- 1Division of Respiratory Therapy and Chest Medicine, Sijhih Cathay General Hospital, Taipei, Taiwan
- 2Division of Pulmonary Medicine, Department of Internal Medicine, Hsinchu Cathay General Hospital, Hsinchu, Taiwan
- 3School of Medicine, National Tsing Hua University, Hsinchu, Taiwan
- 4Department of Health Promotion and Health Education, National Taiwan Normal University, Taipei, Taiwan
Objectives: Treatment beyond progression (TBP) is defined as treatment continuing in spite of disease progression, according to the Response Evaluation Criteria In Solid Tumors. We performed a systematic review and meta-analysis to provide evidence for the effects of TBP on lung cancer survival.
Materials and methods: This study has been conducted following the PRISMA guidelines. A systematic review of PubMed, MEDLINE, Embase, and Cochrane Collaboration Central Register of Controlled Clinical Trials from the inception of each database to December 2021 was conducted. Two authors independently reviewed articles for inclusion and extract data from all the retrieved articles. Random-effects meta-analysis was performed using Comprehensive Meta-Analysis software, version 3 (Biostat, Englewood, NJ, USA). Hazard ratios (HRs) with the corresponding 95% confidence intervals (CI) were used for survival outcomes.
Results: We identified five (15.6%) prospective randomized trials and twenty-seven (84.4%) retrospective observational studies of a total of 9,631 patients for the meta-analysis. 3,941 patients (40.9%) were in a TBP group and 5,690 patients (59.1%) were in a non-TBP group. There is a statistically significant advantage for patients who received TBP compared with those who did not in post progression progression-free survival (ppPFS), post progression overall survival (ppOS), and overall survival (OS) from initiation of drugs (ppPFS: HR, 0.746; 95% CI, 0.644-0.865; P<0.001; ppOS: HR, 0.689; 95% CI, 0.596-0.797; P<0.001; OS from initiation of drugs: HR, 0.515; 95% CI, 0.387-0.685; P<0.001)
Conclusion: This study provides further evidence in support of TBP for NSCLC, however, these results require cautious interpretation. Large, randomized, controlled trials investigating the efficacy of TBP in lung cancer treatment are warranted.
Systemic Review Registration: https://www.crd.york.ac.uk/PROSPERO/ identifier CRD42021285147
Introduction
Lung cancer is the leading cause of cancer related mortality worldwide, with most patients having advanced disease at the time of diagnosis (1). However, much progress has been made recently in treating non-small cell lung cancer (NSCLC), the most common type of lung cancer. Tyrosine kinase inhibitors (TKIs), anti-angiogenesis agents, and immune checkpoint inhibitors have dramatically changed the landscape of NSCLC treatment (2–4).. In addition, combination therapy with different pharmaceuticals has proven highly effective due to the ability to affect multiple pathways involved in the progression of the disease (5).
Drug resistance has been the most important factor limiting the success, in terms of overall survival, of systemic anticancer therapy for advanced lung cancer. Once a cancer has developed resistance to a given chemotherapeutic agent, the usual strategy is to initiate a different therapy using non-cross resistant drugs (6). Since the Response Evaluation Criteria In Solid Tumors (RECIST) was introduced in 2000 (Version 1.0) and updated in 2009 (Version 1.1), to assess tumor response of cancer therapeutics by RECIST and to stop current anti-cancer treatment if evaluated as disease progression has been the standard of care of lung cancer management (7, 8). In a systematic review, Davies et al. described that median overall survival ranged from 4.6 months to 12.8 months from the time of second-line treatment initiation in advanced NSCLC (9). Approximately 30% of patients received third-line treatment and only 2.5 to 17.7% patients received fourth-line therapy (9). Currently, there is an unmet need to prolong the duration of each line of effective therapy.
In oncology, treatment beyond progression (TBP) is an expression that indicates the continuation of ongoing therapy after disease progression has resumed (10). TBP is therefore defined as treatment continuing in spite of disease progression, according to the Response Evaluation Criteria In Solid Tumors (RECIST). TBP is carried on while the patient tolerates the current therapy well, is clinically stable, without main organ dysfunction, and provides updated consent (11).
Randomized studies in which patients either continue or discontinue an anti-cancer agent after disease progression are essential to conclude whether TBP is effective. However, there might have substantial difference of characteristics between patients who continue a treatment and those who discontinue treatment (6). Generally, patients who are doing well are left on their medicine while those who are struggling are moved onto a new therapy. As a result, randomized controlled trials which investigate lung cancer treatment are scant. Nevertheless, there are retrospective observation studies. These studies are not designed to compare TBP or treatment discontinuation, which may result in selection bias, and have produced inconsistent results because of small sample sizes and part of subgroup analysis. However, they are still of great reference value clinically. Therefore, we conducted the present systematic review and meta-analysis to provide evidence for the effects of TBP on lung cancer survival.
Materials and methods
This systematic review and meta-analysis have been performed following the PRISMA checklist. The study protocol was registered with the International Prospective Register of Systematic Reviews (PROSPERO) database (CRD42021285147).
Eligibility criteria
We included clinical trials (i.e., randomized, quasi-randomized) and observational studies which investigated continuing current anti-cancer treatment beyond RECIST progression among patients with lung cancer We included patients receiving four kinds of anti-cancer treatment: targeted therapy, immunotherapy, anti-angiogenesis agents, and chemotherapy. Articles were limited to those available in full text and published in English and Chinese peer-reviewed journals.
Search strategy
We conducted a literature search using these electronic databases: PubMed, MEDLINE, Embase, and Cochrane Collaboration Central Register of Controlled Clinical Trials from the inception of each database to December 2021. We also reviewed the bibliographies of included trials and related review articles for relevant references. The search strategy comprised the following terms (lung cancer) AND (treatment beyond progression) AND ((target therapy) OR (immunotherapy) OR (anti-angiogenesis) OR (chemotherapy)). There was no time restriction on the duration of trials.
Study selection and data collection
Two investigators screened studies independently. All disagreement between investigators was resolved by consulting a third reviewer. Discrepancies in study inclusion were discussed among all authors until consensus was achieved. We screened the titles and abstracts identified from the electronic search and investigated full text articles of those deemed potentially relevant. All retrieved studies were required to contain at least two treatment arms, one of which was the intervention group (TBP group), and the other of which was the control group (non-TBP group). The target population consisted of NSCLC patients receiving systemic therapy, including targeted therapy, immunotherapy, anti-angiogenesis agents, and chemotherapy. Systemic therapy regarding neo-adjuvant or adjuvant settings were excluded.
Data extraction
The two reviewers used a predetermined data extraction sheet to extract data from all the retrieved articles. We recorded study characteristics, including first author, year of publication, study design, type and details of treatment arms. We attempted to contact the corresponding author in cases where the data in the article were incompletely reported.
Quality assessment
The two reviewers evaluated the quality of the enrolled studies independently. For non-randomized studies, we used the Risk of Bias Assessment Tool for Non-randomized Studies (RoBANS) which consists of six domains; these include selection of participants, confounding variables, measurement of exposure, blinding of outcome assessment, incomplete outcome data, and selective outcome reporting (12). We used the Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). It contains five domains, including the randomization process, intended intervention, missing outcome data, measurement of outcomes, and selection of reported results. Based on the RoB 2, we evaluated methodological quality as falling into three categories: low risk of bias, some concerns, and high risk of bias.
Outcome measures
The outcomes of interest were post progression progression-free survival (ppPFS), post progression overall survival (ppOS), and overall survival (OS) from initiation of drugs. ppPFS was defined as the time starting from the first point of disease progression following use of intervention drugs until the second progression or death; ppOS was defined as the period from the date of first disease progression (PD) after use of intervention drugs to the date of death due to any cause; OS from initiation of drugs was defined as the period from the date treatment with the intervention drugs began to the date of death due to any cause.
A priori subgroup analysis for the outcomes of interest were planned based on classification of the TBP intervention drugs (classified as “Epidermal Growth Factor Receptor (EGFR) TKIs”, “Anaplastic lymphoma kinase (ALK) TKIs”, “immunotherapy”, and “anti-angiogenesis agents”), treatment of the non-TBP group (classified as “Other” and “Other and None”), whether add-on therapy was allowed in the TBP group (classified as “With add-on” and “Without add-on”), and region (classified as “America”, “Asia”, “Europe”, and “Worldwide”). “Other” indicates patients who switched from TBP to other anticancer treatments such as chemotherapy and immunotherapy, and “Other and None” indicates patients who switched to other anticancer treatments plus those who received no further anticancer treatment. “Add-on” refers to a treatment strategy of continuing TBP but adding chemotherapy or radiotherapy to that.
Analysis
Meta-analysis was performed using Comprehensive Meta-Analysis (CMA) software, version 3 (Biostat, Englewood, NJ, USA). Hazard ratios (HRs) with the corresponding 95% confidence intervals (CI) were used for survival outcomes. Because of the clinical heterogeneity inherent in the data, we employed a random effects model to pool individual HRs. We used forest plots to graphically display the effect size in each group and the pooled estimates. Between-study heterogeneity was assessed using I2 tests; values greater than 50% were considered significant heterogeneity. We conducted sensitivity analysis to assess the impact of each study on the pooled estimate by removing each study one at a time and recalculating the pooled HR estimates for the remaining ones. We used funnel plots and Egger’s test to examine potential publication bias. We defined statistical significance as a p-value of < 0.05, except for the determination of publication bias, for which we used a p value of < 0.10.
Results
Literature search results
The literature search identified 840 non-duplicate references for a review of their titles and abstracts. After removing references violating the inclusion criteria, we included 76 studies for meticulous evaluation (Figure 1). We excluded 25 of those studies due to their single arm design, 6 review articles, 3 studies which had insufficient data for extraction, and 10 studies which did not meet our outcome of interest. The final quantitative analysis included 32 studies.
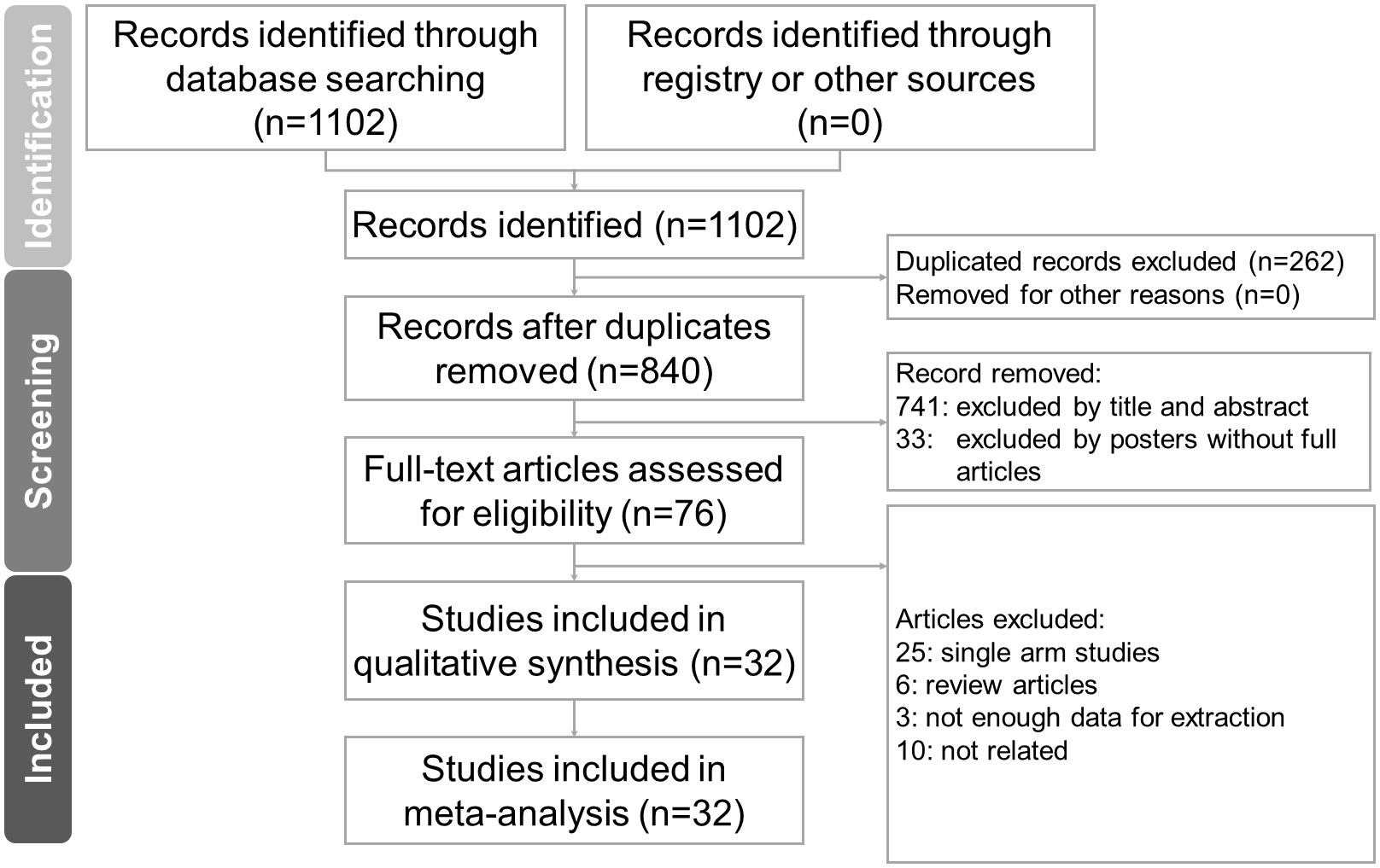
Figure 1 PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analysis) flowchart of article selection process.
Characteristics of included studies
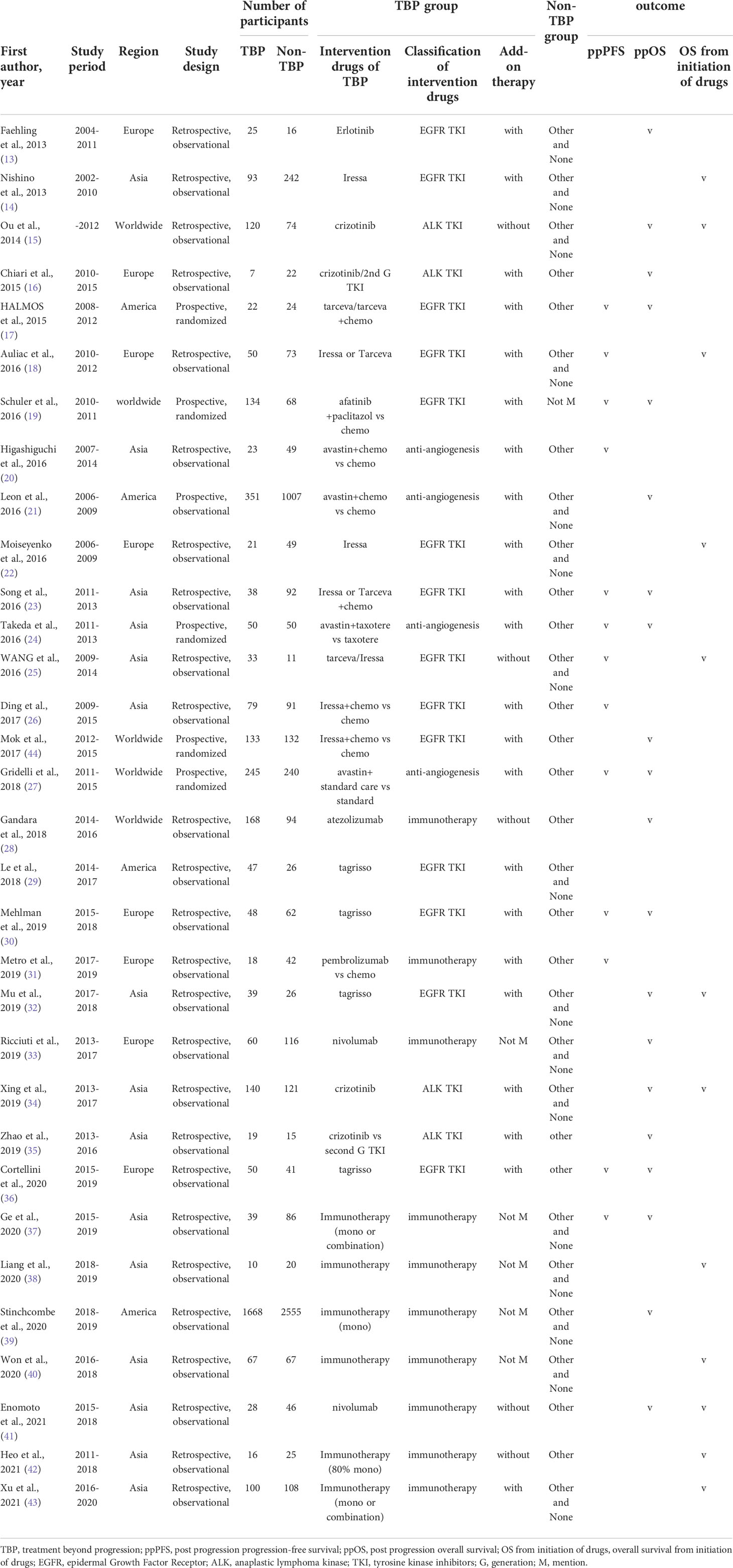
Table 1 lists the main characteristics of the 32 studies. In total, the studies included 9,631 patients, of which 3,941 (40.9%) were in a TBP group and 5,690 (59.1%) were in a non-TBP group. Only 5 (15.6%) prospective randomized studies were identified, and all the others (84.4%) employed a retrospective observational methodology. Most studies enrolled patients within the past 20 years and all studies were published within the past 10 years. There were 13 studies which evaluated ppPFS (1,758 patients) (17–20, 23–27, 30, 31, 36, 37), 20 studies which evaluated ppOS (8,271 patients) (13, 15–17, 19, 21, 23, 24, 27, 28, 30, 32–37, 39, 41, 44), and 12 studies evaluated OS from initiation of drugs (1,579 patients) (14, 15, 18, 22, 25, 32, 34, 38, 40–43). The drugs provided to the TBP groups fell into four categories: 14 studies used EGFR TKIs (43.8%) (13, 14, 17–19, 22, 23, 25, 26, 29, 30, 32, 36, 44), 4 employed ALK TKIs (12.5%) (15, 16, 34, 35), 10 studies used immunotherapy (31.3%) (28, 31, 33, 37–43), and 4 articles used anti-angiogenesis (12.4%) (20, 21, 24, 27).
Risk of bias
The risk of bias assessment for the 27 non-randomized studies used RoBANs (Supplemental Table 1). Performance bias and reporting bias were low in all studies. Only 10 studies had a low risk of detection bias and the remaining 17 studies were judged as unclear or at high risk of inadequate blinding of outcome assessments. Selection and attrition bias were low in most studies. Bias due to confounding variables were high in 10 studies, unclear in 1 study, and low in the remaining 16 studies. Supplemental Table 2 shows risk of bias assessment using RoB 2 for five randomized studies. Two studies were rated as having “high risk of bias,” two as “some concerns,” and one as “low risk of bias.”
Primary analysis
Meta-analysis of the available literature revealed a statistically significant advantage for patients who received TBP compared with those who did not in ppPFS, ppOS, and OS from initiation of drugs (ppPFS: HR, 0.746; 95% CI, 0.644-0.865; P<0.001; ppOS: HR, 0.689; 95% CI, 0.596-0.797; P<0.001; OS from initiation of drugs: HR, 0.515; 95% CI, 0.387-0.685; P<0.001)(Figure 2). Statistically significant between-study heterogeneity was noted among results of ppOS (I2 = 77.5%, P<0.001) and OS starting from initiation of drugs (I2 = 63.436, P=0.002), but not in ppPFS (I2 = 43.4%, P=0.053). In sensitivity analysis, exclusion of any single study did not essentially vary the overall results of the primary analysis. Significant publication bias was detected in analysis of ppOS (Egger’s test, ppOS: P=0.041), but not in ppPFS and OS from initiation of drugs (Egger’s test, ppPFS: P=0.560; OS from initiation of drugs: P=0.550) (Figure 3).
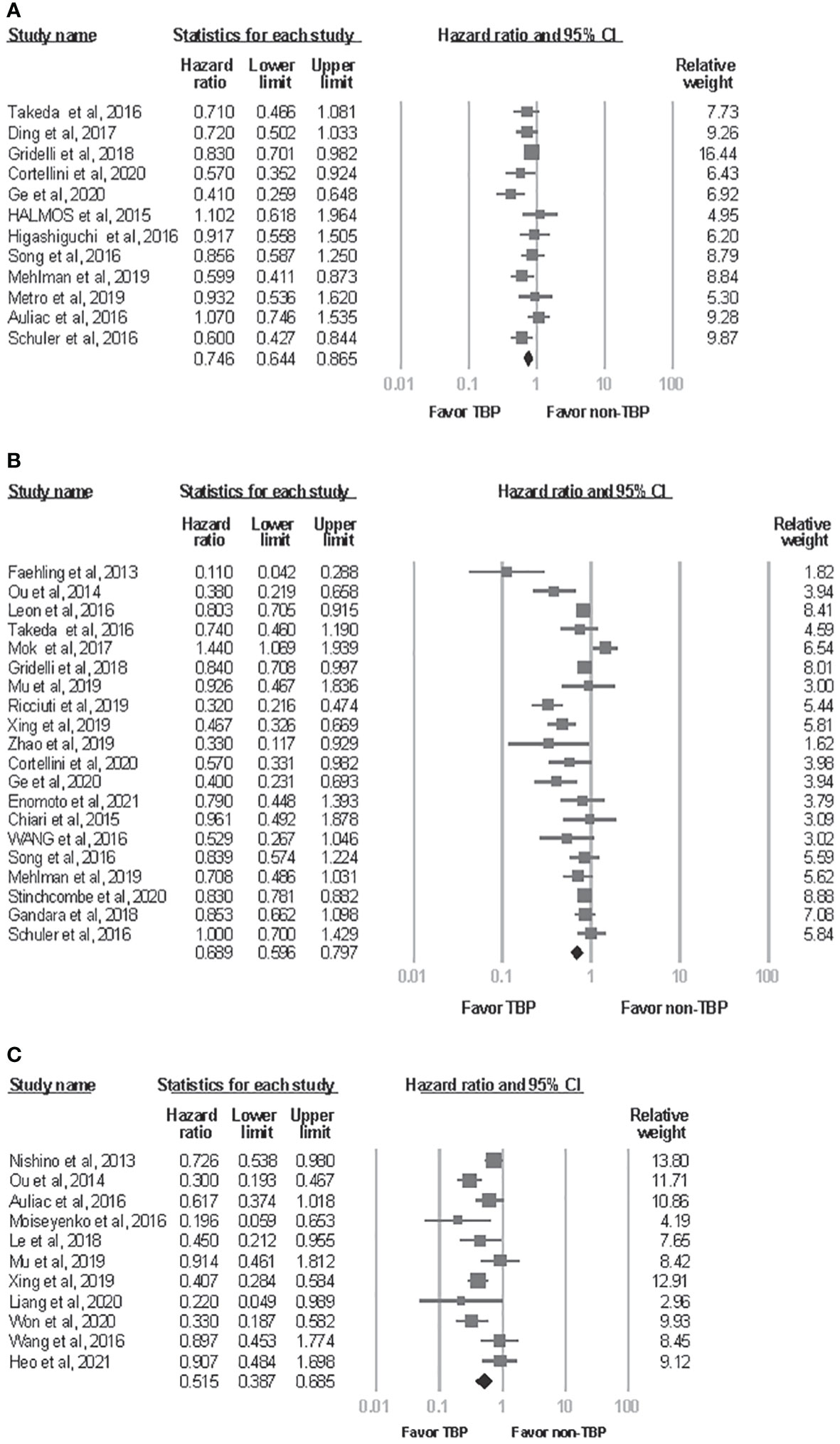
Figure 2 Forest plot of meta-analysis for effects of treatment beyond progression on survival outcome of NSCLC patients. (A) post progression progression-free survival. (B) post progression overall survival. (C) overall survival from initiation of drugs. CI, confidence interval; TBP, treatment beyond progression.
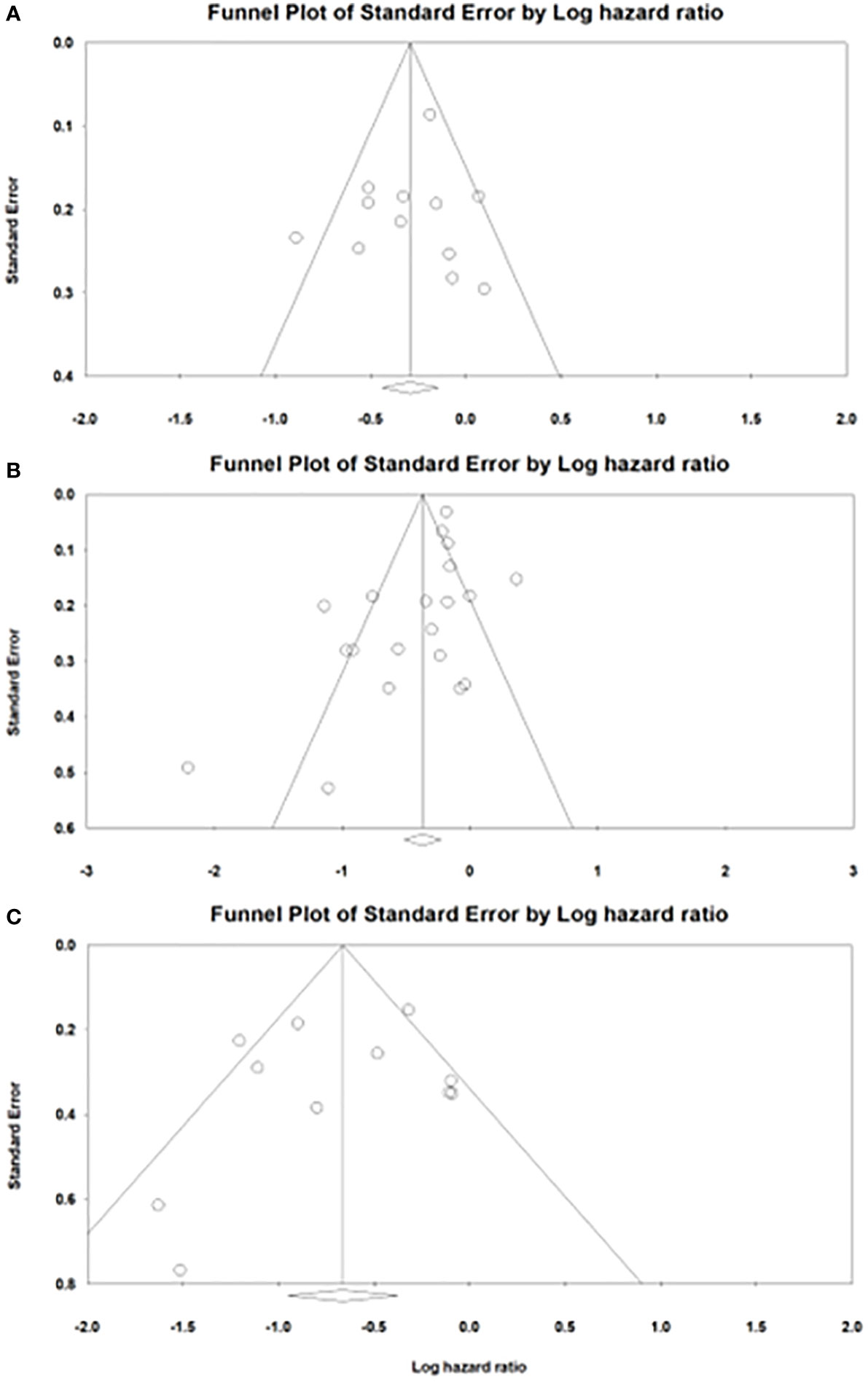
Figure 3 Funnel plots of publication bias in analysis of (A) post progression progression-free survival. (B) post progression overall survival. (C) overall survival from initiation of drugs.
Subgroup analysis
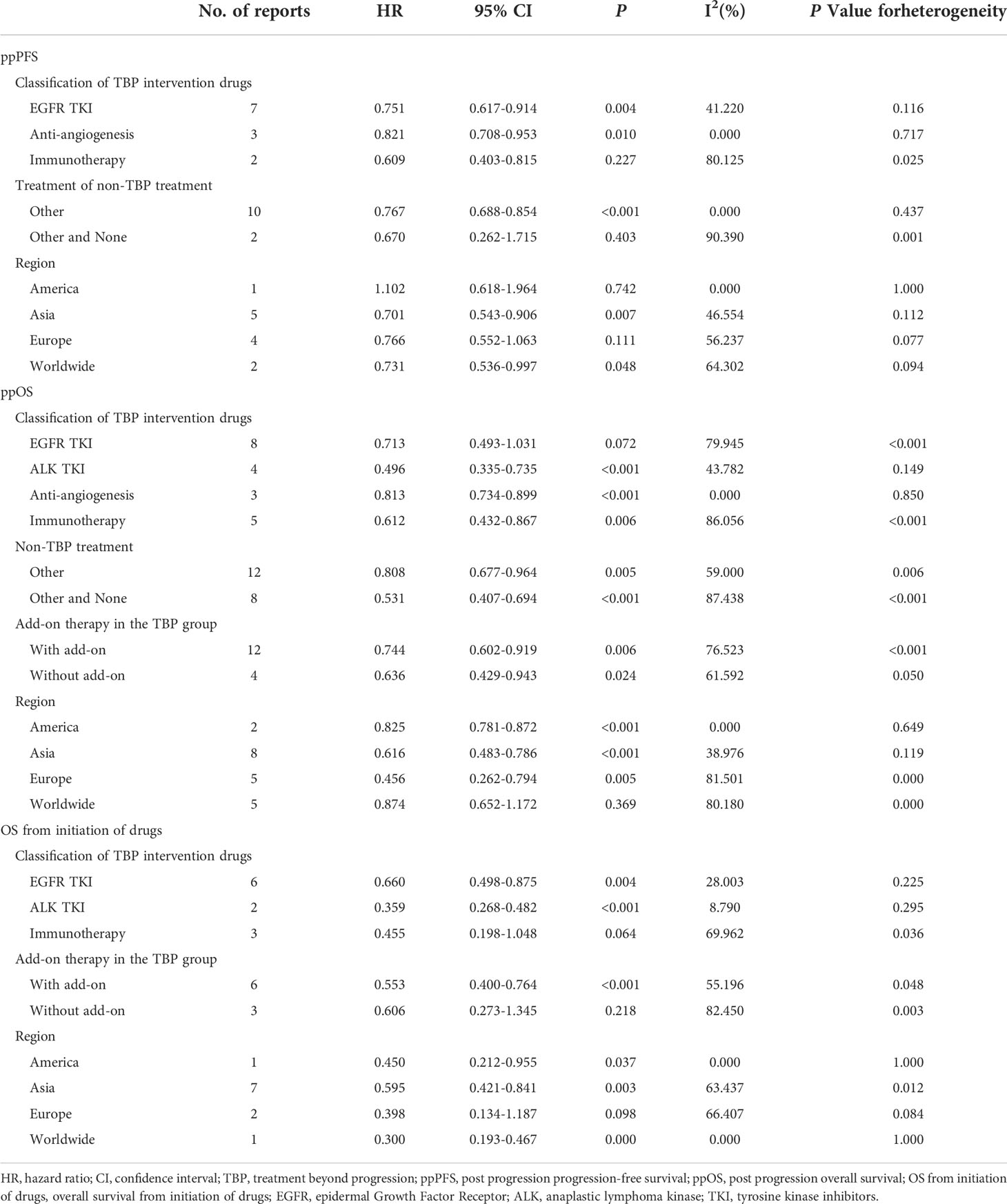
Subgroup analysis according to classification of the TBP drugs, treatment of the non-TBP group, whether add-on therapy was allowed in the TBP group, and region are shown in Table 2 and Supplemental Figure 1A-3C, respectively. Subgroup analysis of the classification of TBP drugs revealed that EGFR TKIs resulted in significantly improved ppPFS (HR, 0.751; 95% CI, 0.617-0.914; I2, 41.2%) and OS from initiation of drugs (HR, 0.660; 95% CI, 0.498-0.875; I2, 28.0%), but not ppOS (HR, 0.713; 95% CI, 0.493-1.031; I2, 79.9%). ALK TKIs showed significantly improved ppOS (HR, 0.496; 95% CI, 0.335-0.735; I2, 43.8%) and OS from initiation of drugs (HR, 0.359; 95% CI, 0.268-0.482; I2, 8.8%). Immunotherapy produced significantly improved ppOS (HR, 0.612; 95% CI, 0.432-0.867; I2, 86.1%), but not ppPFS (HR, 0.609; 95% CI, 0.403-0.815; I2, 80.1%) or OS from initiation of drugs (HR, 0.455; 95% CI, 0.198-1.048; I2, 70.0%). Anti-angiogenesis agents resulted in significantly improved ppPFS (HR, 0.821; 95% CI, 0.708-0.953; I2, 0%) and ppOS (HR, 0.813; 95% CI, 0.734-0.899; I2, 0%).
Subgroup analysis of the non-TBP treatment group showed significantly improved ppPFS in the Other treatment group (HR, 0.767; 95% CI, 0.688-0.854; I2, 0%), but not in the Other and None treatment group(HR, 0.670; 95% CI, 0.262-1.715; I2, 90.4%). Improved ppOS was also evident (Other treatment group: HR, 0.808; 95% CI, 0.677-0.964; I2, 59.0%; Other and None treatment group: HR, 0.531; 95% CI, 0.407-0.694; I2, 87.4%). Subgroup analysis to assess results of add-on therapy with TBP showed that the With add-on group had significantly improved ppOS (HR, 0.744; 95% CI, 0.602-0.919; I2, 76.5%) and OS from initiation of drugs (HR, 0.553; 95% CI, 0.400-0.764; I2, 55.2%). The Without add-on group likewise demonstrated significantly improved ppOS (HR, 0.636; 95% CI, 0.429-0.943; I2, 61.6%), but showed no benefit for OS from initiation of drugs (HR, 0.606; 95% CI, 0.273-1.345; I2, 82.5%). Subgroup analysis of region demonstrated significantly improved ppPFS, ppOS and OS from initiation of drugs in the Asia group but less consistent results of other subgroups, which might be due to limited number of studies or high heterogeneity. When analyzing ppOS and OS from initiation of drugs, subgroup analysis according to classes of drugs decreased heterogeneity between studies.
Discussion
To the best of our knowledge, this is the first systematic review and meta-analysis to focus on whether or not the TBP treatment strategy provided survival benefit for NSCLC patients. Our findings suggest that TBP may improve ppPFS, ppOS and OS from initiation of drugs.
In recent years, immunotherapy, mainly consisting of checkpoint inhibitors including anti-programmed death 1 and anti–programmed death-ligand 1, has dramatically changed cancer treatment paradigms. Immune checkpoint inhibitors stimulate the immune system to attack tumors instead of targeting tumor cells directly, exhibiting different patterns of response to immunotherapy (45). These include alterations in tumor biology reflecting anticancer efficacy following initial radiographic PD (46). Because uncertainty about whether immunotherapy was discontinued and late benefit from treatment continuation among some patients, most clinical trials of immunotherapies permit treatment beyond RECIST-defined PD as long as performance status remains acceptable, the patient provides consent, no serious toxic effects, and no impending end organ damage is observed (47, 48).
Immunotherapy TBP may be a rational treatment choice for the following reasons. First, about 0.6 to 5.8% patients with NSCLC may initially experience increased size of tumor lesions during immunotherapy treatment, followed by a delayed partial response (49). This phenomenon is called “pseudo-progression,” and possibly results from infiltration and recruitment of lymphocytes in the tumor (50). Second, the interaction between the tumor and the immune system may be a long term process which could possibly result in undulant clinical effects, such as undulating tumor growth and shrinkage (48). Third, radiotherapy and chemotherapy may have synergistic effects when combined with immunotherapy via the release of tumor antigen, causing a proinflammatory environment and resulting in activation and clonal expansion of T cells (51, 52). Forth, lesion-level heterogeneity at the time of RECIST-defined PD was common in immunotherapy-treated patients and they these patients may demonstrate ongoing disease control in a subset of tumor sites (53).
In our meta-analysis, TBP significantly prolonged ppOS without statistically significant benefit for ppPFS and OS from initiation of drugs. Enomoto et al. demonstrated no significant difference in ppOS between TBP and the other treatment group. The definition of TBP (nivolumab ≥ 2 weeks after first PD using RECIST v1.1) may explain the less favorable results obtained with nivolumab beyond progression in this study compared with other studies, such as Ricciuti et al. (nivolumab ≥ 6 weeks after first PD using RECIST v1.1) (33, 41). Metro et al. showed no significant difference in ppPFS after comparing pembrolizumab beyond progression and salvage chemotherapy (31). Despite the study’s small sample size, pembrolizumab TBP could be beneficial in select patients. Among the nine patients in the TBP group with the addition of local ablative radiotherapy and PD in no more than two organ sites, the ppPFS rates at 6 and 12 months were high at 88.9% and 71.1%, respectively. Based on previous studies, TBP with immunotherapy may be beneficial in specific circumstances, such as oligo-progression, PD without new lesion, in patients with good performance status, and with add on treatment (41, 43, 53).
For many years, first and second generation EGFR TKIs represented milestones of first line treatment in NSCLC patients with EGFR mutations (54). Osimertinib, a third generation TKI, could overcome treatment resistance acquired after use of first line TKIs, such as T790M. In first line settings, T790M showed better efficacy compared to first and second generation TKIs (55, 56). However, most patients receiving EGFR TKIs eventually developed TKIs resistant progressions. Moreover, EGFR-mutant lung cancer patients showed poor responses to immunotherapy treatment (57). To achieve better survival among EGFR mutant lung cancer patients, we need to prolong treatment duration with EGFR TKIs. In clinical practice, continuing EGFR TKIs still benefited some patients with EGFR mutation who developing acquired resistance to Erlotinib or gefitinib, suggesting that part of tumor cells remained sensitive to EGFR-TKIs (58).
Oxnard et al. explained this phenomenon in vitro using EGFR-mutant NSCLC cell lines; they pointed out that resistant tumors are likely a mixed components of EGFR-TKIs-sensitive and resistant cells (59). In our subgroup analysis, TBP with EGFR TKIs significantly prolonged ppPFS and OS from initiation of drugs but had no significant benefit in ppOS. Mok et al. and Ding et al. showed that gefitinib plus chemotherapy was not beneficial for patients with acquired resistance to first line gefitinib, however, patients with T790M negative tumors may be the select patients who can benefit from continuation of gefitinib beyond progression (26, 44). In previous studies, patients with gradual progression rather than dramatic progression, oligo-progressive disease, and added on therapy using local ablative treatments may also be among the select patients who benefit from TBP (32, 36, 60, 61).
Since the 2007 discovery of ALK rearrangement in NSCLC, tremendous strides have been made in the treatment of ALK positive NSCLC, best exemplified by the approval of six ALK TKIs (62). Our data show that TBP with ALK TKIs may further prolong ppOS and OS from initiation of drugs. Results from Chiari et al. revealed negative results of TBP; unsurprisingly, shifting to second generation ALK TKIs produced better ppPFS than TBP with first generation TKI, because second generation ALK TKIs may overcome some mechanisms of resistance to first generation ALK TKIs (16, 63). Therefore, a reasonable treatment strategy could be to maximize treatment duration of TBP with each line of ALK TKIs, then shifting to the next line ALK TKIs which impact resistance pathways produced by the previous line ALK TKIs.
Anti-angiogenesis agents, such as bevacizumab, which is a recombinant, humanized monoclonal antibody that targets vascular endothelial growth factor (VEGF), have been approved for treatment of non-squamous NSCLC in combination with chemotherapy, target therapies, and immunotherapy (64). Targeted action against angiogenesis can cause normalization or regression of existing tumor vasculature and the inhibition of new and recurrent tumor vessel growth (65). Furthermore, due to the multiple effects of VEGF on the tumor immune microenvironment, targeting VEGF with anti-angiogenesis agents enhances the anti-cancer immune response (66). Given the mechanism of action of anti-angiogenesis agents, there is a rationale for TBP with added-on TKIs or chemotherapy, on purpose to maintain an angiogenesis blockade (67). Our data supports that TBP with anti-angiogenesis agents prolongs ppPFS and ppOS. However, Takeda et al. reported no significant survival benefit of TBP with bevacizumab; subgroup analysis of their data revealed that patients whose disease progressed starting at least six months after the initiation of first-line chemotherapy and those who achieved a complete or partial response to first-line treatment gained more advantage from bevacizumab continuation (24). In another study that observed negative results of TBP, Higashiguchi et al. nonetheless claimed that they could not deny the possibility of the benefits of TBP with bevacizumab, because it was associated with a better response rate and the OS of the TBP group with bevacizumab looked slightly better than that of the non-TBP group (20).
The results of this study have some limitations. First, as in any meta-analysis, analysis of results of the study limited to the data reported by the authors. Precisely, some authors do not present the HRs, and we could only calculate HRs and the associated statistics based on the information given in the study report. Second, most of the studies were observational, not randomized controlled trials. Observational studies are likely to have greater potential biases than randomized studies because randomized studies adjust known and unknown confounders to balance across different groups. Therefore, we should always interpret results cautiously when observational studies are included in reviews and meta-analyses. Third, this meta-analysis did not include data on individual patients. As a result, it was not possible to adjust patient variables such Eastern Cooperative Oncology Group performance status, age, and race. Fourth, the between-study heterogeneity became lower but was still high after subgroup analysis. Factors that could potentially explain the heterogeneity may include the definition of TBP, which differed in each study; moreover, more than half of the studies did not provide one. Fifth, potential bias of recruitment may contribute to meaningful OS differences. Patients who were selected to TBP groups may have, to a varying degree, better condition such as performance status than those who were not.
Conclusions
This study provides further evidence in support of TBP for NSCLC, however, these results require cautious interpretation. Currently, clinicians and patients are left with uncertainty about how best to deal with disease progression. Treatment decisions will continue to depend on many points, including the availability of other therapeutic agents, clinician’s instincts, and the patient’s evaluation of benefits and risks. Large, randomized, prospective controlled trials to investigate the efficacy of TBP in lung cancer treatment and the biomarkers to predict the populations who may benefit from TBP are warranted.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
W-KK and Y-JL designed the study. W-KK and Y-JL designed the statistical plan. W-KK and Y-JL performed the key analyses. W-KK and Y-JL generated and collected the data. C-FW assisted in data interpretation. W-KK wrote the manuscript. C-FW and Y-JL revised the manuscript. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1023894/full#supplementary-material
Supplementary Figure 1 | Forest plot of subgroup analysis of association between post progression progression-free survival and (A) classification of treatment beyond progression (TBP) drugs. (B) treatment of the non-TBP group. (C) region.
Supplementary Figure 2 | Forest plot of subgroup analysis of association between post progression overall survival and (A) classification of treatment beyond progression (TBP) drugs. (B) whether add-on therapy was allowed in the TBP group. (C) treatment of the non-TBP group. (D) region.
Supplementary Figure 3 | Forest plot of subgroup analysis of association between overall survival from initiation of drugs and (A) classification of treatment beyond progression (TBP) drugs. (B) whether add-on therapy was allowed in the TBP group. (C) region.
References
1. Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups 1990 to 2017: a systematic analysis for the global burden of disease study. JAMA Oncol (2019) 5(12):1749–68. doi: 10.1001/jamaoncol.2019.2996
2. Yuan M, Huang L-L, Chen J-H, Wu J, Xu Q. The emerging treatment landscape of targeted therapy in non-small-cell lung cancer. Signal transduction targeted Ther (2019) 4(1):1–14. doi: 10.1038/s41392-019-0099-9
3. Brahmer JR, Govindan R, Anders RA, Antonia SJ, Sagorsky S, Davies MJ, et al. The society for immunotherapy of cancer consensus statement on immunotherapy for the treatment of non-small cell lung cancer (NSCLC). J immunother cancer. (2018) 6(1):1–15. doi: 10.1186/s40425-018-0382-2
4. Villaruz LC, Socinski MA. The role of anti-angiogenesis in non-small-cell lung cancer: an update. Curr Oncol Rep (2015) 17(6):1–13. doi: 10.1007/s11912-015-0448-y
5. Wu L, Leng D, Cun D, Foged C, Yang M. Advances in combination therapy of lung cancer: Rationales, delivery technologies and dosage regimens. J Controlled Release. (2017) 260:78–91. doi: 10.1016/j.jconrel.2017.05.023
6. Kuczynski EA, Sargent DJ, Grothey A, Kerbel RS. Drug rechallenge and treatment beyond progression–implications for drug resistance. Nat Rev Clin Oncol (2013) 10(10):571–87. doi: 10.1038/nrclinonc.2013.158
7. Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Institute. (2000) 92(3):205–16. doi: 10.1093/jnci/92.3.205
8. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1. 1). Eur J Cancer (2009) 45(2):228–47. doi: 10.1016/j.ejca.2008.10.026
9. Davies J, Patel M, Gridelli C, De Marinis F, Waterkamp D, McCusker ME. Real-world treatment patterns for patients receiving second-line and third-line treatment for advanced non-small cell lung cancer: a systematic review of recently published studies. PloS One (2017) 12(4):e0175679. doi: 10.1371/journal.pone.0175679
10. Serra F, Faverio C, Lasagna A, Barruscotti S, Dominioni T, Benazzo M, et al. Treatment beyond progression and locoregional approaches in selected patients with BRAF-mutated metastatic melanoma. Drugs context (2021) 10. doi: 10.7573/dic.2021-3-1
11. Oxnard GR, Morris MJ, Hodi FS, Baker LH, Kris MG, Venook AP, et al. When progressive disease does not mean treatment failure: reconsidering the criteria for progression. J Natl Cancer Institute. (2012) 104(20):1534–41. doi: 10.1093/jnci/djs353
12. Kim SY, Park JE, Lee YJ, Seo H-J, Sheen S-S, Hahn S, et al. Testing a tool for assessing the risk of bias for nonrandomized studies showed moderate reliability and promising validity. J Clin Epidemiol (2013) 66(4):408–14. doi: 10.1016/j.jclinepi.2012.09.016
13. Faehling M, Eckert R, Kamp T, Kuom S, Griese U, Sträter J, et al. EGFR-tyrosine kinase inhibitor treatment beyond progression in long-term Caucasian responders to erlotinib in advanced non-small cell lung cancer: A case-control study of overall survival. Lung Cancer. (2013) 80(3):306–12. doi: 10.1016/j.lungcan.2013.02.010
14. Nishino K, Imamura F, Morita S, Mori M, Komuta K, Kijima T, et al. A retrospective analysis of 335 Japanese lung cancer patients who responded to initial gefitinib treatment. Lung Cancer. (2013) 82(2):299–304. doi: 10.1016/j.lungcan.2013.08.009
15. Ou SH, Jänne PA, Bartlett CH, Tang Y, Kim DW, Otterson GA, et al. Clinical benefit of continuing ALK inhibition with crizotinib beyond initial disease progression in patients with advanced ALK-positive NSCLC. Ann Oncol (2014) 25(2):415–22. doi: 10.1093/annonc/mdt572
16. Chiari R, Metro G, Iacono D, Bellezza G, Rebonato A, Dubini A, et al. Clinical impact of sequential treatment with ALK-TKIs in patients with advanced ALK-positive non-small cell lung cancer: Results of a multicenter analysis. Lung Cancer. (2015) 90(2):255–60. doi: 10.1016/j.lungcan.2015.09.009
17. Halmos B, Pennell NA, Fu P, Saad S, Gadgeel S, Otterson GA, et al. Randomized phase II trial of erlotinib beyond progression in advanced erlotinib-responsive non-small cell lung cancer. Oncologist (2015) 20(11):1298–303. doi: 10.1634/theoncologist.2015-0136
18. Auliac JB, Fournier C, Audigier Valette C, Perol M, Bizieux A, Vinas F, et al. Impact of continuing first-line EGFR tyrosine kinase inhibitor therapy beyond RECIST disease progression in patients with advanced EGFR-mutated non-Small-Cell lung cancer (NSCLC): Retrospective GFPC 04-13 study. Targeted Oncol (2016) 11(2):167–74. doi: 10.1007/s11523-015-0387-4
19. Schuler M, Yang JCH, Park K, Kim JH, Bennouna J, Chen YM, et al. Afatinib beyond progression in patients with non-small-cell lung cancer following chemotherapy, erlotinib/gefitinib and afatinib: Phase III randomized LUX-lung 5 trial. Ann Oncol (2016) 27(3):417–23. doi: 10.1093/annonc/mdv597
20. Higashiguchi M, Kijima T, Morimura O, Osa A, Suzuki H, Inoue T, et al. Assessment of chemotherapy strategy using bevacizumab for non-squamous non-small cell lung cancer in a real-world setting: A multi-institutional observational study. Cancer Treat Commun (2016) 5:1–10. doi: 10.1016/j.ctrc.2015.11.004
21. Leon L, Kosty M, Jahanzeb M, Spigel D, Wozniak AJ, Brahmer J, et al. Effectiveness of bevacizumab exposure beyond disease progression in patients with non-small-cell lung cancer: Analyses of the ARIES observational cohort study. Pharmacoepidemiol Drug Safety. (2016) 25(5):569–77. doi: 10.1002/pds.3948
22. Moiseyenko FV, Moiseyenko VM, Aleksakhina SN, Chubenko VA, Volkov NM, Kozyreva KS, et al. Survival outcomes in EGFR mutation-positive lung cancer patients treated with gefitinib until or beyond progression. Oncol Res Treat (2016) 39(10):605–14. doi: 10.1159/000449024
23. Song Z, Zhang Y. Treatment and prognosis after progression in long-term responders to EGFR-tyrosine kinase inhibitor in advanced non-small cell lung cancer. Arch Med Sci (2016) 12(1):107–11. doi: 10.5114/aoms.2016.57586
24. Takeda M, Yamanaka T, Seto T, Hayashi H, Azuma K, Okada M, et al. Bevacizumab beyond disease progression after first-line treatment with bevacizumab plus chemotherapy in advanced nonsquamous non-small cell lung cancer (West Japan oncology group 5910L): An open-label, randomized, phase 2 trial. Cancer (2016) 122(7):1050–9. doi: 10.1002/cncr.29893
25. Wang P, Zhang D, Li XM, Guo XG, Sun BJ, Fang XQ, et al. Continued EGFR-TKIs treatment promotes the survival of elderly patients with acquired resistance to EGFR-TKIs therapy. Eur Rev Med Pharmacol Sci (2016) 20(11):2450–9. doi: 10.21518/2079-701X-2016-9-28-32
26. Ding T, Zhou F, Chen X, Zhang S, Liu Y, Sun H, et al. Continuation of gefitinib plus chemotherapy prolongs progression-free survival in advanced non-small cell lung cancer patients who get acquired resistance to gefitinib without T790M mutations. J Thorac Dis (2017) 9(9):2923–34. doi: 10.21037/jtd.2017.07.107
27. Gridelli C, De Castro Carpeno J, Dingemans AMC, Griesinger F, Grossi F, Langer C, et al. Safety and efficacy of bevacizumab plus standard-of-Care treatment beyond disease progression in patients with advanced non-small cell lung cancer: The AvaALL randomized clinical trial. JAMA Oncol (2018) 4(12):e183486. doi: 10.1001/jamaoncol.2018.3486
28. Gandara DR, von Pawel J, Mazieres J, Sullivan R, Helland Å, Han JY, et al. Atezolizumab treatment beyond progression in advanced NSCLC: Results from the randomized, phase III OAK study. J Thorac Oncol (2018) 13(12):1906–18. doi: 10.1016/j.jtho.2018.08.2027
29. Le X, Puri S, Negrao MV, Nilsson MB, Robichaux J, Boyle T, et al. Landscape of EGFR-dependent and -independent resistance mechanisms to osimertinib and continuation therapy beyond progression in EGFR-mutant NSCLC. Clin Cancer Res (2018) 24(24):6195–203. doi: 10.1158/1078-0432.CCR-18-1542
30. Mehlman C, Cadranel J, Rousseau-Bussac G, Lacave R, Pujals A, Girard N, et al. Resistance mechanisms to osimertinib in EGFR-mutated advanced non-small-cell lung cancer: A multicentric retrospective French study. Lung Cancer. (2019) 137:149–56. doi: 10.1016/j.lungcan.2019.09.019
31. Metro G, Addeo A, Signorelli D, Gili A, Economopoulou P, Roila F, et al. Outcomes from salvage chemotherapy or pembrolizumab beyond progression with or without local ablative therapies for advanced non-small cell lung cancers with PD-L1 ≥50% who progress on first-line immunotherapy: Real-world data from a European cohort. J Thorac Disease. (2019) 11(12):4972–81. doi: 10.21037/jtd.2019.12.23
32. Mu Y, Hao X, Yang K, Ma D, Wang S, Xu Z, et al. Clinical modality of resistance and subsequent management of patients with advanced non-small cell lung cancer failing treatment with osimertinib. Targeted Oncol (2019) 14(3):335–42. doi: 10.1007/s11523-019-00644-6
33. Ricciuti B, Genova C, Bassanelli M, De Giglio A, Brambilla M, Metro G, et al. Safety and efficacy of nivolumab in patients with advanced non–small-cell lung cancer treated beyond progression. Clin Lung Cancer. (2019) 20(3):178–85.e2. doi: 10.1016/j.cllc.2019.02.001
34. Xing P, Ma D, Wang Q, Hao X, Wang M, Wang Y, et al. Impact of crizotinib on long-term survival of ALK-positive advanced non-small-cell lung cancer: A Chinese multicenter cohort study. Chin J Cancer Res (2019) 31(3):481–8. doi: 10.21147/j.issn.1000-9604.2019.03.10
35. Zhao Y, Zhang B, Wang S, Qiao R, Xu J, Zhang L, et al. Management of central nervous system metastases in patients with advanced anaplastic lymphoma kinase-rearranged non-Small-Cell lung cancer during crizotinib treatment. Clin Lung Cancer. (2019) 20(6):e631–e7. doi: 10.1016/j.cllc.2019.06.013
36. Cortellini A, Leonetti A, Catino A, Pizzutillo P, Ricciuti B, De Giglio A, et al. Osimertinib beyond disease progression in T790M EGFR-positive NSCLC patients: a multicenter study of clinicians' attitudes. Clin Transl Oncol (2020) 22(6):844–51. doi: 10.1007/s12094-019-02193-w
37. Ge X, Zhang Z, Zhang S, Yuan F, Zhang F, Yan X, et al. Immunotherapy beyond progression in patients with advanced non-small cell lung cancer. Trans Lung Cancer Res (2020) 9(6):2391–400. doi: 10.21037/tlcr-20-1252
38. Liang H, Xu Y, Chen M, Zhong W, Wang M, Zhao J. Patterns of response in metastatic NSCLC during PD-1 or PD-L1 inhibitor therapy: Comparison of the RECIST 1.1 and iRECIST criteria. Thorac Cancer (2020) 11(4):1068–75. doi: 10.1111/1759-7714.13367
39. Stinchcombe TE, Miksad RA, Gossai A, Griffith SD, Torres AZ. Real-world outcomes for advanced non-small cell lung cancer patients treated with a PD-L1 inhibitor beyond progression. Clin Lung Cancer. (2020) 21(5):389–94.e3. doi: 10.1016/j.cllc.2020.04.008
40. Won SE, Park HJ, Byun S, Pyo J, Kim JH, Choi CM, et al. Impact of pseudoprogression and treatment beyond progression on outcome in patients with non-small cell lung cancer treated with immune checkpoint inhibitors. OncoImmunology (2020) 9(1):1–10. doi: 10.1080/2162402X.2020.1776058
41. Enomoto T, Tamiya A, Matsumoto K, Adachi Y, Azuma K, Inagaki Y, et al. Nivolumab treatment beyond progressive disease in advanced non-small cell lung cancer. Clin Trans Oncol (2021) 23(3):582–90. doi: 10.1007/s12094-020-02452-1
42. Heo JY, Yoo SH, Suh KJ, Kim SH, Kim YJ, Ock CY, et al. Clinical pattern of failure after a durable response to immune check inhibitors in non-small cell lung cancer patients. Sci Rep (2021) 11(1):2514. doi: 10.1038/s41598-021-81666-x
43. Xu Y, Li H, Fan Y. Progression patterns, treatment, and prognosis beyond resistance of responders to immunotherapy in advanced non-small cell lung cancer. Front Oncol (2021) 11. doi: 10.3389/fonc.2021.642883
44. Mok TSK, Kim SW, Wu YL, Nakagawa K, Yang JJ, Ahn MJ, et al. Gefitinib plus chemotherapy versus chemotherapy in epidermal growth factor receptor mutation-positive non-Small-Cell lung cancer resistant to first-line gefitinib (IMPRESS): Overall survival and biomarker analyses. J Clin Oncol (2017) 35(36):4027–34. doi: 10.1200/JCO.2017.73.9250
45. Horn L, Spigel DR, Vokes EE, Holgado E, Ready N, Steins M, et al. Nivolumab versus docetaxel in previously treated patients with advanced non–small-cell lung cancer: two-year outcomes from two randomized, open-label, phase III trials (CheckMate 017 and CheckMate 057). J Clin Oncol (2017) 35(35):3924. doi: 10.1200/JCO.2017.74.3062
46. Herbst RS, Soria J-C, Kowanetz M, Fine GD, Hamid O, Gordon MS, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature (2014) 515(7528):563–7. doi: 10.1038/nature14011
47. Blumenthal GM, Theoret MR, Pazdur R. Treatment beyond progression with immune checkpoint inhibitors–known unknowns. JAMA Oncol (2017) 3(11):1473–4. doi: 10.1001/jamaoncol.2017.1819
48. Queirolo P, Spagnolo F. Atypical responses in patients with advanced melanoma, lung cancer, renal-cell carcinoma and other solid tumors treated with anti-PD-1 drugs: a systematic review. Cancer Treat Rev (2017) 59:71–8. doi: 10.1016/j.ctrv.2017.07.002
49. Nishino M, Ramaiya NH, Chambers ES, Adeni AE, Hatabu H, Jänne PA, et al. Immune-related response assessment during PD-1 inhibitor therapy in advanced non-small-cell lung cancer patients. J immunother cancer. (2016) 4(1):1–10. doi: 10.1186/s40425-016-0193-2
50. Ma Y, Wang Q, Dong Q, Zhan L, Zhang J. How to differentiate pseudoprogression from true progression in cancer patients treated with immunotherapy. Am J Cancer Res (2019) 9(8):1546.
51. Mondini M, Levy A, Meziani L, Milliat F, Deutsch E. Radiotherapy–immunotherapy combinations–perspectives and challenges. Mol Oncol (2020) 14(7):1529–37. doi: 10.1002/1878-0261.12658
52. Salas-Benito D, Pérez-Gracia JL, Ponz-Sarvisé M, Rodriguez-Ruiz ME, Martínez-Forero I, Castañón E, et al. Paradigms on immunotherapy combinations with chemotherapy. Cancer discovery. (2021) 11(6):1353–67. doi: 10.1158/2159-8290.CD-20-1312
53. Topp B, Thiagarajan K, De Alwis D, Snyder A, Hellmann M. Lesion-level heterogeneity of radiologic progression in patients treated with pembrolizumab. Ann Oncol (2021) 32(12):1618–25. doi: 10.1016/j.annonc.2021.09.006
54. Novello S, Barlesi F, Califano R, Cufer T, Ekman S, Levra MG, et al. Metastatic non-small-cell lung cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2016) 27:v1–v27. doi: 10.1093/annonc/mdw326
55. Soria J-C, Ohe Y, Vansteenkiste J, Reungwetwattana T, Chewaskulyong B, Lee KH, et al. Osimertinib in untreated EGFR-mutated advanced non–small-cell lung cancer. New Engl J Med (2018) 378(2):113–25. doi: 10.1056/NEJMoa1713137
56. Mok TS, Wu Y-L, Ahn M-J, Garassino MC, Kim HR, Ramalingam SS, et al. Osimertinib or platinum–pemetrexed in EGFR T790M–positive lung cancer. New Engl J Med (2017) 376(7):629–40. doi: 10.1056/NEJMoa1612674
57. Yu S, Liu D, Shen B, Shi M, Feng J. Immunotherapy strategy of EGFR mutant lung cancer. Am J Cancer Res (2018) 8(10):2106.
58. Riely GJ, Kris MG, Zhao B, Akhurst T, Milton DT, Moore E, et al. Prospective assessment of discontinuation and reinitiation of erlotinib or gefitinib in patients with acquired resistance to erlotinib or gefitinib followed by the addition of everolimus. Clin Cancer Res (2007) 13(17):5150–5. doi: 10.1158/1078-0432.CCR-07-0560
59. Oxnard GR, Arcila ME, Chmielecki J, Ladanyi M, Miller VA, Pao W. New strategies in overcoming acquired resistance to epidermal growth factor receptor tyrosine kinase inhibitors in lung cancer. Clin Cancer Res (2011) 17(17):5530–7. doi: 10.1158/1078-0432.CCR-10-2571
60. Yang J-J, Chen H-J, Yan H-H, Zhang X-C, Zhou Q, Su J, et al. Clinical modes of EGFR tyrosine kinase inhibitor failure and subsequent management in advanced non-small cell lung cancer. Lung cancer. (2013) 79(1):33–9. doi: 10.1016/j.lungcan.2012.09.016
61. Schmid S, Klingbiel D, Aeppli S, Britschgi C, Gautschi O, Pless M, et al. Patterns of progression on osimertinib in EGFR T790M positive NSCLC: A Swiss cohort study. Lung Cancer. (2019) 130:149–55. doi: 10.1016/j.lungcan.2019.02.020
62. Zhang SS, Nagasaka M, Zhu VW, Ou S-HI. Going beneath the tip of the iceberg. Identifying and understanding EML4-ALK variants and TP53 mutations to optimize treatment of ALK fusion positive (ALK+) NSCLC. Lung Cancer. (2021) 158:126–36. doi: 10.1016/j.lungcan.2021.06.012
63. Gainor JF, Dardaei L, Yoda S, Friboulet L, Leshchiner I, Katayama R, et al. Molecular mechanisms of resistance to first-and second-generation ALK inhibitors in ALK-rearranged lung CancerResistance mechanisms in ALK-positive lung cancer. Cancer discovery. (2016) 6(10):1118–33. doi: 10.1158/2159-8290.CD-16-0596
64. Garcia J, Hurwitz HI, Sandler AB, Miles D, Coleman RL, Deurloo R, et al. Bevacizumab (Avastin®) in cancer treatment: A review of 15 years of clinical experience and future outlook. Cancer Treat Rev (2020) 86:102017. doi: 10.1016/j.ctrv.2020.102017
65. Lauro S, Onesti CE, Righini R, Marchetti P. The use of bevacizumab in non-small cell lung cancer: an update. Anticancer Res (2014) 34(4):1537–45.
66. Hegde PS, Wallin JJ, Mancao C. Predictive markers of anti-VEGF and emerging role of angiogenesis inhibitors as immunotherapeutics. In: Seminars in cancer biology. Elsevier (2018) 52(2):117–24.
Keywords: treatment beyond progression, NSCLC, meta-analysis, systemic review, survival
Citation: Kuo W-K, Weng C-F and Lien Y-J (2022) Treatment beyond progression in non-small cell lung cancer: A systematic review and meta-analysis. Front. Oncol. 12:1023894. doi: 10.3389/fonc.2022.1023894
Received: 20 August 2022; Accepted: 26 October 2022;
Published: 17 November 2022.
Edited by:
Yanguang Cao, University of North Carolina at Chapel Hill, United StatesReviewed by:
Timothy Qi, University of North Carolina at Chapel Hill, United StatesBrian Topp, Merck (United States), United States
Copyright © 2022 Kuo, Weng and Lien. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yin-Ju Lien, eWpsaWVuQG50bnUuZWR1LnR3
 Wei-Ke Kuo1
Wei-Ke Kuo1 Ching-Fu Weng
Ching-Fu Weng Yin-Ju Lien
Yin-Ju Lien