- 1Department of Urology, Guizhou Provincial People’s Hospital, Guiyang, China
- 2Zunyi Medical University, Zunyi, China
Urachal signet ring cell carcinoma is a kind of rare but aggressive tumor, and a few cases have been reported previously. A 49-year-old male patient with primary complaints of increased frequency of urination, urodynia, and hematuria was diagnosed to have primary urachal signet ring cell carcinoma by our department. Multiple metastases were found in the sigmoid colon, terminal ileum, mesentery, and peritoneum during the operation, and palliative surgery involving partial cystectomy with en bloc resection of the urachus was then performed. A chemotherapy regimen of fluorouracil combined with cisplatin was made for this case. In addition, this patient also received anlotinib for targeted therapy. So far, this patient has done well on regular follow-up for 6 months and is in stable condition. We reported this additional urachal signet ring cell carcinoma case and conducted a literature review to strengthen our cognition of this disease.
Introduction
Urachal carcinoma is a kind of rare but aggressive tumor, which is reported with an incidence of one case per million per year and accounts for <1% of bladder-associated malignancy (1). Adenocarcinoma is the most common urachal carcinoma (>90%), and about 20%–40% of bladder adenocarcinoma is of urachal origin (2). Signet ring cell carcinoma is a subtype of urachal adenocarcinoma and only accounts for 7% (3). A few cases regarding urachal signet ring cell carcinoma have been reported (4–13). In the current study, one additional case in a 49-year-old male patient was reported, and a literature review was conducted to strengthen the cognition of urachal carcinoma.
Case presentation
A 49-year-old male patient was admitted to our department due to increased frequency of urination, urodynia, and hematuria for about 11 months. Ultrasonography identified a solid mass with the size of 3.7 cm × 2.6 cm in the anterior wall of the bladder. Computed tomography of the abdomen further showed that the bladder was irregularly thickened. A tumor with the size of 4.0 cm × 2.7 cm × 4.0 cm and progressive annular enhancement was observed in the anterior wall, which extended superiorly up to just below the umbilicus (Figure 1). Cystoscopy identified a cauliflower-like tumor with the size of 4 cm × 4 cm and a broad base on the top wall of the bladder. Diagnostic resection was then performed, and histological analysis revealed that there was bladder adenocarcinoma (predominantly consisting of signet ring cell type) in the excised mass. Immunohistochemistry showed that villin, CK20, CDX-2, and Ki-67 were positively stained in the tumor, while P40, P63, and CK7 were negatively stained. No gastrointestinal tumor was found by gastroscopy, colonoscopy, or gastrointestinal barium.
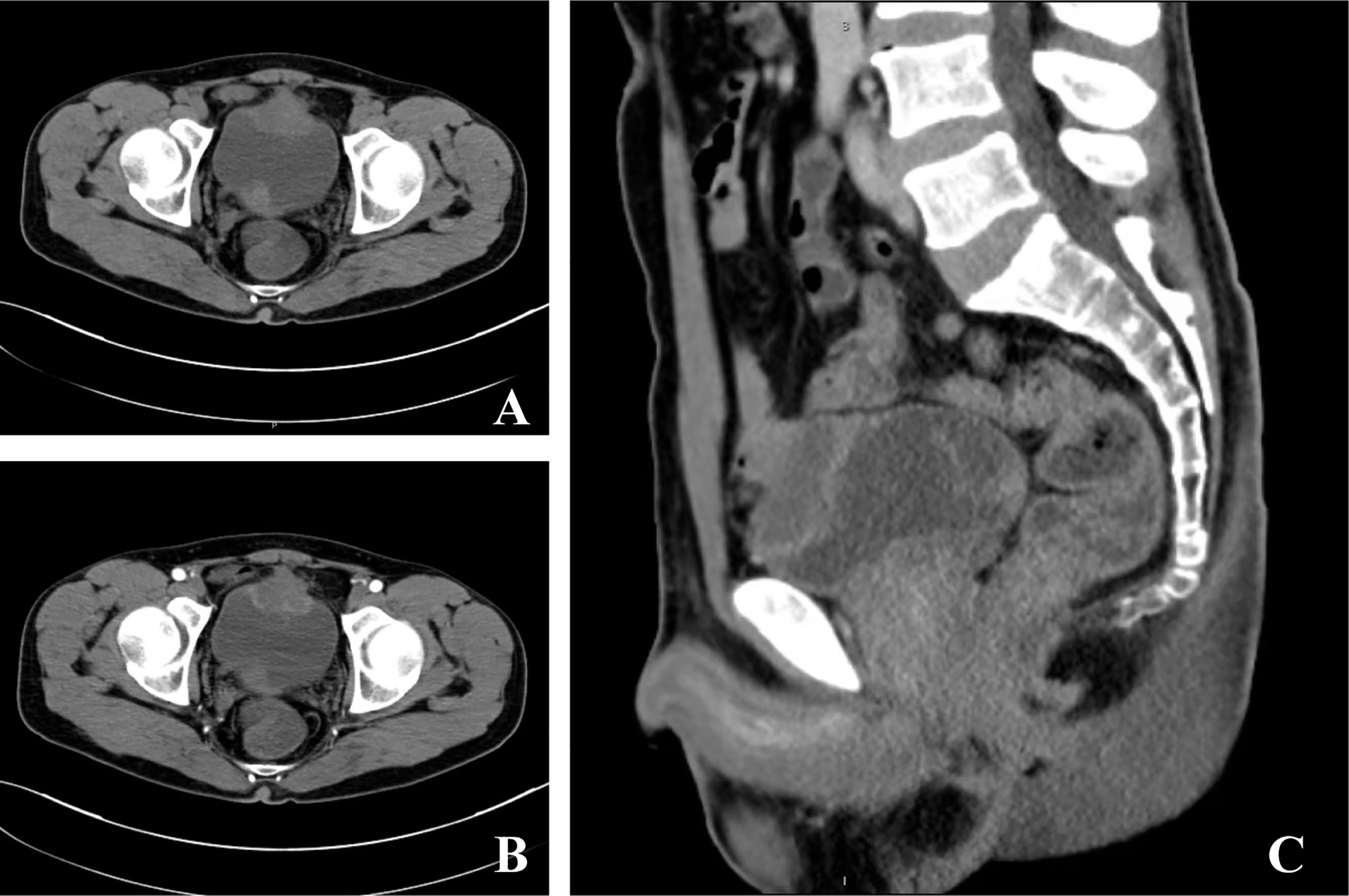
Figure 1 Computed tomography of the current urachal signet ring cell carcinoma case. (A, B) A tumor with the size of 4.0 cm × 2.7 cm × 4.0 cm and progressive annular enhancement was observed in the anterior wall of the bladder. The mass in the trigone of the bladder was confirmed to be a blood clot via cystoscopy. (C) The tumor extended superiorly up to just below the umbilicus.
Radical cystectomy was prepared for this patient according to the above findings. During the surgical exploration, a urachal mass extending from the umbilicus to the dome of the urinary bladder was found. Unfortunately, multiple metastases were seen in the sigmoid colon, terminal ileum, mesentery, and peritoneum, so palliative surgery including partial cystectomy and urachal mass cystectomy was conducted for this patient. Postoperative pathology showed that the tumor consisted of poorly to moderately differentiated adenocarcinoma, including signet ring cell carcinoma and mucinous adenocarcinoma (Figure 2). The carcinoma invaded the subserosal fibrous connective tissue and some area had broken through the serosa. The immunohistochemical results were as follows: CKpan (+), villin (+), CK20 (+), CDX-2 (+), β-catenin (+), SATB (+), cdh17 (+), CK7 (−), GATA-3 (−), CK5/6 (−), P40 (−), P63 (−), PAP (−), PSA (−), CK7 (−), and Ki-67 (+). In addition, the periodic acid–Schiff stain and mucin carmine staining were both positive in the carcinoma tissue. Postoperative PET-CT identified several metastases in the peritoneum and omentum. The clinical stage for this patient was finally referred to as IVb according to the Sheldon staging system.
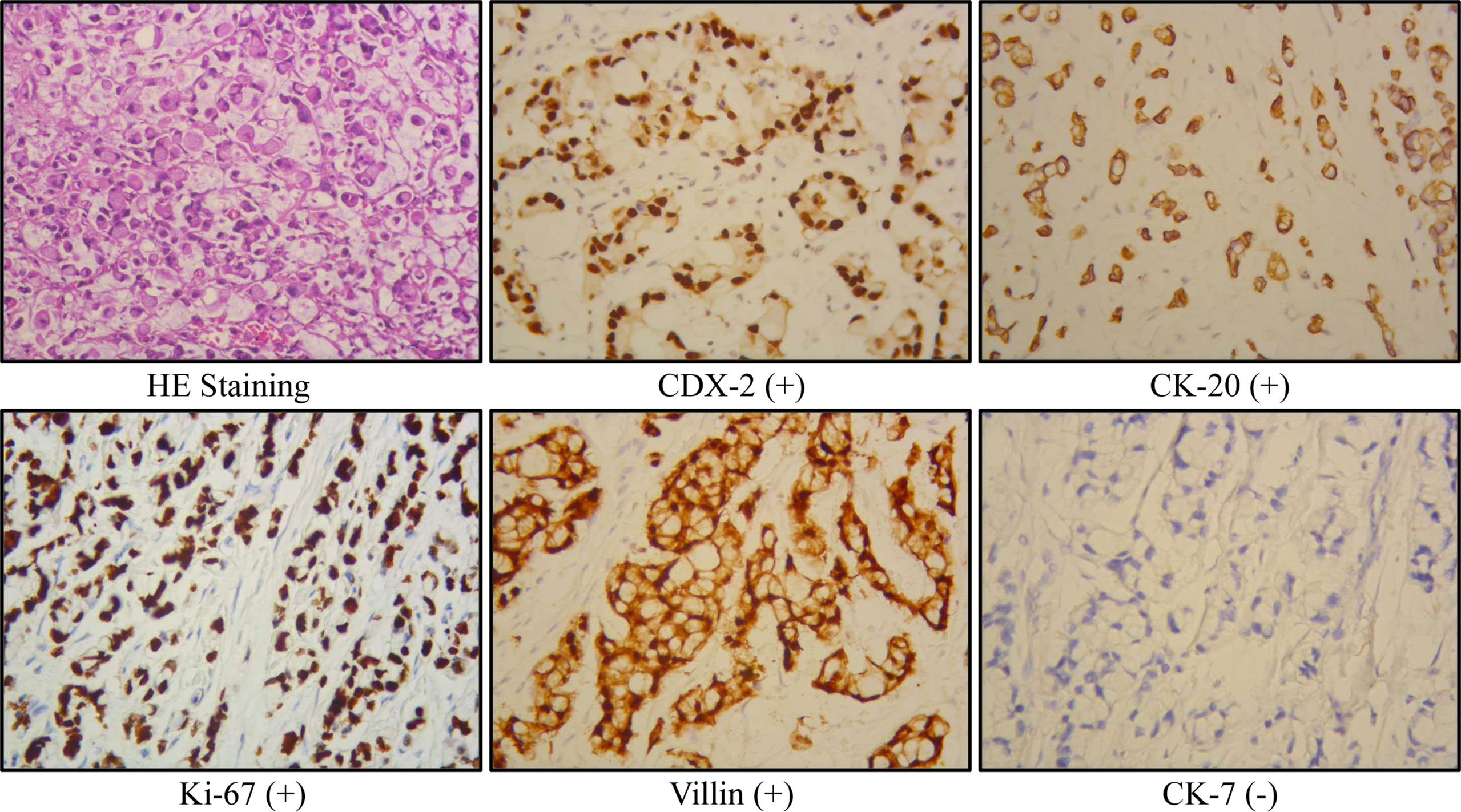
Figure 2 Representative immunohistochemical results of the current urachal signet ring cell carcinoma case.
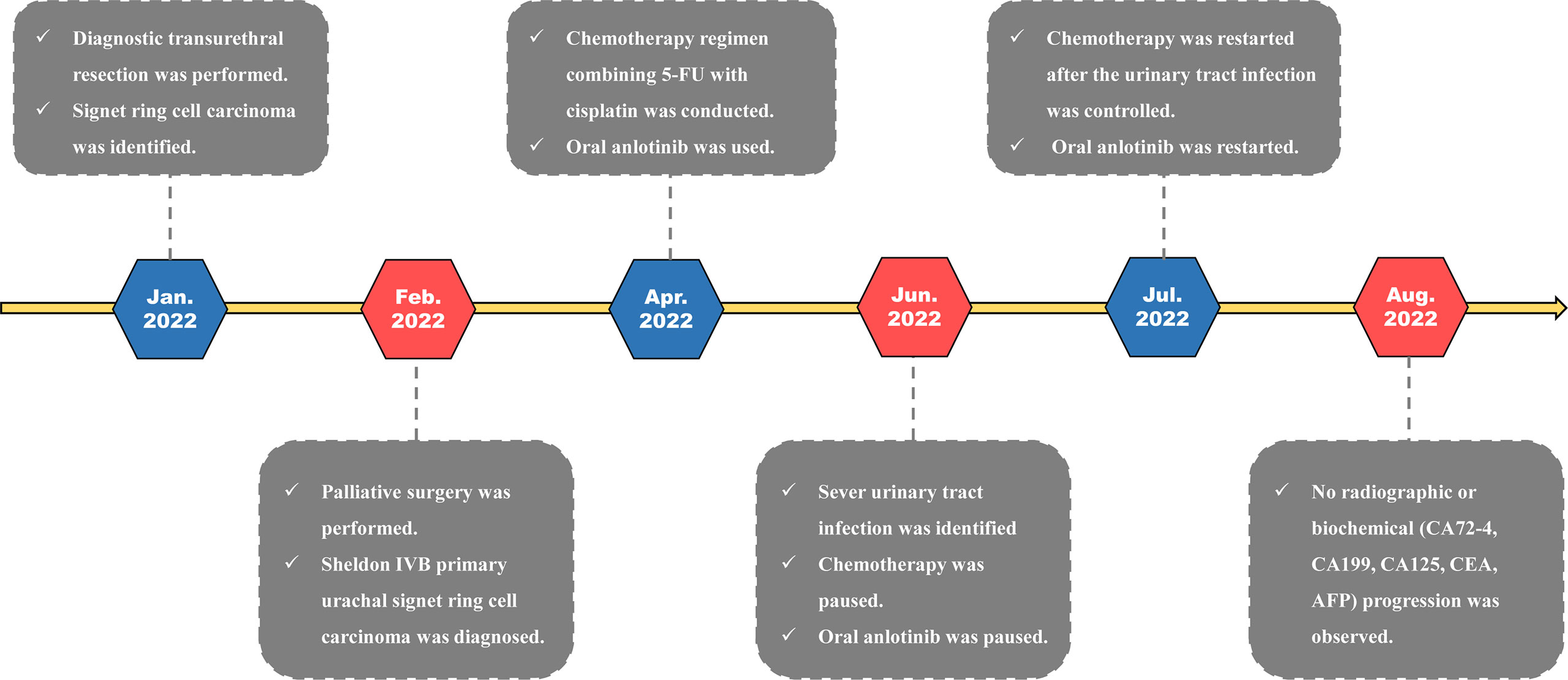
Chemotherapy was conducted for this patient after surgery. A chemotherapy regimen (repeated every 21 days) involving fluorouracil (100 mg/m2, civ 96 h, days 1–4) combined with cisplatin (75 mg/m2, ivgtt, days 1–3) was made for this patient. In addition, the patient also received oral anlotinib (10 mg/day on days 1–14 of a 21-day cycle) for targeted therapy. So far, this patient has received 4 cycles of pharmaceutical therapy and is now doing well on regular follow-up. The treatment timeline for this patient is summarized in Figure 3.
Tumor biomarkers, including carcinoembryonic antigen (CEA), alpha-fetoprotein (AFP), carbohydrate antigen 72-4 (CA72-4), carbohydrate antigen 19-9 (CA19-9), and carbohydrate antigen 125 (CA125), were detected for this case. CEA was 1.2 ng/ml (normal: 0–5), AFP was 1.9 μg/L (normal: <7), CA72-4 was 11.3 U/ml (normal: 0–6.9), CA19-9 was 9.3 U/ml (normal: 0–39), and CA125 was 145 U/ml (normal: 0–35) in the first month after surgery. In the most recent follow-up, CEA was 1.3 ng/ml, AFP was 2.6 μg/L, CA72-4 was 12.8 U/mL, CA19-9 was 10.3 U/mL, and CA125 decreased to 22.2 U/ml.
Discussion
The urachus is a lumen structure connecting the allantois and the bladder during embryonic development. After the third trimester, it obliterates to a 5–10-cm fibromuscular canal within the Retzius space and stretches from the bladder roof to the umbilicus (1). When the urachus obliterates incompletely, it may form a urachal fistula, urachal cyst, and urachal bladder diverticulum (14, 15). Actually, a microscopic residue of the urachus is found in 32% of adults even without the above malformations (16). The urachus can be histologically divided into three layers. The inner layer mainly consisted of the urothelium, while 30% is columnar epithelium or goblet cell (16). The middle layer is submucosal connective tissue and the outer is smooth muscle. Despite the common presence of urothelium in urachal residues, adenocarcinoma is the most common urachal carcinoma (>90%). Glandular metaplasia of the urachal urothelium is postulated to participate in the oncogenesis of urachal adenocarcinoma (17). Another theory suspects that urachal adenocarcinomas may arise from displaced cloacal epithelium (18).
Urachal carcinoma mainly affects patients aged 20–90 years and shows a male predilection (2). The most common symptom of urachal carcinoma is hematuria (2). Other symptoms, such as mucinuria, abdominal pain, urodynia, and dysuria, have also been reported (2, 19). The diagnostic criteria suggested by Gopalan et al. (17) are frequently used for primary urachal tumors, and the clinical classification can be assessed according to the Sheldon, Mayo, and TNM staging systems (20–22). It should be indicated that metastases from other sites (especially the gastrointestinal tract) must be ruled out before a primary urachal carcinoma can be diagnosed. In addition, it is also important to differentiate urachal adenocarcinoma from non-urachal adenocarcinoma of the bladder because they require different treatment strategies. Generally, urachal adenocarcinoma locates in the dome/anterior wall of the bladder and lacks widespread cystitis cystica/glandularis beyond the dome/anterior wall (20). In contrast, primary bladder adenocarcinoma often locates in the lateral/bottom wall of the bladder and presents with cystitis cystica/glandularis.
Partial cystectomy with en bloc resection of the urachus is now the primary surgical option for localized urachal cancer. Whether lymphadenectomy should be conducted remains controversial because lymph node positivity was found in only 17% of cases and lymphadenectomy contributes little to improvement in survival (2, 23). About 21% of urachal carcinoma patients have distant metastasis at first presentation (2). For patients with metastasis, systemic treatment is suggested. There is currently no standard chemotherapy regimen for urachal adenocarcinoma, but the chemotherapy regimens for bladder cancer and colorectal cancer can be referred. It is reported that the combination of 5-fluorouracil (5-FU) with cisplatin provides more benefit than 5-FU or cisplatin alone in metastatic urachal carcinoma (2). In addition, Goss et al. reported that one patient with urachal cancer had a transient 55% decrease in tumor size after receiving 800 mg/day of gefitinib, indicating that an epidermal growth factor receptor (EGFR) inhibitor seems to be an alternative choice for metastatic urachal carcinoma (24). In the current study, the combination of 5-FU with cisplatin and anlotinib is made for the patient and the condition keeps stable after surgery.
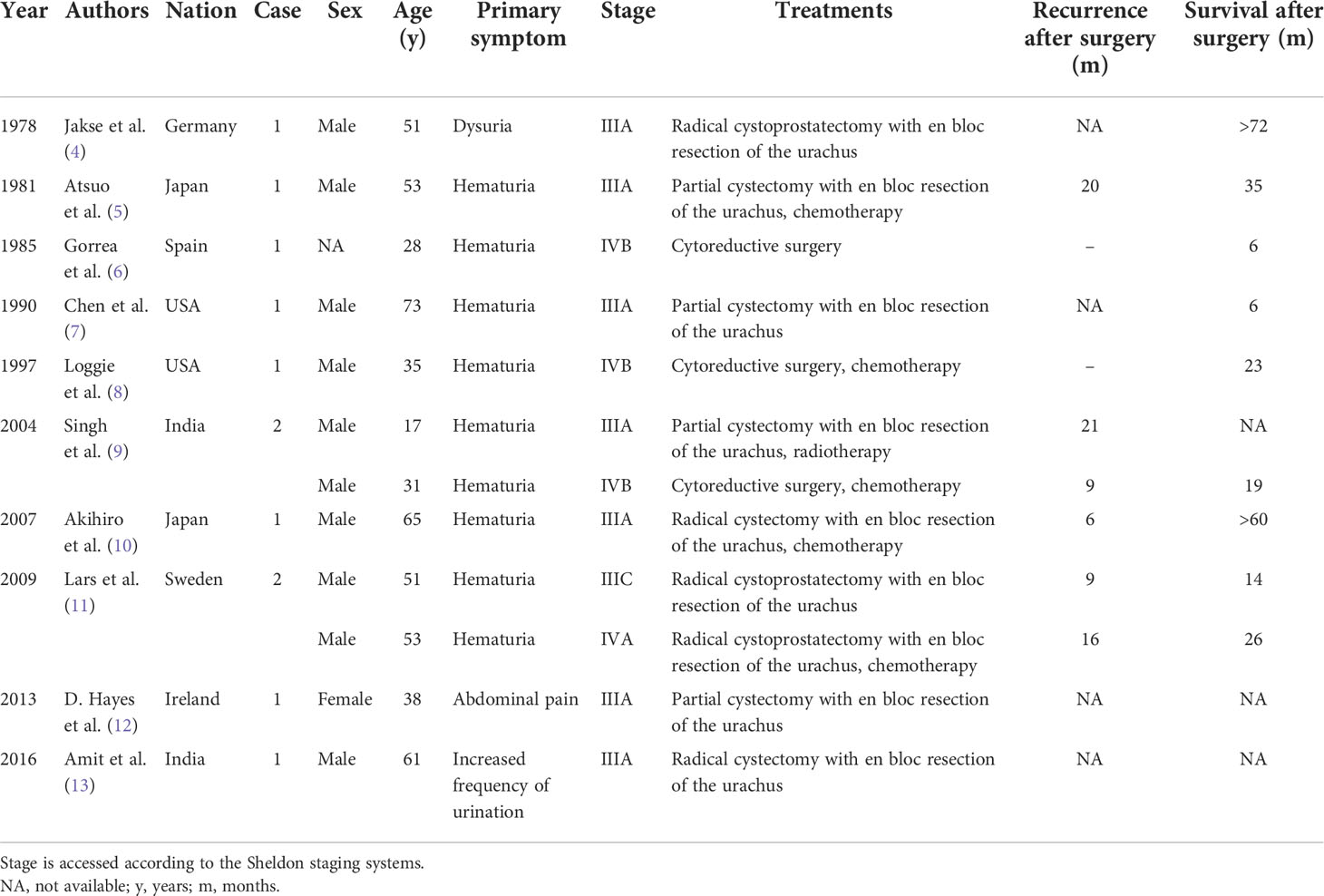
The reported median overall survival (OS) time from the diagnosis of urachal cancer in all stages ranges from 42.9 to 67 months, and the estimated 5-year OS rate is about 50% (2, 23). Sheldon stage >IIIB, Mayo stage >II, the presence of lymph node metastases or distant metastases, and tumor-positive surgical margins have been identified as independent parameters of poor prognosis (2). Although it is unknown whether there are differences in behavior among different urachal adenocarcinoma subtypes, urachal signet ring cell carcinoma often presents with high grade, high stage, and poor prognosis, which may be explained by its more diffuse infiltrative growth (3). We summarized the clinical information of previously reported urachal signet ring cell carcinoma cases in Table 1. As we can see, most patients are men (>80%) and all of them are diagnosed at a late stage (Sheldon ≥III). The OS time after surgery ranges from 6 to 72 months, and the 5-year OS rate is only about 22%. In the current study, no significant radiographic (assessed by CT and magnetic resonance imaging) or biochemical (CEA, AFP, CA72-4, CA19-9, and CA125) progression was observed in the patient during 6 months after surgery. Chemotherapy and regular follow-up will be continually conducted for this patient.
Conclusion
Urachal signet ring cell carcinoma is a kind of rare but aggressive tumor, which often presents with high grade and high stage. Despite the poorer prognosis, the diagnosis, classification, and treatment strategy of urachal signet ring cell carcinoma are currently in accordance with other urachal carcinomas. The early diagnosis method and standard high-level evidence guiding the treatment are lacking for urachal carcinoma. More significant efforts are needed to increase the cognition of this disease.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Guizhou Provincial People’s Hospital ethics committee. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
QW and XC drafted the manuscript. JZ and YL collected the clinical data. KJ did the surgery. All authors read and approved the final manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (Number: 82060462), Science and Technology Plan Project of Guizhou Province (Number [2019]:5405), and the Doctoral Foundation of Guizhou Provincial People’s Hospital (GZSYBS[2018]02).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Reis H, Szarvas T. Urachal cancer-current concepts of a rare cancer. Pathologe (2019) 40(Suppl 1):31–9. doi: 10.1007/s00292-018-0516-9
2. Szarvas T, Módos O, Niedworok C, Reis H, Szendröi A, Szász MA, et al. Clinical, prognostic, and therapeutic aspects of urachal carcinoma-a comprehensive review with meta-analysis of 1,010 cases. Urol Oncol (2016) 34(9):388–98. doi: 10.1016/j.urolonc.2016.04.012
3. Paner GP, Lopez-Beltran A, Sirohi D, Amin MB. Updates in the pathologic diagnosis and classification of epithelial neoplasms of urachal origin. Adv Anat Pathol (2016) 23(2):71–83. doi: 10.1097/PAP.0000000000000110
4. Jakse G, Schneider HM, Jacobi GH. Urachal signet-ring cell carcinoma, a rare variant of vesical adenocarcinoma: incidence and pathological criteria. J Urol (1978) 120(6):764–6. doi: 10.1016/s0022-5347(17)57356-5
5. Kondo A, Ogisu B, Mitsuya H. Signet-ring cell carcinoma involving the urinary bladder. report of a case and review of 21 cases. Urol Int (1981) 36(6):373–9. doi: 10.1159/000280784
6. Alonso-Gorrea M, Mompo-Sanchis JA, Jorda-Cuevas M, Froufe A, Jiménez-Cruz JF. Signet ring cell adenocarcinoma of the urachus. Eur Urol (1985) 11(4):282–4. doi: 10.1159/000472516
7. Chen KT, Workman RD, Rainwater G. Urachal signet-ring cell carcinoma. Urology (1990) 36(4):339–40. doi: 10.1016/0090-4295(90)80243-g
8. Loggie BW, Fleming RA, Hosseinian AA. Peritoneal carcinomatosis with urachal signet-cell adenocarcinoma. Urology (1997) 50(3):446–8. doi: 10.1016/S0090-4295(97)00247-1
9. Daljeet S, Amreek S, Satish J, Raman A, Hara GS, Lovneesh G, et al. Signet ring cell adenocarcinoma of the urachus. Int J Urol (2004) 11(9):785–8. doi: 10.1111/j.1442-2042.2004.00892.x
10. Morii A, Furuya Y, Fujiuchi Y, Akashi T, Ishizawa S, Fuse H. Urachal signet ring cell carcinoma. Int J Urol (2007) 14(4):360–1. doi: 10.1111/j.1442-2042.2007.01676.x
11. Egevad L, Håkansson U, Grabe M, Ehrnstrom R. Urachal signet-cell adenocarcinoma. Scand J Urol Nephrol (2009) 43(1):88–91. doi: 10.1080/00365590802361914
12. Hayes Ryan D, Paramanathan P, Russell N, Coulter J. Primary urachal malignancy: case report and literature review. Ir J Med Sci (2013) 182(4):739–41. doi: 10.1007/s11845-013-0964-4
13. Verma A, Tomar V. Signet ring cell carcinoma of urachal origin presenting as irritative lower urinary tract symptoms and pain abdomen: A rare case report. J Clin Diagn Res (2016) 10(7):PD12–13. doi: 10.7860/JCDR/2016/18851.8129
14. Galati V, Donovan B, Ramji F, Campbell J, Kropp BP, Frimberger D. Management of urachal remnants in early childhood. J Urol (2008) 180(4 Suppl):1824–6. doi: 10.1016/j.juro.2008.03.105
15. Copp HL, Wong IY, Krishnan C, Malhotra S, Kennedy WA. Clinical presentation and urachal remnant pathology: implications for treatment. J Urol (2009) 182(4 Suppl):1921–4. doi: 10.1016/j.juro.2009.03.026
16. Schubert GE, Pavkovic MB, Bethke-Bedürftig BA. Tubular urachal remnants in adult bladders. J Urol (1982) 127(1):40–2. doi: 10.1016/s0022-5347(17)53595-8
17. Gopalan A, Sharp DS, Fine SW, Tickoo SK, Herr HW, Reuter VE, et al. Urachal carcinoma: a clinicopathologic analysis of 24 cases with outcome correlation. Am J Surg Pathol (2009) 33(5):659–68. doi: 10.1097/PAS.0b013e31819aa4ae
18. Grignon DJ, Ro JY, Ayala AG, Johnson DE, Ordóñez NG. Primary adenocarcinoma of the urinary bladder. A clinicopathologic Anal 72 cases. Cancer (1991) 67(8):2165–72. doi: 10.1002/1097-0142(19910415)67:8<2165::aid-cncr2820670827>3.0.co;2-m
19. Mangiacapra FJ, Scheraga JL, Jones LA. Mucinous colloid adenocarcinoma of the urachus. Radiographics (2001) 21(4):965–9. doi: 10.1148/radiographics.21.4.g01jl25965
20. Sheldon CA, Clayman RV, Gonzalez R, Williams RD, Fraley EE. Malignant urachal lesions. J Urol (1984) 131(1):1–8. doi: 10.1016/s0022-5347(17)50167-6
21. Ashley RA, Inman BA, Sebo TJ, Leibovich BC, Blute ML, Kwon ED, et al. Urachal carcinoma: clinicopathologic features and long-term outcomes of an aggressive malignancy. Cancer (2006) 107(4):712–20. doi: 10.1002/cncr.22060
22. Molina JR, Quevedo JF, Furth AF, Richardson RL, Zincke H, Burch PA. Predictors of survival from urachal cancer: a Mayo clinic study of 49 cases. Cancer (2007) 110(11):2434–40. doi: 10.1002/cncr.23070
23. Jia Z, Chang X, Li X, Wang B, Zhang X. Urachal carcinoma: Are lymphadenectomy and umbilectomy necessary? Med Sci Monit (2020) 26:e927913. doi: 10.12659/MSM.927913
24. Goss G, Hirte H, Miller WH Jr, Lorimer IA, Stewart D, Batist G, et al. A phase I study of oral ZD 1839 given daily in patients with solid tumors: IND.122, a study of the investigational new drug program of the national cancer institute of Canada clinical trials group. Invest New Drugs (2005) 23(2):147–55. doi: 10.1007/s10637-005-5860-y
Keywords: case report, diagnosis, signet ring cell carcinoma, treatment, urachal carcinoma
Citation: Wang Q, Chen X, Zhang J, Luo Y and Jiang K (2022) Primary urachal signet ring cell carcinoma: A case report. Front. Oncol. 12:1034245. doi: 10.3389/fonc.2022.1034245
Received: 01 September 2022; Accepted: 20 September 2022;
Published: 06 October 2022.
Edited by:
Haoran Liu, Stanford University, United StatesReviewed by:
Ligang Zhang, First Affiliated Hospital of Anhui Medical University, ChinaBeichen Ding, First Affiliated Hospital of Harbin Medical University, China
Copyright © 2022 Wang, Chen, Zhang, Luo and Jiang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kehua Jiang, dGpqa2hAc2luYS5jb20=
†These authors have contributed equally to this work
 Qing Wang1†
Qing Wang1† Kehua Jiang
Kehua Jiang