
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 14 November 2022
Sec. Cancer Genetics
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1027696
This article is part of the Research Topic Case Reports in Cancer Genetics : 2022 View all 16 articles
Angiosarcoma (AS) is a rare, clinically aggressive tumor with limited treatment options and a poor prognosis. Mutations involving the angiogenesis-related genesTP53, PTPRB, PLCG1, KDR as well as FLT4 amplification have been observed in AS. There is a potential therapeutic value of inhibition of the VEGF pathway against angiosarcoma. Our case first described a patient with two sites of cutaneous angiosarcomas (cASs) that responded differently to anlotinib. And genetic analysis revealed that those two sites had different FLT4 variants, suggesting that FLT4 amplification could be the cause of anlotinib non-response.
Angiosarcomas (ASs) are rare, aggressive tumors of vascular or lymphatic endothelial cell origin that account for about 2%–3% of all adult soft tissue sarcomas. They can arise from anywhere on the body, with cutaneous angiosarcomas (about 60% of all cases) involving the head and neck, particularly the scalp, followed by the breast, extremity, trunk, liver, and other sites (1, 2). ASs can be divided into primary cutaneous (in the absence of lymphoedema or radiation), parenchymal tissue/visceral (which includes primary breast lesions), deep soft tissue, and lymphoedema-associated and post-radiation angiosarcomas, according to lesion location and underlying risk factors (1, 3). The prognosis of ASs is poor, with approximately 16%–44% of advanced or metastatic patients presenting at the time of diagnosis (2, 4). ASs have a 5-year survival rate of 30%–40% with overall survival ranging from 6 to 16 months depending on tumor stage, surgical resection, and distant metastases (5, 6).
When compared to Western countries, approximately 50%–60% of cutaneous angiosarcomas (cASs) originate in the head and neck, with Asians involving up to 90% of the scalp and face (7). cASs are more common in elderly men between the ages of 60 and 70 who have distinct clinical causative factors, such as chronically sun-damaged skin or chronic lymphedema/irradiated skin (8). Patients with angiosarcoma of the head and neck have a higher tumor mutation burden and a dominant UV damage mutational signature (9, 10).
Surgical resection with wide excisional margins is the main therapy for R0 resection cASs, but due to insufficient surgical excision and the infiltrative growth pattern, local recurrences or metastases are common, particularly in head and neck locations, with 5-year overall survival of 10%–15% (11, 12). Several studies revealed that age, tumor size, tumor location, resection with positive margins, and advanced stage are the poor prognosis factors for cASs (13–15).
Amplifications of MYC, KLT4, FLT4, and recurrent mutations in TP53, PLCG1 (R707), and PTPRB, as well as genetic alterations in the mitogen-activated protein kinase (MAPK) pathway, have been reported in ASs (9, 16–19). Many antiangiogenic targeted agents, including vascular endothelial growth factor/receptor inhibitors, such as bevacizumab (20), or multi-kinase inhibitors, pazopanib (21, 22), and sunitinib, have demonstrated clinical efficacy in ASs, including cASs, when used alone or in combination with standard therapies. Clinical success with angiogenesis targeting agents, on the other hand, has so far been lacking.
In December 2018, a 73-year-old male patient discovered on his own a swelling in the left parotid area, initially the size of peanut rice, with local pressure pain but no fever, facial palsy, or taste disturbance. One month later, the swelling had grown to the size of a small date and was diagnosed as a “parotid cyst” at an outside hospital and underwent “left parotid abscess excision.” In March 2019, the patient visited our hospital for a reexamination. The CT scan revealed an irregular mass in the posterior and inferior portion of the left parotid gland, measuring 3.7 × 3.4 cm. This mass had uneven density, mild to moderate enhancement, uneven edges, a poor boundary with the parotid gland, and local skin thickening. Pathological findings postoperatively showed that vascular endothelial derived cells were responsible for the infection, and the immunohistochemical staining showed that CD31 was diffusely positive and the Ki-67 proliferation index was 50%–60%, whereas CD34 was negative (Figures 1A–C), with lymphoid and plasma cell infiltration and bleeding. The presence of two lymph nodes surrounding the tissue led to a preliminary diagnosis of poorly differentiated AS.
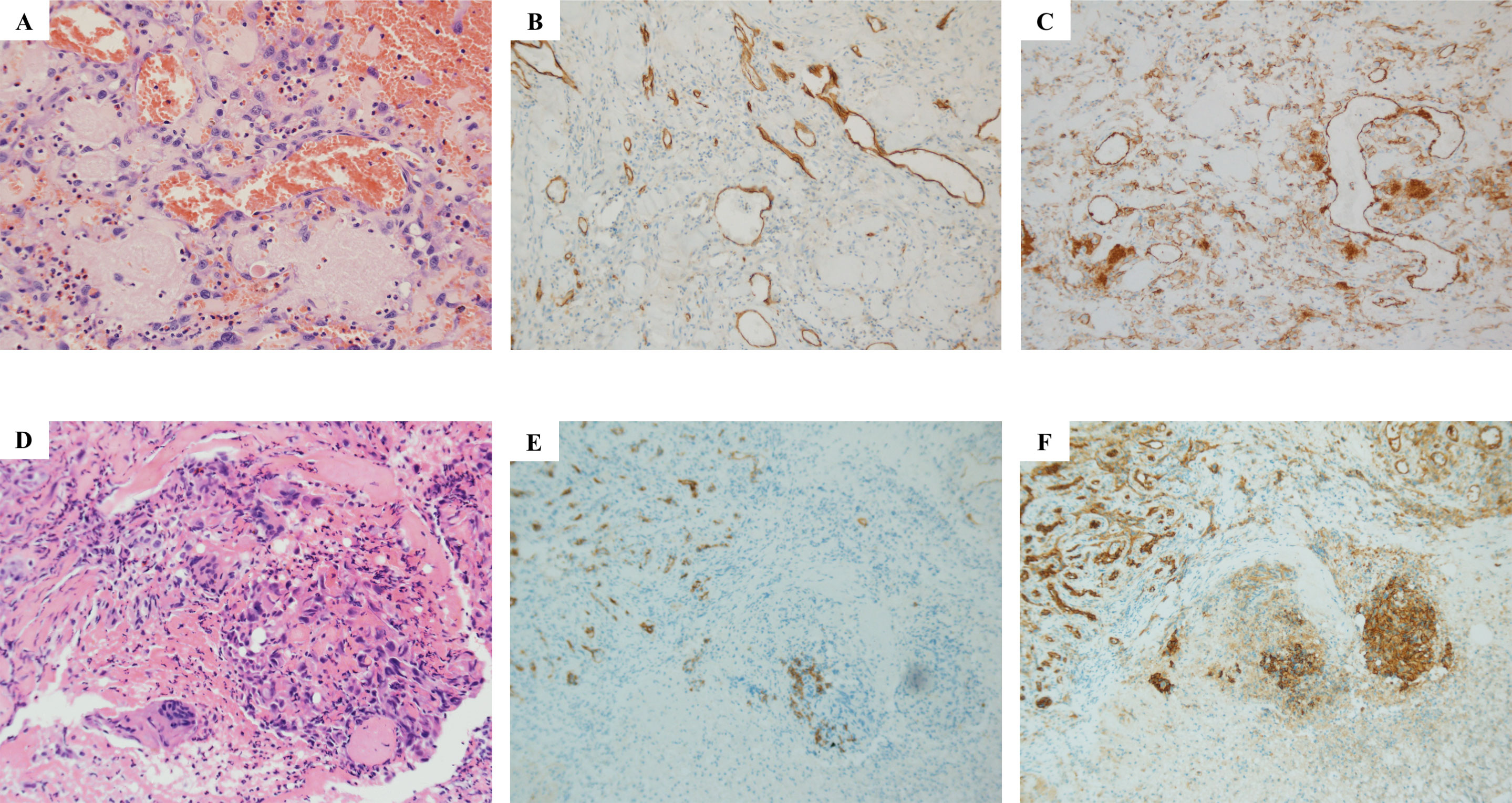
Figure 1 Histopathological examination results of parotid gland and scalp metastasis. Hematoxylin–eosin (HE) staining showed that salivary gland tissue was found in the sample tissue of the left parotid gland mass, and fibrous tissue hyperplasia was observed in some areas (A–C) Atypical cells were seen near the granulation tissue, which was indicated by immunophenotype as endovascular cells, and infiltration of lymphocytes and plasma cells was also observed, accompanied by bleeding (A). Immunohistochemical results showed CD34 negative (B) and CD31 positive (C). As for the HE result of scalp metastasis (D, E), squamous mucosa, epithelial erosion, a small amount of increased cytoplasmic ratio, irregular karyotype, and trace lesion tissue (D) were shown. Immunohistochemical results showed CD34 negative (E) and CD31 positive (F).
However, a relapse of the local bump behind the left ear 2 months after the operation. Meanwhile, a lesion appeared on the scalp. The scalp was broken locally, the wound was poorly healed, and the surrounding tissues were eroded. A local biopsy revealed CD31, ERG, and Ki-67 were positive, P53 was partially positive, while CK, CD34, CD117, CEA, and P40 were negative, indicating that this was a metastasis (Figures 1D–F). Given the size of the lesion, the patient’s old age, and physical condition, surgery and chemotherapy were risky treatments, and the patient made an informed decision to decline them. So anlotinib, a multi-targeted tyrosine kinase inhibitor approved as second-line treatment for advanced soft-tissue sarcoma, was recommended after complete informed consent in July 2019. During the treatment, the tumor behind the left ear was quickly relieved until it disappeared (Figures 2A–E), but anlotinib was not effective for scalp metastasis, and the lesions continued to deteriorate until they were uncontrollable (Figures 2F–O). This patient died in May 2020 without a relapse behind the left ear.
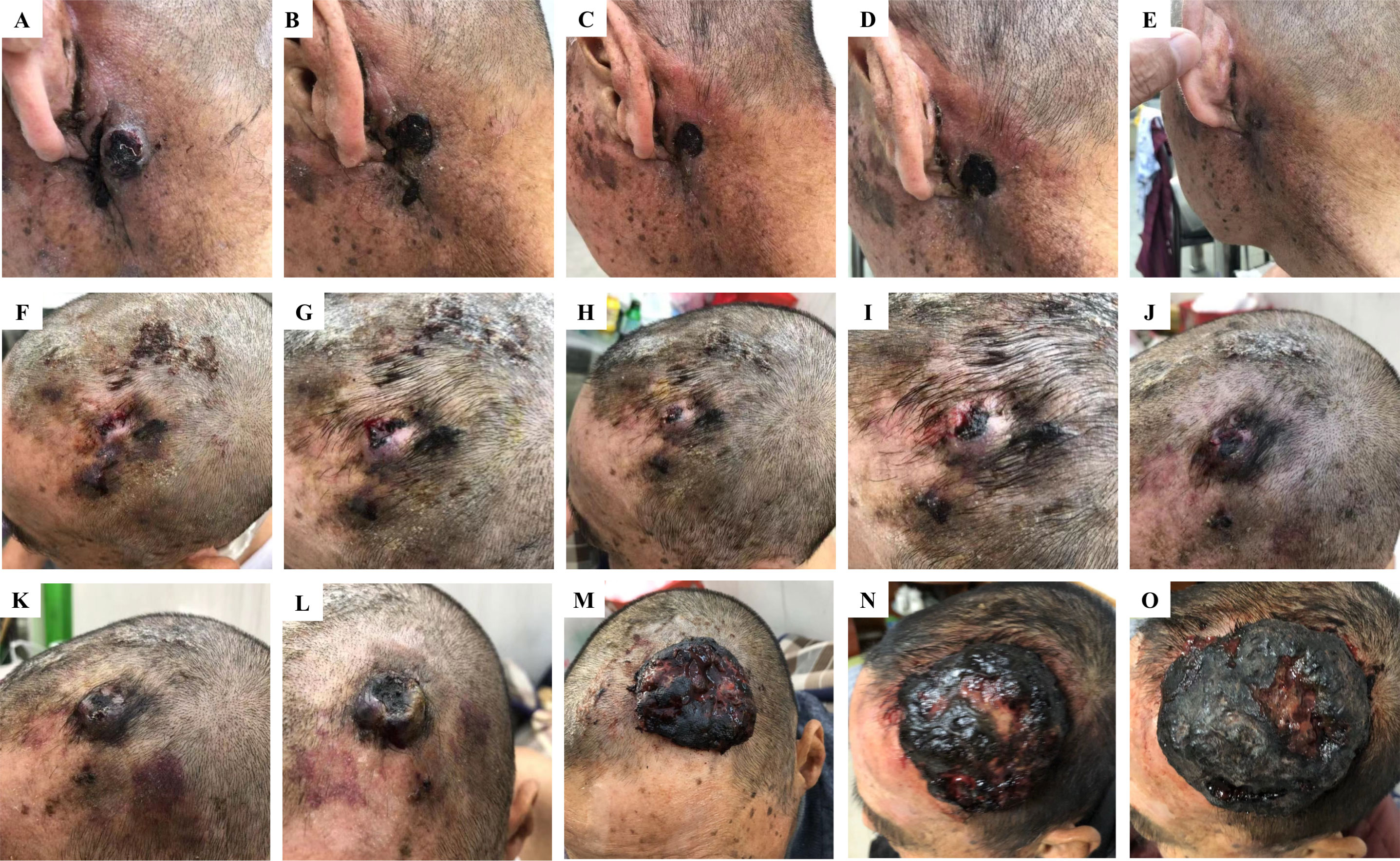
Figure 2 Different responses of retro auricular and scalp angiosarcoma metastases to anlotinib treatment. Changes in retro auricular recurrence and overhead metastases during anlotinib treatment on 13 July, 22 July, 25 July, 28 July, and 1 August 2019, respectively. Among them, the retro auricular tumor was relieved quickly after anlotinib treatment, and the bump was gradually reduced to disappear, achieving recovery (A–E). However, the scalp metastasis of the same period was not effective, and the lesion gradually deteriorated until it was uncontrollable (F–O). Continuous deterioration of the scalp metastatic during anlotinib maintenance on 4 August (K), 12 August (L), 8 October (M), 19 November (N), and 10 December 2019 (O).
There was a significant difference in the response to anlotinib between recurrent parotid lesions and scalp metastases. We sequenced the scalp metastatic and the surgically resected primary tissues by Next Generation Sequencing (NGS). The sequencing results showed that the primary lesions only had the FLT4 p.G1276E mutation. However, in addition to the FLT4 p.G1276E mutation, scalp metastasis also exhibited FLT4 amplification and mutations of FLT1/ARID1A/CHEK2/IRS1 (Table 1).
Identification of genomic alterations to confirm predictive biomarkers for anti-angiogenesis therapy is always on the way. The predictive value of the FLT4 F131S mutation to regorafenib was not ruled out because of its extracellular location and complicated genomic profiles (23). Although a 6-month clinical stable response to pazopanib caught our attention in VEGFR-2 or 3 amplified angiosarcoma (24), we should not ignore the potential responsive mechanism of the VEGFR3 R1070L mutation in the tyrosine kinase domain. In our description, the unique and pure FLT4 G1276E mutation may be a reasonable explanation for this rapid and durable response to anlotinib. The amplification of FLT4 by metastatic lesions may be the reason for the unresponsiveness after constructional analysis of other gene alterations. Further study is ongoing and warranted.
AS is a rare, highly heterogeneous sarcoma with a variety of clinical characteristics. The prognosis of ASs is poor, with a 5-year survival rate of only 30%–40% from primary diagnosis, but once metastasized, the average survival period is less than 1 year. Based on the stage and the location, radical surgery remains the curative treatment for Ass, and adjuvant radiochemotherapy is mainly used to treat locally advanced or progressive patients (6). Targeted agents, including mono-target antibodies, bevacizumab, or multi-target small molecules in tyrosine kinases, pazopanib, and regorafenib, have shown significant clinical efficacy (25). There are some immunotherapy drugs, and combination strategies are being investigated in various subtypes of soft tissue sarcomas, including ASs (26). Due to the complex correlation between drug efficiency and pathological subtype heterogeneity as well as genetic characteristics, treatment options for ASs remain limited (27).
Anlotinib is a new oral molecular multi-target tyrosine kinase inhibitor (TKI) that targets vascular endothelial growth factor receptor (VEGFR) 1, VEGFR3, VEGFR2/KDR, platelet-derived growth factor receptor a (PDGFR-a), c-Kit, and fibroblast growth factor receptor (FGFR) 1–3 and inhibits tumor angiogenesis and tumor cell proliferation. It has been approved as a third-line treatment for patients with advanced non-small-cell lung cancer (NSCLC) (28). Based on a preclinical study, anlotinib showed high selectivity for VEGF family members, especially VEGFR2 and VEGFR3, with IC50 values of 0.2 and 0.7 nmol/L, showing high selectivity and inhibitory potency to sunitinib (29). A phase 2 clinical trial involving 166 patients to evaluate anlotinib in recurrent metastatic soft tissue sarcomas found that the overall 12-week PFS and ORR rates were 68% and 13%, respectively, with easily controllable adverse events (30). More trials are being conducted to investigate the efficacy of anlotinib monotherapy, anlotinib plus immunotherapy, and anlotinib plus chemotherapy in advanced sarcomas, particularly soft tissue sarcomas (31). So far, there have been few reports on the efficacy of anlotinib treatment in ASs, with only a few case reports referring to various lesions in head and neck AS (32) and cardiac AS (33) or combined immunotherapy in metastatic primary splenic AS (34). In the case we present, the patient experienced local recurrence in the ear as well as scalp metastases following surgery. Anlotinib was an option chosen when the patient declined chemotherapy and surgery. However, the two lesions respond differently to treatment, with the lesion behind the ear disappearing quickly and the lesion on the scalp worsening. FLT4 p.G1276E mutation rate doubled, and newly added FLT4 amplification as well as point mutation of FLT1/ARID1A/CHEK2/IRS1, according to genetic alterations from primary and metastatic tissues.
The FLT4 gene encodes VEGFR3 and is involved in lymphatic differentiation. It has been reported that inappropriate genetic abnormalities of VEGFR signaling are critical in the genesis of angiosarcomas (35, 36). FLT4 gene amplification, along with MYC, is present in approximately 25% of secondary angiosarcomas (17). A case report described a 6-month clinically stable response to pazopanib in an angiosarcoma patient with VEGFR-2, VEGFR-3 amplification, and a novel VEGFR3 R1070L mutation (24). Arnaud-Coffin et al. (37) also reported a partial response to pazopanib in an FLT4 amplified angiosarcoma patient, but with a worse PFS of 3.1 months. FLT 4 amplification was also reported to be a poor prognostic factor for ASs (38). Several basket trials with FLT4 gene amplification subgroups are also being conducted (NCT02693535, NCT02029001, NCT03297606). The FLT1 gene encodes VEGFR1 and is a member of the VEGFR family. The point mutation c.542G>A was found in FLT1’s Ig-like domain 2, which is a high-affinity ligand-binding region (29). Some studies have found that soluble FLT1 expression is associated with poor clinical outcomes and may be a biomarker of intrinsic resistance to anti-angiogenic therapies that selectively inhibit the VEGFR-2 signaling pathway (39). However, no studies have been conducted to investigate the impact of FLT1 extracellular domain mutations on the efficacy of kinase inhibitor therapy.
ARID1A encodes BAF250A, which is a member of the SWI/SNF chromatin-remodeling complex. ARID1A is a tumor suppressor, and most mutations seen in cancers are frameshift or nonsense mutations that result in inactivation and reduced expression of ARID1A (40). The overall mutation rate of ARID1A in cancers is about 6%, with clear-cell ovarian cancer (45%) and endometrial cancer (37%) being the most common (41, 42). There is a lot of evidence that ARID1A loss-of-function mutations can activate the PI3K/Akt/mTORpathway (43, 44) and increase the sensitivity of ARID1A-deficient cells to treatment with the PI3K/AKT inhibitor in gastric cancer cells (45) or cholangiocarcinoma (46). Checkpoint kinase 2 (CHEK2) is a cancer susceptibility gene that codes for the serine/threonine CHK2 kinase involved in DNA damage response (DDR). A higher frequency of germline mutations is significantly associated with breast and ovarian cancers (47). Insulin receptor substrate-1 (IRS1) is a mediator of oncogenic IGF signaling and is overexpressed in a variety of malignant tumor types, where it also mediates EGFR or mTOR inhibitor resistance (48–50). Although these mutations are linked to sarcoma development to some extent, there have been no reports of these mutations being linked to sarcoma-targeted therapy resistance.
In our case, FLT4 p.G1276E is a novel mutation localized in the carboxyterminal domain of VEGFR3 that may be the primary reason for the response to anlotinib in the recurrence site, but the FLT4 amplification in the metastatic site may be a worse prognosis factor and showed no response to anlotinib. Our findings may broaden the spectrum of the FLT4 gene mutations while also providing guidance for clinical anlotinib treatment of local recurrence and metastatic ASs.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
YiG treated and observed the patient. YuG, JM, and YiG collected the clinical information, diagnostic information, therapeutic information, and images of the patients. JL performed the histopathological and immunohistochemical examinations. YiG, YuG, JM, and JL prepared the manuscript and the literature search. YJ, XY, and TS performed data analysis and interpretation. YiG and JM reviewed and edited the manuscript. All authors contributed to the article and approved the submitted version.
Authors YJ, XY, and TS are employed by Jiangsu Simcere Diagnostics Co., Ltd.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Young RJ, Brown NJ, Reed MW, Hughes D, Woll PJ. Angiosarcoma. Lancet Oncol (2010) 11(10):983–91. doi: 10.1016/S1470-2045(10)70023-1
2. Singla S, Papavasiliou P, Powers B, Gaughan J, von Mehren M, Watson JC, et al. Challenges in the treatment of angiosarcoma: a single institution experience. Am J Surg (2014) 208(2):254–9. doi: 10.1016/j.amjsurg.2014.01.007
3. Gaballah AH, Jensen CT, Palmquist S, Pickhardt PJ, Duran A, Broering G, et al. Angiosarcoma: Clinical and imaging features from head to toe. Br J Radiol (2017) 90(1075):20170039. doi: 10.1259/bjr.20170039
4. Buehler D, Rice SR, Moody JS, Rush P, Hafez GR, Attia S, et al. Angiosarcoma outcomes and prognostic factors: a 25-year single institution experience. Am J Clin Oncol (2014) 37(5):473–9. doi: 10.1097/COC.0b013e31827e4e7b
5. Cao J, Wang J, He C, Fang M. Angiosarcoma: a review of diagnosis and current treatment. Am J Cancer Res (2019) 9(11):2303–13.
6. Boichard A, Wagner MJ, Kurzrock R. Angiosarcoma heterogeneity and potential therapeutic vulnerability to immune checkpoint blockade: insights from genomic sequencing. Genome Med (2020) 12(1):61. doi: 10.1186/s13073-020-00753-2
7. Farid M, Ong WS, Lee MJ, Jeevan R, Ho ZC, Sairi AN, et al. Cutaneous versus non-cutaneous angiosarcoma: clinicopathologic features and treatment outcomes in 60 patients at a single Asian cancer centre. Oncology (2013) 85(3):182–90. doi: 10.1159/000354215
8. Ronchi A, Cozzolino I, Zito Marino F, De Chiara A, Argenziano G, Moscarella E, et al. Primary and secondary cutaneous angiosarcoma: Distinctive clinical, pathological and molecular features. Ann Diagn Pathol (2020) 48:151597. doi: 10.1016/j.anndiagpath.2020.151597
9. Painter CA, Jain E, Tomson BN, Dunphy M, Stoddard RE, Thomas BS, et al. The angiosarcoma project: enabling genomic and clinical discoveries in a rare cancer through patient-partnered research. Nat Med (2020) 26(2):181–7. doi: 10.1038/s41591-019-0749-z
10. Chan JY, Lim JQ, Yeong J, Ravi V, Guan P, Boot A, et al. Multiomic analysis and immunoprofiling reveal distinct subtypes of human angiosarcoma. J Clin Invest (2020) 130(11):5833–46. doi: 10.1172/JCI139080
11. Holden CA, Spittle MF, Jones EW. Angiosarcoma of the face and scalp, prognosis and treatment. Cancer (1987) 59(5):1046–57. doi: 10.1002/1097-0142(19870301)59:5<1046::aid-cncr2820590533>3.0.co;2-6
12. Lee BL, Chen CF, Chen PC, Lee HC, Liao WC, Perng CK, et al. Investigation of prognostic features in primary cutaneous and soft tissue angiosarcoma after surgical resection: A retrospective study. Ann Plast Surg (2017) 78(3 Suppl 2):S41–6. doi: 10.1097/SAP.0000000000001004
13. Perez MC, Padhya TA, Messina JL, Jackson RS, Gonzalez RJ, Bui MM, et al. Cutaneous angiosarcoma: a single-institution experience. Ann Surg Oncol (2013) 20(11):3391–7. doi: 10.1245/s10434-013-3083-6
14. Dettenborn T, Wermker K, Schulze HJ, Klein M, Schwipper V, Hallermann C. Prognostic features in angiosarcoma of the head and neck: a retrospective monocenter study. J Craniomaxillofac Surg (2014) 42(8):1623–8. doi: 10.1016/j.jcms.2014.05.002
15. Goerdt LV, Schneider SW, Booken N. Cutaneous angiosarcomas: Molecular pathogenesis guides novel therapeutic approaches. J Dtsch Dermatol Ges (2022) 20(4):429–43. doi: 10.1111/ddg.14694
16. Manner J, Radlwimmer B, Hohenberger P, Mössinger K, Küffer S, Sauer C, et al. MYC high level gene amplification is a distinctive feature of angiosarcomas after irradiation or chronic lymphedema. Am J Pathol (2010) 176(1):34–9. doi: 10.2353/ajpath.2010.090637
17. Cornejo KM, Deng A, Wu H, Cosar EF, Khan A, St Cyr M, et al. The utility of MYC and FLT4 in the diagnosis and treatment of postradiation atypical vascular lesion and angiosarcoma of the breast. Hum Pathol (2015) 46(6):868–75. doi: 10.1016/j.humpath.2015.02.014
18. Behjati S, Tarpey PS, Sheldon H, Martincorena I, Van Loo P, Gundem G, et al. Recurrent PTPRB and PLCG1 mutations in angiosarcoma. Nat Genet (2014) 46(4):376–9. doi: 10.1038/ng.2921
19. Murali R, Chandramohan R, Möller I, Scholz SL, Berger M, Huberman K, et al. Targeted massively parallel sequencing of angiosarcomas reveals frequent activation of the mitogen activated protein kinase pathway. Oncotarget (2015) 6(34):36041–52. doi: 10.18632/oncotarget.5936
20. Agulnik M, Yarber JL, Okuno SH, von Mehren M, Jovanovic BD, Brockstein BE, et al. An open-label, multicenter, phase II study of bevacizumab for the treatment of angiosarcoma and epithelioid hemangioendotheliomas. Ann Oncol (2013) 24(1):257–63. doi: 10.1093/annonc/mds237
21. Ogata D, Yanagisawa H, Suzuki K, Oashi K, Yamazaki N, Tsuchida T. Pazopanib treatment slows progression and stabilizes disease in patients with taxane-resistant cutaneous angiosarcoma. Med Oncol (2016) 33(10):116. doi: 10.1007/s12032-016-0831-z
22. Fujiwara S, Nakano E, Nakamura K, Washio K, Ogura K, Nishigori C. Pazopanib as a potential chemotherapy for cutaneous angiosarcoma: A case series of 10 patients from a single institution. J Dermatol (2020) 47(7):e273–4. doi: 10.1111/1346-8138.15381
23. Korphaisarn K, Loree JM, Nguyen V, Coulson R, Holla V, Litzenburger BC, et al. Genomic analysis of exceptional responder to regorafenib in treatment-refractory metastatic rectal cancer: a case report and review of the literature. Oncotarget (2017) 8(34):57882–8. doi: 10.18632/oncotarget.18357
24. Ravi V, Sanford EM, Wang WL, Ross JS, Ramesh N, Futreal A, et al. Antitumor response of VEGFR2- and VEGFR3-amplified angiosarcoma to pazopanib. J Natl Compr Canc Netw (2016) 14(5):499–502. doi: 10.6004/jnccn.2016.0058
25. Florou V, Wilky BA. Current management of angiosarcoma: Recent advances and lessons from the past. Curr Treat Options Oncol (2021) 22(7):61. doi: 10.1007/s11864-021-00858-9
26. Tang F, Tie Y, Wei YQ, Tu CQ, Wei XW. Targeted and immuno-based therapies in sarcoma: mechanisms and advances in clinical trials. Biochim Biophys Acta Rev Cancer (2021) 1876(2):188606. doi: 10.1016/j.bbcan.2021.188606
27. Weidema ME, Versleijen-Jonkers YMH, Flucke UE, Desar IME, van der Graaf WTA. Targeting angiosarcomas of the soft tissues: A challenging effort in a heterogeneous and rare disease. Crit Rev Oncol Hematol (2019) 138:120–31. doi: 10.1016/j.critrevonc.2019.04.010
28. Han B, Li K, Wang Q, Zhang L, Shi J, Wang Z, et al. Effect of anlotinib as a third-line or further treatment on overall survival of patients with advanced non-small cell lung cancer: The ALTER 0303 phase 3 randomized clinical trial. JAMA Oncol (2018) 4(11):1569–75. doi: 10.1001/jamaoncol.2018.3039
29. Tanaka K, Yamaguchi S, Sawano A, Shibuya M. Characterization of the extracellular domain in vascular endothelial growth factor receptor-1 (Flt-1 tyrosine kinase). Jpn J Cancer Res (1997) 88(9):867–76. doi: 10.1111/j.1349-7006.1997.tb00463.x
30. Chi Y, Fang Z, Hong X, Yao Y, Sun P, Wang G, et al. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft-tissue sarcoma. Clin Cancer Res (2018) 24(21):5233–8. doi: 10.1158/1078-0432.CCR-17-3766
31. Li S. Anlotinib: A novel targeted drug for bone and soft tissue sarcoma. Front Oncol (2021) 11:664853. doi: 10.3389/fonc.2021.664853
32. Ren B, Wang W, Tan J, Yuan B, Chen G, Mo X, et al. Efficacy of anlotinib for the treatment of angiosarcoma of the face and neck: A case report. Front Oncol (2021) 11:596732. doi: 10.3389/fonc.2021.596732
33. Fang X, Zheng S. Primary cardiac angiosarcoma: a case report. J Int Med Res (2021) 49(8):3000605211033261. doi: 10.1177/03000605211033261
34. Xu W, Wang K, Gu W, Nie X, Zhang H, Tang C, et al. Case report: Complete remission with anti-PD-1 and anti-VEGF combined therapy of a patient with metastatic primary splenic angiosarcoma. Front Oncol (2022) 12:809068. doi: 10.3389/fonc.2022.809068
35. Antonescu CR, Yoshida A, Guo T, Chang NE, Zhang L, Agaram NP, et al. KDR activating mutations in human angiosarcomas are sensitive to specific kinase inhibitors. Cancer Res (2009) 69(18):7175–9. doi: 10.1158/0008-5472.CAN-09-2068
36. Itakura E, Yamamoto H, Oda Y, Tsuneyoshi M. Detection and characterization of vascular endothelial growth factors and their receptors in a series of angiosarcomas. J Surg Oncol (2008) 97(1):74–81. doi: 10.1002/jso.20766
37. Arnaud-Coffin P, Brahmi M, Vanacker H, Eberst L, Tredan O, Attignon V, et al. Therapeutic relevance of molecular screening program in patients with metastatic sarcoma: Analysis from the ProfiLER 01 trial. Transl Oncol (2020) 13(12):100870. doi: 10.1016/j.tranon.2020.100870
38. Huang SC, Zhang L, Sung YS, Chen CL, Kao YC, Agaram NP, et al. Recurrent CIC gene abnormalities in angiosarcomas: A molecular study of 120 cases with concurrent investigation of PLCG1, KDR, MYC, and FLT4 gene alterations. Am J Surg Pathol (2016) 40(5):645–55. doi: 10.1097/PAS.0000000000000582
39. Meyerhardt JA, Ancukiewicz M, Abrams TA, Schrag D, Enzinger PC, Chan JA, et al. Phase I study of cetuximab, irinotecan, and vandetanib (ZD6474) as therapy for patients with previously treated metastastic colorectal cancer. PloS One (2012) 7(6):e38231. doi: 10.1371/journal.pone.0038231
40. Huang J, Zhao YL, Li Y, Fletcher JA, Xiao S. Genomic and functional evidence for an ARID1A tumor suppressor role. Genes Chromosomes Cancer (2007) 46(8):745–50. doi: 10.1002/gcc.20459
41. Jiang T, Chen X, Su C, Ren S, Zhou C. Pan-cancer analysis of ARID1A alterations as biomarkers for immunotherapy outcomes. J Cancer (2020) 11(4):776–80. doi: 10.7150/jca.41296
42. Mullen J, Kato S, Sicklick JK, Kurzrock R. Targeting ARID1A mutations in cancer. Cancer Treat Rev (2021) 100:102287. doi: 10.1016/j.ctrv.2021.102287
43. Hideshima T, Nakamura N, Chauhan D, Anderson KC. Biologic sequelae of interleukin-6 induced PI3-K/Akt signaling in multiple myeloma. Oncogene (2001) 20(42):5991–6000. doi: 10.1038/sj.onc.1204833
44. Yang Y, Wang X, Yang J, Duan J, Wu Z, Yang F, et al. Loss of ARID1A promotes proliferation, migration and invasion via the akt signaling pathway in NPC. Cancer Manag Res (2019) 11:4931–46. doi: 10.2147/CMAR.S207329
45. Lee D, Yu EJ, Ham IH, Hur H, Kim YS. AKT inhibition is an effective treatment strategy in ARID1A-deficient gastric cancer cells. Onco Targets Ther (2017) 10:4153–9. doi: 10.2147/OTT.S139664
46. Tessiri S, Techasen A, Kongpetch S, Namjan A, Loilome W, Chan-On W, et al. Therapeutic targeting of ARID1A and PI3K/AKT pathway alterations in cholangiocarcinoma. PeerJ (2022) 10:e12750. doi: 10.7717/peerj.12750
47. Weitzel JN, Neuhausen SL, Adamson A, Tao S, Ricker C, Maoz A, et al. Pathogenic and likely pathogenic variants in PALB2, CHEK2, and other known breast cancer susceptibility genes among 1054 BRCA-negative hispanics with breast cancer. Cancer (2019) 125(16):2829–36. doi: 10.1002/cncr.32083
48. Sun Y, Zhou J, Shi L, Li J, Chen J. MicroRNA−466 inhibits cell proliferation and invasion in osteosarcoma by directly targeting insulin receptor substrate 1. Mol Med Rep (2019) 19(4):3345–52. doi: 10.3892/mmr.2019.9956
49. Reuveni H, Flashner-Abramson E, Steiner L, Makedonski K, Song R, Shir A, et al. Therapeutic destruction of insulin receptor substrates for cancer treatment. Cancer Res (2013) 73(14):4383–94. doi: 10.1158/0008-5472.CAN-12-3385
Keywords: cutaneous angiosarcomas, vascular endothelial growth factor receptor (VEGFR), FLT4, anlotinib, soft tissue sarcomas
Citation: Gu Y, Meng J, Ju Y, You X, Sun T, Lu J and Guan Y (2022) Case report: Unique FLT4 variants associated with differential response to anlotinib in angiosarcoma. Front. Oncol. 12:1027696. doi: 10.3389/fonc.2022.1027696
Received: 31 August 2022; Accepted: 25 October 2022;
Published: 14 November 2022.
Edited by:
Athina Markou, National and Kapodistrian University of Athens, GreeceReviewed by:
Brian Van Tine, Washington University in St. Louis, United StatesCopyright © 2022 Gu, Meng, Ju, You, Sun, Lu and Guan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yin Guan, Z3Vhbnlpbl8yMDIxQDE2My5jb20=
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.