratification in EC (19). Cavaliere et al. also identified ncRNA pools that have a prognostic role and impact on the treatment of EC patients based on epigenetic profiles (20). However, the molecular properties of individuals may not be generalized due to the interactions between different ncRNAs. Focusing on only one molecule may not be sufficient, and combining ncRNAs with other well-known risk factors may be the key to achieving better treatment approaches. Researchers demonstrated that in luminal B HER-2 breast cancer, the miR-718 and miR-4516 expressions were lower in the group of responders than in non-responders, whereas the miR-210 expression was the opposite (21). Not come singly but in pairs, miR-195 and miR-26b were also found to be consistently upregulated after NACT (22). Todorova and colleagues found that miR-328-3p expression was downregulated before NACT and suggested that miR-127-3p is a strong predictor of NACT-positive treatment response in triple-negative breast cancer (23). Furthermore, miRNA-21 could be used as an independent predictor of chemotherapy sensitivity not only in breast cancer but also in esophageal SCC, where its levels were remarkably lower in chemotherapy-sensitive patients, whereas levels in the non-responders did not change significantly (24, 25). This indicates that miRNAs that are predictable for chemotherapy response may not only target single cancer.
Plenty of studies have proved that dysregulations of miR-326 are associated with pathological processes such as cellular proliferation and apoptosis (26), differentiation, metastasis (27, 28), and chemotherapy resistance (29, 30). Our study found that circulating miR-326 in LACC patients who were sensitive to chemotherapy significantly downregulated after NACT, whereas the expression in chemotherapy-resistant patients had no statistical difference between before NACT and after NACT. The results implied that circulating miR-326 in newly LACC patients could be a biomarker to predict the response to NACT. Liang et al. showed that miR-326 was downregulated in VP-16-resistant multidrug resistance cell lines and in a panel of advanced breast cancer tissues and consistent reversely with the expression levels of MRP-1. These results demonstrated that miR-326 could be an agent in the mechanism of chemotherapy resistance of breast cancer (29).
There are several studies that focus on exploring biomarkers or others to predict the response to NACT of newly diagnosed cervical cancer patients. Yan Hou et al. identified and verified L-valine and L-tryptophan as the biomarkers to predict the response to NACT by performing plasma metabolite profiling (31). Chun Fu et al. studied in a different angle and found that the axial and sagittal magnetic resonance diffusion-weighted imaging (DWI) could detect the changes in LACC patients after NACT, and the apparent diffusion coefficient values measured could be used to evaluate the response to NACT (8).
The strengths of this study are based on the meta-analysis; the target miRNAs are selected by microarray analysis, and then we screen differentially expressed miRNAs in the serum of SCC patients, which are more stable and have less influence during the collection of samples.
The limitations of our work are that we just found the potential biomarker, and we failed to find the correlation between miRNAs expression and HPV infection, as HPV infection is widely regarded as the primary cause of SCC.
Cervical adenocarcinoma patients should be included in the following relevant studies, which will benefit further clinical application. Moreover, the target gene, protein, and regulating pathway need to be explored. Finally, a large population is also needed in future research to validate the predicted performance of miR-326 in SCC patients.
In summary, our present study provides the first evidence that the circulating miR-326 is significantly upregulated after receiving NACT in responders, whereas the expression has no change in non-responders. The cutoff value of ROC for miR-326 to predict the response of NACT was <0.023. It suggested that the expression of miR-326 was lower than 0.023 in patients with newly diagnosed cervical SCC, indicating that they were likely sensitive to the NACT. It seems that the circulating miR-326 may be a biomarker to predict the response of NACT in SCC patients.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Committee of West China Second University Hospital. The patients/participants provided their written informed consent to participate in this study.
KZ contributed to the design of the study, sample collection and preparation, interpretation of data, manuscript drafting, and final approval. EY contributed to the sample collection and preparation and bioinformatics analysis. TC contributed to sample collection and preparation. ZL contributed to the conception of the study and design, manuscript revision, and critical discussion. All authors contributed to the article and approved the submitted version.
The microarray work and analysis of the data of this study were supported by the Medical Science and Technology Project of Sichuan Province Health Commission (No.21PJ050).
We acknowledge the staff of the Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, People’s Republic of China, for the methods instruction of the study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
LACC, locally advanced cervical cancer; NACT, neoadjuvant chemotherapy; miRNAs, microRNAs; ROC, receiver operating characteristic; qRT-PCR, quantitative reverse transcription–polymerase chain reaction; SCC, squamous cell carcinoma; HPV, human papilloma virus; CR, complete response; PR, partial response; PD, progressive disease; SD, stable disease; DLBCL, diffuse large B-cell lymphoma.
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
2. Walboomers JMM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol (1999) 189(1):12–9. doi: 10.1002/(Sici)1096-9896(199909)189
3. Pimple S, Mishra G. Cancer cervix: Epidemiology and disease burden. Cytojournal (2022) 19:21. doi: 10.25259/cmas_03_02_2021
4. Moore DH. Cervical cancer. Obstet Gynecol (2006) 107(5):1152–61. doi: 10.1097/01.Aog.0000215986.48590.79
5. Friedlander M, Kaye SB, Sullivan A, Atkinson K, Elliott P, Coppleson M, et al. Cervical carcinoma: A drug-responsive tumor–experience with combined cisplatin, vinblastine, and bleomycin therapy. Gynecol Oncol (1983) 16(2):275–81. doi: 10.1016/0090-8258(83)90102-6
6. Lapresa M, Parma G, Portuesi R, Colombo N. Neoadjuvant chemotherapy in cervical cancer: An update. Expert Rev Anticancer Ther (2015) 15(10):1171–81. doi: 10.1586/14737140.2015.1079777
7. Kumar JV, Doval DC, Rao R, Rawal S. A retrospective study of patients with locally advanced cancer of the cervix treated with neoadjuvant chemotherapy followed by radical surgery. Int J Gynecol Cancer (2009) 19(3):417–22. doi: 10.1111/IGC.0b013e3181a1c6df
8. Fu C, Bian D, Liu F, Feng X, Du W, Wang X. The value of diffusion-weighted magnetic resonance imaging in assessing the response of locally advanced cervical cancer to neoadjuvant chemotherapy. Int J Gynecol Cancer (2012) 22(6):1037–43. doi: 10.1097/IGC.0b013e31825736d7
9. Bartel DP. Micrornas: Genomics, biogenesis, mechanism, and function. Cell (2004) 116(2):281–97. doi: 10.1016/s0092-8674(04)00045-5
10. Zhu W, Liu M, Fan Y, Ma F, Xu N, Xu B. Dynamics of circulating micrornas as a novel indicator of clinical response to neoadjuvant chemotherapy in breast cancer. Cancer Med (2018) 7(9):4420–33. doi: 10.1002/cam4.1723
11. Raychaudhuri M, Bronger H, Buchner T, Kiechle M, Weichert W, Avril S. Micrornas mir-7 and mir-340 predict response to neoadjuvant chemotherapy in breast cancer. Breast Cancer Res Treat (2017) 162(3):511–21. doi: 10.1007/s10549-017-4132-9
12. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised recist guideline (Version 1.1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
13. Jadideslam G, Ansarin K, Sakhinia E, Babaloo Z, Abhari A, Ghahremanzadeh K, et al. Diagnostic biomarker and therapeutic target applications of mir-326 in cancers: A systematic review. J Cell Physiol (2019) 234(12):21560–74. doi: 10.1002/jcp.28782
14. Wang Y, Jiang F, Xiong Y, Cheng X, Qiu Z, Song R. Lncrna ttn-As1 sponges mir-376a-3p to promote colorectal cancer progression Via upregulating Klf15. Life Sci (2020) 244:116936. doi: 10.1016/j.lfs.2019.116936
15. Benedetti-Panici P, Bermudez A, Blake P, Cardenas J, Chang TC, Chiara S, et al. Neoadjuvant chemotherapy for locally advanced cervical cancer: A systematic review and meta-analysis of individual patient data from 21 randomised trials. Eur J Cancer (2003) 39(17):2470–86. doi: 10.1016/S0959-8049(03)00425-8
16. Chim SS, Shing TK, Hung EC, Leung TY, Lau TK, Chiu RW, et al. Detection and characterization of placental micrornas in maternal plasma. Clin Chem (2008) 54(3):482–90. doi: 10.1373/clinchem.2007.097972
17. Gilad S, Meiri E, Yogev Y, Benjamin S, Lebanony D, Yerushalmi N, et al. Serum micrornas are promising novel biomarkers. PloS One (2008) 3(9):e3148. doi: 10.1371/journal.pone.0003148
18. Fang C, Zhu DX, Dong HJ, Zhou ZJ, Wang YH, Liu L, et al. Serum micrornas are promising novel biomarkers for diffuse Large b cell lymphoma. Ann Hematol (2012) 91(4):553–9. doi: 10.1007/s00277-011-1350-9
19. Piergentili R, Zaami S, Cavaliere AF, Signore F, Scambia G, Mattei A, et al. Non-coding rnas as prognostic markers for endometrial cancer. Int J Mol Sci (2021) 22(6):3151. doi: 10.3390/ijms22063151
20. Cavaliere AF, Perelli F, Zaami S, Piergentili R, Mattei A, Vizzielli G, et al. Towards personalized medicine: Non-coding rnas and endometrial cancer. Healthc (Basel) (2021) 9(8):965. doi: 10.3390/healthcare9080965
21. Zhang Z, Zhang H, Li C, Xiang Q, Xu L, Liu Q, et al. Circulating micrornas as indicators in the prediction of neoadjuvant chemotherapy response in luminal b breast cancer. Thorac Cancer (2021) 12(24):3396–406. doi: 10.1111/1759-7714.14219
22. Baxter DE, Allinson LM, Al Amri WS, Poulter JA, Pramanik A, Thorne JL, et al. Mir-195 and its target Sema6d regulate chemoresponse in breast cancer. Cancers (Basel) (2021) 13(23):5979. doi: 10.3390/cancers13235979
23. Todorova VK, Byrum SD, Gies AJ, Haynie C, Smith H, Reyna NS, et al. Circulating exosomal micrornas as predictive biomarkers of neoadjuvant chemotherapy response in breast cancer. Curr Oncol (2022) 29(2):613–30. doi: 10.3390/curroncol29020055
24. McGuire A, Casey MC, Waldron RM, Heneghan H, Kalinina O, Holian E, et al. Prospective assessment of systemic micrornas as markers of response to neoadjuvant chemotherapy in breast cancer. Cancers (Basel) (2020) 12(7):1820. doi: 10.3390/cancers12071820
25. Kurashige J, Kamohara H, Watanabe M, Tanaka Y, Kinoshita K, Saito S, et al. Serum microrna-21 is a novel biomarker in patients with esophageal squamous cell carcinoma. J Surg Oncol (2012) 106(2):188–92. doi: 10.1002/jso.23064
26. Li D, Du X, Liu A, Li P. Suppression of nucleosome-binding protein 1 by mir-326 impedes cell proliferation and invasion in non-small cell lung cancer cells. Oncol Rep (2016) 35(2):1117–24. doi: 10.3892/or.2015.4403
27. Cai M, Wang Z, Zhang J, Zhou H, Jin L, Bai R, et al. Adam17, a target of mir-326, promotes emt-induced cells invasion in lung adenocarcinoma. Cell Physiol Biochem (2015) 36(3):1175–85. doi: 10.1159/000430288
28. Cheng Y, Jiang S, Yuan J, Liu J, Simoncini T. Vascular endothelial growth factor c promotes cervical cancer cell invasiveness Via regulation of microrna-326/Cortactin expression. Gynecol Endocrinol (2018) 34(10):853–8. doi: 10.1080/09513590.2018.1458304
29. Liang Z, Wu H, Xia J, Li Y, Zhang Y, Huang K, et al. Involvement of mir-326 in chemotherapy resistance of breast cancer through modulating expression of multidrug resistance-associated protein 1. Biochem Pharmacol (2010) 79(6):817–24. doi: 10.1016/j.bcp.2009.10.017
30. Ma J, Wang T, Guo R, Yang X, Yin J, Yu J, et al. Microrna133a and Microrna326 cocontribute to hepatocellular carcinoma 5fluorouracil and cisplatin sensitivity by directly targeting bcell lymphomaextra Large. Mol Med Rep (2015) 12(4):6235–40. doi: 10.3892/mmr.2015.4134
Keywords: microRNAs, locally advanced cervical cancer, neoadjuvant chemotherapy, predictive biomarker, molecular
Citation: Zou K, Yang E, Cui T and Li Z (2022) Circulating miR-326 could serve as a predictive biomarker for response to neoadjuvant chemotherapy in locally advanced cervical cancer. Front. Oncol. 12:1036710. doi: 10.3389/fonc.2022.1036710
Received: 05 September 2022; Accepted: 13 October 2022;
Published: 09 November 2022.
Edited by:
Gabriella Lillsunde Larsson, Örebro University, SwedenReviewed by:
Federica Perelli, Santa Maria Annunziata Hospital, ItalyCopyright © 2022 Zou, Yang, Cui and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhengyu Li, emhlbmd5dWxpMDFAMTI2LmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.