- 1Merck & Co., Inc., Rahway, NJ, United States
- 2Department of Epidemiology, Richard M. Fairbanks School of Public Health, Indiana University, Indianapolis, IN, United States
- 3Seagen Inc., Bothell, WA, United States
- 4Integrative Precision Health, LLC, Carmel, IN, United States
Background: More than 60% of all stage IV melanoma patients develop brain metastases, while melanoma brain metastases (MBM) is historically difficult to treat with poor prognosis.
Objectives: To summarize clinical outcomes and prognostic factors in MBM patients.
Methods: A systematic review with meta-analysis was conducted, and a literature search for relevant studies was performed on November 1, 2020. Weighted average of median overall survival (OS) was calculated by treatments. The random-effects model in conducting meta-analyses was applied.
Results: A total of 41 observational studies and 12 clinical trials with our clinical outcomes of interest, and 31 observational studies addressing prognostic factors were selected. The most common treatments for MBM were immunotherapy (IO), MAP kinase inhibitor (MAPKi), stereotactic radiosurgery (SRS), SRS+MAPKi, and SRS+IO, with median OS from treatment start of 7.2, 8.6, 7.3, 7.3, and 14.1 months, respectively. Improved OS was observed for IO and SRS with the addition of IO and/or MAPKi, compared to no IO and SRS alone, respectively. Several prognostic factors were found to be significantly associated with OS in MBM.
Conclusion: This study summarizes pertinent information regarding clinical outcomes and the association between patient characteristics and MBM prognosis.
Introduction
Brain metastasis is a frequent and grave complication of melanoma (1). The median overall survival (OS) of patients with melanoma brain metastases (MBM) has historically been approximately 4 months after diagnosis. Recent studies have shown that immune checkpoint inhibitors targeting the programmed cell death protein 1 (PD1) and cytotoxic T-lymphocyte associated protein 4 (CTLA-4) pathways as well as novel small-molecule tyrosine kinase inhibitors targeting BRAF driver mutations, can improve survival in MBM (2). Margolin et al. reported a phase II trial investigating the activity of ipilimumab in MBM patients and showed that it was safe and resulted in tumor regression in some patients (3). Long et al. studied dabrafenib in BRAF mutated MBM in a phase II clinical trial, and demonstrated activity against brain metastases in MBM patients with or without prior local treatment (4). The treatment of MBM has thus shifted significantly in recent years, creating a growing body of research on novel targeted therapies in MBM in the realm of clinical oncology. However, there is still a lack of understanding of the efficacy of newer therapies for patients with MBM.
It has been suggested that patients who present with larger, symptomatic metastases are at higher risk for poorer performance status and worse prognosis, providing a strong rationale for early detection and treatment of MBM (5). An institutional database study of patients with melanoma enrolled on clinical trials from 1986 to 2004 by Davies et al. found that 330 developed MBM and prognostic factors for OS were earlier diagnosis, increased number of MBM, leptomeningeal involvement, and development of MBM after systemic therapy for extracranial metastatic disease (6). Nevertheless, prognostic factors for OS in MBM patients are not well defined.
To address these gaps in the research literature, there is a need to summarize the clinical outcomes and prognostic factors in patients with MBM at diagnosis or who develop MBM during the course of treatment. We performed a systematic review and meta-analysis to examine clinical outcomes, including OS and progression-free survival (PFS), and prognostic factors for patients with MBM, focusing on the most recent research.
Patients and methods
Study design and search strategy
This study was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines. Relevant studies with full text articles published in English in the last five years were searched in the databases: EMBASE, MEDLINE, Cochrane Register of Controlled Trials, and Cochrane Review on November 1, 2020. Search terms included “melanoma”, “brain metastasis” or “cerebral metastasis”, and related terms (e.g. metastases), along with an epidemiology studies filter to include the eligible study designs (Tables S1–S4). Eligible studies were identified and selected according to the following eligibility criteria: 1) Studies published from November 1, 2015 to November 1, 2020; 2) study population are adult patients (>18 years) with melanoma who develop or present with at least one brain metastases; 3) reported clinical outcomes (OS, PFS) or prognostic factors for OS in MBM patients; 4) study designs included prospective and retrospective cohort studies, case-controls, cross-sectional studies, controlled or uncontrolled longitudinal studies; 5) no minimum sample sizes were required. Exclusion criteria included that the study was not published in English language, that the study was in animals or laboratory setting only, did not fall within the date range (published before November 1, 2015), had a duplicate study population, or if the relevant intervention (treatment) or outcomes of interest (OS, PFS, HR) were not available. Two reviewers independently selected studies according to the inclusion and exclusion criteria and extracted data, with a third independent reviewer available to address any discrepancies and perform a quality check. Bibliographies from review articles were reviewed thoroughly to identify relevant additional studies and trial results.
Data extraction
The clinical outcomes of interest for this study were OS and PFS. We extracted median OS/PFS (in months) and the hazard ratios (HR) for OS/PFS along with 95% confidence intervals (CI). Some studies reported OS/PFS from date of diagnosis of MBM (time between diagnosis of brain metastases and death or last follow up), while others reported OS/PFS from start of treatment (time from the first treatment start date to the time of death or last follow-up). We also extracted the HR and 95% CI for each prognostic factor for OS in MBM patients, including age, sex, biomarkers, performance status, intracranial and extracranial disease status, and mutation status.
Data analysis
The weighted average (by sample size) was calculated for the median months of OS by treatments. For studies that presented Kaplan-Meier (K-M) survival data without reporting HR, we used a previously published methodology for estimating HR from time-to-event analyses (7). Meta-HR for OS with corresponding 95% CIs were calculated for clinical outcomes and prognostic factors using random-effects models. Cochrane’s Q test and the I2 statistic were used to assess heterogeneity between studies, with a P-value < 0.05 for Cochrane’s Q test and I2 > 50% considered cut-offs for significant heterogeneity (8, 9). The results from the meta-analysis are presented graphically as forest plots. Publication bias was assessed by contour‐enhanced funnel plots of standard error against the effect estimate. All statistical analyses were performed using STATA (Version 14; Stata Corp., College Station, TX).
For clinical outcomes of observational studies, multiple studies were reported with clear information on treatment assignments for stereotactic radiosurgery (SRS) alone, MAP kinase inhibitor (MAPKi, which includes BRAFi [BRAF inhibitor] and/or MEKi [MEK inhibitor] and is used in patients with a BRAF mutation), SRS+IO, SRS+MAPKi, and SRS+MAPKi+IO. Therefore, we grouped those studies together, and performed meta-analyses for treatment comparisons by separating for those with OS from start of treatment and those with OS from date of diagnosis. However, if one study reported separate results for anti-PD1 and anti-CTLA-4 using a common reference group, these results were not grouped into a single IO group, but instead were reported separately in the summary tables.
For prognostic factors of MBM, the studies with similar definitions were grouped and meta-analysis was performed to summarize their association with OS in MBM patients. However, due to variable cut-off values and different reference groups chosen in some studies, we were not able to perform meta-analysis on all studies.
Results
Study selection
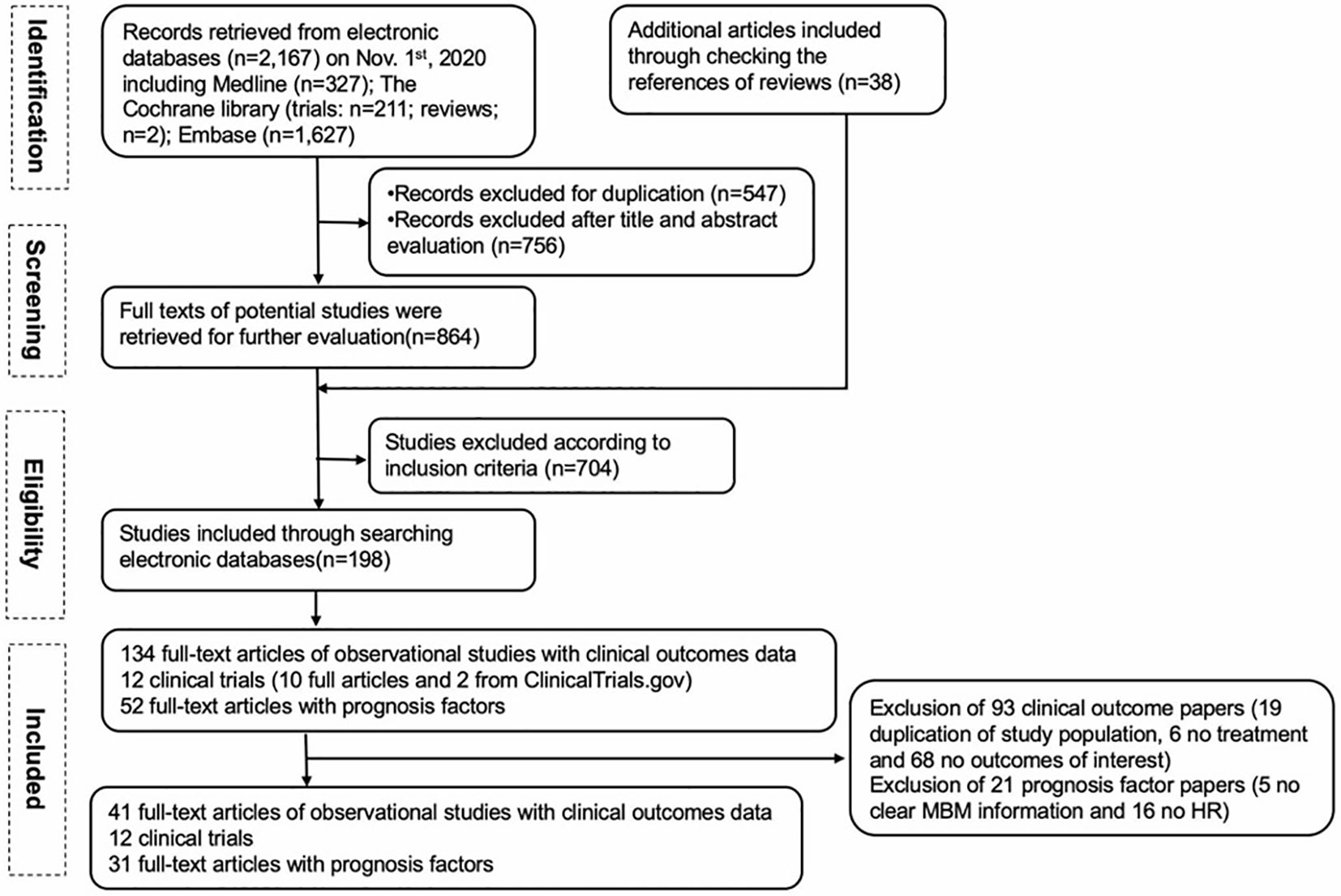
Our PRISMA study protocol is presented schematically in Figure 1. For clinical outcomes, 134 full-text articles of observational studies were screened, and 93 articles were excluded (19 due to duplication of the same population, 6 had no treatment reported, and 68 had no outcomes of interest). Ten full-text articles of clinical trials were included, and two additional clinical trials were identified from ClinicalTrials.gov. Finally, 41 observational studies and 12 clinical trials with our clinical outcomes of interest (OS and/or PFS) were included. For prognostic factors among MBM, 52 full-text articles were screened, and 21 were excluded (5 due to no clear MBM information, and 16 due to no HR). Thirty-one full-text papers for prognostic factors were included in the final analysis.
OS reported in observational studies
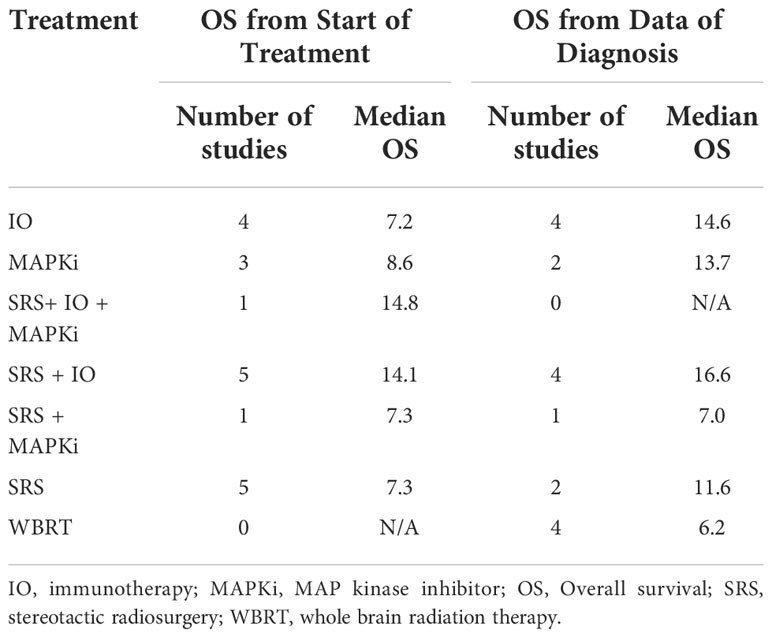
We present the clinical outcomes from 41 observational studies in which median OS or HR for OS were available to extract, ordered either from start of treatment (29 studies) or from date of diagnosis (12 studies) (Supporting Information, Tables S5, S6) (10–50). The median OS averaged across studies utilizing the same treatment combinations is also shown in Table 1, ranging from 7.2-14.8 months from start of treatment and 6.2-16.6 months from date of diagnosis. For SRS+IO, the weighted average median OS was 14.1 months from start of treatment, and 16.6 months from date of diagnosis.
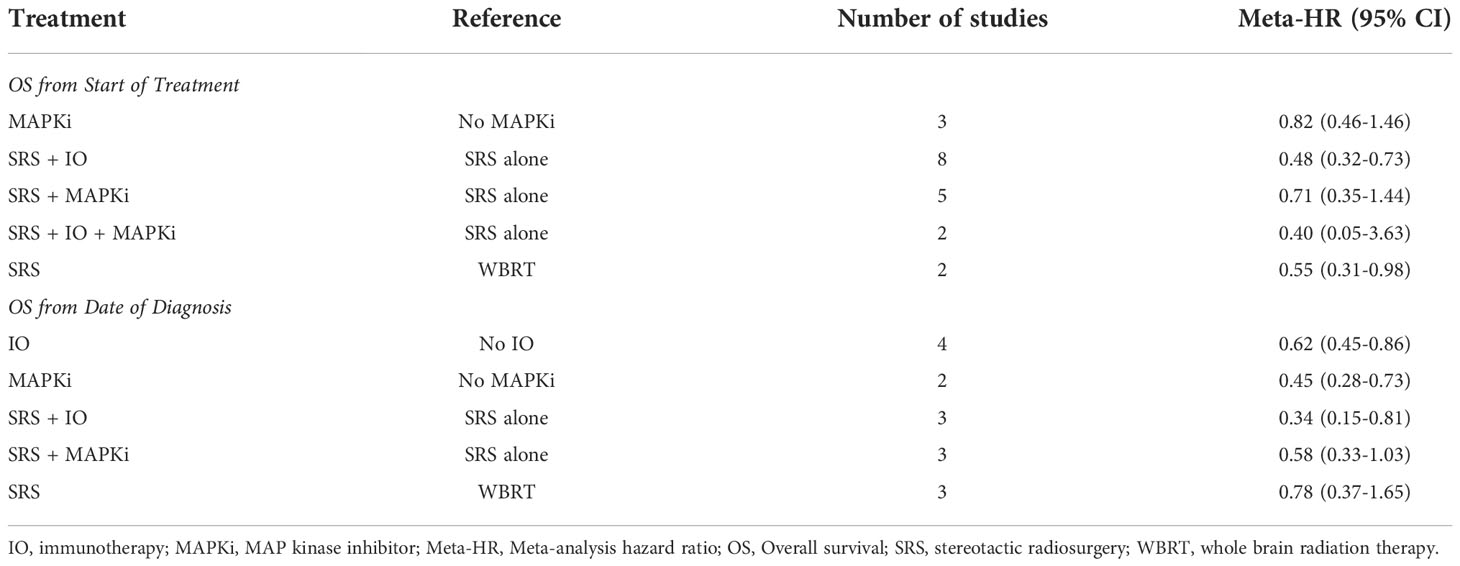
Meta-analysis by treatment for OS in observational studies
Meta-analysis by treatment for OS were summarized in Table 2, and forest plots were provided in Figures S1-S6. The significant benefit of IO on OS from start of treatment was observed by the comparison of SRS+IO vs. SRS alone (n = 8), with meta-HR of 0.48 (95% CI, 0.32-0.73). SRS compared to whole brain radiation therapy (WBRT) had a meta-HR of 0.55 (95% CI, 0.31-0.98) based on analysis of 2 studies (19, 20). Non-significant improvement of OS was observed for SRS+IO+MAPKi vs. SRS alone (meta-HR 0.40; 95% CI, 0.05-3.63; n=2), MAPKi vs. no MAPKi (meta-HR, 0.82; 95% CI, 0.46-1.46; n=3), and SRS+MAPKi vs. SRS alone (meta-HR, 0.71; 95% CI, 0.35-1.44; n=5) (11–13, 15, 16, 20–22).
Meta-analysis results by treatment for OS from date of diagnosis showed similar results. For the OS from date of diagnosis, treatment with SRS+IO vs. SRS alone had meta-HR of 0.34 (95% CI, 0.15-0.81; n=3) (Table 2, Figure S2), and IO alone vs. no IO had a meta-HR of 0.62 (95% CI, 0.45-0.86; n=4) (Table 2, Figure S6) (39, 41, 42). For MAPKi vs. no MAPKi, meta-analysis showed meta-HR of 0.45 (95% CI, 0.28-0.73; n=2) (43, 50). However, no significant improvement OS from date of diagnosis was observed for SRS vs. WBRT or for SRS+MAPKi vs. SRS alone (39–42, 50).
PFS reported in observational studies
Ten selected observational studies contained data on PFS, which ranged from 2-20.3 months from start of treatment or from 3.4-19 months from date of diagnosis (Table S7). Of the 10 studies, 9 also contained OS data, while one study by Robin et al. only included PFS data (51). PFS results were generally consistent with OS results, for example the study by Minniti et. al., 2019 that showed improved OS with SRS+IO found median PFS of 19 months from date of diagnosis of MBM (31).
OS reported in clinical trials
The median OS and HR for OS results in 12 identified clinical trials are summarized in Table S8 (52–63). Eleven clinical trials reported median OS ranging from 2.5 months (in patients who received only WBRT) to OS not reached (NR) in patients who received IO. However, comparison between trials was difficult given the different interventions being tested, the different patient populations (e.g. symptomatic vs. asymptomatic, previously treated vs. untreated, etc), and the relatively small numbers of patients in most trials (8 of the 12 trials had 25 patients or less in a study arm).
Prognostic factors for OS in patients with MBM
The HRs for each prognostic factor extracted from 31 observational studies are summarized in Table S9, meta-HR are summarized in Table 3, and forest plots provided in Figures S7–S15 (1, 27, 47, 48, 50, 64–89). Meta-analysis suggested elevated lactate dehydrogenase (LDH) levels, male gender, BRAF wild-type, increased number of intracranial metastases, presence of active extracranial metastases, lower Karnofsky Performance Scale (KPS), and larger MBM volume were significantly associated with worse prognosis in patients with MBM.
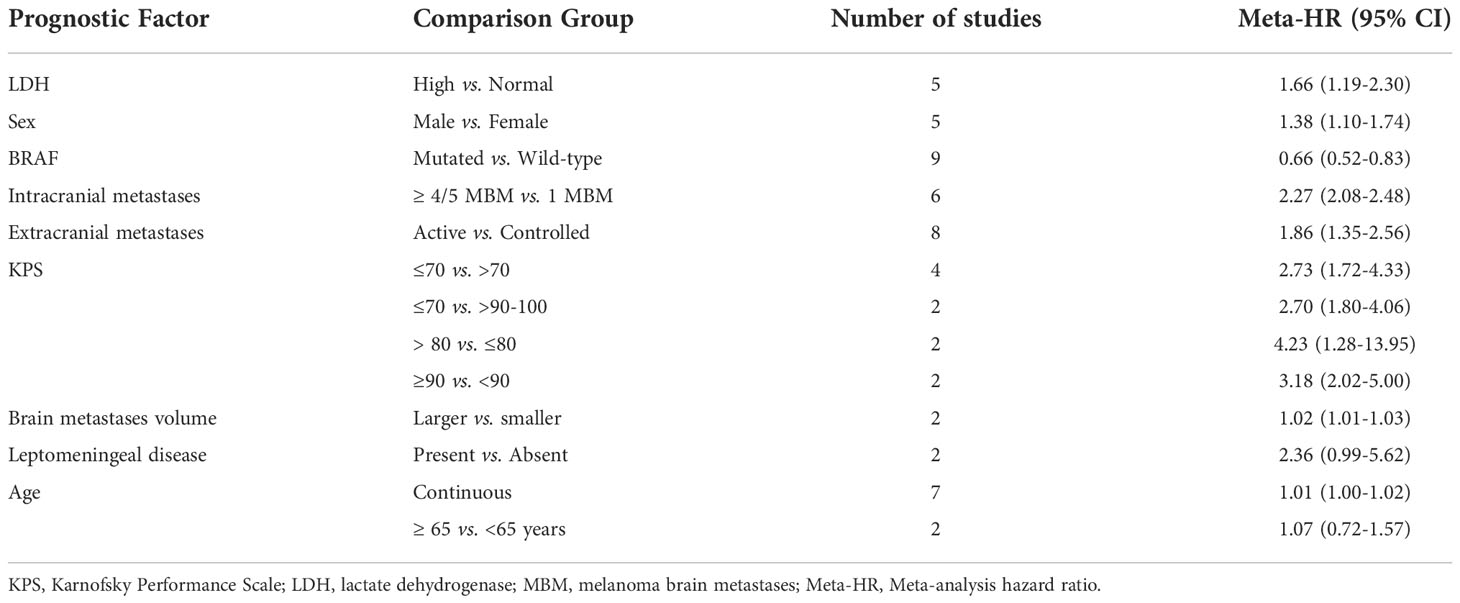
Table 3 Meta-analysis hazard ratios (Meta-HR) for prognostic factors of overall survival (OS) among patients with MBM.
In particular, five studies showed increased LDH was associated with shorter survival (meta-HR, 1.66; 95% CI, 1.19-2.30). Five studies tested for an association between gender and OS and found decreased OS with male gender compared to female gender (meta-HR, 1.38; 95% CI, 1.10-1.74; n=5). Nine studies showed improved outcomes with BRAF mutation compared to BRAF wildtype (meta-HR, 0.66; 95% CI, 0.52-0.83; n=9). Nine studies assessed whether higher burden of MBM was associated with OS. In general, the data supported that more abundant intracranial metastases are associated with decreased OS. Among studies that had a reference group of 1 MBM compared to higher numbers, patients with 2 to 4 or 5 metastases had a meta-HR of 1.41 (95% CI, 1.11-1.80; n=5), and patients with more than 4 or 5 metastases had a meta-HR of 2.27 (95% CI, 2.08-2.48; n=6). Eight studies demonstrated worse survival outcomes with active extracranial disease compared to controlled disease (meta-HR, 1.86; 95% CI, 1.35-2.56). Decreased KPS (worse performance status) was associated with worse prognosis based on the results of thirteen studies, and the meta-HR was 2.73 (95% CI, 1.72-4.33; n=4), 4.23 (95% CI, 1.28-13.95; n=2), or 3.18 (95% CI, 2.02-5.00; n=2), using (≤70 vs. >70), (≤80 vs. >80), or (≤90 vs. >90) as cutoff points, respectively. Compared to those with KPS 90-100, those with KPS of ≤ 70 had a meta-HR of 2.70 (95% CI, 1.80-4.06; n=2). Larger total intracranial tumor volume was found to be associated with worse survival (meta-HR = 1.02; 95% CI, 1.01-1.03; n=2). Presence of leptomeningeal disease and advanced age trended towards association with worse prognosis, however the meta-HRs were non-significant.
Discussion
Overall, evidence from observational studies suggest that SRS with addition of IO or IO plus MAPKi may improve survival outcomes in patients with MBM, compared to SRS alone. When averaged across studies utilizing the same treatment combinations, SRS+ IO had an improved median OS in months from start of treatment of approximately 14.1 months based on 5 studies. Treatment with combined SRS+IO+MAPKi was also promising with one study showing a median OS of 14.8 months. Meta-analyses provided support for the benefit from SRS+IO compared to SRS alone (12, 15). Further meta-analysis for studies that measured OS from date of diagnosis also showed that IO and SRS+IO had significantly improved OS compared to no IO and SRS alone, respectively.
A recent meta-analysis of MBM patients by Tawbi et al. (90) included 13 trials, of which 3 were randomized controlled trials, 9 were single-arm studies, and 1 was a non-randomized comparative study. They calculated median OS through a meta-analysis of K-M curves for selected interventions including IO or TT or as a weighted average of median OS. They observed that median OS was longer with nivolumab plus ipilimumab (28.3 months; 95% CI = 19.7-31.9) than with the other interventions including IO monotherapy or TT (range 5.7-11.8 months), based on pooled K-M curves. Similar OS benefit was also observed with nivolumab plus ipilimumab when the weighted average of the median was used (median OS 29.2 months) compared with the other interventions. This analysis suggested a clinical advantage with this treatment combination, but the heterogeneity of the data with respect to prior therapies (many patients received prior surgery, RT, systemic therapy, IO, or TT) and patient characteristics contributed uncertainty to the analysis.
Studies included in both the Tawbi et al. meta-analysis and our systematic review were a randomized trial by Long et al., 2018 and single-arm studies by McArthur et al., 2017, Davies et al., 2017, Kluger et al., 2019, and Tawbi et al., 2018 (52, 58–60, 62). However, in our analysis, prolonged median OS with IO was not demonstrated to the extent seen in the meta-analysis by Tawbi et al. In our study, average median OS from start of treatment was 7.2 months, 14.1 months, and 14.8 months for IO alone, SRS+IO, and SRS+IO+MAPKi, respectively. This may have been due to the heterogeneity of study populations, with inclusion of patients in the observational studies who had received a variety of prior treatments. Selection bias is also a limitation as healthier patients may be more likely to be selected for combination therapy such as SRS+IO or nivolumab+ipilimumab, and patients that undergo SRS generally have a limited number of brain metastases compared to patients that undergo WBRT or are not recommended for any radiation. It is worth noting that there may be unaccounted-for differences in patients who participated in clinical trials compared to those who did not (91). Given the variable patient populations and interventions, meta-analysis was not performed on the 12 clinical trials identified in our systematic review. More clinical trial data is needed for MBM patients in order to determine the most beneficial interventions.
In addition, our results suggest that elevated LDH levels, male gender, BRAF wild-type, more-numerous intracranial metastases, larger total MBM volume, presence of active extracranial metastases, and lower KPS scores may be prognostic for reduced OS in patients with MBM. While it is not unexpected that worse performance status and higher burden of disease were associated with reduced OS, some of the other associations are less clear. It is possible that an unknown confounding factor or biomarker is related to the association between gender and reduced OS. Limitations for this analysis included heterogeneity in participants, interventions, and outcomes studied (variable definitions in some studies related to the cutoff values and reference groups for some prognostic factors). A limitation of the OS meta-analysis results is that many studies defined date of diagnosis as the start date for OS calculation, rather than defining the start date as the day of treatment start, leading to more variability. Overall, this population is difficult to study given most of the data available is from retrospective reports or small clinical trials. Many of the meta-analyses performed included only a small number of studies. Since immunotherapy was not the primary focus, additional prognostic biomarkers such a neutrophil-lymphocyte ratio and PD-L1 were not included in this review. We have stayed abreast of the new literature on this specific topic after the date of our search execution. However, no major studies fell into our inclusion criteria.
In conclusion, although MBM is known to be associated with poor survival, evidence from our systematic review and meta-analysis of observational studies indicates that IO or combination IO and MAPKi therapy with SRS may lead to improved outcomes compared to patients treated without these therapies. A better understanding of prognostic factors may help clinicians with treatment planning, outcome assessment, and planning of support measures for individual MBM patients. Larger, randomized clinical trials would help to further elucidate the most effective therapy combinations to meet the needs of this understudied population.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
Study design, X-LT, ES, JH, and IS. Data collection, HT, NK, and JH. Data analysis, HT and JH. Manuscript writing, X-LT, AL, and JH. Manuscript review and approval, X-LT, AL, ES, HT, NK, JH, RJ, SD, and IS. All authors contributed to the article and approved the submitted version.
Conflict of interest
X-LT, ES, RJ, SD, and IS are employed by Merck & Co., Inc. JH, HT, and AL are employed by Integrative Precision Health LLC.
The authors declare that this study received funding from Merck & Co., Inc., Rahway, NJ, USA. The funder had the following involvement with the study: study design, collection, analysis, interpretation of data, the writing of this article and the decision to submit it for publication.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1025664/full#supplementary-material
References
1. Kavouridis VK, Harary M, Hulsbergen AFC, Lo YT, Reardon DA, Aizer AA, et al. Survival and prognostic factors in surgically treated brain metastases. J Neuro-Oncol (2019) 143(2):359–67. doi: 10.1007/s11060-019-03171-6
2. Dodson C, Smith DA, Richards TJ, Devita RR, Hoimes CJ, Ramaiya NH. Systemic therapies for melanoma brain metastases: A primer for radiologists. J Comput Assist Tomogr. (2020) 44(3):346–55. doi: 10.1097/RCT.0000000000001006
3. Margolin K, Ernstoff MS, Hamid O, Lawrence D, McDermott D, Puzanov I, et al. Ipilimumab in patients with melanoma and brain metastases: An open-label, phase 2 trial. Lancet Oncol (2012) 13(5):459–65. doi: 10.1016/S1470-2045(12)70090-6
4. Long GV, Trefzer U, Davies MA, Kefford RF, Ascierto PA, Chapman PB, et al. Dabrafenib in patients with Val600Glu or Val600Lys BRAF-mutant melanoma metastatic to the brain (BREAK-MB): A multicentre, open-label, phase 2 trial. Lancet Oncol (2012) 13(11):1087–95. doi: 10.1016/S1470-2045(12)70431-X
5. Raizer JJ, Hwu WJ, Panageas KS, Wilton A, Baldwin DE, Bailey E, et al. Brain and leptomeningeal metastases from cutaneous melanoma: Survival outcomes based on clinical features. Neuro Oncol (2008) 10(2):199–207. doi: 10.1215/15228517-2007-058
6. Davies MA, Liu P, McIntyre S, Kim KB, Papadopoulos N, Hwu WJ, et al. Prognostic factors for survival in melanoma patients with brain metastases. Cancer (2011) 117(8):1687–96. doi: 10.1002/cncr.25634
7. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. (2007) 8:16. doi: 10.1186/1745-6215-8-16
8. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
9. Pereira TV, Patsopoulos NA, Salanti G, Ioannidis JP. Critical interpretation of cochran's q test depends on power and prior assumptions about heterogeneity. Res Synth Methods (2010) 1(2):149–61. doi: 10.1002/jrsm.13
10. Acharya S, Mahmood M, Mullen D, Yang D, Tsien CI, Huang J, et al. Distant intracranial failure in melanoma brain metastases treated with stereotactic radiosurgery in the era of immunotherapy and targeted agents. Adv Radiat Oncol (2017) 2(4):572–80. doi: 10.1016/j.adro.2017.07.003
11. An Y, Jiang W, Kim BYS, Qian JM, Tang C, Fang P, et al. Stereotactic radiosurgery of early melanoma brain metastases after initiation of anti-CTLA-4 treatment is associated with improved intracranial control. Radiotherapy Oncol (2017) 125(1):80–8. doi: 10.1016/j.radonc.2017.08.009
12. Carron R, Gaudy-Marqueste C, Amatore F, Padovani L, Malissen N, Balossier A, et al. Stereotactic radiosurgery combined with anti-PD1 for the management of melanoma brain metastases: A retrospective study of safety and efficacy. Eur J Cancer (2020) 135:52–61. doi: 10.1016/j.ejca.2020.04.028
13. Choong ES, Lo S, Drummond M, Fogarty GB, Menzies AM, Guminski A, et al. Survival of patients with melanoma brain metastasis treated with stereotactic radiosurgery and active systemic drug therapies. Eur J Cancer (2017) 75:169–78. doi: 10.1016/j.ejca.2017.01.007
14. Diao K, Bian SX, Routman DM, Yu C, Ye JC, Wagle NA, et al. Stereotactic radiosurgery and ipilimumab for patients with melanoma brain metastases: Clinical outcomes and toxicity. J Neuro-Oncol (2018) 139(2):421–9. doi: 10.1007/s11060-018-2880-y
15. Gaudy-Marqueste C, Dussouil AS, Carron R, Troin L, Malissen N, Loundou A, et al. Survival of melanoma patients treated with targeted therapy and immunotherapy after systematic upfront control of brain metastases by radiosurgery. Eur J Cancer (2017) 84:44–54. doi: 10.1016/j.ejca.2017.07.017
16. Kaidar-Person O, Zagar TM, Deal A, Moschos SJ, Ewend MG, Sasaki-Adams D, et al. The incidence of radiation necrosis following stereotactic radiotherapy for melanoma brain metastases: The potential impact of immunotherapy. Anti-Cancer Drugs (2017) 28(6):669–75. doi: 10.1097/CAD.0000000000000497
17. Matsunaga S, Shuto T, Yamamoto M, Yomo S, Kondoh T, Kobayashi T, et al. Gamma knife radiosurgery for metastatic brain tumors from malignant melanomas: A Japanese multi-institutional cooperative and retrospective cohort study (JLGK1501). Stereotactic Funct Neurosurgery (2018) 96(3):162–71. doi: 10.1159/000489948
18. Minniti G, Paolini S, D'Andrea G, Lanzetta G, Cicone F, Confaloni V, et al. Outcomes of postoperative stereotactic radiosurgery to the resection cavity versus stereotactic radiosurgery alone for melanoma brain metastases. J Neurooncol (2017) 132(3):455–62. doi: 10.1007/s11060-017-2394-z
19. Rauschenberg R, Bruns J, Brütting J, Daubner D, Lohaus F, Zimmer L, et al. Impact of radiation, systemic therapy and treatment sequencing on survival of patients with melanoma brain metastases. Eur J Cancer. (2019) 110:11–20. doi: 10.1016/j.ejca.2018.12.023
20. Bhatia A, Birger M, Veeraraghavan H, Um H, Tixier F, McKenney AS, et al. MRI Radiomic features are associated with survival in melanoma brain metastases treated with immune checkpoint inhibitors. Neuro-Oncol (2019) 21(12):1578–86. doi: 10.1093/neuonc/noz141
21. Gorka E, Fabó D, Gézsi A, Czirbesz K, Fedorcsák I, Liszkay G. Dabrafenib therapy in 30 patients with melanoma metastatic to the brain: A single-centre controlled retrospective study in Hungary. Pathol Oncol Res (2018) 24(2):401–6. doi: 10.1007/s12253-017-0256-9
22. Iorgulescu JB, Harary M, Zogg CK, Ligon KL, Reardon DA, Hodi FS, et al. Improved risk-adjusted survival for melanoma brain metastases in the era of checkpoint blockade immunotherapies: Results from a national cohort. Cancer Immunol Res (2018) 6(9):1039–45. doi: 10.1158/2326-6066.CIR-18-0067
23. Ahmed KA, Abuodeh YA, Echevarria MI, Arrington JA, Stallworth DG, Hogue C, et al. Clinical outcomes of melanoma brain metastases treated with stereotactic radiosurgery and anti-PD-1 therapy, anti-CTLA-4 therapy, BRAF/MEK inhibitors, BRAF inhibitor, or conventional chemotherapy. Ann Oncol (2016) 27(12):2288–94. doi: 10.1093/annonc/mdw417
24. De La Fuente M, Beal K, Carvajal R, Kaley TJ. Whole-brain radiotherapy in patients with brain metastases from melanoma. CNS Oncol (2015) 3(6):401–6. doi: 10.2217/cns.14.40
25. Drago JZ, Lawrence D, Livingstone E, Zimmer L, Chen T, Giobbie-Hurder A, et al. Clinical experience with combination BRAF/MEK inhibitors for melanoma with brain metastases: A real-life multicenter study. Melanoma Res (2019) 29(1):65–9. doi: 10.1097/CMR.0000000000000527
26. Frakes JM, Figura NB, Ahmed KA, Juan TH, Patel N, Latifi K, et al. Potential role for LINAC-based stereotactic radiosurgery for the treatment of 5 or more radioresistant melanoma brain metastases. J Neurosurgery (2015) 123(5):1261–7. doi: 10.3171/2014.12.JNS141919
27. Geukes Foppen MH, Boogerd W, Blank CU, van Thienen JV, Haanen JB, Brandsma D. Clinical and radiological response of BRAF inhibition and MEK inhibition in patients with brain metastases from BRAF-mutated melanoma. Melanoma Res (2018) 28(2):126–33. doi: 10.1097/CMR.0000000000000429
28. Le Rhun E, Wolpert F, Fialek M, Devos P, Andratschke N, Reyns N, et al. Response assessment and outcome of combining immunotherapy and radiosurgery for brain metastasis from malignant melanoma. ESMO Open (2020) 5(4):e000763. doi: 10.1136/esmoopen-2020-000763
29. Knispel S, Stang A, Zimmer L, Lax H, Gutzmer R, Heinzerling L, et al. Impact of a preceding radiotherapy on the outcome of immune checkpoint inhibition in metastatic melanoma: A multicenter retrospective cohort study of the DeCOG. J ImmunoTherapy Cancer (2020) 8(1):e000395. doi: 10.1136/jitc-2019-000395
30. McHugh FA, Kow CY, Falkov A, Heppner P, Law A, Bok A, et al. Metastatic melanoma: Surgical treatment of brain metastases – analysis of 110 patients. J Clin Neurosci (2020) 73:144–9. doi: 10.1016/j.jocn.2019.12.063
31. Minniti G, Anzellini D, Reverberi C, Cappellini GCA, Marchetti L, Bianciardi F, et al. Stereotactic radiosurgery combined with nivolumab or ipilimumab for patients with melanoma brain metastases: Evaluation of brain control and toxicity. J ImmunoTherapy Cancer (2019) 7(1):102. doi: 10.1186/s40425-019-0588-y
32. Tetu P, Allayous C, Oriano B, Dalle S, Mortier L, Leccia MT, et al. Impact of radiotherapy administered simultaneously with systemic treatment in patients with melanoma brain metastases within MelBase, a French multicentric prospective cohort. Eur J Cancer (2019) 112:38–46. doi: 10.1016/j.ejca.2019.02.009
33. Pomeranz Krummel DA, Nasti TH, Izar B, Press RH, Xu M, Lowder L, et al. Impact of sequencing radiation therapy and immune checkpoint inhibitors in the treatment of melanoma brain metastases. Int J Radiat Oncol Biol Physics (2020) 108(1):157–63. doi: 10.1016/j.ijrobp.2020.01.043
34. Rahman R, Cortes A, Niemierko A, Oh KS, Flaherty KT, Lawrence DP, et al. The impact of timing of immunotherapy with cranial irradiation in melanoma patients with brain metastases: intracranial progression, survival and toxicity. J Neuro-Oncol (2018) 138(2):299–306. doi: 10.1007/s11060-018-2795-7
35. Schmidberger H, Rapp M, Ebersberger A, Hey-Koch S, Loquai C, Grabbe S, et al. Long-term survival of patients after ipilimumab and hypofractionated brain radiotherapy for brain metastases of malignant melanoma: sequence matters. Strahlentherapie und Onkologie (2018) 194(12):1144–51. doi: 10.1007/s00066-018-1356-5
36. Skrepnik T, Sundararajan S, Cui H, Stea B. Improved time to disease progression in the brain in patients with melanoma brain metastases treated with concurrent delivery of radiosurgery and ipilimumab. Oncoimmunology (2017) 6(3):e1283461. doi: 10.1080/2162402X.2017.1283461
37. Stera S, Balermpas P, Blanck O, Wolff R, Wurster S, Baumann R, et al. Stereotactic radiosurgery combined with immune checkpoint inhibitors or kinase inhibitors for patients with multiple brain metastases of malignant melanoma. Melanoma Res (2019) 29(2):187–95. doi: 10.1097/CMR.0000000000000542
38. Yusuf MB, Amsbaugh MJ, Burton E, Chesney J, Woo S. Peri-SRS administration of immune checkpoint therapy for melanoma metastatic to the brain: Investigating efficacy and the effects of relative treatment timing on lesion response. World Neurosurg (2017) 100:632–40.e4. doi: 10.1016/j.wneu.2017.01.101
39. Amaral T, Tampouri I, Eigentler T, Keim U, Klumpp B, Heinrich V, et al. Immunotherapy plus surgery/radiosurgery is associated with favorable survival in patients with melanoma brain metastasis. Immunotherapy (2019) 11(4):297–309. doi: 10.2217/imt-2018-0149
40. Gabani P, Fischer-Valuck BW, Johanns TM, Hernandez-Aya LF, Keller JW, Rich KM, et al. Stereotactic radiosurgery and immunotherapy in melanoma brain metastases: Patterns of care and treatment outcomes. Radiotherapy Oncol (2018) 128(2):266–73. doi: 10.1016/j.radonc.2018.06.017
41. Kotecha R, Miller JA, Venur VA, Mohammadi AM, Chao ST, Suh JH, et al. Melanoma brain metastasis: The impact of stereotactic radiosurgery, BRAF mutational status, and targeted and/or immune-based therapies on treatment outcome. J Neurosurgery (2018) 129(1):50–9. doi: 10.3171/2017.1.JNS162797
42. Martins F, Schiappacasse L, Levivier M, Tuleasca C, Cuendet MA, Aedo-Lopez V, et al. The combination of stereotactic radiosurgery with immune checkpoint inhibition or targeted therapy in melanoma patients with brain metastases: A retrospective study. J Neuro-Oncol (2020) 146(1):181–93. doi: 10.1007/s11060-019-03363-0
43. Wattson DA, Sullivan RJ, Niemierko A, Merritt RM, Lawrence DP, Oh KS, et al. Survival patterns following brain metastases for patients with melanoma in the MAP-kinase inhibitor era. J Neuro-Oncol (2015) 123(1):75–84. doi: 10.1007/s11060-015-1761-x
44. Stokes WA, Binder DC, Jones BL, Oweida AJ, Liu AK, Rusthoven CG, et al. Impact of immunotherapy among patients with melanoma brain metastases managed with radiotherapy. J Neuroimmunol (2017) 313:118–22. doi: 10.1016/j.jneuroim.2017.10.006
45. Tio M, Wang X, Carlino M, Shivalingam B, Fogarty G, Guminski A, et al. Survival and prognostic factors for patients with melanoma brain metastases in the era of modern systemic therapy. Pigment Cell Melanoma Res (2018) 31(4):509–15. doi: 10.1111/pcmr.12682
46. Alvarez-Breckenridge C, Giobbie-Hurder A, Gill CM, Bertalan M, Stocking J, Kaplan A, et al. Upfront surgical resection of melanoma brain metastases provides a bridge toward immunotherapy-mediated systemic control. Oncologist (2019) 24(5):671–9. doi: 10.1634/theoncologist.2018-0306
47. Amaral T, Kiecker F, Schaefer S, Stege H, Kaehler K, Terheyden P, et al. Combined immunotherapy with nivolumab and ipilimumab with and without local therapy in patients with melanoma brain metastasis: A DeCOG* study in 380 patients. J ImmunoTherapy Cancer (2020) 8(1)e000333. doi: 10.1136/jitc-2019-000333
48. Cohen-Inbar O, Shih HH, Xu Z, Schlesinger D, Sheehan JP. The effect of timing of stereotactic radiosurgery treatment of melanoma brain metastases treated with ipilimumab. J Neurosurgery (2017) 127(5):1007–14. doi: 10.3171/2016.9.JNS161585
49. White RJ, Abel S, Horne ZD, Lee J, Edington H, Greenberg L, et al. Melanoma brain metastases: is it time to eliminate radiotherapy? J Neuro-Oncol (2020) 149(1):27–33. doi: 10.1007/s11060-020-03485-w
50. Sloot S, Chen YA, Zhao X, Weber JL, Benedict JJ, Mulé JJ, et al. Improved survival of patients with melanoma brain metastases in the era of targeted BRAF and immune checkpoint therapies. Cancer (2018) 124(2):297–305. doi: 10.1002/cncr.30946
51. Robin TP, Breeze RE, Smith DE, Rusthoven CG, Lewis KD, Gonzalez R, et al. Immune checkpoint inhibitors and radiosurgery for newly diagnosed melanoma brain metastases. J Neuro-Oncol (2018) 140(1):55–62. doi: 10.1007/s11060-018-2930-5
52. Davies MA, Saiag P, Robert C, Grob JJ, Flaherty KT, Arance A, et al. Dabrafenib plus trametinib in patients with BRAFV600-mutant melanoma brain metastases (COMBI-MB): A multicentre, multicohort, open-label, phase 2 trial. Lancet Oncol (2017) 18(7):863–73. doi: 10.1016/S1470-2045(17)30429-1
53. Goldberg SB, Gettinger SN, Mahajan A, Chiang AC, Herbst RS, Sznol M, et al. Pembrolizumab for patients with melanoma or non-small-cell lung cancer and untreated brain metastases: early analysis of a non-randomised, open-label, phase 2 trial. Lancet Oncol (2016) 17(7):976–83. doi: 10.1016/S1470-2045(16)30053-5
54. Gupta A, Roberts C, Tysoe F, Goff M, Nobes J, Lester J, et al. RADVAN: A randomised phase 2 trial of WBRT plus vandetanib for melanoma brain metastases-results and lessons learnt. Br J Cancer (2016) 115(10):1193–200. doi: 10.1038/bjc.2016.318
55. Hauswald H, Bernhardt D, Krug D, Katayama S, Habl G, Bermejo JL, et al. Whole-brain helical tomotherapy with integrated boost for brain metastases in patients with malignant melanoma - final results of the BRAINRT trial. Cancer Manage Res (2019) 11:4669–76. doi: 10.2147/CMAR.S204729
56. Hong AM, Fogarty GB, Dolven-Jacobsen K, Burmeister BH, Lo SN, Haydu LE, et al. Adjuvant whole-brain radiation therapy compared with observation after local treatment of melanoma brain metastases: A multicenter, randomized phase III trial. J Clin Oncol (2019) 37(33):3132–41. doi: 10.1200/JCO.19.01414
57. Roche H-L. A study of vemurafenib in metastatic melanoma participants with brain metastases. (2016). Available at: https://clinicaltrials.gov/ct2/show/NCT01378975.
58. Long GV, Atkinson V, Lo S, Hu S, Guminski AD, Brown MP, et al. Combination nivolumab and ipilimumab or nivolumab alone in melanoma brain metastases: a multicentre randomised phase 2 study. Lancet Oncol (2018) 19(5):672–81. doi: 10.1016/S1470-2045(18)30139-6
59. Kluger HM, Chiang V, Mahajan A, Zito CR, Sznol M, Tran T, et al. Long-term survival of patients with melanoma with active brain metastases treated with pembrolizumab on a phase II trial. J Clin Oncol (2019) 37(1):52–60. doi: 10.1200/JCO.18.00204
60. McArthur GA, Maio M, Arance A, Nathan P, Blank C, Avril MF, et al. Vemurafenib in metastatic melanoma patients with brain metastases: An open-label, single-arm, phase 2, multicentre study. Ann Oncol (2017) 28(3):634–41. doi: 10.1093/annonc/mdw641
61. McQuade JL, Posada LP, Lecagoonporn S, Cain S, Bassett RL, Patel SP, et al. A phase I study of TPI 287 in combination with temozolomide for patients with metastatic melanoma. Melanoma Res (2016) 26(6):604–8. doi: 10.1097/CMR.0000000000000296
62. Tawbi HA, Forsyth PA, Algazi A, Hamid O, Hodi FS, Moschos SJ, et al. Combined nivolumab and ipilimumab in melanoma metastatic to the brain. New Engl J Med (2018) 379(8):722–30. doi: 10.1056/NEJMoa1805453
63. Squibb. A multi-center phase II study to evaluate tumor response to ipilimumab (BMS-734016) monotherapy in subjects with melanoma brain metastases. Available at: https://clinicaltrials.gov/ct2/show/NCT00623766.
64. Kano H, Morales-Restrepo A, Iyer A, Weiner GM, Mousavi SH, Kirkwood JM, et al. Comparison of prognostic indices in patients who undergo melanoma brain metastasis radiosurgery. J Neurosurg (2018) 128(1):14–22. doi: 10.3171/2016.9.JNS161011
65. Zubatkina I, Ivanov P. Early imaging radioresponsiveness of melanoma brain metastases as a predictor of patient prognosis. J Neurosurgery (2018) 129(2):354–65. doi: 10.3171/2017.1.JNS162075
66. Bian SX, Routman D, Liu J, Yang D, Groshen S, Zada G, et al. Prognostic factors for melanoma brain metastases treated with stereotactic radiosurgery. J Neurosurg (2016) 125:31–9. doi: 10.3171/2016.8.GKS161359
67. Badakhshi H, Engeling F, Budach V, Ghadjar P, Zschaeck S, Kaul D. Are prognostic indices for brain metastases of melanoma still valid in the stereotactic era? Radiat Oncol (2018) 13(1):3. doi: 10.1186/s13014-017-0951-4
68. Wilkins A, W Corbett R, Bloomfield A, Porta N, Morris S, Ali Z, et al. The melanoma-specific graded prognostic assessment does not adequately discriminate prognosis in a modern population with brain metastases from malignant melanoma. Br J Cancer (2015) 113(9):1275–81. doi: 10.1038/bjc.2015.357
69. Gummadi T, Valpione S, Kim C, Kottschade LA, Mittapalli RK, Chiarion-Sileni V, et al. Impact of BRAF mutation and BRAF inhibition on melanoma brain metastases. Melanoma Res (2015) 25(1):75–9. doi: 10.1097/CMR.0000000000000133
70. Partl R, Kaiser J, Kronhuber E, Cetin-Strohmer K, Steffal C, Böhmer-Breitfelder B, et al. KPS/LDH index: a simple tool for identifying patients with metastatic melanoma who are unlikely to benefit from palliative whole brain radiotherapy. Supportive Care Cancer (2016) 24(2):523–8. doi: 10.1007/s00520-015-2793-7
71. Chowdhury IH, McMillan MT, Miller D, Kolker JD, Kurtz G, Dorsey JF, et al. Alonso-basanta, m. novel risk scores for survival and intracranial failure in patients treated with radiosurgery alone to melanoma brain metastases. Radiat Oncol (2015) 10:248. doi: 10.1186/s13014-015-0553-y
72. Rodenburg RJ, Hanssens PE, Ho VKY, Beerepoot LV. Validation of the chowdhury overall survival score in patients with melanoma brain metastasis treated with gamma knife radiosurgery. J Neuro-Oncol (2018) 138(2):391–9. doi: 10.1007/s11060-018-2808-6
73. Ramos RI, Wu J, Jones P, Chang SC, Kiyohara E, Tran K, et al. Upregulation of cell surface GD3 ganglioside phenotype is associated with human melanoma brain metastasis. Mol Oncol (2020) 14(8):1760–78. doi: 10.1002/1878-0261.12702
74. Marzese DM, Liu M, Huynh JL, Hirose H, Donovan NC, Huynh KT, et al. Brain metastasis is predetermined in early stages of cutaneous melanoma by CD44v6 expression through epigenetic regulation of the spliceosome. Pigment Cell Melanoma Res (2015) 28(1):82–93. doi: 10.1111/pcmr.12307
75. Farris M, McTyre ER, Cramer CK, Hughes R, olph DM, Ayala-Peacock DN, et al. Brain metastasis velocity: A novel prognostic metric predictive of overall survival and freedom from whole-brain radiation therapy after distant brain failure following upfront radiosurgery alone. Int J Radiat Oncol Biol Physics (2017) 98(1):131–41. doi: 10.1016/j.ijrobp.2017.01.201
76. Mastorakos P, Xu Z, Yu J, Hess J, Qian J, Chatrath A, et al. BRAF V600 mutation and BRAF kinase inhibitors in conjunction with stereotactic radiosurgery for intracranial melanoma metastases: A multicenter retrospective study. Neurosurgery (2019) 84(4):872–80. doi: 10.1093/neuros/nyy203
77. Liu H, Xu YB, Guo CC, Li MX, Ji JL, Dong RR, et al. Predictive value of a nomogram for melanomas with brain metastases at initial diagnosis. Cancer Med (2019) 8(18):7577–85. doi: 10.1002/cam4.2644
78. Ferrel EA, Roehrig AT, Kaya EA, Carlson JD, Carlson JD, Wagner A, et al. Retrospective study of metastatic melanoma and renal cell carcinoma to the brain with multivariate analysis of prognostic pre-treatment clinical factors. Int J Mol Sci (2016) 17(3):400. doi: 10.3390/ijms17030400
79. Rades D, Sehmisch L, Janssen S, Schild SE. Prognostic factors after whole-brain radiotherapy alone for brain metastases from malignant melanoma. Anticancer Res (2016) 36(12):6637–40. doi: 10.21873/anticanres.11271
80. Schaule J, Kroeze SGC, Blanck O, Blanck O, Stera S, Kahl KH, et al. Predicting survival in melanoma patients treated with concurrent targeted- or immunotherapy and stereotactic radiotherapy. Radiat Oncol (2020) 15(1):135. doi: 10.1186/s13014-020-01708-y
81. Sehmisch L, Schild S, Rades D. Development of a survival score for patients with cerebral metastases from melanoma. Anticancer Res (2017) 37(1):249–52. doi: 10.21873/anticanres.11314
82. Gallaher IS, Watanabe Y, DeFor TE, Dusenbery KE, Lee CK, Hunt MA, et al. BRAF mutation is associated with improved local control of melanoma brain metastases treated with gamma knife radiosurgery. Front Oncol (2016) 6. doi: 10.3389/fonc.2016.00107
83. Sperduto PW, Jiang W, Brown PD, Braunstein S, Sneed P, Wattson DA, et al. The prognostic value of BRAF, c-KIT, and NRAS mutations in melanoma patients with brain metastases. Int J Radiat Oncol Biol Phys (2017) 98(5):1069–77. doi: 10.1016/j.ijrobp.2017.03.030
84. Xu Z, Lee CC, Ramesh A, Mueller AC, Schlesinger D, Cohen-Inbar O, et al. BRAF V600E mutation and BRAF kinase inhibitors in conjunction with stereotactic radiosurgery for intracranial melanoma metastases. J Neurosurgery (2017) 126(3):726–34. doi: 10.3171/2016.2.JNS1633
85. Frinton E, Tong D, Tan J, Read G, Kumar V, Kennedy S, et al. Metastatic melanoma: prognostic factors and survival in patients with brain metastases. J Neuro-Oncol (2017) 135(3):507–12. doi: 10.1007/s11060-017-2591-9
86. Zhang M, Rodrigues AJ, Bhambhvani HP, Fatemi P, Pollom EL, Gibbs IC, et al. Intracranial tumor control after immune-related adverse events and discontinuation of immunotherapy for melanoma. World Neurosurg (2020) 144:e316–e325. doi: 10.1016/j.wneu.2020.08.124
87. Maxwell R, Garzon-Muvdi T, Lipson EJ, Sharfman WH, Bettegowda C, Redmond KJ, et al. BRAF-V600 mutational status affects recurrence patterns of melanoma brain metastasis. Int J Cancer (2017) 140(12):2716–27. doi: 10.1002/ijc.30241
88. Hirshman BR, Wilson BR, Ali MA, Schupper AJ, Proudfoot JA, Goetsch SJ, et al. Cumulative intracranial tumor volume augments the prognostic value of diagnosis-specific graded prognostic assessment model for survival in patients with melanoma cerebral metastases. Neurosurgery (2018) 83(2):237–44. doi: 10.1093/neuros/nyx380
89. Bates JE, Youn P, Usuki KY, Walter KA, Huggins CF. Okunieff, p.; milano, m. t. brain metastasis from melanoma: The prognostic value of varying sites of extracranial disease. J Neuro-Oncol (2015) 125(2):411–8. doi: 10.1007/s11060-015-1932-9
90. Tawbi HA-H, Long GV, Meyer N, Breznen B, Vyas C, Leung L, et al. Treatment outcomes in patients (pts) with melanoma brain metastases (MBM) treated with systemic therapy: A systematic literature review (SLR) and meta-analysis. J Clin Oncol (2021) 39(15_suppl):9561. doi: 10.1200/JCO.2021.39.15_suppl.9561
Keywords: melanoma, brain metastasis, immunotherapy, targeted therapy, radiosurgery, prognostic factors, outcomes
Citation: Tan X-L, Le A, Scherrer E, Tang H, Kiehl N, Han J, Jiang R, Diede SJ and Shui IM (2022) Systematic literature review and meta-analysis of clinical outcomes and prognostic factors for melanoma brain metastases. Front. Oncol. 12:1025664. doi: 10.3389/fonc.2022.1025664
Received: 23 August 2022; Accepted: 24 November 2022;
Published: 08 December 2022.
Edited by:
David Kaul, Charité Universitätsmedizin Berlin, GermanyReviewed by:
David Wasilewski, Charité University Medicine Berlin, GermanyWendy Sherman, Mayo Clinic Florida, United States
Copyright © 2022 Tan, Le, Scherrer, Tang, Kiehl, Han, Jiang, Diede and Shui. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiang-Lin Tan, eGlhbmdsaW4udGFuQG1lcmNrLmNvbQ==
†These authors have contributed equally to this work
 Xiang-Lin Tan
Xiang-Lin Tan Amy Le
Amy Le Emilie Scherrer1,3
Emilie Scherrer1,3 Ruixuan Jiang
Ruixuan Jiang