
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 02 November 2022
Sec. Radiation Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1034656
This article is part of the Research Topic Methods in Radiation Oncology View all 9 articles
Objective: This study aimed to analyze whether involved field irradiation (IFI) is associated with improving survival outcomes and reducing adverse events compared with elective nodal irradiation (ENI) in patients of esophageal cancer who underwent definitive radiotherapy or chemoradiotherapy.
Summary background data: Radiotherapy plays an important role for not surgery patients. However, the role of radiation target size is still uncertain.
Methods: We searched Web of Science, Embase, PubMed, and Cochrane Central for English and non-English publications comparing esophageal cancer patients who received radiotherapy with IFI with those with ENI. Primary outcomes included overall survival (OS) and adverse events related to radiotherapy. The risk of bias was assessed using the Cochrane Risk of Bias tool for randomized studies and the Newcastle-Ottawa Scale and Agency for Healthcare Research and Quality Standard for non-randomized studies. We evaluated the certainty of evidence by Grading of Recommendations, Assessment, Development, and Evaluation.
Results: Totally, 23 studies with 4120 patients were included. IFI group demonstrated significant improvement in the OS rates at 5 years, but not at 1, 2, and 3 years, compared with the ENI group (pooled Risk Ratio [RR], 0.78; 95% confidence interval [CI], 0.68–0.90; P = 0.0004; high certainty). In addition, IFI demonstrated a significant decrease in the incidence of grade ≥2 acute esophagitis (AE) (pooled RR, 0.79; 95% CI, 0.69–0.90; P = 0.0005; high certainty) and grade ≥3 AE (pooled RR, 0.51; 95% CI, 0.38–0.69; P < 0.00001; high certainty) compared with ENI, but not in the incidence of grades ≥3 acute pneumonia, late esophagitis, and late pneumonia.
Conclusions: Compared to ENI, IFI demonstrated significant improvement in OS at 5 years. The addition of intensity-modulated radiotherapy (IMRT) to IFI increased the 5-year OS; however, similar results were not observed with the addition of three-dimensional conformal radiotherapy to IFI and ENI. Furthermore, IFI demonstrated a significant decrease in grade ≥2 and grade ≥3 AE, while IMRT demonstrated no difference in the incidence of grade ≥3 AE. IFI and ENI do not differ in the incidence of grades ≥3 acute pneumonia, late esophagitis, and late pneumonia.
Esophageal cancer results in more than half a million cancer-related deaths worldwide each year (1). It ranked sixth in the main cause of cancer-related death, ranked seventh in the incidence of tumor (2). Squamous cell carcinoma and adenocarcinoma are two main subtypes of esophageal cancer, which occupies the majority of all (3, 4). Many esophageal cancers were unresectable, and most eventually returned after radical treatment (5–7). Most patients are diagnosed with late staged disease, not suitable for surgery (8). Radiotherapy is important in the treatment of esophageal cancer. The RTOG 85-01 trial demonstrated that definitive chemoradiotherapy (CRT) is recommended for not surgery patients (9).
Optimal depiction of radiation therapy targets is essential to improve treatment effectiveness and reduce radiotoxicity (10). To reduce tumor metastasis, the usual practice is to provide irradiation to an area that has not been metastasized, called elective lymph node irradiation (ENI). ENI improved local area control but did not improve overall survival (OS). In addition, there is a corresponding increase in treatment-related adverse events. With the progress of treatment technology, the target size of radiotherapy can be reduced to a certain extent. Involved-field irradiation (IFI), which irradiates only the affected area, is a method to reduce the volume of irradiation. For IFI, one common radiation target is that the gross tumor volume (GTV) is the primary focus of esophageal cancer plus metastatic lymph nodes; the clinical target volume (CTV) is the normal esophagus outlined 3 cm above and below the GTV, and the metastatic lymph nodes are not outwardly placed in the CTV; the planned target volume (PTV) is the CTV outwardly expanded 1 cm in all directions. The method of determining the clinical CTV for the primary tumor is much the same in various countries (11–13). The modalities available for determining the CTV, especially the lymph node volume, vary. The treatment modality of ENI advocates irradiation of normal areas of non-metastatic lymph nodes that are also included in the CTV. Thus, the method of setting the CTV is still uncertain. However, In the last few decades, many studies have explored the impact of target volume on clinical prognosis. Some studies suggested that IFI can improve the prognosis of patients (14–17), whereas other studies favored ENI over IFI (18–21).
Moreover, no large prospective RCTs compared the treatment outcomes of IFI with ENI in esophageal cancer patients. Therefore, this systematic review and meta-analysis aimed to explore whether IFI is more beneficial than ENI in terms of survival and incidence of adverse events in a large group of population.
The medical databases, namely, Web of Science, Embase, PubMed, and Cochrane Central, were searched for publications that do not distinguish between languages(last update: April 30, 2022). The search strategy is summarized in Supplementary Table 1. This study was proceeded based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Only those studies that investigated the role of IFI and ENI in definitive radiotherapy or chemoradiotherapy for esophageal cancer were eligible for inclusion. We included RCTs and retrospective studies. Exclusion criteria included (1): studies that investigated either IFI or ENI alone (2), palliative rather than curative radiotherapy (3), unpublished data (4), case reports, conference abstracts, meta-analysis, ongoing clinical trials, academic papers, editorials, letters, review papers, comments, and basic science articles (5); no correlation results; and (6) full text not available. We did not discriminate against articles by language.
Two authors (authors Chunyang Song and Xiaohan Zhao) evaluated each article separately and extract relevant information. If there was any difference in the process, the other person (author Wenzhao Deng) would resolve it. We extracted relevant information from the included studies: baseline study characteristics (author, year, country, and study type), sample size, follow-up time, study period, age, tumor location, pathological type, clinical stage, radiation dose, radiation technology, chemotherapy regimens, and relevant outcomes data.
The primary outcomes in this review were 1-, 2-, 3-, and 5-year OS rates and adverse events related to radiotherapy. Secondary outcomes included 1-, 2-, 3-, and 5-year progression-free survival (PFS) rates and 1-, 2-, and 3-year local control rates (LCRs).
We evaluated randomized studies by the Cochrane Risk of Bias tool and non-randomized studies by the Newcastle-Ottawa Scale (NOS) and Agency for Healthcare Research and Quality (AHRQ) Standard. The scores were from 0 to 9. A score above 6 was considered high quality. Authors Chunyang Song and Xiaohan Zhao scored the included studies respectively. If there was any dispute, Wenzhao Deng would be asked to settle it. We evaluated the quality of the results by the Cochrane Grading of Recommendations Assessment, Development, and Evaluation (GRADE) methodology.
This study was conducted by the software of Cochrane Review Manager, version 5.4 (London, UK). Survival curves were read by Engauge Digitizer, version 12.1 (available from: http://markummitchellgithubio/engauge-digitizer/). Heterogeneity was evaluated by I2 statistic. If I2 ≤50%, which indicated no significant heterogeneity among the studies, a fixed-effects model was used; otherwise, a random-effects model was employed. Publication bias was assessed using a funnel plot for results that included more than 10 studies. The significance level of the results was set to P <0.05. Subgroup analyses were performed based on the study type (RCTs and non-RCTs), radiotherapy used (three-dimensional conformal radiotherapy [3D-CRT], intensity-modulated radiotherapy [IMRT], and 3D-IMRT–mixed for patients who received both 3D-CRT and IMRT), pathology (esophageal squamous cell carcinoma [ESCC] and ESCC-mixed in patients with both ESCC and non-ESCC), and type of chemotherapy (CCRT—patients who received concurrent chemoradiotherapy; CCRT+CT—patients who received concurrent chemoradiotherapy with consolidated chemotherapy, and CRT-mixed—patients who received radiotherapy with or without chemotherapy).
329 potential studies were retrieved, ultimately, we included 23 studies. After removing duplicate studies, 184 records underwent screening. In total, 36 articles were assessed for eligibility, and, finally, 23 (22–44) studies with 4120 patients were ultimately included in this study, including 6 RCTs (23, 25, 29, 41, 43, 44) and 17 non-RCTs (22, 24, 26–28, 30–40, 42) (Figure 1).
All studies were performed in Asian countries (including 20 studies from China, 2 from Japan, and 1 from Korea). Of the 4120 patients, 2279 received IFI and 1841 received ENI. The study publication time ranged from 2011 to 2020. The study period ranged between 2000 to 2017. The median age of the patients ranged from 56.8–75.0 years, and the follow-up duration ranged between 1 and 188 months. Cancer type included ESCC (97.6%; n = 4022) and non-ESCC (2.4%; n = 98). Notably, 18 studies enrolled ESCC patients only, while the other 5 studies included patients with both ESCC and non-ESCC. Tumor locations included the cervical and upper, middle, and lower thoracic regions. Only one study included one specific tumor location, and eight studies included various tumor locations. The stage included I to IV. Only one study included one specific stage, and five studies included tumors with various TNM stages. The most commonly used radiotherapeutic modalities were 3D-CRT and IMRT. The radiation dose delivered ranged from 38 to 72 Gy, and the per fraction ranged from 1.6 to 2.5 Gy. Patients in one study received radiotherapy alone, whereas those in the other 4, 6, and 12 studies received CCRT, CCRT+CT, CRT-mixed, respectively. The detailed treatment are summarized in Supplementary Table 2, and the summary of outcomes are detailed in Supplementary Tables 3 and 4. The characteristics are presented in Table 1.
A total of 18 studies (22–29, 31, 32, 34, 36–38, 41–44) analyzed the 1-year OS rates (Figure 2A), including 6 RCTs (23, 25, 29, 41, 43, 44) and 12 non-RCTs (22, 24, 26–28, 31, 32, 34, 36–38, 42). No significant differences were observed between the IFI and ENI groups (pooled Risk Ratio [RR], 0.97; 95% confidence interval [CI], 0.94–1.01; P = 0.14, high certainty) with no significant heterogeneity (P = 0.34; I2 = 10%). As the studies performed by Q. F. Li et al. (26) and Zhu et al. (22) contributed significantly and weighted similarly, we conducted a sensitivity analysis. Removing these did not change the 1-year OS rates. With regard to subgroup, the study type (Supplementary Figure 1A for RCTs group, and Supplementary Figure 1B for non-RCTs group), the type of radiotherapy (Supplementary Figure 2A for the 3D-CRT group, Supplementary Figure 2B for IMRT group, and Supplementary Figure 2C for 3D-IMRT–mixed group), the type of pathology (Supplementary Figure 3A for the ESCC group, and Supplementary Figure 3B for ESCC-mixed group), and the type of chemotherapy received (Supplementary Figure 4A for the CCRT group, Supplementary Figure 4B for CCRT+CT group, and Supplementary Figure 4C for CRT-mixed group), there were no substantial differences between them.
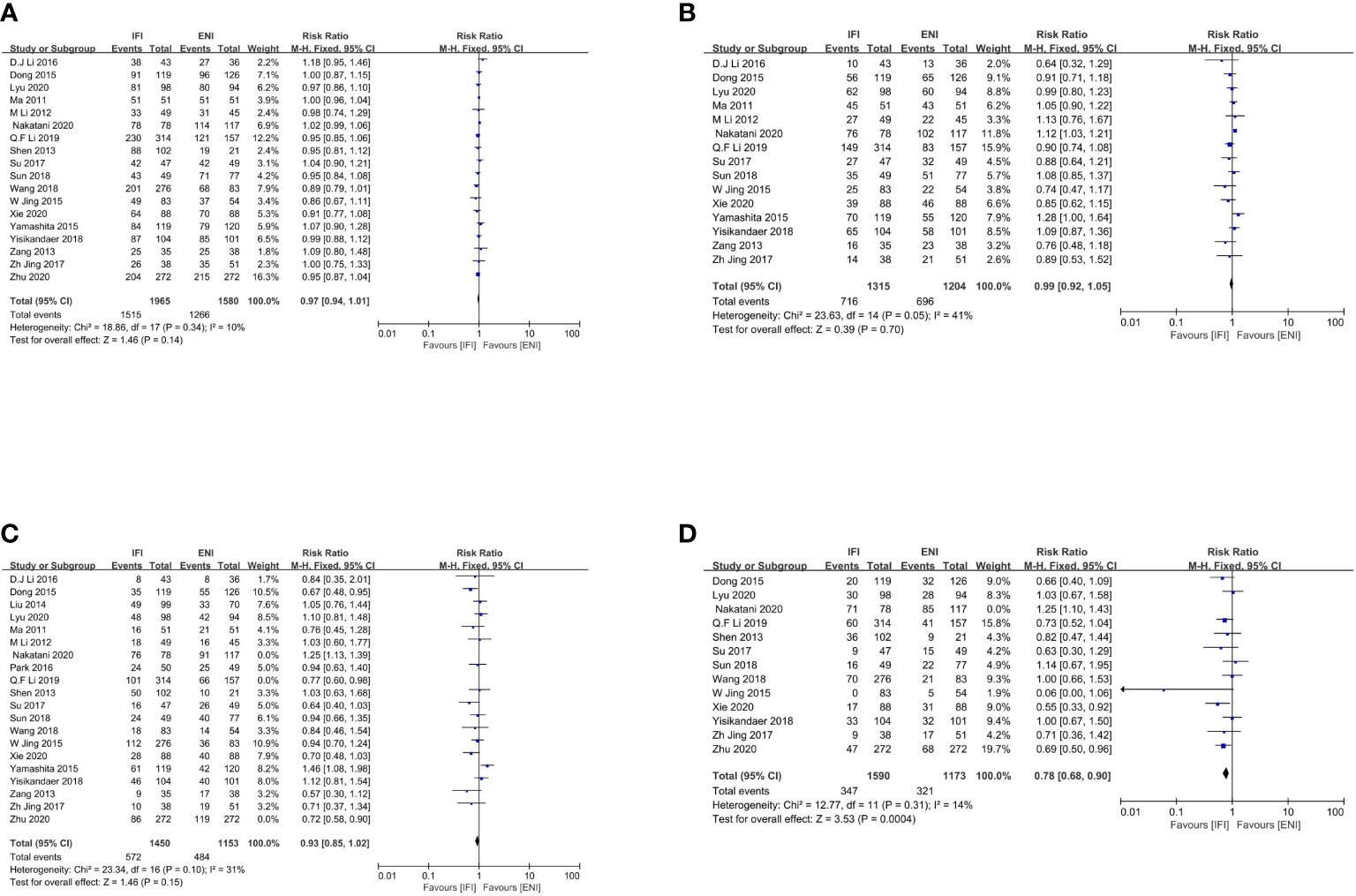
Figure 2 Forest plot of 1- (A), 2- (B), 3- (C) and 5-years (D) overall survival rate. IFI, involved field irradiation; ENI, elective nodal irradiation; M-H, Mantel-Haenszel; CI, confidence interval.
Overall, 15 studies (23–26, 28, 29, 31, 32, 34, 36–38, 41, 43, 44) analyzed the 2-year OS rates (Figure 2B), including 6 RCTs (23, 25, 29, 41, 43, 44) and 9 non-RCTs (24, 26, 28, 31, 32, 34, 36–38). No significant differences were observed between two groups (pooled RR, 0.99; 95% CI, 0.92–1.05; P = 0.70; high certainty) with no significant heterogeneity (P = 0.05; I2 = 41%). As the study by Q. F. Li et al. (26) contributed significantly, we conducted a sensitivity analysis. Removing this did not change the 2-year OS rates. With regard to subgroup, the study type (Supplementary Figure 5A for RCTs group, and Supplementary Figure 5B for non-RCTs group), the type of radiotherapy (Supplementary Figure 6A for the 3D-CRT group, Supplementary Figure 6B for IMRT group, and Supplementary Figure 6C for 3D-IMRT–mixed group), the type of pathology (Supplementary Figure 7A for the ESCC group, and Supplementary Figure 7B for ESCC-mixed group), and the type of chemotherapy received (Supplementary Figure 8A for the CCRT group, Supplementary Figure 8B for CCRT+CT group, and Supplementary Figure 8C for CRT-mixed group), there were no substantial differences between them.
Notably, 20 studies (22–29, 31–34, 36–38, 40–44) analyzed the 3-year OS rates, including 6 RCTs (23, 25, 29, 41, 43, 44) and 14 non-RCTs (22, 24, 26–28, 31–34, 36–38, 40, 42). No significant differences were observed, although obvious heterogeneities were found among these studies (P < 0.00001; I2, 71%). As studies by Nakatani et al. (24), Q. F. Li et al. (26), and Zhu et al. (22) contributed significantly and weighted similarly, we excluded these three studies and found that the 3-year OS rates remained unchanged (pooled RR, 0.93; 95% CI, 0.85–1.02; P = 0.15; moderate certainty), with no significant heterogeneity (P = 0.15; I2, 31%; Figure 2C). With regard to subgroup, the study type (Supplementary Figure 9A for RCTs group, and Supplementary Figure 9B for non-RCTs group), the type of radiotherapy (Supplementary Figure 10A for the 3D-CRT group, Supplementary Figure 10B for IMRT group, and Supplementary Figure 10C for 3D-IMRT–mixed group), the type of pathology (Supplementary Figure 11A for the ESCC group, and Supplementary Figure 11B for ESCC-mixed group), and the type of chemotherapy received (Supplementary Figure 12A for the CCRT group, Supplementary Figure 12B for CCRT+CT group, and Supplementary Figure 12C for CRT-mixed group), there were no substantial differences between them.
In total, 13 studies (22–29, 31, 32, 36, 38, 41) analyzed 5-year OS rates, including 3 RCTs (23, 25, 29) and 10 non-RCTs (22, 24, 26–28, 31, 32, 36, 38, 41). No significant differences were observed, although obvious heterogeneities were found among these studies (P < 0.00001; I2, 74%). Subsequently, we excluded the study by Nakatani et al. (24) and found that the IFI group had a significant advantage over the ENI group in terms of 5-year OS rates (pooled RR, 0.78; 95% CI, 0.68–0.90; P = 0.0004; high certainty; Figure 2D), with no significant heterogeneity (P= 0.31; I2, 14%). With regard to subgroup, the type of radiotherapy, the IFI group had a significant advantage over the ENI group in the IMRT subgroup (Supplementary Figure 13A), whereas no differences were observed in the 3D-IMRT–mixed subgroup regarding the 5-year OS rate (Supplementary Figure 13B). With regard to subgroup, the type of pathology, the ESCC-mixed subgroup (Supplementary Figure 14B) showed a significant 5-year OS benefit but not the ESCC subgroup; however, obvious heterogeneities were found among these studies (P = 0.0003; I2, 73%). Therefore, we excluded the study by Nakatani et al. (24) and found that the IFI group had an advantage over the ENI group in 5-year OS rates, with no significant heterogeneity (Supplementary Figure 14A). Analysis of other subgroups was not conducted due to the lack of interested outcomes.
A total of 10 studies (22, 25, 26, 29, 31, 38, 41–44) analyzed the incidence of grade ≥2 acute esophagitis (AE), including 5 RCTs (25, 29, 41, 43, 44) and 5 non-RCTs (22, 26, 31, 38, 42). No significant differences were observed, although obvious heterogeneities were found among these studies (P = 0.03; I2, 53%). Therefore, we excluded the study by Lyu et al. (25) and found that IFI demonstrated a significant decrease in the incidence of grade ≥2 AE compared with ENI (pooled RR, 0.79; 95% CI, 0.68–0.91; P = 0.001; high certainty; Figure 3A), with no obvious heterogeneity (P = 0.33; I2, 13%). With regard to subgroup of the study type, no significant differences were observed in the RCTs; however, obvious heterogeneities were found among these studies. Therefore, we excluded the study by Lyu et al. (25) and found that the IFI group had an advantage over the ENI group in the RCTs, with no significant heterogeneity (Supplementary Figure 15A), similar to that of the non-RCT group (Supplementary Figure 15B). With regard to subgroup, the type of radiotherapy (Supplementary Figure 16A for the 3D-CRT group, and Supplementary Figure 16B for IMRT group), there were no substantial differences between them.
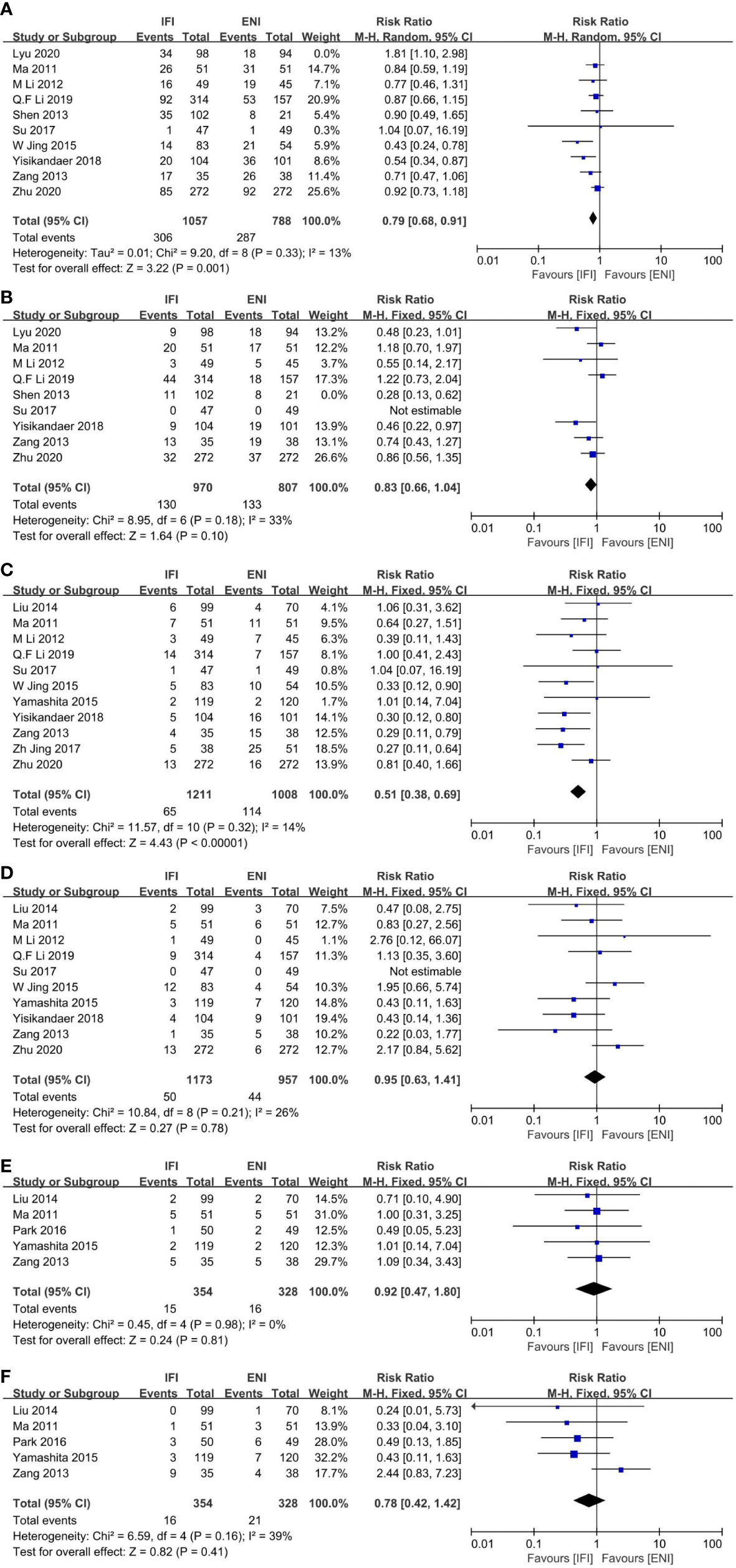
Figure 3 Forest plots of ≥ grade 2 acute esophagitis (A), ≥ grade 2 acute pneumonitis (B), ≥ grade 3 acute esophagitis (C), grade 3 acute pneumonitis (D), ≥ grade 3 late esophagitis (E) and ≥ grade 3 late pneumonitis (F). IFI, involved field irradiation; ENI, elective nodal irradiation; M-H, Mantel-Haenszel; CI, confidence interval.
Nine studies (22, 25, 26, 29, 31, 41–44) analyzed grade ≥2 acute pneumonia (AP), including five RCTs (25, 29, 41, 43, 44) and four non-RCTs (22, 26, 31, 42). No significant differences were observed. Although obvious heterogeneities were found among these studies (P = 0.03; I2, 56%). Hence, we removed Shen et al. (42) and found that the results were unchanged (pooled RR, 0.83; 95% CI, 0.66–1.04; P = 0.10; high certainty; Figure 3B). With regard to subgroup of the study type, the IFI group showed a significant decrease in the incidence in RCTs (Supplementary Figure 17A), but not in non-RCTs (Supplementary Figure 17B). With regard to subgroup, the type of radiotherapy (Supplementary Figure 18A for the 3D-CRT group, and Supplementary Figure 18B for IMRT group), there were no substantial differences between them.
Overall, 11 studies (22, 26, 29, 31, 32, 37, 38, 40, 41, 43, 44) analyzed grade ≥3 acute esophagitis (AE) (Figure 3C), including 4 RCTs (29, 41, 43, 44) and 7 non-RCTs (22, 26, 31, 32, 37, 38, 40). IFI showed a significant reduction in the incidence compared with ENI (pooled RR, 0.51; 95% CI 0.38–0.69; P < 0.00001; high certainty) with no heterogeneity (P = 0.32; I2, 14%). With regard to subgroup, the type of radiotherapy, the IFI group demonstrated a significant reduction in the incidence in the 3D-CRT subgroup (Supplementary Figure 20A) and 3D-IMRT–mixed subgroup (Supplementary Figure 20C), whereas there was no difference in the IMRT subgroup (Supplementary Figure 20B). With regard to subgroup, the study type (Supplementary Figure 19A for RCTs group, and Supplementary Figure 19B for non-RCTs group), and the type of pathology (Supplementary Figure 21A for the ESCC group, and Supplementary Figure 21B for ESCC-mixed group), there were no substantial differences between them.
Ten studies (22, 26, 29, 31, 37, 38, 40, 41, 43, 44) analyzed grade ≥3 acute pneumonia (AP) (Figure 3D), including four RCTs (29, 41, 43, 44) and six non-RCTs (22, 26, 31, 37, 38, 40). No significant differences were observed between two groups (pooled RR, 0.95; 95% CI, 0.63–1.41; P = 0.78; high certainty) with no heterogeneity (P = 0.21; I2, 26%). With regard to subgroup, the study type (Supplementary Figure 22A for RCTs group, and Supplementary Figure 22B for non-RCTs group), and the type of radiotherapy (Supplementary Figure 23A for the 3D-CRT group, and Supplementary Figure 23B for IMRT group), the type of pathology (Supplementary Figure 24A for the ESCC group, and Supplementary Figure 24B for ESCC-mixed group), there were no substantial differences between them.
Five studies (33, 37, 40, 41, 44) analyzed grade ≥3 late esophagitis (LE) (Figure 3E), including two RCTs (41, 44) and three non-RCTs (33, 37, 40). No differences were observed (pooled RR, 0.92; 95% CI, 0.47–1.80; P= 0.81; high certainty). Furthermore, five studies (33, 37, 40, 41, 44) analyzed grade ≥3 late pneumonia (LP) (Figure 3F), including two RCTs (41, 44) and three non-RCTs (33, 37, 40), and no differences were observed (pooled RR, 0.78; 95% CI, 0.42–1.42; P = 0.41; high certainty).
Totally, 12 (22, 24–26, 28, 29, 31–33, 36, 38, 43), 11 (24–26, 28, 29, 31–33, 36, 38, 43), 12 (22, 24–26, 28, 29, 31–33, 36, 38, 43), and 12 (22, 24–29, 31, 32, 36, 38, 42) studies were to analyze 1-, 2-, 3-, and 5-years PFS rates. IFI group had an advantage over ENI at 1-, and 3-year PFS rates (1-year PFS: pooled RR, 0.90; 95% CI, 0.85–0.95; P = 0.0004; high certainty; Figure 4A; 3-year PFS: pooled RR, 0.86; 95% CI, 0.78–0.96; P = 0.007; moderate certainty; Figure 4C). No differences were observed at 2- and 5-year PFS rates (2-year PFS: pooled RR, 0.92; 95% CI, 0.84–1.01; P = 0.09; high certainty; Figure 4B). However, obvious heterogeneities were found among these studies at 5-year PFS rate (P < 0.0001; I2, 71%). Subsequently, we excluded the study by Nakatani et al. (24) and re-evaluated the 5-year PFS rate. We showed that the IFI group had an advantage over the ENI group (pooled RR, 0.81; 95% CI, 0.70–0.93; P = 0.003; high certainty; Figure 4D) with no heterogeneity (P= 0.40; I2, 5%).
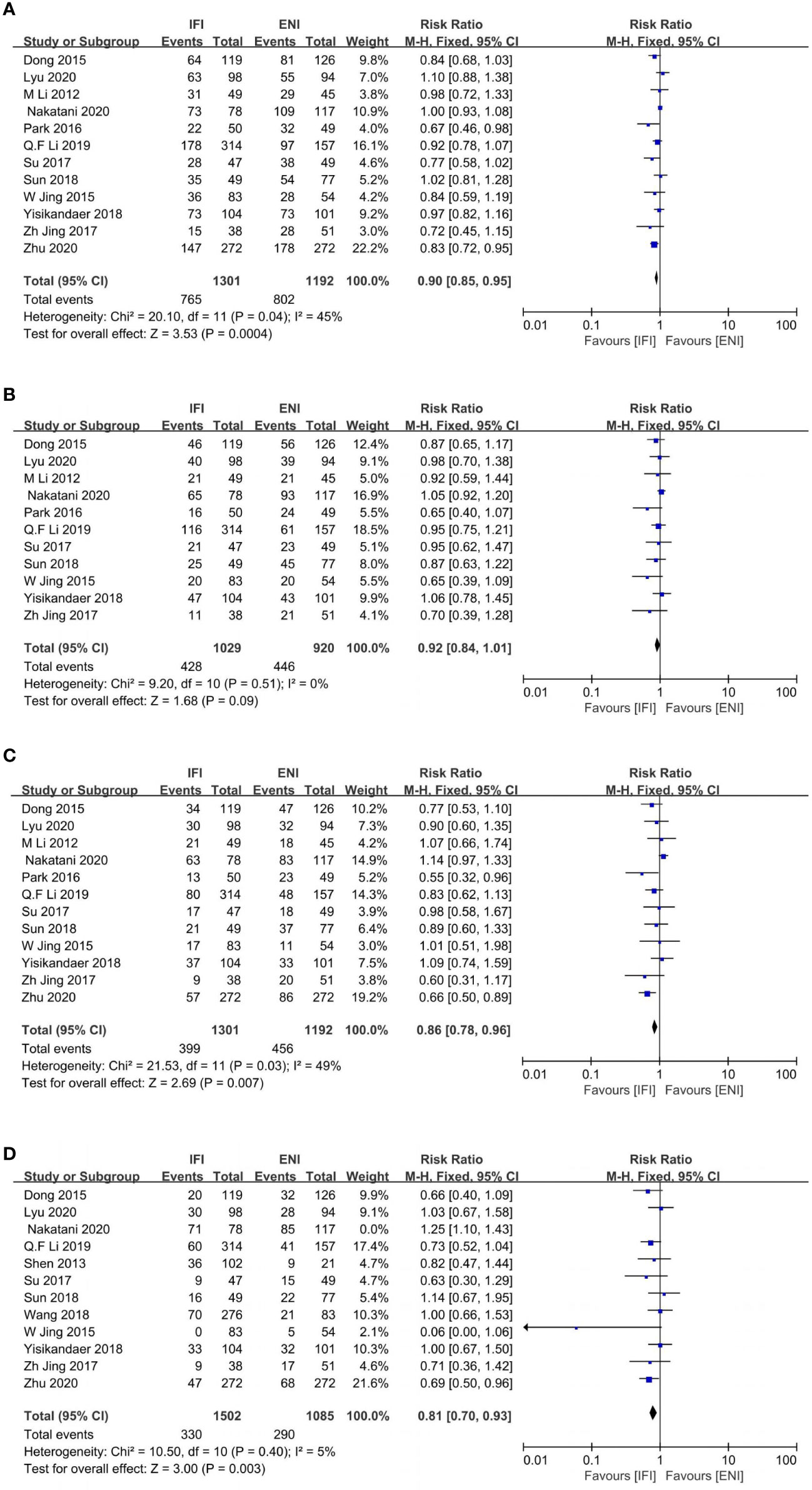
Figure 4 Forest plot of 1- (A), 2- (B), 3- (C) and 5-years (D) progression free survival rate. IFI, involved field irradiation; ENI, elective nodal irradiation; M-H, Mantel-Haenszel; CI, confidence interval.
Five (31, 36, 41, 43, 44), four (31, 41, 43, 44), and five (31, 36, 41, 43, 44) studies analyzed the 1-, 2-, and 3-year LCRs. The IFI group had a significant advantage over the ENI group at 2- and 3-year LCRs (2-year LCR: pooled RR, 0.87; 95% CI, 0.77–0.99; P = 0.04; moderate certainty; Figure 5B; 3-year LCR: pooled RR, 0.87; 95% CI, 0.76–1.00; P = 0.04; high certainty; Figure 5C), while no differences at 1-year LCR (pooled RR, 0.94; 95% CI, 0.85–1.02; P = 0.15; high certainty; Figure 5A).
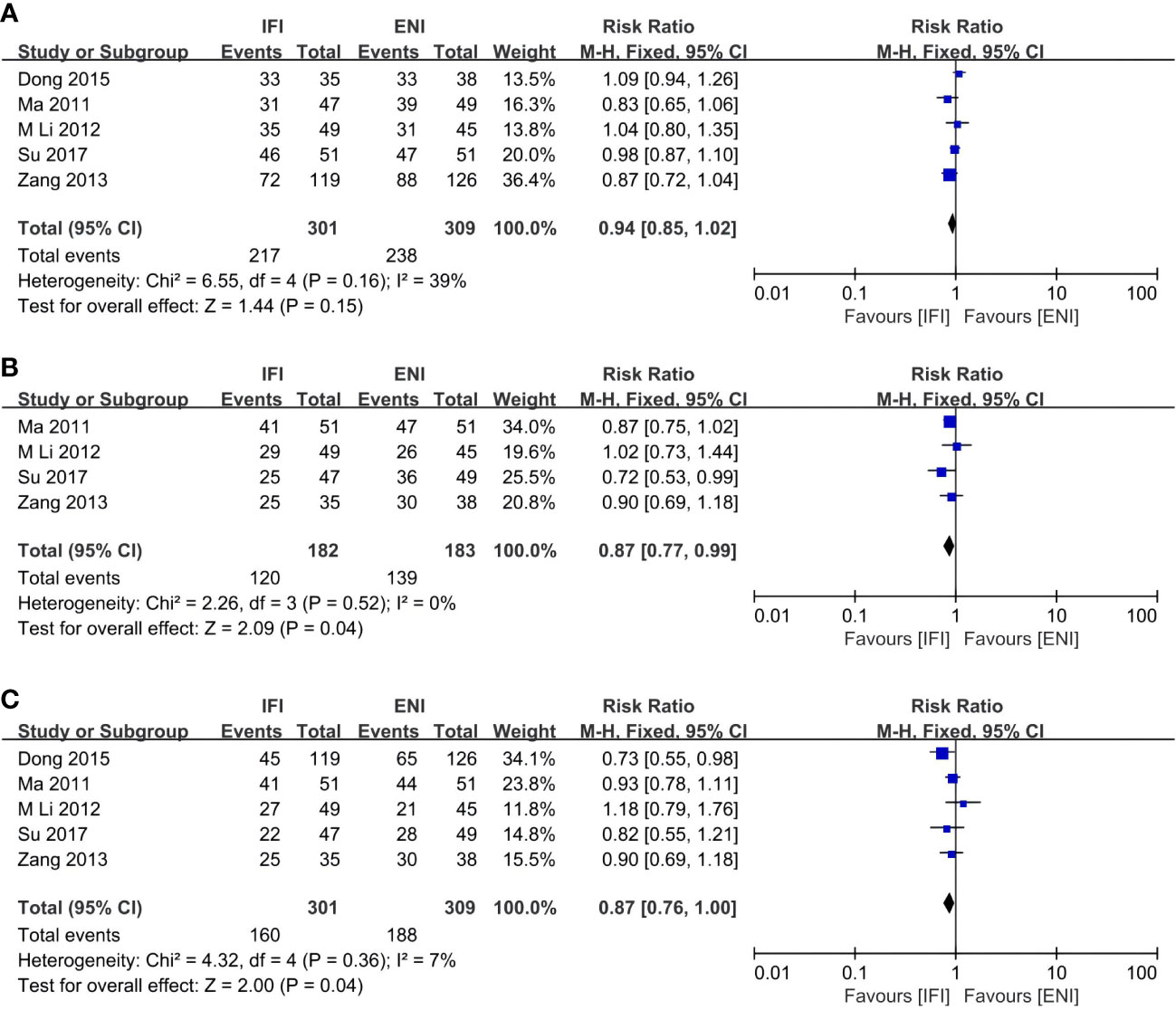
Figure 5 Forest plots of 1- (A), 2- (B) and 3-years (C) local control rate. IFI, involved field irradiation; ENI, elective nodal irradiation; M-H, Mantel-Haenszel; CI, confidence interval.
Details of the evaluation for RCTS and non-RCTs are provided in Supplementary Tables 5 and 6. The Cochrane Risk of Bias tool showed that equality was high in the included studies. In the included RCTs, only one RCT had a bias in blinding participants and health care providers, and no other bias was observed. The AHRQ Standards were all good. The NOS scores of non-RCTs were between 7 and 9. The average score was 8.3. All 17 studies had selection scores of 4. Comparability scores were rated as 2 and 1 in 58.8% (10/17) and 41.2% (7/17) of the studies, respectively. The outcome scores were rated as 3 and 2 in 70.6% (12/17) and 29.4% (5/17) of the studies, respectively.
As a result, the overall quality is excellent. Supplementary Table 7 summarizes all outcomes with GRADE quality evaluation. Funnel plots for outcomes were evaluated in Supplementary Figures 25–33.
As far as we know, this is the first systematic review and meta-analysis that included the highest number of patients and RCTs. In this study including 4120 patients, IFI significantly improved OS at 5 years (RR, 0.78) but not at 1, 2, and 3 years with high-to-moderate certainty of evidence. For subgroup analyses performed based on the study type, pathology, radiotherapy, and chemotherapy, the findings observed in each subgroup were consistent with the general finding that OS unchanged at 1, 2, and 3 years. However, regarding the 5-year OS rate, the IMRT subgroup demonstrated improvement, whereas no difference was observed in the 3D-IMRT–mixed subgroup. Regarding treatment-related adverse events, IFI was associated with a significant improvement in grade ≥2 AE (RR, 0.79), similar to the findings observed in the subgroup analyses. Furthermore, there was no differences in the incidence of grade ≥2 AP, and only RCTs reported that the IFI group demonstrated an improvement in the grade ≥2 AP (RR, 0.69). The IFI group demonstrated significant improvements in grade ≥3 AE events compared with the ENI group (RR, 0.51), while no difference was observed in the IMRT subgroup compared with the 3D-CRT and 3D-IMRT–mixed subgroups. Additionally, no differences were observed in the incidence of grade ≥3 AP (RR, 0.95), grade ≥3 LE (RR, 0.91) and grade ≥3 LP (RR, 0.89) between two groups. Regarding PFS, IFI had an improvement in 1-, 3-, and 5-year PFS rates (RR, 0.90, 0.86, and 0.81, respectively) but not in 2-year PFS rates (RR, 0.92; 95% CI, 0.84–1.01). IFI had an improvement in the 2- and 3-year LCR (RR, 0.87 and 0.87, respectively) but not in the 1-year LCR (RR, 0.94; 95% CI, 0.85–1.02).
Although this is the largest meta-analysis to explored the role of IFI and ENI in definitive radiotherapy or chemoradiotherapy for esophageal cancer, previous studies have made a few attempts. A meta-analysis conducted by H.P. Zhu er al (45). demonstrated no difference in the OS rates and the incidence of AE and LP between those who underwent IFI and ENI; however, this meta-analysis only included seven articles in which there were two abstracts submitted to conferences without full texts and only one RCT, and the study only compared the 1-, 2-, and 3-year OS rates and the incidence of grade ≥3 AE/AP and grade ≥3 LE, which showed the same conclusions as our study. Our systematic review also demonstrated that IFI had an improvement in the 5-year OS rate and grade in ≥2 AE compared with ENI; however, no difference in the incidence of grade ≥2 AP was observed. Although we showed that no differences were observed in the incidence of grade ≥3 LP, this finding differed from that reported by H.P. Zhu er al (45).; the difference can be explained that we included one more RCT, which provided a higher level of evidence. For subgroup analyses performed by the study type, there was no difference in the 1-, 2-, 3-year OS rates and grade ≥3 AE/AP events between RCTs and non-RCTs, similarly to H.P. Zhu et al. (45). While most studies demonstrated no differences in the survival benefit between two groups, our study challenges these results; although we found no difference in the short-term survival, IFI demonstrated improved long-term survival compared with ENI. Two ongoing randomized controlled clinical phase III trials, the CSWOG 003 trial by T. Li et al. (46) and the NROG 001 trial by B. Li et al. (47), showed in the interim analysis that there was no difference in the 1- and 2-year OS rates, similar to ours. In 2018, S. Tsuruoka et al. (48) reported that there was no difference in the 3-year OS, similarly our study; although the patients included in their study all had esophageal cancer in the thoracic region. As shown in the subgroup analyses, the type of radiotherapy, chemotherapy, and pathology were not associated with the 1-, 2-, and 3-year OS. Furthermore, the clinical outcomes of RCTs were consistent with those of non-RCTs. Regarding the analysis of 5-year OS rates in the subgroups, the IFI group demonstrated significant improvement compared with the ENI group in the IMRT subgroup, while there were no differences in the 3D-IMRT–mixed subgroup. Owing to the missing data regarding the 5-year OS rates in the 3D-CRT subgroup, we performed an indirect comparison between IMRT and 3D-CRT. Differences in survival were observed, which may be attributed to technical differences, indicating that, in the era of 3D-CRT, there is no difference in 5-year OS rates following IFI and ENI. Although the popularity of IMRT is increasing, IFI offers a better improvement in 5-year OS rates than ENI, which may be explained by the fact that IMRT can increase the therapeutic effect and reduce the size of the radiotherapy target compared with 3D-CRT. Moreover, treatment-related adverse events associated with 3D-CRT may show no differences in the 5-year OS between two groups. The more favorable results of IMRT compared with the old 3D technique confirmed the superiority of IMRT also for esophageal cancer. The outcomes by the type of pathology were consistent with the overall conclusion.
Regarding treatment-related adverse events, although E. Jean-Mary et al. (19) reported that it was feasible for ENI when the surrounding tissues were not in high doses, they only reported this finding from the viewpoint of the target volume and the exposure dose to the organ at risk without considering the actual clinical outcomes. Our study reported that IFI was associated with a significant improvement in grade ≥2 AE, similarly to T. Li et al. (46), and the findings of the subgroup analyses were also consistent with this conclusion. There was no difference in the incidence of grade ≥2 AP between two groups. Only RCTs reported that IFI showed a significant improvement in grade ≥2 AP, which varies from the overall conclusion and those derived from the other subgroup analyses, which may be explained by the fact that a retrospective analysis is more accurate than an RCT for evaluating the adverse events. Therefore, more prospective studies are needed to validate this finding. In our study, IFI showed significant reductions in the incidence of grade ≥3 AE compared with ENI, which is similar to that reported in most studies (45, 49, 50). Compared with the 3D-CRT and 3D-IMRT–mixed subgroups, there were no differences in the incidence of grade ≥3 AE in the IMRT subgroup, which could be explained by the fact that IMRT may reduce the difference in the incidence rates of grade ≥3 AE between two groups. Another challenging finding from our systematic review was that there were no differences in the incidence of grade ≥3 AP between them and the other subgroups, similarly to Zhu et al. (45) and different from Cheng et al. (49) and Jing et al. (50). The databases these two authors searched included the China National Knowledge Infrastructure (CNKI) and Chinese Biomedical Literature Database, without including the Web of Science database, which differed from the databases included in the present study. Moreover, studies by Jing et al. (50) and Cheng et al. (49) were performed before 2015 except for one study by Cheng et al. Most of their treatment regimens included radiotherapy alone, in which the patient received a larger dose of radiation, which resulted in difference in the incidence rates of grade in ≥3 AP between those undergoing IFI and ENI. A few studies reported on late treatment side effects. Our systematic review showed that there were no differences in the incidence of grade ≥3 LE, which is consistent with Zhu et al. (45). Zhu et al. (45) reported that IFI demenstrated a decrease in the incidence of grade ≥3 LP compared with ENI, this finding differed from that reported in our study where we reported no difference. Zhu et al. (45) included four studies to analyze the incidence of grade ≥3 LP; in contrast, we included five studies, four of which were the same as Zhu et al. (45). We included one other study by Zang et al. (41), which reported an RR value of 2.44 (95% CI, 0.83–7.23) with very high upper limit value. As both Zhu et al. and the present study included only a few studies, conclusions regarding the incidence of grade ≥3 LP could not be provided.
As this review included retrospective studies, it was difficult to accurately determine the time of tumor recurrence; therefore, we evaluated PFS and LCR as secondary outcomes, although the secondary outcomes were not analyzed in the subgroup. We reported that IFI had an improvement in PFS at 1, 3, and 5 years, but not at 2 years, and the pooled RR for the 2-year PFS was 0.92 (95% CI, 0.84–1.01), where the upper line of the CI of the RR value is only slightly above 1.00. Furthermore, we showed that IFI had an improvement in the LCR at 2 and 3 years, but not at 1 year (RR, 0.94; 95% CI 0.85–1.02), where the upper line of the CI of the RR value is also slightly above 1.00. The reason why we could not draw conclusions regarding the time of tumor recurrence may be attributed to the bias and errors observed in retrospective studies, which is also a limitation of the present study. Additionally, the time of tumor recurrence and control differed in previous studies. Zhou et al. (51) reported that no differences were reported in the 1- and 2-year LCRs (75% and 57%; 72% and 45%, respectively; χ2, 0.79; P = 0.376) in those undergoing IFI and ENI, similar to those reported by Li et al. (52) (1- and 2-year LCRs—66% and 48% and 68% and 49%, respectively; χ2, 0.56; P = 0.78). However, Zhu et al. (53) reported that ENI showed a significant improvement in LCRs at 1, 3, and 5 years compared with IFI (70.5% and 53.3%; 51.7% and 63.0%; 39.1% and 27.2%, respectively; χ2, 6.22; P = 0.013). Therefore, the advantages and disadvantages of IFI and ENI in tumor control and recurrence cannot be concluded.
There were also some limitations to this meta-analysis. First, most publications in this study were retrospective studies, with only six RCTs. Although subgroup analyses were performed based on the study type, the results of 1-, 2-, and 3-year OS rates and the incidence of grade 2 AE and grade ≥3 AE/AP did not differ from the overall conclusion; however, differences in the incidence of grade ≥2 AP were observed. Furthermore, very few RCTs reported 5-year OS rates and grade ≥3 LE/LP events; hence, we could not fully analyze the impact on these results because the evidence for these results was limited. Though the OS is necessary for efficacy evaluation, PFS and LCRs are also needed to refine the effectiveness of treatment (54, 55). Nevertheless, IFI demonstrated a meaningful improvement in OS at 5 years but not at 1, 2, and 3 years; this is an important discovery that should be further investigated. Treatment-related adverse events related to radiotherapy must be evaluated in future prospective studies to consolidate the evidence. Second, only a few studies investigated a single subgroup included in this study and reported relevant outcomes. Therefore, in the subgroup analysis, in addition to including relevant subgroups, mixed groups were also included for indirect comparison in the present study. Third, in conducting this study, there were five head-to-head ongoing RCTs investigating IFI and ENI, none of which have reported the final results; only the interim results were published. Therefore, we excluded these studies. Forth, due to the inability to obtain data separately and the lack of comprehensive reporting in the included literature, we also included a small proportion of patients with stage IV, which would also have a small impact on this study. Fifth, although the patients included in this article were by far the largest under the relevant topic, the total number of patients was still not very large. Moreover, the articles included in this study were all from Asia, on the one hand, the incidence of esophageal cancer was much higher in Asia than in other regions, and on the other hand, when we were screening and including the articles, we only included head-to-head articles of IFI and ENI to ensure the high quality of the study. Many articles from other regions were excluded by us in this process. In addition to this, we only included articles that provided full texts in order to ensure the comprehensiveness of the study. Therefore, conference abstracts without full texts presented at academic conferences such as ASCO and ESMO, for example, were not included in this study.
To sum up, this meta-analysis demonstrated that IFI had improvements in the 5-year OS rate, but not at 1, 2, and 3 years, compared with ENI. We showed that the addition of IMRT to IFI improves the 5-year OS, whereas the same cannot be observed by the addition of 3D-CRT to IFI and ENI. IFI reduced the incidence of grade ≥2 and ≥3 AE compared with ENI, while IMRT showed no difference in the incidence of grade ≥3 AE. Furthermore, IFI and ENI showed no difference in the incidence of grades ≥3 AP, LE, and LP. There is a limited amount of data on which to draw conclusions regarding PFS and LCRs. Our systematic review presents interesting information in terms of the potential survival improvements imparted by IFI in esophageal cancer patients receiving radical radiotherapy or CRT. Long-term follow-up prospective study should be performed to further validate IFI implementation.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
All authors read and approved the final manuscript prior to submission. WS is responsible for the conception and design of the study. HW is responsible for analyzing and interpreting data, drafting the article, and revising it. CS and XZ are responsible for the acquisition of data. WD is responsible for data checks. All authors contributed to the article and approved the submitted version.
We would like to express our sincere thanks to Department of Radiation Oncology, Fourth Hospital of Hebei Medical University. We thank Jingyuan Wen and Luanying Wu for their assistance during this work.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1034656/full#supplementary-material
IFI, involved field irradiation; ENI, elective nodal irradiation; GTV, gross tumor volume; CTV, clinical target volume; PTV, planned target volume; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; NOS, Newcastle-Ottawa Scale; AHRQ, Agency for Healthcare Research and Quality; GRADE, Grading of Recommendations Assessment, Development, and Evaluation; CT, chemotherapy; CRT, chemoradiotherapy; CCRT, concurrent chemoradiotherapy; 3D-CRT, three-dimensional conformal radiotherapy; IMRT, intensity-modulated radiotherapy; ESCC, esophageal squamous cell carcinoma; RCT, randomized controlled trial; RR, risk ratio; CI, confidence interval; OS, overall survival; PFS, progression-free survival; LCR, local control rate; AE, acute esophagitis; AP, acute pneumonia; LE, late esophagitis; LP, late pneumonia.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Enzinger PC, Mayer RJ. Esophageal cancer. New Engl J Med (2003) 349:2241–52. doi: 10.1056/NEJMra035010
4. Graham AJ, Shrive FM, Ghali WA, Manns BJ, Grondin SC, Finley RJ, et al. Defining the optimal treatment of locally advanced esophageal cancer: a systematic review and decision analysis. Ann Thorac Surg (2007) 83(4):1257–64. doi: 10.1016/j.athoracsur.2006.11.061
5. Patel N, Benipal B. Incidence of esophageal cancer in the united states from 2001-2015: A united states cancer statistics analysis of 50 states. Cureus (2018) 10(12):e3709. doi: 10.7759/cureus.3709
6. Smyth EC, Lagergren J, Fitzgerald RC, Lordick F, Shah MA, Lagergren P, et al. Oesophageal cancer. Nat Rev Dis Primers. (2017) 3:17048. doi: 10.1038/nrdp.2017.48
7. Tachimori Y, Ozawa S, Numasaki H, Ishihara R, Matsubara H, Muro K, et al. Comprehensive registry of esophageal cancer in Japan, 2012. Esophagus (2019) 16(3):221–45. doi: 10.1007/s10388-019-00674-z
8. Paul S, Altorki N. Outcomes in the management of esophageal cancer. J Surg Oncol (2014) 110(5):599–610. doi: 10.1002/jso.23759
9. Cooper JS, Guo MD, Herskovic A, Macdonald JS, Martenson JA Jr., Al-Sarraf M, et al. Chemoradiotherapy of locally advanced esophageal cancer: long-term follow-up of a prospective randomized trial (RTOG 85-01). Radiat Ther Oncol Group Jama. (1999) 281(17):1623–7. doi: 10.1001/jama.281.17.1623
10. Fokas E, Rodel C. Definitive, preoperative, and palliative radiation therapy of esophageal cancer. Viszeralmedizin (2015) 31(5):347–53. doi: 10.1159/000440638
11. Minsky BD, Pajak TF, Ginsberg RJ, Pisansky TM, Martenson J, Komaki R, et al. (Radiation therapy oncology group 94-05) phase III trial of combined-modality therapy for esophageal cancer: High-dose versus standard-dose radiation therapy. J Clin Oncol (2002) 20(5):1167–74. doi: 10.1200/JCO.2002.20.5.1167
12. Gao XS, Qiao X, Wu F, Cao L, Meng X, Dong Z, et al. Pathological analysis of clinical target volume margin for radiotherapy in patients with esophageal and gastroesophageal junction carcinoma. Int J Radiat Oncol Biol Phys (2007) 67(2):389–96. doi: 10.1016/j.ijrobp.2006.09.015
13. Lesueur P, Servagi-Vernat S. [Definition of accurate planning target volume margins for esophageal cancer radiotherapy]. Cancer Radiother. (2016) 20(6-7):651–6. doi: 10.1016/j.canrad.2016.07.065
14. Zhu HC, del Campo ER, Ye JJ, Simone CB, Zhu ZF, Zhao WX, et al. Involved-field irradiation in definitive chemoradiotherapy for locoregional esophageal squamous cell carcinoma: Results from the ESO-shanghai 1 trial. Int J Radiat Oncol Biol Physics. (2021) 110(5):1396–406. doi: 10.1016/j.ijrobp.2021.02.053
15. Tsuruoka S, Kataoka M, Makita K, Ishikawa H, Takada N, Nagasaki K, et al. The role of elective nodal irradiation in radiotherapy for stage I esophageal cancer. Radiotherapy Oncol (2018) 127:S780. doi: 10.1016/S0167-8140(18)31744-4
16. Li M, Zhao F, Zhang X, Shi F, Zhu H, Han A, et al. Involved-field irradiation in definitive chemoradiotherapy for T4 squamous cell carcinoma of the esophagus. Curr Oncol (2016) 23(2):e131-7. doi: 10.3747/co.23.2846
17. Kono S, Ishii Y, Matsubara H, Izumi S, Hashimoto Y, Karasawa K. Is involved-field irradiation sufficient for superficial esophageal carcinoma? Int J Radiat Oncol (2016) 96(2):E162. doi: 10.1016/j.ijrobp.2016.06.998
18. Sekii S, Ito Y, Kato K, Umezawa R, Takahashi K, Inaba K, et al. Recurrence pattern after definitive chemoradiation therapy for cervical esophageal cancer. Int J Radiat Oncol Biol Physics. (2015) 93(3):E158. doi: 10.1016/j.ijrobp.2015.07.953
19. Jean-Mary E, Dalban C, Serre AA, Maingon P, Petitfils A, Mirjolet C, et al. Elective nodal irradiation during exclusive chemoradiation for locally-advanced esophageal cancer: IMRT better spares critical organs at risk. Eur J Cancer. (2015) 51:S351. doi: 10.1016/S0959-8049(16)30992-3
20. Nishibuchi I, Murakami Y, Adachi Y, Imano N, Takeuchi Y, Takahashi I, et al. Long-term results of definitive chemoradiotherapy with elective nodal irradiation using modern radiotherapy technique for resectable locally advanced esophageal cancer. Int J Radiat Oncol Biol Physics. (2019) 105(1):E193. doi: 10.1016/j.ijrobp.2019.06.2110
21. Lee DY, Moon SH, Cho KH, Kim TH, Kim MS, Lee JY, et al. Treatment outcomes of extended-field radiation therapy for thoracic superficial esophageal cancer. Radiat Oncol J (2017) 35(3):241–8. doi: 10.3857/roj.2017.00458
22. Zhu S, Li Q, Zhang X, Deng W, Song C, Wang X, et al. Clinical outcomes of different irradiation ranges in definitive intensity-modulated radiotherapy for esophageal cancer. Chin J Oncol (2020) 42(12):1040–7. doi: 10.3760/cma.j.cn112152-20191225-00842
23. Xie C, Jing Z, Luo H, Jiang W, Ma L, Hu W, et al. Chemoradiotherapy with extended nodal irradiation and/or erlotinib in locally advanced oesophageal squamous cell cancer: long-term update of a randomised phase 3 trial. Br J cancer. (2020) 123(11):1616–24. doi: 10.1038/s41416-020-01054-6
24. Nakatani Y, Kato K, Shoji H, Iwasa S, Honma Y, Takashima A, et al. Comparison of involved field radiotherapy and elective nodal irradiation in combination with concurrent chemotherapy for T1bN0M0 esophageal cancer. Int J Clin Oncol (2020) 25(6):1098–104. doi: 10.1007/s10147-020-01652-7
25. Lyu J, Yisikandaer A, Li T, Zhang X, Wang X, Tian Z, et al. Comparison between the effects of elective nodal irradiation and involved-field irradiation on long-term survival in thoracic esophageal squamous cell carcinoma patients: A prospective, multicenter, randomized, controlled study in China. Cancer Med (2020) 9(20):7460–8. doi: 10.1002/cam4.3409
26. Li Q, Zhu S, Li S, Deng W. Elective nodal irradiation provides a superior therapeutic modality for lymph node positivity esophageal squamous cell carcinoma patients receiving definitive radiotherapy versus involved-field irradiation. Med (Baltimore). (2019) 98(3):e14080. doi: 10.1097/MD.0000000000014080
27. Wang LL, Wang J, Yu B, Liu HL, Hu LJ, Zhou JY. Analysis of side effects and prognosis of radiotherapy alone in treatment of senile esophageal cancer. Chin J Cancer Prev Treat (2018) 25(23):1638–42. doi: 10.16073/j.cnki.cjcpt.2018.23.005
28. Sun Y, Zhang XL, Mao QF, Liu YH, Kong L, Li MH. Elective nodal irradiation or involved-field irradiation in definitive chemoradiotherapy for esophageal squamous cell cancer: a retrospective analysis in clinical N0 patients. Curr Oncol (2018) 25(5):e423-e9. doi: 10.3747/co.25.3895
29. Yisikandaer A, Lyu JH, Li T, Zhang XZ, Tian ZG, Wang XH, et al. Involvedfield irradiation (IFI) versus elective nodalirradiation (ENI) in combination with concurrent chemotherapy for esophageal thoracic squam ous cell cancer : a prospective, randomized, multicenter, controlled study. Chin J Radiat Oncol (2018) 27(3):245–9. doi: 10.3760/cma.j.issn.1004-4221.2018.03.004
30. Zhao L, Zhou Y, Mu Y, Chai G, Xiao F, Tan L, et al. Patterns of failure and clinical outcomes of definitive radiotherapy for cervical esophageal cancer. Oncotarget (2017) 8(13):21852–60. doi: 10.18632/oncotarget.15665
31. Su J, Zhu S, Liu Z, Zhao Y, Song C. Target volume delineation for radical radiotherapy of early oesophageal carcinoma in elderly patients. Cancer Radiother. (2017) 21(1):34–9. doi: 10.1016/j.canrad.2016.08.129
32. Jing Z, Chen T, Zhang XB, Wu SX. Long-term outcome of concurrent chemoradiotherapy with elective nodal irradiation for inoperable esophageal cancer. Cancer Science. (2017) 108(9):1828–33. doi: 10.1111/cas.13308
33. Park JH, Kim WC, Kim HJ. Comparison of the treatment results of involved-field and elective nodal irradiation in locally advanced esophageal cancer. Esophagus (2016) 13(4):361–8. doi: 10.1007/s10388-016-0545-5
34. Li DJ, Li HW, He B, Wang GM, Cai HF, Duan SM, et al. Patterns of failure after involved field radiotherapy for locally advanced esophageal squamous cell carcinoma. J buon. (2016) 21(5):1268–73.
35. Bai WW, Song YZ, Liu M, Zhou ZG, Zhang RH, Li J, et al. Clinical application of simultaneous integrated boost intensity-modulated radiation therapy in cervical and upper esophageal carcinoma. Chin J Cancer Prev Treat (2016) 23(4):248–52. doi: 10.16073/j.cnki.cjcpt.2016.04.010
36. Dong H, Zhu S, Su J, Liu Z, Shen W, Li S, et al. Comparative study of the failure model of esophageal cancer with elective nodal prophylactic and involved-field irradiation. Int J Radiat Oncol Biol Physics. (2015) 93(3):E142–E3. doi: 10.1016/j.ijrobp.2015.07.911
37. Yamashita H, Takenaka R, Omori M, Imae T, Okuma K, Ohtomo K, et al. Involved-field radiotherapy (IFRT) versus elective nodal irradiation (ENI) in combination with concurrent chemotherapy for 239 esophageal cancers: a single institutional retrospective study. Radiat Oncol (2015) 10:171. doi: 10.1186/s13014-015-0482-9
38. Jing W, Zhu H, Guo H, Zhang Y, Shi F, Han A, et al. Feasibility of elective nodal irradiation (ENI) and involved field irradiation (IFI) in radiotherapy for the elderly patients (Aged ≥ 70 years) with esophageal squamous cell cancer: A retrospective analysis from a single institute. PloS One (2015) 10(12):e0143007. doi: 10.1371/journal.pone.0143007
39. Cao YK, Li RX, Tian ZH, Gao HM. Analysis of prognosis and related factors in esophageal squamous cell carcinoma treated with chemotherapy or radiotherapy. Chin J Cancer Prev Treat (2015) 22(16):1297–302. doi: 10.16073/j.cnki.cjcpt.2015.16.011
40. Liu MN, Zhao KL, Chen Y, Jiang GL. Evaluation of the value of ENI in radiotherapy for cervical and upper thoracic esophageal cancer: a retrospective analysis. Radiat Oncol (2014) 9:232. doi: 10.1186/s13014-014-0232-4
41. Zang RK, Ma JB, Song YP, Wang DW, Liu P. Comparation of involved-field conformal irradiation and elective nodal irradiation for middle-thoracic esophageal cancer. Chin J Cancer Prev Treat (2013) 20(24):1917–20. doi: 10.16073/j.cnki.cjcpt.2013.24.016
42. Shen W, Gao H, Zhu S, Li Y, Li J, Su J, et al. Preliminary comparative study between elective nodal irradiation and involved field radiation therapy for clinical early-stage esophageal carcinoma. Chin J Clin Oncol (2013) 40(17):1047–50. doi: 10.3969/j.issn.1000-8179.20130161
43. Li M, Qiao X, Zhou Z, Zhen C, Song Y. A prospectively randomized study of clinical target volume margins for three-dimensional conformal radiotherapy in patients with thoracic esophageal squamous cell carcinoma. Chin J Clin Oncol (2012) 39(17):1294–8. doi: 10.3969/j.issn.1000-8179.2012.17.008
44. Ma JB, Song YP, Yu JM, Zhou W, Cheng EC, Zhang XQ, et al. Feasibility of involved-field conformal radiotherapy for cervical and upper-thoracic esophageal cancer. Onkologie (2011) 34(11):599–604. doi: 10.1159/000334194
45. Zhu H, Pan W, Chen Y, Chen H, Zuo Y, Sun X. What is the optimal radiotherapy target size for non-operable esophageal cancer? a meta-analysis. Oncol Res Treat (2019) 42(9):470–9. doi: 10.1159/000501594
46. Li T, Yisikandaer A, Zhang X, Wang X, Ma Y, Chen L, et al. Involved-field irradiation vs elective nodal irradiation for locally advanced thoracic esophageal squamous cell carcinoma: A comparative interim analysis of clinical outcomes and toxicities (NCT01551589, CSWOG 003). Int J Radiat Oncol Biol Physics. (2015) 93(3):S3–4. doi: 10.1016/j.ijrobp.2015.07.015
47. Li B, Zhang J, Zhang K, Li G, Zheng A, Li J, et al. Chemoradiation with ENI versus IFI, high-dose versus standard-dose radiation therapy for locally advanced esophageal squamous cell carcinoma: Preliminary results of multicenter, phase III clinical trial (NROG 001-northern radiation oncology group of China). Int J Radiat Oncol Biol Physics. (2019) 105(1):E188–E9. doi: 10.1016/j.ijrobp.2019.06.2096
48. Tsuruoka S, Hamamoto Y, Kataoka M, Makita K, Ishikawa H, Takata N, et al. Incidence of regional recurrence outside of radiation fields in patients with or without elective nodal irradiation for stage I middle thoracic esophageal cancer. Int J Radiat Oncol Biol Physics. (2018) 102(3):E42–E. doi: 10.1016/j.ijrobp.2018.07.546
49. Cheng YJ, Jiy SW, Zhu LL, Wang J, Wang L, Liu Q, et al. Comparison of elective nodal irradiation and involved-field irradiation in esophageal squamous cell carcinoma: a meta-analysis. J Radiat Res (2018) 59(5):604–15. doi: 10.1093/jrr/rry055
50. Jing SW, Wang J, Liu Q, Cheng YJ, Yang CR, Wang Y, et al. Involved-field irradiation and elective nodal irradiation under precise radiotherapy in esophageal squamous cell carcinoma: A meta analysis. Chin J Cancer Prev Treat (2017) 24(2):136–42. doi: 10.16073/j.cnki.cjcpt.2017.02.011
51. Zhou ZG, Qiao XY, Gao XS, Wan X, Zhang J, Zhang P, et al. Clinical target volume of 3 d conformal radiotherapy for esophageal cancer. Chin J Radiat Oncol (2009) 18(2):86–7. doi: 10.3760/cma.j.issn.1004-4221.2009.02.086
52. Li DJ, Li HW, He B, Wang GM, Cai HF, Duan SM, et al. Comparison of involved field radiotherapy and extended field radiotherapy of definitive radiotherapy in patients with esophageal squamous cell carcinoma. Chin J Clin Oncol (2013) 40(20):1248–51. doi: 10.3969/j.issn.1000-8179.20130671
53. Zhu SC, Xu JR, Liu ZK, Su JW, Li J. A preliminary study of three-dimensional conformal radiotherapy wih different clinical target volumes for esophageal cancer. Chin J Radiat Oncol (2014) 23(2):127–30. doi: 10.3760/cma.j.issn.1004-4221.2014.02.012
54. Li SQ, Pan XF, Kashaf MS, Xue QP, Luo HJ, Wang YY, et al. Five-year survival is not a useful measure for cancer control in the population: an analysis based on UK data. Asian Pac J Cancer Prev (2017) 18(2):571–6. doi: 10.22034/APJCP.2017.18.2.571
Keywords: involved field irradiation, elective nodal irradiation, IFI, ENI, radiotherapy, esophageal neoplasms, esophageal carcinoma, meta-analysis
Citation: Wang H, Song C, Zhao X, Deng W and Shen W (2022) The role of involved field irradiation versus elective nodal irradiation in definitive radiotherapy or chemoradiotherapy for esophageal cancer- a systematic review and meta-analysis. Front. Oncol. 12:1034656. doi: 10.3389/fonc.2022.1034656
Received: 01 September 2022; Accepted: 17 October 2022;
Published: 02 November 2022.
Edited by:
Xuanfeng Ding, William Beaumont Hospital, United StatesReviewed by:
Martin Leu, University Medical Center Göttingen, GermanyCopyright © 2022 Wang, Song, Zhao, Deng and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wenbin Shen, V2JzaGVuMTk3OUBzaW5hLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.