- 1Department of Gastroenterology, Beijing Key Laboratory of Functional Gastrointestinal Disorders Diagnosis and Treatment of Traditional Chinese Medicine, Wangjing Hospital, China Academy of Chinese Medical Sciences, Beijing, China
- 2Department of Internal Medicine, MetroHealth Medical Center/Case Western Reserve University, Cleveland, OH, United States
- 3Department of Clinical Medicine, Chengdu University of Traditional Chinese Medicine, Chengdu, China
- 4Department of Gastroenterology and Hepatology, MetroHealth Medical Center/Case Western Reserve University, Cleveland, OH, United States
Background and aim: Patients with gastric intestinal metaplasia (IM) are at increased risk of gastric cancer (GC). The endoscopic grading of gastric intestinal metaplasia (EGGIM) with high-definition endoscopes has shown the potential to facilitate GC risk stratification. However, a comprehensive review and meta-analysis of published articles are lacking. We conducted a meta-analysis to access the value of EGGIM in the assessment of histological IM.
Materials: Studies were selected from PubMed, Medline, Embase, and Cochrane (last selection, Jun 2022). We extracted relevant data to calculate the accuracy of EGGIM compared with the operative link of gastric intestinal metaplasia (OLGIM) and to calculate pooled odds ratio (OR) with a 95% confidence interval (CI) assessing GC risk with different grading.
Results: Four diagnostic studies and three case-control clinical trials were included in the analysis, which included 665 patients and 738 patients, respectively. Compared with OLGIM III/IV, EGGIM(5-10) had a pooled sensitivity and specificity of 0.92(95%CI 0.86-0.96) and 0.90(95%CI 0.88-0.93), and the area under the curve(AUC) was 0.9702. In assessing early GC, the pooled OR of patients with EGGIM(5-10) was 7.46(95%CI 3.41-16.31) compared with that of EGGIM(0-4).
Conclusions: EGGIM is highly consistent with OLGIM, and patients with EGGIM(5-10) are at a higher risk for early GC. Some heterogeneity in the current research suggests that we need to carry out more strict control of confounding factors.
Systematic Review Registration: [https://www.crd.york.ac.uk/PROSPERO/display_record.php?RecordID=248691], (Prospero registration number: 248691)
Introduction
Gastric cancer (GC) is the fifth most commonly diagnosed cancer worldwide and the fourth most common cause of cancer-related death (1). More than 95% of GC are adenocarcinomas (2). According to Lauren’s classification, gastric adenocarcinomas can be classified into diffuse and intestinal types based on histology (3). The intestinal type is the most common type of gastric adenocarcinoma (4), which develops through a multistep process from gastritis, atrophy, intestinal metaplasia (IM), dysplasia to intestinal-type GC (5). Among those steps, IM is widely recognized as a precancerous stomach mucosal lesion, and patients with IM are at increased risk for intestinal-type GC.
Operative Link on Gastric Intestinal Metaplasia (OLGIM) is a grading standard to risk stratify IM, which involves five biopsy specimens: two from the antrum two from the corpus, and one from the angle of the stomach. Patients with OLGIM stage III-IV are at an increased risk of early gastric neoplasia (HR 20.7; 95%CI 5.04 to 85.6) and may develop early gastric neoplasia within two years (6). A meta-analysis that included three case-control studies reported a 3.99-fold odds ratio(95%CI 3.05 to 5.21) for GC of OLGIM stage III-IV (7). Although studies have supported that OLGIM can effectively risk-stratify GC (8–10), there are still some limitations. There are chances that biopsies may miss certain gastric mucosal lesions because they can present multifocally. Furthermore, the histopathology risk stratification tool can bring an extra burden to the healthcare system (11).
In recent years, image-enhanced endoscopy (IEE), including narrow-band imaging (NBI), linked color imaging (LCI), and blue laser imaging (BLI), has improved the performance of endoscopy (12). IEE can present a better characterization of the mucosal pattern, which is more accurate for endoscopists to make diagnoses based on images. Above this foundation, the endoscopic grading of gastric intestinal metaplasia (EGGIM) score system using IEE technology has been proposed to assess the GC risk based on endoscopic visualization of IM, which shows high concordance with OLGIM. EGGIM is performed by endoscopic evaluation of greater and lesser curvature of the antrum, angulus, and greater and lesser curvature of corpus. Each area was scored according to the extent of intestinal metaplasia (0, no intestinal metaplasia; 1, an area less than or equal to 30%; 2, a size greater than 30%), with a total score of 10 (8). Several studies have shown its potential to facilitate GC risk stratification. However, a comprehensive review and meta-analysis of published articles are lacking.
In this study, we conducted a systematic review and meta-analysis to explore the value of the EGGIM system in the assessment of histological IM and the risk classification of early gastric cancer.
Materials and methods
Study design
We performed a systematic search for diagnostic studies and case-control studies that evaluate the diagnostic accuracy of the EGGIM system using the OLGIM system or GC incidence as a gold standard. The protocol for this study has previously been registered with PROSPERO (registration no. 248691). The protocol followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (13) guidelines.
Search strategy
Four scientific databases (PubMed, Medline, Embase, and Cochrane) were searched by two researchers (WL and QXL) independently from their inception through Oct 14, 2021. Eligible articles published after this date were added manually. The last elicitation date was Jun 25, 2022. The references of identified articles were also checked to retrieve potential missed articles. The detailed search strategy is shown in Supplementary Materials 1. Variables were combined with “Endoscopic grading of gastric intestinal metaplasia” or “EGGIM”. Titles and abstracts were read to exclude irrelevant records. Articles not excluded were read through by two reviewers. Disagreements were resolved with a third reviewer (DSJ).
Inclusion/exclusion criteria
The inclusion criteria were as follows: 1) Diagnostic studies whose primary or secondary outcome was the evaluation of the accuracy of EGGIM using OLGIM as a gold standard, or case-control studies using EGGIM to stratify GC risk. 2) True positive (TP), true negative (TN), false positive (FP), false negative (FN), or the number of GC events were included in the paper. 3) The papers were published in English. We excluded study types, including letters, reviews, expert opinions, study protocols, animal studies, preclinical trials, and studies with fewer than 10 cases.
Data extraction and quality evaluation
Two reviewers (YY and DSJ) read the included articles and extracted data independently. The following variables were collected: the first author, publication year, country, study period, sample size, gender, the experience of endoscopists, endoscopic technology, and details of the measurement data. All collected data were double-checked by a third reviewer (FSS). Two reviewers(FSS and WL) scored the included diagnostic studies independently for risk of basis according to Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) (14) and scored the included case-control studies independently for risk of basis according to The Newcastle–Ottawa Scale (NOS) (15). Disagreements were resolved with a third reviewer(WW).
Data synthesis and statistical analysis
Meta-DiSc 1.4 and RevMan 5.4.1 (Cochrane, London, UK) were used to analyze the data. Heterogeneity was assessed with Cochrane’s Q test and Higgins’s I2 statistics. A fixed-effects model was used when heterogeneity was absent (I2 <50% or p > 0.1) and a random-effects model was used if heterogeneity existed (50% <I2 <80% or p < 0.05). When heterogeneity was substantial (I2 >80% or p < 0.01), a descriptive analysis was used, and subgroup analyses implement for different image-enhanced technology when the necessary data were available. All P-values were two-tailed, and P values < 0.05 were deemed to be statistically significant.
Results
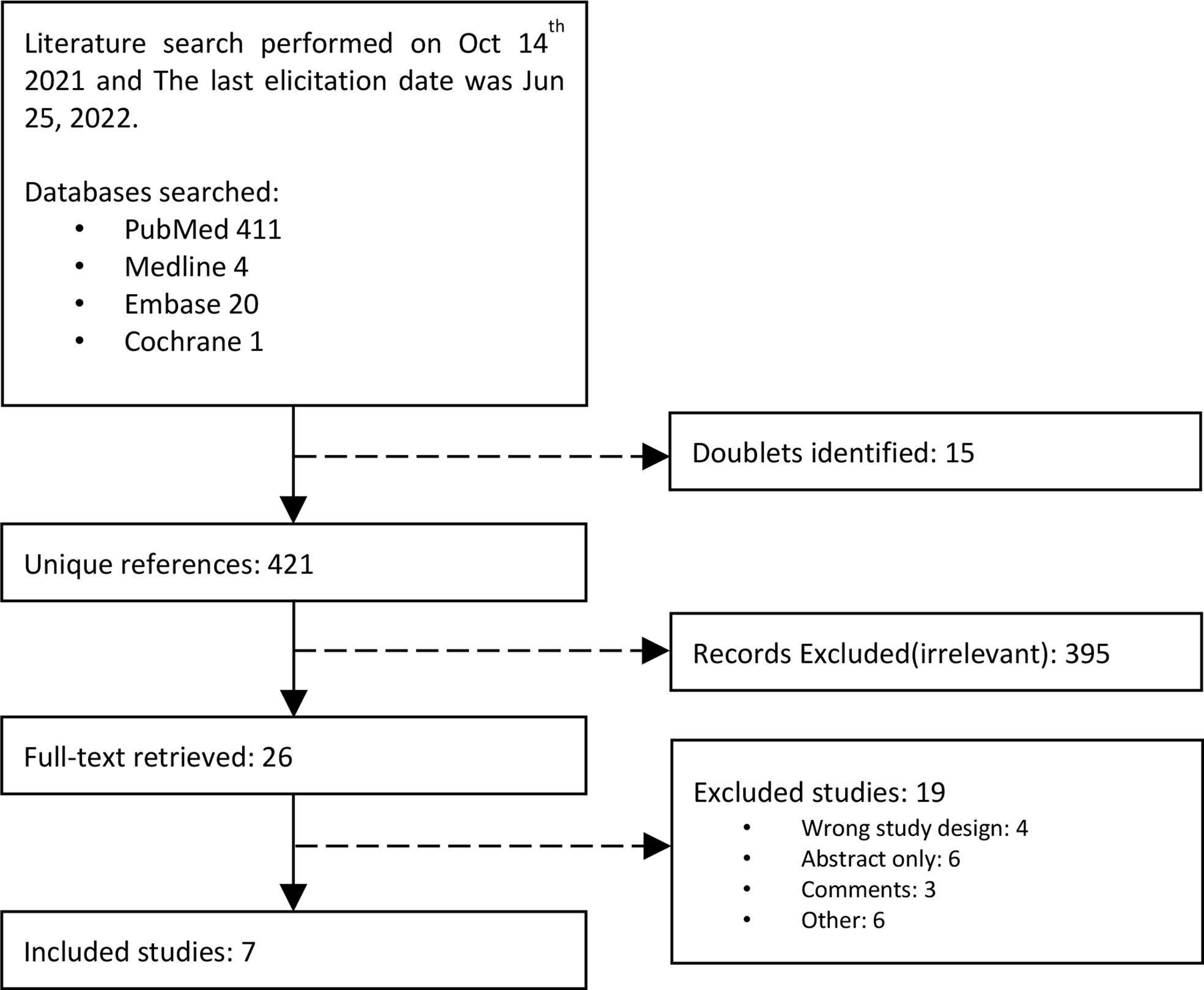
We identified a total of 435 studies. Four diagnostic studies with 665 patients (8, 10, 16, 17) and three case-control studies with 728 patients met the selection criteria (9, 18, 19) (Figure 1).
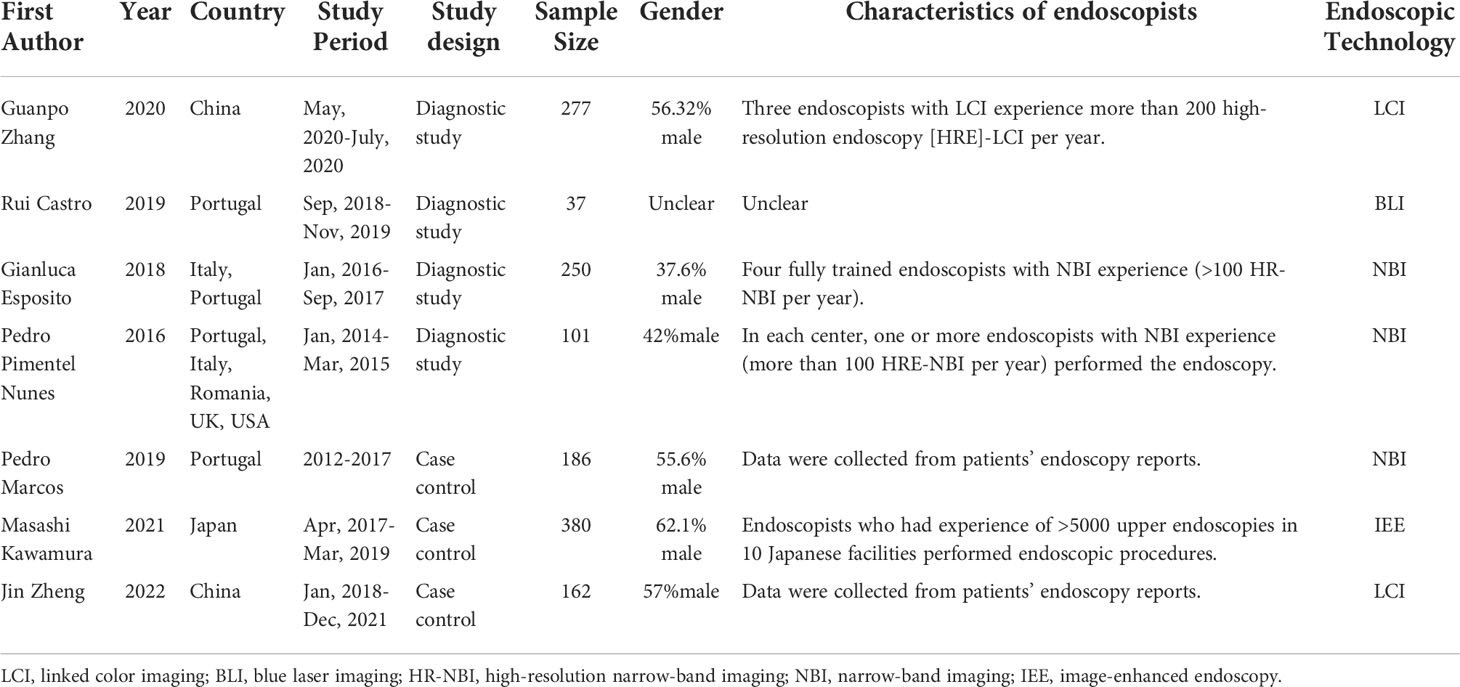
Table 1 summarizes the characteristics of the studies included in the meta-analysis. Two of the diagnostic studies compared NBI-assisted EGGIM with OLGIM and the other two comparisons were made LCI and BLI respectively. One case-control study compared NBI-assisted EGGIM with OLGIM to classify GC and non-GC patients; one used LCI and one used multiple IEE technologies risks stratify early gastric neoplasia. Most of the trials were conducted in Portugal, of which two were multinational studies and the remaining three were conducted in China and Japan. Four studies stipulate endoscopists’ experience. Two studies required endoscopists who have operated more than 100 NBI per year, one study required endoscopists who performed 200 LCI per year, and the other study required endoscopists who have experience in performing more than 5000 upper endoscopies.
Quality assessment
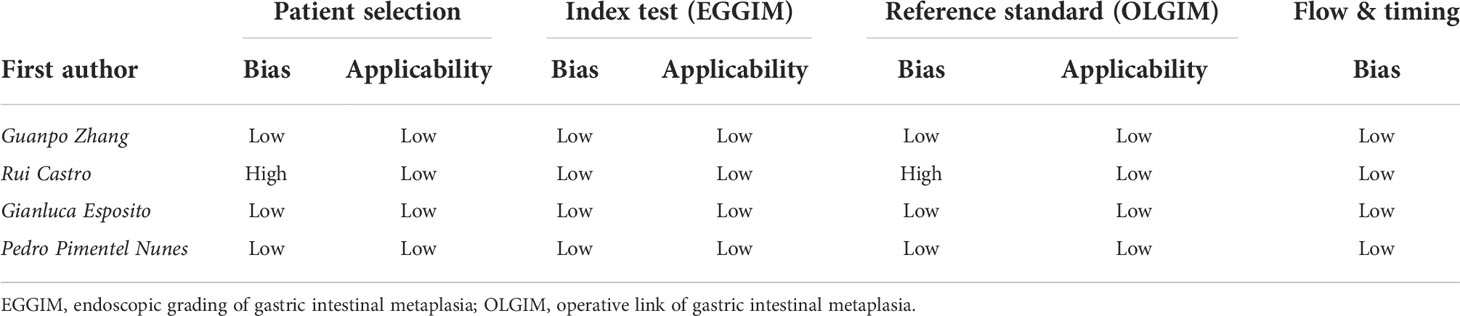
The assessment of the risk of bias and applicability concerns for the four diagnostic studies are presented in Table 2. The survey conducted by Rui Castro et al. (17) was determined to have a high risk of bias in patient selection and reference standards since they failed to enroll consecutive patients and to include all cases in the analysis. The remaining studies were deemed to have a low risk of bias and applicability concern because the cases were included consecutively, EGGIM was graded before the pathological examination, and the pathologists were blinded when determining the gold standard OLGIM.
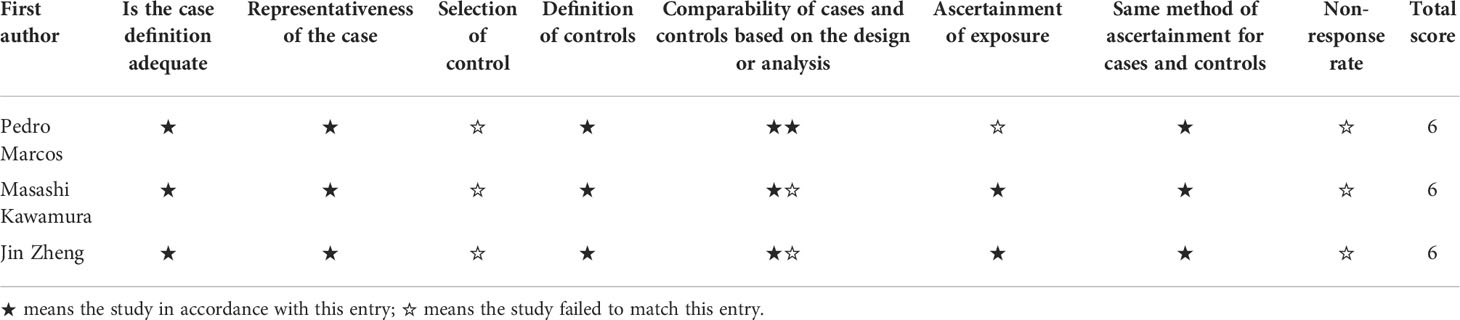
According to the NOS assessment, three case-control studies (9, 18, 19) obtained 6 stars, which represents acceptable study quality shown in Table 3.
EGGIM compared to OLGIM
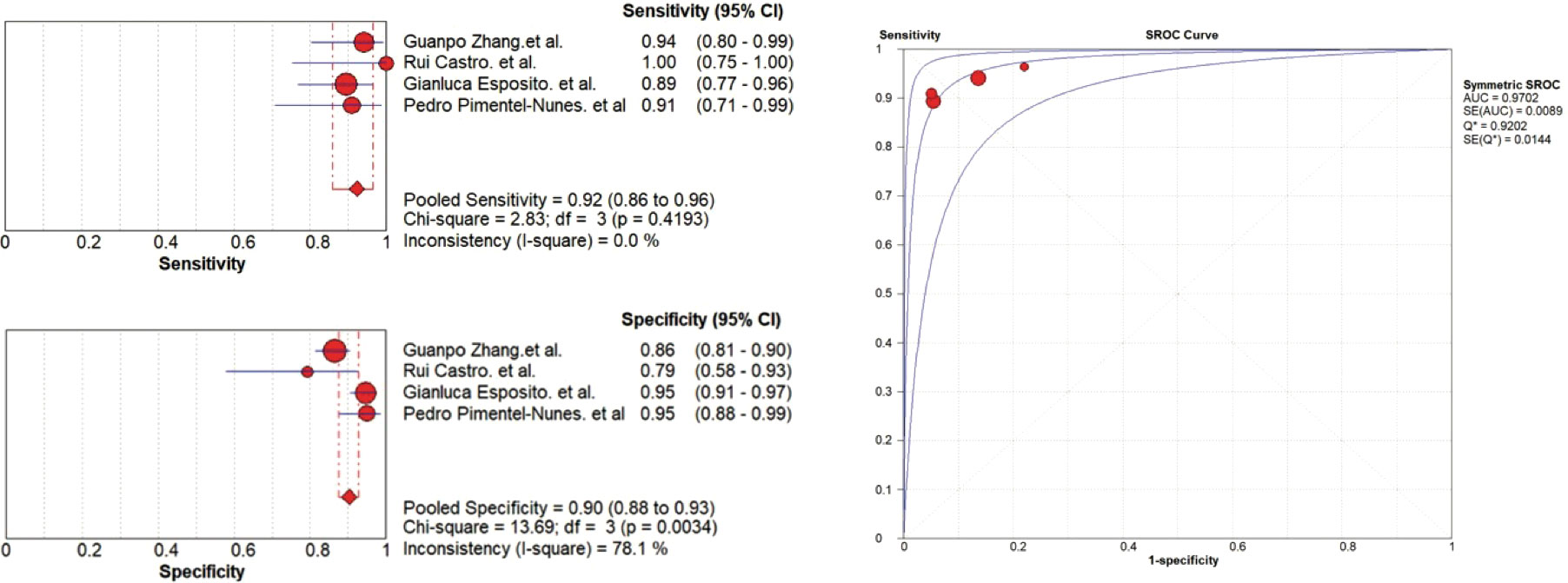
Compared with OLGIM III/IV, EGGIM(5-10) obtained a pooled sensitivity and specificity of 0.92(95%CI 0.86-0.96) and 0.90(95%CI 0.88-0.93), and the area under the curve(AUC) was 0.9702 (Figure 2).
The results showed heterogeneity in the specificity(P=0.0034, I2 =78.1%), between the studies, which means there are differences in the diagnosis of true negatives between studies. Rui Castro et al. (13) who compared BLI with OLGIM reported five false positives among 37 patients(13.51%), and Guanpo Zhang et al. (16) who used LCI reported 33 false positives among 277 patients(11.91%). However, two studies (8, 10) that compared NBI with OLGIM reported a lower false positives rate, 11 out of 250 (4.40%) and 4 out of 101 (3.96%) were false positives, respectively.
EGGIM for gastric cancer risk stratification
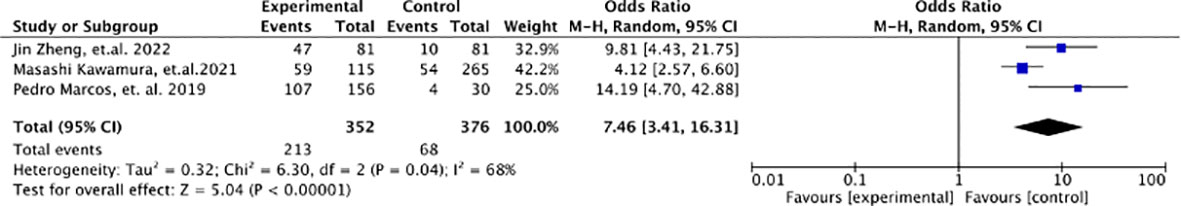
A high EGGIM score (5-10) has a significantly higher risk for GC compared with a low EGGIM score (0-4), with an odds ratio of 7.46 (95%CI 2.06-23.05; P=0.04, I2 = 68%) (Figure 3).
Discussion
The OLGIM system based on histological examination to assess GC risk has been reported in the Kyoto global consensus (20). However, the clinical utility of OLGIM remains controversial especially in low-risk populations since it requires 5 biopsies. The frequency of OLGIM assessment remains low in daily clinical practice (21). The extent of mucosal lesions positively correlates with GC risk (22), and pathological assessment usually focuses on local areas of the stomach and fails to assess the whole stomach. Furthermore, endoscopic risk stratification tools, such as the Kimura-Takemoto classification, are highly correlated with pathological atrophy, and multi-point biopsies are not required (23). Therefore, endoscopic risk stratification tools play a significant role in gastric cancer risk stratification compared with histology-based risk assessment tools.
Our meta-analysis evaluated the effectiveness of EGGIM as a GC risk stratification tool. EGGIM was compared with the gold standard- the pathology-based OLGIM system. We found that EGGIM was in high concordance with OLGIM with an AUC of 0.9702, and patients with EGGIM (5-10 points) had a 7.46-fold higher risk of developing early GC than those with EGGIM (0-4 points). The EGGIM system as a tool for endoscopic GC risk stratification may be helpful for daily clinical practice.
Meta-analysis of the EGGIM system compared with OLGIM shows heterogeneity in specificity between different studies. The results suggest that there are differences in the number of false-positive cases in the various studies. The EGGIM system overestimated the GC risk for some patients with OLGIM stages 0-II. On the other hand, the EGGIM system has a high sensitivity with no apparent heterogeneity, indicating that EGGIM can identify high-risk patients well. Therefore, EGGIM can identify the high-risk population with an acceptable rate of false positives, which can serve as the first step in risk stratification. An endoscopic-guided biopsy will improve the efficiency of GC screening and reduce the need for pathological examinations.
In addition to EGGIM, Kimura-Takemoto’s endoscopic atrophy classification, which assesses the transition of the fundic-pyloric border (24), is widely used in GC risk stratification. It was consistent with the Operative link for gastritis assessment(OLGA) (25–27). Patients with Kimura-Takemoto endoscopic atrophy classification stages O2-O3 had a higher risk of gastric cancer HR=9.3 (95%CI 1.7-174, P=0.007) (28). Kimura-Takemoto’s endoscopic atrophy classification assesses the border of atrophy, and EGGIM assesses the extent of intestinal metaplasia in five gastric sites. Both Kimura-Takemoto classification and EGGIM can predict GC risk, and the combination of the two may be able to improve the accuracy and consistency of GC risk stratification.
The Kyoto classification risk scoring system, which adds observations of intestinal metaplasia, Fhypertrophic fold enlargement, and nodularity to the Kimura-Takemoto system, is also widely used to assess GC risk (29, 30). The study by Masashi Kawamura et al. (19) found that OLGIM stage III/IV, high EGGIM score (5-10), and Kimura-Takemoto open type were risk factors for GC in multivariate analysis; however, the Kyoto classification risk scoring system combined with endoscopic features are not significant(P=0.315). The Kyoto classification risk scoring system based on white light endoscopy may result in a lower diagnostic power than IEE. Meanwhile, it incorporates multiple endoscopic features that may fail to provide new risk predictors. Further studies are recommended to explore the clinical utility of these endoscopic features. We hope these endoscopic and pathological risk assessment tools would be complementary to each other and optimally applied in practice.
There are some limitations of this meta-analysis. First, the number of included studies was small, with only 4 diagnostic studies and 3 case-control studies. Second, the specificity of EGGIM compared to OLGIM is heterogeneous, and there is a heterogeneity of EGGIM in predicting GC risk. Due to insufficient literature, subgroup analysis could not be performed for the operating physicians, the endoscopic techniques, etc. The EGGIM stage in one of the included case-control studies (9) was based on the reports, and there may be a significant bias in the evaluation of the EGGIM. Rui Castro’s study (17) did not report the essential characteristics of the included patients and the endoscopist’s experience, which reduced the study’s reliability.
Conclusion
In summary, our meta-analysis is the first one to synthesize multiple studies to assess the effectiveness of EGGIM. The results showed that EGGIM was a reliable tool for gastric cancer risk stratification. Even though EGGIM carries a false-positive rate, it can still be complementary to pathological biopsy in risk stratification. In addition, with the development of endoscopic technology, endoscopic diagnosis of GC precancerous mucosa has shown great potential, and a unified diagnostic standard may be helpful to promote its application in daily practice. Finally, further prospective studies on endoscopic GC risk stratification systems such as EGGIM are needed to clarify their clinical utility.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
Author contributions
SF: Conceptualization, Methodology, Software, Validation, Formal analysis, Investigation, Data curation, Writing – original draft, Supervision. YF: Investigation, Writing. SD: Validation, Visualization. LW: Investigation, Data curation. XQ: Investigation, Data curation. XL: Investigation, Data curation. GS: Supervision, Project administration,. YY: Supervision, Project administration, Data curation. WW: Conceptualization, Methodology, Supervision, Project administration, Funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
National Traditional Chinese Medicine Inheritance and Innovation Team Project (No. ZYYCXTD-C-202210). Scientific and technological innovation project of China Academy of Chinese Medical Science (No. CI2021A01008 and CI2021A01820). Science Research Program for TCM Industry (No. 201507001-09).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1018248/full#supplementary-material
References
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: A Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. de Martel C, Georges D, Bray F, Ferlay J, Clifford GM. Global burden of cancer attributable to infections in 2018: a worldwide incidence analysis. Lancet Global Health (2020) 8(2):e180–90. doi: 10.1016/S2214-109X(19)30488-7
3. Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. An attempt at a histo-clinical classification. Acta pathol microbiol Scandinavica (1965) 64:31–49. doi: 10.1111/apm.1965.64.1.31
4. Ajani JA, Lee J, Sano T, Janjigian YY, Fan D, Song S. Gastric adenocarcinoma. Nat Rev Dis primers (2017) 3:17036. doi: 10.1038/nrdp.2017.36
5. Piazuelo MB, Correa P. Gastric cáncer: Overview. Colombia Med (Cali Colombia) (2013) 44(3):192–201.
6. Lee JWJ, Zhu F, Srivastava S, Tsao SK, Khor C, Ho KY, et al. Severity of gastric intestinal metaplasia predicts the risk of gastric cancer: a prospective multicentre cohort study (GCEP). Gut (2022) 71(5):854–63. doi: 10.1136/gutjnl-2021-324057
7. Yue H, Shan L, Bin L. The significance of OLGA and OLGIM staging systems in the risk assessment of gastric cancer: a systematic review and meta-analysis. Gastric cancer : Off J Int Gastric Cancer Assoc Japanese Gastric Cancer Assoc (2018) 21(4):579–87. doi: 10.1007/s10120-018-0812-3
8. Pimentel-Nunes P, Libânio D, Lage J, Abrantes D, Coimbra M, Esposito G, et al. A multicenter prospective study of the real-time use of narrow-band imaging in the diagnosis of premalignant gastric conditions and lesions. Endoscopy (2016) 48(8):723–30. doi: 10.1055/s-0042-108435
9. Marcos P, Brito-Gonçalves G, Libânio D, Pita I, Castro R, Sá I, et al. Endoscopic grading of gastric intestinal metaplasia on risk assessment for early gastric neoplasia: can we replace histology assessment also in the West? Gut (2020) 69(10):1762–8. doi: 10.1136/gutjnl-2019-320091
10. Esposito G, Pimentel-Nunes P, Angeletti S, Castro R, Libânio D, Galli G, et al. Endoscopic grading of gastric intestinal metaplasia (EGGIM): a multicenter validation study. Endoscopy (2019) 51(6):515–21. doi: 10.1055/a-0808-3186
11. Quach DT, Hiyama T, Gotoda T. Identifying high-risk individuals for gastric cancer surveillance from western and eastern perspectives: Lessons to learn and possibility to develop an integrated approach for daily practice. World J Gastroenterol (2019) 25(27):3546–62. doi: 10.3748/wjg.v25.i27.3546
12. East JE, Vleugels JL, Roelandt P, Bhandari P, Bisschops R, Dekker E, et al. Advanced endoscopic imaging: European society of gastrointestinal endoscopy (ESGE) technology review. Endoscopy (2016) 48(11):1029–45. doi: 10.1055/s-0042-118087
13. Moher D, Liberati A, Tetzlaff J, Altman DG, , PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PloS Med (2009) 6(7):e1000097. doi: 10.1371/journal.pmed.1000097
14. Whiting PF, Rutjes AWS, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Internal Med (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
15. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
16. Zhang G, Zheng J, Zheng L, Yu S, Jiang C, Lin W, et al. Gastric intestinal metaplasia assessment between linked color imaging based on endoscopy and pathology. Scandinavian J Gastroenterol (2021) 56(1):103–10. doi: 10.1080/00365521.2020.1849385
17. Castro R, Rodriguez M, Libânio D, Esposito G, Pita I, Patita M. Reliability and accuracy of blue light imaging for staging of intestinal metaplasia in the stomach. Scand J Gastroenterol (2019) 54(11):1301–05. doi: 10.1055/s-0039-1681615
18. Zheng J, Zhang G, Gao C, Xu G, Lin W, Jiang C, et al. Linked color imaging-based endoscopic grading of gastric intestinal metaplasia and histological gastritis staging in the assessment of gastric cancer risk. Scandinavian J Gastroenterol (2022) 14:1–7. doi: 10.1080/00365521.2022.2085061
19. Kawamura M, Uedo N, Koike T, Kanesaka T, Hatta W, Ogata Y, et al. Kyoto Classification risk scoring system and endoscopic grading of gastric intestinal metaplasia for gastric cancer: Multicenter observation study in Japan. Digest Endosc (2022) 34(3):508–16. doi: 10.1111/den.14114
20. Sugano K, Tack J, Kuipers EJ, Graham DY, El-Omar EM, Miura S, et al. Kyoto Global consensus report on helicobacter pylori gastritis. Gut (2015) 64(9):1353–67. doi: 10.1136/gutjnl-2015-309252
21. Shah SC, Piazuelo MB, Kuipers EJ, Li D. AGA clinical practice update on the diagnosis and management of atrophic gastritis: Expert review. Gastroenterology (2021) 161(4):1325–1332.e7. doi: 10.1053/j.gastro.2021.06.078
22. Gawron AJ, Shah SC, Altayar O, Davitkov P, Morgan D, Turner K, et al. AGA technical review on gastric intestinal metaplasia-natural history and clinical outcomes. Gastroenterology (2020) Vol. 158:705–731.e5. doi: 10.1053/j.gastro.2019.12.001
23. Quach DT, Hiyama T, Gotoda T. Do subjects with mild or moderate atrophic gastritis or intestinal metaplasia confined to the antrum benefit from gastric cancer surveillance? Gut England (2020) Vol. 69:968–9. doi: 10.1136/gutjnl-2019-318720
24. Kimura K. Chronological transition of the fundic-pyloric border determined by stepwise biopsy of the lesser and greater curvatures of the stomach. Gastroenterology (1972) 63(4):584–92. doi: 10.1016/S0016-5085(19)33241-X
25. Ebigbo A, Marienhagen J, Messmann H. Regular arrangement of collecting venules and the kimura-takemoto classification for the endoscopic diagnosis of helicobacter pylori infection: Evaluation in a Western setting. Digest endoscopy : Off J Japan Gastroenterol Endosc Soc (2021) 33(4):587–91. doi: 10.1111/den.13808
26. Kim SJ, Choi CW, Kang DH, Kim HW, Park SB. Comparison of biannual and annual endoscopic gastric cancer surveillance after endoscopic resection. Surg endosc (2022) 36(3):1806–13. doi: 10.1007/s00464-021-08460-8
27. Maric L, Castaneda D, Singh H, Bejarano P, Jimenez Cantisano B, Castro FJ, et al. Kimura-takemoto classification: A tool to predict gastric intestinal metaplasia progression to advanced gastric neoplasia. Digest Dis Sci (2022) 67(8):4092–99. doi: 10.1007/s10620-021-07212-x
28. Shichijo S, Hirata Y, Niikura R, Hayakawa Y, Yamada A, Ushiku T, et al. Histologic intestinal metaplasia and endoscopic atrophy are predictors of gastric cancer development after helicobacter pylori eradication. Gastrointest endosc (2016) 84(4):618–24. doi: 10.1016/j.gie.2016.03.791
29. Toyoshima O, Nishizawa T, Yoshida S, Sakaguchi Y, Nakai Y, Watanabe H, et al. Endoscopy-based Kyoto classification score of gastritis related to pathological topography of neutrophil activity. World J gastroenterol (2020) 26(34):5146–55. doi: 10.3748/wjg.v26.i34.5146
Keywords: endoscopic grading of gastric intestinal metaplasia, EGGIM, intestinal metaplasia, accuracy, Sensitivity
Citation: Fang S, Fu Y, Du S, Wang L, Qing X, Luo X, Song G, Yang Y and Wei W (2022) The role of the endoscopic grading of gastric intestinal metaplasia in assessing gastric cancer risk: A systematic review and meta-analysis. Front. Oncol. 12:1018248. doi: 10.3389/fonc.2022.1018248
Received: 24 August 2022; Accepted: 17 October 2022;
Published: 08 November 2022.
Edited by:
Zhendong Jin, Medical University, ChinaReviewed by:
Gianluca Esposito, Sapienza University Of Rome, ItalyJun-Hyung Cho, Soonchunhyang University Hospital Seoul, South Korea
Copyright © 2022 Fang, Fu, Du, Wang, Qing, Luo, Song, Yang and Wei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gengqing Song, c29uZ2dhdmluMjAxMEBnbWFpbC5jb20=; Yang Yang, cGZka3NAc2luYS5jb20=; Wei Wei, d2Vpd2VpMzgxNkBtYWlsLmNpbnRjbS5hYy5jbg==
 Shuangshuang Fang1
Shuangshuang Fang1 Lin Wang
Lin Wang Gengqing Song
Gengqing Song Wei Wei
Wei Wei