- 1Department of Internal Medicine, Brody School of Medicine, East Carolina University, Greenville, NC, United States
- 2Department of Internal Medicine, College of Medicine, University of Oklahoma, Oklahoma City, OK, United States
- 3Circulogene, Birmingham, AL, United States
Immunotherapy has changed the paradigm of cancer treatment, yet immune checkpoint inhibitors (ICIs) such as PD-1/PD-L1 monoclonal antibodies may cause immune-related adverse events (irAEs) in some patients. In this report, two non-small cell lung cancer (NSCLC) patients treated with nivolumab presented with checkpoint inhibitor-induced thyroid dysfunction (CITD), followed by a second irAE of pneumonitis and intestinal perforation, respectively. Increases in peripheral CD8+ T cells correlated with the onset of CITD in the patients. Intriguingly, common inflammatory biomarkers, including C-reactive protein (CRP) and neutrophil/lymphocyte ratio (NLR), were not consistently increased during the onset of CITD but were substantially increased during the onset of pneumonitis and intestinal perforation irAEs. The observations suggest that unlike other irAEs such as pneumonitis, CRP levels and NLR were non-contributory in diagnosing CITD, whereas T cell expansion may be associated with immunotherapy-induced thyroiditis.
Introduction
Immune checkpoint inhibitors (ICIs) are currently the first-line treatment for multiple late-stage malignancies including non-small cell lung cancer (NSCLC). However, systemic immunostimulation by ICIs may lead to immune-related adverse events (irAEs) in some patients (1–4). Patients receiving ICIs are closely monitored for clinical symptoms and signs of irAEs as well as laboratory tests, including C reactive protein (CRP) levels, to detect occult, subclinical irAEs (1, 5).
Checkpoint inhibitor-induced thyroid dysfunction (CITD) is one of the most common irAEs, along with rash, pruritis, and diarrhea (1–3). While CITD is often detected by regular measurements of thyroid-stimulating hormone (TSH), it is unclear whether other common biomarkers also have utility in understanding the molecular mechanism and prognosis of CITD (6, 7).
Here we present two cases of patients with NSCLC treated with ICIs that developed CITD, followed by a second irAE. We describe the levels of peripheral blood biomarkers associated with CITD’s respective disease courses, including TSH, CRP, immune cell subsets, and cytokine levels. In two patients with CITD, peripheral blood CD8+ T cells increased in number at or preceding the time of CITD, yet the patients did not consistently demonstrate an acute change in systemic inflammatory biomarkers that were observed with the second irAEs (pneumonitis and intestinal perforation, respectively) they developed later. This study provides data that may enhance the management of CITD and prompt further investigations on the mechanisms of CITD.
Case presentation
Case #1
The first patient was a 55-year-old male with a five-year history of inoperable stage IIIA (T1aN2M0) right upper lobe (RUL) lung adenocarcinoma treated with chemoradiation. He remained asymptomatic for three years at which time surveillance chest CT showed a new left upper lobe (LUL) lesion diagnosed as stage I metachronous LUL lung adenocarcinoma and treated with CyberKnife Radiosurgery (CKRS). After two years, patient reported symptoms of fatigue, progressive weight loss, and generalized lymph node swelling. Chest CT showed a new right lower lobe (RLL) lesion with PET scan showing increased metabolic activity in draining lymph nodes and the liver. Image-guided core biopsy of the right supraclavicular lymph node was consistent with lung adenocarcinoma. PD-L1 expression was 0% by immunohistochemical (IHC) staining. He was enrolled in the trial described herein and blood samples were procured at baseline and after each cycle of the immunotherapy. The patient was started on two-week cycles of 240 mg nivolumab with 8 planned cycles.
After receiving the second dose of Nivolumab, TSH values showed thyrotoxicosis (TSH = 0.09 and 0.04 mIU/L) at cycles 2 and 3 of Nivolumab treatment. Subclinical hypothyroidism (TSH = 5.1 mIU/L) at the 4th cycle followed by symptomatic hypothyroidism (TSH = 58.37, 89.96, and 96.16) at the 5th, 6th, and 7th treatment cycles, respectively was observed (Figure 1A and Supplementary Figure 1A). The patient was diagnosed with CITD and daily levothyroxine was started. The patient’s symptoms were fatigue and constipation. The dose of levothyroxine was subsequently escalated over the course of 6 months to 150 mcg daily for symptoms and thyroid function optimization. Anti-TPO antibodies were not measured.
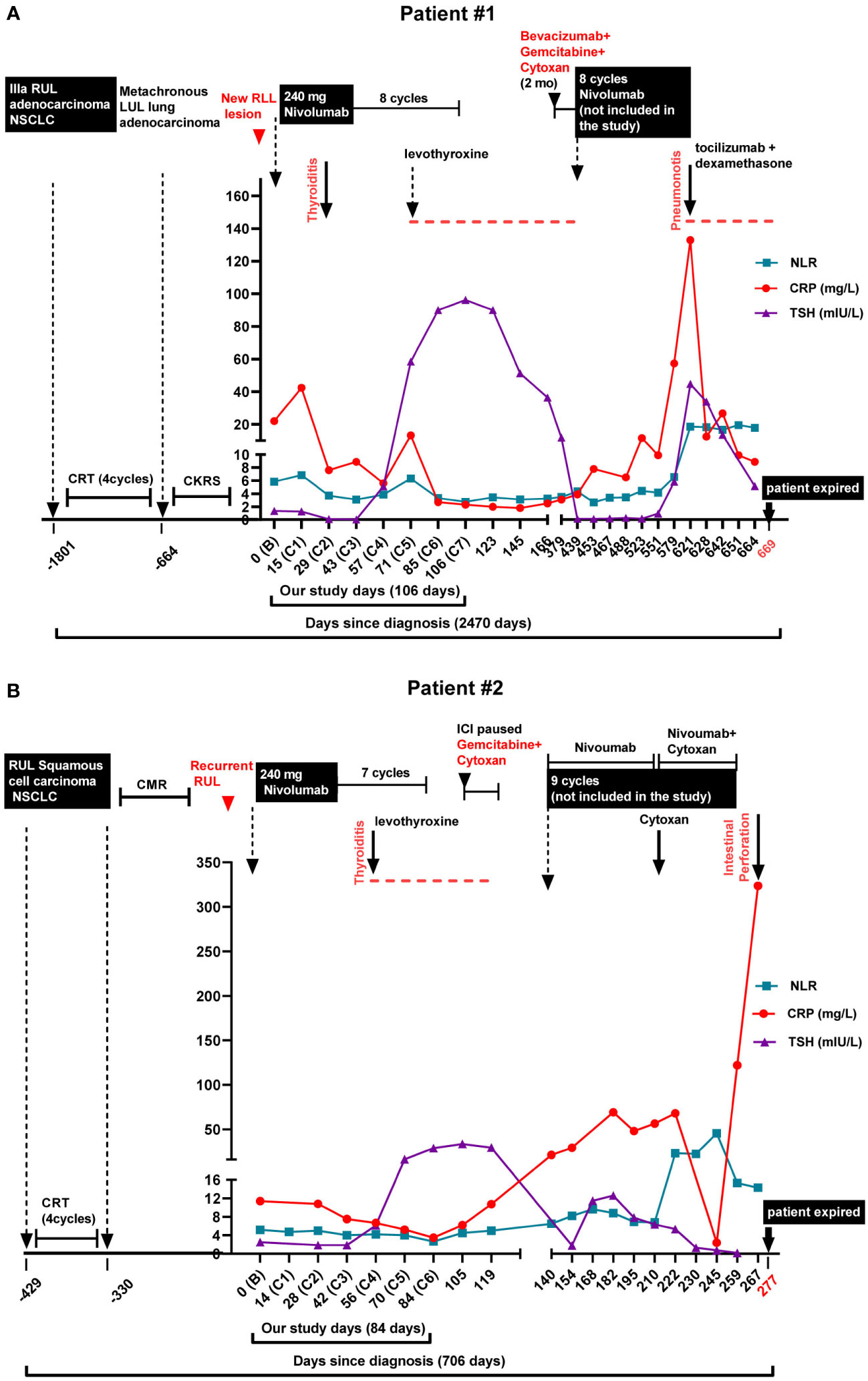
Figure 1 Diagnosis, treatment regimens, immune-related adverse events, and biomarker levels in the lung cancer patients. Neutrophile to Lymphocyte Ratio (NLR), C-Reactive Protein (CRP), and Thyroid Stimulating Hormone (TSH) levels are shown along with the treatment and immune-related adverse events (irAEs). (A) Patient #1. The patient had a 5-year history (-1801 days) of inoperable stage IIIA (T1aN2M0) right upper lobe (RUL) lung adenocarcinoma. He was initially treated with chemoradiation, followed by disease progression. He then received 8 doses of bi-weekly 240 mg Nivolumab (days 0 to 106) and was enrolled in the biomarker study. Checkpoint inhibitor-induced thyroid dysfunction (CITD, thyroiditis) was observed as an immune-related adverse event (irAE) after the 2nd dose of Nivolumab (C2). Later, the patient received another round of Nivolumab treatment (days 439 to 621, not enrolled in our biomarker trial) in which pneumonitis as another irAE occurred (day 621). (B) Patient #2. The patient was diagnosed with RUL squamous cell carcinoma (-429 days) and received chemoradiation. Subsequent PET scan demonstrated a complete metabolic response (CMR). Unfortunately, 7 months later he had recurrence of RUL mass. The patient received 7 cycles of bi-weekly 240 mg Nivolumab treatment (days 0 to 84) when enrolled in our biomarker study. CIDT (thyroiditis) was detected after the 4th dose of Nivolumab (C4). Later, the patient received another round of Nivolumab treatment (days 140 to 259, not enrolled in our biomarker trial) in which another irAE, intestinal perforation, occurred (day 267).
The patient’s cancer progressed after 11 months, when PET scan showed further metastasis within the liver. The patient started on 2 months of immune modulating gemcitabine, cyclophosphamide and bevacizumab. Monthly nivolumab 480 mg cycles were restarted, but unfortunately patient was not enrolled in the trial and blood samples were not collected for T cell and cytokine analyses. The patient demonstrated an excellent response after 6 cycles of nivolumab yet developed checkpoint inhibition-induced pneumonitis (CIP). After his hospitalization for CIP, the patient began to have recurrent diarrhea and extensive workup interestingly revealed elevated levels of chromogranin and serotonin. Biopsy of the liver showed metastatic adenocarcinoma with neuroendocrine differentiation with small cell morphology, raising the concern for transformation of his primary NSCLC to SCLC. Unfortunately, a corresponding brain MRI demonstrated extensive central nervous system (CNS) disease progression and the patient expired shortly thereafter (Figure 1A).
Case #2
The second patient was a 61-year-old male with history of smoking, who was initially presented with progressive neck swelling and diagnosed with superior vena cava syndrome after CT chest showed right paratracheal mass with extension into the anterior mediastinum, and SVC compression. He subsequently underwent bronchoscopy with biopsy which revealed squamous cell carcinoma and tumor cells were strongly positive for p63 and negative for TTF-1. Molecular analysis of the tumor was not performed. The patient received chemoradiation, and subsequent PET scan demonstrated CMR. Unfortunately, 7 months later the patient was admitted for post obstructive pneumonia and was found to have recurrence of RUL mass. Bronchoscopy with biopsy showed recurrent squamous cell carcinoma, with 50% expression of PD-L1 by IHC staining. He was enrolled in the trial described herein and blood samples were procured at baseline and after each cycle of the immunotherapy. The patient was started on two-week cycles of 240 mg nivolumab with 8 planned cycles.
After 4 cycles of nivolumab, routine TSH measurements revealed an increase above the upper limit of normal to 28.82 mIU/L on cycle 6, with a slight prior decrease in TSH on cycle 2 and 3 (Figure 1B and Supplementary Figure 1B). Anti-TPO antibodies were negative. The patient initially reported fatigue, and patient was started on levothyroxine 75 mcg daily. After 7 cycles of nivolumab, repeat PET scan showed progression of his disease and new liver and brain metastases. The patient was switched to gemcitabine and cytoxan, but later had hemoptysis, and nivolumab was restarted. He received an additional 9 cycles of nivolumab of which the patient was not enrolled in the trial and no blood samples were collected for our cytokine and T cell study. While on the second round of nivolumab, the patient’s dose of levothyroxine was increased to 100 mcg daily, yet TSH remained intermittently elevated ranging from 5.31 to 12.58 mIU/L while on treatment. During this time, the patient also reported constipation. Upon completing the 9th cycle of nivolumab, the patient developed an intestinal perforation of undifferentiated etiology and succumbed to the complications of his disease (Figure 1B and Supplementary Figure 2).
Discussion
Thyroid dysfunction (TD) is a common irAE that occurs in cancer patients treated with immune checkpoint inhibitors (1, 2). Subtypes and clinical manifestations of CITD are discussed elsewhere (8, 9). Multiple studies have noted that patients on ICI therapy often have a thyrotoxicosis phase prior to hypothyroidism (6, 10). Yet patients can often be asymptomatic, with one study stating that 67% of patients on ICI therapy were asymptomatic during the thyrotoxicosis phase (10). The manifestations of CITD are usually not severe, and normally grade 1 to 3 by CTCAE v5 (11). The most common clinical symptoms include fatigue, constipation, cold intolerance, swelling, and weight gain (12). Notably, both patients reported fatigue and constipation after CITD, and patient #2 had associated intestinal perforation (Figure 1B and Supplementary Figure 2). In patients with existing thyroid disease, especially with hyperthyroidism, the risk of worsening TD increases after ICI initiation (13, 14). Notably Kim et al. showed a statistically significant increase in overall survival and progression-free survival among in patients with TD when compared to euthyroid patients (15). Overall, CITD is quite common and has prognostic implications for patients’ disease. Although mild symptoms are common, severe TD is associated with significant morbidity; therefore, an increased focus on the mechanisms of CITD may improve the diagnosis, management, and care of patients.
The pathogenesis of CITD is unclear, yet the pathologic mechanisms are likely distinct from autoimmune thyroid disease, e.g., Hashimoto, subacute, and silent thyroiditis (16). While thyroid peroxidase (TPO) antibodies (Abs) are often positive in autoimmune thyroid disease, several reports have shown the TPO Abs are not necessarily associated with immunotherapy induced thyroiditis (14, 17). By contrast, Osorio et. al., showed in NSCLC receiving pembrolizumab, 80% of patients who developed CITD had TPO Abs present at diagnosis compared with 8% who did not develop CITD (6). The role of humoral immunity may have a distinct role in CITD, yet emerging data strongly implicates T cells as a primary pathologic actor (16, 18).
Other mechanistic indicators of CITD include a higher incidence with PD-1 inhibitors compared to CTLA-4 or PD-L1 inhibitors (19). Possible explanations include polymorphisms in either CTLA-4, PD-1, or PD-L1 molecules or genetic variations that predispose patients to autoimmune thyroid disease (20–22). Recent studies suggested the difference may be from the widespread T cell activation by anti-CTLA-4, whereas PD-1 or PD-L1 blockade polarizes the activation of pre-existing CD8+ T cells that have reactivity to thyroid antigens (16, 23, 24). The importance of T cells rather than B cells in CITD is reinforced by the reported comparison of T cells from thyroid fine needle aspiration to blood analysis showed that immunotherapy induced thyroiditis had a T lymphocyte-mediated process with intrathyroidal predominance of CD8+ and CD4-CD8- T lymphocytes (18). Furthermore, Delivanis et al. suggested that ICI-related thyroid destruction may be independent of thyroid autoantibodies but involve T cell, NK cell, and monocyte-mediated pathways (14).
Interestingly, for patients described here, we observed an increase in peripheral T cells associated with the onset of CITD. For patient #1, both CD4+ and CD8+ T cells more than doubled between cycles 2 and 3 of treatment (Figure 2A), corresponding with a decrease in TSH to 0.09 mIU/L at cycle 2 and 0.04 mIU/L at cycle 3 (Figure 1A and Supplementary Figure 1A). For patient #2, CD8+ T cells more than doubled between his 1st and 2nd cycles of treatment (Figure 2B), and subsequently his TSH increased from 1.88 to 6.11 mIU/L (Figure 1B and Supplementary Figure 1B). While this observation may be in part secondary to a treatment effect as we and others have previously described, it may also go along with previous reports of T cell expansion associated with immunotherapy induced thyroiditis (25–28).
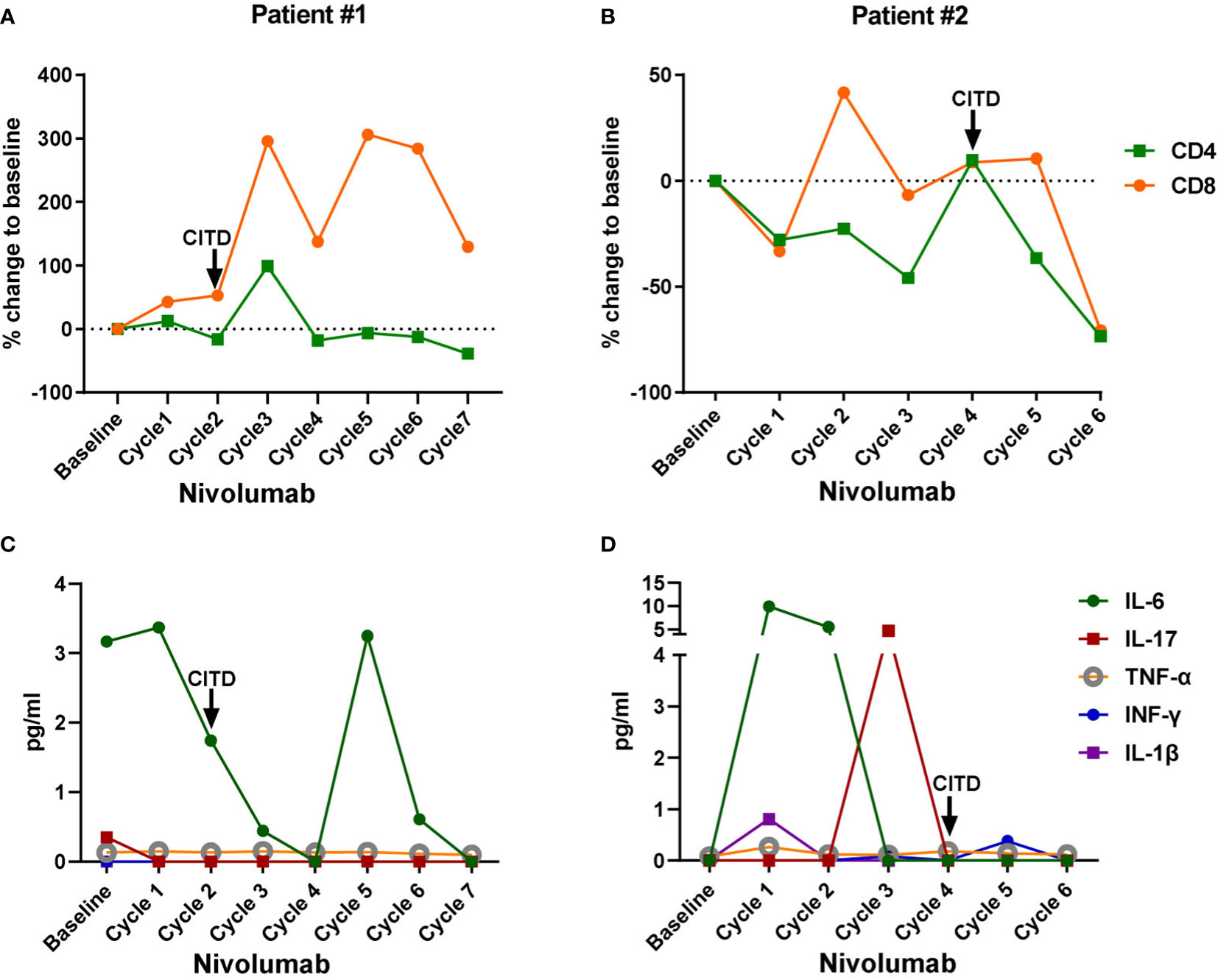
Figure 2 Dynamic changes of peripheral blood CD4+ T cells, CD8+ T cells, and inflammatory cytokines in the lung cancer patients treated with Nivolumab. Blood samples were collected at the baseline and after each cycle of Nivolumab treatment during the biomarker trial. Baseline: values from the blood samples collected before the Nivolumab treatment initiation; Cycle 1: values after the 1st dose of Nivolumab, from the blood samples collected right before the second dose administration; Cycle 2: values after the 2nd dose of Nivolumab, from the blood samples collected right before the third dose administration, and so on. (A, B) Percent change of peripheral CD4+ T cells and CD8+ T cells from baseline after each dose of Nivolumab in patient #1 and #2, respectively. (C, D) Plasma IL-6, IL-17, TNF-α, INF-γ, and IL-1β levels in patient #1 and #2, respectively. Onset of checkpoint inhibitor-induced thyroid dysfunction (CITD) is indicated by arrows on the graphs.
Next, we examined potential inflammatory mediators that may be associated with CITD. We were interested in measuring levels of IL-6 that we and others have found to be elevated during pneumonitis and neutropenia irAEs (25, 29). Interestingly, patient #1 had a decrease in IL-6 levels during his episode of CITD (Figure 2C). Patient #2 had a mildly elevated level in IL-6 to 9.97 pg/mL after the first ICI dose (Figure 2D), consistent with previous reports that hypothyroidism (HT) patients had elevated serum IL-6 levels (30). It is also noteworthy that patient #2 had a transient increase in IL-17 to 4.7 pg/mL (Figure 2D). Increased levels of IL-17 have been observed in patients with HT compared to healthy patients (31, 32). However, patient #2’s IL-6 and IL-17 levels were very low during his episode of CITD (Figure 2D). Furthermore, the levels of inflammatory molecules TNF-α, INF-γ, and IL-1β were very low at the onset of CITD in patients #1 and #2 (Figures 2C, D).
Additional proinflammatory biomarkers associated with irAEs are CRP and neutrophil to lymphocyte ratio (NLR) (33–35). We recently report a case of checkpoint inhibitor pneumonitis with increases in CRP and NLR associated with CIP onset and resolution (25). Furthermore, in patients with autoimmune hypothyroidism, a correlation was found between levels of high-sensitive CRP and thyroglobulin autoantibody and TSH levels (36). We suspected a similar observation may be consistent in CITD, yet neither patient had dynamic increases from baseline in CRP or NLR at CITD onset (Figure 1 and Supplementary Figure 1). Although both patients had elevated baseline CRP levels, 22.0 and 11.4 mg/mL, respectively, after ICI treatment CRP levels decreased. However, patient #1 had a transient elevation of CRP (42.4 mg/L) after the first dose of ICI, whereas the CRP level decreased below the baseline after the second and third doses of ICI (Figure 1A and Supplementary Figure 1C). Interestingly, this patient later developed pneumonitis as an irAE while receiving Nivolumab for a second eight cycle treatment phase (not included in our biomarker trial). During this second irAE, CRP levels spiked to 132.9 mg/L, suggesting CRP as a more valuable biomarker for pneumonitis than for CITD (Figure 1A). Moreover, for patient#2, CRP levels continued to decrease from baseline after ICI initiation (Figure 1B and Supplementary Figure 1D). Similar to patient #1, this patient also received a second phase of Nivolumab (not included in our trial) and also developed a second irAE, intestinal perforation. On the day the patient presented with intestinal perforation, a spike in CRP level of 323.7 mg/L was observed, in contrast to the decreased CRP levels previously observed with CITD (Figure 1B and Supplementary Figure 1D). Likewise, NLR decreased during the CITD episodes after ICI treatment for both patients; however, later on during both of their second reported irAE, NLR levels elevated (Figures 1A, B). CRP and NLR are often appreciated as sensitive biomarkers for irAEs which are consistent with our results of the second irAEs in both patients (pneumonitis and intestinal perforation, respectively), yet these cases suggest further investigation is needed to define the utility of CRP and NLR in diagnosing and understanding CITD. It is still not fully understood why CRP and NLR levels in these two patients did not significantly elevate at the onset of CITD but spiked during the events of pneumonitis and intestinal perforation. Further studies are required to better understand the commonalities and differences among various irAEs.
CITD is a common irAE, and although cases are usually mild, and can be treated with thyroid hormone supplementation. The diagnosis remains clinically meaningful, and if left unmanaged can result in significant morbidity. Mechanistically, CITD is not fully understood, yet the histological predominance of T cells and lack of consistently elevated TPO antibodies suggest the overall importance a T cell-mediated process (5, 7, 16, 18). Consistently, our observations indicate an increase in peripheral CD8+ T cells during or preceding the onset of CITD in both patients (Figures 2A, B). Monitoring peripheral CD8+ T cells, in conjunction with TSH measurement, may be useful for the research and diagnosis of CITD. Recent studies demonstrate that a dynamic increase in peripheral CD8+ T cells is a valuable indicator of immune responses to ICIs in cancer patients (26, 27, 37). Further research is warranted to investigate the temporal and subtype changes of peripheral CD8+ T cells in order to elucidate immunotherapy-specific responses versus autoimmune responses in CITD.
Conclusion
Our case studies demonstrate that increases in peripheral CD8+ T cells may correlate with onset of CITD. Unlike previous reports on other irAEs such as pneumonitis and neutropenia from our group and others (25, 38, 39), CRP levels and NLR were non-contributory in the diagnosis of CITD, yet larger cohorts are needed to confirm this observation. Despite the limitations of two case reports, these observations may aid in a more robust understanding of the mechanisms of CITD.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Ethics statement
The studies involving human participants were reviewed and approved by The East Carolina University Institutional Review Board (IRB). The patients/participants provided their written informed consent to participate in this study.
Author contributions
MAM: designed study, conducted, analyzed, and interpreted experiments, and wrote manuscript. JM: designed study, conducted, analyzed, and interpreted experiments, and wrote manuscript. ZH: gathered clinical data and assisted in assembling manuscript. AN: gathered clinical data, critical edits and revisions to manuscript. AH: assisted in assembling manuscript. DA: processed patient blood samples and performed experiments. SA: gathered clinical data, critical edits and revisions to manuscript. MM: enrolled patients, critical edits and revisions to manuscript. PW: supervised, enrolled patient, involved in the clinical care of patients, critical edits and revisions to manuscript. LY: supervised, designed study, analyzed and interpreted data, and wrote manuscript. All authors contributed to the article and approved the submitted version.
Funding
The study was supported in part by grants from the Lung Cancer Initiative of North Carolina and the Coach Rock Foundation.
Conflict of interest
Author PW was employed by company Circulogene.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1023545/full#supplementary-material
Supplementary Figure 1 | Levels of thyroid stimulating hormone (TSH), neutrophil to lymphocyte ratio (NLR), and C-reactive protein (CRP) in the lung cancer patients treated with Nivolumab. Blood samples were collected at the baseline and after each cycle of Nivolumab treatment during the biomarker trial. (A&B) TSH levels (mIU/L), (C&D) CRP levels (mg/L), and (E&F) NLR levels, in patient #1 and #2, respectively. Onset of checkpoint inhibitor-induced thyroid dysfunction (CITD) is indicated by arrows on the graphs.
Supplementary Figure 2 | Radiographic imaging of patient #2 during the event of intestinal perforation. (A) The anteroposterior chest X-ray image shows free air under the diaphragm and elevation of hemidiaphragm on the right side. (B) The lateral chest X-ray image shows free air below the diaphragm (red arrowhead).
References
1. Kennedy LB, Salama AKS. A review of cancer immunotherapy toxicity. CA Cancer J Clin (2020) 70(2):86–104. doi: 10.3322/caac.21596
2. Naqash AR, Ricciuti B, Owen DH, Florou V, Toi Y, Cherry C, et al. Outcomes associated with immune-related adverse events in metastatic non-small cell lung cancer treated with nivolumab: a pooled exploratory analysis from a global cohort. Cancer Immunol Immunother (2020) 69(7):1177–87. doi: 10.1007/s00262-020-02536-5
3. Sonpavde GP, Grivas P, Lin Y, Hennessy D, Hunt JD. Immune-related adverse events with PD-1 versus PD-L1 inhibitors: a meta-analysis of 8730 patients from clinical trials. Future Oncol (2021) 17(19):2545–58. doi: 10.2217/fon-2020-1222
4. Weinstein A, Gordon RA, Kasler MK, Burke M, Ranjan S, Hodgetts J, et al. Understanding and managing immune-related adverse events associated with immune checkpoint inhibitors in patients with advanced melanoma. J advanced practitioner Oncol (2017) 8(1):58–72. doi: 10.6004/jadpro.2017.8.1.5
5. Bhattacharya S, Goyal A, Kaur P, Singh R, Kalra S. Anticancer drug-induced thyroid dysfunction. Eur Endocrinol (2020) 16(1):32–9. doi: 10.17925/EE.2020.16.1.32
6. Osorio JC, Ni A, Chaft JE, Pollina R, Kasler MK, Stephens D, et al. Antibody-mediated thyroid dysfunction during T-cell checkpoint blockade in patients with non-small-cell lung cancer. Ann Oncol Off J Eur Soc Med Oncol (2017) 28(3):583–9. doi: 10.1093/annonc/mdw640
7. Kurimoto C, Inaba H, Ariyasu H, Iwakura H, Ueda Y, Uraki S, et al. Predictive and sensitive biomarkers for thyroid dysfunctions during treatment with immune-checkpoint inhibitors. Cancer Sci (2020) 111(5):1468–77. doi: 10.1111/cas.14363
8. Scott ES, Long GV, Guminski A, Clifton-Bligh RJ, Menzies AM, Tsang VH. The spectrum, incidence, kinetics and management of endocrinopathies with immune checkpoint inhibitors for metastatic melanoma. Eur J Endocrinol (2018) 178(2):173–80. doi: 10.1530/EJE-17-0810
9. Lee H, Hodi FS, Giobbie-Hurder A, Ott PA, Buchbinder EI, Haq R, et al. Characterization of thyroid disorders in patients receiving immune checkpoint inhibition therapy. Cancer Immunol Res (2017) 5(12):1133–40. doi: 10.1158/2326-6066.CIR-17-0208
10. Iyer PC, Cabanillas ME, Waguespack SG, Hu MI, Thosani S, Lavis VR, et al. Immune-related thyroiditis with immune checkpoint inhibitors. Thyroid Off J Am Thyroid Assoc (2018) 28(10):1243–51. doi: 10.1089/thy.2018.0116
11. Mahmood SS, Fradley MG, Cohen JV, Nohria A, Reynolds KL, Heinzerling LM, et al. Myocarditis in patients treated with immune checkpoint inhibitors. J Am Coll Cardiol (2018) 71(16):1755–64. doi: 10.1016/j.jacc.2018.02.037
12. Stelmachowska-Banaś M, Czajka-Oraniec I. Management of endocrine immune-related adverse events of immune checkpoint inhibitors: an updated review. Endocrine connections (2020) 9(10):R207–r28. doi: 10.1530/EC-20-0342
13. Kaleta F, Brody H, Namireddy P. 639 immune-related thyroid dysfunction in patients with existing thyroid dysfunction. J ImmunoTherapy Cancer (2020) 8(Suppl 3):A382–A3. doi: 10.1136/jitc-2020-SITC2020.0639
14. Delivanis DA, Gustafson MP, Bornschlegl S, Merten MM, Kottschade L, Withers S, et al. Pembrolizumab-induced thyroiditis: Comprehensive clinical review and insights into underlying involved mechanisms. J Clin Endocrinol Metab (2017) 102(8):2770–80. doi: 10.1210/jc.2017-00448
15. Kim HI, Kim M, Lee SH, Park SY, Kim YN, Kim H, et al. Development of thyroid dysfunction is associated with clinical response to PD-1 blockade treatment in patients with advanced non-small cell lung cancer. Oncoimmunology (2017) 7(1):e1375642. doi: 10.1080/2162402X.2017.1375642
16. Zhan L, Feng HF, Liu HQ, Guo LT, Chen C, Yao XL, et al. Immune checkpoint inhibitors-related thyroid dysfunction: Epidemiology, clinical presentation, possible pathogenesis, and management. Front Endocrinol (2021) 12:649863. doi: 10.3389/fendo.2021.649863
17. Yamauchi I, Yasoda A, Matsumoto S, Sakamori Y, Kim YH, Nomura M, et al. Incidence, features, and prognosis of immune-related adverse events involving the thyroid gland induced by nivolumab. PloS One (2019) 14(5):e0216954. doi: 10.1371/journal.pone.0216954
18. Kotwal A, Gustafson MP, Bornschlegl S, Kottschade L, Delivanis DA, Dietz AB, et al. Immune checkpoint inhibitor-induced thyroiditis is associated with increased intrathyroidal T lymphocyte subpopulations. Thyroid Off J Am Thyroid Assoc (2020) 30(10):1440–50. doi: 10.1089/thy.2020.0075
19. Wang PF, Chen Y, Song SY, Wang TJ, Ji WJ, Li SW, et al. Immune-related adverse events associated with anti-PD-1/PD-L1 treatment for malignancies: A meta-analysis. Front Pharmacol (2017) 8:730. doi: 10.3389/fphar.2017.00730
20. Tomer Y. Genetic susceptibility to autoimmune thyroid disease: past, present, and future. Thyroid Off J Am Thyroid Assoc (2010) 20(7):715–25. doi: 10.1089/thy.2010.1644
21. Jacobson EM, Huber A, Tomer Y. The HLA gene complex in thyroid autoimmunity: from epidemiology to etiology. J Autoimmun (2008) 30(1-2):58–62. doi: 10.1016/j.jaut.2007.11.010
22. Hadj-Kacem H, Rebuffat S, Mnif-Féki M, Belguith-Maalej S, Ayadi H, Péraldi-Roux S. Autoimmune thyroid diseases: genetic susceptibility of thyroid-specific genes and thyroid autoantigens contributions. Int J immunogenetics (2009) 36(2):85–96. doi: 10.1111/j.1744-313X.2009.00830.x
23. Chen DS, Mellman I. Elements of cancer immunity and the cancer-immune set point. Nature (2017) 541(7637):321–30. doi: 10.1038/nature21349
24. Elia G, Ferrari SM, Galdiero MR, Ragusa F, Paparo SR, Ruffilli I, et al. New insight in endocrine-related adverse events associated to immune checkpoint blockade. Best Pract Res Clin Endocrinol Metab (2020) 34(1):101370. doi: 10.1016/j.beem.2019.101370
25. McCallen JD, Naqash AR, Marie MA, Atwell DC, Muzaffar M, Sharma N, et al. Peripheral blood interleukin 6, interleukin 10, and T lymphocyte levels are associated with checkpoint inhibitor induced pneumonitis: a case report. Acta Oncol (2021) 60(6):813–7. doi: 10.1080/0284186X.2021.1917001
26. Fairfax BP, Taylor CA, Watson RA, Nassiri I, Danielli S, Fang H, et al. Peripheral CD8(+) T cell characteristics associated with durable responses to immune checkpoint blockade in patients with metastatic melanoma. Nat Med (2020) 26(2):193–9. doi: 10.1038/s41591-019-0734-6
27. Kamphorst AO, Pillai RN, Yang S, Nasti TH, Akondy RS, Wieland A, et al. Proliferation of PD-1+ CD8 T cells in peripheral blood after PD-1-targeted therapy in lung cancer patients. Proc Natl Acad Sci United States America (2017) 114(19):4993–8. doi: 10.1073/pnas.1705327114
28. Valpione S, Pasquali S, Campana LG, Piccin L, Mocellin S, Pigozzo J, et al. Sex and interleukin-6 are prognostic factors for autoimmune toxicity following treatment with anti-CTLA4 blockade. J Trans Med (2018) 16(1):94. doi: 10.1186/s12967-018-1467-x
29. Naqash AR, Yang LV, Sanderlin EJ, Atwell DC, Walker PR. Interleukin-6 as one of the potential mediators of immune-related adverse events in non-small cell lung cancer patients treated with immune checkpoint blockade: Evidence from a case report. Acta Oncol (2018) 57(5):705–8. doi: 10.1080/0284186X.2017.1406668
30. Liu Y, Tang X, Tian J, Zhu C, Peng H, Rui K, et al. Th17/Treg cells imbalance and GITRL profile in patients with hashimoto's thyroiditis. Int J Mol Sci (2014) 15(12):21674–86. doi: 10.3390/ijms151221674
31. Qin Q, Liu P, Liu L, Wang R, Yan N, Yang J, et al. The increased but non-predominant expression of Th17- and Th1-specific cytokines in hashimoto's thyroiditis but not in graves' disease Brazilian Journal of medical and biological research = revista brasileira de pesquisas medicas e biologicas. Braz J Med Biol Res (2012) 45(12):1202–8. doi: 10.1590/s0100-879x2012007500168
32. Esfahanian F, Ghelich R, Rashidian H, Jadali Z. Increased levels of serum interleukin-17 in patients with hashimoto's thyroiditis. Indian J Endocrinol Metab (2017) 21(4):551–4. doi: 10.4103/ijem.IJEM_412_16
33. Naqash AR, Stroud CRG, Butt MU, Dy GK, Hegde A, Muzaffar M, et al. Co-Relation of overall survival with peripheral blood-based inflammatory biomarkers in advanced stage non-small cell lung cancer treated with anti-programmed cell death-1 therapy: Results from a single institutional database. Acta Oncol (2018) 57(6):867–72. doi: 10.1080/0284186X.2017.1415460
34. Abolhassani AR, Schuler G, Kirchberger MC, Heinzerling L. C-reactive protein as an early marker of immune-related adverse events. J Cancer Res Clin Oncol (2019) 145(10):2625–31. doi: 10.1007/s00432-019-03002-1
35. Pavan A, Calvetti L, Dal Maso A, Attili I, Del Bianco P, Pasello G, et al. Peripheral blood markers identify risk of immune-related toxicity in advanced non-small cell lung cancer treated with immune-checkpoint inhibitors. oncologist (2019) 24(8):1128–36. doi: 10.1634/theoncologist.2018-0563
36. Czarnywojtek A, Owecki M, Zgorzalewicz-Stachowiak M, Woliński K, Szczepanek-Parulska E, Budny B, et al. The role of serum c-reactive protein measured by high-sensitive method in thyroid disease. Archivum immunologiae therapiae experimentalis (2014) 62(6):501–9. doi: 10.1007/s00005-014-0282-1
37. Carlisle JW, Jansen CS, Cardenas MA, Sobierajska E, Reyes AM, Greenwald R, et al. Clinical outcome following checkpoint therapy in renal cell carcinoma is associated with a burst of activated CD8 T cells in blood. J Immunother Cancer (2022) 10(7):e004803. doi: 10.1136/jitc-2022-004803
38. Naqash AR, Appah E, Yang LV, Muzaffar M, Marie MA, McCallen JD, et al. Isolated neutropenia as a rare but serious adverse event secondary to immune checkpoint inhibition. J Immunother Cancer (2019) 7(1):169. doi: 10.1186/s40425-019-0648-3
39. Lauwyck J, Beckwée A, Santens A, Schwarze JK, Awada G, Vandersleyen V, et al. C-reactive protein as a biomarker for immune-related adverse events in melanoma patients treated with immune checkpoint inhibitors in the adjuvant setting. Melanoma Res (2021) 31(4):371–7. doi: 10.1097/CMR.0000000000000748
Keywords: immune checkpoint inhibitor (ICI), thyroid dysfunction and thyroiditis, lung cancer, immune-related adverse events, irAEs
Citation: Marie MA, McCallen JD, Hamedi ZS, Naqash AR, Hoffman A, Atwell D, Amara S, Muzaffar M, Walker PR and Yang LV (2022) Case Report: Peripheral blood T cells and inflammatory molecules in lung cancer patients with immune checkpoint inhibitor-induced thyroid dysfunction: Case studies and literature review. Front. Oncol. 12:1023545. doi: 10.3389/fonc.2022.1023545
Received: 19 August 2022; Accepted: 23 November 2022;
Published: 07 December 2022.
Edited by:
Luca Roncati, University of Modena and Reggio Emilia, ItalyReviewed by:
Ada Gabriela Blidner, Instituto de Biología y Medicina Experimental (IBYME) (CONICET), ArgentinaYusuke Okuma, National Cancer Center Hospital, Japan
Copyright © 2022 Marie, McCallen, Hamedi, Naqash, Hoffman, Atwell, Amara, Muzaffar, Walker and Yang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li V. Yang, eWFuZ2xAZWN1LmVkdQ==; Paul R. Walker, d2Fsa2VycEBlY3UuZWR1
 Mona A. Marie
Mona A. Marie Justin D. McCallen1
Justin D. McCallen1 Zahra S. Hamedi
Zahra S. Hamedi Mahvish Muzaffar
Mahvish Muzaffar Paul R. Walker
Paul R. Walker Li V. Yang
Li V. Yang