- 1Department of Neurosurgery, Tangdu Hospital, The Fourth Military Medical University, Xi’an, China
- 2Evidence-Based Social Sciences Research Centre, School of Public Health, Lanzhou University, Lanzhou, China
- 3Innovation Center for Advanced Medicine, Tangdu Hospital, The Fourth Military Medical University, Xi’an, China
Introduction: Secondary gliosarcomas (SGS) are rare malignancies that are diagnosed subsequent to pre-existing glioma. Clinical features and optimal treatment strategies for SGS have not been conclusively established. This study aimed to assess the clinicopathological features and outcomes of SGS.
Methods: We assessed the clinicopathological features and outcomes of SGS via retrospective analysis of data for SGS patients at Tangdu Hospital. Data from SGS patients in prior publications were also analyzed in accordance with PRISMA guidelines.
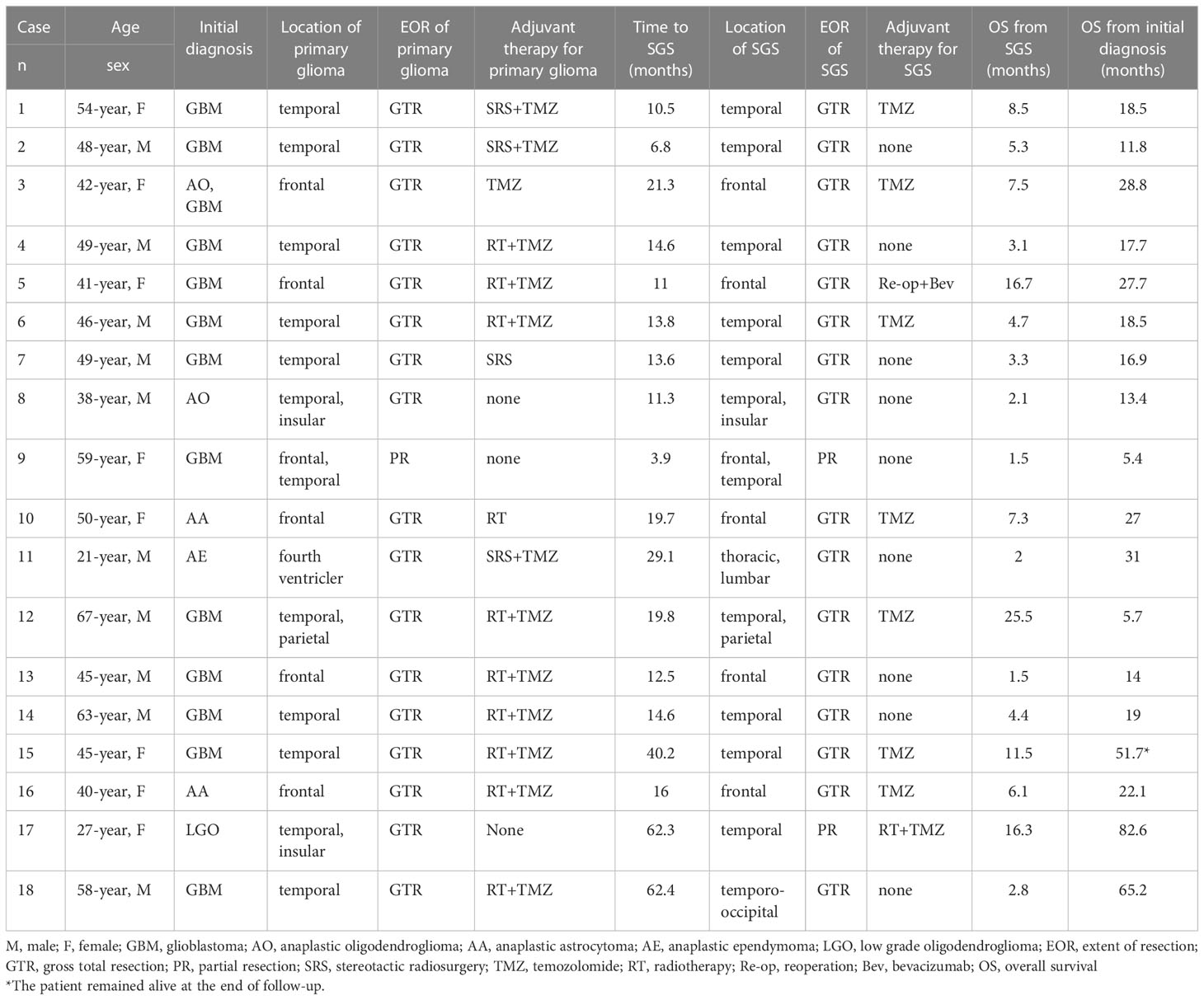
Results: Eighteen SGS patients who had been treated at Tangdu Hospital between 2013 and 2020 were enrolled in this study. Additional 89 eligible SGS patients were identified from 39 studies. The median age for the patients was 53 years old, and the most common location was the temporal lobe. The most common initial diagnosis was glioblastoma (GBM) (72.0%). Radiology revealed enhanced masses in 94.8% (73/77) of patients. Ten patients (10/107, 9.35%) had extracranial metastases at or after SGS diagnosis. Patients with initial diagnosis of non-GBM and who were younger than 60 years of age were significantly associated with a long duration of disease progression to SGS. After SGS diagnosis, patients with initial non-GBM diagnosis, gross total resection and chemoradiotherapy exhibited prolonged survival outcomes. Patients who had been initially diagnosed with GBM and received both chemoradiotherapy and active therapy after disease progression to SGS, had a significantly longer overall survival than patients who did not.
Conclusion: Initial diagnosis of GBM was a poor prognostic factor for SGS. Patients who underwent gross total resection and chemoradiation had better overall survival outcomes than those who did not. However, during treatment, clinicians should be cognizant of possible extracranial metastases.
Introduction
Gliosarcomas (GS) are rare malignant central nervous system (CNS) tumors that are characterized by a mixture of gliomatous and sarcomatous elements (1). In the 2016 & 2021 World Health Organization (WHO) classification of tumors of the CNS, GS was classified as a subtype of isocitrate dehydrogenase (IDH)-wildtype GBM (2) and a variant of GBM (3, 4) respectively. Therefore, a similar therapeutic regimen for GS and GBM was recommended by the National Comprehensive Cancer Network (NCCN) (5) and the European Association of Neuro-Oncology (EANO) (6) guidelines. In clinical practice, GS and GBM are also perceived as the same type of lesion and the prognosis of GS patients has been postulated to be comparable to that of GBM patients (7–9). Other studies found that GS has worse prognostic outcomes than GBM (1, 10, 11), with a distinct genomic landscape, indicating that GS are distinctly different tumors from GBM (12).
Among the GBM patients, about 2% are GS cases (1, 13), which are divided into the predominant primary gliosarcomas (PGS) that are de novo in origin and secondary gliosarcomas (SGS) that arise from pre-existing gliomas (14–17) and constitute 21% of GS (18, 19). Extremely low incidences of SGS have resulted in a few case reports and studies, creating a paucity of information on its clinical features and optimal treatment strategies. To elucidate on the disease and inform the design of effective treatment strategies for its management, it is important to investigate the prognosis and associated risk factors of SGS.
In this study, data for SGS patients at Tangdu Hospital were retrospectively analyzed, and data for SGS patients in prior published studies were also analyzed. Based on these analyses, we comprehensively elucidate on SGS, specifically its clinical and radiological presentations, pathological diagnosis, and treatment outcomes.
Methods
Patient enrollment and data collection
A retrospective analysis was conducted on data from patients treated at Tangdu hospital between 2013 and 2020. The inclusion criteria were: (1) Patients with a history of glioma, (2) Pathological confirmation of GS from subsequent resection. The exclusion criteria were patients with a previously diagnosed intracranial malignant glioma that had GS components. Data from 18 SGS patients were finally analyzed. The ethics committee of Tangdu Hospital approved this study, which had been pre-registered on PROSPERO (Registration number: CRD42022303335).
To obtain patient data from prior studies on GS patients, the following criteria were used: (1) present clinical data of patients, (2) no time restrictions on studies, (3) studies published in English were reviewed by two independent investigators, (4) studies were identified by searching for the terms “Secondary gliosarcoma,” “Recurrence gliosarcoma,” “postirradiation gliosarcoma,” and “post radiotherapy gliosarcoma” alone or in combination in PubMed, EMBASE, Cochrane and Ovid/Medline databases. The reference lists of identified articles were also screened to identify potentially relevant articles.
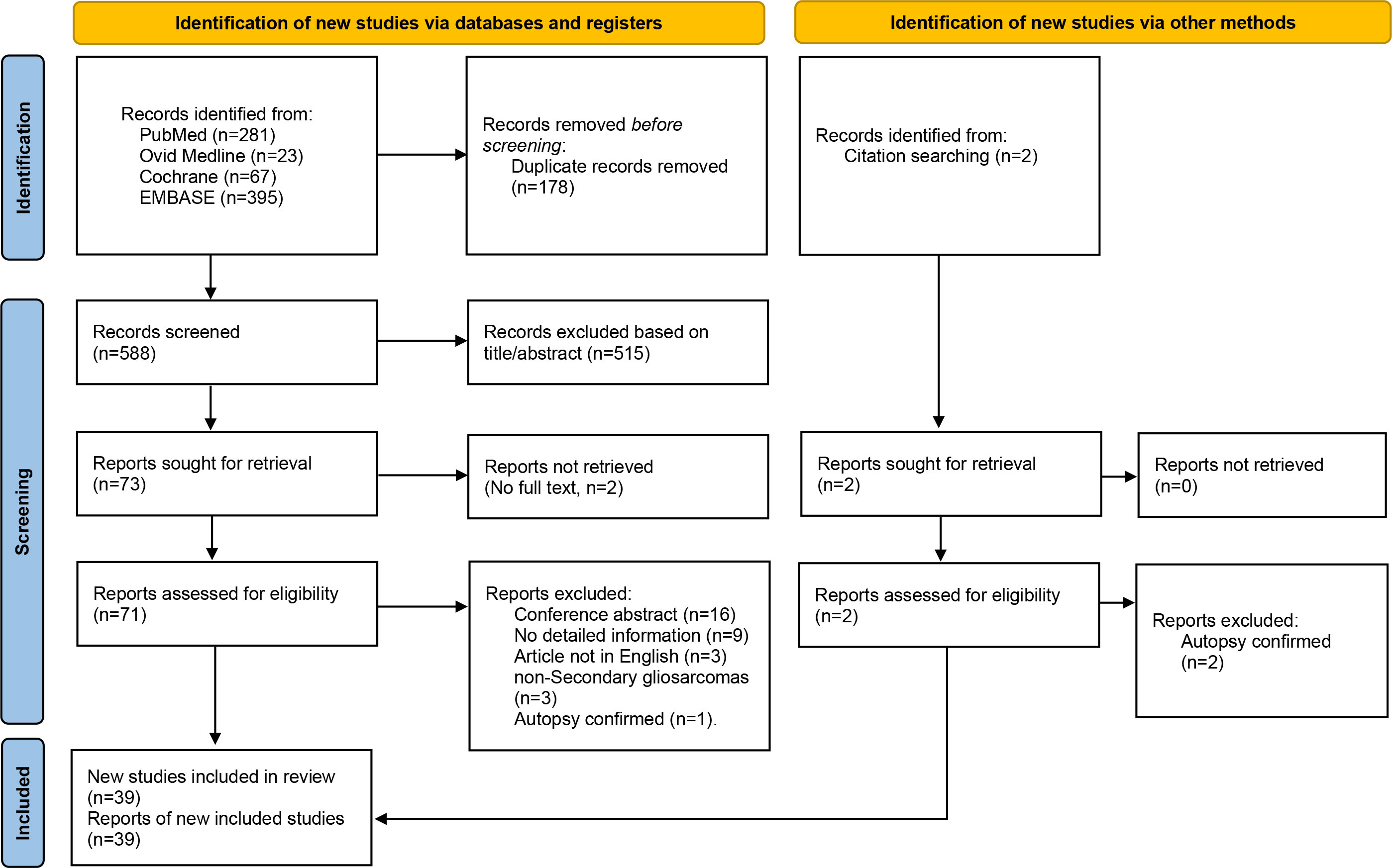
The titles and abstracts of the identified studies were independently screened by two investigators. Studies that did not meet the inclusion criteria were excluded. Then, full articles were screened and those that did not meet the entire inclusion criteria eliminated, leaving 39 studies, from which data on 89 eligible SGS patients were included in the final analysis. These data were reported as per the PRISMA guidelines (Figure 1). A total of 107 patients were included in the final analysis. Data that were extracted from patients’ records included: age at diagnosis, sex, tumor location, radiological features of SGS, initial pathological diagnosis, adjuvant therapy for glioma, time from initial diagnosis to SGS, extent of resection for SGS, adjuvant therapy for SGS, survival from SGS, and overall survival after initial diagnosis.
Quality assessment
To determine the risk of bias in prior studies, two investigators independently assessed the following characteristics: treatment allocation concealment; completeness of outcome data and selective outcome reporting. Disagreements between investigators regarding the risk of bias was resolved by discussion, and when necessary, mediated by a third investigator.
Statistical analysis
Univariate survival analysis was conducted using the Kaplan Meier method with the logrank test. Factors with p<0.10 on univariate analysis were included in multivariable analyses. Multivariate survival analysis was conducted using the Cox proportional-hazards regression model. Notably, p ≤ 0.05 was set as the threshold for statistical significance. The SPSS® software (Version 20.0) was used for statistical analyses.
Results
Demographic characteristics
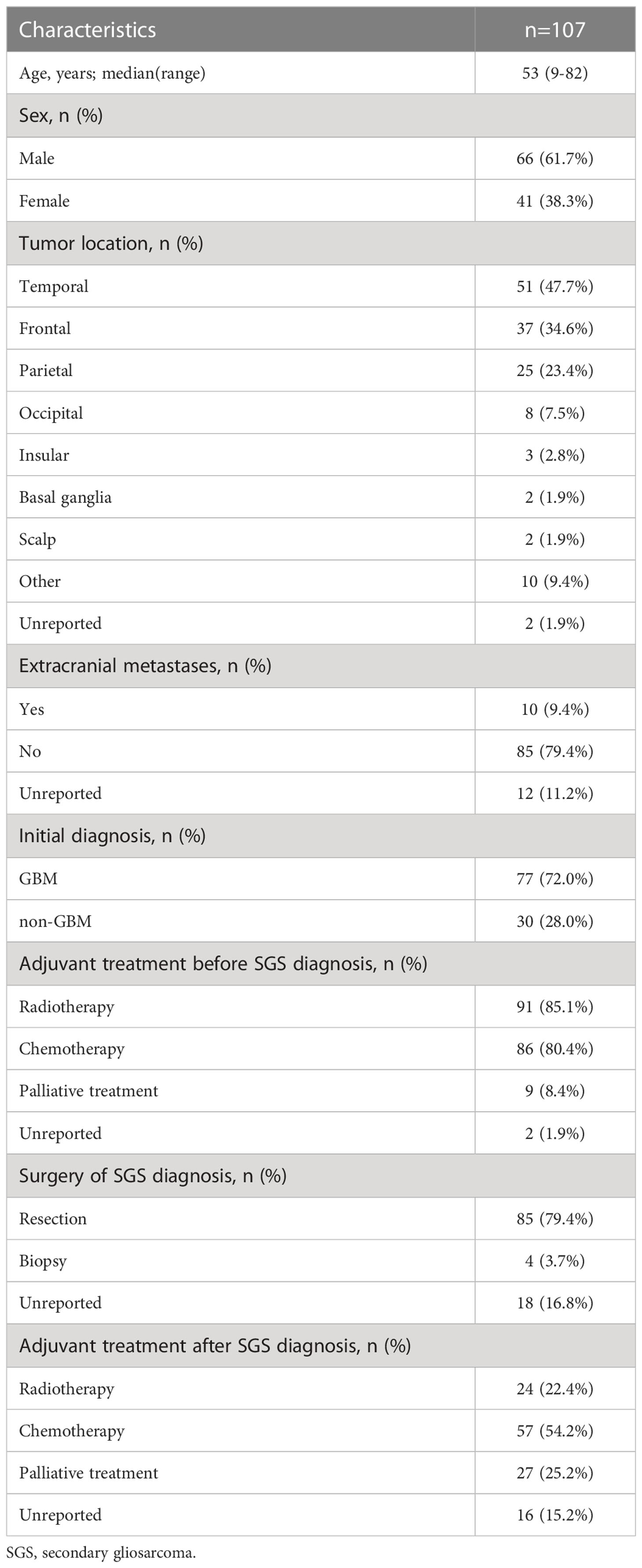
Clinical records for 18 SGS patients who had diagnosed between 2013 and 2020 at Tangdu Hospital were analyzed. Their clinical information is presented in Table 1. Data from these patients were pooled with those from 89 patients in prior SGS studies, totaling to 107 patients. The demographic data for these patients are summarized in Table 2. In summary. There were 66 men and 41 women, 93.5% (100/107) of whose records had age data. Median age at SGS diagnosis was 53 years (range 9–82 years). About 72.0% (77/107) of the patients had their radiological data presented, among them, 94.8% (73/77) had enhancing masses. Moreover, 98.1% (105/107) of patients had SGS in known locations; in the temporal lobe (n=51), frontal lobe (n=37), parietal (n=25), and occipital lobe (n=8). Low frequency tumor locations were the insular lobe (n=3), basal ganglia (n=2), and scalp (n=2, 1.9%). In one patient, tumors were located in the cerebellum, brainstem, corpus callosum, dura, subdural, pterygomaxillary region, skull, spinal cord and paranasal sinus. Ten patients had extracranial metastases at or after SGS diagnosis (Supplementary Table).
Most of the patients (82, 76.6%) had prior GBM diagnoses, 77 of which were initial GBM diagnoses. At initial diagnosis, 97 patients were subjected to surgical resection, 4 only received biopsies while 6 patients had unreported treatments. Before SGS diagnosis, 91 and 86 patients had received radiotherapy and chemotherapy, respectively. For chemotherapy, temozolomide (TMZ) was administered to 66 patients. At SGS diagnosis, 85 patients underwent surgical resection, 4 received biopsies only, while 18 had unreported treatments. After SGS diagnosis, 6 patients received radiotherapy only, 40 received chemotherapy only, 18 received chemoradiotherapy, while 16 had unreported treatments (Supplementary Table).
Time to progression to SGS
For 105 patients (98.1%), the median disease progression duration from initial disease diagnosis to SGS was 14.0 months (range 0.5–156 months). Gender and chemotherapy before SGS diagnosis were not significantly associated with duration of disease progression to SGS, as per univariate analysis. Compared with patients younger than 60 years, patients who were aged over 60 years had longer durations of disease progression to SGS (15.0 vs. 11.0 months, p=0.003) (Figure 2A). A significantly long duration of disease progression to SGS was seen in patients with initial pathological diagnosis non-GBM, relative to GBM (40.3 vs. 12.0 months, p<0.001) (Figure 2B). Furthermore, multivariate analysis revealed that patients with initial diagnosis of non-GBM had significantly longer duration of disease progression to SGS (HR 3.651, 95%CI: 2.269-5.876, p<0.001).
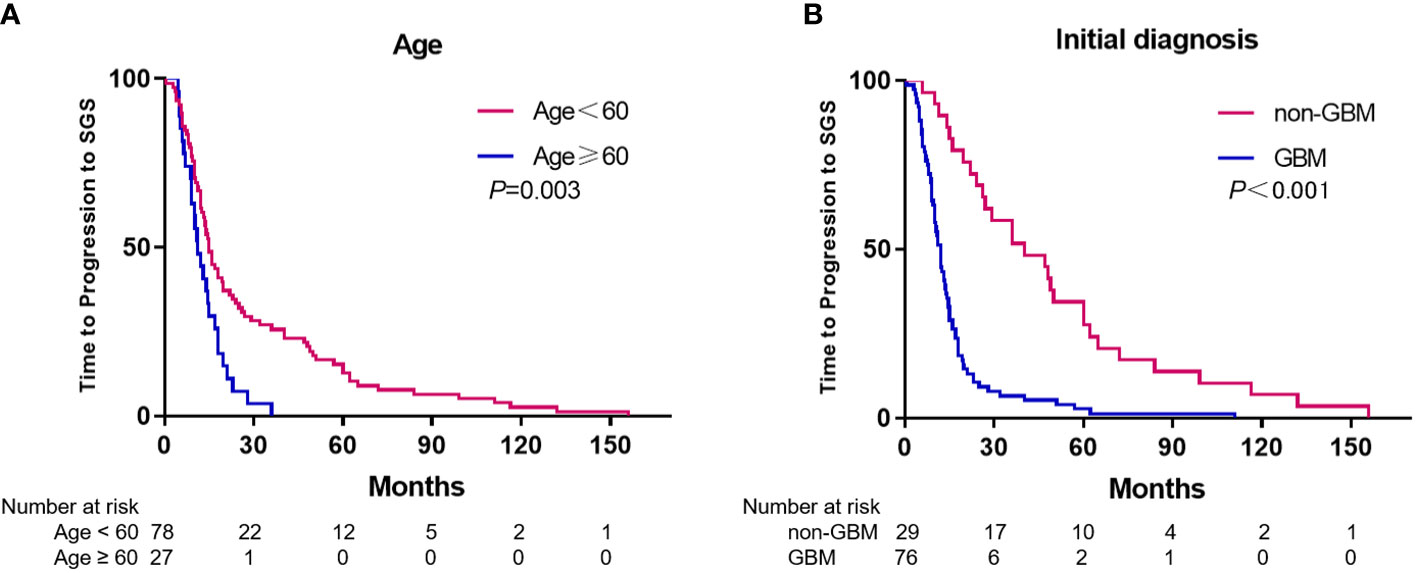
Figure 2 Kaplan-Meier estimates of time to progression to SGS stratified by age (A) and initial diagnosis (B).
Survival outcomes post SGS diagnosis
For 92 patients (86.0%), survival duration post SGS diagnosis was known and had a median of 6.0 months (95% CI, 4.72-7.28). Univariate analysis revealed that post SGS diagnosis, gender, age <60 years and chemotherapy before SGS diagnosis were not significantly associated with survival duration. A significantly longer survival duration post SGS diagnosis was observed in patients with initial diagnoses of non-GBM, compared to GBM (8.0 vs. 5.0 months, p=0.004) (Figure 3A). Compared to patients who had not been subjected to radiotherapy before SGS diagnosis, we observed a significantly worse survival duration for patients with radiotherapy before SGS diagnosis (7.5 vs. 5.0 months, p=0.022) (Figure 3B). To analyze the effects of resection of SGS, only data for patients from Tangdu Hospital were used, as that from prior studies often lacked the resection extent. After SGS diagnosis all patients underwent surgical resection and gross total resection (GTR) was achieved in 16 (88.9%) of the patients. Compared to subtotal resection (STR), GTR had a significantly longer median overall survival (OS) time (5.3 vs 1.5 months, p=0.003).
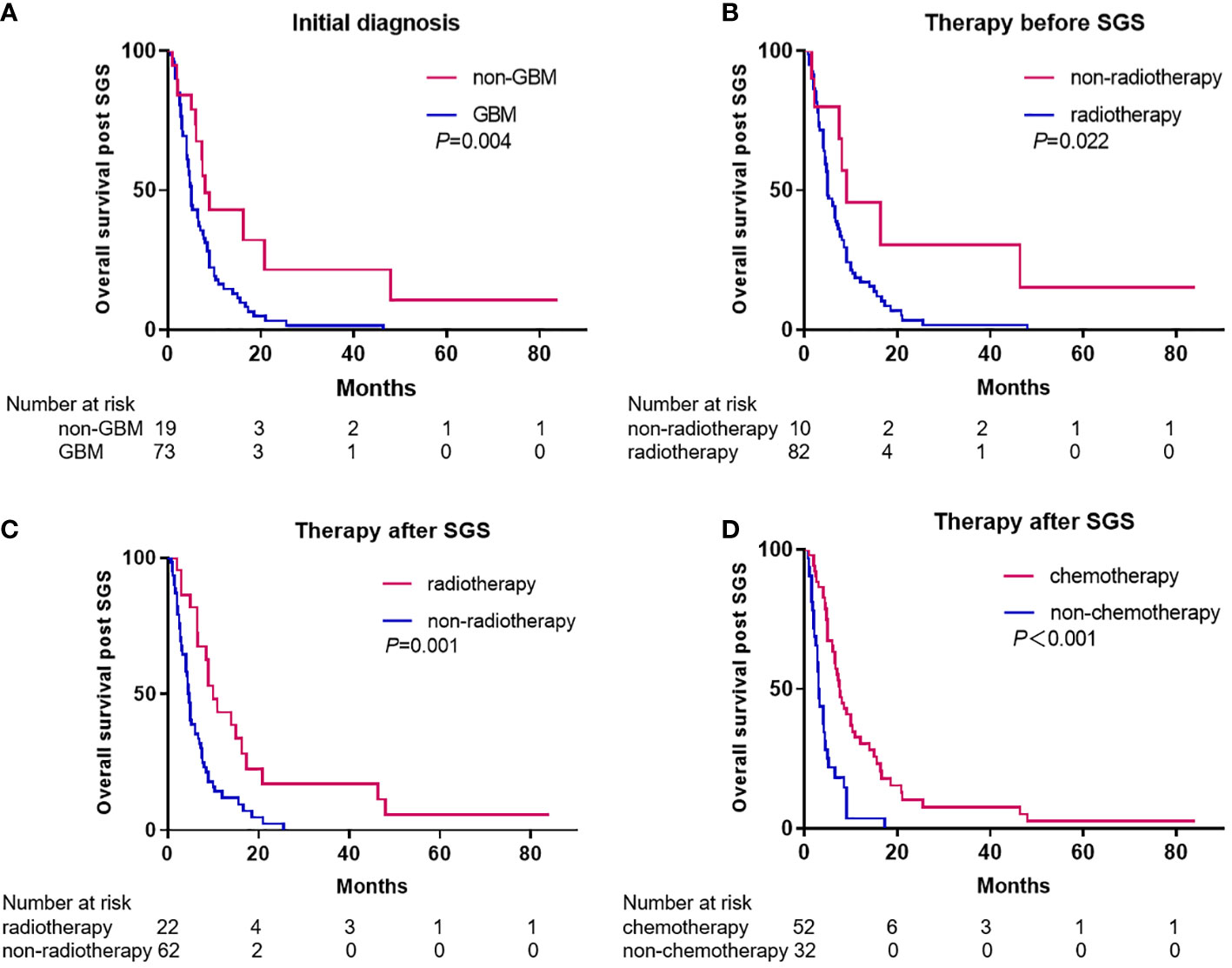
Figure 3 Kaplan-Meier estimates of overall survival post SGS diagnosis stratified by initial diagnosis (A), therapy before SGS (B), and therapy after SGS (C, D).
For patients who received radiotherapy after SGS diagnosis, their survival duration was longer than that of patients that were not subjected to radiotherapy after SGS (10.0 vs. 4.6 months, p=0.001) (Figure 3C). A longer survival duration was also observed in patients who received chemotherapy after SGS diagnosis, compared to those who did not receive chemotherapy after SGS (7.6 vs. 3.0 months, p<0.001) (Figure 3D). Compared to patients who received chemotherapy or radiotherapy alone, those who received chemoradiotherapy had longer survival durations (14.0 vs. 6.7 months, p=0.006). Notably, among patients with extracranial metastases, the median survival duration from diagnosis of metastasis to death was 3 months (range 1–8 months). Multivariate analysis revealed that either chemotherapy (HR 3.282, 95%CI: 1.987-5.420, p<0.001) or radiotherapy (HR 2.737, 95%CI: 1.562-4.796, p<0.001) after SGS diagnosis were independent prognostic factors for survival outcomes.
Survival outcomes of patients with initial GBM diagnosis
For 72 patients (93.5%), the median OS time for patients with initial GBM diagnosis was known and had a median of 18.5 months (range 5.4–65.2 months). For treatment, 67 patients (88.3%) received radiotherapy and chemotherapy. The median survival time post SGS diagnosis was known for 73 patients and had a median of 5.0 months (range 0.73–46.4 months). After SGS diagnosis, 46 patients received adjuvant radiotherapy and/or chemotherapy while seven patients were re-operated on due to SGS recurrence. Compared with patients who did not receive any treatment after SGS diagnosis, patients who treated with adjuvant radiotherapy, chemotherapy and/or re-operated had longer survival outcomes after SGS diagnosis (6.7 vs 2.8 months, p<0.001). Patients who had received radiotherapy and chemotherapy for GBM and active therapy for SGS had a median survival time of 18.6 months.
Discussion
Gliosarcoma is a rare tumor that is classified as either primary or secondary gliosarcoma. In a recent meta-analysis, incidences of IDH1/2 mutation, EGFR mutation, and MGMT methylation between PGS and SGS were found to be comparable, however, survival analysis revealed that compared with PGS, SGS is associated with significantly worse PFS and OS outcomes (20). A retrospective study from the MD Anderson Cancer Center showed that the median OS outcome from pathological diagnosis of primary and secondary GS were 17.3 months and 10.2 months, respectively (p < 0.01) (21). A retrospective analysis found that PGS patients had significantly high PFS (p < 0.03) and OS (p < 0.031), compared to SGS patients (9). To gain a better understanding of SGS and design effective treatment strategies for its management, apart from our cases, we performed a systematic review and analysis of literature.
To the best of our knowledge, with a total of 107 patients, this is the largest SGS study. Analysis of patient data revealed disease characteristics and optimal treatment strategies. Lesions were most often located in the temporal lobe (48.6%), and GBM was the most common initial diagnosis (72.0%). After SGS diagnosis, aggressive radiotherapy and chemotherapy were most effective therapeutic options.
Clinically, SGS have been defined in different ways, one of which is tumors diagnosed at recurrence after initial GBM diagnosis (14). Another is tumors detected after a high-grade glioma was either resected or irradiated (16, 22, 23). Other studies defined SGS as those arising from non-irradiated WHO grade II glioma (15, 24). Gliosarcoma originating from grade II oligodendroglioma that had been pretreated with radiotherapy has also been reported in other studies (17, 25). Based on the above studies, we propose the definition of SGS as tumors that originate from a pre-existing glioma, usually after radiation treatment.
Extracranial metastasis of CNS tumors is rare due to the blood-brain barrier and the absence of lymphatic vessels in the CNS (26, 27). The reported incidences of extracranial metastases for GBM vary between 0.4–0.5% (26), which is comparatively low than the 11% frequency for GS, which is commonly known to metastasize to the lungs, liver, and lymph nodes (28). In 2010, Han et al. (14) reported 30 cases of confirmed SGS, of which one patient had scalp/subgaleal metastasis. In 2013, 44 SGS cases were reported, of which five patients had extra-cranial metastases (29). In this study, ten patients developed extracranial metastases at or after SGS diagnosis with the lungs being the most common metastatic site (three patients). This implies that SGS is likely to undergo extracranial metastasis, therefore, identification of potential extracranial diseases, during initial diagnosis and continued surveillance is necessary.
The association between extracranial metastases of glioma and prognosis has been previously investigated. In a meta-analysis of 88 cases of extracranial glioblastoma (five were GS) (26), the median time from diagnosis of primary glioblastoma to detection of extracranial metastasis was 8.5 months, while from metastasis to death was 1.5 months, with lung metastasis patients having the worst survival outcomes. Sun et al. (30) reported cases of two patients who developed extracranial metastases after surgery for primary glioma, and died within 2 months of metastasis diagnoses. In this study, the median survival time from diagnosis of metastasis to death was 3 months (range 1–8 months). Therefore, when patients present with dyspnea or physical pain without deterioration of their neurologic status, clinicians should be cognizant of the possibility of metastatic disease.
In this study, among non-GBM patients at initial diagnosis, median durations from initial diagnosis to SGS and median OS post SGS diagnosis were 36 months and 8 months, respectively. These survival durations were comparable to those of patients with secondary glioblastoma (sGBM), which have been reported to be 158.9 weeks (31), and 7.8 months (32), respectively. In contrast, patients with initial GBM diagnosis had significantly shorter survival outcomes as the median duration from initial diagnosis to SGS and median OS post SGS diagnosis was 12.0 and 5.0 months, respectively. This was comparable to that of recurrent glioblastoma (rGBM) patients, who had a median survival time of approximately 6 months (33, 34). This disparity indicates that different initial diagnoses have potentially different clinical and molecular characteristics, such as sensitivity to treatment and IDH mutation rates.
In this study, the extent of resection was a significant prognostic factor for GS and this corroborated extent of resection as a crucial prognostic factor for primary GBM (35, 36), rGBM (37, 38) and sGBM (31, 39). Smith et al. (23) analyzed 22 PGS patients and showed that the extent of resection was a significant prognostic factor in univariate but not in multivariate analysis. Moreover, for 34 GS patients (24 PGS and 10 SGS), those who had GTR at the time of first diagnosis lived longer than those with STR (40), however, this study did not analyze the SGS separately. In tandem with previous studies, we found that the median OS was significantly longer in GTR patients than STR patients, demonstrating that GTR can significantly prolong the OS outcomes of SGS patients.
In this study, for patients whose initial diagnoses were non-GBM and treated with radiotherapy, the median survival time after SGS diagnosis was longer, compared to those treated with only chemotherapy or palliative care (20.9 vs 7.3 vs 2.0 months p<0.001). However, all SGS cases were recurrent gliomas, and thus, some patients were ineligible for re-irradiation. Prior studies have noted the importance of active treatment on survival time of sGBM patients. In a single-center retrospective study of 39 sGBM patients, patients who had been subjected to adjuvant treatment exhibited longer OS, compared to patients without adjuvant treatment (18.3 vs 8.8 months, p=0.003) (39). Moreover, Gessler F et al. (31) conducted a retrospective study of 45 sGBM patients and found that radiotherapy and chemotherapy are associated with prolonged OS. Further, patients treated with chemoradiotherapy had significantly longer survival outcomes, compared with those treated with a standalone treatment (87.3 vs 54.3 weeks, p<0.001). These results are in tandem with our findings.
A retrospective study from the MD Anderson Cancer Center showed that the median OS time for PGS was 17.5 months (40), which was similar to that of GBM. Conversely, a multi-center study conducted by Castelli J et al. (41) found that the median OS time for PGS was only 13 months and TMZ chemotherapy was not associated with improved OS, compared to patients who received radiation therapy only. Another study involving 30 SGS patients, who relapsed after progression to GBM, found that the median OS after original GBM diagnosis was 12.6 months (14). Therefore, GS patients tend to have poor prognostic outcomes. However, a study involving 10 SGS patients (9 patients with initial GBM diagnoses and one with anaplastic oligodendroglioma) showed the median OS post original diagnosis as 18.6 months (23). This corroborates our results where the median OS was 18.6 months in patients who had received chemoradiotherapy and active therapy for treatment GBM and SGS respectively. Compared with previous studies, we enrolled a large number of SGS patients, which increased the degree of accuracy and robustness. We show that recurrence of GBM as SGS does not affect the OS time.
The O6-methylguanine DNA methyltransferase (MGMT) promoter methylation is the most important prognostic factor in GBM, especially in relation to temozolomide efficacy (42). The MGMT promoter methylation is also a significant prognostic factor for temozolomide rechallenge in rGBM (43).The MGMT status is significantly associated with OS in temozolomide-treated PGS patients, yet the frequency of MGMT promoter methylation is significantly low in PGS (26.1%) than GBM (54.6%) (8). Furthermore, the median OS time for GS patients with MGMT promoter methylation is 16.4 months versus 9.4 months for those with unmethylated MGMT promoter (44). Singh et al. detected MGMT promoter methylation in five of 16 GS patients who had been treated with temozolomide, however, the MGMT status did not significantly affect OS. Singh et al. (45) detected MGMT promoter methylation in five of 16 GS patients who had been treated with temozolomide, however, the MGMT status did not significantly affect OS. There are no relevant studies on the association between MGMT promoter methylation and OS of SGS patients. Despite this study having 16 patients with known MGMT status, no further statistical analyses were performed as only four and seven of the sixteen patients had MGMT methylation and treatment with temozolomide post SGS diagnosis, respectively. Further studies should investigate whether TMZ rechallenge is a treatment option for SGS, especially for those with MGMT promoter methylation.
Limitation
Although our findings are generally encouraging, this study has some limitations. First, this was a retrospective study, which has its inherent limitations. Second, given that most cases were based on previously published articles, it was inevitable that some clinical data were not available in all studies, such as pre- and postoperative KPS scores, extent of resection and number of chemotherapy cycles. Third, for data from studies that spanned long durations, treatment regimens often differed between patients and treatment-related adverse effects were not always recorded. Fourth, several important molecular markers, such as IDH and telomerase reverse transcriptase (TERT) promoter mutations as well as epidermal growth factor receptor (EGFR) amplification were not available. Studies should aim at elucidating the clinicopathologic features, treatment strategies, and outcomes of SGS patients.
Conclusion
Despite the rarity of SGS, 107 SGS patients were included in the final analysis, making this the largest study of SGS patients to date. Patients with an initial non-GBM diagnosis had favorable prognostic outcomes. After SGS diagnosis, there was a high risk of extracranial metastasis, and the lung was the most common metastatic site. Extracranial metastases were associated with poor prognoses. Patients with GTR and chemoradiation after SGS diagnosis exhibited better overall survival outcomes, therefore, we recommend that the most suitable SGS treatment strategy is maximal safe resection combined with adjuvant chemoradiotherapy. However, during treatment, clinicians should be cognizant of possible extracranial metastases.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
JL performed the data analyses and drafted the manuscript. CL performed the clinical analyses (imaging data) with YW and contributed to writing of the manuscript. PJ, SG and YZ contributed to data analyses. NW and MX performed the clinical follow up. LW performed the clinical analyses and designed the study with JW. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (81772661), the Natural Science Foundation of Shaanxi Province (2020JZ-30).
Acknowledgments
We greatly appreciate the assistance of Ms. Binfang Zhao, who provided help with the data extraction.
Conflict of interest
The authors declare that this study was performed in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1026747/full#supplementary-material
References
1. Kozak KR, Mahadevan A, Moody JS. Adult gliosarcoma: Epidemiology, natural history, and factors associated with outcome. Neuro Oncol (2009) 11(2):183–91. doi: 10.1215/15228517-2008-076
2. Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 world health organization classification of tumors of the central nervous system: A summary. Acta Neuropathol (2016) 131(6):803–20. doi: 10.1007/s00401-016-1545-1
3. Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro Oncol (2021) 23(8):1231–51. doi: 10.1093/neuonc/noab106
4. Chen Y, Zhou S, Zhou X, Dai X, Wang L, Chen P, et al. Gliosarcoma with osteosarcomatous component: A case report and short review illustration. Pathol Res Pract (2022) 232:153837. doi: 10.1016/j.prp.2022.153837
5. Nabors LB, Portnow J, Ahluwalia M, Baehring J, Brem H, Brem S, et al. Central nervous system cancers, version 3.2020, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw (2020) 18(11):1537–70. doi: 10.6004/jnccn.2020.0052
6. Weller M, van den Bent M, Preusser M, Le Rhun E, Tonn JC, Minniti G, et al. EANO guidelines on the diagnosis and treatment of diffuse gliomas of adulthood. Nat Rev Clin Oncol (2021) 18(3):170–86. doi: 10.1038/s41571-020-00447-z
7. Lutterbach J, Guttenberger R, Pagenstecher A. Gliosarcoma: A clinical study. Radiother Oncol (2001) 61(1):57–64. doi: 10.1016/s0167-8140(01)00415-7
8. Frandsen S, Broholm H, Larsen VA, Grunnet K, Møller S, Poulsen HS, et al. Clinical characteristics of gliosarcoma and outcomes from standardized treatment relative to conventional glioblastoma. Front Oncol (2019) 9:1425. doi: 10.3389/fonc.2019.01425
9. Hong B, Lalk M, Wiese B, Merten R, Heissler HE, Raab P, et al. Primary and secondary gliosarcoma: Differences in treatment and outcome. Br J Neurosurg (2021), 1–8. doi: 10.1080/02688697.2021.1872773
10. Damodaran O, van Heerden J, Nowak AK, Bynevelt M, McDonald K, Marsh J, et al. Clinical management and survival outcomes of gliosarcomas in the era of multimodality therapy. J Clin Neurosci (2014) 21(3):478–81. doi: 10.1016/j.jocn.2013.07.042
11. Pierscianek D, Ahmadipour Y, Michel A, Rauschenbach L, Darkwah Oppong M, Deuschl C, et al. Demographic, radiographic, molecular and clinical characteristics of primary gliosarcoma and differences to glioblastoma. Clin Neurol Neurosurg (2021) 200:106348. doi: 10.1016/j.clineuro.2020.106348
12. Zaki MM, Mashouf LA, Woodward E, Langat P, Gupta S, Dunn IF, et al. Genomic landscape of gliosarcoma: Distinguishing features and targetable alterations. Sci Rep (2021) 11(1):18009. doi: 10.1038/s41598-021-97454-6
13. Walker GV, Gilbert MR, Prabhu SS, Brown PD, McAleer MF. Temozolomide use in adult patients with gliosarcoma: An evolving clinical practice. J Neurooncol (2013) 112(1):83–9. doi: 10.1007/s11060-012-1029-7
14. Han SJ, Yang I, Otero JJ, Ahn BJ, Tihan T, McDermott MW, et al. Secondary gliosarcoma after diagnosis of glioblastoma: Clinical experience with 30 consecutive patients. J Neurosurg (2010) 112(5):990–6. doi: 10.3171/2009.9.Jns09931
15. Rech F, Rigau V, Fabbro M, Kerr C, Gauchotte G, Taillandier L, et al. A nonradiated grade II glioma that underwent delayed malignant transformation to a gliosarcoma with meningeal growth and dissemination. J Neurol Surg A Cent Eur Neurosurg (2014) 75(6):485–90. doi: 10.1055/s-0034-1372437
16. Niu H, Wang K, Song Z, Sun W. Secondary gliosarcoma arising from an anaplastic astrocytoma: A case report and review of the literature. Neurosurg Q (2015) 25(2):271–4. doi: 10.1097/WNQ.0000000000000042
17. Yasuda T, Nitta M, Komori T, Kobayashi T, Masui K, Maruyama T, et al. Gliosarcoma arising from oligodendroglioma, IDH mutant and 1p/19q codeleted. Neuropathology (2018) 38(1):41–6. doi: 10.1111/neup.12406
18. Perry JR, Ang LC, Bilbao JM, Muller PJ. Clinicopathologic features of primary and postirradiation cerebral gliosarcoma. Cancer (1995) 75(12):2910–8. doi: 10.1002/1097-0142(19950615)75:12<2910::aid-cncr2820751219>3.0.co;2-a
19. Jin MC, Liu EK, Shi S, Gibbs IC, Thomas R, Recht L, et al. Evaluating surgical resection extent and adjuvant therapy in the management of gliosarcoma. Front Oncol (2020) 10:337. doi: 10.3389/fonc.2020.00337
20. Vuong HG, Dunn IF. Primary versus secondary gliosarcoma: A systematic review and meta-analysis. J Neurooncol (2022) 159(1):195–200. doi: 10.1007/s11060-022-04057-w
21. Amer A, Khose S, Alhasan H, Pokhylevych H, Fuller G, Chasen N, et al. Clinical and survival characteristics of primary and secondary gliosarcoma patients. Clin Neurol Neurosurg (2022) 214:107146. doi: 10.1016/j.clineuro.2022.107146
22. Romeike BF, Chen Y, Walter J, Petersen I. Diagnostic utility of IDH1- and p53-mutation analysis in secondary gliosarcoma. Clin Neuropathol (2011) 30(5):231–4. doi: 10.5414/np300375
23. Smith DR, Wu CC, Saadatmand HJ, Isaacson SR, Cheng SK, Sisti MB, et al. Clinical and molecular characteristics of gliosarcoma and modern prognostic significance relative to conventional glioblastoma. J Neurooncol (2018) 137(2):303–11. doi: 10.1007/s11060-017-2718-z
24. Tanaka S, Hitotsumatsu T, Sugita Y, Ishido K, Ito O, Hatae R, et al. Gliosarcoma arising from oligodendroglioma (Oligosarcoma): A case report with genetic analyses. Pathol Int (2018) 68(10):567–73. doi: 10.1111/pin.12723
25. Hiniker A, Hagenkord JM, Powers MP, Aghi MK, Prados MD, Perry A. Gliosarcoma arising from an oligodendroglioma (oligosarcoma). Clin Neuropathol (2013) 32(3):165–70. doi: 10.5414/np300577
26. Lun M, Lok E, Gautam S, Wu E, Wong ET. The natural history of extracranial metastasis from glioblastoma multiforme. J Neurooncol (2011) 105(2):261–73. doi: 10.1007/s11060-011-0575-8
27. Briones-Claudett KH, Briones-Claudett MH, Villacrés Garcia F, Ortega Almeida C, Escudero-Requena A, Benítez Solís J, et al. Early pulmonary metastasis after a surgical resection of glioblastoma multiforme. A case report. Am J Case Rep (2020) 21:e922976. doi: 10.12659/ajcr.922976
28. Beaumont TL, Kupsky WJ, Barger GR, Sloan AE. Gliosarcoma with multiple extracranial metastases: Case report and review of the literature. J Neurooncol (2007) 83(1):39–46. doi: 10.1007/s11060-006-9295-x
29. Dawar R, Fabiano AJ, Qiu J, Khushalani NI. Secondary gliosarcoma with extra-cranial metastases: a report and review of the literature. Clin Neurol Neurosurg (2013) 115(4):375–80. doi: 10.1016/j.clineuro.2012.06.017
30. Sun Q, Xu R, Xu H, Wang G, Shen X, Jiang H. Extracranial metastases of high-grade glioma: The clinical characteristics and mechanism. World J Surg Oncol (2017) 15(1):181. doi: 10.1186/s12957-017-1249-6
31. Gessler F, Zappi J, Konczalla J, Bernstock JD, Forster MT, Wagner M, et al. Secondary glioblastoma: Molecular and clinical factors that affect outcome after malignant progression of a lower grade tumor. World Neurosurg (2017) 102:49–55. doi: 10.1016/j.wneu.2017.02.104
32. Ohgaki H, Kleihues P. The definition of primary and secondary glioblastoma. Clin Cancer Res (2013) 19(4):764–72. doi: 10.1158/1078-0432.Ccr-12-3002
33. Stupp R, Wong ET, Kanner AA, Steinberg D, Engelhard H, Heidecke V, et al. NovoTTF-100A versus physician’s choice chemotherapy in recurrent glioblastoma: a randomised phase III trial of a novel treatment modality. Eur J Cancer (2012) 48(14):2192–202. doi: 10.1016/j.ejca.2012.04.011
34. Lombardi G, De Salvo GL, Brandes AA, Eoli M, Rudà R, Faedi M, et al. Regorafenib compared with lomustine in patients with relapsed glioblastoma (REGOMA): A multicentre, open-label, randomised, controlled, phase 2 trial. Lancet Oncol (2019) 20(1):110–9. doi: 10.1016/s1470-2045(18)30675-2
35. Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, DeMonte F, et al. A multivariate analysis of 416 patients with glioblastoma multiforme: Prognosis, extent of resection, and survival. J Neurosurg (2001) 95(2):190–8. doi: 10.3171/jns.2001.95.2.0190
36. Slotty PJ, Siantidis B, Beez T, Steiger HJ, Sabel M. The impact of improved treatment strategies on overall survival in glioblastoma patients. Acta Neurochir (Wien) (2013) 155(6):959–63. doi: 10.1007/s00701-013-1693-1
37. Bloch O, Han SJ, Cha S, Sun MZ, Aghi MK, McDermott MW, et al. Impact of extent of resection for recurrent glioblastoma on overall survival: Clinical article. J Neurosurg (2012) 117(6):1032–8. doi: 10.3171/2012.9.Jns12504
38. Oppenlander ME, Wolf AB, Snyder LA, Bina R, Wilson JR, Coons SW, et al. An extent of resection threshold for recurrent glioblastoma and its risk for neurological morbidity. J Neurosurg (2014) 120(4):846–53. doi: 10.3171/2013.12.Jns13184
39. Hamisch C, Ruge M, Kellermann S, Kohl AC, Duval I, Goldbrunner R, et al. Impact of treatment on survival of patients with secondary glioblastoma. J Neurooncol (2017) 133(2):309–13. doi: 10.1007/s11060-017-2415-y
40. Cachia D, Kamiya-Matsuoka C, Mandel JJ, Olar A, Cykowski MD, Armstrong TS, et al. Primary and secondary gliosarcomas: Clinical, molecular and survival characteristics. J Neurooncol (2015) 125(2):401–10. doi: 10.1007/s11060-015-1930-y
41. Castelli J, Feuvret L, Haoming QC, Biau J, Jouglar E, Berger A, et al. Prognostic and therapeutic factors of gliosarcoma from a multi-institutional series. J Neurooncol (2016) 129(1):85–92. doi: 10.1007/s11060-016-2142-9
42. Gittleman H, Lim D, Kattan MW, Chakravarti A, Gilbert MR, Lassman AB, et al. An independently validated nomogram for individualized estimation of survival among patients with newly diagnosed glioblastoma: NRG oncology RTOG 0525 and 0825. Neuro Oncol (2017) 19(5):669–77. doi: 10.1093/neuonc/now208
43. Weller M, Tabatabai G, Kästner B, Felsberg J, Steinbach JP, Wick A, et al. MGMT promoter methylation is a strong prognostic biomarker for benefit from dose-intensified temozolomide rechallenge in progressive glioblastoma: The DIRECTOR trial. Clin Cancer Res (2015) 21(9):2057–64. doi: 10.1158/1078-0432.Ccr-14-2737
44. Frandsen J, Orton A, Jensen R, Colman H, Cohen AL, Tward J, et al. Patterns of care and outcomes in gliosarcoma: An analysis of the national cancer database. J Neurosurg (2018) 128(4):1133–8. doi: 10.3171/2016.12.Jns162291
45. Singh G, Mallick S, Sharma V, Joshi N, Purkait S, Jha P, et al. A study of clinico-pathological parameters and O6-methylguanine DNA methyltransferase (MGMT) promoter methylation status in the prognostication of gliosarcoma. Neuropathology (2012) 32(5):534–42. doi: 10.1111/j.1440-1789.2012.01297.x
Keywords: secondary gliosarcoma, prognosis, glioblastoma, extracranial metastasis, chemoradiation
Citation: Liu J, Li C, Wang Y, Ji P, Guo S, Zhai Y, Wang N, Xu M, Wang J and Wang L (2023) Prognostic and predictive factors of secondary gliosarcoma: A single-institution series of 18 cases combined with 89 cases from literature. Front. Oncol. 12:1026747. doi: 10.3389/fonc.2022.1026747
Received: 24 August 2022; Accepted: 27 December 2022;
Published: 31 January 2023.
Edited by:
Christine Marosi, Medical University of Vienna, AustriaReviewed by:
Huy Gia Voung, University of Iowa Hospitals and Clinics, United StatesWei Hua, Fudan University, China
Copyright © 2023 Liu, Li, Wang, Ji, Guo, Zhai, Wang, Xu, Wang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Julei Wang, d2FuZ19qdWxlaUBzaW5hLmNu; Liang Wang, RHJ3YW5nbGlhbmdAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Jinghui Liu
Jinghui Liu Chen Li
Chen Li Yuan Wang
Yuan Wang Peigang Ji
Peigang Ji Shaochun Guo1
Shaochun Guo1 Yulong Zhai
Yulong Zhai Na Wang
Na Wang Meng Xu
Meng Xu Julei Wang
Julei Wang Liang Wang
Liang Wang