- 1Department of Thoracic Surgery, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
- 2Department of Thoracic Surgery, The First Affiliated Hospital of Shantou University Medical College, Shantou, China
- 3Research Center of Medical Sciences, Guangdong Provincial People’s Hospital, Guangdong Academy of Medical Sciences, Guangzhou, China
- 4Shantou University Medical College, Shantou, China
Background: Lymphovascular invasion (LVI) is recognized as an unfavorable prognostic factor for many solid tumors. However, its staging value has not been adequately illustrated in esophageal squamous cell carcinoma (ESCC).
Methods: The clinicopathologic relevance and prognostic impact of LVI were retrospectively analyzed in 822 patients with surgically treated ESCC. Univariate and multivariate analyses were used to determine the independent prognostic factors. Subgroup analyses stratified by pathological stages, nodal status and invasive depth were conducted using Kaplan–Meier method and log-rank test. Multiple staging models based on overall survival (OS) were constructed using Cox regression and evaluated by Harrell’s concordance index (C-index), integrated discrimination improvement (IDI), and net reclassification index (NRI).
Results: LVI was detected in 24.6% of ESCC patients, and its prevalence increased with a higher pathological stage (p < 0.001). In multivariate analysis, LVI was found to be an independent prognostic factor for OS [Hazard ratio (HR) = 1.545, 95% CI, 1.201–1.986), and was associated with unfavorable outcomes in stage I to III ESCC, regardless of nodal status and invasive depth. The staging model that incorporated LVI as an independent factor achieved the greatest improvement in accuracy (ΔC-index: 2.9%), and the greatest added value (IDI 2.8%, p < 0.01; NRI 13.7%, p < 0.05) for prediction of OS in ESCC patients.
Conclusions: LVI can facilitate further survival stratification in ESCC patients. The adoption of LVI as an independent staging factor in the current cancer staging system should be considered and further validated.
Introduction
Esophageal cancer is one of the leading causes of cancer-related-death worldwide (1). It is characterized by extensive treatment requirements, considerable decrease in quality of life, and unfavorable clinical outcomes (2). Advancement in multidisciplinary therapeutic approaches has gradually improved its prognosis and survivability (2, 3). However, an optimal and individualized treatment usually requires accurate pathological staging and prognostication of patients. The tumor–node–metastasis (TNM) classification system has been continuously updated by the American Joint Committee on Cancer (AJCC) for better prognostication and determination of treatment strategies (4, 5). Staging factors including grade and location of esophageal cancer were once incorporated into the practice of TNM classification system (4, 5); however, their prognostic significance remains controversial (6–10). Therefore, the identification and validation of more sensitive biological factors are essential to further subclassify the patients and facilitate individualized therapeutic strategies (11).
Lymphovascular invasion (LVI), an aggressive tumor histopathological feature, might increase the risk of lymphatic or hematogenous micrometastases for localized diseases (12). LVI is defined as a cluster of neoplastic cells within the lumen of lymphatic or blood vessels on tissue slides (13), and can be easily and reliably assessed microscopically. The adverse prognostic effect of LVI in various solid tumors, including those of lung cancer (14–16), breast cancer (17, 18), urothelial cancer (12, 19), gastric cancer (20), and esophageal squamous cell carcinoma (ESCC) (13, 21–25), has been evaluated and validated by many well-conducted retrospective studies. LVI was proposed as a supplementary factor for pathological nodal (pN) staging system in two prior large sample studies of ESCC (3, 22); however, only the risk of lymphatic metastasis was explored. Other studies were usually limited by their small sample sizes and thus heterogeneity of patients (23, 26), or by the inclusion of only early stage diseases (21, 24, 25). Thus, the practical value of LVI in inadequately examined.
We therefore conducted a retrospective study to characterize the prognostic effect of LVI in ESCC patients with different pathological stages, nodal status, and invasive depth. Predictive models were also constructed to evaluate the predictive accuracy and to investigate the potential utilization of LVI as an independent staging factor in ESCC patients.
Methods
Study population
A study cohort was identified from an ESCC database, which included 1,265 consecutive cases from January 2009 to October 2019 in Guangdong Provincial People’s Hospital, China. Information in this database has been maintained by a regular collection and review of the sociodemographic and clinicopathologic data from electronic medical records (EMR), and the follow-up information of individual patients was collected mostly through phone call or in our outpatient clinic. The selection criteria for patient enrollment included: (i) pathologically confirmed ESCC; (ii) surgically treated ESCC; (iii) pathological stage I to stage IVA; (iv) aged 18–80 years old; (v) no major postoperative complications such as respiratory clinicopathological information was retrieved from the ESCC database by one researcher and confirmed in the EMR system by another. Surgically treated patients who had no description of LVI status in their pathological reports were excluded from the statistical analysis (n=52). Finally, 822 patients were eligible for retrospective analysis (see Supplementary Figure S1). This study was approved by the Institutional Review Board of Guangdong Provincial People’s Hospital, with patient informed consent waived (No. GDREC2019687H).
Preoperative evaluation and surgical procedures
All patients had an Eastern Cooperative Oncology Group (ECOG) performance status score of 0–2 and had routinely undergone preoperative workup, including esophageal endoscopy, barium swallow, cardiopulmonary function evaluation, computed tomography of chest and abdomen, as well as biochemical profile of blood. Endoscopic ultrasound or whole-body flurodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) were offered to selected patients only. The surgical types included transthoracic open esophagectomy (open procedure), hybrid minimally invasive esophagectomy (hybrid procedure), or totally minimally invasive esophagectomy (TMI procedure). Surgical approaches included thoracotomy, Ivor Lewis esophagectomy and lymphadenectomy was performed in patients with a curative intent.
Histopathologic assessment
The pathological evaluation of all resected specimens was performed by one pathologist and confirmed by another according to the institutional diagnostic protocol. Differentiation between lymphatic or vascular invasion using immunohistochemical (IHC) staining was not required in our institution. Pathological reports with typical hematoxylin and eosin (H&E) stained slides on EMR were reassessed by two researchers independently. Any disagreement on LVI status was resolved by reexamining the stored paraffin-embedded specimens in the archive room. Pathological staging of all the patients was reassessed following the 8th edition of the AJCC TNM staging system (5).
Neoadjuvant and adjuvant therapies
Platinum-based neoadjuvant or adjuvant chemotherapy or chemoradiotherapy was recommended for selected patients according to the National Comprehensive Cancer Network (NCCN) guideline for esophageal cancer. Chemotherapy regimens usually included platinum plus docetaxel or 5-fluorouracil. Most patients had received at least two cycles of neoadjuvant chemotherapy before assessing the eligibility for surgery.
Patient follow-up
The patients were followed up at our outpatient clinics every 3 months after esophagectomy for the first 2 years and every 6 months for the following 3 years. Follow-up examinations included serum tumor biomarkers, chest radiography, thoracic and abdominal CT scans, abdominal ultrasound, bone scan, or a cranial MRI scan for the surveillance of recurrence or metastasis. Patients who did not comply with the follow-up plan were contacted regularly via telephone to renew their vital status. If the patient died, the date of death or any information on cancer recurrence would be collected from their family members. Patients who were lost to follow-up or still alive after the cut-off date for follow-up were classified as censored. Overall survival (OS) was defined as the time from esophagectomy to the date of death. The patient follow-up was cut off on March 31, 2020 for the final data analysis, and the median follow-up time was 53.9 months (95% CI, 50.5–57.4 months).
Statistical analysis
The categorical and ordinal variables between the groups with or without LVI were compared with the Chi-square test and Mann-Whitney U test, respectively. Data not recorded in EMR were treated as missing values in the statistical analysis. Survival curves were depicted using Kaplan-Meier method and compared by the log-rank test. A univariate Cox regression analysis was conducted with its significance level cross-validated by log-rank test. All covariates with a significance level of p < 0.15 in the univariate analysis were included in the multivariate analysis using the Cox proportional hazards model. Outcome prediction models combining different independent prognostic factors were generated using Cox regression with 1000-bootstrap resampling and compared by C-index, with a larger value indicating better predictive accuracy. The C-index was calculated using R software version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria) with the “rms” and “survcomp” packages. Subsequently, the “survIDINRI” package (https://cran.r-project.org/web/packages/survIDINRI/index.html) was used for the calculation of integrated discrimination improvement (IDI) and net reclassification index (NRI) of different modified staging systems, with 1,000 iterations for each calculation. The p-value of IDI and NRI was obtained through Z statistics (27). The cut-off point for the NRI calculation was set as 5 years. IDI and NRI can help to quantify the net added value contributed by LVI, and they represent the new metrics to supplement the C-index in the model assessment. NRI is more sensitive than C-index and easier to understand. However, NRI fails to evaluate the overall improvement across different time points, which can be overcome by calculating the IDI (28). If NRI or IDI >0, this indicates that the new model has a positive improvement of predictive value compared to the original one. A larger difference means that the new model is better. The calculation method and interpretation of IDI and NRI have been described in detail in previous studies (28, 29). Other statistical analyses were performed using SPSS 23.0 software for Windows (IBM Corp., Armonk, NY, USA), and a two-sided p value < 0.05 was considered statistically significant.
Results
Patient characteristics
The demographic and clinicopathologic characteristics of the 822 patients are summarized in Table 1. LVI was found in 24.6% of all patients (202 out of 822) and 22.0% of these patients receiving neoadjuvant therapies (24 out of 109), which was similar to the results of previous studies (24.6%–33.8%) (13, 22, 23). Patients with a more advanced pathological stage (p < 0.001) were found to have a higher prevalence of LVI. Interestingly, LVI was not associated with the histological differentiation of ESCC (p = 0.355). Otherwise, the LVI-positive and LVI-negative groups were similar regarding age, sex, body mass index (BMI), Charlson comorbidity index (CCI) and surgical approaches, with all p values > 0.05 (Table 1).
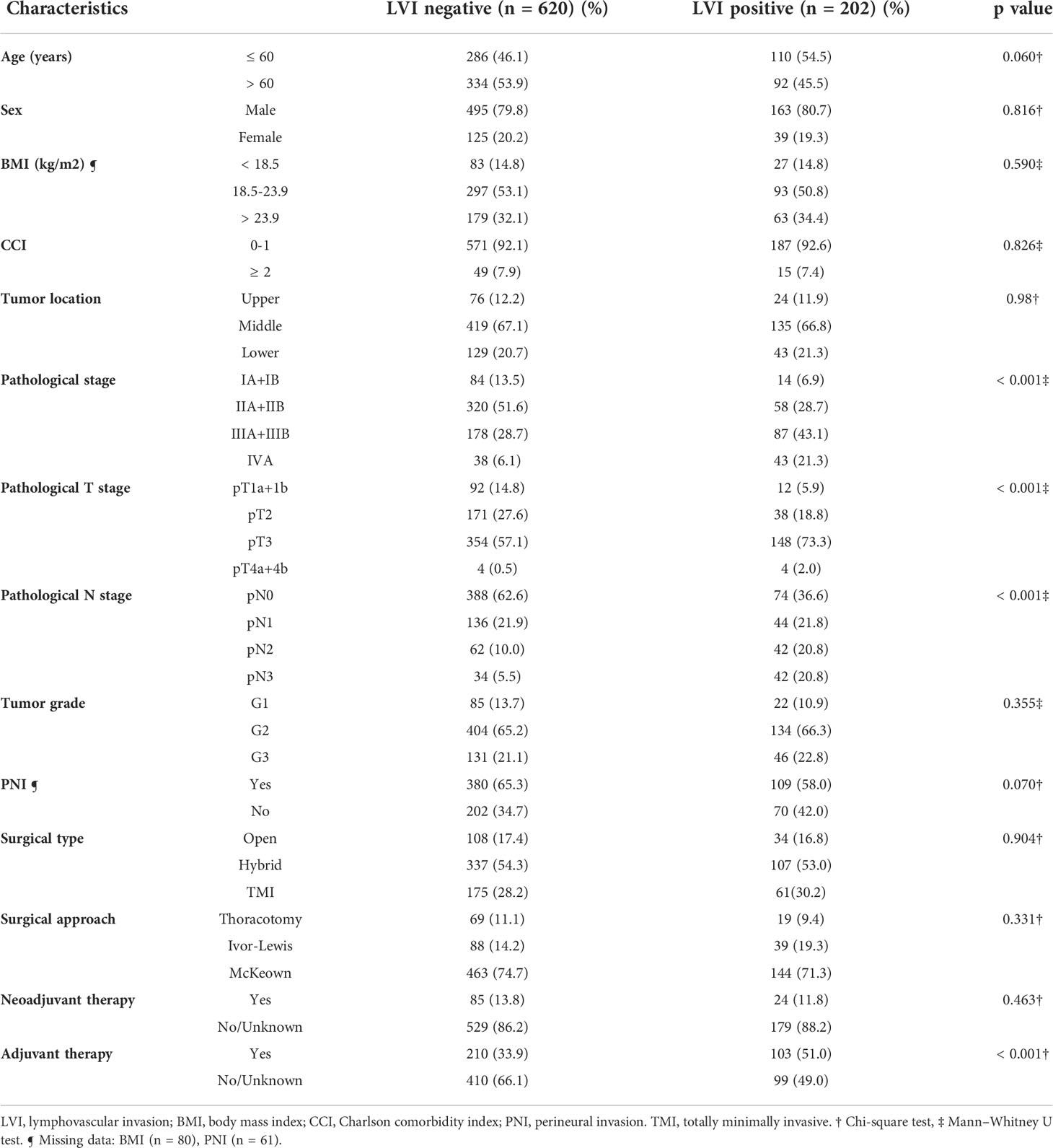
Table 1 Association of lymphovascular invasion with clinicopathologic characteristics of patients with resected esophageal squamous cell carcinoma.
Prognostic importance of LVI
In the overall study cohort, the 5-year OS rate was significantly lower in patients with LVI than in patients without LVI (36.9% vs. 58.5%, p < 0.001). Among the 109 patients receiving neoadjuvant therapies, a similar trend of unfavorable survival outcome was observed in the LVI-positive group (median OS: 60.93 months vs. 27.67 months, p=0.106), albeit not reaching a level of statistically significance (see Supplementary Figure S2). The univariate analysis suggested that sex, BMI, tumor grade, pathological T or N classification, perineural invasion (PNI), LVI, administration of neoadjuvant or adjuvant therapies were the potential prognostic candidates of ESCC (all p < 0.15, see Supplementary Table S1). In the multivariate analysis adjusted for the abovementioned covariates (Table 2), LVI was found to be an independent prognostic factors of OS [hazard ratio (HR) = 1.561, 95% CI, 1.214–2.007, p = 0.001].
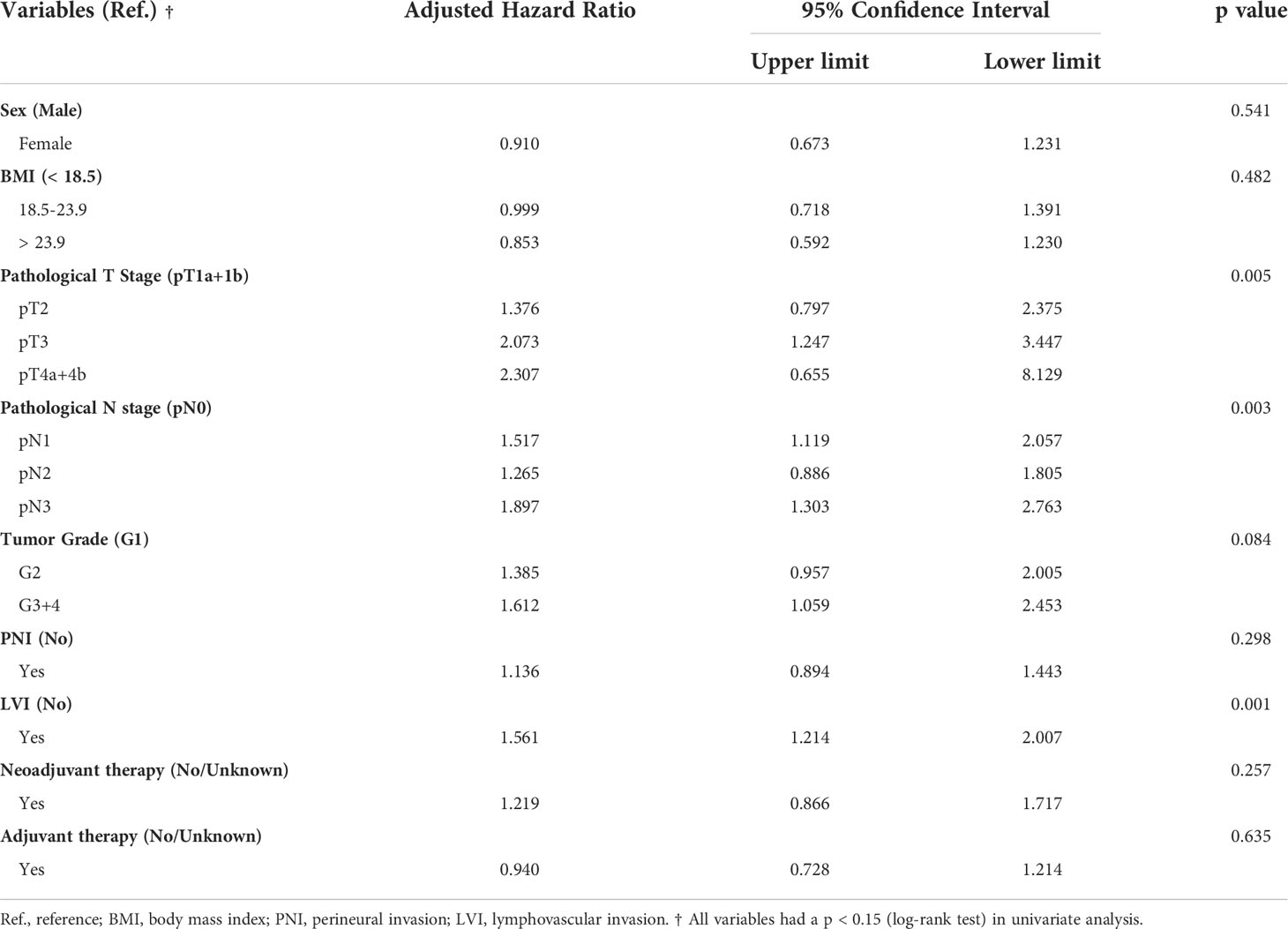
Table 2 Multivariate Cox regression analysis for overall survival in patients with resected esophageal squamous cell carcinoma.
Subgroup analysis stratified by pathological stages
A subgroup analysis was conducted to investigate the staging value of LVI based on the 8th edition of the AJCC TNM staging system (5). The OS among patients with a pathological stage I to III could be further subclassified by LVI status (Figure 1). LVI-positive patients with lower pathological stages were found to consistently achieve a similar survivorship as the patients with one stage higher (Figure 1, Table 3). For example, the 5-year OS among patients with LVI-positive stage II ESCC was 43.77%, versus 43.00% in stage III diseases (p = 0.755). Similar results were obtained when comparing the OS among patients of LVI-positive stage I with stage II (p = 0.673), and when comparing LVI-positive stage III with stage IV diseases (p = 0.585).
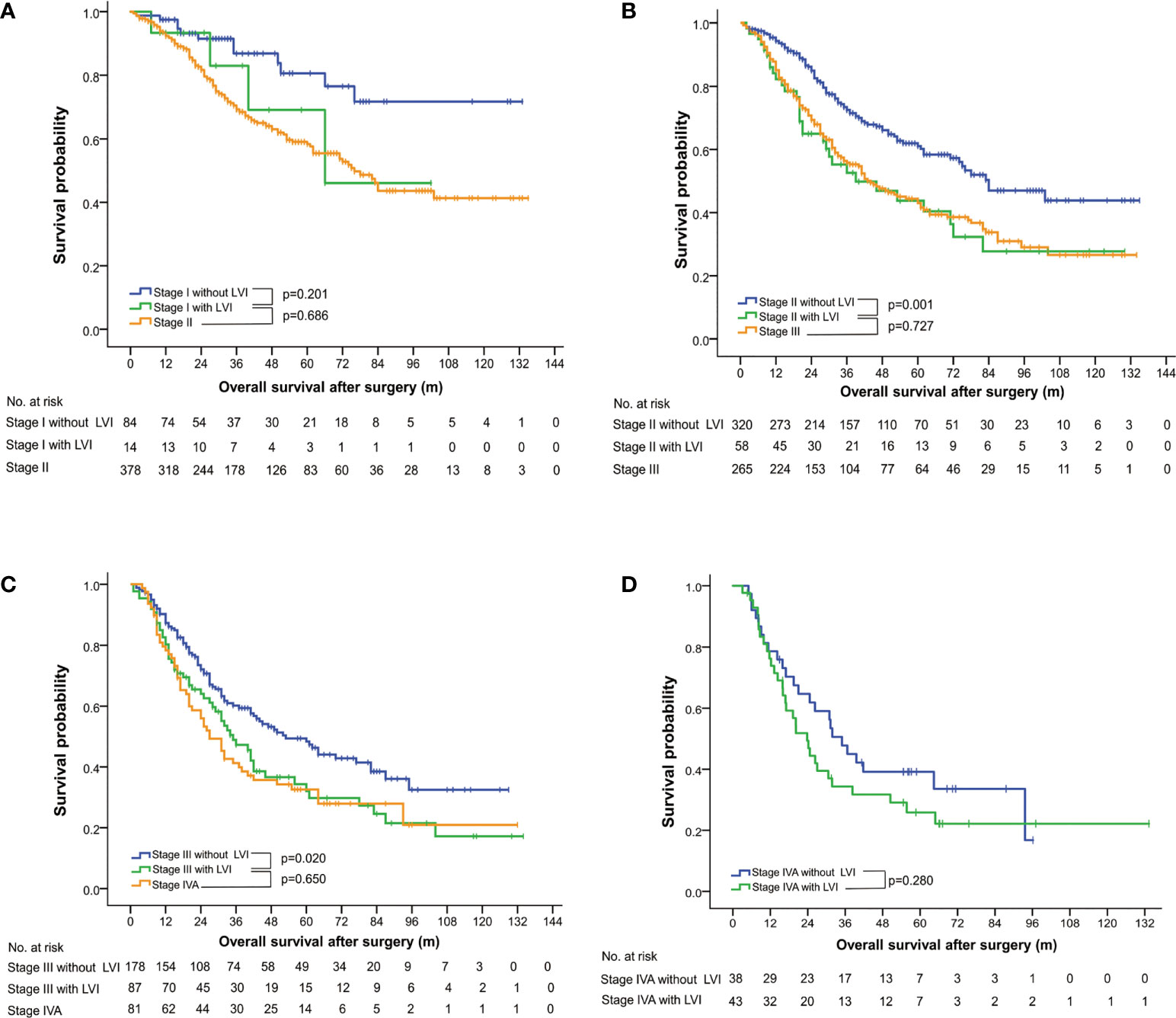
Figure 1 Overall survival curves stratified by status of lymphovascular invasion and pathological stages, based on the 8th edition of the AJCC TNM classification system for esophageal squamous cell carcinoma. LVI, lymphovascular invasion.
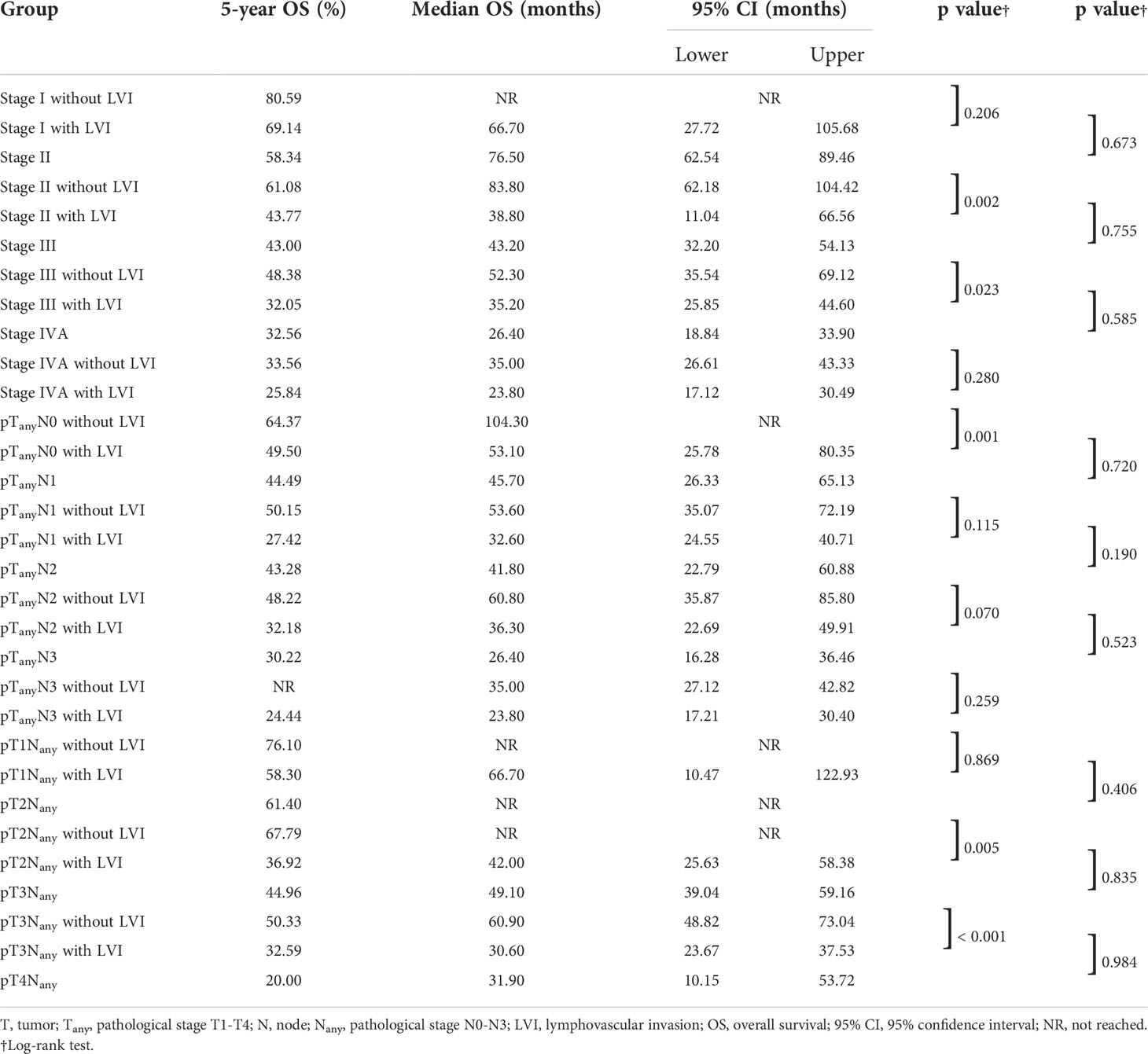
Table 3 Subgroup analysis of overall survival stratified by lymphovascular invasion, pathological stages, N classifications and T classifications.
Subgroup analysis stratified by nodal status
In the LNM-negative group, 16.0% (74 out of 462) of patients had LVI. In contrast, 35.6% (128 out of 360) of LNM-positive patients were found to have LVI (Table 1). Survival analysis of both groups suggested an unfavorable outcome of positive-LVI regardless of nodal status (Figure 2A). The 5-year OS rates in LNM-negative diseases with or without LVI were 49.5% versus 64.2% (p = 0.001). Similarly, the 5-year OS rates in LNM-positive diseases with or without LVI were 29.4% versus 47.3% (p = 0.002). It is worth noting that the LNM-negative diseases with LVI had demonstrated a similar survival outcome to LNM-positive diseases without LVI (OS: p = 0.954) (Figure 2A). However, further analysis of OS on pathological N classifications did not distinguish the subgrouping value of LVI among patients with N1 (p = 0.115), N2 (p = 0.070) and N3 (p = 0.259) diseases (Table 3, Figure 2B).
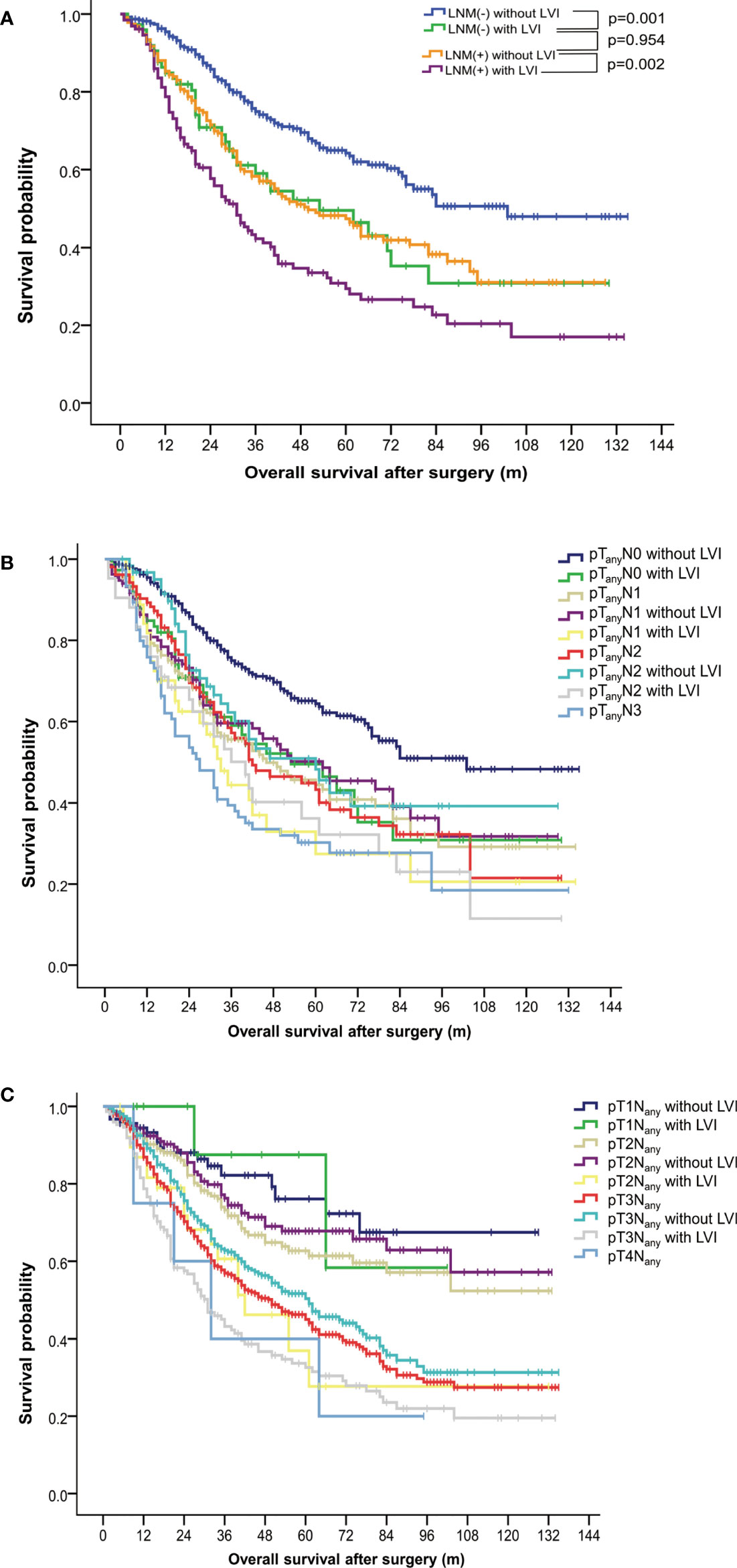
Figure 2 Subgroup analysis of overall survival in patients with esophageal squamous cell carcinoma. (A) Survival curves stratified by status of lymphovascular invasion and lymph node metastasis. (B) Survival curves stratified by status of lymphovascular invasion and pathological N classifications. (C) Survival curves stratified by status of lymphovascular invasion and pathological T classifications. LNM, lymph node metastasis; LVI, lymphovascular invasion.
Subgroup analysis stratified by invasive depth
The 5-year OS rates among patients with and without LVI were 58.30% versus 76.10% (p = 0.869), 36.92% versus 67.79% (p = 0.005), 32.59% versus 50.33% (p < 0.001) in pT1, pT2, and pT3 ESCC, respectively. ESCC with positive-LVI also demonstrated an inferior OS comparable to those with a higher pT stage (with all p > 0.05), which was also similar to the findings in a subgroup analysis of the pathological stage (Table 3, Figure 2C).
Accuracy and improvement of prognostic prediction models incorporating LVI status
To further determine the staging value of LVI, prognostic prediction models were generated using Cox regression with 1000-bootstrap resampling. The involvement of LVI status as an independent predictive variable in the TNM system (Model II) increased the C-index by 2.9% for OS (Table 4), which was superior to the direct upstaging of TNM stage (Model III, increased by 1.6%) or modification of T classification (Model IV, increased by 1.8%) and N classification (Model V, increased by 2.0%). The IDI and NRI demonstrated that Model II (OS: IDI 2.8%, p < 0.01; NRI 13.7%, p < 0.05) provided the greatest net improvement to the original TNM staging system in the prediction of OS compared with Model III, Model IV and Model V (Table 4). Internal validation of Model II by the bootstrap method supported LVI as a predictor independent of the current TNM staging system (p = 0.001, see Supplementary Table S2).
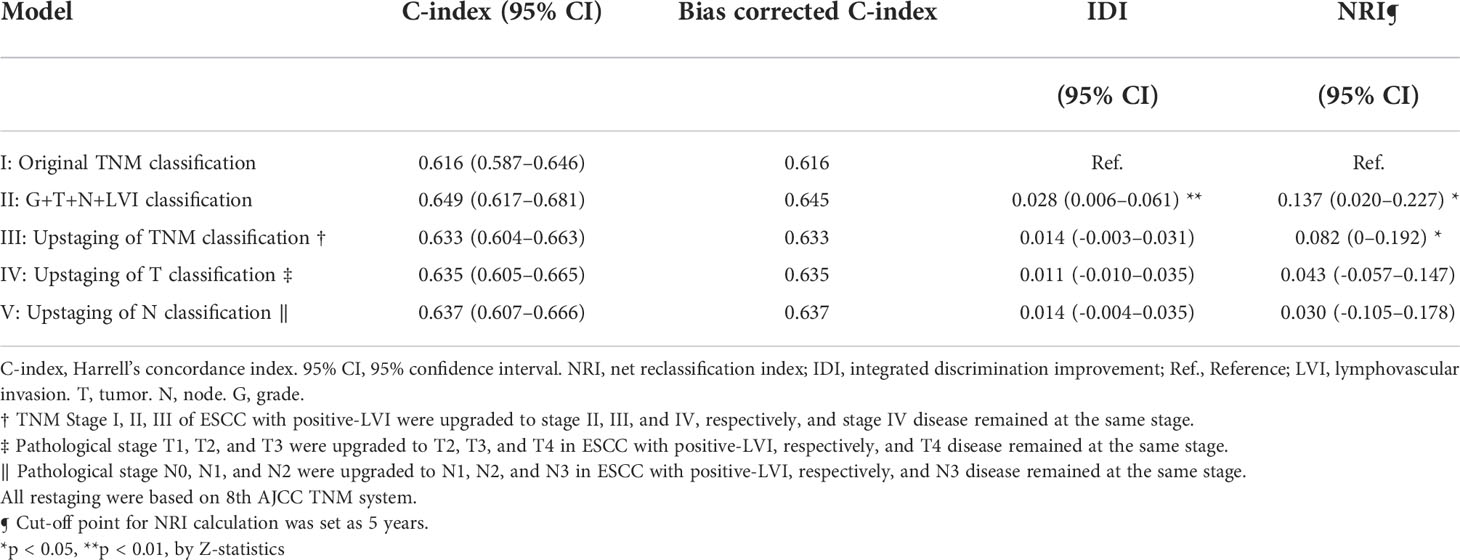
Table 4 Assessment of accuracy and improvement of clinical outcome of different predictive models based on overall survival.
Discussion
Lymphatic and hematogenous metastasis are two of the most critical mechanisms of locoregional and distant recurrence of cancer (27). Although they have been well characterized, the microscopic events of cancer metastasis have not been fully understood (28). LVI appears to be an early microscopic feature to predict regional or distant recurrence in various solid tumors (29–32). Nevertheless, its clinical significance is usually underestimated in ESCC.
In the current study, a thorough analysis of the large-scale ESCC database was performed to investigate the practical significance of LVI regarding prognostic grouping and pathological staging. The association between a more advanced stage of ESCC and a higher prevalence of LVI in our study might suggest its aggressive biological feature. In the multivariate analysis, LVI was found to be an independent predictor of survival outcome, which was in parallel to pathological T and N parameters (Table 2). In contrast, tumor grade, a controversial staging factor (6–10), was not an independent prognostic factor in our study. This indicates the higher sensitivity of LVI in prognostication and supports its possible use as a staging factor in resectable ESCC.
A thorough subgroup analysis regarding OS further validated the prognostic grouping value of LVI among ESCC patients in different pathological stages and T classifications, except in patients with stage IV diseases (Table 2, Figure 1). Interestingly, LVI was found to be an unfavorable factor for OS not only in LNM-negative ESCC, but also in LNM-positive ones (Figure 2A), which was in accordance to the findings of a previous large-scale study (22), but in conflict with the results of the other one (13). The results of our study might imply the underlying risks of hematogenous metastasis in LVI-positive patients, which was not discussed in the aforementioned study (13). A simultaneous lymphatic and vascular invasion had been found to associate with a poorer prognosis than lymphatic or vascular invasion alone (33). Therefore, differentiation between lymphatic and vascular invasion by immunohistochemical staining may facilitate further subclassification of LNM-positive patients.
Given the significant prognostic value of LVI, several researchers had proposed to upstage the N classification of ESCC in the presence of this pathological feature (13, 22). In our study, the construction of various predictive models with modified pathological stage, N parameter, or T parameter was carried out to explore the role of LVI as an upstaging indicator. We further computed the NRI and IDI for all modified staging systems, with original TNM system (Model I) serving as the control to further quantify the additional survival-prediction value attributed to LVI. As demonstrated, Model III, Model IV, and Model IV did not achieve significant improvement in the prediction of survival outcome, while Model II had the best and significant performance, which was internally validated by 1000-bootstrap resampling (Table 4, Supplementary Table S2). These results support the hypothesis that LVI increases the risk of both lymphatic and hematogenous metastasis, and even represents a more aggressive histological subtype with unknown molecular mechanisms. Therefore, direct upstaging or integration of LVI as a supplementary factor to N or T classification is not recommended in purpose of tailoring treatment strategies. However, it might be a simple way for coarse prognostication and to identify patients at elevated risk of micrometastases. Besides, the presence of LVI may also guide the clinical practice of blood test for minimal residual disease (MRD).
In the past two decades, dozens of studies regarding LVI have been conducted in esophageal cancers (13, 21–24, 26, 33, 34), and the prognostic significance of LVI in LNM-negative or superficial esophageal cancers has been well recognized. Nonetheless, only a few studies have examined its prognostic value in LNM-positive or higher staged esophageal cancers, and these have offered controversial and inconclusive results (13, 21, 22). Moreover, patients in previous studies were mostly staged by an older version of TNM staging system (13, 22). To obtain a more accurate result, our study was conducted on a large sample of ESCC patients restaged by the newest TNM system. An adverse impact of LVI was found in patients with positive node or higher stage diseases, which indicates that a closer surveillance of cancer recurrence is warranted.
LVI was found to be associated with occult lymph node metastasis in various solid tumors including esophageal cancers (29, 32, 35, 36), and therefore, LVI-positive patients might benefit from a multidisciplinary treatment (15, 20). Data on neoadjuvant and adjuvant therapies were presented in our study to address concerns about patient heterogeneity and to provide an insight into real-world practice. Although neoadjuvant chemoradiation followed by surgical resection is the standard treatment for patients with locally advanced ESCC, the number of patients with neoadjuvant therapies and positive LVI was regretfully small to allow further subgroup analysis by TNM stage, nodal status or invasive depth.
Despite the impressive prognostic effects in our study, the incorporation of LVI into the TNM staging system is more for prognostication rather than for decision-making at the current stage. To the best of our knowledge, no prospective studies have been conducted to validate LVI’s practical value. Well-designed prospective trials are warranted to advance the clinical application of LVI. Furthermore, clarifying the molecular mechanisms of LVI may help to identify potential targets for comprehensive treatment (37–42).
Our study also has several limitations. First, this was a retrospective study on a single-institution database. However, this database has been prospectively maintained. The consistency of the institutional procedures of surgery, pathological diagnosis and patient managements could also reduce potential confounding effects. Second, the administration of adjuvant therapies was not balanced between LVI-positive and LVI-negative groups in our study, which might have further complicated the heterogeneity of patients and influence the survival outcomes. Additionally, the number of stage I and pT1 ESCC patients was small in this study, and these results should be interpreted cautiously.
In conclusion, LVI can help with further survival stratification and increase the accuracy of prognostic prediction of the current TNM staging system. Incorporation of LVI as an independent factor into the staging system should be considered and validated by prospective multi-center clinical trials.
Data availability statement
The raw data supporting the conclusions of this article can be shared per specific institutional review board (IRB) requirements. Upon reasonable request to each respective author/institution, a data sharing agreement can be initiated between the interested parties and the clinical institution following institution-specific guidelines.
Ethics statement
The studies involving human participants were reviewed and approved by Institutional Review Board of Guangdong Provincial People’s Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
I) Study design: WZ, GQ, YT (II) Data collection: WZ, HW, SH (III) Statistical analysis: WZ, YT(IV) Data interpretation: WZ, RC, XB (V)Literature search: WZ, ZZ, JW (VI) Manuscript writing: All authors (VII) Final approval of manuscript: All authors.
Funding
This study was funded by the Science and Technology Program of Guangzhou, China (202206010103); and Natural Science Foundation of Guangdong Province (2022A1515012469). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1018827/full#supplementary-material
References
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin (2018) 68:394–424. doi: 10.3322/caac.21492
2. Lagergren J, Smyth E, Cunningham D, Lagergren P. Oesophageal cancer. Lancet (2017) 390:2383–96. doi: 10.1016/S0140-6736(17)31462-9
3. Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med (2014) 371:2499–509. doi: 10.1056/NEJMra1314530
4. Rice TW, Blackstone EH, Rusch VW. 7th edition of the AJCC cancer staging manual: Esophagus and esophagogastric junction. Ann Surg Oncol (2010) 17:1721–4. doi: 10.1245/s10434-010-1024-1
5. Rice TW, Patil DT, Blackstone EH. 8th edition AJCC/UICC staging of cancers of the esophagus and esophagogastric junction: application to clinical practice. Ann Cardiothorac Surg (2017) 6:119–30. doi: 10.21037/acs.2017.03.14
6. Hsu PK, Wu YC, Chou TY, Huang CS, Hsu WH. Comparison of the 6th and 7th editions of the American joint committee on cancer tumor-node-metastasis staging system in patients with resected esophageal carcinoma. Ann Thorac Surg (2010) 89:1024–31. doi: 10.1016/j.athoracsur.2010.01.017
7. Situ D, Wei W, Lin P, Long H, Zhang L, Fu J, et al. Do tumor grade and location affect survival in esophageal squamous cell carcinoma? Survival analysis of 302 cases of pT3N0M0 esophageal squamous cell carcinoma. Ann Surg Oncol (2013) 20:580–5. doi: 10.1245/s10434-012-2656-0
8. Tan Z, Chen Y, Ma G, Meng Y, Fu J, Zhang L, et al. Validation of the 7th edition American joint committee on cancer staging system for esophageal squamous cell carcinoma. Thorac Cancer (2013) 4:410–5. doi: 10.1111/1759-7714.12039
9. Wang Q, Zhang W, Liu X, Zhang X, He J, Feng Q, et al. Prognosis of esophageal squamous cell carcinoma patients with preoperative radiotherapy: Comparison of different cancer staging systems. Thorac Cancer (2014) 5:204–10. doi: 10.1111/1759-7714.12079
10. Zhang D, Zheng Y, Wang Z, Huang Q, Cao X, Wang F, et al. Comparison of the 7th and proposed 8th editions of the AJCC/UICC TNM staging system for esophageal squamous cell carcinoma underwent radical surgery. Eur J Surg Oncol (2017) 43:1949–55. doi: 10.1016/j.ejso.2017.06.005
11. Amin MB, Greene FL, Edge SB, Compton CC, Gershenwald JE, Brookland RK, et al. The eighth edition AJCC cancer staging manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin (2017) 67:93–9. doi: 10.3322/caac.21388
12. Kikuchi E, Margulis V, Karakiewicz PI, Roscigno M, Mikami S, Lotan Y, et al. Lymphovascular invasion predicts clinical outcomes in patients with node-negative upper tract urothelial carcinoma. J Clin Oncol (2009) 27:612–8. doi: 10.1200/JCO.2008.17.2361
13. Huang Q, Luo K, Chen C, Wang G, Jin J, Kong M, et al. Identification and validation of lymphovascular invasion as a prognostic and staging factor in node-negative esophageal squamous cell carcinoma. J Thorac Oncol (2016) 11:583–92. doi: 10.1016/j.jtho.2015.12.109
14. Okiror L, Harling L, Toufektzian L, King J, Routledge T, Harrison-Phipps K, et al. Prognostic factors including lymphovascular invasion on survival for resected non-small cell lung cancer. J Thorac Cardiovasc Surg (2018) 156:785–93. doi: 10.1016/j.jtcvs.2018.02.108
15. Wang S, Xu J, Wang R, Qian F, Yang W, Qiao R, et al. Adjuvant chemotherapy may improve prognosis after resection of stage I lung cancer with lymphovascular invasion. J Thorac Cardiovasc Surg (2018) 156:2006–15.e2002. doi: 10.1016/j.jtcvs.2018.06.034
16. Higgins KA, Chino JP, Ready N, D'Amico TA, Berry MF, Sporn T. Lymphovascular invasion in non-small-cell lung cancer: Implications for staging and adjuvant therapy. J Thorac Oncol (2012) 7:1141–7. doi: 10.1097/JTO.0b013e3182519a42
17. Zhang S, Zhang D, Gong M, Wen L, Liao C, Zou L. High lymphatic vessel density and presence of lymphovascular invasion both predict poor prognosis in breast cancer. BMC Cancer (2017) 17:335. doi: 10.1186/s12885-017-3338-x
18. Liu YL, Saraf A, Lee SM, Zhong X, Hibshoosh H, Kalinsky K, et al. Lymphovascular invasion is an independent predictor of survival in breast cancer after neoadjuvant chemotherapy. Breast Cancer Res Treat (2016) 157:555–64. doi: 10.1007/s10549-016-3837-5
19. Novara G, Matsumoto K, Kassouf W, Walton TJ, Fritsche HM, Bastian PJ, et al. Prognostic role of lymphovascular invasion in patients with urothelial carcinoma of the upper urinary tract: an international validation study. Eur Urol (2010) 57:1064–71. doi: 10.1016/j.eururo.2009.12.029
20. Lu J, Dai Y, Xie JW, Wang JB, Lin JX, Chen QY, et al. Combination of lymphovascular invasion and the AJCC TNM staging system improves prediction of prognosis in N0 stage gastric cancer: Results from a high-volume institution. BMC Cancer (2019) 19:216. doi: 10.1186/s12885-019-5416-8
21. Oguma J, Ozawa S, Kazuno A, Yamamoto M, Ninomiya Y, Yatabe K, et al. Prognostic impact of lymphovascular invasion in lymph node-negative superficial esophageal squamous cell carcinoma. Dis Esophagus (2019) 32(11):doz001. doi: 10.1093/dote/doz001
22. Wang Z, Chen P, Wang F, Lin L, Liu S. Lymphovascular invasion as an independent prognostic indicator in radically resected thoracic esophageal squamous cell carcinoma. Thorac Cancer (2019) 10:150–5. doi: 10.1111/1759-7714.12922
23. Gu YM, Yang YS, Hu WP, Wang WP, Yuan Y, Chen LQ. Prognostic value of lymphovascular invasion in patients with esophageal squamous cell carcinoma. Ann Transl Med (2019) 7:256. doi: 10.21037/atm.2019.05.23
24. Hsu CP, Chuang CY, Hsu PK, Chien LI, Lin CH, Yeh YC, et al. Lymphovascular invasion as the major prognostic factor in node-negative esophageal cancer after primary esophagectomy. J Gastrointest Surg (2020) 24:1459–68. doi: 10.1007/s11605-019-04310-0
25. Cen P, Hofstetter WL, Correa AM, Wu TT, Lee JH, Ross WA. Lymphovascular invasion as a tool to further subclassify T1b esophageal adenocarcinoma. Cancer (2008) 112:1020–7. doi: 10.1002/cncr.23265
26. Lagarde SM, Phillips AW, Navidi M, Disep B, Immanuel A, Griffin SM. The presence of lymphovascular and perineural infiltration after neoadjuvant therapy and oesophagectomy identifies patients at high risk for recurrence. Br J Cancer (2015) 113:1427–33. doi: 10.1038/bjc.2015.354
27. Uno H, Tian L, Cai T, Kohane IS, Wei LJ. A unified inference procedure for a class of measures to assess improvement in risk prediction systems with survival data. Stat Med (2013) 32:2430–42. doi: 10.1002/sim.5647
28. Zhou ZR, Wang WW, Li Y, Jin KR, Wang XY, Wang ZW, et al. In-depth mining of clinical data: The construction of clinical prediction model with r. Ann Transl Med (2019) 7:796. doi: 10.21037/atm.2019.08.63
29. Deng J, Zhao M, Wang T, She Y, Wu J, E H. A modified T categorization for part-solid lesions in Chinese patients with clinical stage I non-small cell lung cancer. Lung Cancer (2020) 145:33–9. doi: 10.1016/j.lungcan.2020.04.028
31. Lambert AW, Pattabiraman DR, Weinberg RA. Emerging biological principles of metastasis. Cell (2017) 168:670–91. doi: 10.1016/j.cell.2016.11.037
32. Han G, Lim D, Leitao MM Jr, Abu-Rustum NR, Soslow RA. Histological features associated with occult lymph node metastasis in FIGO clinical stage I, grade I endometrioid carcinoma. Histopathology (2014) 64:389–98. doi: 10.1111/his.12254
33. Hsu PK, Chien LI, Wang LC, Chou TY, Taipei Veterans General Hospital Esophageal Cancer P. Lymphovascular invasion and extracapsular invasion are risk factors for distant recurrence after preoperative chemoradiotherapy and oesophagectomy in patients with oesophageal squamous cell carcinoma. Eur J Cardiothorac Surg (2017) 51:1188–94. doi: 10.1093/ejcts/ezx029
34. Sung SY, Kwak YK, Lee SW, Jo IY, Park JK, Kim KS, et al. Lymphovascular invasion increases the risk of nodal and distant recurrence in node-negative stage I-IIA non-Small-Cell lung cancer. Oncology (2018) 95:156–62. doi: 10.1159/000488859
35. Lotan Y, Gupta A, Shariat SF, Palapattu GS, Vazina A, Karakiewicz PI, et al. Lymphovascular invasion is independently associated with overall survival, cause-specific survival, and local and distant recurrence in patients with negative lymph nodes at radical cystectomy. J Clin Oncol (2005) 23:6533–9. doi: 10.1200/JCO.2005.05.516
36. Zhang H, Chen X, Wang S, Fan J, Lu L. Poorer prognosis associated with simultaneous lymphatic and vascular invasion in patients with squamous carcinoma of the thoracic oesophagus. Eur J Cardiothorac Surg (2017) 52:378–84. doi: 10.1093/ejcts/ezx081
37. Wang A, Tan Y, Geng X, Chen X, Wang S. Lymphovascular invasion as a poor prognostic indicator in thoracic esophageal carcinoma: A systematic review and meta-analysis. Dis Esophagus (2019) 32(2). doi: 10.1093/dote/doy083
38. Goldsmith B, Baumann BC, He J, Tucker K, Bekelman J, Deville C, et al. Occult pelvic lymph node involvement in bladder cancer: Implications for definitive radiation. Int J Radiat Oncol Biol Phys (2014) 88:603–10. doi: 10.1016/j.ijrobp.2013.11.211
39. Semenkovich TR, Yan Y, Subramanian M, et al. A clinical nomogram for predicting node-positive disease in esophageal cancer. Ann Surg (2020) 273:e214–21. doi: 10.1097/SLA.0000000000003450
40. Klahan S, Wong HS, Tu SH, Chou WH, Zhang YF, Ho TF, et al. Identification of genes and pathways related to lymphovascular invasion in breast cancer patients: A bioinformatics analysis of gene expression profiles. Tumour Biol (2017) 39:1010428317705573. doi: 10.1177/1010428317705573
41. Mohamed HT, El-Husseiny N, El-Ghonaimy EA, Ibrahim SA, Bazzi ZA, Cavallo-Medved D, et al. IL-10 correlates with the expression of carboxypeptidase B2 and lymphovascular invasion in inflammatory breast cancer: The potential role of tumor infiltrated macrophages. Curr Probl Cancer (2018) 42:215–30. doi: 10.1016/j.currproblcancer.2018.01.009
Keywords: esophageal cancer, lymphovascular invasion, staging, prognosis, pathology
Citation: Zhuang W, Wu H, Chen R, Ben X, Huang S, Zhou Z, Wu J, Tang Y and Qiao G (2022) The staging performance of a modified tumor-node-metastasis staging system incorporated with lymphovascular invasion in patients with esophageal squamous cell carcinoma. Front. Oncol. 12:1018827. doi: 10.3389/fonc.2022.1018827
Received: 14 August 2022; Accepted: 07 September 2022;
Published: 13 October 2022.
Edited by:
Mingzhou Guo, People’s Liberation Army General Hospital, ChinaReviewed by:
Hanlu Zhang, Sichuan University, ChinaFernando A.M. Herbella, Federal University of São Paulo, Brazil
Copyright © 2022 Zhuang, Wu, Chen, Ben, Huang, Zhou, Wu, Tang and Qiao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Guibin Qiao, Z3VpYmlucWlhb0AxMjYuY29t; Yong Tang, eXd6MTExMkBzaW5hLmNvbQ==
†ORCID:Weitao Zhuang, orcid.org/0000-0002-8679-4109
Guibin Qiao, orcid.org/0000-0001-9200-9317
 Weitao Zhuang
Weitao Zhuang Hansheng Wu
Hansheng Wu Rixin Chen1,3
Rixin Chen1,3 Shujie Huang
Shujie Huang Yong Tang
Yong Tang Guibin Qiao
Guibin Qiao