- 1Department of Urology, University Hospital Basel, Basel, Switzerland
- 2Department of Radiology, University Hospital Basel, Basel, Switzerland
- 3University of Basel, Basel, Switzerland
- 4Department of Medicine, Faculty of Medicine and Dentistry, Danube Private University, Krems, Austria
Introduction: Robotic-assisted transperineal MRI-US-fusion guided biopsy of the prostate is a novel and highly accurate procedure. The aim of this study was to evaluate the MonaLisa prostate biopsy system in terms of safety, tolerability, and patient-related outcomes.
Methods: This prospective study included 228 patients, who had undergone Robotic-assisted transperineal MRI-US-fusion guided biopsy of the prostate at the University Hospital Basel between January 2020 and June 2022. Peri-operative side effects, functional outcomes and patient satisfaction were assessed.
Results: Mean pain score on the day of biopsy was 1.3 points on VAS, which remained constant on the day after biopsy. Overall, 32 of 228 patients (14%) developed grade I complications according to Clavien-Dindo classification. No higher-grade complications occurred. Gross haematuria, hematospermia and acute urinary retention occurred in 145/228 (63.6%), 98/228 (43%) and 32/228 (14%) patients, respectively. One patient (0.4%) developed urinary tract infection.
Conclusions: Robotic-assisted transperineal MRI-US-fusion guided biopsy of the prostate performed under general anesthesia is a safe and well tolerated procedure. This technique allows to omit perioperative prophylaxis and at the same time minimizes the risk of infectious complications. We attribute the favorable risk profile and tolerability to the minimal invasive approach via two entry points.
Introduction
Prostate cancer (PCa) is the second most common malignant disease in men worldwide (1) Suspicion for PCa is based on pathological digital rectal examination (DRE), prostate specific antigen (PSA) or magnetic resonance image (MRI) findings and indicates, as standard of care, a biopsy of the prostate (PBx) for histopathological verification (2). PBx represents one of the most common urological procedures, with more than 1 million interventions performed in Europe and the United States every year (3). PBx can be performed via a transrectal (TR) or transperineal (TP) route, each approach being associated with specific benefits and limitations. TR offers practicability in the in-office setup due to feasibility under local anesthesia reflected by the majority of PBx being performed via the TR approach in the US (93.1 – 99.2%) (4). However, punction of the prostate through the rectum ampulla is associated with a significant risk for infectious complications (5). The incidence for infectious complications after TR-PBx ranges between 5 and 7% with a hospitalization rate of about 2% (2, 3). Rising rates of fluorchinolone-resistance organisms, which could be found in up to 30% of rectal swab cultures prior to TR-PBx, possibly aggravate the situation (2). With the TP approach infectious complications are significantly lower, even negligible (2, 6, 7). Technological advances in diagnostics of PCa, like the implementation of multiparametric MRI (mpMRI) and MRI-targeted PBx have increased the detection rate of significant PCa, simultaneously decreasing the detection rate of clinical insignificant PCa (8). Newly available robotic-assisted biopsy systems like MonaLisa combine the robotic precision with the preferable transperineal approach. Furthermore, this system allows for a minimal-invasive and gentle sampling requiring only two puncture sites and thus promising lower complication rates and patient tolerability. The robotic-assisted MRI-TRUS-fusion allows for highly precise biopsies with maximal reproducibility, while safely sparing the neurovascular bundle. So far there are no prospective reports on patient related outcomes in terms of tolerability and complications after robotic-assisted transperineal MRI-US-fusion guided biopsy of the prostate (RA-TP-PBx). An upcoming PBx bearing uncertainty regarding a suspected malignant disease as well as the interventional risks, poses a physical and psychological burden for patients. Therefore, the ideal biopsy technique is as painless as possible and combines low complication rates with upmost diagnostic precision. The aim of this study was to evaluate the MonaLisa prostate biopsy system in terms of safety, tolerability, and patient-related outcomes.
Materials and methods
This prospective study analyses the safety profile and functional results of 228 patients, who had undergone RA-TP-PBx at the University Hospital Basel between January 2020 and June 2022. Indication for biopsy resulted from suspicious DRE, elevated PSA values or suspicious lesions in mpMRI. Imaging was performed in all patients prior to biopsy, suspicious lesions were classified according to PI-RADS v2.1. The study was approved by the local ethics committee (ID 2020-01381) and was performed in accordance with the Declaration of Helsinki. All patients provided written informed consent. Side effects, clinical, functional, histological, and demographic data were collected and assessed. In addition, medication for male urinary dysfunction, type of anticoagulation and immunodeficiency, including diabetes mellitus type 2, immunosuppressants or acquired immune deficiency syndrome (AIDS), were recorded.
Biopsy technique
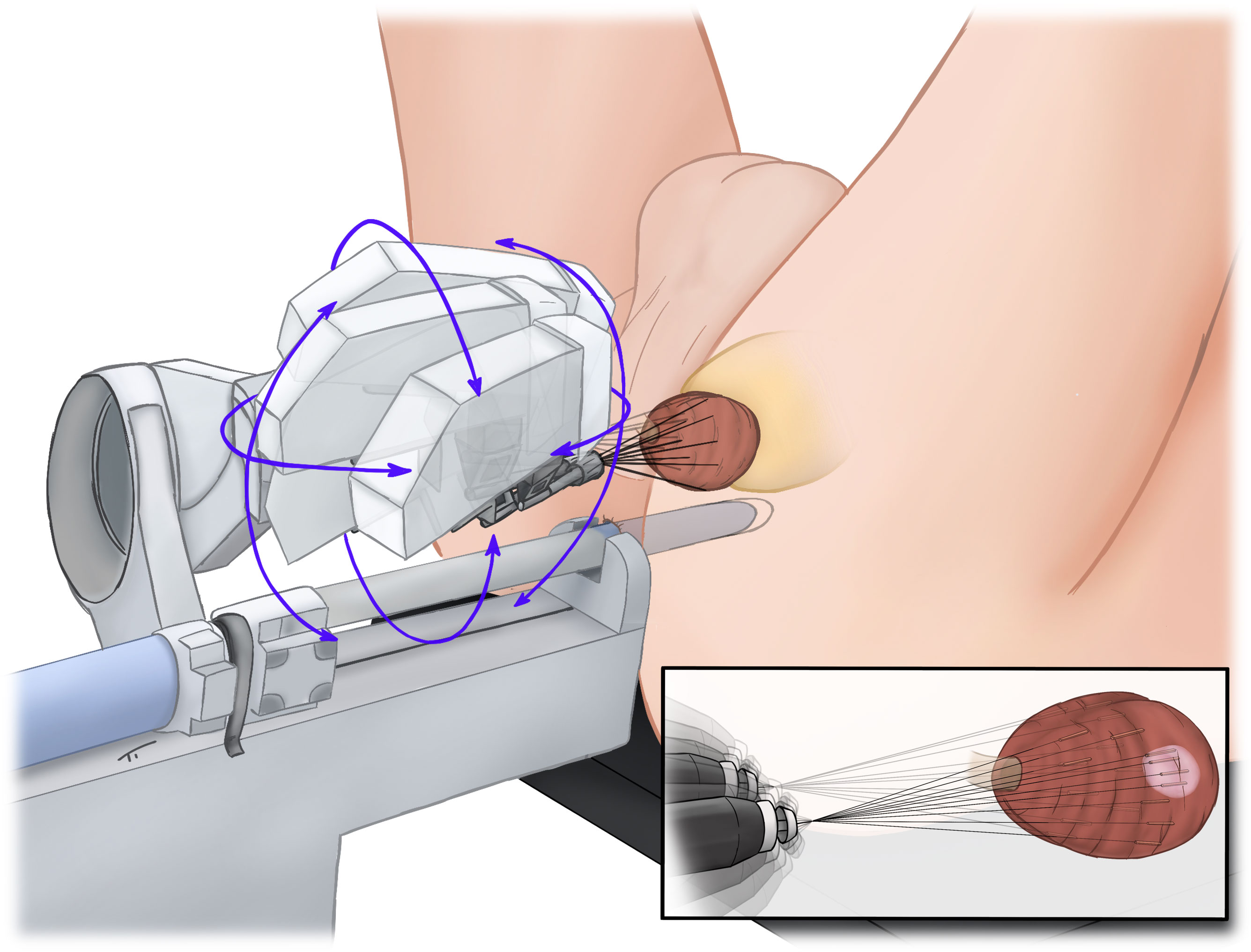
A 3D model of the prostate, including suspicious lesions, was generated by a skilled team of radiologists (DJW, PB) and RA-TP-PBx was performed with an iSR’obot™ MonaLisa device (Biobot©) (Figure 1) by one experienced surgeon (CW). Anticoagulation with factor Xa inhibitors and phenprocoumon was discontinued and bridged with low-molecular-weight heparin according to the individual risk of a thromboembolic event. Therapy with acetylsalicylic acid was continued and was used to bridge patients under therapy with clopidogrel. Standardized, anti-infective prophylaxis was administered to the first 60 (26.3%) patients. After the initial implementation phase of the new biopsy technique anti-infective prophylaxis was omitted if not indicated by positive findings in preoperative urine culture. After RA-TP-PBx no transurethral catheter was used by default. A detailed description of our procedure has already been published previously (9).
Analysis and statistical methods
Validated questionnaires, including “International Prostate Symptom Score” (IPSS) with quality of life (QoL), “International Consultation on Incontinence Questionnaire – Urinary Incontinence” (ICIQ), and “National Institutes of Health - Chronic Prostatitis Symptom Index” (NIH-CPSI) were used to assess functional outcome before and about one week after biopsy. Additionally, the occurrence of side-effects including acute urinary retention (AUR), gross hematuria, hematospermia, pain according to visual analog scale for pain (VAS, 1 – 10 points), urinary tract infections (UTI), local complications and patient satisfaction were collected and analyzed.
Database was created using Excel (Microsoft©), statistical analyses were performed with SPSS Statistics 24.0 (IBM©). The Chi-squared and Fisher`s exact tests were used to compare nominal data. For determination of significant differences among the normally distributed data the Student`s t test (dependent/independent) was applied. Logistic regression was used for binary classification, i.e. to estimate the posterior probability of a binary response based on a list of independent predictor variables. This probability is described by a generalized linear model. Odd`s ratio was performed for risk assessment. All tests were performed at a two-sided significance level of α = 0.05.
Results
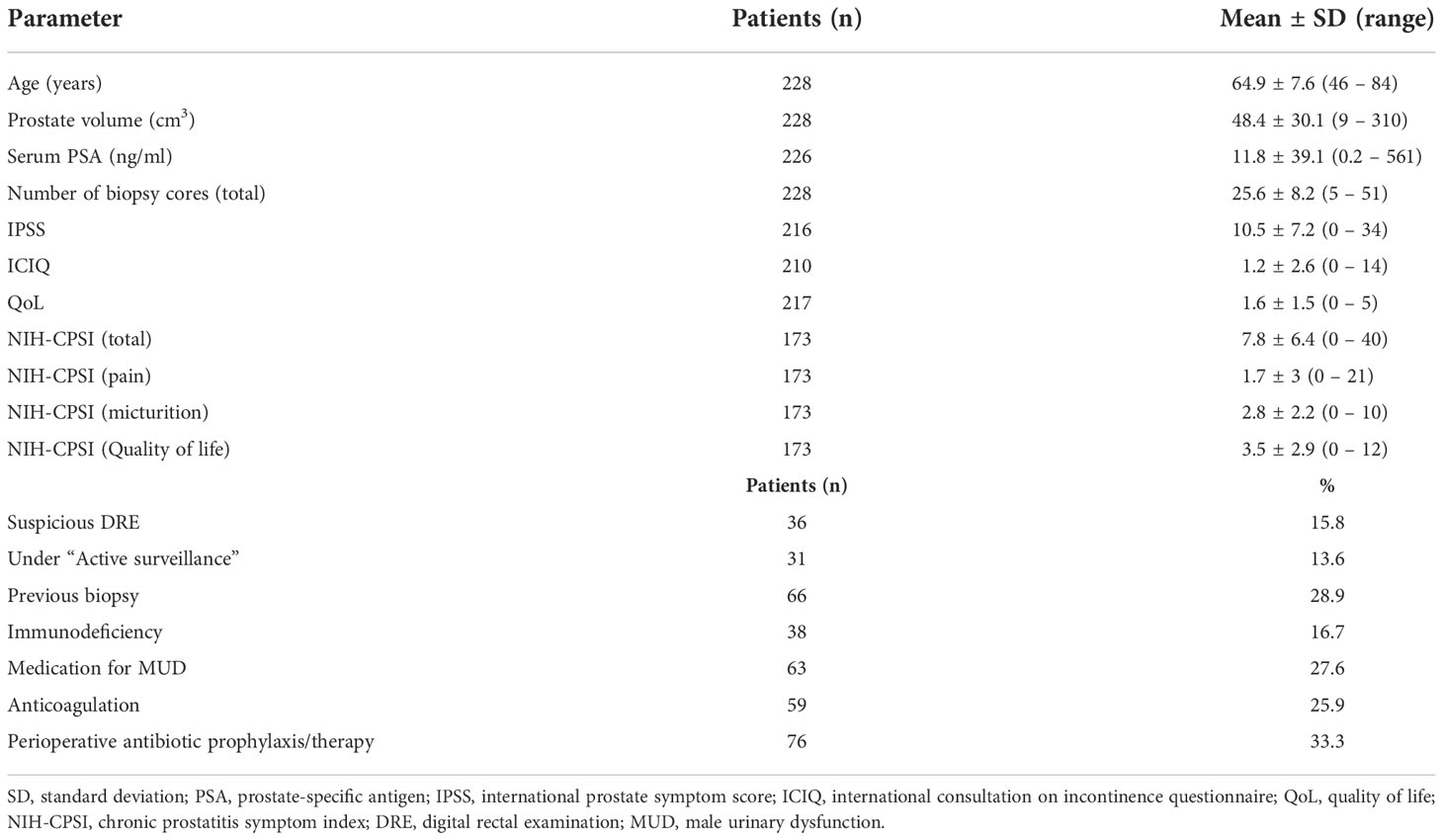
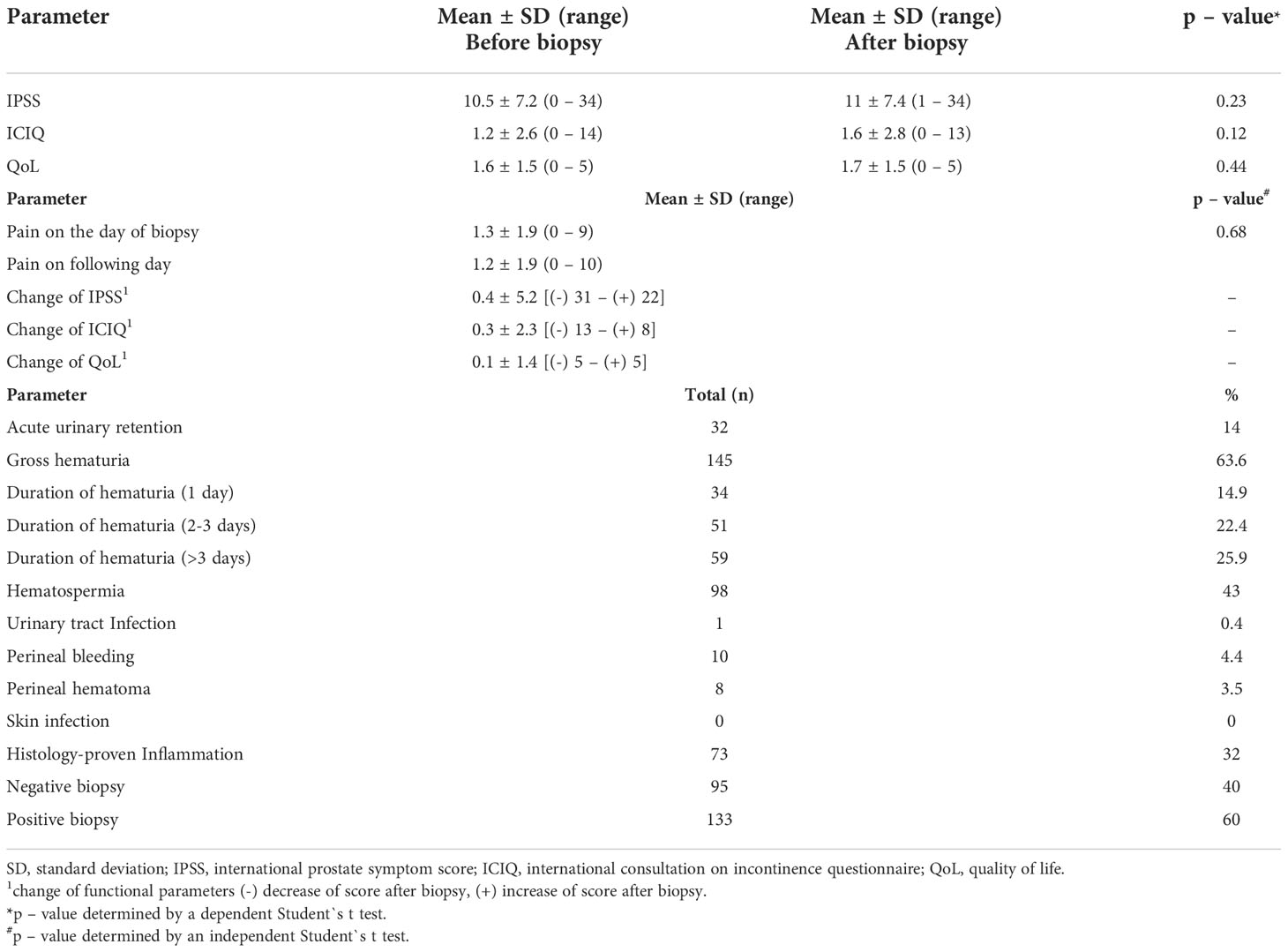
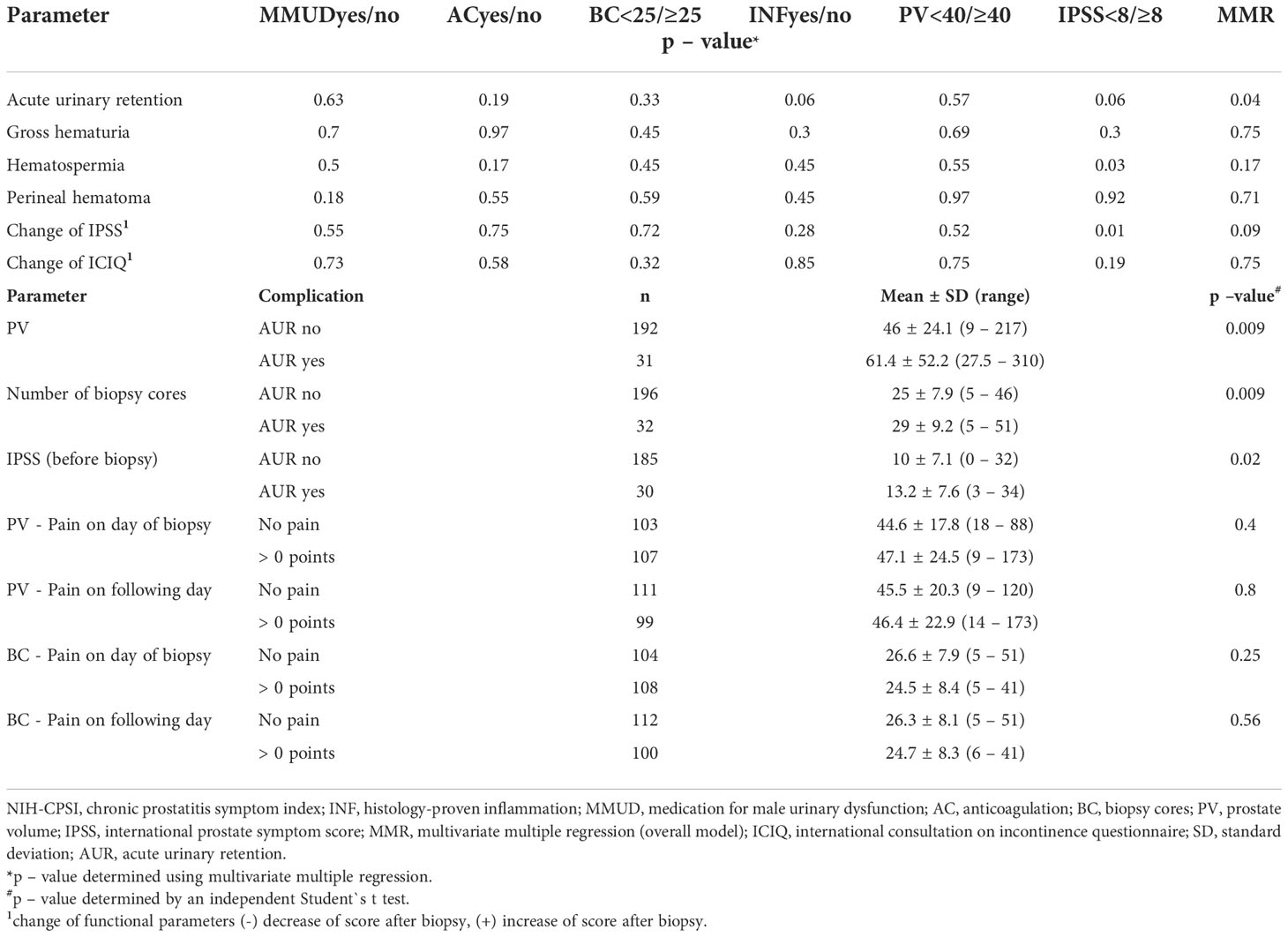
Transperineal robotic-assisted biopsy of the prostate was successfully performed in 228 men with suspicious mpMRI-lesions and/or PSA-constellation. Mean (range) age, PSA, and prostate volume (PV) were 64.9 years (46 – 84), 11.8 ng/ml (0.2 - 561) and 48.4 ml (9 – 310), respectively. Detailed patient baseline characteristics are summarized in Table 1. At the time of biopsy, 63/228 (27.6%) patients took regular medication for male urinary dysfunction. 59/228 (25.9%) took anticoagulant medication, of which 38 patients had biopsy under ongoing antiplatelet therapy. 38/228 (16.7%) patients presented a form of immunodeficiency as stated in the methods. Mean pain score on the day of biopsy was 1.3 points on VAS, which remained constant on the day after biopsy (1.2 points). Overall, 32 of 228 patients (14%) developed grade I complications according to Clavien-Dindo classification. No higher-grade complications occurred. The most common side-effect observed after biopsy was gross haematuria 145/228 (63.6%), which was self-limiting and none of these patients required treatment. 59/145 (25.9%) patients reported gross haematuria duration of more than 3 days. Hematospermia occurred in 98/228 (43%) patients. Anticoagulant therapy, continued antiplatelet medication, PV (≥ 40 ml), biopsy proven inflammation or number of biopsy cores (≥ 25) had no significant influence on occurrence of haematuria/-spermia. Acute urinary retention (AUR) occurred in 32/228 (14%) patients. Patients who developed AUR had a significant higher baseline IPSS-Score (13.2 vs. 10; p 0.02), a bigger prostate volume (61.4 vs. 46 ml; p 0.008) and more biopsy cores taken (29 vs. 25; p 0.009). However, number of biopsy cores (≥ 25), PV (≥ 40 ml) and medication for male urinary dysfunction couldn`t be identified as individual risk factors for the occurrence of AUR. IPSS ≥ 8 (moderate/severe symptoms) and biopsy proven inflammation showed only a tendential association to an increased risk of AUR (OR = 2.49 and 2.29, respectively). Using multivariate multiple regression, only for AUR a significant overall model (p = 0.04) was demonstrated, with none of the predictors providing a clear prediction. Significant influence was shown for IPSS ≥ 8 on “Change of IPSS”, although this result is considered random with regard to the insignificant overall model. No statistically significant change of functional scores (IPSS, QoL and ICIQ) occurred in our cohort shortly after biopsy. One patient (0.4%) developed urinary tract infection (UTI). 66/228 (28.9%) had undergone prostate biopsy priorly. 48/66 (84.2%) of these patients favored transperineal robotic-assisted biopsy over all other methods and rated transperineal robotic-assisted biopsy as the most pleasant biopsy approach. Regarding local conditions, haematoma at puncture, local skin infection and bleeding from puncture site occurred in 8/228 (3.5%), 0/228 (0%) and 10/228 (4.4%), respectively. Detailed data for functional outcome and side-effects are summarized in Table 2. Notably, no patients with immunodeficiency developed any infectious complications.
Sub-group-analysis for the functional outcome and side effects and subgroup specifications are summarized in Table 3 and Supplementary Table 1, respectively.
Discussion
To the best of our knowledge, this is the first prospective study to evaluate safety, tolerability, side effects, and functional outcome of transperineal robotic-assisted prostate biopsy. Transrectal ultrasound-guided biopsy of the prostate still is used as the standard approach for obtaining representative samples for identification and classification of PCa (10). However, the current EAU Guidelines 2022 clearly favor the perineal access route, due to the lower risk of infectious complications (1). Our study reports the outcomes of robotic-assisted perineal biopsy, that requires only two puncture sites. The applied sampling strategy provides histologic evaluation of the entire gland including suspicious lesions (9). Overall, 14% of our patients developed grade I complications according to Clavien-Dindo classification. The superior tolerability of the RA-TP-PBx is highlighted by the mean value of 1.3 points on VAS for pain on the day of and 1.2 points on the days after biopsy. TP-PBx performed under general anesthesia also displays a favorable pain profile (VAS 1.3) as compared to TP-PBx (VAS 2) and TR-PBx (VAS 2) in local anesthesia (11). Furthermore, most patients (84.2%) of our cohort having undergone conventional non-robotic biopsy, preferred RA-TP-PBx. Although, feasibility of the TP-PBx in local anesthesia was shown in various studies (6, 12), general anesthesia is recommended in RA-TP-PBx in order to enable maximum diagnostic accuracy. Hematuria and hematospermia were identified as most common side effects. Rates of occurrence were comparable to other studies reporting sides effects of TP-PBx and TR-PBx (13). Notably, none of our patients developed significant gross hematuria requiring bladder irrigation. A further advantage of the TP-PBx is the absence of hematochezia or rectal bleeding, which is described with an incidence of up to 45% in transrectal biopsy (3). In our cohort, the rate of AUR after RA-TP-PBx was 14%, which is comparable to the study of Pepe et al. with 11.1% on saturation TP-PBx and > 24 cores taken (14), yet higher than in studies with lower number of biopsy cores taken (10–18) with rates of AUR ranging from 1.4% to 6.7% (15, 16). Even though the number of biopsy cores is considered a risk factor for AUR (14), the number of cores (≥25) had no significant impact on the risk of appearance of an AUR in our cohort applying a target saturation approach (9). Using multivariate multiple regression, an significant overall model (p = 0.04) for AUR was shown, with none of the predictors providing a clear prediction. RA-TP-PBx allows for complete diagnostic coverage of the prostate via only two puncture sites. This sterile and minimally invasive approach resulted in the occurrence of only one UTI (0.4%) requiring intravenous antibiotic treatment. Notably, this patient had received antibiotic treatment with oral cephalosporine according to resistency profile, however the duration of pre-treatment (single dose) turned out to be insufficient given the histopathology also revealed acute inflammation. The rate of UTI is comparable to other studies reporting rates of UTI after TP-PBx between 0 – 0.7% (15–17). In contrast, TR- PBx is associated with higher rates of infectious complications ranging between 2 - 5% despite antibiotic prophylaxis (11, 18, 19). In line with the study of Günzel et al. (11), omission of standard perioperative antibiotic prophylaxis in TP- PBx did not result in a significant increase of infections. Notably, none of the immunodeficient patients developed infectious complications indicating that the sterile and minimally invasive biopsy technique enables to safely omit perioperative antibiotic prophylaxis even in patients at special risk for the development of infectious complications. Requiring no antibiotic prophylaxis helps to reduce the risk of antibiotic related complications and the development of drug resistant bacteria. Our results corroborate the findings from other groups (20). However, single center data, limited patient number and non-randomized trial design without a control group represent limitations of this study. Further studies are required to confirm our results. Nevertheless, this work indicates the superior safety profile of robotic assisted transperineal prostate biopsy as compared to a transrectal approach. We assume that the minimally invasive biopsy technique via only two entry points diminished local tissue trauma and subsequently reduced the risk for infectious complications.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Ethikkommission Nordwest- und Zentralschweiz. The patients/participants provided their written informed consent to participate in this study.
Author contributions
All authors have conjointly designed the study, and MW, PT, and CW interpreted the data and drafted the manuscript. AM supported data collection and patient care. All authors designed and critically revised the manuscript for important intellectual content. MW, PT, and CW were involved in the statistical analysis. All authors contributed to the article and approved the submitted version.
Conflict of interest
Author CW was supported by grants from Siemens Healthineers and Uromed.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1025355/full#supplementary-material
Supplementary Table 1 | Subgroups specifications.
Abbreviations
5-ARI, 5-alpha-reductase inhibitor; AC, anticoagulation; AIDS, acquired immune deficiency syndrome; AUR, acute urinary retention; BC, biopsy cores; DRE, digital rectal examination; ICIQ, International Consultation on Incontinence Questionnaire – Urinary Incontinence; IPSS, International Prostate Symptom Score; i.v., intravenous; INF, histology-proven inflammation; LUTS, lower urinary tract symptoms; mpMRI, multiparametric MRI; MRI, magnetic resonance image; MUD, male urinary disfunction; MMUD, medication for male urinary dysfunction; NIH-CPSI, National Institutes of Health - Chronic Prostatitis Symptom Index; PBx, biopsy of the prostate; PCa, Prostate cancer; p.o., per os; PSA, prostate specific antigen; PV, prostate volume; QoL, quality of life; RA-TP-PBx, Robotic-assisted transperineal MRI-US-fusion guided biopsy of the prostate; SD, standard deviation; TP, transperineal; TP-PBx, transperineal biopsy of the prostate; TR, transrectal; TR-PBx, transrectal biopsy of the prostate; TRUS, transrectal ultrasound; UTI, urinary tract infections; VAS, visual analog scale.
References
1. Mottet N, Bellmunt J, Bolla M, Briers E, Cumberbatch MG, De Santis M, et al. EAU-ESTRO-SIOG guidelines on prostate cancer. part 1: Screening, diagnosis, and local treatment with curative intent. Eur Urol (2017) 71(4):618–29. doi: 10.1016/j.eururo.2016.08.003
2. Borghesi M, Ahmed H, Nam R, Schaeffer E, Schiavina R, Taneja S, et al. Complications after systematic, random, and image-guided prostate biopsy. Eur Urol (2017) 71(3):353–65. doi: 10.1016/j.eururo.2016.08.004
3. Loeb S, Vellekoop A, Ahmed HU, Catto J, Emberton M, Nam R, et al. Systematic review of complications of prostate biopsy. Eur Urol (2013) 64(6):876–92. doi: 10.1016/j.eururo.2013.05.049
4. Liu W, Patil D, Howard DH, Moore RH, Wang H, Sanda MG, et al. Adoption of prebiopsy magnetic resonance imaging for men undergoing prostate biopsy in the united states. Urology (2018) 117:57–63. doi: 10.1016/j.urology.2018.04.007
5. Shigemura K, Fujisawa M. Prevention and management of infectious complications in prostate biopsy: A review. Int J Urol Off J Jpn Urol Assoc (2021) 28(7):714–9. doi: 10.1111/iju.14572
6. Wetterauer C, Shahin O, Federer-Gsponer JR, Keller N, Wyler S, Seifert HH, et al. Feasibility of freehand MRI/US cognitive fusion transperineal biopsy of the prostate in local anaesthesia as in-office procedure-experience with 400 patients. Prostate Cancer Prostatic Dis (2020) 23(3):429–34. doi: 10.1038/s41391-019-0201-y
7. Symons JL, Huo A, Yuen CL, Haynes AM, Matthews J, Sutherland RL, et al. Outcomes of transperineal template-guided prostate biopsy in 409 patients. BJU Int (2013) 112(5):585–93. doi: 10.1111/j.1464-410X.2012.11657.x
8. Kasivisvanathan V, Rannikko AS, Borghi M, Panebianco V, Mynderse LA, Vaarala MH, et al. MRI-Targeted or standard biopsy for prostate-cancer diagnosis. N Engl J Med (2018) 378(19):1767–77. doi: 10.1056/NEJMoa1801993
9. Wetterauer C, Trotsenko P, Matthias MO, Breit C, Keller N, Meyer A, et al. Diagnostic accuracy and clinical implications of robotic assisted MRI-US fusion guided target saturation biopsy of the prostate. Sci Rep (2021) 11(1):20250. doi: 10.1038/s41598-021-99854-0
10. Lee MS, Moon MH, Kim CK, Park SY, Choi MH, Jung SI. Guidelines for transrectal ultrasonography-guided prostate biopsy: Korean society of urogenital radiology consensus statement for patient preparation, standard technique, and biopsy-related pain management. Korean J Radiol (2020) 21(4):422–30. doi: 10.3348/kjr.2019.0576
11. Günzel K, Heinrich S, Schlegel J, Ri C, Schostak M, Magheli A, et al. [Initial results of perineal MRI/ultrasound fusion biopsies under local anesthesia without standard perioperative antibiotic prophylaxis]. Urol Ausg A. (2020) 59(10):1225–30. doi: 10.1007/s00120-020-01164-2
12. Thurtle D, Starling L, Leonard K, Stone T, Gnanapragasam VJ. Improving the safety and tolerability of local anaesthetic outpatient transperineal prostate biopsies: A pilot study of the CAMbridge PROstate biopsy (CAMPROBE) method. J Clin Urol (2018) 11(3):192–9. doi: 10.1177/2051415818762683
13. Wegelin O, Exterkate L, van der Leest M, Kelder JC, Bosch JLHR, Barentsz JO, et al. Complications and adverse events of three magnetic resonance imaging-based target biopsy techniques in the diagnosis of prostate cancer among men with prior negative biopsies: Results from the FUTURE trial, a multicentre randomised controlled trial. Eur Urol Oncol (2019) 2(6):617–24. doi: 10.1016/j.euo.2019.08.007
14. Pepe P, Aragona F. Prostate biopsy: results and advantages of the transperineal approach–twenty-year experience of a single center. World J Urol (2014) 32(2):373–7. doi: 10.1007/s00345-013-1108-1
15. Stefanova V, Buckley R, Flax S, Spevack L, Hajek D, Tunis A, et al. Transperineal prostate biopsies using local anesthesia: Experience with 1,287 patients. prostate cancer detection rate, complications and patient tolerability. J Urol (2019) 201(6):1121–6. doi: 10.1097/JU.0000000000000156
16. Pepe P, Aragona F. Morbidity after transperineal prostate biopsy in 3000 patients undergoing 12 vs 18 vs more than 24 needle cores. Urology (2013) 81(6):1142–6. doi: 10.1016/j.urology.2013.02.019
17. Grummet JP, Weerakoon M, Huang S, Lawrentschuk N, Frydenberg M, Moon DA, et al. Sepsis and ‘superbugs’: should we favour the transperineal over the transrectal approach for prostate biopsy? BJU Int (2014) 114(3):384–8. doi: 10.1111/bju.12536
18. Wagenlehner FME, van Oostrum E, Tenke P, Tandogdu Z, Çek M, Grabe M, et al. Infective complications after prostate biopsy: outcome of the global prevalence study of infections in urology (GPIU) 2010 and 2011, a prospective multinational multicentre prostate biopsy study. Eur Urol (2013) 63(3):521–7. doi: 10.1016/j.eururo.2012.06.003
19. Batura D, Gopal Rao G. The national burden of infections after prostate biopsy in England and Wales: a wake-up call for better prevention. J Antimicrob Chemother (2013) 68(2):247–9. doi: 10.1093/jac/dks401
Keywords: biopsy, prostate, robotic-assisted, safety, transperineal
Citation: Walter M, Trotsenko P, Breit H-C, Keller N, Meyer A, Winkel DJ, Seifert HH and Wetterauer C (2022) Safety profile of robotic-assisted transperineal MRI-US-fusion guided biopsy of the prostate. Front. Oncol. 12:1025355. doi: 10.3389/fonc.2022.1025355
Received: 22 August 2022; Accepted: 14 November 2022;
Published: 01 December 2022.
Edited by:
Shomik Sengupta, Eastern Health Clinical School, Monash University, AustraliaReviewed by:
Jianbo Li, Case Western Reserve University, United StatesKazumi Kamoi, Saiseikai Shigaken Hospital, Japan
Copyright © 2022 Walter, Trotsenko, Breit, Keller, Meyer, Winkel, Seifert and Wetterauer. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christian Wetterauer, Y2hyaXN0aWFuLndldHRlcmF1ZXJAdXNiLmNo
†These authors have contributed equally to this work and share first authorship
 Manuel Walter
Manuel Walter Pawel Trotsenko1†
Pawel Trotsenko1† David Jean Winkel
David Jean Winkel Christian Wetterauer
Christian Wetterauer