- 1Nursing Department, Tianjin Medical University Cancer Institute & Hospital, National Clinical Research Center for Cancer, Tianjin, China
- 2Radiotherapy Department, Shaanxi Provincial Cancer Hospital, Xian, China
- 3Nursing Department, Shandong Cancer Hospital, Qingdao, China
- 4Nursing Department, Cangzhou People's Hospital, Cangzhou, China
- 5Nursing Department, Chongqing Cancer Hospital, Chongqing, China
- 6Nursing Department, Shanxi Provincial Cancer Hospital, Taiyuan, China
- 7Nursing Department, The Fourth Hospital of Hebei Medical University, Shijiazhuang, China
Objectives: To investigate the short-term efficacy and radiotoxicity 3.543of chronoradiotherapy in patients with cervical cancer. We also examined the overall symptom score and quality of life (QOL) of patients who underwent morning radiotherapy and evening radiotherapy.
Methods: We conducted a multicenter randomized controlled trial to compare the effects of morning radiotherapy (9:00–11:00 AM) with evening radiotherapy (7:00–9:00 PM) in cervical cancer patients receiving radiotherapy. From November 2021 to June 2022, 114 cervical cancer patients admitted to eight cancer center hospitals in Tianjin, Chongqing, Hubei, Shanxi, Shandong, Shaanxi, Hebei, and Cangzhou were randomly divided into the morning radiotherapy group (MG; N = 61) and the evening radiotherapy group (EG; N = 53). The short-term efficacy of radiotherapy on cervical cancer patients at different time points and the occurrence of radiotoxicity were explored after patients had undergone radiotherapy.
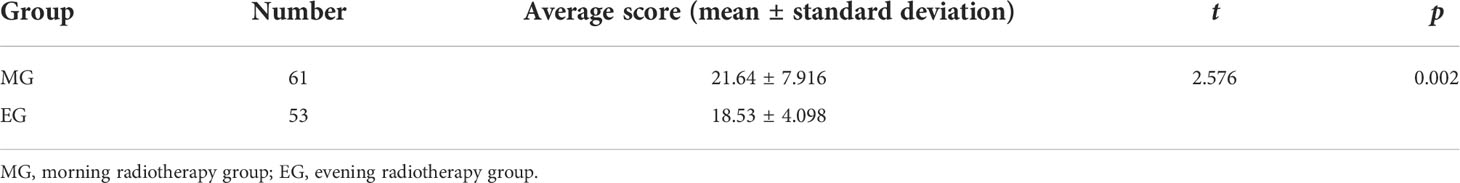
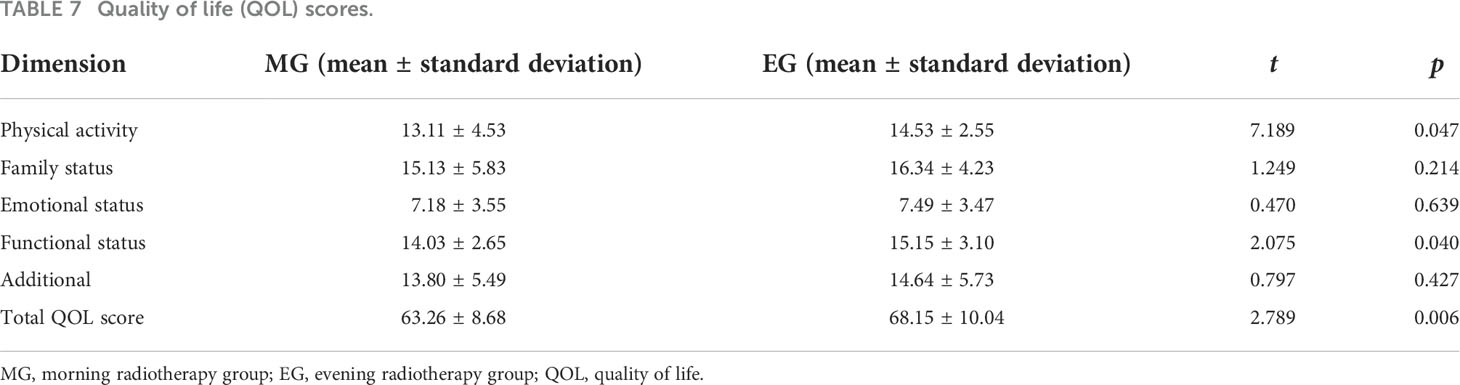
Results: The total effective response (partial remission [PR] + complete remission [CR]) rate was similar across the two groups (93.5% vs. 96.3%, p > 0.05). However, the incidence of bone marrow suppression and intestinal reaction in the two groups were significantly different (p < 0.05). The patients in the MG had significantly higher Anderson symptom scores than patients in the EG (21.64 ± 7.916 vs. 18.53 ± 4.098, p < 0.05). In terms of physical activity, functional status, and overall QOL, the MG had significantly lower scores than the EG (p < 0.05). No other measures showed a significant difference between the groups.
Conclusion: The radiotherapy effect of the MG was consistent with that of the EG. The incidence of radiation enteritis and radiation diarrhea in the MG was significantly higher than that in the EG; however, bone marrow suppression and blood toxicity in the EG were more serious than in the MG. Because of the small sample size of the study, we only examined the short-term efficacy of radiotherapy. Therefore, further clinical trials are needed to verify the efficacy and side effects of chronoradiotherapy.
Clinical Trial Registration: http://www.chictr.org.cn/searchproj.aspx, Registration Number: ChiCTR2100047140.
Introduction
A recent analysis revealed that cervical cancer remains a major threat to women. In 2020, there were an estimated 604,000 new cases of cervical cancer globally, which was the second most diagnosed cancer in women (1). Although cervical cancer is one of the leading causes of cancer-related death in women worldwide (2), nearly 90% of cervical cancer deaths occur in developing countries, with India and China accounting for 35% of the total cervical cancer burden (3).
Radiotherapy, alone or in combination with surgery or chemotherapy, is the main treatment for cervical cancer (4). Almost 80% of patients with cervical cancer undergo radiation therapy as part of their treatment (5). The aim of radiotherapy is to irradiate malignant tumors via ionizing radiation, and the cumulative effect of the irradiation dose destroys tumor cells (6). However, during the process of radiotherapy, although tumor cells are killed, the surrounding normal tissues are also damaged, which causes a series of toxic side effects.
Exposure to ionizing radiation during radiotherapy of the abdominopelvic region is associated with the development of treatment-limiting untoward symptoms. The consequences of damaging healthy cells can result in a series of adverse reactions ranging from acute radiation toxicity to organ damage and secondary cancers (7). Approximately 84% of patients undergo some form of acute radiation toxicity during radiation therapy for cervical cancer (7). The most common symptoms are hematological toxicity, gastrointestinal mucositis, diarrhea, nausea, and vomiting, which may lead to treatment interruptions, increased healthcare costs, and impaired quality of life (QOL) in patients undergoing irradiation. These adverse reactions are attributed to various factors, such as therapeutic, environmental, and genetic factors. In recent years, studies have explored how the time of day of radiotherapy administration affects radiation therapy outcomes to determine whether chrono-modulation may be beneficial (6–8).
The circadian rhythm is governed by an internal timing system that is regulated at the transcriptional level, creating networks of genes that oscillate on a 24-hour cycle (8). The cell cycle, proliferation, and cell death are closely intertwined with the circadian rhythm. Several recent studies have provided compelling evidence on the association between the circadian cycle and cancer; similar to healthy cells, tumor cells are rhythmic (9, 10), and their growth depends on circadian rhythms (11). It has been reported that each phase of the cell cycle corresponds to a different degree of radiosensitivity (12). Cells in or near mitosis (G2 and M phases) have the highest radiosensitivity, whereas cells in the S and G1 phases are less radiosensitive. Tumor cells also show time rhythms in metabolism and proliferation, which differ from those of healthy tissue cells. According to the different sensitivities of cells to radiation during different mitosis cycles, studies have investigated the time law of radiation sensitivity of tumor tissue and healthy tissue cells (12, 13). In line with circadian rhythm regularity, selecting a specific time to apply radiation therapy to tumors can significantly improve the efficacy of tumor radiation therapy (12, 13).
Chronoradiotherapy involves selecting the optimal radiotherapy time according to the body’s rhythm changes. It is aimed at protecting normal tissues as much as possible while killing tumor cells to the greatest extent to attenuate toxicity and increase efficiency. Radiotherapy can achieve a good curative effect, but the dose is roughly the maximum that the body can tolerate, which significantly limits the treatment of tumors. Therefore, ways in which to further improve the curative effect and minimize radiotoxicity is an important topic that requires urgent study. In this study, we investigated the radiation effects, radiotoxicity, and QOL in inoperable cervical cancer patients irradiated at different times of the day. Although chronoradiotherapy may be offered to cervical cancer patients as a new method, its efficacy and toxicity must be established. Current prospective randomized clinical data are lacking, and the use of chronoradiotherapy for the treatment of cervical carcinoma has not yet been established. Therefore, we conducted a multicenter prospective randomized study to assess the effectiveness of chronoradiotherapy in cervical cancer patients and to explore the relationship between the severity of acute gastrointestinal mucositis and the time of radiation in patients with carcinoma of the cervix.
Materials and methods
Study design
This was a multicenter randomized controlled trial (RCT) comparing morning radiotherapy (9:00–11:00 AM) with evening radiotherapy (7:00–9:00 PM) for cervical cancer patients undergoing radiotherapy. The study was registered with the Chinese Clinical Trial Registry (ref. Chi-CTR-2100047140) and was conducted from November 2021 to June 2022 at eight cancer center hospitals in the cities and provinces of Tianjin, Chongqing, Hubei, Shanxi, Shandong, Shaanxi, and Hebei. The Tianjin Medical University Cancer Institute and the Hospital Institutional Review Board approved the study protocol (approval number: bc2020185), and all caregivers provided informed consent. A total of 114 patients were registered during this period and were included in the study.
Study participants
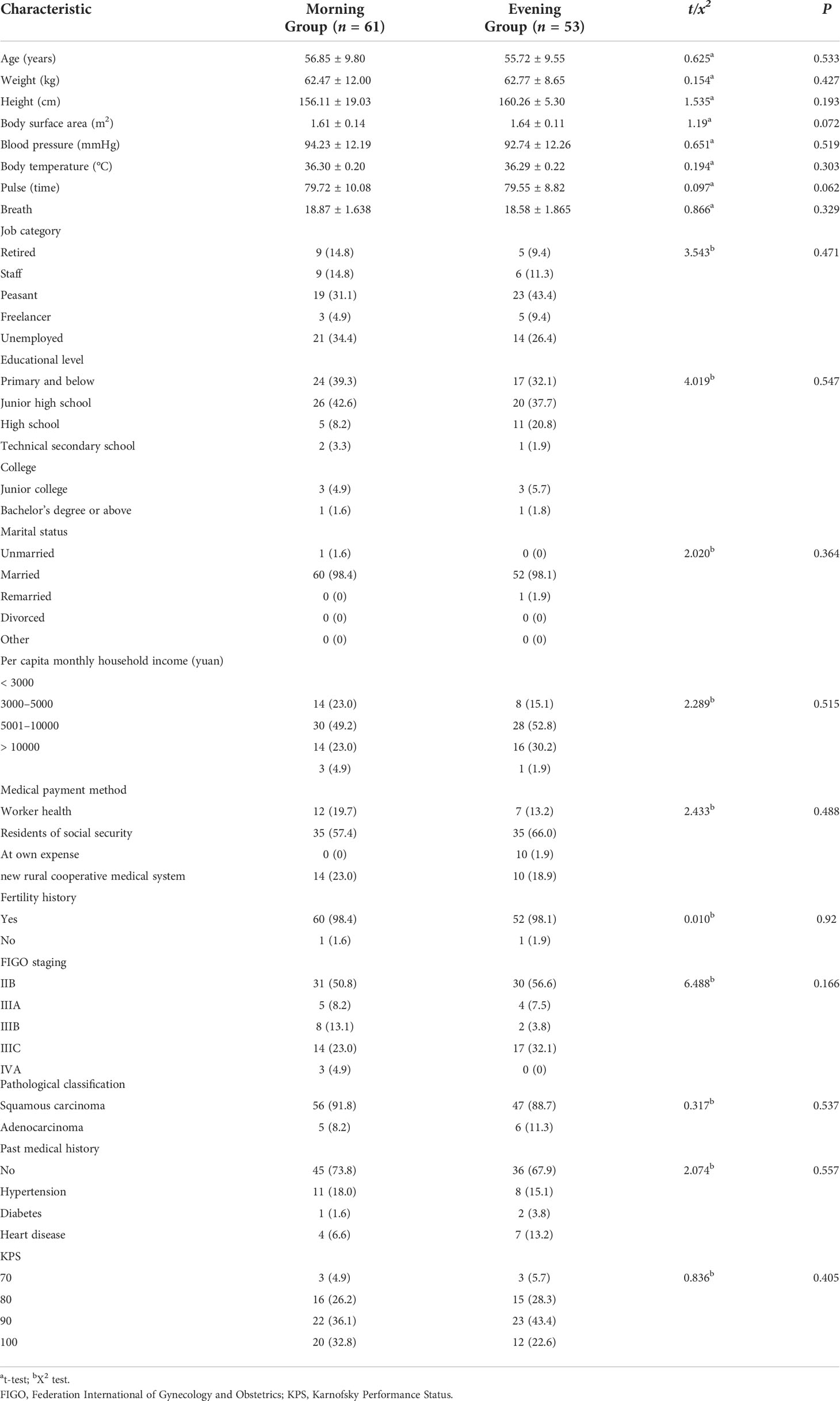
Patients were eligible if they fulfilled the inclusion and exclusion criteria. The inclusion criteria were as follows: 1) aged between 18 and 65 years; 2) cervical cancer patients with Federation International of Gynecology and Obstetrics (FIGO) stage IIB-IVA tumors confirmed by pathological biopsy to be nonmetastatic cervical cell carcinoma (see Table 1 for details) (14); 3) a Karnofsky Performance Status (KPS) score of ≥ 70 points; 4) patients participated voluntarily and provided written informed consent.

Table 1 2018 Federation International of Gynecology and Obstetrics (FIGO) Staging System for uterine cervical cancer.
The exclusion criteria were as follows: 1) clinically significant diseases (e.g., second primary tumor, severe infection, acute and chronic intestinal diseases or hemorrhoids, mental diseases, and systemic immune diseases) that might interfere with the primary endpoint assessment; 2) patients who had undergone major surgery within the 14 days before enrollment; 3) patients with serious liver, kidney, or another organ dysfunction.
Randomization, allocation concealment, and blinding
Randomization was performed before the beginning of the intervention using a random number table technique to ensure an equal number of participants in each group. The random allocation sequence was produced using the Statistical Analysis Software (SAS), version 9.4 (SAS Institute, Inc., Cary, NC, USA). Eight sets of random sequences with a sample size of 114 cases were generated by a computer, randomly grouped in a 1:1 ratio, and each center was divided into two sets of random sequences. After the participants provided informed consent and underwent baseline assessments, they were randomly assigned to receive either morning radiotherapy (i.e., the morning radiotherapy group [MG]) or evening radiotherapy (i.e., the evening radiotherapy group [EG]). Allocation concealment was assured by using sequentially numbered, opaque, sealed, and stapled envelopes that were distributed to the participants by the project manager. To avoid the disclosure of group assignment, aluminum foil was used to keep the envelope invisible, even under intense light. The group assignment (intervention or control group) was replaced by group A or B, so that the research assistant who collected and entered the study data into a database remained blinded to group allocation throughout the study.
Radiotherapy regimen
Both the MG and EG were treated with a uniform treatment combining external beam irradiation and high dose-rate (HDR) brachytherapy, without low dose-rate (LDR) brachytherapy. The external irradiation area has a large area to primarily address the problem of lymph node metastasis in the abdomen and pelvis. We used high-energy 6 MV and above X-rays for irradiation, and the irradiation dose was (50.4 Gy, 5–6 weeks, 28 fractions). HDR brachytherapy with iridium 192 HDR at a dose rate of 12–70/h, was initiated when the external radiation dose reached 30 Gy, and short-range radiation was added (30 Gy, five fractions). The samples in the MG received radiotherapy from 9:00 to 11:00 AM, whereas those in the EG received radiotherapy from 7:00 to 9:00 PM. In addition to radiotherapy, patients received a cisplatin chemotherapy regimen, (25 mg/m2 intravenously Guttae for 4–6 weeks). All the samples of this study in both groups received chemotherapy over the same time period, which was from 9:00 to 11:00 AM. We assessed the QOL of samples at baseline and the end of treatment. In addition, patients recorded any complaints of discomfort in a booklet we developed during this period to improve the compliance rate of patients.
Interventions
Before enrollment, patients in both groups received unified dietary guidance and radiotherapy-related health education. One day before radiotherapy, patients underwent blood tests, imaging examinations, such as chest X-ray, pelvic ultrasound, and electrocardiogram, and other baseline assessments. During radiation therapy, blood tests and radiotoxicity were assessed weekly by a trained observer blinded to group assignment. In addition, during radiotherapy, patients were instructed to use a uniform douche for vaginal douches twice per week. For radiotherapy-related symptoms, such as diarrhea and enteritis, we provided standardized treatments in strict accordance with the requirements of the protocol and maintained a complete record of the course of treatments. Finally, patients were evaluated for efficacy on the day following the final radiotherapy session (i.e., the day after the final brachytherapy).
Outcome measures
All outcomes were measured at baseline before the treatment and at the end of treatment. The curative effect of radiotherapy was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST) 1.1 (see Table 2 for details) (15). Toxicity was assessed using the Radiation Therapy Oncology Group’s common toxicity criteria (16). Myelosuppression was assessed using the myelosuppression grading of the World Health Organization. The Functional Assessment of Cancer Therapy-Cervix scale (17) was used to assess the QOL of patients during radiotherapy. Other radiation-related adverse reactions, such as pain, vomiting, sadness, and insomnia, were assessed using the M.D. Anderson Symptom Inventory (MDASI) (18). The case collection period was from August 2021 to December 2021. A trained observer assessed the results of patient assessments and completed unified case report forms, which included general information and relevant assessment results if patients visited the hospital for surveillance as an outpatient.
Sample size calculation
Before conducting the study, we calculated the approximate sample size considering the incidence of diarrhea in the two groups of chronoradiotherapy in relevant literature using the Power Analysis and Sample Size software version 15.0 (NCSS, Inc., USA). sample size calculation software: MG: 87.39%, EG: 68.18%, β = 0.1, test efficiency 1 – β = 80%, α = 0.05, N1 = N2 = 47, a total of 94 cases. Accounting for a 10% dropout rate, we determined that a minimum sample size of 104 cases would be required with N1 = N2 = 52, respectively.
Statistical analysis
The data were analyzed using the Statistical Product and Service Solution version 21.0 (IBM Institute, Inc., Stanford, CA, USA). The count data are expressed as frequencies and percentages. Data that conformed to a normal distribution are described as means ± standard deviations, and those that were not normally distributed are described as medians and interquartile ranges. Baseline characteristics in the control and intervention groups were analyzed to assess whether there were between-group differences. To assess differences in mean scores between the intervention and control groups, we used a parametric test (t-test) for scores with a normal distribution and a non-parametric test (Mann–Whitney U test) for scores with non-normal distribution. Chi-square analysis was used to compare differences between the two groups after radiotherapy. Statistical significance was defined as a two-sided p < 0.05. Excel (Microsoft Office Home and Student 2019) was used for the analysis.
Results
Patients
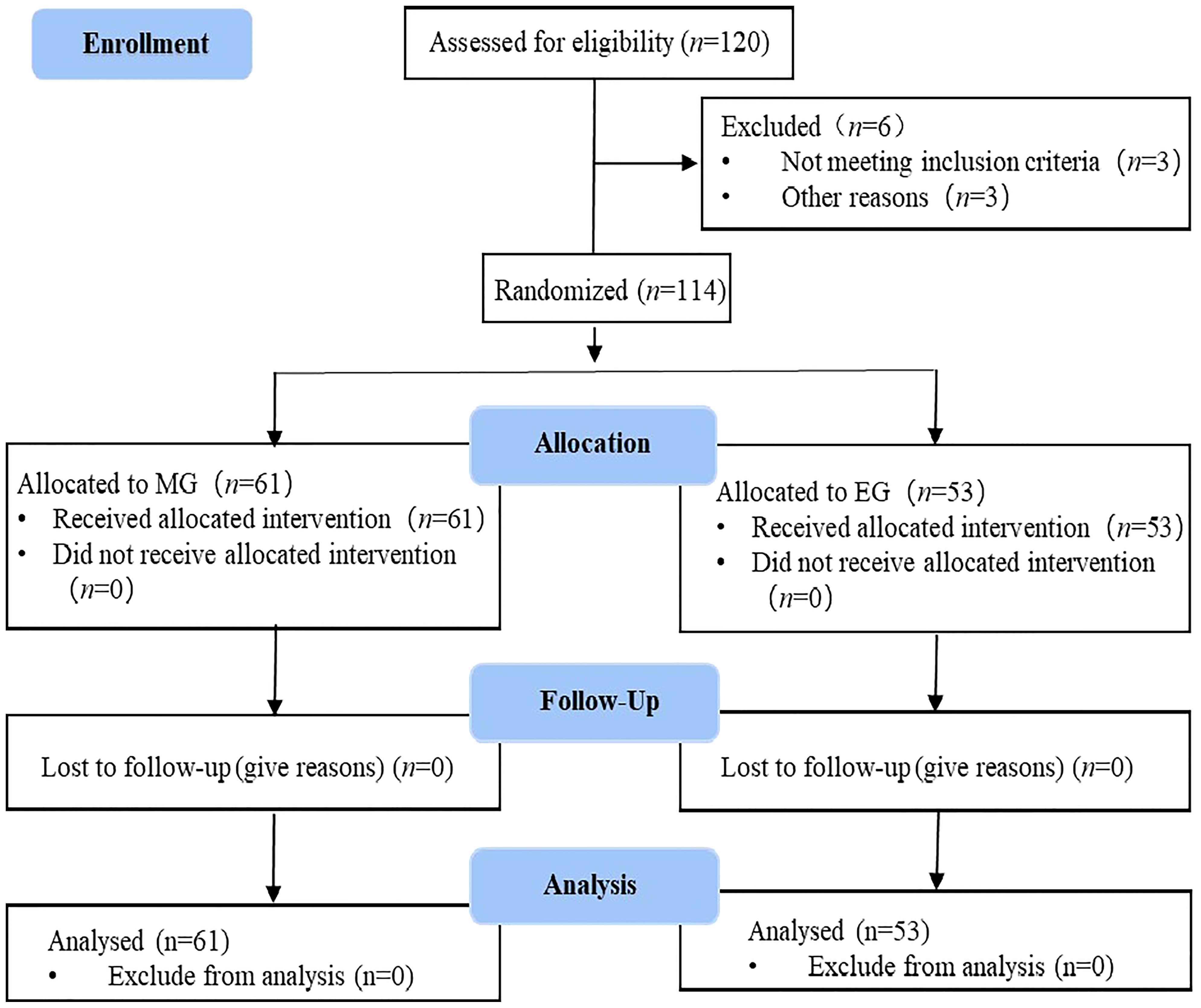
Between November 2021 and June 2022, this study initially included 120 patients. However, three cases were excluded because they did not meet the inclusion criteria, and three cases dropped out of the study before the follow-up. Finally, 114 cases were included, which comprised 61 patients in the MG and 53 patients in the EG (Figure 1). All patients were pathologically diagnosed with cervical cancer with clinical stage IIB-IVA and had no indication for surgery. All the samples in this study had the same circadian rhythm sleeping at night and doing daily activities on daytime. Before the intervention, there were no statistical differences between the groups in terms of baseline characteristics, including general demographic, disease, or social data (p < 0.05). The baseline characteristics of MG and EG are shown in Table 3.
Efficacy of radiotherapy
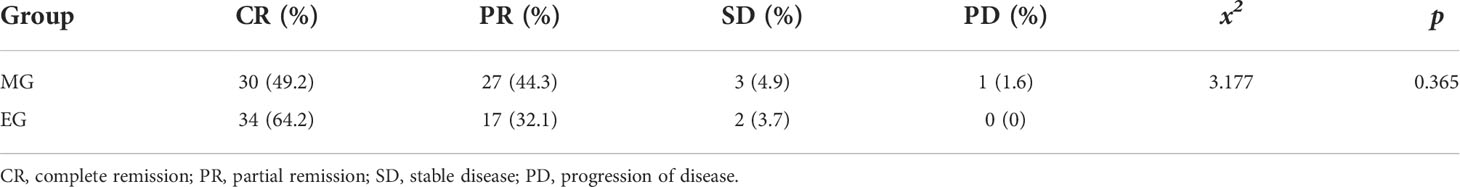
The complete remission (CR) rate was 49.2%, and the partial remission (PR) rate was 44.3% in the MG. The CR rate was 64.2% and the PR rate was 32.1% in the EG. The total effective rates (PR + CR) of the MG and EG were 93.5% and 96.3%, respectively. The CR rate of the EG was slightly higher than that of the MG, although further analysis showed that there was no significant difference in the CR rate between the two groups (p > 0.05). The results are described in Table 4.
Radiotherapy toxicity, symptoms, and related QOL outcomes
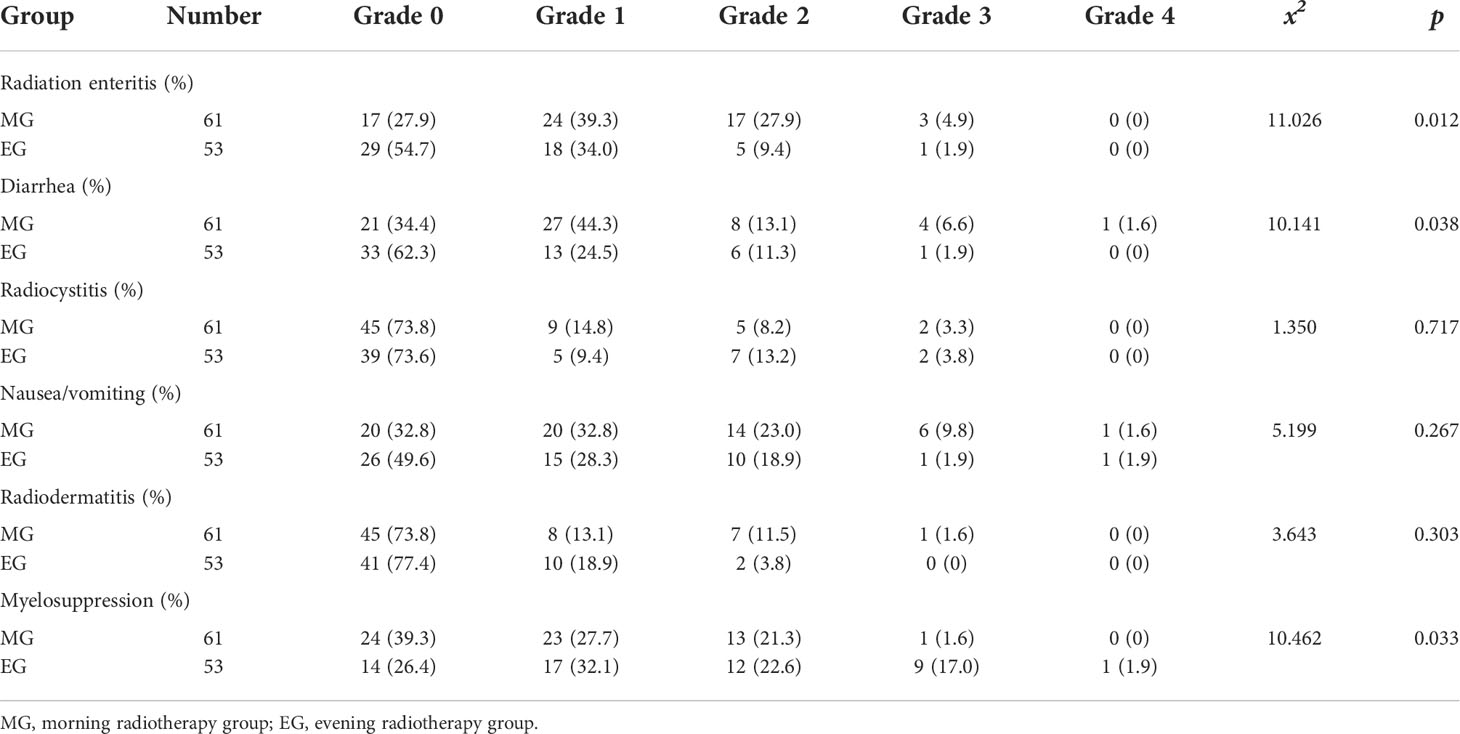
In this study, the main toxic reactions were radioactive gastrointestinal reactions and myelosuppression, and the toxicity levels were 0, I, II, III, and IV. There were significant differences in the incidence of myelosuppression and intestinal reaction between the MG and EG (p < 0.05). The MDASI score of the MG was slightly higher than that of the EG (21.64 ± 7.916 vs. 18.53 ± 4.098, p < 0.05). In terms of QOL, physical activity, functional status, and overall QOL of the MG were significantly poorer than those of the EG (p < 0.05). No other measures showed a significant difference between the groups. The results are described in Tables 5–7.
Discussion
Radiotherapy toxicity
In nature, from simple single-celled organisms to complex mammals and humans, there are certain periodic life activities. Circadian rhythm is a special internal timing mechanism with a 24-hour cycle that is produced by the body, and it can self-regulate and change from day to night (19). The growth of normal human tissues and cells is precisely regulated by the circadian rhythm. Studies have shown that the cell cycle, proliferation, and cell death are closely related to the circadian clock; thus, the disruption of the circadian rhythm is likely involved in cancer development and progression (20). Radiotherapy remains the main treatment for cervical cancer at present (2). Basic research results have confirmed that the sensitivity of different cells to radiation varies significantly depending on its cycle, and each stage of the cell cycle corresponds to different degrees of radiation sensitivity (21, 22). The sensitivity of cells to radiation varies with the cell cycle; therefore, selecting an appropriate radiotherapy time is crucial. Radiotherapy aimed at the sensitive period of tumor cells while avoiding the sensitive period of healthy tissues can achieve the maximum killing effect on cancer cells and minimize damage to healthy cells (22).
In this study, we implemented chronoradiotherapy under the condition of ethical review. For observations of acute radiation adverse reactions, we found that the incidence of radiation enteritis in the morning was higher than that in the evening (above grade II: 32.8% vs. 11.3%, p < 0.05). Diarrhea in the MG was more serious than that in the EG, and the diarrhea of grade II and above was significantly more serious in the MG than in the EG (above grade II: 21.3% vs. 13.2%, p < 0.05). Additionally, the degree of myelosuppression was more severe in the EG than in the MG (above grade II: 22.9% vs. 41.5%, p < 0.05). Chang et al. (23) randomly divided 67 patients into MG (9:00–11:00 AM) and EG (9:00–11:00 PM) groups, and results showed that the incidence of grade III–IV diarrhea in the MG and EG was 12.5% and 6.1%, respectively. In the EG, the incidence of serious hematological toxicity was significantly higher than that in the MG, which is consistent with our results. A systematic Cochrane review in 2018 included two RCTs with a total sample size of 294 patients treated with radiotherapy for cervical cancer (24). Results showed that the incidence of grade I–II diarrhea in cancer patients was lower in the EG than in MG.
Diarrhea caused by radiotherapy in the pelvic region is mainly caused by intestinal crypt cell apoptosis (25). In a study of the intestinal crypt in mice, an obvious circadian rhythm was observed in the number of apoptotic cells in the intestinal crypt during the administration of radiotherapy at different times, which indicated that radiotherapy-induced apoptosis occurs in a time-dependent manner (26, 27). Studies on the effects of radiation therapy on mice have shown that the induction of apoptosis peaks between 9:00 AM and 11:00 AM and troughs between 7:00 PM and 9:00 PM. Therefore, the occurrence of toxic reactions, such as diarrhea and mucositis, is more serious in the morning than in the evening (13). Myelosuppression and hematological toxicity are more serious in the evening after radiotherapy, which may be because proliferation and apoptosis of bone marrow cells exhibit circadian rhythm changes; indeed, apoptosis in the evening group was significantly higher than that in the morning group (28, 29).
Radiotherapy effect
Before radiotherapy, there were no significant differences in general demographic, disease, or social data between the two groups of patients. In this study, RECIST 1.1 was used to evaluate the efficacy of radiotherapy in patients with cervical cancer. After radiotherapy, the total effective rates (CR + PR) of the MG and EG were similar (93.5% vs. 96.3%, p > 0.05). Moreover, no significant differences in treatment response or disease progression were found between the MG and EG.
The results of this study are consistent with the report of Chang et al. (23) on the treatment of patients with cervical cancer by chronoradiotherapy. Chang et al. (23) randomized 67 cervical cancer patients to evaluate the efficacy of radiotherapy delivered using RECIST 1.1 in the morning and evening. Results showed that the effects were similar in the MG and the EG, and the total effective rates (CR + PR) were 100%, which was consistent with the results of our study. However, Guo et al. (30) evaluated the short-term efficacy of chronoradiotherapy using RECIST 1.1 in 25 cervical cancer patients and found that the effective rates of the MG and EG were 61.5% and 80.0%, respectively, which were significantly different. This finding is inconsistent with the results of our study. Possible reasons for this discrepancy are our sample size was too small, affecting the statistical analyses; or the efficacy of chronoradiotherapy was evaluated after the treatment, and the tumors of some patients regressed, which may have affected the evaluation results. Therefore, we plan to follow up patients over a longer period to evaluate long-term efficacy.
General condition of symptoms and QOL
The results of this study showed that the MDASI score in the MG was significantly higher than that in the EG (21.64 ± 7.916 vs. 18.53 ± 4.098, p < 0.05). Cervical cancer patients experience different degrees of symptoms during radiotherapy, including fatigue, nausea, vomiting, diarrhea, and pain (31). This may be because, during radiotherapy for cervical cancer, the deep penetration of radiation and the numerous organs in the pelvic cavity with similar anatomical positions results in normal tissues and organs being affected by radiation, inducing a series of corresponding symptoms and reactions (32). The MDASI scores were slightly higher in the MG than in the EG, which may be attributed to the higher incidence of radiation enteritis and diarrhea in the MG. Most patients experience nausea, vomiting, loss of appetite, pain, fatigue, and other symptoms (2, 33), which seriously affect the daily lives and QOL of patients, which may explain the higher total MDASI score in the MG than in the EG.
In terms of QOL, the total QOL score in the MG (63.26 ± 8.68) was lower than that in the EG (68.15 ± 10.04). In addition, the scores of physical activity and functional activity in the MG were also slightly lower than those in the EG. A previous study (34, 35) on the application of chronoradiation in patients with head and neck cancer found that the QOL score of the EG was higher than that of the MG during the first and second weeks after the start of radiotherapy, although the difference was not significant. The discrepancy between the results of the two studies may be due to the different biological rhythms and time points of radiosensitivity between the two cancer types. Patients with cervical cancer have a higher incidence of gastrointestinal reactions in the morning, whereas head and neck cancer patients have a higher incidence of oral mucosa in the evening. Alternatively, the time points for the QOL life assessments may have been inconsistent between the two studies.
Limitations
In this study, the sample size was small, and the observation time for efficacy was short. We only examined the short-term efficacy of radiotherapy. Thus, further longitudinal investigations of the long-term efficacy and toxicity of radiotherapy are needed.
Conclusion
This multicenter randomized controlled trial focused on the short-term efficacy and side effects of chronoradiotherapy in patients with cervical cancer. We verified that the efficacy of radiotherapy was similar irrespective of whether it was administered in the morning or the evening. However, toxicity and side effects differed depending on the time of radiotherapy administration. That is, more severe hematologic toxicity and greater bone marrow suppression were observed in the EG, whereas more severe gastrointestinal toxicity was observed in the MG. Post-radiation assessment revealed that the overall severity of symptoms in the MG was greater than that in the EG; moreover, the QOL of the MG was lower than that of the EG.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Medical Ethics Committee of Tianjin Medical University Cancer Institute & Hospital, China. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
Study design: WY, QWM. Clinical subject recruitment: ZBZ. Clinical data collection: CXC, LXF, XJ, YR, LXH, ZZL, WCL and LYL. Primary outcome assessor: LJQ and SAM. Data interpretation: All authors. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by the Basic Scientific Research Fund of Tianjin Medical University (general project 2020KJ144).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet (2019) 393(10167):169–82. doi: 10.1016/S0140-6736(18)32470-X
3. Arbyn M, Weiderpass E, Bruni L, de Sanjosé S, Saraiya M, Ferlay J, et al. Estimates of incidence and mortality of cervical cancer in 2018: A worldwide analysis. Lancet Glob Health (2020) 8(2):e191–203. doi: 10.1016/S2214-109X(19)30482-6
4. Marth C, Landoni F, Mahner S, McCormack M, Gonzalez MA, Colombo N, et al. Cervical cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol (2017) 28(4):72–83. doi: 10.1093/annonc/mdx220
5. Li Z, Zhang Y, Sui S, Hua Y, Zhao A, Tian X, et al. Targeting HMGB3/hTERT axis for radio resistance in cervical cancer. J Exp Clin Cancer Res (2020) 39(1):1–17. doi: 10.1186/s13046-020-01737-1
6. Shen H, Cook K, Gee HE, Hau E. Hypoxia, metabolism, and the circadian clock: New links to overcome radiation resistance in high-grade gliomas. J Exp Clin Cancer Res (2020) 39(1):129. doi: 10.1186/s13046-020-01639-2
7. Rivina L, Davoren M, Schiestl RH. Radiation-induced myeloid leukemia in murine models. Hum Genomics (2014) 8(1):13. doi: 10.1186/1479-7364-8-13
8. Radojevic MZ, Tomasevic A, Karapandzic VP, Milosavljevic N, Jankovic S, Folic M. Acute chemoradiotherapy toxicity in cervical cancer patients. Open Med (2020) 15(1):822–32. doi: 10.1515/med-2020-0222
9. Sato F, Bhawal UK, Yoshimura T, Muragaki Y. DEC1 and DEC2 crosstalk between circadian rhythm and tumor progression. J Cancer (2016) 7(2):153–9. doi: 10.7150/jca.13748
10. Nelson N, Lombardo J, Matlack L, Smith A, Hines K, Shi WY, et al. Chronoradiobiology of breast cancer: The time is now to link circadian rhythm and radiation biology. Int J Mol Sci (2022) 23(3):1331. doi: 10.3390/ijms23031331
11. Liu X, Yu R, Zhu L, Hou X, Zou K. Bidirectional regulation of circadian disturbance and inflammation in inflammatory bowel disease. Inflammation Bowel Dis (2017) 23(10):1741–51. doi: 10.1097/MIB.0000000000001265
12. Pawlik TM, Keyomarsi K. Role of cell cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol Biol Phys (2004) 59(4):928–42. doi: 10.1016/j.ijrobp.2004.03.005
13. Bermúdez GL, Blanco SA, Ramírez ZJ, Lovo E. The time for chronotherapy in radiation oncology. Front Oncol (2021) 11:687672. doi: 10.3389/fonc.2021.687672
14. Lee SI, Atri M. 2018 FIGO staging system for uterine cervical cancer: Enter cross-sectional imaging. Radiol (2019) 292(1):15–24. doi: 10.1148/radiol.2019190088
15. Armato SG, Anna KN. Revised modified response evaluation criteria in solid tumors for assessment of response in malignant pleural mesothelioma (Version 1.1). J Thorac Oncol (2018) 13(7):1012–21. doi: 10.1016/j.jtho.2018.04.034
16. Cox JD, Stetz J, Pajak TF. Toxicity criteria of the radiation therapy oncology group (RTOG) and the European organization for research and treatment of cancer (EORTC). Int J Radiat Oncol Biol Phys (1995) 31(5):1341–6. doi: 10.1016/0360-3016(95)00060-C
17. Cella DF, Tulsky DS, Gray G, Sarafian B, Linn E, Bonomi A, et al. The functional assessment of cancer therapy scale: Development and validation of the general measure. J Clin Oncol (1993) 11(3):570–9. doi: 10.1200/JCO.1993.11.3.570
18. Cleeland CS, Mendoza TR, Wang XS, Chou C, Harle MT, Morrissey M, et al. Assessing symptom distress in cancer patients: The M.D. Anderson symptom inventory. Cancer (2000) 89(7):1634–46. doi: 10.1002/1097-0142(20001001)89:7<1634:aid-cncr29>3.0.co;2-v
19. Sancar A, Van Gelder RN. Clocks, cancer, and chronochemotherapy. Science (2021) 371:6524.eabb0738. doi: 10.1126/science.abb0738
20. Shilts J, Chen G, Hughey JJ. Evidence for widespread dysregulation of circadian clock progression in human cancer. PeerJ (2018) 31:6:e4327. doi: 10.7717/peerj.4327
21. Zhang Y, Chen X, Ren P, Su Z, Cao H, Zhou J, et al. Synergistic effect of combination topotecan and chronomodulated radiation therapy on xenografted human nasopharyngeal carcinoma. Int J Radiat Oncol Biol Phys (2013) 87(2):356–62. doi: 10.1016/j.ijrobp.2013.05.047
22. Chan S, Rowbottom L, McDonald R, Bjarnason GA, Tsao M, Danjoux C, et al. Does the time of radiotherapy affect treatment outcomes? a review of the literature. Clin Oncol (2017) 29(4):231–8. doi: 10.1016/j.clon.2016.12.005
23. Chang L, Li L, Li W, Jiang M, Jv Y, Wang L, et al. Research on radiotherapy at different times of the day for inoperable cervical cancer. Int J Clin Pharmacol Ther (2016) 54(11):856–64. doi: 10.5414/CP202654
24. Lawrie TA, Green JT, Beresford M, Wedlake L, Burden S, Davidson SE, et al. Interventions to reduce acute and late adverse gastrointestinal effects of pelvic radiotherapy for primary pelvic cancers. Cochrane Database Syst Rev (2018) 1(1):CD012529. doi: 10.1002/14651858.CD012529.pub2
25. Wang L, Wang X, Zhang G, Ma Y, Zhang Q, Zheng L, et al. The impact of pelvic radiotherapy on the gut microbiome and its role in radiation-induced diarrhoea: A systematic review. Radiat Oncol (2021) 16(1):187. doi: 10.1186/s13014-021-01899-y
26. Harper E, Talbot CJ. Is it time to change radiotherapy: The dawning of chronoradiotherapy? Clin Oncol (2019) 31(5):326–35. doi: 10.1016/j.clon.2019.02.010
27. Qiu G, Yu Y, Wang Y, Wang X. The significance of probiotics in preventing radiotherapy-induced diarrhea in patients with cervical cancer: A systematic review and meta-analysis. Int J Surg (2019) 65:61–9. doi: 10.1016/j.ijsu.2019.03.015
28. Benderitter M, Reyes EH, Gigov Y, Souleau B, Huet C, Trompier F, et al. Hematopoietic recovery using multi-cytokine therapy in 8 patients presenting radiation-induced myelosuppression after radiological accidents. Radiat Res (2021) 196(6):668–79. doi: 10.1667/RADE-21-00169.1
29. Corbeau A, Kuipers SC, Boer SM, Horeweg N, Hoogeman MS, Godart J, et al. Correlations between bone marrow radiation dose and hematologic toxicity in locally advanced cervical cancer patients receiving chemoradiation with cisplatin: A systematic review. Radiother Oncol (2021) 164:128–37. doi: 10.1016/j.radonc.2021.09.009
30. Guo P, Wang H, Jiang R, Wang Z. The clinical effect study on malignant tumors with chronoradiotherapy. Biol Rhythm Res (2015) 46(2):249–55. doi: 10.1080/09291016.2014.985001
31. Chargari C, Peignaux K, Escande A, Renard S, Lafond C, Petit A, et al. Radiotherapy of cervical cancer. Cancer Radiother (2022) 26(1-2):298–308. doi: 10.1016/j.canrad.2021.11.009
32. Vande Wetering FT, Verleye L, Andreyev HJN, Maher J, Vlayen J, Pieters BR, et al. Non-surgical interventions for late rectal problems (Proctopathy) of radiotherapy in people who have received radiotherapy to the pelvis. Cochrane Database Syst Rev (2016) 4(4):CD003455. doi: 10.1002/14651858.CD003455.pub2
33. Moezian GSA, Javadinia SA, Sales SS, Fanipakdel A, Elyasi S, Karimi G. Oral silymarin formulation efficacy in management of AC-T protocol induced hepatotoxicity in breast cancer patients: A randomized, triple blind, placebo-controlled clinical trial. J Oncol Pharm Pract (2022) 28(4):827–35. doi: 10.1177/10781552211006182
34. Bjarnason GA, Mackenzie RG, Nabid A, Hodson ID, Sayed SE, Grimard L, et al. Comparison of toxicity associated with early morning versus late afternoon radiotherapy in patients with head-and-neck cancer: A prospective randomized trial of the national cancer institute of Canada clinical trials group (HN3). Int J Radiat Oncol Biol Phys (2009) 73(1):166–72. doi: 10.1016/j.ijrobp.2008.07.009
35. Salek R, Dehghani M, Mohajeri SA, Talaei A, Fanipakdel A, Javadinia SA. Amelioration of anxiety, depression, and chemotherapy related toxicity after crocin administration during chemotherapy of breast cancer: A double blind, randomized clinical trial. Phytother Res (2021) 35(9):5143–53. doi: 10.1002/ptr.7180
Keywords: radiotherapy, chronoradiotherapy, cervical cancer, radiation toxicity, radiotherapy effects
Citation: Wang Y, Qiang W-M, Li J-Q, Shen A-M, Chen X-C, Li X-F, Zhang B-Z, Xie J, Yan R, Li X-H, Zhang Z-L, Wang C-L and Li L-Y (2022) The effect of chronoradiotherapy on cervical cancer patients: A multicenter randomized controlled study. Front. Oncol. 12:1021453. doi: 10.3389/fonc.2022.1021453
Received: 17 August 2022; Accepted: 24 October 2022;
Published: 15 November 2022.
Edited by:
John Varlotto, Marshall University, United StatesReviewed by:
DeeDee Smart, Radiation Oncology Branch, Center for Cancer Research, National Cancer Institute (NIH), Bethesda, United StatesSeyed Alireza Javadinia, Sabzevar University of Medical Sciences, Iran
Copyright © 2022 Wang, Qiang, Li, Shen, Chen, Li, Zhang, Xie, Yan, Li, Zhang, Wang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wan-Min Qiang, UWlhbmd3YW5taW5Ac2luYS5jbg==
 Ying Wang
Ying Wang Wan-Min Qiang1*
Wan-Min Qiang1*