
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 07 November 2022
Sec. Cancer Genetics
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1030232
This article is part of the Research Topic New Therapeutic Approaches in Microsatellite Stable Colorectal Cancer Patients. View all 7 articles
A correction has been applied to this article in:
Corrigendum: Case report: MAP2K1 K57N mutation is associated with primary resistance to anti-EGFR monoclonal antibodies in metastatic colorectal cancer
 Gianluca Mauri1,2,3†
Gianluca Mauri1,2,3† Giorgio Patelli1,3†
Giorgio Patelli1,3† Viviana Gori1,3
Viviana Gori1,3 Calogero Lauricella3
Calogero Lauricella3 Benedetta Mussolin4
Benedetta Mussolin4 Alessio Amatu3
Alessio Amatu3 Katia Bencardino3
Katia Bencardino3 Federica Tosi3
Federica Tosi3 Erica Bonazzina3
Erica Bonazzina3 Emanuela Bonoldi3
Emanuela Bonoldi3 Alberto Bardelli4,5
Alberto Bardelli4,5 Salvatore Siena1,3
Salvatore Siena1,3 Andrea Sartore-Bianchi1,3*
Andrea Sartore-Bianchi1,3*Background: We aim to identify the prevalence and the role of the MAP2K1 K57N mutation in predicting resistance to anti-EGFR agents in metastatic colorectal cancer (mCRC) patients.
Methods: We retrospectively reviewed tumor-based next generation sequencing (NGS) results from mCRC patients screened for enrollment in the GO40872/STARTRK-2 clinical trial between July 2019 and March 2021. Then, in patients harboring microsatellite stable (MSS) RAS and BRAF wild-type MAP2K1 mutant mCRC, we reviewed outcome to treatment with anti-EGFR monoclonal antibodies.
Results: A total of 246 mCRC patients were screened. Most of them, 215/220 (97.7%), were diagnosed with MSS mCRC and 112/215 (52.1%) with MSS, RAS and BRAF wild-type mCRC. Among the latter, 2/112 (1.8%) had MAP2K1 K57N mutant mCRC and both received anti-EGFR monotherapy as third line treatment. In both patients, MAP2K1 K57N mutant tumors proved primary resistant to anti-EGFR agent panitumumab monotherapy. Of interest, one of these patients was treated with anti-EGFR agents three times throughout his course of treatment, achieving some clinical benefit only when associated with other cytotoxic agents (FOLFOX or irinotecan).
Conclusion: We verified in a clinical real-world setting that MAP2K1 K57N mutation is a resistance mechanism to anti-EGFR agents in mCRC. Thus, we suggest avoiding the administration of these drugs to MSS RAS and BRAF wild-type MAP2K1 N57K mutant mCRC.
Anti-epidermal growth factor receptor (EGFR) monoclonal antibodies (MoAbs) are recommended as standard treatment options for patients affected with RAS wild-type (WT), mainly left-sided, metastatic colorectal cancer (mCRC) (1). Anti-EGFR MoAbs prevent EGFR activation, thus blocking the downstream mitogen-activated protein kinase (MAPK) pathway and the resulting proliferative signal (2). Mutations occurring in KRAS and NRAS exons 2, 3, and 4 represent the main mechanisms of resistance to these anticancer agents, countering the MAPK pathway blockade and even entailing detrimental survival in RAS mutant mCRC patients receiving anti-EGFR drugs (3). Accumulating evidence also suggests BRAF mutations being a resistance mechanism to anti-EGFR MoAbs, even though treatment is allowed as per label (4). Over the years, several other molecular biomarkers beyond RAS and BRAF have been associated to primary and/or acquired resistance to anti-EGFR MoAbs, such as gene mutations of ERBB2, EGFR, FGFR1, PDGFRA, PIK3CA, PTEN, AKT1, and MAP2K1/MEK1, amplifications of KRAS, ERBB2, and MET, and fusions of ALK, ROS1, NTRK1-3, and RET (5–11). However, given the low prevalence of such alterations and the confounding effect of cytotoxic agents administered together with anti-EGFR MoAbs, no definitive consensus on therapeutic implications has been derived so far.
The increasing availability of next generation sequencing (NGS) panels in the clinical setting allows frequent detection of these alterations, thus requiring evaluation of whether available evidence is strong enough to preclude patients from a potential effective treatment option, therefore posing an emergent therapeutic challenge. Here we provide a molecular tumor board (MTB) discussion of two emblematic cases of uncommon MAP2K1 K57N mutated mCRC patients, commenting upon treatment decision making focused on anti-EGFR drug administration.
At Niguarda Cancer Center, we retrospectively reviewed tumor-based NGS results of mCRC patients screened for enrollment in the GO40872/STARTRK-2 clinical trial (NCT02568267) between July 2019 and March 2021. Through this process, we checked for mCRC samples harboring MAP2K1 mutations. After the identification of microsatellite stable (MSS), RAS and BRAF WT, MAP2K1 mutant mCRC, we retrospectively reviewed patients’ records to evaluate treatment outcome to anti-EGFR MoAb.
The molecular results presented in this manuscript are based on FoundationOne CDx NGS (Foundation Medicine, Inc.) panel results performed on archival formalin-fixed paraphing-embedded (FFPE) tumor tissue; data from NGS and allele frequency were made available by the sponsor of the GO40872/STARTRK-2 trial upon personalized request, following the approval by the local ethical committee. Concerning mismatch repair (MMR) assessment, results from FoundationOne CDx NGS panels were integrated by immunohistochemistry (IHC) testing performed on archival FFPE tumor tissue, if the NGS data was not available.
After MAP2K1 mutant mCRC patients’ identification, previously collected plasma samples were retrospectively analyzed by looking for MAP2K1 K57N circulating tumor DNA (ctDNA). The ctDNA analysis was performed by droplet-digital PCR (dd-PCR).
Both patients consented to the submission and publication of the following article reports.
A total of 246 mCRC patients were screened. Most of them, 215/220 (97.7%), were diagnosed with MSS mCRC and 112/215 (52.1%) with MSS, RAS and BRAF WT mCRC. Among the latter, 2/112 (1.8%) had MAP2K1 K57N mutant mCRC and both received anti-EGFR monotherapy as third line treatment.
In February 2015, a 41-year-old man was diagnosed with stage IV, RAS and BRAF WT, ERBB2 not-amplified, MSS rectal cancer with synchronous liver metastases. As presented in Figure 1 and Table 1, the patient underwent multimodal treatment with initial first-line therapy with FOLFOX and panitumumab, achieving partial response (PR) after 4 cycles, followed by short-course radiotherapy (RT) on the pelvis and radical surgery on both the primary tumor and liver metastases in June 2015. Thereafter, the patient received post-surgical treatment with FOLFOX for further 8 cycles. After 11 months of follow-up, a liver recurrence occurred and the patient underwent a second metastasectomy in November 2016. However, due to nodal relapse soon after surgery, FOLFOX and bevacizumab was then reintroduced as second line therapy, achieving stable disease (SD) as best response. Treatment had to be interrupted after 6 courses due to persistent G4 thrombocytopenia, that was interpreted as secondary to 5-fluorouracil infusion. Due to oligo-progressive disease (PD) in a single lymph node, the patient underwent stereotactic RT in October 2017, with no further PD until July 2018. Then, the patient received panitumumab monotherapy as a reintroduction strategy with PD after 6 cycles, developing a severe mediastinal syndrome requiring palliative thoracic surgery and RT. At this stage, aiming to extent the spectrum of druggable therapeutic targets, the patient was screened for the GO40872/STARTRK-2 clinical trial (NCT02568267) at Niguarda Cancer Center, Milan, Italy. Thus, a FoundationOne CDx NGS assay (https://www.foundationmedicine.com/test/foundationone-cdx, Foundation Medicine, Inc.) was obtained from the archival formalin-fixed paraffin-embedded (FFPE) tissue derived from the first liver metastasectomy. The case was presented for MTB discussion, revealing no actionable targets. Of note, a somatic tumor MAP2K1 K57N mutation was found on tissue with 17.1% variant allelic frequency (VAF), and confirmed also on liquid biopsy (LB) at progression to anti-EGFR therapy, assessed as reported elsewhere (12) through droplet digital polymerase chain reaction (ddPCR), with a 16.9% minor allelic frequency (MAF). Further treatment with weekly irinotecan and cetuximab was administered, achieving SD as best response. Finally, regorafenib was initiated after PD but soon interrupted due to the deterioration of the clinical conditions. The patient died on March 31th, 2020.
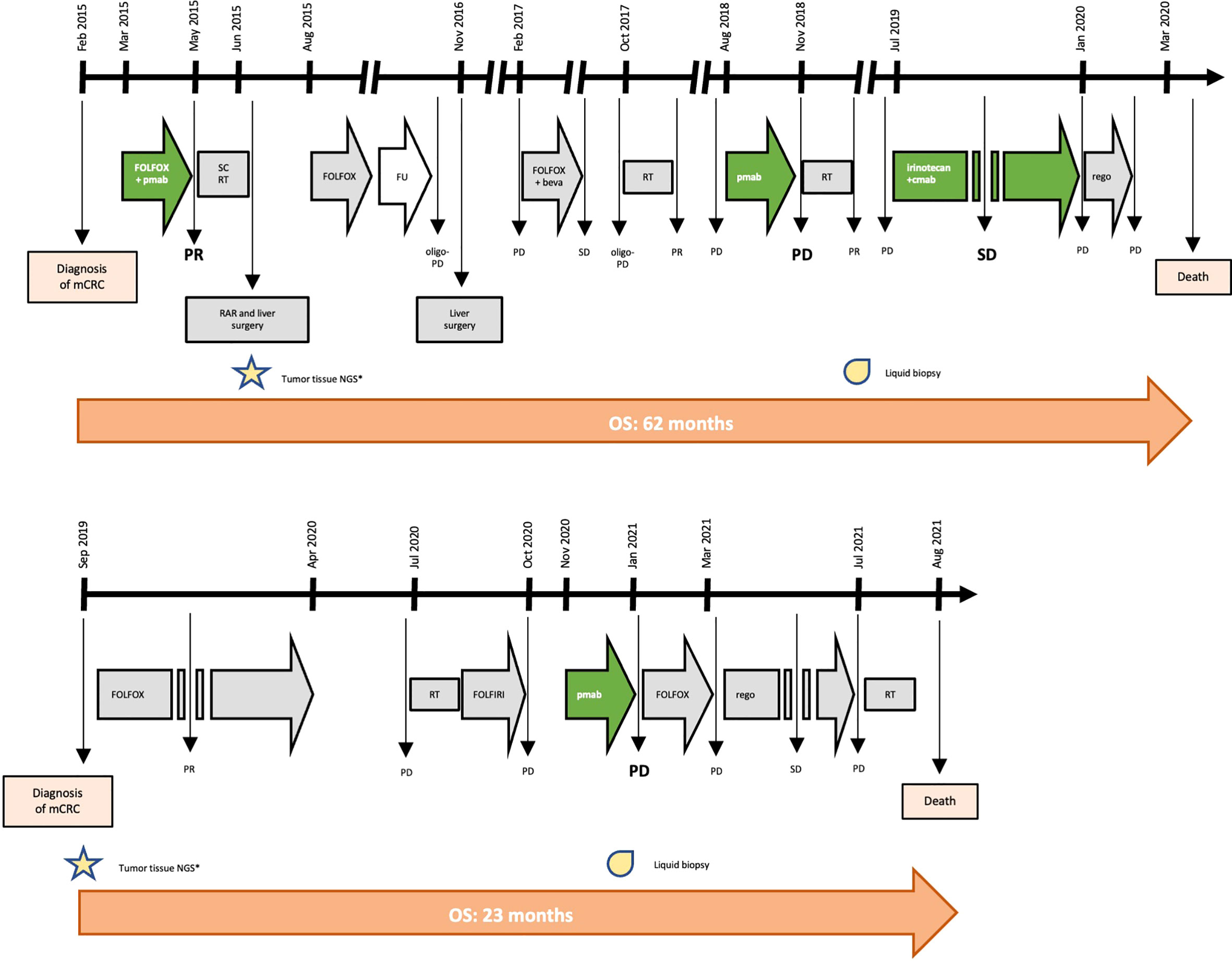
Figure 1 Timeline of treatment for patient #1 and #2. Keys: beva, bevacizumab; cmab, cetuximab; FU, follow-up; mCRC, metastatic colorectal cancer; NGS, next genome sequencing; OS, overall survival; pmab, panitumumab; PD, progressive disease; PR, partial response; RAR, rectal anterior resection; rego, regorafenib; RT, radiotherapy; SC, short-course; SD, stable disease.
In September 2019, a 62-year-old man was diagnosed with RAS and BRAF WT, ERBB2 not-amplified, MSS, right-sided mCRC with synchronous liver and bone metastases. Initially, the patient underwent FOLFOX first-line therapy for 12 cycles achieving PR, followed by a therapeutic holiday due to an occurring, non-treatment related intracranial hemorrhagic event. Following systemic PD, the patient received palliative bone RT and FOLFIRI as second line therapy, with further PD. Given the absence of available clinical trials and according to the molecular status, the patient started third line panitumumab monotherapy in October 2020. After 3 cycles, due to worsening clinical conditions, we performed a CT scan demonstrating dimensional and numerical PD. At this stage, aiming to expand druggable therapeutic targets, a FoundationOne CDx NGS panel was performed on archival FFPE tissue from the primary tumor biopsy undergone at baseline. The case was presented at MTB: although no druggable targets were retrieved, a tumor somatic MAP2K1 N57K mutation was found, with a 14.7% VAF, thus potentially entailing anti-EGFR resistance. This was confirmed by LB through ddPCR showing a 0.51% MAF. Subsequently, the patient was treated with FOLFOX reintroduction achieving PD as best response, and then regorafenib with SD. In July 2021, further palliative RT was provided for worsening gonalgia secondary to a knee bone metastasis. However, the patient died on August 20th 2021 (Figure 1 and Table 1).
We present two cases of patients affected by RAS and BRAF WT, MSS mCRC, both harboring a MAP2K1 K57N mutation, and both treated with anti-EGFR monotherapy with early symptomatic PD. Particularly, patient #1 achieved clinical benefit from anti-EGFR agents only when administered in combination with cytotoxic agents (FOLFOX or irinotecan, respectively), while he rapidly progressed to anti-EGFR monotherapy (Table 1 and Figure 1). Similarly, patient #2 had a right-sided mCRC that proved primary resistant to panitumumab monotherapy as third line of treatment. Altogether, overall survival was consistent with tumor sidedness and other molecular characteristics for both patients.
The presence of a MAP2K1 mutation was the only anti-EGFR resistance identified in both cases presented; indeed, no other MAPK pathway alterations known as putative mechanisms of resistance to anti-EGFR drugs were found (Table 1). For patient #2, we can allege that MAP2K1 activation exerted a primary mechanism of resistance, as the NGS analysis preceded the administration of the first anti-EGFR therapy. Following, LB confirmed the persistence of this alteration also at the time of PD. Differently, it was not possible to distinguish whether MAP2K1 K57N was primary or acquired in patient #1, since archival tissue from initial tissue biopsy was insufficient for NGS analysis; indeed, in this case the NGS analysis was performed on the surgical specimen collected after an initial anti-EGFR based therapy (FOLFOX plus panitumumab). Despite this limitation, we can speculate that it is unlikely that the MAP2K1 K57N mutation in patient #1 was acquired upon anti-EGFR exposure, as it has been reported that acquired MAPK pathway alterations are uncommon [6.8% of mCRC patients progressing to first line anti-EGFR containing regimens (13)] and that patient #1 received only 4 courses of FOLFOX and panitumumab (thus having a time-limited anti-EGFR exposure for resistance acquisition).
Notwithstanding, these data provide retrospective evidence that MAP2K1 K57N mutation is a potential mechanism of resistance to anti-EGFR MoAbs in mCRC patients. Hence, despite the lack of high level of evidence, MAP2K1 alterations should be taken into consideration in clinical practice and reviewed through MTB discussion. Indeed, the Mitogen-Activated Protein Kinase Kinase 1 gene, also known as MAP2K1, MAPKK1 or MEK1, is a well-known oncogene encoding for the protein kinase MEK, and exerting its function downstream of the RAS and BRAF proteins in the MAPK signaling cascade, thus conceivably capable of resistance to MAPK pharmacologic silencing (Figure 2) (14). The prevalence of MAP2K1 mutations in CRC is 1-2%, mainly enriched in RAS/BRAF WT tumors (https://www.cbioportal.org/). Most common alterations are found in the hotspots K57 (as in both the reported cases) and Q56 codons (15). A previously published mechanism study identified MAP2K1 K57N mutations as a class II category mutation, which are partially dependent on upstream RAF activation (16). In clinical practice, the role of MAP2K1 mutations is seldom addressed in mCRC patients, given the absence of specific targeted therapy. However, a contribution to carcinogenesis and resistance to EGFR inhibition has been highlighted in preclinical and translational studies, making MAP2K1 alterations relevant for MTB discussion before treating these patients with anti-EGFR drugs (at least as monotherapy) (6, 11, 17, 18).

Figure 2 MAP2K1 K57N in the MAPK pathway drives resistance to anti-EGFR therapy in metastatic colorectal cancer. Keys: MoAb, monoclonal antibodies. Created with BioRender.com.
Even if the clinical characterization of patients affected by mCRC harboring this alteration is still partial and not conclusive, comparable cases were previously reported by other authors (15, 19). However, in this prior reports patients with MAP2K1 mutant tumors were treated with anti-EGFR drugs combined with other cytotoxic or targeted agents, thus hampering definitive clinical conclusions concerning resistance to anti-EGFR MoAbs (15, 19). Besides, only 1 case of MAP2K1 K57N was reported in these studies (15, 19). Differently, both our patients were affected by MAP2K1 K57N mutant mCRC experiencing PD to panitumumab monotherapy. Given the infrequent occurrence of these alterations, taking into account MAP2K mutations in clinical practice and then gathering further clinical evidence in form of scientific reports is needed to translate preclinical discoveries to real-world clinical practice and refine the spectrum of mCRC patients not benefitting from anti-EGFR MoAbs.
In conclusion, we discuss the role of MAP2K1 K57N mutation as a negative predictive factor of response and mechanisms of primary resistance to anti-EGFR MoAbs, occurring in 1.8% of RAS and BRAF WT, MSS mCRC. No assumption can be made on the prognostic value of these alterations given the restricted number of patients, although survival data were in line with expectations in both cases. Therefore, based on previously available literature and the present MTB discussion, anti-EGFR agents should be omitted in MAP2K1 K57N mutant mCRC, regardless of primary tumor sidedness, to spare toxicities in patients likely not benefitting from such treatment.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
All patients consented to the submission and publication of the following article. This study respected ethical principles as established by the Helsinki Declaration and the Good Clinical Practice: Consolidated Guideline approved by the International Conference on Harmonisation (ICH); a study protocol was presented to the local ethics committee Milano Area 3 (Italy). In no way therapeutic changes took place secondary to the results of this study, in conformity with the aforementioned clinical guidelines for the treatment and management of vulnerable patients. The collection, recording, and reporting of data was accurate and ensured the privacy, health, and welfare of research subjects during and after the study. All collected data will be preserved anonymously and with respect to the patients’ privacy.
GM, GP, and VG were major contributors in writing the manuscript; CL and EBono performed the pathology procedures that were preparatory to the NGS analysis; BM and AB performed the molecular analyses on plasma samples; GM, GP, VG, AA, KB, FT, EBona, AS-B, and SS provided clinical care to the patients; AS-B and SS supervised oncological care and critically reviewed this article. All authors contributed to the article and approved the submitted version.
Authors are supported by Fondazione Oncologia Niguarda Onlus. FONDAZIONE AIRC under 5 per Mille 2018 (ID 21091 program) (AB and SS).
Gianluca Mauri is a PhD student within the European School of Molecular Medicine (SEMM).
AS-B is an advisory board member for Amgen, Bayer, Novartis, Sanofi and Servier. AA is an advisory board member for Roche and Bayer and received honoraria from CheckmAb. SS is an advisory board member for Agenus, AstraZeneca, Bayer, BMS, CheckmAb, Daiichi-Sankyo, Guardant Health, Menarini, Merck, Novartis, Roche-Genentech, and Seagen. AB is an advisory board member for NeoPhore and Inivata, and a shareholder of NeoPhore, and received research support from Astrazeneca and Boehringer Ingelheim. GM received honoraria from COR2ED.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Benson AB, Venook AP, Al-Hawary MM, Arain MA, Chen Y, Ciombor K, et al. Colon cancer, version 2.2021, NCCN clinical practice guidelines in oncology. J Natl Compr Cancer Netw JNCCN (2021) 19:329–59. doi: 10.6004/jnccn.2021.0012
2. Di Nicolantonio F, Vitiello PP, Marsoni S, Siena S, Tabernero J, Trusolino L, et al. Precision oncology in metastatic colorectal cancer - from biology to medicine. Nat Rev Clin Oncol (2021) 18:506–25. doi: 10.1038/s41571-021-00495-z
3. Bardelli A, Siena S. Molecular mechanisms of resistance to cetuximab and panitumumab in colorectal cancer. J Clin Oncol Off J Am Soc Clin Oncol (2010) 28:1254–61. doi: 10.1200/JCO.2009.24.6116
4. Mauri G, Bonazzina E, Amatu A, Tosi F, Bencardino F, Gori V, et al. The evolutionary landscape of treatment for BRAFV600E mutant metastatic colorectal cancer. Cancers (2021) 13. doi: 10.3390/cancers13010137
5. Sartore-Bianchi A, Di Nicolantonio F, Nichelatti M, Molinari F, De Dosso S, Saletti P, et al. Multi-determinants analysis of molecular alterations for predicting clinical benefit to EGFR-targeted monoclonal antibodies in colorectal cancer. PloS One (2009) 4:e7287. doi: 10.1371/journal.pone.0007287
6. Bertotti A, Papp E, Jones S, Adleff V, Anagnostou V, Lupo B, et al. The genomic landscape of response to EGFR blockade in colorectal cancer. Nature (2015) 526:263–7. doi: 10.1038/nature14969
7. Misale S, Di Nicolantonio F, Sartore-Bianchi A, Siena S, Bardelli A. Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution. Cancer Discovery (2014) 4:1269–80. doi: 10.1158/2159-8290.CD-14-0462
8. Cremolini C, Morano F, Moretto R, Berenato R, Tamborini E, Perrone F, et al. Negative hyper-selection of metastatic colorectal cancer patients for anti-EGFR monoclonal antibodies: the PRESSING case-control study. Ann Oncol Off J Eur Soc Med Oncol (2017) 28:3009–14. doi: 10.1093/annonc/mdx546
9. Sartore-Bianchi A, Amatu A, Porcu L, Ghezzi S, Lonardi S, Leone F, et al. HER2 positivity predicts unresponsiveness to EGFR-targeted treatment in metastatic colorectal cancer. Oncol (2019) 24:1395–402. doi: 10.1634/theoncologist.2018-0785
10. Morano F, Corallo S, Lonardi S, Raimondi A, Cremolini C, Rimassa L, et al. Negative hyperselection of patients with RAS and BRAF wild-type metastatic colorectal cancer who received panitumumab-based maintenance therapy. J Clin Oncol Off J Am Soc Clin Oncol (2019) 37:3099–110. doi: 10.1200/JCO.19.01254
11. Russo M, Siravegna G, Blaszkowsky LS, Corti G, Crisafulli G, Ahronian LG, et al. Tumor heterogeneity and lesion-specific response to targeted therapy in colorectal cancer. Cancer Discovery (2016) 6:147–53. doi: 10.1158/2159-8290.CD-15-1283
12. Siravegna G, Mussolin B, Buscarino M, Corti G, Cassingena A, Crisafulli G, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med (2015) 21:827. doi: 10.1038/nm0715-827b
13. Parseghian CM, Sun R, Napolitano S, et al. Rarity of acquired mutations (MTs) after first-line therapy with anti-EGFR therapy (EGFRi) (2022). Available at: https://meetinglibrary.asco.org/record/195999/abstract.
14. Dhillon AS, Hagan S, Rath O, Kolch W. MAP kinase signalling pathways in cancer. Oncogene (2007) 26:3279–90. doi: 10.1038/sj.onc.1210421
15. Chuang J, Wang C, Guo Y, Valenzuela V, Wu J, Fakih M. MAP2K1 mutations in advanced colorectal cancer predict poor response to anti-EGFR therapy and to vertical targeting of MAPK pathway. Clin Colorectal Cancer (2021) 20:72–8. doi: 10.1016/j.clcc.2020.12.003
16. Gao Y, Chang MT, McKay D, Na N, Zhou B, Yaeger R, et al. Allele-specific mechanisms of activation of MEK1 mutants determine their properties. Cancer Discovery (2018) 8:648–61. doi: 10.1158/2159-8290.CD-17-1452
17. Parikh AR, Leshchiner I, Elagina L, Goyal L, Levovitz C, Siravegna G, et al. Liquid versus tissue biopsy for detecting acquired resistance and tumor heterogeneity in gastrointestinal cancers. Nat Med (2019) 25:1415–21. doi: 10.1038/s41591-019-0561-9
18. Choi YL, Soda M, Ueno T, Hamada T, Haruta H, Yamato A, et al. Oncogenic MAP2K1 mutations in human epithelial tumors. Carcinogenesis (2012) 33:956–61. doi: 10.1093/carcin/bgs099
Keywords: MAP2K1, MEK, panitumumab, resistance mechanisms, colorectal cancer
Citation: Mauri G, Patelli G, Gori V, Lauricella C, Mussolin B, Amatu A, Bencardino K, Tosi F, Bonazzina E, Bonoldi E, Bardelli A, Siena S and Sartore-Bianchi A (2022) Case Report: MAP2K1 K57N mutation is associated with primary resistance to anti-EGFR monoclonal antibodies in metastatic colorectal cancer. Front. Oncol. 12:1030232. doi: 10.3389/fonc.2022.1030232
Received: 28 August 2022; Accepted: 19 October 2022;
Published: 07 November 2022.
Edited by:
Andreas Seeber, Innsbruck Medical University, AustriaReviewed by:
John Mariadason, Olivia Newton-John Cancer Research Institute, AustraliaCopyright © 2022 Mauri, Patelli, Gori, Lauricella, Mussolin, Amatu, Bencardino, Tosi, Bonazzina, Bonoldi, Bardelli, Siena and Sartore-Bianchi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Sartore-Bianchi, YW5kcmVhLnNhcnRvcmViaWFuY2hpQHVuaW1pLml0
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.