
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol., 28 October 2022
Sec. Molecular and Cellular Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1026278
This article is part of the Research TopicAldehyde Dehydrogenase in Clinical Settings: Potential Biomarker and Therapeutic Target in Solid TumorsView all 5 articles
 Yaolu Wei1†
Yaolu Wei1† Yan Li1†
Yan Li1† Yenan Chen2
Yenan Chen2 Pei Liu2
Pei Liu2 Sheng Huang2
Sheng Huang2 Yuping Zhang1
Yuping Zhang1 Yanling Sun1
Yanling Sun1 Zhe Wu1
Zhe Wu1 Meichun Hu1
Meichun Hu1 Qian Wu1
Qian Wu1 Hongnian Wu1
Hongnian Wu1 Fuxing Liu1*
Fuxing Liu1* Tonghui She1*
Tonghui She1* Zhifeng Ning1*
Zhifeng Ning1*Solid tumors can be divided into benign solid tumors and solid malignant tumors in the academic community, among which malignant solid tumors are called cancers. Cancer is the second leading cause of death in the world, and the global incidence of cancer is increasing yearly New cancer patients in China are always the first. After the concept of stem cells was introduced in the tumor community, the CSC markers represented by ALDH1 have been widely studied due to their strong CSC cell characteristics and potential to be the driving force of tumor metastasis. In the research results in the past five years, it has been found that ALDH1 is highly expressed in various solid cancers such as breast cancer, lung cancer, colorectal cancer, liver cancer, gastric cancer, cervical cancer, esophageal cancer, ovarian cancer, head,and neck cancer. ALDH1 can activate and transform various pathways (such as the USP28/MYC signaling pathway, ALDH1A1/HIF-1α/VEGF axis, wnt/β-catenin signaling pathway), as well as change the intracellular pH value to promote formation and maintenance, resulting in drug resistance in tumors. By targeting and inhibiting ALDH1 in tumor stem cells, it can enhance the sensitivity of drugs and inhibit the proliferation, differentiation, and metastasis of solid tumor stem cells to some extent. This review discusses the relationship and pathway of ALDH1 with various solid tumors. It proposes that ALDH1 may serve as a diagnosis and therapeutic target for CSC, providing new insights and new strategies for reliable tumor treatment.
According to global cancer data, 9.96 million people worldwide will die by 2020, of which China ranks first in the world in terms of cancer deaths (1). The cause of cancer death is still unclear and is currently mainly related to cancer stem cells and drug resistance. In recent years, with the introduction of the stem cell concept into cancer research, researchers have found that cancer heterogeneity is signficant source of disease progression and treatment failure, and cancer stem cells (CSCs) are the source of heterogeneity (2). Although the number is scarce, it has a solid carcinogenic, robust carcinogenic, carcinogenic solid ability and the potential to generate various types of cells that constitute tumors (3). The tumor is a stem cell disease and acetaldehyde dehydrogenase 1 (ALDH1) is one of the most essential markers in CSCs. The expression level of ALDH1 in solid tumor tissues is higher than in normal tissues. Therefore, in recent years, more and more researchers have studied the possibility of ALDH1 as a potential therapeutic target for CSCs.
ALDH1 is one of the aldehyde dehydrogenases, located mainly on chromosome 9q21 (4). As an isoenzyme of acetaldehyde dehydrogenase, ALDH1 exists mainly in the cytoplasm of liver cells. It is responsible for further oxidation of acetaldehyde as a substrate by alcohol dehydrogenase to harmless acetic acids. As a cellular lipase, ALDH1 plays a vital role in gene expression and tissue differentiation in many tissues. Current studies have found that ALDH1 is very likely to be a stem cell marker for various solid tumors (5–7). With in-depth study by researchers, ALDH1 is highly expressed in lung cancer (8), invasive cervical cancer (9), breast cancer (10), ovarian cancer (7), colorectal cancer (11), gastric cancer (5), esophageal cancer (12), head and neck cancer (13) and other solid cancers from clinical research. ALDH1A1 is the main component of ALDH1, and the activation of ALDH1 depends mainly on ALDH1A1. Recent studies have shown that the higher the level of ALDH1A1, the worse the prognosis for patients, especially for tumors of the digestive system. Furthermore, ALDH1 is not only involved in many critical biological functions such as cell differentiation and resistance to radiation therapy and chemotherapy but also clinical research found that high expression of the ALDH1 gene signature in cancer tissue is positively correlated with malignant progression in cancer patients (14). Therefore, it should be clear whether ALDH1 can be used as a therapeutic target for various solid tumor stem cells? When targeting ALDH1, can it inhibit the proliferation and differentiation of tumor stem cell markers and reduce the recurrence and metastasis of malignant tumors? Thus, it can provide a new target and basis for the treatment of solid tumors.
Acetaldehyde dehydrogenase (ALDH) is a randomly assembled tetramer in the body and contains 19 functional ALDH genes in the human genome (15), and ALDH1 introduced in this study is one of the more critical subgroups. The ALDH1 family consists of six human ALDH genes, including ALDH1A1, ALDH1A2, ALDH1A3, ALDH1B1, ALDH1L1, and ALDH1L2. The rat and mouse genomes contain an additional gene, ALDH1A7, which is 92% identical to the mouse ALDH1A1 (16). ALDH1 can not only be used as a marker of cancer stem cells but also plays an irreplaceable role in promoting physiological functions such as alcohol metabolism and synthesis of retinoic acid (RA).
In normal human stem cells, ALDH1 can irreversibly convert the retinal to RA in the cytoplasm. Then RA will be transferred to the nucleus, activating the retinoic acid receptor (RAR), retinoic acid X receptor (RXR), and nuclear hormone receptor peroxisome proliferator-activated receptor β/δ (PPARβ/δ) to regulate the transcriptional activity of more than 500 genes (17), which play an essential role in human development and maintain homeostasis of human organs.
ALDH1 is considered a marker of CSCs, which can induce cancer by maintaining the characteristics of CSCs, modifying metabolism, and promoting DNA repair. ALDH1 is lowly expressed in normal tissues and highly expressed in cancer the expression level of ALDH1is a marker that distinguishes normal stem cells from cancer stem cells. Clinical research studies have found that the prognostic ratio of the ALDH1 gene family can be used as a robust poor predictor of various solid cancers, including breast cancer, colon cancer, esophageal squamous cell carcinoma, non-small cell lung cancer, ovarian cancer, and other cancers (18, 19). As a strong predictor, ALDH1 is also involved in deriving drug resistance in solid cancers. Although still controversial, it is undeniable that cancer cells with high ALDH activity and other stem cell-like characteristics are closely related to drug resistance and tumor recurrence. The underlying mechanism is currently unclear but may involve RA biosynthesis, scavenging of reactive oxygen species (ROS) and toxic aldehydes (20), USP28/MYC signaling pathway (21), ALDH1A1/HIF-1α/VEGF axis (22), wnt/β-catenin signaling pathway (23). As shown in Figure 1. From the above, we can boldly propose ALDH1 as a potential therapeutic target for solid cancer and provide a new ideas for further and more accurate prognosis and the development of new therapeutic targets.
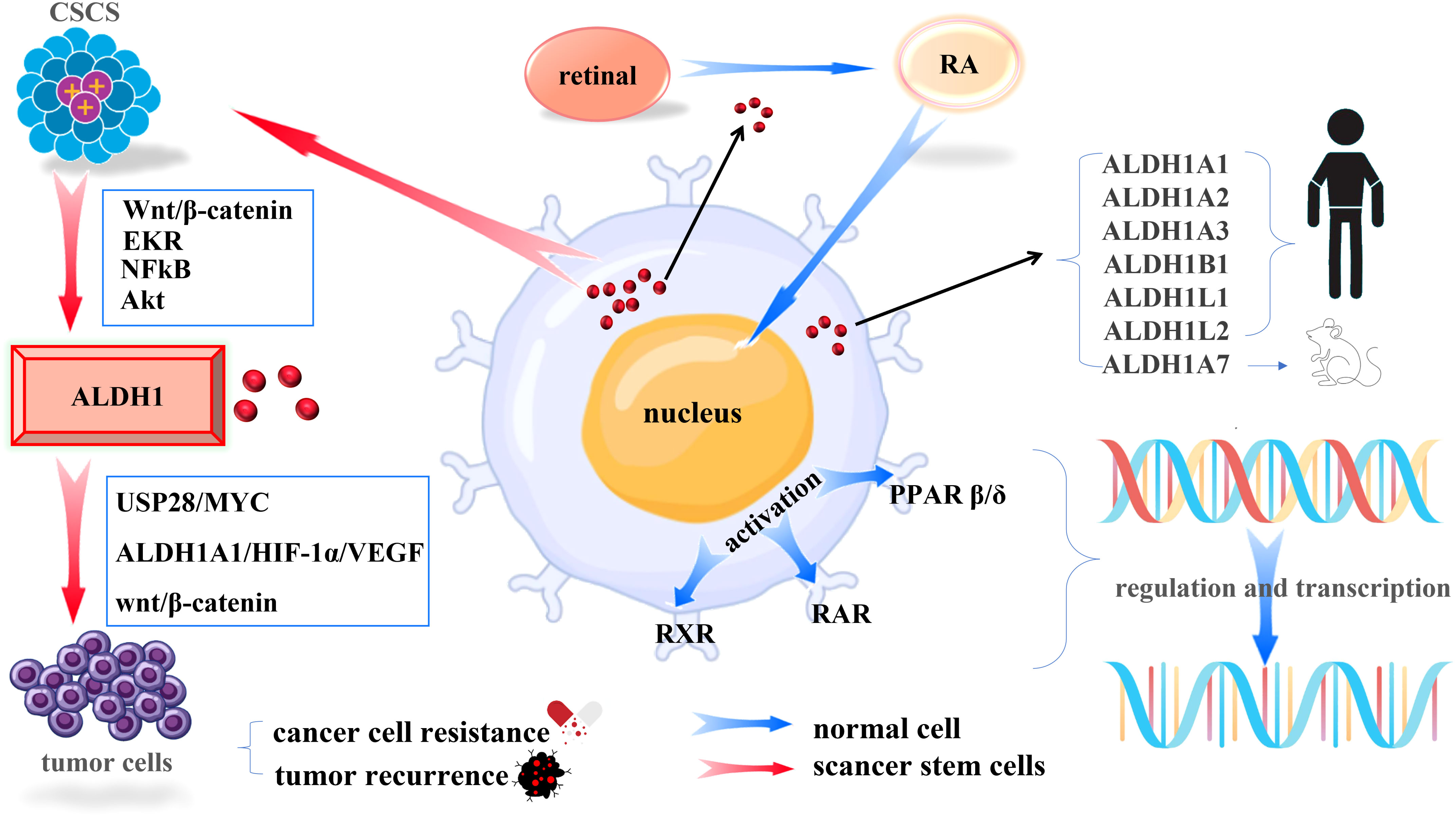
Figure 1 Mechanisms of ALDH1 in Cells. (Blue arrows indicate the mechanism of action of ALDH1 in normal tissues, red arrows indicate the mechanism of action of ALDH1 in cancer stem cells).
Breast cancer is the most common cancer in the world and the leading cause of cancer death in women. IARC estimates that by 2040, new breast cancer cases will exceed 3 million each year (1). Although endocrine therapy, radiotherapy, and chemotherapy have greatly improved the overall survival rate of breast cancer patients, the limitation of these treatment options is that they cannot target CSCs, leading to drug resistance and tumor recurrence, which is still incurable (24, 25). Elevated ALDH1 levels are associated with resistance to chemotherapy in breast cancer patients treated with a taxane-doxorubicin-cyclophosphamide regimen (26). ALDH1 contributes to normal and tumor stem cell differentiation and breast cancer invasion and metastasis are mediated by tumor cell subsets that exhibit stem cell-like featcharacteristics express ALDH1 (10). ALDH1 functions primarily by regulating vitamin A oxidation, and the expression level of ALDHI has become a marker for distinguishing normal stem cells from tumor stem cells in breast tissue (27, 28) and is significantly correlated with a poor prognosis (29–31). ALDH1A1 and ALDH1A3 can dramatically enhance ALDH1 activity and are associated with a poor prognosis in patients with breast cancer (32). The elimination of ALDH1A1, ALDH1A3 inhibits ALDH1 activity, increases chemosensitivity, and reverses chemoresistance in breast cancer (33).
Based on its enzymatic activity, ALDH1A1 reduces the intracellular pH of breast cancer cells and upregulates GMCSF by activating the TAK1-NFkB signaling pathway. It induces MDSC expansion, thus decreasing antitumor immunity and promoting breast cancer progression (34). ALDH1A1 also enhances the USP28/MYC signaling pathway to promote breast cancer stem cells by maintaining a local acid microenvironment (35). The breast cancer stemness marker ALDH1A1 activates ALDH1A1/HIF-1α/VEGF axis through retinoic acid conduction, upstream HIF-1α is activated, induces VEGF expression and release, and promotes tumor angiogenesis (22). ALDH1 activation and transcription are is mainly related to the MUC1-C/TWIST1/EMT pathway (36), MUC1-C→ERK→CEBPβ→ALDH1A1 pathway (20, 37), Nanog signaling (38), Wnt/β-catenin pathway (39), Notch, TGF-β pathway (40), SIRT1-PRRX1-KLF4-ALDH1 pathway (41), IL-6/STAT3/ALDH1 path (42) and so on.
Studies have shown that targeting or inhibiting ALDH1 can alleviate breast cancer. CAP targets ALDH1 breast CSCs by regulating AQP3-19Y-mediated ubiquitination of AQP3-5K and FOXO1 K48, which can improve therapeutic efficacy (43). Targeting ALDH1 in breast CSCs with ATRA or N,N-diethylaminobenzaldehyde (DEAB) combined with doxorubicin or paclitaxel therapy and radiation therapy significantly reduced tumor cell viability (38). The ALDH1 inhibitor disulfiram inhibited breast tumor growth and occurrence (34). Limonin (44), quercetin (45), and curcumin (46) inhibit breast cancer stem cells by downregulating ALDH1A1. ALDH1 may serve as a potential therapeutic target for breast CSCs (47), so it can provide a new therapeutic target and a basis for breast CSCs.
The mortality rate of lung cancer ranks first among malignant tumors worldwide (48). Although surgery and chemotherapy have a specice, particular effect on their treatment, the 5-year prognosis of patients is severe, mainly due to tumor metastasis and drug resistance (49–51). ALDH1, as a lung CSC marker (52, 53), is associated with a poor prognosis and resistanceto treatment in lung cancer patients (54, 55). High expression of ALDH1 is negatively correlated with patient survival (56). Targeting ALDH1 could be a new strategy to overcome drug resistance (51).
The study shows that ALDH1 promotes functional changes in the glutathione redox system and enhances chemosensitivity in non-small cell lung cancer (57). ALDH1A1 confers resistance to erlotinib by promoting a ROS-active carbonyl species metabolic pathway in lung adenocarcinoma (51). Lung adenocarcinoma cells overexpressing ALDHA1A1 can meet rapid growth of tumor cells or respond to drug stress through the Warburg effect (58). High expression of ALDH1 can be achieved by activating the MEK/ERK signaling pathway (59). S100A9 upregulates ALDH1A1 expression and activates the RA signaling pathway in lung cancer cells (60). Regulation and expression of ALDH1 in lung cancer are mainly related to TSPYL5 (61), STAT3 (62), SOX9 (63), β-catenin (64), MiR-34a/IL-629 (54), miR-326/GNB1 (65), RNAMACC1-AS143 (66), PFKFB346 (67) and other signals and pathways.
Several studies on the treatment of lung cancer have shown that treatment strategies that reduce ALDH1 or target ALDH1 can reduce chemotherapeutic drug resistance and malignant proliferation of lung cancer. The vitamin A/retinoic acid axis depletes ALDH1 positive CSCs and resensitizes drug resistant lung cancer cells to cisplatin (53). Targeting the s100A9-ALDH1A1-retinoic acid signaling pathway inhibits brain recurrence in EGFR-mutant lung cancer (60). Standard drugs for lung cancer treatment, cisplatin/gemcitabine,and menadione, reduced ALDH1 expression (68), while the elimination of ALDH1A1 significantly increased apoptosis and decreased resistance to cisplatin (69). Fat1 overexpression (70), aerobic exercise (71), nilotinib, erlotinib (72), all-trans retinoic acid (73), itraconazole (74), cryptotanshinone (55), CFTR31 (75), fluorescent interleukin (76), pomegranate (77), ginsenoside Rg3 (78), puerarin 6″-O-xyloside (79), glycolysis inhibitor PFK158 (67), globulin (80) can reduce expression of ALDH1 and drug resistance in lung cancer, mainly involved NF-κB pathway, Wnt pathway, Src-STAT3 signaling axis, Wnt/β-catenin/STAT3 axis, Akt/c-Myc signaling pathway. Therefore, in the treatment of lung cancer, targeting ALDH1 has broad prospects and should be further studied and explored.
Colorectal cancer (CRC) is the third most common cancer worldwide and the second leading cause of cancer-related death worldwide (81). Most CRC patients die from recurrence, distant metastasis, and chemotherapy resistance (82, 83), mainly due to a small subpopulation of cells within the tumor called CSCs. Increased expression of ALDH1 is associated with tumor progression and poorer outcomes in CRC patients (11). ALDH1 is abundant in tumor samples from patients with CRC (21, 84), which can be used as a particular marker for CSCs of colorectal cancer (85), mainly including ALDH1A1, ALDH1B1, and ALDH1A3, are associated with poor prognosis and resistance to chemotherapy in colorectal cancer (86–88).
ALDH1 plays a vital role in CRC and promotes the metastasis and proliferation of CRC stem cells through various classical pathways. Studies have suggested that the high expression of ALDH1 in CRC is related to the Wnt/β-catenin pathway (82, 89, 90), hsa_circ_0001806/miR-193a-5p/, COL1A1 axis (89), PI3K/AKT/mTOR signaling pathway (91), CXCL2/CXCR2 axis (92), miR-200-ZEB1/SANI2 axis (88). Additionally, ALDH1B1 may maintain the CSC phenotype and promote cancer cell growth by protecting cells from DNA damage (87). Its presence is closely related to the activation of Wnt/β-catenin (93). LncRNA NEAT1 increases H3K27ac by affecting chromatin remodeling, leading to increased levels of acetylation in the ALDH1 and c-Myc promoter regions to improve the stemness of colorectal cancer cells (83). p53 (94), P2X7R (95), and lncRNA B4GALT1-AS1 (96) can up-regulate ALDH1 expression in CRC.
In an effort to address the abnormal expression and related pathways of ALDH1 in colorectal cancer, targeting or inhibiting ALDH1 has become a new direction for treating colorectal cancer. The study proposes that physciosporin inhibits the stemness of colon cancer cells and the expression of ALDH1 through the Sonic Hedgehog and Notch signaling pathways (97). Similarly, silibinin down-regulates the cancer stemness marker ALDH1 by modulating the E-Cadherin/β-Catenin pathway (98). Furthermore, tumidulin reduces ALDH1 in CRC cells by inhibiting The Hh signaling pathway (99). Inhibiting of ALDH1A1 expression can down-regulate oxidative phosphorylation, mitochondrial function, the sirtuin signaling pathway, cholesterol biosynthesis, and the vitamin A (retinol) metabolism pathway (93). Numerous studies point to inhibition of DCLK1 (100), down-regulation of MUC1-C (101), down-regulation of ALDH1B1 (102), down-regulation of KDM2B (103), down-regulation of NEAT1 (83), polymethoxylated flavones (104), resveratrol (105), ALDH1A3 inhibitor (88), 5-fluorouracil (106), grape pomace (107), montelukast (108), celecoxib targeted therapy (109), puerarin (110), can both down-regulate the expression of ALDH1 in CRC and reduce cell migration, invasion, and chemotherapy resistance. Therefore, the potential value of targeting ALDH1 to improve the efficacy of standard treatment and thus prevent recurrence of colorectal cancer remains to be further investigated.
Liver cancer is the third largest cancer in the world and the leading cause of cancer-related death (111). Liver cancer has a high degree of malignancy and is easy to metastasize, and most patients are in the middle and late stages when diagnosed (112). Most patients with advanced liver cancer are treated with chemotherapy, but are, incredibly prone to drug resistance, leading to failure of treatment, mainly related to liver CSCs (113). ALDH1 is considered a marker of liver cancer stem cells, and the high expression of ALDH1A1 is closely related to recurrence of liver cancer (114). Studies have found that PFKP can promote reverse transcriptional activation of β-catenin, leading to the expression of ALDH1 in liver cancer stem (115). Abnormally expressed PDK1 can covalently bind to the inactive ALDH1A1 apoenzyme (apoALDH1A1), forming the catalytically active ALDH1A1 holoenzyme (holoALDH1A1), thus activating ALDH1, leading to desensitization of liver cancer to radiation therapy (116). LncRNASNHG5 promotes hepatocellular carcinoma proliferation and tumor stem cell-like ALDH1 properties by regulating the UPF1 and Wnt signaling pathways (117). LncRNA LINC00460 regulates liver cancer cell proliferation by targeting the miR-503-5p/miR-654-3p/TCP1 axis (118). Long noncoding RNA MACC1-AS1 promotes ALDH1 in stem cells from hepatocellular carcinoma by antagonizing miR-145 (119). Carboxypeptidase A4 upregulates ALDH1 expression and promotes proliferation of hepatocellular carcinoma (120).
Studies have found that inhibiting or targeting ALDH1 liver cancer stem cells can reduce cancer proliferation and resistance to treatment. Silencing PFKP can inhibit the liver cancer stemness marker ALDH1 (121). Silencing shRNA-mediated MALAT1 significantly inhibited ALDH1 activity, partly due to the inhibition of the MALAT1/Wnt/β-Catenin pathway (122). STARD13 overexpression can reduce ALDH1 activity and improve 5-FU sensitivity in liver cancer, which is positively correlated with a good prognosis (123). Limonin reduces cell quiescence and reduces ALDH1 stem in liver cancer cells by activating PI3K/Akt signaling (124). UTI, inhibits Wnt/β-catenin signaling and attenuates the ALDH1-sensitivity of the liver cancer stem to FU (113). The box protein FBXO11 reduces ALDH1 activity and hepatocellular carcinoma stemness by promoting ubiquitin-mediated inhibition of snail degradation (125). Overexpression of TRPV2 minimizes the liver cancer stem cell marker ALDH1 (126). Therefore, targeting or inhibiting ALDH1 in liver cancer can enhance chemosensitivity, and provide potential novel therapeutic strategies and new insights for developing new therapeutic targets for liver cancer.
Gastric cancer (GC) ranks fifth in incidence (5) and is one of the leading causes of cancer-related deaths. GC is usually diagnosed at an advanced stage when the tumor is inoperable and only chemotherapy may be a helpful method (127). Although traditional chemotherapy can significantly improve the survival rate of gastric cancer patients, chemotherapy alone is still very limited and has reached a bottleneck (128). Therefore, it is urgent to find new targets for molecularly targeted therapy of GC. GCs with histological diversity show a poor prognosis and characteristic expression of the cancer stem cell-associated molecule ALDH1 (129). High ALDH1 expression is associated with poor prognosis in gastric neuroendocrine carcinoma (130, 131). The presentation of ALDH1 in gastric cancer tissue was significantly higher than in normal tissue, The manifestation of ALDH1 was significantly correlated with tumor grade, tumor stage, lymph node metastasis, tumor metastasis stage, and overall survival of patients (132).
The present results suggest that ALDH1 overexpression may be involved in the occurrence, invasion, and metastasis of GAC, leading to a poor prognosis (132). High expression of ALDH1 in GC cells improves stem cell properties and antagonizes the action of macrophages, thus affecting cell viability, anti-apoptosis, invasion, migration, and cloning ability. ALDH1 is positively correlated with helicobacter pylori infection. When ALDH1 is overexpressed, it mainly restores the reduction of drug resistance caused by miR-625 overexpression. It is involved in the regulation of various genes and proteins in the process of GC occurrence and development (5). Furthermore, GC cells with high expression of ALDH1 can evade the deadly effect of macrophages by antagonizing tumor necrosis factor α and other effector molecules secreted by macrophages and increase tumor proliferation and invasion ability (133). Studies have shown that overexpression of TAZ (134), upregulation of A1 in cancer-testes (135), and knockdown of HMGA2 (136) can promote the high expression of ALDH1 in the GC and tumor growth.
With extensive research on ALDH1 in recent years, it has been found that targeting drugs can inhibit the growth of GC and reverse drug resistance by inhibiting ALDH1expression. All-trans retinoic acid appears to inhibit tumor growth, target gastric CSCs, and reduce ALDH1 expression (137). ALDH1A1 silencing inhibits cell viability by modulating Wnt signaling in the migration and invasion of MKN-45 cells. Small interference RNA, high expression of Ror β, salinomycin, and FoxM1 siRNA transfection inhibited the expression and invasive capacity of ALDH1 in GC (5). Silencing miR-95 or overexpressing a miR-95 inhibitor (dual specificity phosphatase 5) inhibited ALDH1 expression in GC cells (138). BRD4 promotes the stemness of gastric cancer cells and reduces ALDH1 activity by attenuating the mir-216a-3p-mediated inhibition of the Wnt/β-catenin signaling pathway (134). Therefore, ALDH1 may be a new target for related tumor suppressors and stem cells.
Cervical cancer is the fourth most common cancer and the most common gynecological malignancy (139), of which squamous cell carcinoma (SCC) is the most common type, accounting for approximately 80% of all cervical cancers (140). Treatment of advanced cervical cancer includes surgery, chemotherapy, and radiation therapy, but cervical cancer mortality remains high (141), mainly related to CSCs (142). Studies have suggested that ALDH1 can be used as a cervical CSC marker (143, 144), which can lead to resistance to chemotherapy and is closely related to a poor prognosis (145).
Studies have shown that ALDH1 is the target of miR-222, and miR-222 can bind to the 3’untranslated seed region of ALDH1 mRNA to regulate its expression, resulting in an elevated expression level of ALDH1 in cervical cancer (146). At the same time, studies have indicated that ALDH1 expression in cervical cancer is closely related to the Erk1/2 and Akt signaling pathways (147). Hypoxia promotes ALDH-1 expression in radioresistant cells, and ALDH-1-positive cells promote radioresistance in cervical cancer by preferentially activating DNA damage checkpoint responses and increasing DNA repair capacity (143). ALDH1 expression is associated with higher cell proliferation, spheroid formation, migration, and tumor incidence in cervical cancer cells, which exhibit chemo and radioresistance (148).
In recent years, some achievements have been made in inhibitory drugs or targeted therapy for ALDH1. For example, PM01183 (149), zoledronic acid (147), and limonin (150) can inhibit the activity of the cervical CSC marker ALDH1, weaken the stemness of cervical cancer cells, and waste their chemoresistance. Therefore, ALDH1 may be a new target for cervical cancer, which may provide a new and promising strategy for anticancer therapy, which is worthy of further exploration.
Esophageal cancer is the sixth most common cause of cancer death worldwide (151), and 90% of esophageal cancers are esophageal squamous cell carcinoma (ESCC), which has an inferior, an abysmal prognosis and high mortality (152). Esophageal cancer has a low 5-year survival rate and lacks effective therapeutic targets (153). In ESCC, ALDH1 is a more reliable CSC marker, and high expression of ALDH1 is associated with poor differentiation from ESCC and poor prognosis (154, 155).
Studies have shown that ALDH1A1 can activate AKT, interact with β-catenin, and start the wnt/β-catenin signaling pathway to maintain the CSC properties of ESCC (23). The study suggested that the high expression of ALDH1A1 is also associated with the promoting EMT in ESCC (156). ALDH1 plays a vita role in tumor aggressiveness and is associated with the pro-tumor microenvironment of esophageal cancer, mainly involving the IL-6/STAT3 pathway and factors such as p-STAT3, MDSC2 (12). Similarly, ALDH1A1 knockdown or the use of the small molecule inhibitor NCT-501 reduced the level of AKT phosphorylationin in A549/DDP cells and inhibited the AKT-β-catenin signaling pathway (23). Animal experiments demonstrated that COX-2 inhibition reduced ALDH1 and IL-6 expression levels, attenuated MDSC recruitment, and subsequently slowed esophageal tumors (12). Tranilast significantly reduced the strong expression of ALDH1A1 in TE8 cells (157). Additionally, CA3 and LEE011 can jointly inhibit the YAP1 and CDK6 pathways, significantly reduce the growth of esophageal cancer cells and cancer stem cell ALDH1, sensitize cells to radiation, and show strong antitumor effects in vivo (158). Therefore, ALDH1 is expected to become a new direction for esophageal cancer stem cell research.
Ovarian cancer (OC) is the most lethal gynecological malignancy and ranks first in cancer mortality among female malignancies (159). Epithelial ovarian cancer (EOC) accounts for 95% of ovarian malignancies, and is the most common OC (160). The prognosis of OC treatment is unsatisfactory mainly, probably due to the presence of ovarian cancer stem cells (OCSC) and chemoresistance (7, 160). In OC, high ALDH1 is a hallmark of OCSC (161–163) and is strongly associated with poor prognosis, and resistance to chemotherapy (164–166).
Studies support that OC patients with high expression of the ALDH1A1 stemness gene have an attenuated response to platinum-containing chemotherapy (167) and that ALDH1A1 may be involved in acquired resistance to cisplatin through upregulation of NEK-2 in OC (166). Platinum-induced secretion of IL-6 from cancer-associated fibroblasts in the tumor microenvironment promotes the enrichment of OCSC in residual tumors after chemotherapy through activation of STAT3 and up-regulation of ALDH1A1 expression (45). ALDH1 activity has also been reported to be positively regulated by the BET family protein BRD4, which is capable of to up-regulate ALDH1A1 transcription through super-enhancer elements (168). AhR can also mediate OC progression, a stem ALDH1 signature, by activating PI3K/Akt, Wnt/β-catenin, and EMT (169). Furthermore, the expression and activity of ALDH1A1 can be regulated by β-Catenin, the EZH2 enhancer, and the bromodomain and extra terminal (BET) protein family (170).
Recent studies have suggested that customized therapy targeting ALDH1 can reduce resistance to chemotherapy and improve the survival rate of OC. ALDH1A1 knockdown can reverse the resistance to OC chemotherapy (168). Dual inhibition of Src and MEK reduces OC growth and targets ALDH1 (171). Consistent with these, ALDH1 inhibition effectively blocks the proliferation and survival of OC spheroids (167), and aldehyde dehydrogenase inhibitors promote OC DNA damage (172). IL-6-Nab combined with HMA completely eradicated OCSCs, and this combination blocked IL-6/IL6-R/pSTAT3-mediated ALDH1A1 expression, providing a strategy for tumor recurrence after chemotherapy (173). NAMPT inhibitors and 4-MU treatment reduce ALDH1 protein expression, which reduces chemoresistance and OC cell growth (174, 175). BET inhibitors inhibit ALDH activity by abrogating BRD4-mediated expression of ALDH1A (176). DDB2 inhibits OC cell dedifferentiation by inhibiting ALDH1A1 (170). A selective inhibitor of the ALDH1A family (ALDH1Ai) reduces tumor-initiating capacity and chemoresistance (177). With the continuous in-depth understanding of ALDH1 and drug resistance mechanisms in OC, the development of drugs targeting ALDH1 is expected to become a new direction for treating OC.
Head and neck carcinomas (HNCs) are the sixth most common cancer worldwide (178), which are characterized by the unregulated growth of tumor cells in various parts of the head and neck, such as the buccal mucosa, floor of the mouth, tongue, oropharynx, hypopharynx, esophagus, nasopharynx, and salivary glands (179), and more than 90% of HNCs are squamous cell carcinomas (HNSCC) (180). HNCs have poor survival, and poor prognosis (181), and CSCs have been attributed to poor treatment outcomes and survival in HNSCC (13). ALDH1 is associated with tumor malignancy and self-renewal properties of stem cells in HNCs (182) and has been associated with poorer prognosis and failure of chemoradiotherapy in HNCs (13, 183). ALDH1 acts as a stem cell marker for HNCs such as oral cancer (184), eyelid sebaceous gland carcinoma (185), benign epithelial odontogenic lesions (186), salivary gland tumor (187, 188), lip cancer (189), sublingual adenoma (190).
Studies have shown that in HNCs, ALDH1 is mainly regulated by retinoic acid compounds and other oncogenic pathways such as MUC1-C/ERK and WNT/β-catenin (191), but also by the AKT signaling pathway (192, 193). ALDH1A1 increases TUBB3 expression, which down-regulates PTEN and promotes cell proliferation, migration, and invasion (194). Inhibition of miR-30a and miR-379 upregulates DNMT3B expression, which leading to hypermethylation of the ALDH1A gene, and promotes oncogenic activity (195, 196). Expression of ATAD2 (154), VIM, or ZEB2 (197) promotes ALDH1 expression. Similarly, the ovatodiolide can inhibit ALDH1 activity and stemness properties by inhibiting JAK2/STAT3/JARID1B signaling (198). The soy isoflavone genistein hinders stem cells in HNCs by activating the miR-34a/RTCB axis and reducing ALDH1 activity (199). Butylene phthalide (200), honokiol (201), elimination of long noncoding RNA MACC1-AS1 (202), resveratrol (203), can reduce ALDH1 activity and inhibit the stemness of HNCs. Therefore, ALDH1 is a possible drug target in HNCs.
In recent years, a large number of studies have shown that ALDH1 is also closely related to other solid tumors.The overexpression of ALDH1 is associated with a poor prognosis and malignant tumor development, such as melanoma (204), glioma (205), prostate cancer (206, 207), endometrial cancer (6, 208), bladder cancer (209), Osteosarcoma (210), pancreatic cancer (211, 212), kidney cancer (213), etc. ALDH1, overexpressed in melanoma, promotes tumor angiogenesis mainly by activating IL-8/Notch signaling (214). Additionally, ALDH1A1 can metabolize retinal RA, thus activating RAR-mediated transcription of downstream targets, such as TUBB3, in bladder cancer cells (209). ALDH1A3 promotes pancreatic cancer metastasis through its metabolic effects on glucose metabolism, and PPARγ and its downstream PI3K/AKT/mTOR signaling pathway may be involved in this process (215). Furthermore, the expression of ALDH1 in these solid tumors is also affected by Notch1 signaling (216), ILK signaling (217), Hippo signaling (218), miR-761 (219), ARID1A deficiency (220), IL17 (221), etc.
Similarly, inhibition or targeting of ALDH1 can alleviate the progression of these solid tumors, such as acid sensor ASIC1a (222), depletion of TP73-AS1 (223), eprinomectin (224), silencing of YAP (218), MicroRNA-487b-3p (225), miR-199 (226), CTSB knockdown (227). Therefore, ALDH1 is expected to become a corresponding therapeutic target for the solid tumors mentioned above.
By combing all articles on ALDH1 and solid tumors in the last five years, it is found that ALDH1 can be used as a marker of tumor stem cells, expressed in a variety of malignant tumor tissues; the expression level of ALDH1 is a marker that distinguishes normal stem cells from cancer stem cells. ALDH1 can maintain stem cell characteristics and lead to drug resistance, which is the key to tumor recurrence and is difficult to cure. Preclinical studies have shown that ALDH1 plays an essential role in the occurrence, invasion, and metastasis of different cancers through various pathways (20) (220) (22) (23). In recent years, many scholars have studied ALDH1 inhibitors and targeting ALDH1, but there is no clinical evidence on the efficacy and safety of their inhibition in solid tumors. Although none of these newer compounds has entered clinical trials and are still in the early stages of ALDH1-targeted therapy development, they have shown promising effects and have an encouraging future for ALDH1-targeted drugs. Therefore, further research will be crucial to make ALDH1 a therapeutic target.
YW and YL drafted the manuscript. All authors contributed to the article and approved the submitted version.
This study was supported by the following grants: Hubei Department of Education Science Research project (grant no. Q20192802) for QW, National Natural Science Foundation of China Youth Science Foundation project (grant no. 81902937) for YS.
The authors thank the School of Basic Medicine Sciences, Xianning Medical College, Hubei University of Science and Technology.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CSCs, cancer stem cells; ALDH1, acetaldehyde dehydrogenase 1; RA, retinoic acid; RAR, retinoic acid receptor; RXR, retinoic acid X receptor; PPAR/β/δ, peroxisome proliferator-activated receptor β/δ; ROS, reactive oxygen species; ATRA, all-trans retinoic acid; DEAB, N,N-diethylaminobenzaldehyde; CRC, colorectal cancer; GC, gastric cancer; ESCC, esophageal squamous cell carcinoma; OC, ovarian cancer; EOC, Epithelial ovarian cancer; OCSC, ovarian cancer stem cells; BET, bromodomain and extraterminal; HNSCC, Headneck squamous cell carcinoma.
1. International Agency for Research on Cancer Statistics. (2020). (Lyon, French). Available at: https://www.iarc.who.int/research-home/.
2. Jagust P, de Luxán-Delgado B, Parejo-Alonso B, Sancho P. Metabolism-based therapeutic strategies targeting cancer stem cells. Front Pharmacol (2019) 10:203. doi: 10.3389/fphar.2019.00203
3. Yi L, Zhou X, Li T, Liu P, Hai L, Tong L, et al. Notch1 signaling pathway promotes invasion, self-renewal and growth of glioma initiating cells via modulating chemokine system CXCL12/CXCR4. J Exp Clin Cancer Res: CR (2019) 38(1):339. doi: 10.1186/s13046-019-1319-4
4. Raghunathan L, Hsu LC, Klisak I, Sparkes RS, Yoshida A, Mohandas T. Regional localization of the human genes for aldehyde dehydrogenase-1 and aldehyde dehydrogenase-2. Genomics (1988) 2(3):267–9. doi: 10.1016/0888-7543(88)90012-2
5. Wang L, Wang L, Yu Y, Su R, Zhang Y, Li Y, et al. Aldehyde dehydrogenase 1 in gastric cancer. J Oncol (2022) 2022:5734549. doi: 10.1155/2022/5734549
6. Mah V, Elshimali Y, Chu A, Moatamed NA, Uzzell JP, Tsui J, et al. ALDH1 expression predicts progression of premalignant lesions to cancer in type I endometrial carcinomas. Sci Rep (2021) 11(1):11949. doi: 10.1038/s41598-021-90570-3
7. Srivastava M, Ahlawat N, Srivastava A. Ovarian cancer stem cells: Newer horizons. J Obstetrics Gynaecol India (2021) 71(2):115–7. doi: 10.1007/s13224-020-01412-7
8. Roudi R, Korourian A, Shariftabrizi A, Madjd Z. Differential expression of cancer stem cell markers ALDH1 and CD133 in various lung cancer subtypes. Cancer Invest (2015) 33(7):294–302. doi: 10.3109/07357907.2015.1034869
9. Javed S, Sood S, Rai B, Bhattacharyya S, Bagga R, Srinivasan R. ALDH1 & CD133 in invasive cervical carcinoma & their association with the outcome of chemoradiation therapy. Indian J Med Res (2022) 54(2):367–74. doi: 10.4103/ijmr.IJMR_709_20
10. Sarkar P, Basu K, Sarkar P, Chatterjee U, Mukhopadhyay M, Choudhuri MK, et al. Correlations of aldehyde dehydrogenase-1 (ALDH1) expression with traditional prognostic parameters and different molecular subtypes of breast carcinoma. Clujul Med (2018) 91(2):181–7. doi: 10.15386/cjmed-925
11. Rezaee M, Gheytanchi E, Madjd Z, Mehrazma M. Clinicopathological significance of tumor stem cell markers ALDH1 and CD133 in colorectal carcinoma. Iranian J Pathol (2021) 16(1):40–50. doi: 10.30699/ijp.2020.127441.2389
12. Chen MF, Chen PT, Lu MS, Chen WC. Role of ALDH1 in the prognosis of esophageal cancer and its relationship with tumor microenvironment. Mol Carcinog (2018) 57(1):78–88. doi: 10.1002/mc.22733
13. Dubey P, Kumar N, Gupta R, Mishra A, Bhadauriya S, Kumar V, et al. Association of ALDH1 with response to radiotherapy and its impact on survival in patients with advanced stage of head and neck squamous cell carcinoma (HNSCC). Asian Pacific J Cancer Prevention: APJCP (2022) 23(2):419–27. doi: 10.31557/APJCP.2022.23.2.419
14. Tiwari N, Srivastava AN, Tandon N, Lal N, Yadav S, Kant S, et al. A prospective study of association of cancer stem cell marker aldehyde dehydrogenase 1 with clinicopathological profile in lung carcinoma patients. Indian J Pathol Microbiol (2018) 61(4):489–94. doi: 10.4103/IJPM.IJPM_318_17
15. Marcato P, Dean CA, Giacomantonio CA, Lee PW. Aldehyde dehydrogenase: its role as a cancer stem cell marker comes down to the specific isoform. Cell Cycle (Georgetown Tex.) (2011) 10(9):1378–84. doi: 10.4161/cc.10.9.15486
16. Black WJ, Stagos D, Marchitti SA, Nebert DW, Tipton KF, Bairoch A, et al. Human aldehyde dehydrogenase genes: alternatively spliced transcriptional variants and their suggested nomenclature. Pharmacogenetics Genomics (2009) 19(11):893–902. doi: 10.1097/FPC.0b013e3283329023
17. Rodriguez-Torres M, Allan AL. Aldehyde dehydrogenase as a marker and functional mediator of metastasis in solid tumors. Clin Exp Metastasis (2016) 33(1):97–113. doi: 10.1007/s10585-015-9755-9
18. Ding M. Aldehyde dehydrogenase 1 (aldh1) genes in cancer clinical prognosis outcomes (2016). Available at: https://elischolar.library.yale.edu/ysphtdl/1066.
19. Yue H, Hu Z, Hu R, Guo Z, Zheng Y, Wang Y, et al. ALDH1A1 in cancers: Bidirectional function, drug resistance, and regulatory mechanism. Front Oncol (2022) 12:918778. doi: 10.3389/fonc.2022.918778
20. Vassalli G. Aldehyde dehydrogenases: Not just markers, but functional regulators of stem cells. Stem Cells Int (2019) 2019:3904645. doi: 10.1155/2019/3904645
21. Liu L, Liu Y, Xia Y, Wang G, Zhang X, Zhang H, et al. Synergistic killing effects of PD-L1-CAR T cells and colorectal cancer stem cell-dendritic cell vaccine-sensitized T cells in ALDH1-positive colorectal cancer stem cells. J Cancer (2021) 12(22):6629–39. doi: 10.7150/jca.62123
22. Ciccone V, Terzuoli E, Donnini S, Giachetti A, Morbidelli L, Ziche M. Stemness marker ALDH1A1 promotes tumor angiogenesis via retinoic acid/HIF-1α/VEGF signalling in MCF-7 breast cancer cells. J Exp Clin Cancer Res: CR (2018) 37(1):311. doi: 10.1186/s13046-018-0975-0
23. Wang W, He S, Zhang R, Peng J, Guo D, Zhang J, et al. ALDH1A1 maintains the cancer stem-like cells properties of esophageal squamous cell carcinoma by activating the AKT signal pathway and interacting with β-catenin. Biomed Pharmacother = Biomed Pharmacotherapie (2020) 125:109940. doi: 10.1016/j.biopha.2020.109940
24. Gyan E, Green A, Ahenkorah-Fondjo L, Jackson A, Toss MS, Akakpo PK, et al. The role of ALDH1A1 in contributing to breast tumour aggressiveness: A study conducted in an African population. Ann Diagn Pathol (2021) 51:151696. doi: 10.1016/j.anndiagpath.2020.151696
25. Plichta JK, Thomas SM, Sergesketter AR, Greenup RA, Fayanju OM, Rosenberger LH, et al. Clinical and pathological stage discordance among 433,514 breast cancer patients. Am J Surg (2019) 218(4):669–76. doi: 10.1016/j.amjsurg.2019.07.016
26. Hapidah H, Djabir YY, Prihantono P. Increased aldehyde dehydrogenase 1 (ALDH1) levels are associated with chemo-responsiveness in breast cancer patients treated with taxane-adriamycin-cyclophosphamide regimen. Breast Dis (2021) 40(S1):S33–7. doi: 10.3233/BD-219005
27. Lee JS, Kim WG. Cutaneous metastases of breast cancer during adjuvant chemotherapy correlates with increasing CD44+/CD24-and ALDH-1 expression: a case report and literature review. Stem Cell Invest (2018) 5:7. doi: 10.21037/sci.2018.03.04
28. Köy Y, Dirilenoglu F, Tetikkurt Ü., Muhammedoğlu A, Çelik A. Aldehyde dehydrogenase-1 positivity is associated with ER negativity in patients with invasive ductal carcinoma of the breast. Polish J Pathol (2020) 71(3):254–60. doi: 10.5114/pjp.2020.99792
29. Louhichi T, Ziadi S, Saad H, Dhiab MB, Mestiri S, Trimeche M. Clinicopathological significance of cancer stem cell markers CD44 and ALDH1 expression in breast cancer. Breast Cancer (Tokyo Japan) (2018) 25(6):698–705. doi: 10.1007/s12282-018-0875-3
30. Xing P, Dong H, Liu Q, Zhao T, Yao F, Xu Y, et al. ALDH1 expression and vasculogenic mimicry are positively associated with poor prognosis in patients with breast cancer. Cell Physiol Biochem (2018) 49(3):961–70. doi: 10.1159/000493227
31. Shima H, Kida K, Adachi S, Yamada A, Sugae S, Narui K, et al. Lnc RNA H19 is associated with poor prognosis in breast cancer patients and promotes cancer stemness. Breast Cancer Res Treat (2018) 170(3):507–16. doi: 10.1007/s10549-018-4793-z
32. Ozaki A, Motomura H, Tamori S, Onaga C, Nagashima Y, Kotori M, et al. High expression of p62 and ALDH1A3 is associated with poor prognosis in luminal b breast cancer. Anticancer Res (2022) 42(7):3299–312. doi: 10.21873/anticanres.15818
33. Cole AJ, Fayomi AP, Anyaeche VI, Bai S, Buckanovich RJ. An evolving paradigm of cancer stem cell hierarchies: therapeutic implications. Theranostics (2020) 10(7):3083–98. doi: 10.7150/thno.41647
34. Liu C, Qiang J, Deng Q, Xia J, Deng L, Zhou L, et al. ALDH1A1 activity in tumor-initiating cells remodels myeloid-derived suppressor cells to promote breast cancer progression. Cancer Res (2021) 81(23):5919–34. doi: 10.1158/0008-5472.CAN-21-1337
35. Cui B, Luo Y, Tian P, Peng F, Lu J, Yang Y, et al. Stress-induced epinephrine enhances lactate dehydrogenase a and promotes breast cancer stem-like cells. J Clin Invest (2019) 129(3):1030–46. doi: 10.1172/JCI121685
36. Hata T, Rajabi H, Yamamoto M, Jin C, Ahmad R, Zhang Y, et al. Targeting MUC1-c inhibits TWIST1 signaling in triple-negative breast cancer. Mol Cancer Ther (2019) 18(10):1744–54. doi: 10.1158/1535-7163.MCT-19-0156
37. Poturnajova M, Kozovska Z, Matuskova M. Aldehyde dehydrogenase 1A1 and 1A3 isoforms - mechanism of activation and regulation in cancer. Cell Signalling (2021) 87:110120. doi: 10.1016/j.cellsig.2021.110120
38. Dehghan Harati M, Rodemann HP, Toulany M. Nanog signaling mediates radioresistance in ALDH-positive breast cancer cells. Int J Mol Sci (2019) 20(5):1151. doi: 10.3390/ijms20051151
39. Tang W, Li M, Qi X, Li J. β1,4-galactosyltransferase V modulates breast cancer stem cells through wnt/β-catenin signaling pathway. Cancer Res Treat (2020) 52(4):1084–102. doi: 10.4143/crt.2020.093
40. Kumari M, Krishnamurthy PT, Sola P. Targeted drug therapy to overcome chemoresistance in triple-negative breast cancer. Curr Cancer Drug Targets (2020) 20(8):559–72. doi: 10.2174/1568009620666200506110850
41. Shi L, Tang X, Qian M, Liu Z, Meng F, Fu L, et al. A SIRT1-centered circuitry regulates breast cancer stemness and metastasis. Oncogene (2018) 37(49):6299–315. doi: 10.1038/s41388-018-0370-5
42. Hao J, Zhang Y, Yan X, Yan F, Sun Y, Zeng J, et al. Circulating adipose fatty acid binding protein is a new link underlying obesity-associated Breast/Mammary tumor development. Cell Metab (2018) 28(5):689–705.e5. doi: 10.1016/j.cmet.2018.07.006
43. Dai X, Cai D, Wang P, Nan N, Yu L, Zhang Z, et al. Cold atmospheric plasmas target breast cancer stemness via modulating AQP3-19Y mediated AQP3-5K and FOXO1 K48-ubiquitination. Int J Biol Sci (2022) 18(8):3544–61. doi: 10.7150/ijbs.72296
44. Su Z, Wang C, Chang D, Zhu X, Sai C, Pei J. Limonin attenuates the stemness of breast cancer cells via suppressing MIR216A methylation. Biomed Pharmacother = Biomed Pharmacotherapie (2019) 112:108699. doi: 10.1016/j.biopha.2019.108699
45. Wang R, Yang L, Li S, Ye D, Yang L, Liu Q, et al. Quercetin inhibits breast cancer stem cells via downregulation of aldehyde dehydrogenase 1A1 (ALDH1A1), chemokine receptor type 4 (CXCR4), mucin 1 (MUC1), and epithelial cell adhesion molecule (EpCAM). Med Sci Monitor (2018) 24:412–20. doi: 10.12659/msm.908022
46. Attia YM, El-Kersh DM, Ammar RA, Adel A, Khalil A, Walid H, et al. Inhibition of aldehyde dehydrogenase-1 and p-glycoprotein-mediated multidrug resistance by curcumin and vitamin D3 increases sensitivity to paclitaxel in breast cancer. Chemico-biological Interact (2020) 315:108865. doi: 10.1016/j.cbi.2019.108865
47. O’Conor CJ, Chen T, González I, Cao D, Peng Y. Cancer stem cells in triple-negative breast cancer: a potential target and prognostic marker. Biomarkers Med (2018) 12(7):813–20. doi: 10.2217/bmm-2017-0398
48. Mattiuzzi C, Lippi G. Cancer statistics: a comparison between world health organization (WHO) and global burden of disease (GBD). Eur J Public Health (2020) 30(5):1026–7. doi: 10.1093/eurpub/ckz216
49. Ying J, Yang J, Liu Y. LncARSR promotes non-small-cell lung cancer progression via regulating PTEN/Akt. Am J Trans Res (2020) 12(3):857–66.
50. Khan P, Bhattacharya A, Sengupta D, Banerjee S, Adhikary A, Das T. Aspirin enhances cisplatin sensitivity of resistant non-small cell lung carcinoma stem-like cells by targeting mTOR-akt axis to repress migration. Sci Rep (2019) 9(1):16913. doi: 10.1038/s41598-019-53134-0
51. Lei HM, Zhang KR, Wang CH, Wang Y, Zhuang GL, Lu LM, et al. Aldehyde dehydrogenase 1A1 confers erlotinib resistance via facilitating the reactive oxygen species-reactive carbonyl species metabolic pathway in lung adenocarcinomas. Theranostics (2019) 9(24):7122–39. doi: 10.7150/thno.35729
52. Kanayama T, Taniguchi T, Tomita H, Niwa A, Noguchil K, Matsuo M, et al. ALDH1 and SALL4 expression in cell block samples from patients with lung adenocarcinoma and malignant pleural effusion. Diagnostics (Basel Switzerland) (2021) 11(8):1463. doi: 10.3390/diagnostics11081463
53. MacDonagh L, Santiago RM, Gray SG, Breen E, Cuffe S, Finn SP, et al. Exploitation of the vitamin a/retinoic acid axis depletes ALDH1-positive cancer stem cells and re-sensitises resistant non-small cell lung cancer cells to cisplatin. Trans Oncol (2021) 14(4):101025. doi: 10.1016/j.tranon.2021.101025
54. Li Y, Wang B, Gui S, Ji J. Multiple copies in T-cell malignancy 1 (MCT-1) promotes the stemness of non-small cell lung cancer cells via activating interleukin-6 (IL-6) signaling through suppressing MiR-34a expression. Med Sci Monitor (2019) 25:10198–204. doi: 10.12659/MSM.919690
55. Jin L, Wu Z, Wang Y, Zhao X. Cryptotanshinone attenuates the stemness of non-small cell lung cancer cells via promoting TAZ translocation from nuclear to cytoplasm. Chin Med (2020) 15:66. doi: 10.1186/s13020-020-00348-4
56. Prabavathy D, Swarnalatha Y, Ramadoss N. Lung cancer stem cells-origin, characteristics and therapy. Stem Cell Invest (2018) 5:6. doi: 10.21037/sci.2018.02.01
57. Rebollido-Rios R, Venton G, Sánchez-Redondo S, Iglesias I Felip C, Fournet G, González E, et al. Dual disruption of aldehyde dehydrogenases 1 and 3 promotes functional changes in the glutathione redox system and enhances chemosensitivity in nonsmall cell lung cancer. Oncogene (2020) 39(13):2756–71. doi: 10.1038/s41388-020-1184-9
58. Wang Y, Wang CH, Zhang YF, Zhu L, Lei HM, Tang YB. UPLC-MS-based metabolomics reveals metabolic dysregulation in ALDH1A1-overexpressed lung adenocarcinoma cells. Metabolomics (2019) 15(4):52. doi: 10.1007/s11306-019-1514-5
59. Tian S, Liu DH, Wang D, Ren F, Xia P. Aldehyde dehydrogenase 1 (ALDH1) promotes the toxicity of TRAIL in non-small cell lung cancer cells via post-transcriptional regulation of MEK-1 expression. Cell Physiol Biochem (2018) 51(1):217–27. doi: 10.1159/000495202
60. Biswas AK, Han S, Tai Y, Ma W, Coker C, Quinn SA, et al. Targeting S100A9-ALDH1A1-Retinoic acid signaling to suppress brain relapse in EGFR-mutant lung cancer. Cancer Discovery (2022) 12(4):1002–21. doi: 10.1158/2159-8290.CD-21-0910
61. Kim IG, Lee JH, Kim SY, Heo CK, Kim RK, Cho EW. Targeting therapy-resistant lung cancer stem cells via disruption of the AKT/TSPYL5/PTEN positive-feedback loop. Commun Biol (2021) 4(1):778. doi: 10.1038/s42003-021-02303-x
62. Lee JH, Choi SI, Kim RK, Cho EW, Kim IG. Tescalcin/c-Src/IGF1Rβ-mediated STAT3 activation enhances cancer stemness and radioresistant properties through ALDH1. Sci Rep (2018) 8(1):10711. doi: 10.1038/s41598-018-29142-x
63. Voronkova MA, Rojanasakul LW, Kiratipaiboon C, Rojanasakul Y. The SOX9-aldehyde dehydrogenase axis determines resistance to chemotherapy in non-Small-Cell lung cancer. Mol Cell Biol (2020) 40(2):e00307–19. doi: 10.1128/MCB.00307-19
64. Choi SI, Lee JH, Kim RK, Jung U, Kahm YJ, Cho EW, et al. HSPA1L enhances cancer stem cell-like properties by activating IGF1Rβ and regulating β-catenin transcription. Int J Mol Sci (2020) 21(18):6957. doi: 10.3390/ijms21186957
65. Fan Z, Bai Y, Zhang Q, Qian P. CircRNA circ_POLA2 promotes lung cancer cell stemness via regulating the miR-326/GNB1 axis. Environ Toxicol (2020) 35(10):1146–56. doi: 10.1002/tox.22980
66. Wang X, Yu X, Wei W, Liu Y. Long noncoding RNA MACC1-AS1 promotes the stemness of nonsmall cell lung cancer cells through promoting UPF1-mediated destabilization of LATS1/2. Environ Toxicol (2020) 35(9):998–1006. doi: 10.1002/tox.22936
67. Thirusangu P, Ray U, Sarkar Bhattacharya S, Oien DB, Jin L, Staub J, et al. PFKFB3 regulates cancer stemness through the hippo pathway in small cell lung carcinoma. Oncogene (2022) 41(33):4003–17. doi: 10.1038/s41388-022-02391-x
68. Soltanian S, Sheikhbahaei M. Effect of menadione and combination of gemcitabine and cisplatin on cancer stem cells in human non-small cell lung cancer (NSCLC) cell line A549. Iranian J Pharm Res: IJPR (2021) 20(1):105–17. doi: 10.22037/ijpr.2020.112373.13715
69. Fei X, Wang G, Shen H, Gu X. Placenta-specific 8 is a potential novel target for osimertinib resistance in non-small cell lung cancer. Oncol Lett (2019) 18(1):955–61. doi: 10.3892/ol.2019.10344
70. Li M, Zhong Y, Wang M. Fat1 suppresses the tumor-initiating ability of nonsmall cell lung cancer cells by promoting yes-associated protein 1 nuclear-cytoplasmic translocation. Environ Toxicol (2021) 36(11):2333–41. doi: 10.1002/tox.23347
71. Yang H, Liu Y, Kong J. Effect of aerobic exercise on acquired gefitinib resistance in lung adenocarcinoma. Trans Oncol (2021) 14(11):101204. doi: 10.1016/j.tranon.2021.101204
72. Li Z, Tian J, Du L, Gao Y, Wang Y, You F, et al. Anlotinib exerts anti-cancer efficiency on lung cancer stem cells in vitro and in vivo through reducing NF-κB activity. J Cell Mol Med (2021) 25(12):5547–59. doi: 10.1111/jcmm.16564
73. Yao W, Wang L, Huang H, Li X, Wang P, Mi K, et al. All-trans retinoic acid reduces cancer stem cell-like cell-mediated resistance to gefitinib in NSCLC adenocarcinoma cells. BMC Cancer (2020) 20(1):315. doi: 10.1186/s12885-020-06818-0
74. Chen C, Zhang W. Itraconazole alters the stem cell characteristics of A549 and NCI-H460 human lung cancer cells by suppressing wnt signaling. Med Sci Monito (2019) 25:9509–16. doi: 10.12659/MSM.919347
75. Li H, Wang Y, Yang J, Liu X, Shi J. Impact of cystic fibrosis transmembrane conductance regulatoron malignant properties of KRAS mutant lung adenocarcinoma cancer A549 cells. Zhongguo fei ai za zhi = Chin J Lung Cancer (2018) 21(2):89–98. doi: 10.3779/j.issn.1009-3419.2018.02.03
76. Bhummaphan N, Petpiroon N, Prakhongcheep O, Sritularak B, Chanvorachote P. Lusianthridin targeting of lung cancer stem cells via src-STAT3 suppression. Phytomedicine (2019) 62:152932. doi: 10.1016/j.phymed.2019.152932
77. Huang WC, Kuo KT, Adebayo BO, Wang CH, Chen YJ, Jin K, et al. Garcinol inhibits cancer stem cell-like phenotype via suppression of the wnt/β-catenin/STAT3 axis signalling pathway in human non-small cell lung carcinomas. J Nutr Biochem (2018) 54:140–50. doi: 10.1016/j.jnutbio.2017.12.008
78. Tan Q, Lin S, Zeng Y, Yao M, Liu K, Yuan H, et al. Ginsenoside Rg3 attenuates the osimertinib resistance by reducing the stemness of non-small cell lung cancer cells. Environ Toxicol (2020) 35(6):643–51. doi: 10.1002/tox.22899
79. Tao X, Yin Y, Lian D, Gu H, Chen W, Yang L, et al. Puerarin 6″-o-xyloside suppresses growth, self-renewal and invasion of lung cancer stem-like cells derived from A549 cells via regulating akt/c-myc signalling. Clin Exp Pharmacol Physiol (2020) 47(7):1311–9. doi: 10.1111/1440-1681.13294
80. Li W, Zihan X, Yizhe W, Yanyang L, Zhixi L, Xi Y. Trilobatin induces apoptosis and attenuates stemness phenotype of acquired gefitinib resistant lung cancer cells via suppression of NF-κB pathway. Nutr Cancer (2022) 74(2):735–46. doi: 10.1080/01635581.2021.1912368
81. Shinji S, Yamada T, Matsuda A, Sonoda H, Ohta R, Iwai T, et al. Recent advances in the treatment of colorectal cancer: A review. J Nippon Med School = Nippon Ika Daigaku zasshi (2022) 89(3):246–54. doi: 10.1272/jnms.JNMS.2022_89-310
82. Yin H, Gao T, Xie J, Huang Z, Zhang X, Yang F, et al. FUBP1 promotes colorectal cancer stemness and metastasis via DVL1-mediated activation of wnt/β-catenin signaling. Mol Oncol (2021) 15(12):3490–512. doi: 10.1002/1878-0261.13064
83. Zhu Y, Hu H, Yuan Z, Zhang Q, Xiong H, Hu Z, et al. LncRNA NEAT1 remodels chromatin to promote the 5-fu resistance by maintaining colorectal cancer stemness. Cell Death Dis (2020) 11(11):962. doi: 10.1038/s41419-020-03164-8
84. Vishnubalaji R, Manikandan M, Fahad M, Hamam R, Alfayez M, Kassem M, et al. Molecular profiling of ALDH1+colorectal cancer stem cells reveals preferential activation of MAPK, FAK, and oxidative stress pro-survival signalling pathways. Oncotarget (2018) 9(17):13551–64. doi: 10.18632/oncotarget.24420
85. Ning K, Ning W, Ning X, Wang X, Zhou F. Effect of As2O3 on colorectal CSCs stained with ALDH1 in primary cell culture in vitro. Oncol Lett (2018) 16(3):4008–12. doi: 10.3892/ol.2018.9110
86. Yang W, Wang Y, Wang W, Chen Z, Bai G. Expression of aldehyde dehydrogenase 1A1 (ALDH1A1) as a prognostic biomarker in colorectal cancer using immunohistochemistry. Med Sci Monitor (2018) 24:2864–72. doi: 10.12659/MSM.910109
87. Tsochantaridis I, Kontopoulos A, Voulgaridou GP, Tsifintaris M, Triantafyllou C, Pappa A. Aldehyde dehydrogenase 1B1 is implicated in DNA damage response in human colorectal adenocarcinoma. Cells (2022) 11(13):2017. doi: 10.3390/cells11132017
88. Duan JJ, Wang D, Cai J, Chen JJ, Zheng XX, Chen TQ, et al. An aldehyde dehydrogenase 1A3 inhibitor attenuates the metastasis of human colorectal cancer. Cancer Lett (2022) 536:215662. doi: 10.1016/j.canlet.2022.215662
89. Sun J, Liu J, Zhu Q, Xu F, Kang L, Shi X. Hsa_circ_0001806 acts as a ceRNA to facilitate the stemness of colorectal cancer cells by increasing COL1A1. OncoTargets Ther (2020) 13:6315–27. doi: 10.2147/OTT.S255485
90. Tanaka H, Kawaguchi M, Shoda S, Miyoshi T, Iwasaki R, Hyodo F, et al. Nuclear accumulation of β-catenin in cancer stem cell radioresistance and stemness in human colon cancer. Anticancer Res (2019) 39(12):6575–83. doi: 10.21873/anticanres.13873
91. Tan X, Zhang Z, Yao H, Shen L. Tim-4 promotes the growth of colorectal cancer by activating angiogenesis and recruiting tumor-associated macrophages via the PI3K/AKT/mTOR signaling pathway. Cancer Lett (2018) 436:119–28. doi: 10.1016/j.canlet.2018.08.012
92. Chen MC, Baskaran R, Lee NH, Hsu HH, Ho TJ, Tu CC, et al. CXCL2/CXCR2 axis induces cancer stem cell characteristics in CPT-11-resistant LoVo colon cancer cells via gαi-2 and Gαq/11. J Cell Physiol (2019) 234(7):11822–34. doi: 10.1002/jcp.27891
93. Charkoftaki G, Thompson DC, Golla JP, Garcia-Milian R, Lam TT, Engel J, et al. Integrated multi-omics approach reveals a role of ALDH1A1 in lipid metabolism in human colon cancer cells. Chemico-biological Interact (2019) 304:88–96. doi: 10.1016/j.cbi.2019.02.030
94. Solomon H, Dinowitz N, Pateras IS, Cooks T, Shetzer Y, Molchadsky A, et al. Mutant p53 gain of function underlies high expression levels of colorectal cancer stem cells markers. Oncogene (2018) 37(12):1669–84. doi: 10.1038/s41388-017-0060-8
95. Yang C, Shi S, Su Y, Tong JS, Li L. P2X7R promotes angiogenesis and tumour-associated macrophage recruitment by regulating the NF-κB signalling pathway in colorectal cancer cells. J Cell Mol Med (2020) 24(18):10830–41. doi: 10.1111/jcmm.15708
96. Zhang Y, Fang Z, Guo X, Dong H, Zhou K, Huang Z, et al. lncRNA B4GALT1-AS1 promotes colon cancer cell stemness and migration by recruiting YAP to the nucleus and enhancing YAP transcriptional activity. J Cell Physiol (2019) 234(10):18524–34. doi: 10.1002/jcp.28489
97. Yang Y, Nguyen TT, Pereira I, Hur JS, Kim H. Lichen secondary metabolite physciosporin decreases the stemness potential of colorectal cancer cells. Biomolecules (2019) 9(12):797. doi: 10.3390/biom9120797
98. Sameri S, Saidijam M, Bahreini F, Najafi R. Cancer chemopreventive activities of silibinin on colorectal cancer through regulation of e-Cadherin/β-Catenin pathway. Nutr Cancer (2021) 73(8):1389–99. doi: 10.1080/01635581.2020.1800764
99. Yang Y, Bhosle SR, Yu YH, Park SY, Zhou R, Taş İ., et al. Tumidulin, a lichen secondary metabolite, decreases the stemness potential of colorectal cancer cells. Molecules (Basel Switzerland) (2018) 23(11):2968. doi: 10.3390/molecules23112968
100. Mohammadi C, Mahdavinezhad A, Saidijam M, Bahreini F, Sedighi Pashaki A, Gholami MH, et al. DCLK1 inhibition sensitizes colorectal cancer cells to radiation treatment. Int J Mol Cell Med (2021) 10(1):23–33. doi: 10.22088/IJMCM.BUMS.10.1.23
101. Li W, Zhang N, Jin C, Long MD, Rajabi H, Yasumizu Y, et al. MUC1-c drives stemness in progression of colitis to colorectal cancer. JCI Insight (2020) 5(12):e137112. doi: 10.1172/jci.insight.137112
102. Golla JP, Kandyliari A, Tan WY, Chen Y, Orlicky DJ, Thompson DC, et al. Interplay between APC and ALDH1B1 in a newly developed mouse model of colorectal cancer. Chemico-biological Interact (2020) 331:109274. doi: 10.1016/j.cbi.2020.109274
103. Sanches J, Song B, Zhang Q, Cui X, Yabasin IB, Ntim M, et al. The role of KDM2B and EZH2 in regulating the stemness in colorectal cancer through the PI3K/AKT pathway. Front Oncol (2021) 11:637298. doi: 10.3389/fonc.2021.637298
104. Pereira CV, Duarte M, Silva P, Bento da Silva A, Duarte C, Cifuentes A, et al. Polymethoxylated flavones target cancer stemness and improve the antiproliferative effect of 5-fluorouracil in a 3D cell model of colorectal cancer. Nutrients (2019) 11(2):326. doi: 10.3390/nu11020326
105. Buhrmann C, Yazdi M, Popper B, Shayan P, Goel A, Aggarwal BB, et al. Resveratrol chemosensitizes TNF-β-Induced survival of 5-FU-Treated colorectal cancer cells. Nutrients (2018) 10(7):888. doi: 10.3390/nu10070888
106. Lee JH, Yun CW, Han YS, Kim S, Jeong D, Kwon HY, et al. Melatonin and 5-fluorouracil co-suppress colon cancer stem cells by regulating cellular prion protein-Oct4 axis. J Pineal Res (2018) 65(4):e12519. doi: 10.1111/jpi.12519
107. Tian Q, Xu Z, Sun X, Deavila J, Du M, Zhu M. Grape pomace inhibits colon carcinogenesis by suppressing cell proliferation and inducing epigenetic modifications. J Nutr Biochem (2020) 84:108443. doi: 10.1016/j.jnutbio.2020.108443
108. Bellamkonda K, Satapathy SR, Douglas D, Chandrashekar N, Selvanesan BC, Liu M, et al. Montelukast, a CysLT1 receptor antagonist, reduces colon cancer stemness and tumor burden in a mouse xenograft model of human colon cancer. Cancer Lett (2018) 437:13–24. doi: 10.1016/j.canlet.2018.08.019
109. Salim EI, Mahfouz ME, Kang JS, Hegazi MM, Helmy HM, Eltonouby EA. Celecoxib targeted therapy attenuates mouse colon carcinogenesis through modulation of expression patterns of cancer stem cells. J Environ Pathol Toxicol Oncol (2019) 38(4):329–43. doi: 10.1615/JEnvironPatholToxicolOncol.2019030130
110. Li J, Guo C, Lu X, Tan W. Anti-colorectal cancer biotargets and biological mechanisms of puerarin: Study of molecular networks. Eur J Pharmacol (2019) 858:172483. doi: 10.1016/j.ejphar.2019.172483
111. Rumgay H, Ferlay J, de Martel C, Georges D, Ibrahim AS, Zheng R, et al. Global, regional and national burden of primary liver cancer by subtype. Eur J Cancer (Oxford England: 1990) (2022) 161:108–18. doi: 10.1016/j.ejca.2021.11.023
112. Meng YC, Lou XL, Yang LY, Li D, Hou YQ. Role of the autophagy-related marker LC3 expression in hepatocellular carcinoma: a meta-analysis. J Cancer Res Clin Oncol (2020) 146(5):1103–13. doi: 10.1007/s00432-020-03174-1
113. Hu X, Ding J, Wang G, Zhang X. The combination of ulinastatin and 5-fluorouracil synergistically inhibits hepatocellular carcinoma growth. J Int Med Res (2020) 48(3):300060520909776. doi: 10.1177/0300060520909776
114. Zhan J, Shi LL, Wang Y, Wei B, Yang SL. In vivo study on the effects of xiaoaiping on the stemness of hepatocellular carcinoma cells. Evidence-Based Complementary Altern Medicine: eCAM (2019) 2019:4738243. doi: 10.1155/2019/4738243
115. Lee JH, Shao F, Ling J, Lu S, Liu R, Du L, et al. Phosphofructokinase 1 platelet isoform promotes β-catenin transactivation for tumor development. Front Oncol (2020) 10:211. doi: 10.3389/fonc.2020.00211
116. Bamodu OA, Chang HL, Ong JR, Lee WH, Yeh CT, Tsai JT. Elevated PDK1 expression drives PI3K/AKT/MTOR signaling promotes radiation-resistant and dedifferentiated phenotype of hepatocellular carcinoma. Cells (2020) 9(/3):746. doi: 10.3390/cells9030746
117. Li Y, Hu J, Guo D, Ma W, Zhang X, Zhang Z, et al. LncRNA SNHG5 promotes the proliferation and cancer stem cell-like properties of HCC by regulating UPF1 and wnt-signaling pathway. Cancer Gene Ther (2022) 29(10):1373–83. doi: 10.1038/s41417-022-00456-3
118. Chen B, Zhang K, Han Q, Zhong W, Yi J, Zhu H, et al. LncRNA LINC00460 takes a stimulating role on hepatocellular carcinoma stemness property. Cell Cycle (Georgetown Tex.) (2021) 20(20):2102–13. doi: 10.1080/15384101.2021.1940627
119. Guo Y, Zhong J, Wu F, Zhan Z. Long noncoding RNA MACC1-AS1 promotes the stemness of hepatocellular carcinoma cells by antagonizing miR-145. J Int Med Res (2020) 48(4):300060520920411. doi: 10.1177/0300060520920411
120. Zhang H, Hao C, Wang H, Shang H, Li Z. Carboxypeptidase A4 promotes proliferation and stem cell characteristics of hepatocellular carcinoma. Int J Exp Pathol (2019) 100(2):133–8. doi: 10.1111/iep.12315
121. Sha X, Wang K, Wang F, Zhang C, Yang L, Zhu X. Silencing PFKP restrains the stemness of hepatocellular carcinoma cells. Exp Cell Res (2021) 407(1):112789. doi: 10.1016/j.yexcr.2021.112789
122. Chang HL, Bamodu OA, Ong JR, Lee WH, Yeh CT, Tsai JT. Targeting the epigenetic non-coding RNA MALAT1/Wnt signaling axis as a therapeutic approach to suppress stemness and metastasis in hepatocellular carcinoma. Cells (2020) 9(4):1020. doi: 10.3390/cells9041020
123. Gao F, Yu X, Meng R, Wang J, Jia L. STARD13 is positively correlated with good prognosis and enhances 5-FU sensitivity via suppressing cancer stemness in hepatocellular carcinoma cells. OncoTargets Ther (2018) 11:5371–81. doi: 10.2147/OTT.S170775
124. Tang Z, Tang Y, Li L, Liu T, Yang J. Limonin provokes hepatocellular carcinoma cells with stemness entry into cycle via activating PI3K/Akt signaling. Biomed Pharmacother = Biomed Pharmacotherapie (2019) 117:109051. doi: 10.1016/j.biopha.2019.109051
125. Shao L, Zhang X, Yao Q. The f-box protein FBXO11 restrains hepatocellular carcinoma stemness via promotion of ubiquitin-mediated degradation of snail. FEBS Open Bio (2020) 10(9):1810–20. doi: 10.1002/2211-5463.12933
126. Hu Z, Cao X, Fang Y, Liu G, Xie C, Qian K, et al. Transient receptor potential vanilloid-type 2 targeting on stemness in liver cancer. Biomed Pharmacother = Biomed Pharmacotherapie (2018) 105:697–706. doi: 10.1016/j.biopha.2018.06.029
127. Biagioni A, Skalamera I, Peri S, Schiavone N, Cianchi F, Giommoni E, et al. Update on gastric cancer treatments and gene therapies. Cancer Metastasis Rev (2019) 38(3):537–48. doi: 10.1007/s10555-019-09803-7
128. Baiocchi GL, Giacopuzzi S, Marrelli D, Reim D, Piessen G, Matos da Costa P, et al. International consensus on a complications list after gastrectomy for cancer. Gastric Cance (2019) 22(1):172–89. doi: 10.1007/s10120-018-0839-5
129. Sentani K, Imai T, Kobayashi G, Hayashi T, Sasaki N, Oue N, et al. Histological diversity and molecular characteristics in gastric cancer: relation of cancer stem cell-related molecules and receptor tyrosine kinase molecules to mixed histological type and more histological patterns. Gastric Cancer (2021) 24(2):368–81. doi: 10.1007/s10120-020-01133-w
130. Brungs D, Lochhead A, Iyer A, Illemann M, Colligan P, Hirst NG, et al. Expression of cancer stem cell markers is prognostic in metastatic gastroesophageal adenocarcinoma. Pathology (2019) 51(5):474–80. doi: 10.1016/j.pathol.2019.03.009
131. Ye Y, Zhang S, Chen Y, Wang X, Wang P. High ALDH1A1 expression indicates a poor prognosis in gastric neuroendocrine carcinoma. Pathology Res Pract (2018) 214(2):268–72. doi: 10.1016/j.prp.2017.10.015
132. Lu G, Wang X, Wang Y, Cheng Z, Zhou L. Value of CagA, HER2, ALDH1, and KiSS-1 in predicting metastasis and prognosis for gastric adenocarcinoma. Int J Clin Exp Pathol (2018) 11(7):3628–37.
133. Zhang GQ. The effects of high expression of ALDH1 in gastric tumor cells escaping the killing by mφ. Bengbu, China: Bengbu Medical College (2020).
134. Song H, Shi L, Xu Y, Xu T, Fan R, Cao M, et al. BRD4 promotes the stemness of gastric cancer cells via attenuating miR-216a-3p-mediated inhibition of wnt/β-catenin signaling. Eur J Pharmacol (2019) 852:189–97. doi: 10.1016/j.ejphar.2019.03.018
135. Kong Y. The role and mechanism of CT45A1 promoting gastric cancer cells genesis and malignant progression. Suzhou, China: Suzhou University (2018).
136. Wang Y. Effect of lentivirus-mediated overexpression of PAQR3 gene on biological behavior and stem cell characteristics of gastric cancer. Jinzhou, China: Jinzhou Medical University (2018). p. 2018.
137. Giraud J, Molina-Castro S, Seeneevassen L, Sifré E, Izotte J, Tiffon C, et al. Verteporfin targeting YAP1/TAZ-TEAD transcriptional activity inhibits the tumorigenic properties of gastric cancer stem cells. Int J Cancer (2020) 146(8):2255–67. doi: 10.1002/ijc.32667
138. Gong X, Xu B, Zi L, Chen X. miR-625 reverses multidrug resistance in gastric cancer cells by directly targeting ALDH1A1. Cancer Manage Res (2019) 11:6615–24. doi: 10.2147/CMAR.S208708
139. Touhami O, Plante M. Minimally invasive surgery for cervical cancer in light of the LACC trial: What have we learned? Curr Oncol (Toronto Ont.) (2022) 29(2):1093–106. doi: 10.3390/curroncol29020093
140. Martín-Vallejo J, Laforga JB, Molina-Bellido P, Clemente-Pérez PA. Superficial spreading cervical squamous cell carcinoma in situ involving the endometrium: a case report and review of the literature. J Med Case Rep (2022) 16(1):196. doi: 10.1186/s13256-022-03433-4
141. Mayadev JS, Ke G, Mahantshetty U, Pereira MD, Tarnawski R, Toita T. Global challenges of radiotherapy for the treatment of locally advanced cervical cancer. Int J Gynecological Cancer (2022) 32(3):436–45. doi: 10.1136/ijgc-2021-003001
142. D’Oria O, Corrado G, Laganà AS, Chiantera V, Vizza E, Giannini A. New advances in cervical cancer: From bench to bedside. Int J Environ Res Public Health (2022) 19(12):7094. doi: 10.3390/ijerph19127094
143. Yao T, Weng X, Yao Y, Huang C, Li J, Peng Y, et al. ALDH-1-positive cells exhibited a radioresistant phenotype that was enhanced with hypoxia in cervical cancer. BMC Cancer (2020) 20(1):891. doi: 10.1186/s12885-020-07337-8
144. Fahmi MN, Hertapanndika IN, Kusuma F. The prognostic value of cancer stem cell markers in cervical cancer: A systematic review and meta-analysis. Asian Pacific J Cancer Prevention: APJCP (2021) 22(12):4057–65. doi: 10.31557/APJCP.2021.22.12.4057
145. Tulake W, Yuemaier R, Sheng L, Ru M, Lidifu D, Abudula A. Upregulation of stem cell markers ALDH1A1 and OCT4 as potential biomarkers for the early detection of cervical carcinoma. Oncol Lett (2018) 16(5):5525–34. doi: 10.3892/ol.2018.9381
146. Liu C, Zhang Y, Liang S, Ying Y. Aldehyde dehydrogenase 1, a target of miR-222, is expressed at elevated levels in cervical cancer. Exp Ther Med (2020) 19(3):1673–80. doi: 10.3892/etm.2020.8425
147. Wang L, Liu Y, Zhou Y, Wang J, Tu L, Sun Z, et al. Zoledronic acid inhibits the growth of cancer stem cell derived from cervical cancer cell by attenuating their stemness phenotype and inducing apoptosis and cell cycle arrest through the Erk1/2 and akt pathways. J Exp Clin Cancer Res: CR (2019) 38(1):93. doi: 10.1186/s13046-019-1109-z
148. Organista-Nava J, Gómez-Gómez Y, Garibay-Cerdenares OL, Leyva-Vázquez MA, Illades-Aguiar B. Cervical cancer stem cell-associated genes: Prognostic implications in cervical cancer. Oncol Lett (2019) 18(1):7–14. doi: 10.3892/ol.2019.10307
149. Yokoi E, Mabuchi S, Shimura K, Komura N, Kozasa K, Kuroda H, et al. Lurbinectedin (PM01183), a selective inhibitor of active transcription, effectively eliminates both cancer cells and cancer stem cells in preclinical models of uterine cervical cancer. Investigational New Drugs (2019) 37(5):818–27. doi: 10.1007/s10637-018-0686-6
150. Zhao W, Wu M, Cui L, Du W. Limonin attenuates the stemness of cervical carcinoma cells by promoting YAP nuclear-cytoplasmic translocation. Food Chem Toxicol (2019) 125:621–8. doi: 10.1016/j.fct.2019.02.011
151. Kassab J, Saba L, Kassab R, Kourie HR. Tsunami of immunotherapies in the management of esophageal cancer. Immunotherapy (2022) 14(11):879–84. doi: 10.2217/imt-2022-0035
152. Yang T, Hui R, Nouws J, Sauler M, Zeng T, Wu Q. Untargeted metabolomics analysis of esophageal squamous cell cancer progression. Journal Trans Med (2022) 20(1):127. doi: 10.1186/s12967-022-03311-z
153. He S, Xu J, Liu X, Zhen Y. Advances and challenges in the treatment of esophageal cancer. Acta Pharm Sinica. B (2021) 11(11):3379–92. doi: 10.1016/j.apsb.202103.008
154. Wang XL, Wang S, Wu ZZ, Yang QC, Li H, Xiong HG, et al. Overexpression of ATAD2 indicates poor prognosis in oral squamous cell carcinoma. Int J Med Sci (2020) 17(11):1598–609. doi: 10.7150/ijms.46809
155. Islam F, Gopalan V, Lam AK. Detention and identification of cancer stem cells in esophageal squamous cell carcinoma. Methods Mol Biol (Clifton N.J.) (2020) 2129:177–91. doi: 10.1007/978-1-0716-0377-2_14
156. Tsunedomi R, Yoshimura K, Suzuki N, Hazama S, Nagano H. Clinical implications of cancer stem cells in digestive cancers: acquisition of stemness and prognostic impact. Surg Today (2020) 50(12):1560–77. doi: 10.1007/s00595-020-01968-x
157. Shiozaki A, Kudou M, Ichikawa D, Fujiwara H, Shimizu H, Ishimoto T, et al. Esophageal cancer stem cells are suppressed by tranilast, a TRPV2 channel inhibitor. J Gastroenterol (2018) 53(2):197–207. doi: 10.1007/s00535-017-1338-x
158. Li F, Xu Y, Liu B, Singh PK, Zhao W, Jin J, et al. YAP1-mediated CDK6 activation confers radiation resistance in esophageal cancer - rationale for the combination of YAP1 and CDK4/6 inhibitors in esophageal cancer. Clin Cancer Res (2019) 25(7):2264–77. doi: 10.1158/1078-0432.CCR-18-1029
159. Ho CM, Lee FK, Yen TL, Huang SH, Cheng WF. Everolimus combined with 5-aza-2-deoxycytidine generated potent anti-tumor effects on ovarian clear cell cancer stem-like/spheroid cells by inhibiting the COL6A3-AKT-mTOR pathway. Am J Cancer Res (2022) 12(4):1686–706.
160. Sharbatoghli M, Shamshiripour P, Fattahi F, Kalantari E, Habibi Shams Z, Panahi M, et al. Co-Expression of cancer stem cell markers, SALL4/ALDH1A1, is associated with tumor aggressiveness and poor survival in patients with serous ovarian carcinoma. J Ovarian Res (2022) 15(1):17. doi: 10.1186/s13048-021-00921-x
161. Huddle BC, Grimley E, Buchman CD, Chtcherbinine M, Debnath B, Mehta P, et al. Structure-based optimization of a novel class of aldehyde dehydrogenase 1A (ALDH1A) subfamily-selective inhibitors as potential adjuncts to ovarian cancer chemotherapy. J Medicinal Chem (2018) 61(19):8754–73. doi: 10.1021/acs.jmedchem.8b00930
162. Nowacka M, Ginter-Matuszewska B, Świerczewska M, Sterzyńska K, Nowicki M, Januchowski R. Effect of ALDH1A1 gene knockout on drug resistance in paclitaxel and topotecan resistant human ovarian cancer cell lines in 2D and 3D model. Int J Mol Sci (2022) 23(6):3036. doi: 10.3390/ijms23063036
163. Kaipio K, Chen P, Roering P, Huhtinen K, Mikkonen P, Östling P, et al. ALDH1A1-related stemness in high-grade serous ovarian cancer is a negative prognostic indicator but potentially targetable by EGFR/mTOR-PI3K/aurora kinase inhibitors. J Pathol (2020) 250(2):159–69. doi: 10.1002/path.5356
164. Ruscito I, Darb-Esfahani S, Kulbe H, Bellati F, Zizzari IG, Rahimi Koshkaki H, et al. The prognostic impact of cancer stem-like cell biomarker aldehyde dehydrogenase-1 (ALDH1) in ovarian cancer: A meta-analysis. Gynecologic Oncol (2018) 150(1):151–7. doi: 10.1016/j.ygyno.2018.05.006
165. Roy M, Connor J, Al-Niaimi A, Rose SL, Mahajan A. Aldehyde dehydrogenase 1A1 (ALDH1A1) expression by immunohistochemistry is associated with chemo-refractoriness in patients with high-grade ovarian serous carcinoma. Hum Pathol (2018) 73:1–6. doi: 10.1016/j.humpath.2017.06.025
166. Uddin MH, Kim B, Cho U, Azmi AS, Song YS. Association of ALDH1A1-NEK-2 axis in cisplatin resistance in ovarian cancer cells. Heliyon (2020) 6(11):e05442. doi: 10.1016/j.heliyon.2020.e05442
167. Kim D, Choi BH, Ryoo IG, Kwak MK. High NRF2 level mediates cancer stem cell-like properties of aldehyde dehydrogenase (ALDH)-high ovarian cancer cells: inhibitory role of all-trans retinoic acid in ALDH/NRF2 signaling. Cell Death Dis (2018) 9(9):896. doi: 10.1038/s41419-018-0903-4
168. Liu L, Cai S, Han C, Banerjee A, Wu D, Cui T, et al. ALDH1A1 contributes to PARP inhibitor resistance via enhancing DNA repair in BRCA2-/- ovarian cancer cells. Mol Cancer Ther (2020) 19(1):199–210. doi: 10.1158/1535-7163.MCT-19-0242/4
169. Therachiyil L, Krishnankutty R, Ahmad F, Mateo JM, Uddin S, Korashy HM. Aryl hydrocarbon receptor promotes cell growth, stemness like characteristics, and metastasis in human ovarian cancer via activation of PI3K/Akt, β-catenin, and epithelial to mesenchymal transition pathways. Int J Mol Sci (2022) 23(12):6395. doi: 10.3390/ijms23126395
170. Cui T, Srivastava AK, Han C, Wu D, Wani N, Liu L, et al. DDB2 represses ovarian cancer cell dedifferentiation by suppressing ALDH1A1. Cell Death Dis (2018) 9(5):561. doi: 10.1038/s41419-018-0585-y
171. Simpkins F, Jang K, Yoon H, Hew KE, Kim M, Azzam DJ, et al. Dual src and MEK inhibition decreases ovarian cancer growth and targets tumor initiating stem-like cells. Clin Cancer Res (2018) 24(19):4874–86. doi: 10.1158/1078-0432.CCR-17-3697
172. Grimley E, Cole AJ, Luong TT, McGonigal SC, Sinno S, Yang D, et al. Aldehyde dehydrogenase inhibitors promote DNA damage in ovarian cancer and synergize with ATM/ATR inhibitors. Theranostics (2021) 11(8):3540–51. doi: 10.7150/thno.51885
173. Wang Y, Zong X, Mitra S, Mitra AK, Matei D, Nephew KP. IL-6 mediates platinum-induced enrichment of ovarian cancer stem cells. JCI Insight (2018) 3(23):e122360. doi: 10.1172/jci.insight.122360
174. Lokman NA, Price ZK, Hawkins EK, Macpherson AM, Oehler MK, Ricciardelli C. 4-methylumbelliferone inhibits cancer stem cell activation and overcomes chemoresistance in ovarian cancer. Cancers (2019) 11(8):1187. doi: 10.3390/cancers11081187
175. Nacarelli T, Fukumoto T, Zundell JA, Fatkhutdinov N, Jean S, Cadungog MG, et al. NAMPT inhibition suppresses cancer stem-like cells associated with therapy-induced senescence in ovarian cancer. Cancer Res (2020) 80(4):890–900. doi: 10.1158/0008-5472.CAN-19-2830
176. Testa U, Petrucci E, Pasquini L, Castelli G, Pelosi E. Ovarian cancers: Genetic abnormalities, tumor heterogeneity and progression, clonal evolution and cancer stem cells. Medicines (Basel Switzerland) (2018) 5(1):16. doi: 10.3390/medicines5010016
177. Chefetz I, Grimley E, Yang K, Hong L, Vinogradova EV, Suciu R, et al. A pan-ALDH1A inhibitor induces necroptosis in ovarian cancer stem-like cells. Cell Rep (2019) 26(11):3061–3075.e6. doi: 10.1016/j.celrep.2019.02.032
178. Raj S, Kesari KK, Kumar A, Rathi B, Sharma A, Gupta PK, et al. Molecular mechanism(s) of regulation(s) of c-MET/HGF signaling in head and neck cancer. Mol Cancer (2022) 21(1):31. doi: 10.1186/s12943-022-01503-1
179. Shetty SS, Nalilu SK, Shetty PK, Patil P. Tetraspanin CD9: A friend or foe of head and neck cancer (Review). Oncol Rep (2022) 47(5):88. doi: 10.3892/or.2022.8299. P C, S.
180. Schlachtenberger G, Doerr F, Menghesha H, Lauinger P, Wolber P, Sabashnikov A, et al. Patients with pulmonary metastases from head and neck cancer benefit from pulmonary metastasectomy, a systematic review. Medicina (Kaunas Lithuania) (2022) 58(8):1000. doi: 10.3390/medicina58081000
181. Cirillo N, Wu C, Prime SS. Heterogeneity of cancer stem cells in tumorigenesis, metastasis, and resistance to antineoplastic treatment of head and neck tumours. Cells (2021) 10(11):3068. doi: 10.3390/cells10113068
182. Wu J, Zhao W, Wang Z, Xiang X, Zhang S, Liu L. Long non-coding RNA SNHG20 promotes the tumorigenesis of oral squamous cell carcinoma via targeting miR-197/LIN28 axis. J Cell Mol Med (2019) 23(1):680–8. doi: 10.1111/jcmm.13987
183. Vieira V, Campos LH, Jesus LH, Klabunde C, Gamba TD, Flores IL. Overexpression of ALDH1 and EMT marker profile are linked with unfavorable outcome in head and neck cancer. Medicina Oral Patologia Oral y Cirugia Bucal (2020) 25(6):e752–61. doi: 10.4317/medoral.23777
184. Feng J, Zhou Z, Shi L, Yang X, Liu W. Cancer stem cell markers ALDH1 and Bmi1 expression in oral erythroplakia revisited: Implication for driving the process of field cancerization. J Oral Pathol Med (2020) 49(1):96–9. doi: 10.1111/jop.12955
185. Yu SS, Cirillo N. The molecular markers of cancer stem cells in head and neck tumors. J Cell Physiol (2020) 235(1):65–73. doi: 10.1002/jcp.28963
186. da Trindade GA, da Silva LP, de Andrade Santos PP, Pinto LP, de Souza LB. Expression of a tumor stem cell marker (Aldehyde dehydrogenase 1-ALDH1) in benign epithelial odontogenic lesions. Head Neck Pathol (2022) 16(3):785–91. doi: 10.1007/s12105-022-01430-z
187. Bertlich M, Kitz J, Kruizenga M, Spiegel JL, Canis M, Ihler F, et al. Cancer stem cell markers in squamous cell carcinomas of the salivary glands. Oncology (2021) 99(6):402–12. doi: 10.1159/000514101
188. da Silva LP, Lopes M, Sarmento A, de Albuquerque Borges M, de Moura S, Sobral A, et al. Increased expression of ALDH-1 is associated with clinical parameters of salivary glands neoplasms. Exp Mol Pathol (2020) 117:104552. doi: 10.1016/j.yexmp.2020.104552
189. Custódio M, Pelissari C, Santana T, Trierveiler M. Expression of cancer stem cell markers CD44, ALDH1 and p75NTR in actinic cheilitis and lip cancer. Eur Arch oto-rhino-laryngology (2018) 275(7):1877–83. doi: 10.1007/s00405-018-5002-8
190. Iorio B, Ronchi A, Montella M, Cozzolino I, De Luca R, Rusciano M, et al. Malignant extrapleural solitary fibrous tumor arising in the sublingual gland: A case report and review of literature. Oral Oncol (2019) 90:141–4. doi: 10.1016/j.oraloncology.2018.12.013
191. Rao RS, Raju K L, Augustine D, Patil S. Prognostic significance of ALDH1, Bmi1, and OCT4 expression in oral epithelial dysplasia and oral squamous cell carcinoma. Cancer Control: J Moffitt Cancer Center (2020) 27(1):1073274820904959. doi: 10.1177/1073274820904959
192. Zhou JJ, Xiao Y, Li H, Wu CC, Chen DR, Chen L, et al. Overexpression of malic enzyme 2 indicates pathological and clinical significance in oral squamous cell carcinoma. Int J Med Sci (2020) 17(6):799–806. doi: 10.7150/ijms.43832
193. Zhao Q, Sun P, Qin S, Liu J. Acylglycerol kinase promotes the stemness of nasopharyngeal carcinoma cells by promoting β-catenin translocation to the nucleus through activating PI3K/Akt pathway. Environ Toxicol (2020) 35(12):1299–307. doi: 10.1002/tox.22994
194. Fan Y, Fan X, Yan H, Liu Z, Wang X, Yuan Q, et al. Long non-coding ROR promotes the progression of papillary thyroid carcinoma through regulation of the TESC/ALDH1A1/TUBB3/PTEN axis. Cell Death Dis (2022) 13(2):157. doi: 10.1038/s41419-021-04210-9
195. Shiah SG, Hsiao JR, Chang HJ, Hsu YM, Wu GH, Peng HY, et al. MiR-30a and miR-379 modulate retinoic acid pathway by targeting DNA methyltransferase 3B in oral cancer. J Biomed Sci (2020) 27(1):46. doi: 10.1186/s12929-020-00644-z
196. Wang HF, Wang SS, Zheng M, Dai LL, Wang K, Gao XL, et al. Hypoxia promotes vasculogenic mimicry formation by vascular endothelial growth factor a mediating epithelial-mesenchymal transition in salivary adenoid cystic carcinoma. Cell proliferation (2019) 52(3):e12600. doi: 10.1111/cpr.12600
197. Tada H, Takahashi H, Ida S, Mito I, Matsuyama T, Chikamatsu K. The blood microenvironment influences the molecular phenotypes of circulating tumor cells in head and neck squamous cell carcinoma. Anticancer Res (2021) 41(2):885–93. doi: 10.21873/anticanres.14841
198. Lin CS, Bamodu OA, Kuo KT, Huang CM, Liu SC, Wang CH, et al. Investigation of ovatodiolide, a macrocyclic diterpenoid, as a potential inhibitor of oral cancer stem-like cells properties via the inhibition of the JAK2/STAT3/JARID1B signal circuit. Phytomedicine (2018) 46:93–103. doi: 10.1016/j.phymed.2018.04.016
199. Hsieh PL, Liao YW, Hsieh CW, Chen PN, Yu CC. Soy isoflavone genistein impedes cancer stemness and mesenchymal transition in head and neck cancer through activating miR-34a/RTCB axis. Nutrients (2020) 12(7):1924. doi: 10.3390/nu12071924
200. Chen PY, Chao SC, Hsieh PL, Liao YW, Chu PM, Harn HJ, et al. Butylidenephthalide abrogates the snail-induced cancer stemness in oral carcinomas. Int J Mol Sci (2022) 23(11):6157. doi: 10.3390/ijms23116157
201. Chang MT, Lee SP, Fang CY, Hsieh PL, Liao YW, Lu MY, et al. Chemosensitizing effect of honokiol in oral carcinoma stem cells via regulation of IL-6/Stat3 signaling. Environ Toxicol (2018) 33(11):1105–12. doi: 10.1002/tox.22587
202. Chen S, Luo X, Wu W, Li Y, Yu H, Wang Y, et al. The long non-coding RNA MACC1-AS1 promotes nasopharyngeal carcinoma cell stemness via suppressing miR-145-mediated inhibition on SMAD2/MACC1-AS1 axis. Biomed Pharmacother = Biomed Pharmacotherapie (2020) 125:109986. doi: 10.1016/j.biopha.2020.109986
203. Pradhan R, Chatterjee S, Hembram KC, Sethy C, Mandal M, Kundu CN. Nano formulated resveratrol inhibits metastasis and angiogenesis by reducing inflammatory cytokines in oral cancer cells by targeting tumor associated macrophages. J Nutr Biochem (2021) 92:108624. doi: 10.1016/j.jnutbio.2021.108624
204. Dias Câmara DA, Luiz de Sá Junior P, Alexandre de Azevedo R, Figueiredo CR, Araldi RP, Levy D, et al. Identification of very small cancer stem cells expressing hallmarks of pluripotency in B16F10 melanoma cells and their reoccurrence in B16F10-derived clones. Exp Cell Res (2020) 391(2):111938. doi: 10.1016/j.yexcr.2020.111938
205. Fauß J, Sprang B, Leukel P, Sommer C, Nikolova T, Ringel F, et al. ALDH1A3 segregated expression and nucleus-associated proteasomal degradation are common traits of glioblastoma stem cells. Biomedicines (2021) 10(1):7. doi: 10.3390/biomedicines10010007
206. Verma S, Shankar E, Kalayci F, Mukunda A, Alassfar M, Singh V, et al. Androgen deprivation induces transcriptional reprogramming in prostate cancer cells to develop stem cell-like characteristics. Int J Mol Sci (2020) 21(24):9568. doi: 10.3390/ijms21249568
207. Cao D, Meng Y, Li S, Xin J, Ben S, Cheng Y, et al. Association study between genetic variants in retinol metabolism pathway genes and prostate cancer risk. Cancer Med (2020) 9(24):9462–70. doi: 10.1002/cam4.3538
208. Huang HH, Wang YC, Chou YC, Yu MH, Chao TK. The combination of aldehyde dehydrogenase 1 (ALDH1) and CD44 is associated with poor outcomes in endometrial cancer. PloS One (2018) 13(10):e0206685. doi: 10.1371/journal.pone.0206685
209. Namekawa T, Ikeda K, Horie-Inoue K, Suzuki T, Okamoto K, Ichikawa T, et al. ALDH1A1 in patient-derived bladder cancer spheroids activates retinoic acid signaling leading to TUBB3 overexpression and tumor progression. Int J Cancer (2020) 146(4):1099–113. doi: 10.1002/ijc.32505
210. Bao Z, Cheng Z, Chai D. The expressions of CD133, ALDH1, and vasculogenic mimicry in osteosarcoma and their clinical significance. Int J Clin Exp Pathol (2018) 11(7):3656–63.
211. Noguchi K, Konno M, Koseki J, Nishida N, Kawamoto K, Yamada D, et al. The mitochondrial one-carbon metabolic pathway is associated with patient survival in pancreatic cancer. Oncol Lett (2018) 16(2):1827–34. doi: 10.3892/ol.2018.8795
212. Hsu CC, Liao WY, Chan TS, Chen WY, Lee CT, Shan YS, et al. The differential distributions of ASPM isoforms and their roles in wnt signaling, cell cycle progression, and pancreatic cancer prognosis. J Pathol (2019) 249(4):498–508. doi: 10.1002/path.5341
213. Schoenfeld DA, Zhou R, Zairis S, Su W, Steinbach N, Mathur D, et al. Loss of PBRM1 alters promoter histone modifications and activates ALDH1A1 to drive renal cell carcinoma. Mol Cancer Res: MCR (2022) 20(8):1193–207. doi: 10.1158/1541-7786.MCR-21-1039
214. Ciccone V, Terzuoli E, Ristori E, Filippelli A, Ziche M, Morbidelli L, et al. ALDH1A1 overexpression in melanoma cells promotes tumor angiogenesis by activating the IL-8/Notch signaling cascade. Int J Mol Med (2022) 50(1):99. doi: 10.3892/ijmm.2022.5155
215. Nie S, Qian X, Shi M, Li H, Peng C, Ding X, et al. ALDH1A3 accelerates pancreatic cancer metastasis by promoting glucose metabolism. Front Oncol (2020) 10:915. doi: 10.3389/fonc.2020.00915
216. Wan J, Guo AA, King P, Guo S, Saafir T, Jiang Y, et al. TRPM7 induces tumorigenesis and stemness through notch activation in glioma. Front Pharmacol (2020) 11:590723. doi: 10.3389/fphar.2020.590723
217. Tahara S, Nojima S, Ohshima K, Hori Y, Kurashige M, Wada N. Serum deprivation-response protein regulates aldehyde dehydrogenase 1 through integrin-linked kinase signaling in endometrioid carcinoma cells. Cancer Sci (2019) 110(5):1804–13. doi: 10.1111/cas.14007
218. Zhao AY, Dai YJ, Lian JF, Huang Y, Lin JG, Dai YB, et al. YAP regulates ALDH1A1 expression and stem cell property of bladder cancer cells. OncoTargets Ther (2018) 11:6657–63. doi: 10.2147/OTT.S170858
219. Wang X, Li C, Yao W, Tian Z, Liu Z, Ge H. MicroRNA-761 suppresses tumor progression in osteosarcoma via negatively regulating ALDH1B1. Life Sci (2020) 262:118544. doi: 10.1016/j.lfs.2020.118544
220. Liu S, Cao W, Niu Y, Luo J, Zhao Y, Hu Z, et al. Single-PanIN-seq unveils that ARID1A deficiency promotes pancreatic tumorigenesis by attenuating KRAS-induced senescence. eLife (2021) 10:e64204. doi: 10.7554/eLife.64204
221. Zhang Y, Zoltan M, Riquelme E, Xu H, Sahin I, Castro-Pando S, et al. Immune cell production of interleukin 17 induces stem cell features of pancreatic intraepithelial neoplasia cells. Gastroenterology (2018) 155(1):210–223.e3. doi: 10.1053/j.gastro.2018.03.041
222. King P, Wan J, Guo AA, Guo S, Jiang Y, Liu M. Regulation of gliomagenesis and stemness through acid sensor ASIC1a. Int J Oncol (2021) 59(4):82. doi: 10.3892/ijo.2021.5262
223. Mazor G, Levin L, Picard D, Ahmadov U, Carén H, Borkhardt A, et al. The lncRNA TP73-AS1 is linked to aggressiveness in glioblastoma and promotes temozolomide resistance in glioblastoma cancer stem cells. Cell Death Dis (2019) 10(3):246. doi: 10.1038/s41419-019-1477-5
224. Samy A, Bakthavachalam V, Vudutha M, Vinjamuri S, Chinnapaka S, Munirathinam G. Eprinomectin, a novel semi-synthetic macrocylic lactone is cytotoxic to PC3 metastatic prostate cancer cells via inducing apoptosis. Toxicol Appl Pharmacol (2020) 401:115071. doi: 10.1016/j.taap.2020.115071
225. Cheng M, Duan PG, Gao ZZ, Dai M. MicroRNA-487b-3p inhibits osteosarcoma chemoresistance and metastasis by targeting ALDH1A3. Oncol Rep (2020) 44(6):2691–700. doi: 10.3892/or.2020.7814
226. Wei W, Wang L, Xu L, Liang J, Teng L. MiR-199 reverses the resistance to gemcitabine in pancreatic cancer by suppressing stemness through regulating the epithelial-mesenchymal transition. ACS Omega (2021) 6(47):31435–46. doi: 10.1021/acsomega.1c02945
227. Chen CH, Bhasin S, Khanna P, Joshi M, Joslin PM, Saxena R, et al. Study of cathepsin b inhibition in VEGFR TKI treated human renal cell carcinoma xenografts. Oncogenesis (2019) 8(3):15. doi: 10.1038/s41389-019-0121-7
228. International Agency for Research on Cancer statistics. (2020). Lyon. Available at: https://www.iarc.who.int/research-home/.
229. International Agency for Research on Cancer Breast cancer statistics. . Lyon. Available at: https://www.iarc.who.int/featured-news/breast-cancer-awareness-month-2021/#about_breast_cancer.
Keywords: ALDH1, stem cell markers, solid tumors, targeted therapy, cancer resistance
Citation: Wei Y, Li Y, Chen Y, Liu P, Huang S, Zhang Y, Sun Y, Wu Z, Hu M, Wu Q, Wu H, Liu F, She T and Ning Z (2022) ALDH1: A potential therapeutic target for cancer stem cells in solid tumors. Front. Oncol. 12:1026278. doi: 10.3389/fonc.2022.1026278
Received: 25 August 2022; Accepted: 12 October 2022;
Published: 28 October 2022.
Edited by:
Martin Barr, St. James’s Hospital & Trinity College, IrelandReviewed by:
Sandra Donnini, University of Siena, ItalyCopyright © 2022 Wei, Li, Chen, Liu, Huang, Zhang, Sun, Wu, Hu, Wu, Wu, Liu, She and Ning. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fuxing Liu, TGl1Zng2NTA1QDEyNi5jb20=; Tonghui She, eG5zdGhAaGJ1c3QuZWR1LmNu; Zhifeng Ning, bmluZ3poaWZlbmcxOTc2QDE2My5jb20=
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.