
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
SYSTEMATIC REVIEW article
Front. Oncol. , 20 January 2023
Sec. Thoracic Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1029738
This article is part of the Research Topic Neoadjuvant Therapy in Non-Small Cell Lung Cancer: Clinical, Pathological and Translational Research View all 15 articles
Objective: The study evaluated the effects of high-intensity interval training (HIIT) on postoperative complications and lung function in patients with lung cancer compared to usual care.
Methods: We searched electronic databases in April 2022, including PubMed, Embase, the Cochrane Library, Web of Science, and the China National Knowledge Infrastructure (CNKI). Two authors independently applied the Cochrane Risk of Bias tool to assess the quality of RCTs. The postoperative complications, length of hospitalization, and cardiopulmonary functions from the studies were pooled for statistical analysis.
Results: A total of 12 randomized controlled trials were eligible for inclusion and were conducted in the meta-analysis. HIIT significantly increased VO2peak (MD = 2.65; 95% CI = 1.70 to 3.60; I2 = 40%; P <0.001) and FEV1 (MD = 0.12; 95% CI = 0.04 to 0.20; I2 = 51%; P = 0.003) compared with usual care. A subgroup analysis of studies that applied HIIT perioperatively showed significant improvement of HIIT on FEV1 (MD = 0.14; 95% CI = 0.08 to 0.20; I2 = 36%; P <0.0001). HIIT significantly reduced the incidence of postoperative atelectasis in lung cancer patients compared with usual care (RD = −0.16; 95% CI = −0.24 to −0.08; I2 = 24%; P <0.0001). There was no statistically significant effect of HIIT on postoperative arrhythmias (RD = −0.05; 95% CI = −0.13 to 0.03; I2 = 40%; P = 0.22), length of hospitalization (MD = −1.64; 95% CI = −3.29 to 0.01; P = 0.05), and the six-minute walk test (MD = 19.77; 95% CI = −15.25 to 54.80; P = 0.27) compared to usual care.
Conclusion: HIIT may enhance VO2peak and FEV1 in lung cancer patients and reduce the incidence of postoperative atelectasis. However, HIIT may not reduce the incidence of postoperative arrhythmia, shorten the length of hospitalization, or improve the exercise performance of patients with lung cancer.
Systematic review registration: PROSPERO, CRD42022335441
According to global cancer statistics, lung cancer is one of the most diagnosed cancers, with an estimated 2.2 million new cases and 1.8 million deaths in 2022 (1). Smoking is the major cause of lung cancer, with about 80% to 90% of lung cancer cases related to smoking (2, 3). Lung cancer is divided into small-cell lung cancer (SCLC) and non-small-cell lung cancer (NSCLC), and the prevalence of NSCLC is higher, accounting for about 85% (4). There are many treatments for lung cancer, such as surgical resection, chemotherapy, and radiotherapy (5). Surgical intervention is most applicable to early-stage lung cancer diagnoses and is considered the best curative option (6). Complications adversely affect survival after lung cancer surgery. Fernandez et al. showed that complications including delirium, blood transfusion, reintubation, and pneumonia are associated with worse survival in the early period (0–180 days) (7). Respiratory problems were found to be the most common cause of lung cancer readmission within 30 days after surgery, and postoperative pulmonary complications were strongly associated with mortality within 90 days after surgery (8). A prospective observational study found that patients who underwent a lung resection with postoperative pulmonary complications had a significantly prolonged length of hospital stay post-surgery and reduced overall survival in months compared with patients without postoperative pulmonary complications (9).
Exercise has been found to be effective in improving the health condition, quality of life, and exercise capacity of patients with lung cancer after surgery (10, 11). High-intensity interval training (HIIT) is a unique training method that consists of short (<45 s) to long (2–4 min) physical activity at submaximal to all-out intensity, interspersed with passive or active recovery sessions (12). HIIT was initially used as a physical training method for athletes to improve cardiopulmonary function and has been gradually applied in the field of disease prevention and rehabilitation recently (13). Previous meta-analyses focused on the effect of HIIT on cardiorespiratory fitness in lung cancer patients, especially on peak oxygen uptake (VO2peak) (14, 15). There is a lack of studies on postoperative complications, length of hospitalization, and other cardiopulmonary function indicators in lung cancer patients (14). Therefore, in our meta-analysis, we evaluated the effects of HIIT on postoperative complications and lung function in patients with lung cancer compared to usual care.
This systematic review and meta-analysis strictly followed the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (16). We registered a protocol for this systematic review and meta-analysis on PROSPERO (registration ID is CRD42022335441).
We conducted a literature search on April 23, 2022. Five electronic databases were searched, including PubMed, Embase, Cochrane Library, Web of Science, and China National Knowledge Infrastructure (CNKI).
Identified studies were screened for eligibility if they met the following inclusion criteria: (1) patients who had been diagnosed with lung cancer; (2) the exercise protocol was defined as high-intensity interval training; (3) the HIIT group was compared with usual care or standard care; (4) only randomized controlled trials were included; and (5) all languages were available.
The exclusion criteria were as follows: (1) lack of usual care or standard care; (2) studies with missing data or outliers; (3) repeated publications; and (4) meeting abstracts.
Pulmonary function and postoperative complications were the primary outcomes of this study. Secondary outcomes included the length of hospitalization and the six-minute walk test.
Based on the inclusion and exclusion criteria, data were extracted independently by two authors. The data were extracted from each study using a standard form that included the first author, year, country, number of patients, sex percentage, age, TNM cancer stage, intervention (including HIIT protocol and the timing of HIIT), control, primary outcomes, and secondary outcomes. Any disagreements can be resolved through discussion or by having a third researcher reviewing.
Two authors independently applied the Cochrane Risk of Bias tool to assess the quality of RCTs. It contains the following aspects to assess random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases. Any disagreement was solved by consensus or by asking another researcher to reassess.
Review Manager 5.4 software was used to analyze the extracted data. For continuous outcomes, we used the mean difference (MD) and their 95% confidence intervals for the study. The risk difference (RD) and their 95% confidence intervals were applied to dichotomous outcomes. The imported data were evaluated for statistical heterogeneity. Heterogeneity was tested using the I2 statistic and the Cochrane Q statistic, as recommended by the Cochrane Handbook. I2 and the p-value for Q statistics were applied to assess the heterogeneity across included trials, and I2 >50.0% or P <0.10 was considered significant heterogeneity (17).
A total of 4,141 articles were retrieved and identified after completing the search strategy, including one additional record identified through a manual search. The number was reduced to 3,793 after removing 348 duplicates. A total of 3,746 articles were excluded by two authors who independently read the title and abstract of each article. After assessing the remaining 47 articles, 32 articles were excluded by screening the full text; the excluded reasons were as follows: no RCT (n = 1), no HIIT intervention (n = 22), review (n = 8), repeated publication (n = 3), and non-lung cancer patient (n = 1). Finally, it resulted in the inclusion of 12 articles (Figure 1).
The main characteristics of the HIIT intervention studies included in this review are presented in Table 1. The studies originated in China (18–23), Denmark (24, 25), Spain (26), Norway (27), Australia (28), and Switzerland (29). The trial sample size ranged from 15 to 218. Clinical data were collected from 926 lung cancer-related patients in our meta-analysis. The gender of patients was predominantly male (95.5% to 28.6%). However, one study did not accurately report the age and gender characteristics of the patients (25). Two studies recruited patients with non-small-cell lung cancer (NSCLC) or small-cell lung cancer (SCLC) (21, 25). One study enrolled patients with both NSCLS and chronic obstructive pulmonary disease (COPD) (22). The rest of the studies enrolled patients in the early stages of non-small-cell lung cancer (18–20, 23, 24, 26–29).
Five studies conducted HIIT interventions preoperatively (18–20, 22, 29). In contrast, three studies conducted HIIT interventions postoperatively (21, 26, 27). One study applied HIIT during post-operation or post-chemotherapy (28). Three studies applied HIIT programs during targeted therapy (23), radiotherapy (24), and chemotherapy (25), respectively.
Most studies used high-intensity interval bicycling as the intervention method; a few studies used high-intensity interval walking; and a study used high-intensity interval respiratory muscle training (26). Seven studies applied high-intensity interval training solely (18–23, 29). Three studies combined HIIT with resistance training (25, 27, 28). One study combined HIIT with aerobic training (24). In particular, one study combined high-intensity interval respiratory muscle training, resistance training, and aerobic training together (26).
(Figure 2) presents the risk of bias assessments for all 12 included studies. Four studies were deemed to have a low risk of bias (24–26, 28), and eight studies were considered to have a moderate risk of bias (18–23, 27, 29).

Figure 2 Risk of bias summary review authors’ judgments about each risk of bias item for each included study.
Seven studies supported the finding that HIIT increased VO2peak in patients with lung cancer. The result of the meta-analysis was statistically significant and favored the HIIT intervention (MD = 2.65; 95% CI = 1.70 to 3.60; I2 = 40%; P <0.001) (Figure 3). Five studies examined the effect of HIIT intervention on forced expiratory volume in 1 s (FEV1). The results showed statistical significance and favored the HIIT intervention (MD = 0.12; 95% CI = 0.04 to 0.20; I2 = 51%; P = 0.003). We conducted a subgroup analysis to solve the heterogeneity. Only studies that conducted HIIT perioperatively were included (20–22). The results still support that HIIT benefits FEV1 among patients with lung cancer compared with usual care (MD = 0.14; 95% CI = 0.08 to 0.20; I2 = 36%; P <0.0001) (Figure 4).
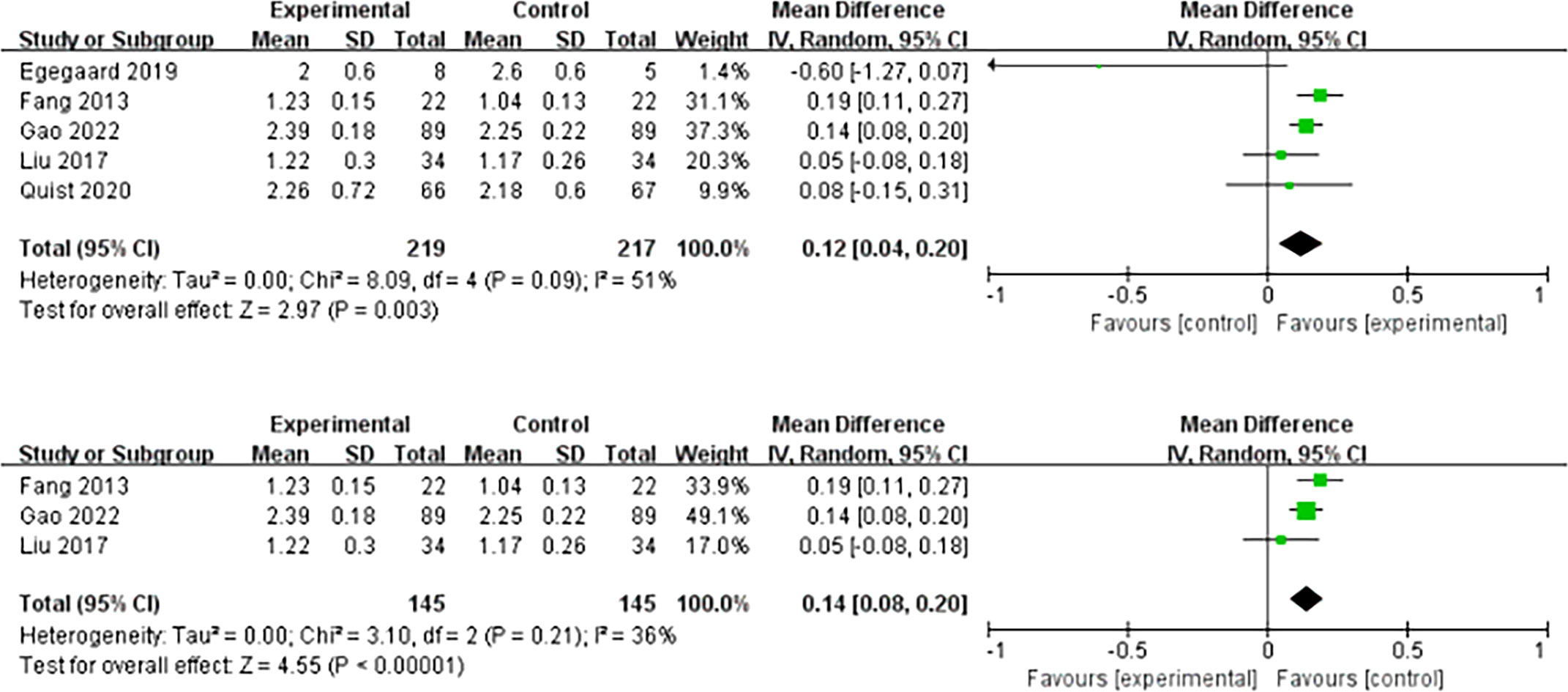
Figure 4 Meta-analysis of the effect of HIIT on FEV1 (L) and subgroup analysis among lung cancer patients.
A total of 59 patients in five studies reported atelectasis events, and the results showed that the HIIT intervention was beneficial to reduce postoperative atelectasis events in lung cancer patients compared with usual care (RD = −0.16; 95% CI = −0.24 to −0.08; I2 = 24%; P <0.0001) (Figure 5). Five studies reported arrhythmia events among 50 patients. There was no effect that HIIT had on postoperative arrhythmias in lung cancer patients compared to usual care (RD = −0.05; 95% CI = −0.13 to 0.03; I2 = 40%; P = 0.22) (Figure 6).
Five studies with 346 patients who reported length of hospitalization were included in our meta-analysis. The results showed no significant difference between the HIIT group and the usual care group (MD = −1.64; 95% CI = −3.29 to 0.01; P = 0.05). Heterogeneity was high (I2 = 77%) (Figure 7).
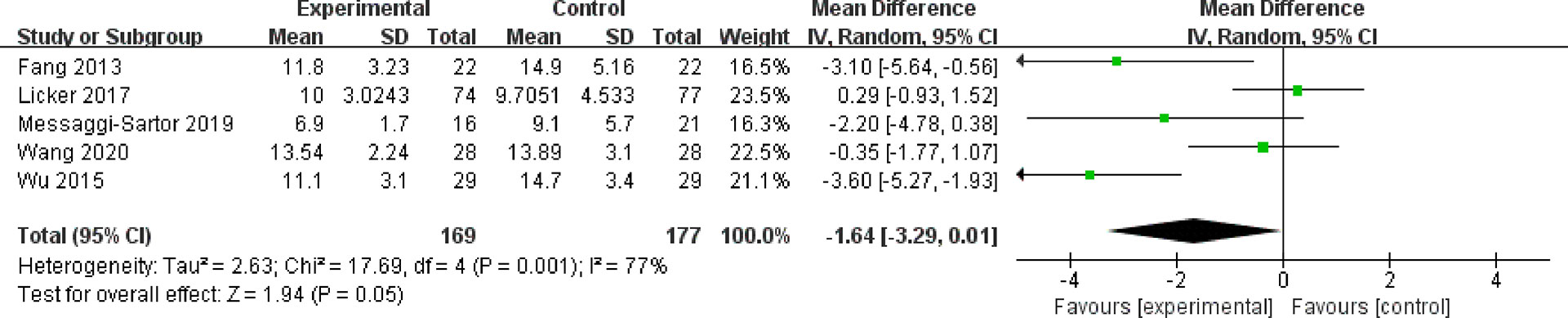
Figure 7 Meta-analysis of the effect of HIIT on length of hospitalization (days) among lung cancer patients.
In our study, five studies presented 393 patients’ 6MWT performance. The results proved no difference between the HIIT group and the usual group (MD = 19.77; 95% CI = −15.25 to 54.80; P = 0.27). Heterogeneity was high (I2 = 76%) (Figure 8).
This study examines the functional and postoperative outcomes of a high-intensity interval training intervention in lung cancer patients. With regard to pulmonary function, our results showed that both VO2peak and FEV1 improved with the application of HIIT among lung cancer patients. With regard to postoperative outcomes, the postoperative incidence of atelectasis was significantly reduced. However, there is limited evidence that HIIT does not reduce the incidence of arrhythmias. Due to the high heterogeneity of the results, it is still unclear whether the length of hospitalization was shortened, and the six-minute walk performance increased in lung cancer patients.
Previous studies mentioned that VO2peak played a key role in predicting surgical outcomes and survival in NSCLC patients (30, 31). It has been reported that HIIT induces VO2peak enhancement (32). A meta-analysis that included 305 lung cancer patients from eight studies showed that VO2peak was significantly increased by HIIT compared to usual care (14). Another study demonstrated that HIIT had a greater impact on VO2peak than usual care (15). The same results were also observed in our meta-analysis. However, the difference is that our study focused not only on lung function in lung cancer patients after HIIT rehabilitation but also on postoperative outcomes. Our results showed that HIIT could also effectively improve FEV1 and reduce the postoperative incidence of atelectasis. Interestingly, our study showed that HIIT did not reduce the incidence of arrhythmia, possibly due to postoperative arrhythmia being associated with surgical inflammation, autonomic nerve injury, and cardiac overload (33). More research is still needed.
HIIT can effectively increase muscle metabolic capacity and promote increases in muscle strength and hypertrophy, thus improving lung respiratory function and mobility in lung cancer patients (27, 28). A study demonstrated that HIIT may increase skeletal muscle mitochondrial capacity, leading to improvements in whole-body metabolic homeostasis by improving several classical markers of mitochondrial biogenesis, including the maximal activity of citrate synthase (CS) and cytochrome c oxidase (COX) as well as the total protein content of CS and COX subunits II and IV (34). Another study found that six sessions of HIIT expanded skeletal muscle mitochondria, as assessed by cytochrome c oxidase activity (35). In terms of exercise capacity, cancer-induced cachexia causes muscle atrophy in cancer patients by inhibiting muscle protein synthesis and enhancing muscle catabolism (36). In two rat models, researchers found that HIIT could lead to muscle hypertrophy by improving the IGF-I/Akt/FoxO and myostatin/Smad signal transduction pathways (37), activating the mTOR pathway, altering the expression of MuRF-1 and MAFbx proteins, and improving autophagic flux (38).
The benefits of HIIT may be influenced by the timing of exercise. Perioperative exercise training included preoperative exercise, acute post-operative (in-hospital) exercise, and postoperative exercise (39). It has been indicated that lung cancer patients who have undergone resection can benefit from preoperative exercise, which includes the improvement of both pulmonary function and exercise capacity, a lower incidence rate of postoperative complications, a shorter length of hospital stay, and a lower degree of dyspnea (40–42). Acute post-operative exercise (in-hospital) involves sitting out of bed and walking around the hospital ward, with the aim of discharge from the hospital as soon as possible (43, 44). There is no consensus on whether acute post-operative exercise improved post-operative physical activity level, mobility, or lung function (45, 46). Cavalheri et al. found that postoperative exercise enhanced exercise performance but not HRQoL and FEV1 in patients with lung cancer (47). Interestingly, a randomized controlled trial showed that early rehabilitation avoided a temporary decline in HRQoL by comparing the effect of early rehabilitation (14 days after surgery) with the late rehabilitation group (14 weeks after surgery) (48). It can be inferred that preoperative HIIT is more beneficial for patients with lung cancer (10). However, there are few studies on preoperative HIIT, and further prospective studies with large samples are needed to explore the benefits of preoperative HIIT. Limited evidence has suggested that exercise training enhances mobility and physical fitness in lung cancer patients during chemotherapy (49). Larger randomized controlled trials are warranted to prove the effect of combining exercise with targeted therapy, chemotherapy, or radiotherapy.
The exercise type of HIIT may also influence the rehabilitation of lung cancer patients after surgery. Most studies included in this meta-analysis used cycling as a type of high-intensity training. Only one study used respiratory muscle training as a type of high-intensity training (26). Laurent et al. (50) showed that lung cancer patients who accepted resection surgery decreased pulmonary postoperative complications by applying respiratory muscle training. However, further studies are needed to determine which exercise type HIIT is more favorable for patients with lung cancer. Meanwhile, the stage, subtype, and smoking habits of patients with lung cancer may also affect postoperative rehabilitation and should be considered. Considering safety and practicality, it is possibly harmful to apply HIIT at all-out intensity for individuals with severe disease (34). Low-volume HIIT is safer and has a similar improvement effect as high-volume HIIT in improving individual cardiopulmonary function (51). Low-volume HIIT is defined as repetitions range from 1 to 10 times with an active interval time of fewer than 15 min, whereas high-volume HIIT requires active intervals and repetitions of more than 15 min and four times, respectively (52). Seven studies included in our meta-analysis used low-volume HIIT (20, 21, 23–26, 29). Therefore, we assume that low-volume HIIT may be a safer way to treat patients with lung cancer, and this needs to be confirmed. In this meta-analysis, three included studies combined HIIT with resistance training (25, 27, 28), and one combined HIIT with aerobic training (24). It is still unclear whether HIIT combined with other training will have a larger effect on lung cancer patients than using HIIT solely.
In conclusion, HIIT improved pulmonary function and reduced postoperative atelectasis in patients with lung cancer. However, the incidence of postoperative arrhythmias was not decreased by HIIT. Due to high heterogeneity, shortening the length of hospitalization and enhanced exercise capacity in lung cancer patients after HIIT intervention were not supported in this meta-analysis.
As a timesaving, effective, and applicable rehabilitation method, high-intensity interval training could open a new perspective for treating lung cancer patients (14). However, the high-intensity interval training protocol remains unclear. It is necessary to determine the optimal types of exercise, the timing of using HIIT, intensity, and interval time in the future. Although some studies have obtained some results, future studies with large samples are still needed. At the same time, the different stages, subtypes, types of surgery, and smoking habits of lung cancer patients should be considered in HIIT rehabilitation, which significantly affects postoperative outcomes. Finally, the mechanism of HIIT for improving cardiopulmonary function and its effect on postoperative outcomes in patients with lung cancer should be more concentrated on by researchers.
HIIT has great potential for clinical rehabilitation of patients with lung cancer. Because of its ease of operation and low economic cost, it can effectively improve the postoperative rehabilitation of lung cancer patients and reduce their economic burden. In clinical practice, clinicians and nurses can integrate HIIT into the treatment process as a beneficial measure to improve the health status of lung cancer patients. Personalized HIIT protocols should be developed based on the different treatment methods and health status of lung cancer patients.
There are some limitations to our study, and further research is needed. First, most of the literature included in our study is a small sample, and the conclusions of these studies need to be treated with caution. Second, lung cancer patients are mainly male, and there may be differences between male and female patients. But we did not study them separately in our study. Third, although the included kinds of literature all adopted high-intensity interval training plans, the intensity and interval time of high-intensity training were different, which might affect the final results. Fourth, cycling was the main type of exercise in most of the included studies, while walking was also used. Bias can also be caused by different types of movement. Finally, warm-up and rest are equally important in HIIT planning, and some studies did not report warm-up and rest programs.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
ZC and JT conceived and planned the review, assessed the methodologic quality of the studies, verified the data, and drafted and revised the manuscript. JJ assessed the methodologic quality of the studies. DG extracted data. FL and JL conducted the literature search and selected studies. JT provided methodologic advice, content expertise, and revised the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Popat S, Liu SV, Scheuer N, Gupta A, Hsu GG, Ramagopalan SV, et al. Association between smoking history and overall survival in patients receiving pembrolizumab for first-line treatment of advanced non-small cell lung cancer. JAMA Netw Open (2022) 5(5):e2214046. doi: 10.1001/jamanetworkopen.2022.14046
3. Condoluci A, Mazzara C, Zoccoli A, Pezzuto A, Tonini G. Impact of smoking on lung cancer treatment effectiveness: A review. Future Oncol (London England) (2016) 12(18):2149–61. doi: 10.2217/fon-2015-0055
4. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2020. CA: Cancer J Clin (2020) 70(1):7–30. doi: 10.3322/caac.21590
5. Miller M, Hanna N. Advances in systemic therapy for non-small cell lung cancer. BMJ (Clinical Res ed) (2021) 375:n2363. doi: 10.1136/bmj.n2363
6. Hoy H, Lynch T, Beck M. Surgical treatment of lung cancer. Crit Care Nurs Clinics North America (2019) 31(3):303–13. doi: 10.1016/j.cnc.2019.05.002
7. Fernandez FG, Kosinski AS, Furnary AP, Onaitis M, Kim S, Habib RH, et al. Differential effects of operative complications on survival after surgery for primary lung cancer. J Thorac Cardiovasc Surg (2018) 155(3):1254–64 e1. doi: 10.1016/j.jtcvs.2017.09.149
8. Oswald N, Halle-Smith J, Kerr A, Webb J, Agostini P, Bishay E, et al. Perioperative immune function and pain control may underlie early hospital readmission and 90 day mortality following lung cancer resection: A prospective cohort study of 932 patients. Eur J Surg Oncol: J Eur Soc Surg Oncol Br Assoc Surg Oncol (2019) 45(5):863–9. doi: 10.1016/j.ejso.2019.02.001
9. Lugg ST, Agostini PJ, Tikka T, Kerr A, Adams K, Bishay E, et al. Long-term impact of developing a postoperative pulmonary complication after lung surgery. Thorax (2016) 71(2):171–6. doi: 10.1136/thoraxjnl-2015-207697
10. Ni HJ, Pudasaini B, Yuan XT, Li HF, Shi L, Yuan P. Exercise training for patients pre- and postsurgically treated for non-small cell lung cancer: A systematic review and meta-analysis. Integr Cancer Therapies (2017) 16(1):63–73. doi: 10.1177/1534735416645180
11. Zhou W, Woo S, Larson JL. Effects of perioperative exercise interventions on lung cancer patients: An overview of systematic reviews. J Clin Nurs (2020) 29(23-24):4482–504. doi: 10.1111/jocn.15511
12. Buchheit M, Laursen PB. High-intensity interval training, solutions to the programming puzzle. Sports Med (2013) 43(10):927–54. doi: 10.1007/s40279-013-0066-5
13. Guiraud T, Nigam A, Gremeaux V, Meyer P, Juneau M, Bosquet L. High-intensity interval training in cardiac rehabilitation. Sports Med (2012) 42(7):587–605. doi: 10.2165/11631910-000000000-00000
14. Heredia-Ciuró A, Fernández-Sánchez M, Martín-Núñez J, Calvache-Mateo A, Rodríguez-Torres J, López-López L, et al. High-intensity interval training effects in cardiorespiratory fitness of lung cancer survivors: A systematic review and meta-analysis. Supportive Care Cancer: Off J Multinational Assoc Supportive Care Cancer (2022) 30(4):3017–27. doi: 10.1007/s00520-021-06647-2
15. Wallen MP, Hennessy D, Brown S, Evans L, Rawstorn JC, Wong Shee A, et al. High-intensity interval training improves cardiorespiratory fitness in cancer patients and survivors: A meta-analysis. Eur J Cancer Care (2020) 29(4):e13267. doi: 10.1111/ecc.13267
16. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The prisma statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ (Clinical Res ed) (2009) 339:b2700. doi: 10.1136/bmj.b2700
17. Deeks JJ, Higgins JP, Altman DG. Chapter 10: Analysing data and undertaking meta-analyses. Cochrane Handbook for Systematic Reviews of Interventions, Version 6.2 Higgins J. P. T., Thomas J., Chandler J., Cumpston M., Li T., Page M. J., et al Eds. (2008). (Lancaster, PA, Cochrane: University of Lancaster) Available at: www.training.cochrane.org/handbook.
18. Wu WB, Lyu WQ, Huang HD, Zeng GQ. Effect of short term medium and high-intensity lower limb exercise training before surgery on the tolerance of low lung function lung cancer. Modern Diagnosis Treat (2015) 26(23):5436–8.
19. Wang YQ. Effect of preoperative high-intensity interval training combined with team empowerment education on patients with lung cancer [master’s thesis]. (Changchun (Jilin), China: Jilin University) (2020).
20. Liu LF, Sha YS, Sun XN, Wang HN. Effect of surgical tolerance in lung cancer patients with impaired pulmonary function for rehabilitation training stairs exercise. Tianjin J Nurs (2017) 25(02):113–5.
21. Gao ML, Gu YJ, Wu Q. Application of high intensity interval training in patients with lung cancer. J Clin Pulmon Med (2022) 27(02):272–5+89.
22. Fang Y, Zhao QY, Huang DF, Guan SF, Shen J. The impact of exercise training on surgery tolerability in lung cancer patients with impaired pulmonary function. Chin J Rehabil Med (2013) 28(07):619–23.
23. Hwang CL, Yu CJ, Shih JY, Yang PC, Wu YT. Effects of exercise training on exercise capacity in patients with non-small cell lung cancer receiving targeted therapy. Supportive Care Cancer: Off J Multinational Assoc Supportive Care Cancer (2012) 20(12):3169–77. doi: 10.1007/s00520-012-1452-5
24. Egegaard T, Rohold J, Lillelund C, Persson G, Quist M. Pre-radiotherapy daily exercise training in non-small cell lung cancer: A feasibility study. Rep Pract Oncol Radiother (2019) 24(4):375–82. doi: 10.1016/j.rpor.2019.06.003
25. Quist M, Langer SW, Lillelund C, Winther L, Laursen JH, Christensen KB, et al. Effects of an exercise intervention for patients with advanced inoperable lung cancer undergoing chemotherapy: A randomized clinical trial. Lung Cancer (Amsterdam Netherlands) (2020) 145:76–82. doi: 10.1016/j.lungcan.2020.05.003
26. Messaggi-Sartor M, Marco E, Martinez-Tellez E, Rodriguez-Fuster A, Palomares C, Chiarella S, et al. Combined aerobic exercise and high-intensity respiratory muscle training in patients surgically treated for non-small cell lung cancer: A pilot randomized clinical trial. Eur J Phys Rehabil Med (2019) 55(1):113–22. doi: 10.23736/S1973-9087.18.05156-0
27. Edvardsen E, Skjonsberg OH, Holme I, Nordsletten L, Borchsenius F, Anderssen SA. High-intensity training following lung cancer surgery: A randomised controlled trial. Thorax (2015) 70(3):244–50. doi: 10.1136/thoraxjnl-2014-205944
28. Cavalheri V, Jenkins S, Cecins N, Gain K, Phillips MJ, Sanders LH, et al. Exercise training for people following curative intent treatment for non-small cell lung cancer: A randomized controlled trial. Braz J Phys Ther (2017) 21(1):58–68. doi: 10.1016/j.bjpt.2016.12.005
29. Licker M, Karenovics W, Diaper J, Fresard I, Triponez F, Ellenberger C, et al. Short-term preoperative high-intensity interval training in patients awaiting lung cancer surgery: A randomized controlled trial. J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer (2017) 12(2):323–33. doi: 10.1016/j.jtho.2016.09.125
30. Brunelli A, Kim AW, Berger KI, Addrizzo-Harris DJ. Physiologic evaluation of the patient with lung cancer being considered for resectional surgery: Diagnosis and management of lung cancer, 3rd Ed: American college of chest physicians evidence-based clinical practice guidelines. Chest (2013) 143(5 Suppl):e166S–e90S. doi: 10.1378/chest.12-2395
31. Gravier FE, Smondack P, Prieur G, Medrinal C, Combret Y, Muir JF, et al. Effects of exercise training in people with non-small cell lung cancer before lung resection: A systematic review and meta-analysis. Thorax (2022) 77(5):486–96. doi: 10.1136/thoraxjnl-2021-217242
32. Astorino TA, Edmunds RM, Clark A, King L, Gallant RA, Namm S, et al. High-intensity interval training increases cardiac output and V O2max. Med Sci Sports Exerc (2017) 49(2):265–73. doi: 10.1249/MSS.0000000000001099
33. Raman T, Roistacher N, Liu J, Zhang H, Shi W, Thaler HT, et al. Preoperative left atrial dysfunction and risk of postoperative atrial fibrillation complicating thoracic surgery. J Thorac Cardiovasc Surg (2012) 143(2):482–7. doi: 10.1016/j.jtcvs.2011.08.025
34. Little JP, Safdar A, Wilkin GP, Tarnopolsky MA, Gibala MJ. A practical model of low-volume high-intensity interval training induces mitochondrial biogenesis in human skeletal muscle: Potential mechanisms. J Physiol (2010) 588(Pt 6):1011–22. doi: 10.1113/jphysiol.2009.181743
35. Jacobs RA, Fluck D, Bonne TC, Burgi S, Christensen PM, Toigo M, et al. Improvements in exercise performance with high-intensity interval training coincide with an increase in skeletal muscle mitochondrial content and function. J Appl Physiol (1985) 2013) 115(6):785–93. doi: 10.1152/japplphysiol.00445.2013
36. Cortiula F, Hendriks LEL, van de Worp W, Schols A, Vaes RDW, Langen RCJ, et al. Physical exercise at the crossroad between muscle wasting and the immune system: Implications for lung cancer cachexia. J Cachexia Sarcopenia Muscle (2022) 13(1):55–67. doi: 10.1002/jcsm.12900
37. Biglari S, Afousi AG, Mafi F, Shabkhiz F. High-intensity interval training-induced hypertrophy in gastrocnemius muscle Via improved igf-I/Akt/Foxo and Myostatin/Smad signaling pathways in rats. Physiol Int (2020) 107(2):220–30. doi: 10.1556/2060.2020.00020
38. Cui X, Zhang Y, Wang Z, Yu J, Kong Z, Ruzic L. High-intensity interval training changes the expression of muscle ring-finger protein-1 and muscle atrophy f-box proteins and proteins involved in the mechanistic target of rapamycin pathway and autophagy in rat skeletal muscle. Exp Physiol (2019) 104(10):1505–17. doi: 10.1113/EP087601
39. Cavalheri V, Granger CL. Exercise training as part of lung cancer therapy. Respirol (Carlton Vic) (2020) 25 Suppl 2:80–7. doi: 10.1111/resp.13869
40. Sebio Garcia R, Yáñez Brage MI, Giménez Moolhuyzen E, Granger CL, Denehy L. Functional and postoperative outcomes after preoperative exercise training in patients with lung cancer: A systematic review and meta-analysis. Interact Cardiovasc Thorac Surg (2016) 23(3):486–97. doi: 10.1093/icvts/ivw152
41. Rosero ID, Ramirez-Velez R, Lucia A, Martinez-Velilla N, Santos-Lozano A, Valenzuela PL, et al. Systematic review and meta-analysis of randomized, controlled trials on preoperative physical exercise interventions in patients with non-Small-Cell lung cancer. Cancers (Basel) (2019) 11(7). doi: 10.3390/cancers11070944
42. Cavalheri V, Granger C. Preoperative exercise training for patients with non-small cell lung cancer. Cochrane Database Syst Rev (2017) 6(6):Cd012020. doi: 10.1002/14651858.CD012020.pub2
43. Granger CL. Physiotherapy management of lung cancer. J Physiother (2016) 62(2):60–7. doi: 10.1016/j.jphys.2016.02.010
44. Agostini P, Lugg ST, Adams K, Vartsaba N, Kalkat MS, Rajesh PB, et al. Postoperative pulmonary complications and rehabilitation requirements following lobectomy: A propensity score matched study of patients undergoing video-assisted thoracoscopic surgery versus thoracotomydagger. Interact Cardiovasc Thorac Surg (2017) 24(6):931–7. doi: 10.1093/icvts/ivx002
45. Jonsson M, Hurtig-Wennlof A, Ahlsson A, Vidlund M, Cao Y, Westerdahl E. In-hospital physiotherapy improves physical activity level after lung cancer surgery: A randomized controlled trial. Physiotherapy (2019) 105(4):434–41. doi: 10.1016/j.physio.2018.11.001
46. Jonsson M, Ahlsson A, Hurtig-Wennlof A, Vidlund M, Cao Y, Westerdahl E. In-hospital physiotherapy and physical recovery 3 months after lung cancer surgery: A randomized controlled trial. Integr Cancer Therapies (2019) 18:1534735419876346. doi: 10.1177/1534735419876346
47. Cavalheri V, Tahirah F, Nonoyama M, Jenkins S, Hill K. Exercise training for people following lung resection for non-small cell lung cancer - a cochrane systematic review. Cancer Treat Rev (2014) 40(4):585–94. doi: 10.1016/j.ctrv.2013.11.001
48. Sommer MS, Vibe-Petersen J, Staerkind MB, Langer SW, Larsen KR, Trier K, et al. Early initiated postoperative rehabilitation enhances quality of life in patients with operable lung cancer: Secondary outcomes from a randomized trial. Lung Cancer (Amsterdam Netherlands) (2020) 146:285–9. doi: 10.1016/j.lungcan.2020.06.023
49. Rutkowska A, Jastrzebski D, Rutkowski S, Zebrowska A, Stanula A, Szczegielniak J, et al. Exercise training in patients with non-small cell lung cancer during in-hospital chemotherapy treatment: A randomized controlled trial. J Cardiopulmon Rehabil Prev (2019) 39(2):127–33. doi: 10.1097/HCR.0000000000000410
50. Laurent H, Aubreton S, Galvaing G, Pereira B, Merle P, Richard R, et al. Preoperative respiratory muscle endurance training improves ventilatory capacity and prevents pulmonary postoperative complications after lung surgery. Eur J Phys Rehabil Med (2020) 56(1):73–81. doi: 10.23736/S1973-9087.19.05781-2
51. Tjonna AE, Leinan IM, Bartnes AT, Jenssen BM, Gibala MJ, Winett RA, et al. Low- and high-volume of intensive endurance training significantly improves maximal oxygen uptake after 10-weeks of training in healthy men. PloS One (2013) 8(5):e65382. doi: 10.1371/journal.pone.0065382
Keywords: HIIT, high-intensity interval training, lung cancer, postoperative outcome, lung function
Citation: Chen Z, Jia J, Gui D, Liu F, Li J and Tu J (2023) Functional and postoperative outcomes after high-intensity interval training in lung cancer patients: A systematic review and meta-analysis. Front. Oncol. 12:1029738. doi: 10.3389/fonc.2022.1029738
Received: 27 August 2022; Accepted: 31 December 2022;
Published: 20 January 2023.
Edited by:
Fan Yang, Peking University People’s Hospital, ChinaCopyright © 2023 Chen, Jia, Gui, Liu, Li and Tu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiayuan Tu, dGp5dGp5ODhAMTYzLmNvbQ==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.