- 1School of Basic Medicine, Gansu University of Traditional Chinese Medicine, Lanzhou, Gansu, China
- 2Department of Pathology, Gansu Provincial Hospital, Lanzhou, Gansu, China
- 3Department of Pathology, The 940th Hospital of Joint Logistics Support Force of Chinese People´s Liberation Army, Lanzhou, China
- 4The Department of Pathology, Hainan Provincial Hospital, Haikou, Hainan, China
- 5Evidence Based Social Science Research Center, School of Public Health, Lanzhou University, Lanzhou, China
Objective: Whether lymph node micrometastasis (LNM) increases the risk in esophageal cancer patients remains controversial. We conducted a systematic review and meta-analysis to explore the prognosis value of LNM in esophageal cancer patients.
Methods: Two reviewers independently searched electronic databases, including PubMed, Embase, and the Cochrane Library, for eligible citations until February 2022. We calculated pooled estimates of the hazards ratio with a random-effects model. The certainty of evidence was determined by the Grade of Recommendations Assessment, Development, and Evaluation (GRADE) method. A sensitivity analysis was performed to assess the stability. Publication bias was assessed using funnel plots and Egger’s test. We also performed subgroup analysis to explore the source of heterogeneity.
Results: A total of 16 studies, with 1,652 patients, were included. The overall survival (OS) was significantly increased with LNM negativity compared with LNM positivity (HR 1.95; 95% CI, 1.53–2.49; P < 0.001; I2 = 0.0%, P = 0.930; certainty of evidence: low). Relapse-free survival (RFS) was significantly increased with LNM negativity compared with LNM positivity (HR 3.39; 95% CI, 1.87–6.16; P < 0.001; I2 = 50.18%, P = 0.060; certainty of evidence: moderate). No significant difference was observed in recurrence between the two groups (certainty of evidence: low). Sensitivity analysis revealed a stable trend. In addition, the funnel plot and Egger’s test did not show significant publication bias.
Conclusion: LNM positivity worsens the prognosis in esophageal cancer, and the evidence for RFS is moderate. Future relevant high-quality studies are warranted to validate our results further and provide a reference for guidelines.
Systematic review registration: https://www.crd.york.ac.uk/prospero, identifier (CRD42022321768).
1 Introduction
The occurrence of esophageal cancer has increased in the Western world over the past few years and is expected to further rise (1, 2). Despite improvements in diagnostic methods and treatment, many patients are at the risk of recurrence post-surgery. Recurrence is likely to be associated with lymph node involvement as this is the strongest prognostic factor in esophageal cancer, with a 5-year survival rate in patients with pN3 ranging from 2% to 17% and that in patients with pN0 (no lymph node metastasis) being up to 83% (3). Lymph node micrometastasis (LNM) can be detected in the pN0 stage. In the presence of LNM, the 5-year survival rate for patients with esophageal cancer varies from less than 1% to 30% (4). Therefore, LNM may be a good survival predictor.
LNM is challenging to identify with certainty by routine Hematoxylin and Eosin (HE) staining. However, immunohistochemical (IHC) staining for cytokeratin can highlight small tumor cells, making them more easily detectable. A previous study (5) has reported that patients with LNM had significantly lower disease-free survival rates than those with negative lymph node metastasis in esophageal cancer. Another study (6) has reported that patients with LNM have a higher local recurrence rate than those without LNM. Another study (7) performed multivariate cox regression analysis, which showed that LNM was an independent prognostic factor for 5-year relapse-free survival (RFS) rate; however, no statistical differences were found in the 5-year overall survival (OS) rate between patients with LNM and those without LNM. It has been reported (8) that patients with LNM have significantly lower disease-free survival rates, indicating a worse prognosis. Although LNM has been evaluated in esophageal cancer, it is not included in TNM staging of esophageal cancer because there are no vital pieces of evidence indicating that LNM has a negative prognostic impact on esophageal cancer (9).
Nonetheless, Union for International Cancer Control (UICC) and American Joint Committee on Cancer (AJCC) are watchful toward LNM (10, 11). Moreover, there is no meta-analysis quantifying the value of LNM in the prognosis of esophageal cancer. Therefore, we have performed this meta-analysis to assess the prognostic value of LNM in patients with esophageal cancer.
2 Methods
2.1 Data sources and search strategy
This meta-analysis was reported following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Statement (12). The study has been registered at the International Prospective Register of Systematic Reviews (PROSPERO) (CRD42022321768) (13).
We searched for eligible studies in the electronic databases PubMed, Embase, and the Cochrane Library up to February 2022. We used the following combined text and MeSH terms: “Esophageal Neoplasms” and “Neoplasm Micrometastasis”. The complete literature search strategy for PubMed is provided in Supplementary Appendices 1. We also conducted a manual search using the reference lists of critical articles published in English.
2.2 Study selection and data extraction
The articles included in the analysis were selected based on the following eligibility criteria: (1) all patients were diagnosed with esophageal cancer and (2) studies included information on the prognostic value of LNM in esophageal cancer. Articles were excluded from the analysis if (1) they were not presented in English or Chinese; (2) they were review articles, meta-analyses, or conference abstracts; and (3) they did not include the available data.
Two independent investigators (JY and QQL) reviewed all relevant and eligible literature using standardized data-extraction forms. Disagreements were solved by consulting with a third investigator (MZ). The following data from each selected article: author names, year of publication, country/region, study design, total number of participants, age, sex, clinical outcomes, effect size with 95% CI, follow-up duration, and drop-out percentage were extracted.
2.3 Assessment of study quality
To assess the quality of each included study, two authors independently assessed the risk of bias using Quality In Prognosis Studies (QUIPS) tool (14). The studies were finally evaluated as “high risk of bias,” “moderate risk of bias,” and “low risk of bias”. The QUIPS tool included six crucial areas to evaluate validity and bias in studies of prognostic factors, including participation, attrition, measurement of prognostic factors, outcomes, confounding factors, statistical analysis, and reporting.
2.4 Assessment of quality of evidence
The certainty of evidence was determined following the Grade of Recommendations Assessment, Development and Evaluation (GRADE) method (15, 16). The assessment of evidence quality was based on five aspects: limitations, inconsistencies, indirectness, inaccuracies, and publication bias. The evidence quality of each outcome was rated as “high,” “moderate,” “low,” and “very low”.
2.5 Study outcomes
We assessed the effect of the prognostic value of LNM in esophageal cancer on three outcomes: OS, RFS, and recurrence. In five of the included studies (5, 8, 17–19), disease-free survival was regarded as RFS because they have the same definition.
2.6 Statistical analyses
All statistical analyses were performed using Stata (version 16.0). We calculated pooled estimates of the hazards ratio (HR) and odds ratio (OR) with a random-effects model (Dersimonian-Laird method). In the included articles (6, 20, 21), the HR of OS was transformed by a survival curve (22). The I2 statistic and P-value of Cochrane’s Q test were used to assess the heterogeneity of effects, with I2 = 25%–50% indicating mild, 50%–75% indicating moderate, and >75% indicating severe heterogeneity (23, 24).
We specified subgroups to explore the source of heterogeneity. Several subgroups were also analyzed, including study design, which was divided into retrospective, prospective, and RCT; region, which was divided into Asia and non-Asia; antibody type, which was divided into Ber-EP4 and AE1/AE3; follow-up duration, which was divided into less than five years and more than five years; tumor type, which was divided into squamous cell carcinoma (SCC) and esophageal adenocarcinoma (EAC); pN status, which was divided into pN+ (studies including LNM in pN+ patients) and pN0 (studies restricted to LNM in pN0 patients); single-center/multicenter studies; and univariate/multivariate analysis.
A P-value < 0.05 indicated statistical significance. In addition, we assessed asymmetry using funnel plots and Egger’s test and defined significant publication bias if P value is <0.05. Finally, we conducted sensitivity analyses to evaluate the stability of the results. Sensitivity analysis was conducted for all studies except three wherein the HRs were transformed by a survival curve to verify the stability of our results (6, 20, 21).
3 Results
3.1 Study selection and baseline characteristics
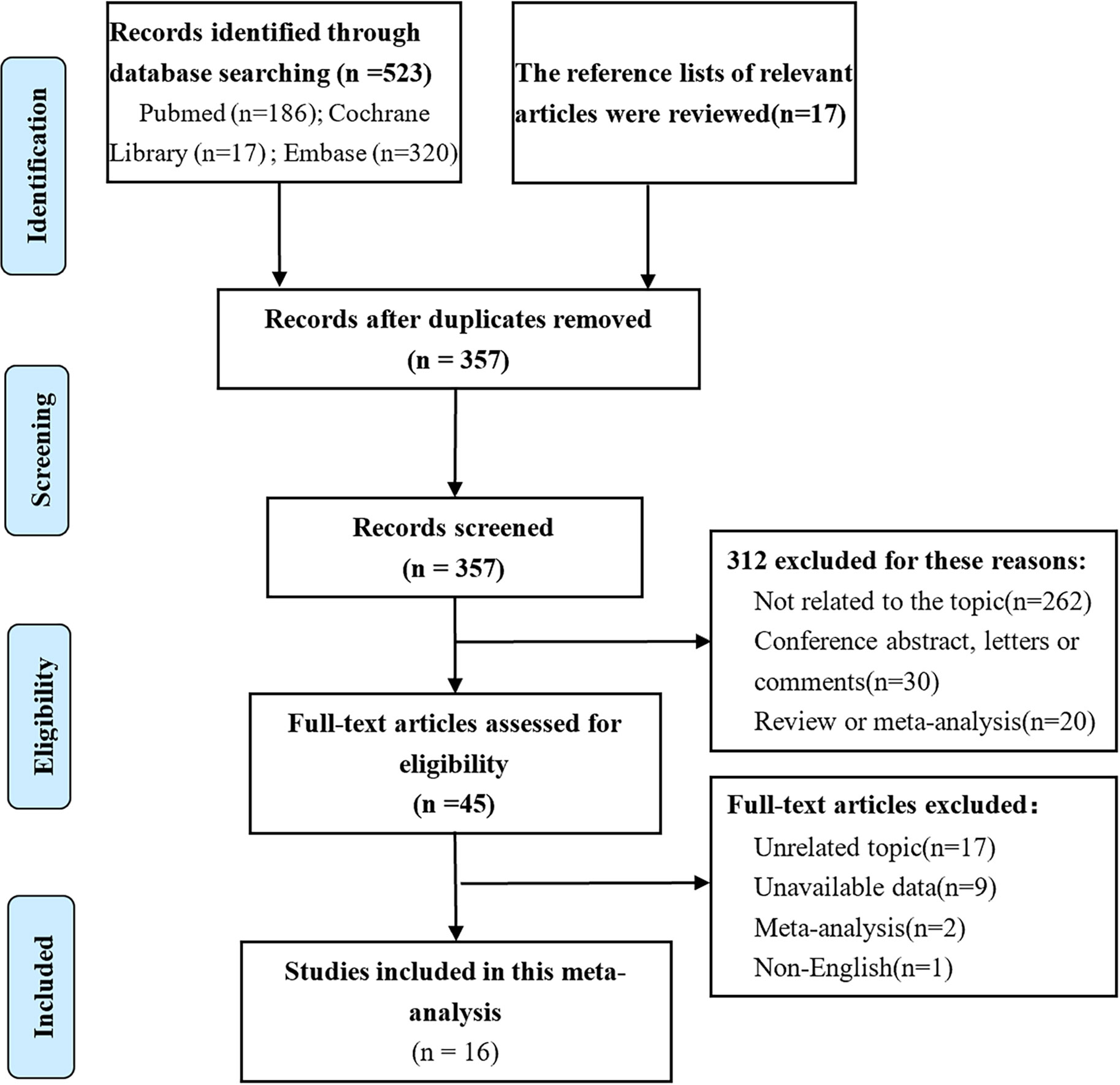
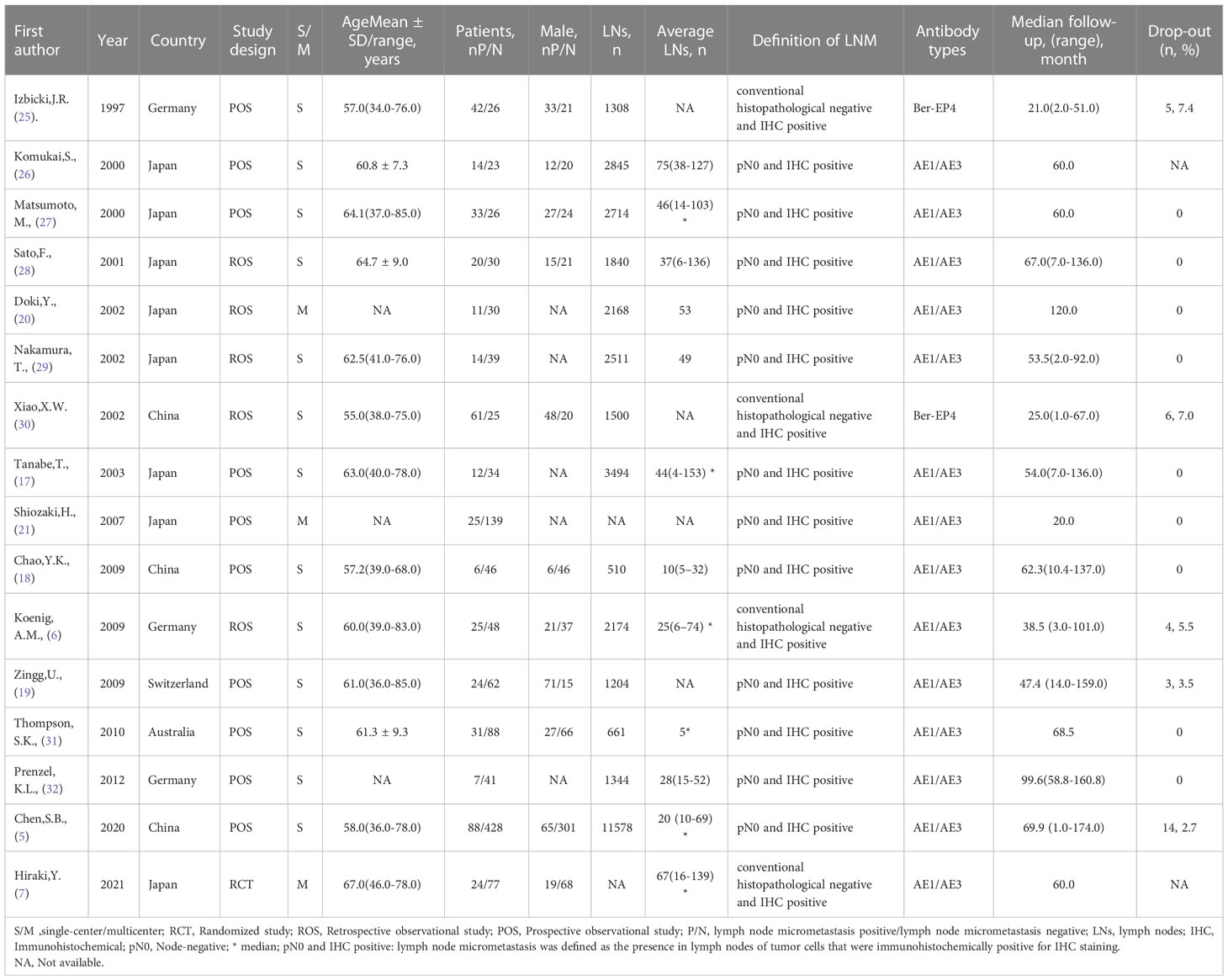
Of the 540 studies identified in our analysis, and 495 abstracts were retrieved and reviewed for possible inclusion after removing duplicates. Subsequently, 45 full-text manuscripts were assessed for eligibility, from which 29 did not meet the inclusion criteria and were excluded. Accordingly, only 16 studies including 1,652 patients were included (5–7, 17–21, 25–32) (Figure 1). The study characteristics and baseline demographics are shown (Table 1). The majority of studies were based in Asia. The study designs were randomized, retrospective, or prospective studies. LNM evaluation was performed via IHC staining with different antibody types that were classified as AE1/AE3 and Ber-EP4. Follow-up durations ranged from 1 month to 174 months. The basic features of esophageal cancer are shown in Supplementary Appendices 2.
3.2 Quality assessment
Observational studies had low-to-moderate bias using the QUIPS tool (14) (Supplementary Appendices 3). Furthermore, confounding factors were not assessed in three studies (18, 27, 29); the drop-out time was not assessed in two studies (7, 26).
3.3 LNM and prognosis of esophageal cancer
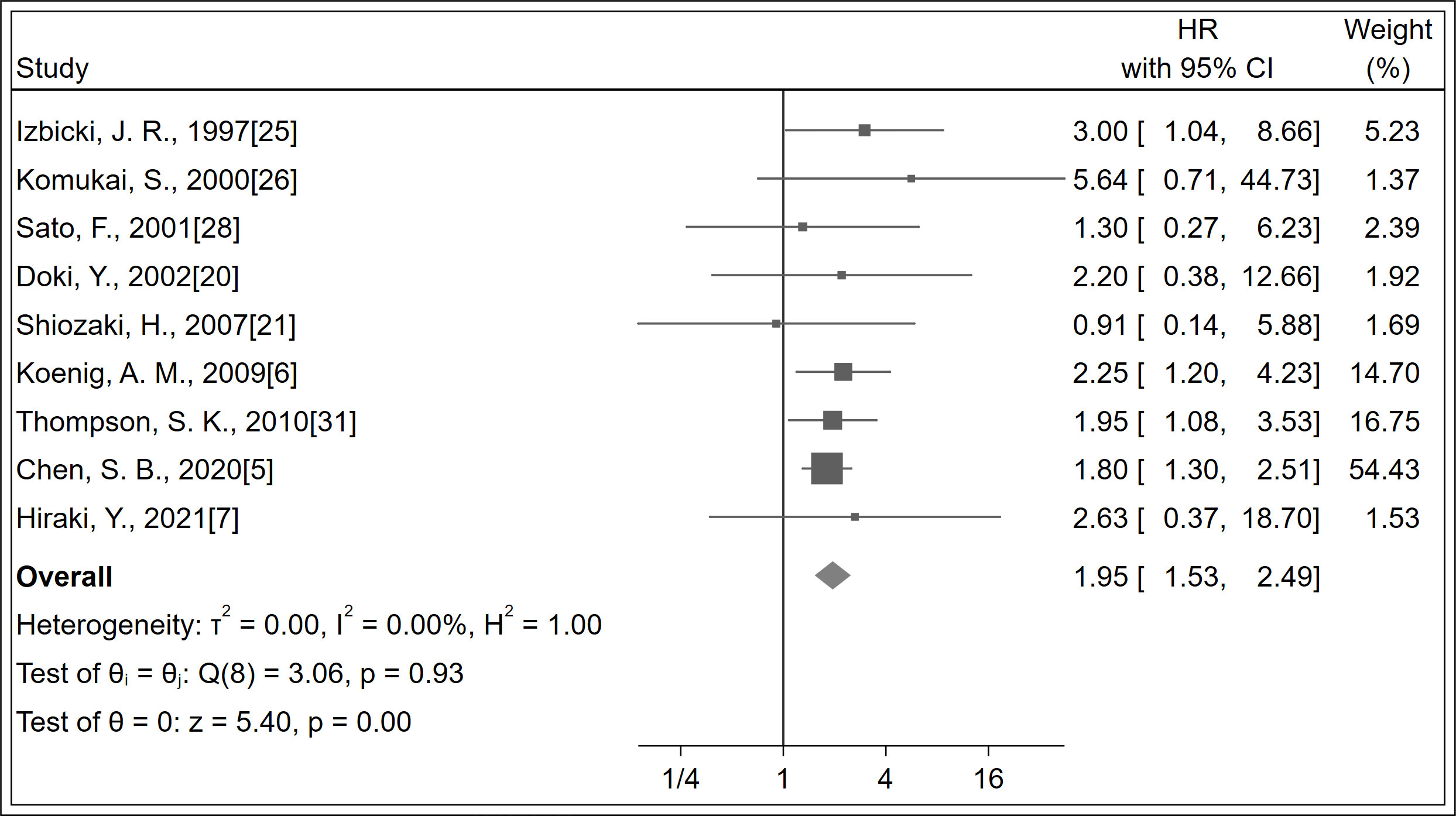
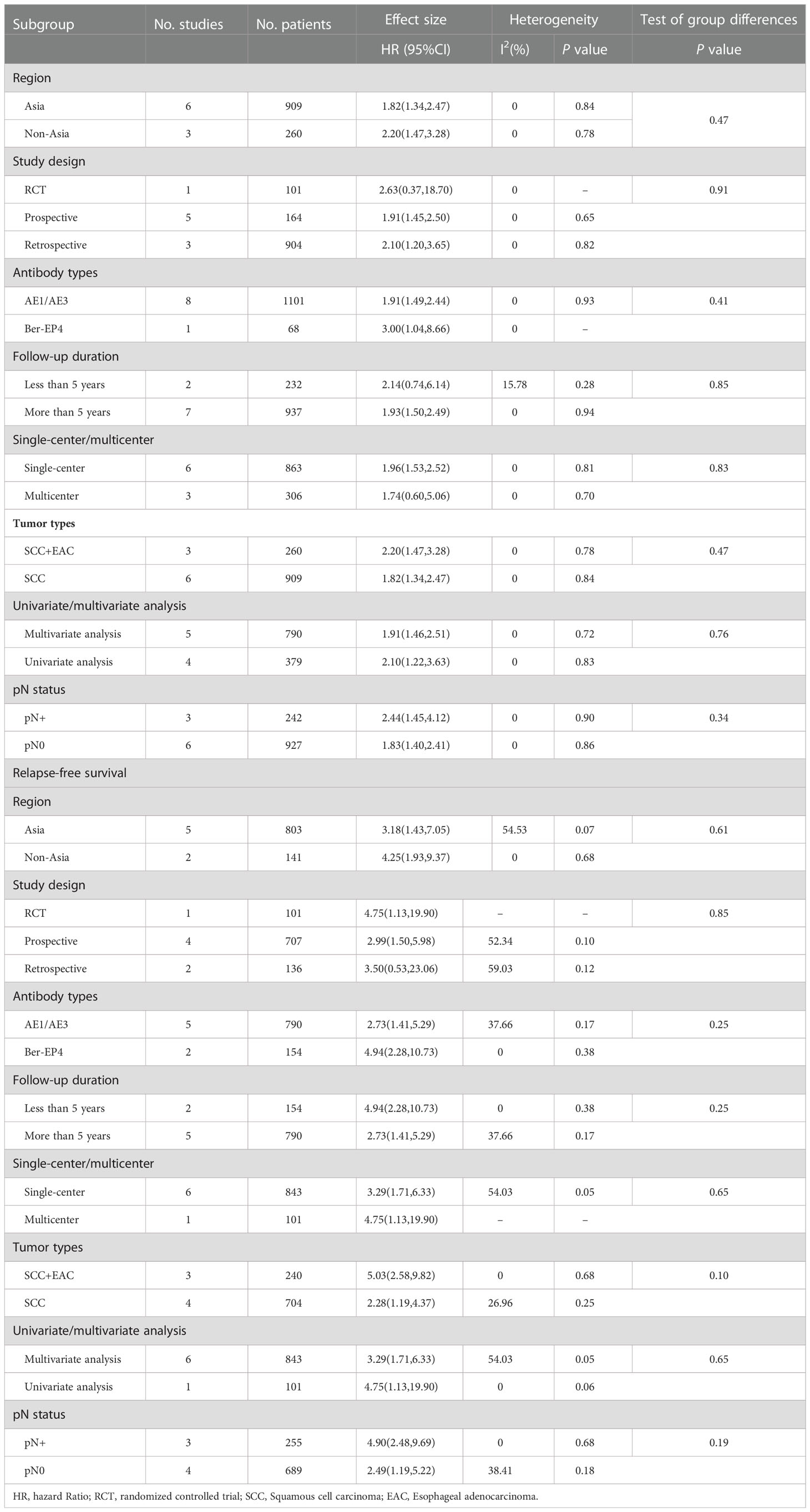
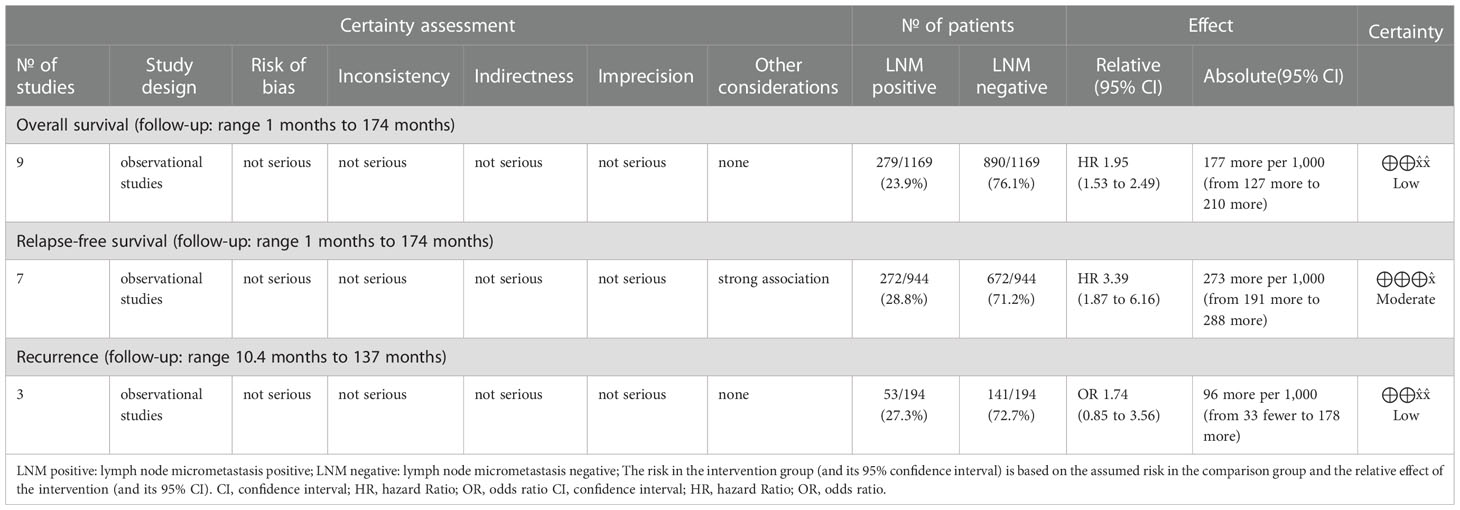
A total of 1,169 patients were identified in nine studies (5–7, 20, 21, 25, 26, 28, 31). The OS was significantly increased with LNM negativity compared with LNM positivity in esophageal cancer patients (HR 1.95; 95% CI, 1.53–2.49; P < 0.001; I2 = 0.0%, P = 0.930; Figure 2; certainty of evidence, low). The results of subgroup analysis showed that there was a trend for OS in Asians (HR 1.82, 95% CI, 1.34–2.47; I2 = 0.0%, P = 0.840) to be lower than in non-Asians (HR 2.20, 95% CI, 1.47–3.28; I2 = 0.0%, P = 0.780), although no significant difference was observed between these subgroups (P=0.47) (Table 2) (Supplementary Appendices 4). Similarly, no significant differences were observed in outcomes between subgroups of study design, antibody types, follow-up duration, pN status, single-center/multicenter, univariate/multivariate analysis and tumor type (Table 2) (Supplementary Appendices 5-11).
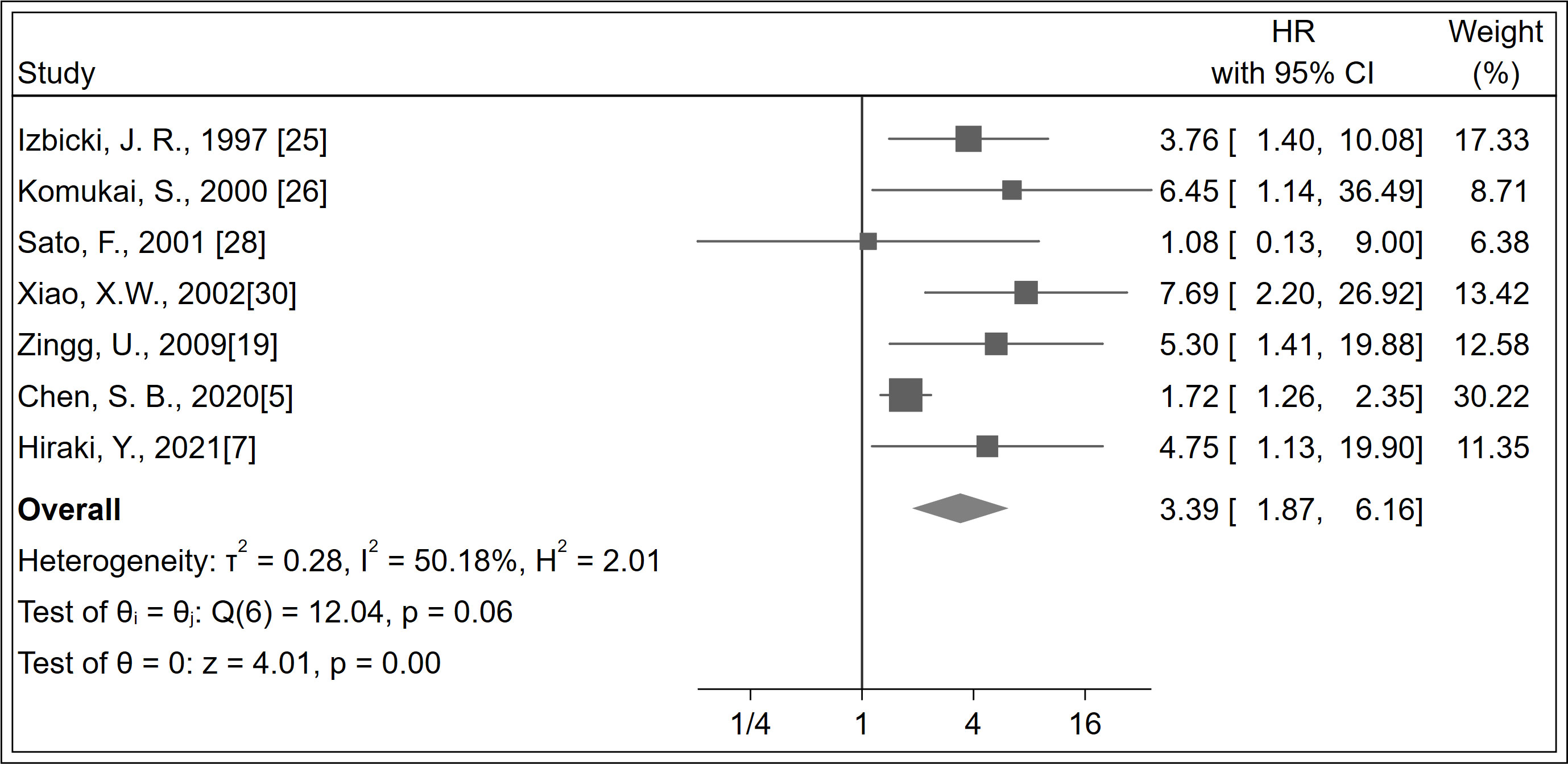
A total of 944 patients were identified in seven studies (5, 7, 19, 25, 26, 28, 30). The RFS was significantly increased with LNM negativity compared with LNM positivity in esophageal cancer patients (HR 3.39; 95% CI, 1.87–6.16; P < 0.001; I2 = 50.18%, P = 0.060; Figure 3; certainty of evidence, moderate). The results of subgroup analysis showed that there was a trend for RFS in Asians (HR 3.18, 95% CI, 1.43–7.05; I2 = 54.53%, P = 0.070) to be lower than in non-Asians (HR 4.25, 95% CI, 1.93–9.37; I2 = 0.0%, P = 0.680), although no significant difference was seen between these subgroups (P = 0.610) (Table 2) (Supplementary Appendices 12). Further, no significant differences were observed between subgroups of study design, region, antibody types, follow-up duration, pN status, single-center/multicenter, univariate/multivariate analysis, and tumor types (Table 2) (Supplementary Appendices 13-19).
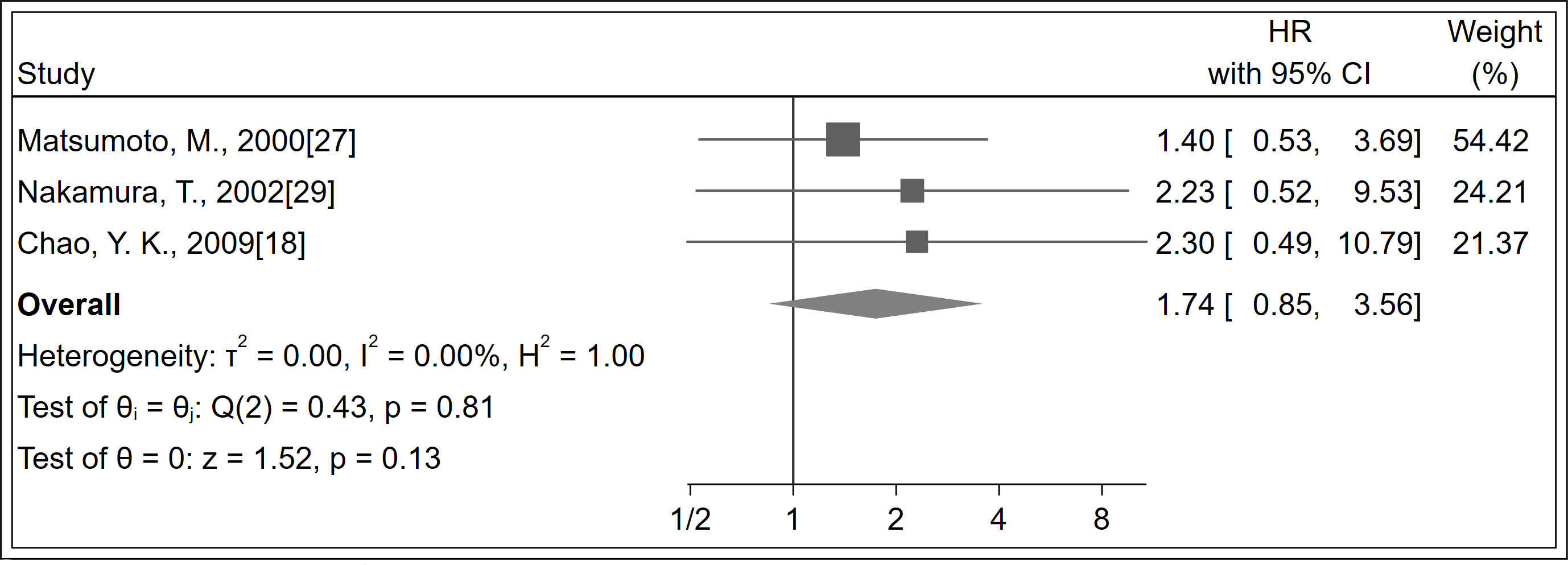
A total of 184 patients were identified in three studies (18, 27, 29). No significant difference was observed in recurrence between LNM positivity and LNM negativity groups (HR 1.74; 95% CI, 0.85–3.56; P = 0.130; I2 = 0%, P = 0.810; Figure 4; certainty of evidence, low). Further, no significant differences were observed between subgroups of follow-up duration (Supplementary Appendices 20).
3.4 Sensitivity analysis and publication bias
Sensitivity analysis with the exclusion of one trial at a time revealed a stable trend (Supplementary Appendices 21-23). In addition, after excluding three articles (6, 20, 21) for which the HR was transformed by a survival curve, it was observed that OS was significantly increased with LNM negativity compared with LNM positivity (HR 1.93; 95% CI, 1.47–2.52; P < 0.001; I2 = 0.0%, P = 0.820), and the trend was still stable.
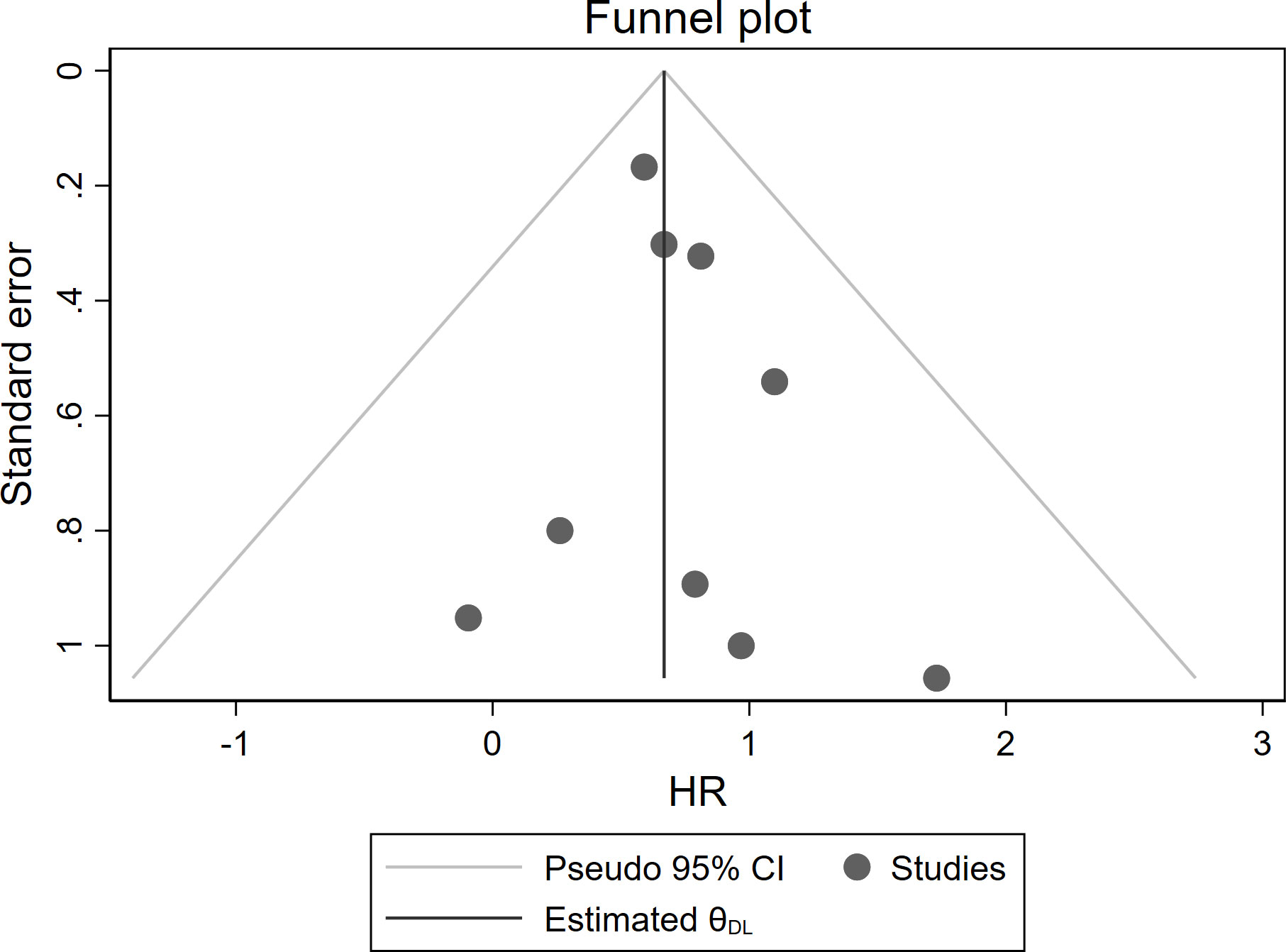
Moreover, no significant asymmetry was observed by visual inspection of the funnel plot of studies reporting OS (Figure 5). The Egger’s test did not show significant publication bias (P = 0.445).
3.5 Evidence quality
The original studies were observational studies that provided low-quality evidence. The OS and recurrence data showed low certainty, indicating that our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect (33, 34). The outcome of RFS was upgraded because of the large effect size; therefore, it shows moderate certainty, indicating that we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different (33, 34). Further details are provided in Table 3.
4 Discussion
In this meta-analysis, moderate-quality evidence showed that RFS was significantly increased with LNM negativity compared with LNM positivity in esophageal cancer patients. The OS was significantly increased with LNM negativity compared with LNM positivity in esophageal cancer patients, although the quality of evidence was low. However, no significant difference was observed in recurrence. Regretfully, several subgroups were not found to be a source of heterogeneity.
According to the UICC Tumor, Node, Metastasis-classification, eighth editions guidelines on lymph node involvement in esophageal cancer, the presence of tumor cells exceeding 0.2 mm in greatest extent is categorized as metastasis (9). LNM is a tumor lesion in a lymph node between 0.2 and 2 mm in diameter and/or a microscopic collection of more than 200 tumor cells in a lymph node (35–37).
Although most studies (19, 31, 32) have found that LNM positivity indicates a worse prognosis than LNM negativity, studies (7, 28) have found no statistically significant difference between prognosis between the two. Further, these studies (27, 29, 38) have found that recurrence with LNM positivity has a different clinical significance in esophageal cancer. Most of the included observational trials had small statistical power in this meta-analysis. Meta-analysis is an ideal statistical tool that increases the statistical power and the precision of comparisons and offers more powerful evidence for clinical decision-making. Thus, this meta-analysis was conducted to assess the clinical significance of LNM in esophageal cancer. Our meta-analysis showed that LNM is a strong prognostic factor in esophageal cancer.
We also conducted subgroup analyses in this meta-analysis including subgroups of study design, region, antibody type, follow-up duration, pN status, single-center/multicenter study, univariate/ultivariate analysis, and tumor type to explore heterogeneity. However, we could not identify the source of heterogeneity. Among the subgroups, it found that OS and RFS may be a worse trend for non-Asians compared with Asians. The incidence of esophageal cancer has increased over the past years in the Western world and is predicted to increase further given a rise in alcohol consumption and lack of physical exercise (39). Compared with Eastern patients, Western patients have a larger BMI, making it relatively difficult to achieve the minimal number of harvested lymph nodes. This is associated with a worse prognosis for esophageal cancer in Western patients (40, 41).
The results of this study are of clinical significance. Here we provide a more objective appraisal of the evidence than traditional narrative reviews and a more precise estimate of the prognostic value of LNM than that currently available. The data presented here may help plan future clinical trials and may help determine whether LNM can be used as a prognostic factor in esophageal cancer. For example, LNM was included in the current AJCC staging system for breast cancer (35); however, LNM in esophageal cancer is not designated as a staging parameter. The data from observational studies suggest that LNM has a clinically significant detrimental effect on OS and RFS in esophageal cancer. It has been shown that control of LNM by neoadjuvant chemotherapy (NAC) in esophageal squamous cell carcinoma (ESCC) is significantly associated with improved RFS at pN0 stage (7). Patients with ESCC who underwent surgery after receiving NAC with Adriamycin + cisplatin + 5-fluorouracil (ACF) or docetaxel + cisplatin + 5-fluorouracil (DCF) have shown a better RFS in the DCF group, and DCF controlled LNM better than ACF (7, 42). In addition to controlling distant metastasis recurrence, LNM control is an important requirement in NAC regimens, and DCF is effective for LNM control (42, 43). We ventured to speculate whether LNM can be included in the staging of N in esophageal cancer. At the same time, LNM was a poor prognostic factor in esophageal cancer, which provided a reference for the treatment and prognostic value of LNM in other gastrointestinal cancers.
There were some limitations to this meta-analysis. First, because the definition of LNM itself has not been standardized, the original research reported some differences in the definition of LNM, which may be the source of heterogeneity. However, further exploration could not be carried out because of the limitation of the original data. Second, an estimated moderate degree of heterogeneity was found for RFS among studies. However, secondary analyses including subgroup analysis and sensitivity analysis were performed to partly explore this limitation. Third, the results of this study needed to be interpreted with caution because a majority of studies were Asian, although there were no restrictions on the study population in this study. Lastly, the evidence is low-to-moderate quality because most studies included in the analysis were observational in nature. Therefore, future relevant high-quality studies with a large sample size are needed to confirm the prognostic value of LNM.
5 Conclusion
LNM positivity has a worse prognosis in esophageal cancer, although the evidence for this is low to moderate. Future relevant high-quality studies are needed to further validate our results and provide a reference for guidelines.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
JY and QL: literature search, screening, data extraction, data analysis and results visualization. The manuscript was written with the contributions of all authors. MZ: fund acquirement. All authors have approved the final version of the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (grant no 81860059). Basic Research Innovation Group Project of Gansu Province (No.22JR5RA709)
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1025855/full#supplementary-material
References
1. Ajani JA, D'Amico TA, Bentrem DJ, Chao J, Corvera C, Das P, et al. Esophageal and Esophagogastric Junction Cancers, Version 2.2019, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw (2019) 17(7):855–83. doi: 10.6004/jnccn.2019.0033
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
3. Okholm C, Svendsen LB, Achiam MP. Status and prognosis of lymph node metastasis in patients with cardia cancer - a systematic review. Surg Oncol (2014) 23(3):140–6. doi: 10.1016/j.suronc.2014.06.001
4. Enzinger PC, Mayer RJ. Esophageal cancer. N Engl J Med (2003) 349(23):2241–52. doi: 10.1056/NEJMra035010
5. Chen SB, Liu DT, Huang SJ, Weng HR, Wang G, Li H, et al. Prognostic value of occult lymph node metastases in patients with completely resected esophageal squamous cell carcinoma. Sci Rep (2020) 10(1):22007. doi: 10.1038/s41598-020-79073-9
6. Koenig AM, Prenzel KL, Bogoevski D, Yekebas EF, Bubenheim M, Faithova L, et al. Strong impact of micrometastatic tumor cell load in patients with esophageal carcinoma. Ann Surg Oncol (2009) 16(2):454–62. doi: 10.1245/s10434-008-0169-7
7. Hiraki Y, Kimura Y, Imano M, Kato H, Iwama M, Shiraishi O, et al. Controlling lymph node micrometastases by neoadjuvant chemotherapy affects the prognosis in advanced esophageal squamous cell carcinoma. Surg Today (2021) 51(1):118–26. doi: 10.1007/s00595-020-02059-7
8. Heeren PAM, Kelder W, Blondeel I, van Westreenen HL, Hollema H, Plukker JT. Prognostic value of nodal micrometastases in patients with cancer of the gastro-oesophageal junction. Eur J Surg Oncol (2005) 31(3):270–6. doi: 10.1016/j.ejso.2004.12.001
9. Brierley JDGM, Wittekind C eds. The TNM classification of malignant tumours. 8th edn. Oxford: Wiley Blackwell (2017).
10. Rice TW DTP, Blackstone EH. AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice, 8th edition, Ann Cardiothorac. Surg (2017) 6:119–30. doi: 10.21037/acs.2017.03.14
11. Odze RD LA, Ochiai A, Washington MK. WHO classification of tumors of the oesophagus. 5th. WHO Classification of Tumours Editorial Board (2019).
12. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Rev Esp Cardiol (Engl Ed) (2021) 74(9):790–9. doi: 10.1016/j.rec.2021.07.010
14. Hayden JA, van der Windt DA, Cartwright JL, Cote P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med (2013) 158(4):280–6. doi: 10.7326/0003-4819-158-4-201302190-00009
15. Pollock A, Farmer SE, Brady MC, Langhorne P, Mead GE, Mehrholz J, et al. An algorithm was developed to assign GRADE levels of evidence to comparisons within systematic reviews. J Clin Epidemiol (2016) 70:106–10. doi: 10.1016/j.jclinepi.2015.08.013
16. Brunetti M, Shemilt I, Pregno S, Vale L, Oxman AD, Lord J, et al. GRADE guidelines: 10. Considering resource Use rating Qual economic evidence. J Clin Epidemiol (2013) 66(2):140–50. doi: 10.1016/j.jclinepi.2012.04.012
17. Tanabe T, Nishimaki T, Watanabe H, Ajioka Y, Akazawa K, Komukai S, et al. Immunohistochemically detected micrometastasis in lymph nodes from superficial esophageal squamous cell carcinoma. J Surg Oncol (2003) 82(3):153–9. doi: 10.1002/jso.10207
18. Chao YK, Yeh CJ, Chuang WY, Fan KH, Hsieh MJ, Chu Y, et al. Prognostic significance of immunohistochemically detected lymph node micrometastases in pT0N0 esophageal squamous cell carcinoma. J Surg Oncol (2009) 100(7):559–62. doi: 10.1002/jso.21362
19. Zingg U, Montani M, Busch M, Metzger U, Went P, Oertli D. Prognostic influence of immunohistochemically detected lymph node micrometastasis and histological subtype in pN0 oesophageal cancer. Eur J Surg Oncol (2009) 35(6):593–9. doi: 10.1016/j.ejso.2008.12.001
20. Doki Y, Ishikawa O, Mano M, Hiratsuka M, Sasaki Y, Kameyama M, et al. Cytokeratin deposits in lymph nodes show distinct clinical significance from lymph node micrometastasis in human esophageal cancers. J Surg Res (2002) 107(1):75–81. doi: 10.1006/jsre.2002.6506
21. Shiozaki H, Fujiwara Y, Hirai T, Matsubara H, Mori M, Nakamura T, et al. Clinical significance of immunohistochemically detected lymph node micrometastasis in patients with histologically node-negative esophageal carcinoma: A multi-institutional study. Esophagus (2007) 4(1):35–9. doi: 10.1007/s10388-006-0098-0
22. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials (2007) 8:16. doi: 10.1186/1745-6215-8-16
23. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
24. Ioannidis JP. Interpretation of tests of heterogeneity and bias in meta-analysis. J Eval Clin Pract (2008) 14(5):951–7. doi: 10.1111/j.1365-2753.2008.00986.x
25. Izbicki JR, Hosch SB, Pichlmeier U, Rehders A, Busch C, Niendorf A, et al. Prognostic value of immunohistochemically identifiable tumor cells in lymph nodes of patients with completely resected esophageal cancer. N Engl J Med (1997) 337(17):1188–94. doi: 10.1056/NEJM199710233371702
26. Komukai S, Nishimaki T, Watanabe H, Ajioka Y, Suzuki T, Hatakeyama K. Significance of immunohistochemically demonstrated micrometastases to lymph nodes in esophageal cancer with histologically negative nodes. Surgery (2000) 127(1):40–6. doi: 10.1067/msy.2000.102754
27. Matsumoto M, Natsugoe S, Nakashima S, Sakamoto F, Okumura H, Sakita H, et al. Clinical significance of lymph node micrometastasis of pN0 esophageal squamous cell carcinoma. Cancer Lett (2000) 153(1-2):189–97. doi: 10.1016/s0304-3835(00)00374-8
28. Sato F, Shimada Y, Li Z, Watanabe G, Maeda M, Imamura M. Lymph node micrometastasis and prognosis in patients with oesophageal squamous cell carcinoma. Br J Surg (2001) 88(3):426–32. doi: 10.1046/j.1365-2168.2001.01687.x
29. Nakamura T, Ide H, Eguchi R, Hayashi K, Ota M, Takasaki K. Clinical implications of lymph node micrometastasis in patients with histologically node-negative (pN0) esophageal carcinoma. J Surg Oncol (2002) 79(4):224–9. doi: 10.1002/jso.10080
30. Xiao XW. Relationship between lymph node metastases in esophageal carcinoma and its prognosis. Chi J Cancer Res (2002) 14(4):297–301. doi: 10.1007/s11670-002-0065-9
31. Thompson SK, Ruszkiewicz AR, Jamieson GG, Sullivan TR, Devitt PG. Isolated tumor cells in esophageal cancer: implications for the surgeon and the pathologist. Ann Surg (2010) 252(2):299–306. doi: 10.1097/SLA.0b013e3181e61e15
32. Prenzel KL, Hölscher AH, Drebber U, Agavonova M, Gutschow CA, Bollschweiler E. Prognostic impact of nodal micrometastasis in early esophageal cancer. Eur J Surg Oncol (2012) 38(4):314–8. doi: 10.1016/j.ejso.2012.01.007
33. Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol (2011) 64(4):383–94. doi: 10.1016/j.jclinepi.2010.04.026
34. Schunemann HJ, Cuello C, Akl EA, Mustafa RA, Meerpohl JJ, Thayer K, et al. GRADE guidelines: 18. How ROBINS-I and other tools to assess risk of bias in nonrandomized studies should be used to rate the certainty of a body of evidence. J Clin Epidemiol (2019) 111:105–14. doi: 10.1016/j.jclinepi.2018.01.012
35. MB A, American Joint Committee on Cancer. AJCC Cancer Staging Manual. 8th edn. New York: Springer-Verlag (2017).
36. Hermanek P, Hutter RV, Sobin LH, Wittekind C. International Union Against Cancer. Classification of isolated tumor cells and micrometastasis. Cancer (1999) 86(12):2668–73.
37. Xu X, Zheng G, Zhang T, Zhao Y, Zheng Z. Clinical Significance of Metastasis or Micrometastasis to the Lymph Node Along the Superior Mesenteric Vein in Gastric Carcinoma: A Retrospective Analysis. Front Oncol (2021) 11:707249. doi: 10.3389/fonc.2021.707249
38. Buskens CJ, Ten Kate FJ, Obertop H, Izbicki JR, van Lanschot JJ. Analysis of micrometastatic disease in histologically negative lymph nodes of patients with adenocarcinoma of the distal esophagus or gastric cardia. Dis Esophagus (2008) 21(6):488–95. doi: 10.1111/j.1442-2050.2007.00805.x
39. Napier KJ, Scheerer M, Misra S. Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol (2014) 6(5):112–20. doi: 10.4251/wjgo.v6.i5.112
40. Zhang H, Yan X, Yang YS, Yang H, Yuan Y, Tian D, et al. The Least Nodal Disease Burden Defines the Minimum Number of Nodes Retrieved for Esophageal Squamous Cell Carcinoma. Front Oncol (2022) 12:764227. doi: 10.3389/fonc.2022.764227
41. Wu LL, Zhong JD, Zhu JL, Kang L, Huang YY, Lin P, et al. Postoperative survival effect of the number of examined lymph nodes on esophageal squamous cell carcinoma with pathological stage T1-3N0M0. BMC Cancer (2022) 22(1):118. doi: 10.1186/s12885-022-09207-x
42. Yamasaki M, Yasuda T, Yano M, Hirao M, Kobayashi K, Fujitani K, et al. Multicenter randomized phase II study of cisplatin and fluorouracil plus docetaxel (DCF) compared with cisplatin and fluorouracil plus Adriamycin (ACF) as preoperative chemotherapy for resectable esophageal squamous cell carcinoma (OGSG1003). Ann Oncol (2017) 28(1):116–20. doi: 10.1093/annonc/mdw439
43. Shiraishi O, Yamasaki M, Makino T, Motoori M, Miyata H, Shinkai M, et al. Feasibility of Preoperative Chemotherapy with Docetaxel, Cisplatin, and 5-Fluorouracil versus Adriamycin, Cisplatin, and 5-Fluorouracil for Resectable Advanced Esophageal Cancer. Oncology (2017) 92(2):101–8. doi: 10.1159/000452765
Keywords: esophageal cancer, lymph node micrometastasis, prognosis, meta-analysis, systematic review
Citation: Yang J, Liu Q, Bai Y, Zhao H, He T, Zhao Z, Huang M, Jiang M, Zhang R and Zhang M (2023) Prognostic value of lymph node micrometastasis in esophageal cancer: A systematic review and meta-analysis. Front. Oncol. 12:1025855. doi: 10.3389/fonc.2022.1025855
Received: 23 August 2022; Accepted: 06 December 2022;
Published: 04 January 2023.
Edited by:
Harushi Udagawa, Toranomon Hospital Kajigaya, JapanReviewed by:
Satoru Motoyama, Akita Red Cross Hospital, JapanXi Yang, Fudan University, China
Justine Lequesne, Centre François Baclesse, France
Copyright © 2023 Yang, Liu, Bai, Zhao, He, Zhao, Huang, Jiang, Zhang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Min Zhang, c2FsbHl6aGFuZ21pbkAxMjYuY29t
†These authors have contributed equally to this work
 Jing Yang
Jing Yang Qianqian Liu1,2,3†
Qianqian Liu1,2,3† Yuping Bai
Yuping Bai Haitong Zhao
Haitong Zhao Min Zhang
Min Zhang