
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 24 October 2022
Sec. Cancer Imaging and Image-directed Interventions
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1017618
Objective: This study aimed to evaluate the 18F-FDG PET/CT in differentiating lung metastasis(LM) from primary lung cancer(LC) in patients with colorectal cancer (CRC).
Methods: A total of 120 CRC patients (80 male, 40 female) who underwent 18F-FDG PET/CT were included. The diagnosis of primary lung cancer or lung metastasis was based on histopathology The patients were divided into a training cohort and a validation cohort randomized 1:1. Independent risk factors were extracted through the clinical information and 18F-FDG PET/CT imaging characteristics of patients in the validation cohort, and then a diagnostic model was constructed and a nomograms was made. ROC curve, calibration curve, cutoff, sensitivity, specificity, and accuracy were used to evaluate the prediction performance of the diagnostic model.
Results: One hundred and twenty Indeterminate lung lesions (ILLs) (77 lung metastasis, 43 primary lung cancer) were analyzed. No significant difference in clinical characteristics and imaging features between the training and the validation cohorts (P > 0. 05). Using uni-/multivariate analysis, pleural tags and contour were identified as independent predictors. These independent predictors were used to establish a diagnostic model with areas under the receiver operating characteristic curves (AUCs) of 0.92 and 0.89 in the primary and validation cohorts, respectively. The accuracy rate of the diagnostic model for differentiating LM from LC were higher than that of subjective diagnosis (P < 0.05).
Conclusions: Pleural tags and contour were identified as independent predictors. The diagnostic model of ILLs in patients with CRC could help differentiate between LM and LC.
Colorectal cancer (CRC) is the third most commonly diagnosed malignancy and the fourth leading cause of cancer-related deaths in the world (1). In patients with CRC, the lung is the second most common site of metastasis, and pulmonary metastasis has been detected in 10%-22% of all CRC patients (2, 3). Indeterminate lung lesions (ILLs) includes a malignancy (either lung metastasis or primary lung cancer) or a benign. The definition of the nature of ILLs is important for accurate staging and decision-making further diagnostic workup and therapeutic strategy. In addition, surgical strategies for treating primary lung cancer (LC) and lung metastasis (LM) are very different. The treatment option for LM is minimally invasive surgical resection in order to preserve as much healthy lung parenchyma as possible in case repeated surgery is required. In contrast, complete surgical resection with lobotomy and mediastinal lymph node dissection is the gold standard for LC (4).
It is sometimes difficult to determine whether an ILL is a primary LC or a LM. There are reports that the incidence of LM is higher in patients with CRC, the incidence of primary lung cancer in CRC patients was reported to be 2.8%-23% (5–7). There are also reports that primary LC is similar to LM (5–7). Bronchoscopy and/or transthoracic fine-needle aspiration biopsy can help distinguish primary LC from LM before surgical. However, sometimes these strategies are difficult and dangerous, especially for those with small lesions or its deep parenchymal location. Therefore, the clinical and imaging characteristics of the patients, which are of great importance for a physician to make judgment whether it is an primary LC or LM, especially when a patient faces those who can’t accomplish biopsy, because treatment strategies may be completely different. However, studies using clinical and radiological findings to discriminate LM from LC are limited (8). Imaging characteristics of ILLs can be used as a noninvasive alternative to determine whether it is primary LC or LM. 18F-fluorodeoxyglucose(FDG)-positron emission tomography/computed tomography (PET/CT) is an accurate and non-invasive imaging method for evaluating pulmonary nodules and mass lesions (9–11). This noninvasive functional imaging test takes advantage of the observed increase in glucose metabolism in malignant cells and is gaining acceptance in oncology for tumor diagnosis. Over the past decades, 18F-FDG-PET/CT has been identified as the main component of the management of cancer patients (12).PET images are mainly analyzed by vision, but quantitative analysis can also be performed. Tissue glucose utilization can be assessed semi-quantitatively by standard uptake value (SUVmax) in the lesion, providing an observer-independent and repeatability measurement (13, 14). The significance of 18F-FDG PET/CT in the diagnosis or prognosis of ILLs has been reported previously (15, 16). Unfortunately, few studies provide the clinical features and 18F-FDG PET/CT features to discriminate LC from LM in CRCs patients. Therefore, the purpose of this study is attempt to establish a diagnostic model to distinguish primary LC and LM of CRC patients.
We retrospectively reviewed CRC patients underwent 18F-FDG PET/CT from January 2012 to December 2018 at our hospital. The inclusion criteria included the following: (1) CRC was diagnosed by pathology; (2) pulmonary lesions SUVmax>0.5 and diameter>0.8cm (17); (3) pulmonary lesions were pathologically confirmed by surgery or needle biopsy. A total of 123 patients were eligible, of which 3 benign patients were excluded because the sample size was too small. The complete patient enrollment process is shown in Figure 1. Finally, 120 CRC patients(40 female and 80 male) with a mean age of 62.68 ± 9.70 years (range: 35–85 years) were enrolled in this study. Since this study is retrospective, national laws require neither institutional review board approval nor informed consent.
All patients underwent whole-body PET/CT acquisition 60 ± 10 min after injection 18F-FDG by 3.7 MBq/kg. Prior to FDG injection, all patients fasted for at least 6 h. In all cases, the serum glucose concentration met the institutional requirement (≤120 mg/dL).
PET/CT scans were conducted by a Siemens Biograph mCT Flow 64 scanner (Siemens, Erlangen, Germany) or Gemini TF scanner (Philips Medical Systems, The Netherlands) which covered the length from the top of skull to the mid-thigh. A low-dose CT scan (120 kV, 35 mA, slice 3 mm) was first performed, and PET acquisition speed was 1.5 mm/s (slice 3 mm, filter: Gaussian, FWHM: 5 mm) or scanning at a total of 9–10 bed positions, a 90-s acquisition time for each bed position. A Siemens Biograph mCT Flow 64 scanner PET images were reconstructed using a three-dimensional iterative reconstruction with the time-of-flight algorithm, and the low-dose CT scans were acquired in CARE Dose 4D mode. A Gemini TF scanner PET reconstruction parameters included use of 3D model, and use of ordered-subcohorts expectation maximization(OSEM) method (two iterations, four subcohorts, 128×128 pixels of 5.15 mm). Attenuation corrections of the PET images were performed using data from CT imaging.
A Siemens workstation (MultiModality Workplace, Siemens, Germany) was used for post processing. Two experienced nuclear medicine practitioners reviewed and analyzed the 18F-FDG PET/CT independently, and any inconsistencies were resolved by consensus.
Volumes of interest (VOIs) were manually drawn for each lesion and the SUVmax values were automatically calculated. The axial, coronal, and sagittal 18F-FDG PET/CT images were qualitatively analyzed by nuclear medicine physicians. The clinical characters included patients: age, gender, smoking history, index tumor location, index tumor stage(TNM, The pathologic staging system was based on the 8th edition of the Union for International Cancer Control/American Joint Committee on Cancer (UICC/AJCC)), whether exist extrapulmonary metastasis or vascular tumor thrombus, carcinoembryonic antigen (CEA) level when ILLs were detected, disease free interval (DFI) considered as the time between the diagnosis of CRC and the lung lesions by 18F-FDG PET/CT detection. Radiological characteristics of the lung lesions in terms of size, SUVmax, contour, location (left or right, upper,middle or lower, central or peripheral), margin(smooth, lobulated, or spiculated), presence of ground-glass opacity (GGO),air bronchogram, cavitation, pleural tags. Lesios were classified as smooth, lobulated, or spiculated based on margin characteristics. An air bronchogram was defined as a gas-filled bronchus surrounded by abnormal lung parenchyma. Pleural tags were defined as linear strands that extended between nodule surface and adjacent pleural surface. In addition,we compared with the uptake intensity in the mediastinum, the intensity of FDG uptake was also visually analyzed to try to further optimize the characterization of ILL.
Statistical analysis were conducted by using IBM SPSS (Version 22.0; IBM Corp., New York, USA) and R package 3.6.2 (R Foundation for Statistical Computing, Vienna, Austria). A two-sided P less than 0.05 indicated statistical significance.
The measurement data were described as means ± standard deviation, and analyzed using Student’s t-test or Mann-Whitney U-test. The enumeration data were described as numbers and percentages, and analyzed using the chi-square test. Multivariate analysis for predicting lung cancer and lung metastasis was performed using logistic regression by incorporating variables with P < 0.05 in univariate analysis. The nomogram and calibration curve were drawn for the multivariate model. The receiver operating characteristic (ROC) curve was used to assess the diagnostic performance. The area under the curve (AUC) was calculated, and the cutoff was determined using the maximum Youden’s method. Sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV) and accuracy were calculated. The accuracy between the model and nuclear medicine physicians were compared using McNemar test. Inter-reader agreement for diagnosis results was assessed by kappa of agreement. A value of kappa lower than 0.20 was interpreted as poor agreement, 0.41–0.60 as moderate, 0.61–0.80 as substantial, and 0.81–1as almost perfect agreement.
After exclusion of ILLs for those SUVmax <0.5,and diameter<0.8cm,and no pathological findings, 123 lesions with pathological results remained for analysis. Among 123 lesions, 3 benign lesions were excluded in the final. The clinical information of the 120patients are summarized in Table 1, 80 males and 40 females, the mean age 62.68 ± 9.70 years old, 77 lung metastasis (64.2%) and 43 primary lung cancer (35.8%), respectively. Simultaneous lung metastasis was observed in 16 patients, and 5 patients with synchronous multiple primary carcinomas. ILLs characteristics are presented in Table 1. There was no statistically significant difference in the clinical characteristics and ILLs imaging of the patients between the training cohort and the validation cohort (P > 0.05, Table 1).
The training cohort was divided into two groups, one for LM and one for LC. Except for age, the difference in clinical characteristics between the two groups was not statistically significant in univariate analysis (Supplementary Table S1). 18F-FDG PET/CT features of ILLs were compared between LM and LC groups (Supplementary Table S2). The mean size of ILLs was significantly greater in the LC group (2.28 ± 1.12cm) than in the LM group (1.71 ± 0.84cm) (P<0.05). The presence of ILLs with contour, margin(smooth, lobulated, or spiculated), an air bronchogram, pleural tags,and GGO were significantly between LC and LM group (P< 0.005). On multivariate analysis including these 9 factors as variables of interest, contour, pleural tags were identified as significant independent factors for discriminating primary LC from LM (Table 2). Table 3 shows the logistic regression models for differentiating LM from LC of CRC patients. The results revealed that ILLs with pleural tags had a higher rate of diagnosing LC(odds ratio (OR) =28.504; 95% confidence interval (CI): 3.966-204.865) and ILLs with circular contour had a higher rate of diagnosing LM(odds ratio (OR) =0.059; 95% confidence interval (CI): 0.006-0.581).
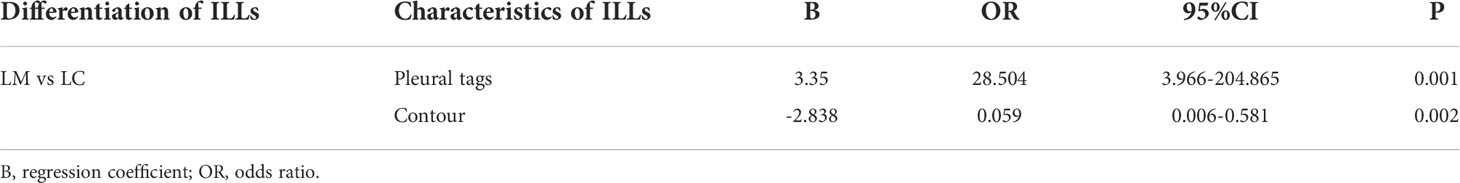
Table 3 Multivariate analysis results of diagnostic model for differentiation LM from LC in training cohort.
Based on multivariate analysis results the diagnostic model were used to construct for discriminating primary LC from LM with CRC. The differential diagnosis prediction model was presented as: Y1 = 3.35* pleural tags -2.838* contour +0.844*size. The area under ROC curve of logistic differentiation model of training cohort was 0.89 (95%CI,0.808-0.973), Y1≥3, which was diagnosed as lung cancer, and Y1<3, which was diagnosed as lung metastasis. The area under ROC curve of validation cohort was 0.86 (95%CI, 0.756-0.963). Based on the predictive factors in the multivariable analysis, nomogram was constructed to differentiate LM from LC of CRCs (Figure 2). The calibration curves of differentiate LM from LC of CRCs suggested a good agreement between the training cohort and validation cohort (Figure 3). The diagnostic model showed an AUC of 0.891(95%CI, 0.808-0.973) and an accuracy of 85% in the training cohort (Figure 4A), and an AUC of 0.859(95%CI,0.756-0.963) and an accuracy of 80.0% in the validation cohort (Figure 4B). Detailed information on the performance of the diagnostic models for differentiating LM from LC of ILLs with CRC is shown in Table 3. A typical case is shown in Figure 5.
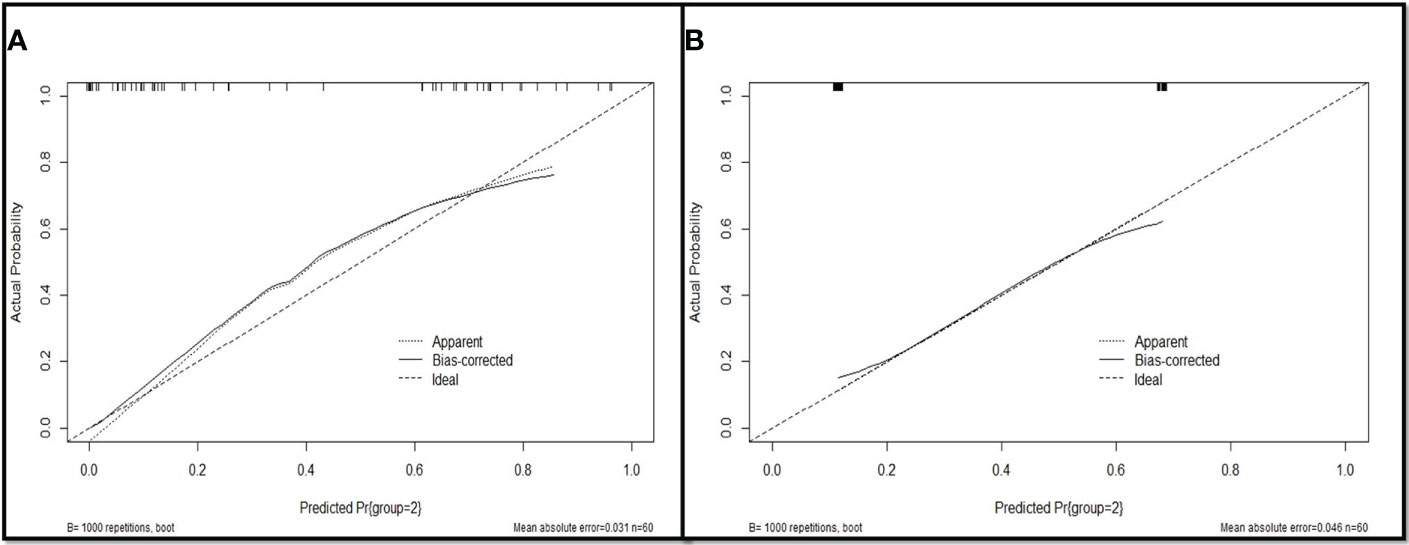
Figure 3 Evaluation of the nomogram for the diagnostic model for differentiating LM from LC of CRC patients. (A) The calibration curves of training cohort; (B) The calibration curves of validation cohort.

Figure 4 Receiver operating characteristics (ROC) curves analysis of diagnostic model for differentiating LM from LC. (A)The AUC of diagnostic model for diagnosing LM and LC was 0.891 in the training cohort; (B) The AUC of diagnostic model for diagnosing LM and LC was 0.859 in the validation cohort.

Figure 5 18F-FDG PET/CT findings of LM and LC of CRC patients. (A, B) 49y female was diagnosis sigmoid colon cancer. 18F-FDG PET/CT showed a circular soft tissue nodule in upper lobe of the left lung, which with smooth margin and high FDG uptake on CT and integrated PET-CT. The nodule was histopathologically confirmed to be LM; (C, D) The hematoxylin-eosin staining (200x) showed that the adenocarcinoma cells grew infiltratively with clear cytoplasm. Immunohistochemistry was negative for TTF-1; (E, F) 71y male was diagnosis sigmoid colon cancer. 18F-FDG PET/CT showed a circular soft tissue nodule of high FDG uptake in inferior lobe of right lung, which with spiculated margin and pleural tags on CT and integrated PET/CT.; The nodule was histopathologically confirmed to be LC; (G, H) The hematoxylin-eosin staining (200x) showed that adenocarcinoma cells are confluent, cribriform infiltrative, and necrotic tissue can be seen. Immunohistochemistry showed TTF-1 positive.
Inter-observer agreement for diagnosis LM with LC was near perfect (kappa=0.850).In the training and validation cohort, both nuclear medicine physicians were less effective at differentiating LM and LC(both P<0.05) than diagnostic model. The sensitivity, specificity, PPV, NPV and accuracy of diagnostic model and experts for diagnosis in differentiating LM from LC of ILLs are shown in Table 4 and Supplementary Tables S3, S4. In the training and validation cohort, accuracy rates were significant by using diagnostic model than experts (both P <0.05) (Figure 6).
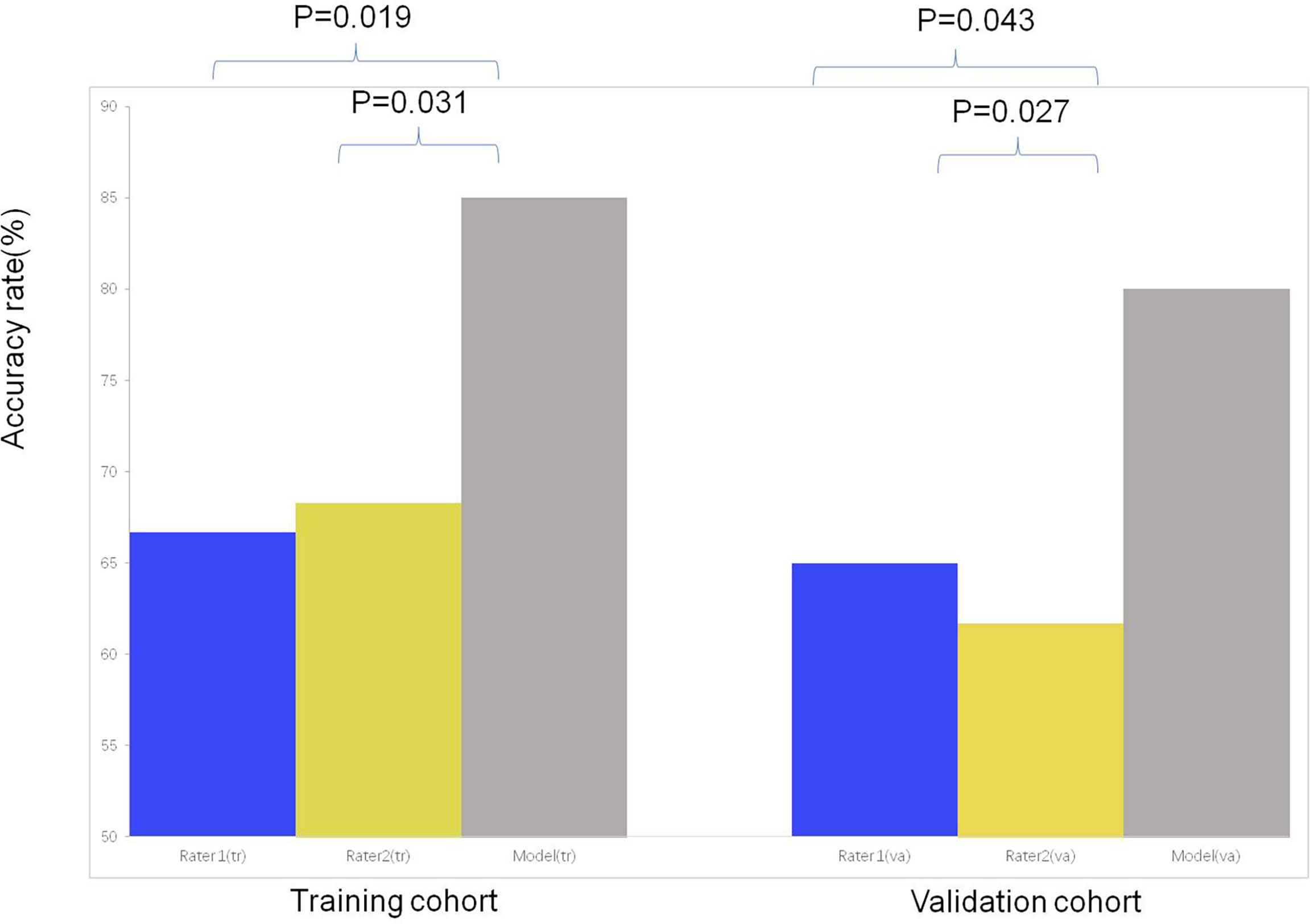
Figure 6 The comparison of the accuracy among subjective evaluation and subjective evaluation with the assistance of diagnostic model for differentiating LC from LM of CRC.
Lung is generally considered to be one of the most common organs for secondary primary tumors (18, 19). However, lung metastasis is also very common after various tumors are cured (20, 21). In CRC patients, the assessment of lung metastasis is important for accurate initial staging and subsequent treatment decisions. As we know, Lung metastases grow relatively slowly and have a good prognosis. Surgical resection of lung metastases is considered the best possible treatment (22). Numerous studies have reported that lung metastases resection is associated with favorable 5-year overall survival rates ranging from 24% to 67.8% (23–25). At present, the main difficulties are how to characterize the lung lesions found in the initial stage or postoperative review of CRC patients and how to determine whether they are metastatic or primary lung cancer. Hence, a more accurate, effective and non-invasion prognostic model for ILL with CRC is urgently needed.
Our diagnostic model was built by clinical characteristics and 18F-FDG PET/CT features of CRC patients with ILL. Most previous studies have focused on the differential diagnosis of benign and malignant for ILLs with CRC. In our study, The size and SUVmax of lesions were controlled during screening of enrolled patients, which may lead to the diagnosis of more malignant lung lesions. Although this reduces the scope of application of the model, it may improve the diagnostic specificity of the model. Meanwhile, all ILLs in our final analysis were pathologically confirmed, which were also lacking in previous studies.
18F-FDG PET/CT is a more accurate, non-invasive method for detecting metastasis of CRC, and has a higher sensitivity than conventional CT in detecting extra-hepatic metastasis (26). 18F-FDG PET/CT has an overall accuracy of 90% in diagnosing malignant lung lesions in patients with a history of cancer (27). Our study assessed the clinical value of 18F-FDG PET/CT features and Clinical characteristics for distinguishing LM and LC of CRC. Clarification of these characteristics may reduce patient consumption and the use of unnecessary diagnosis, treatment, and follow-up. A diagnostic model using the pleural tags and contour had AUCs of 0.89 and 0.86 in the training and validation sets for distinguishing LM and LC, respectively. The accuracy rate of the subjective evaluation was improved obviously with the assistance of 18F-FDG PET/CT diagnostic model for differentiating LM from LC compared to subjective diagnosis alone.
The analysis of nodule morphology may provide important information about the etiology of the lesion. For example, smooth edges and circular are theoretically more indicative of benign lesions or LM, while irregular edges may indicate LC. ILLs with circular contour suggest LM, and ILLs with pleural tags indicate LC in both univariate and multivariate analyses of our study. The results were consist with previous study. Margin characteristics of ILLs were significantly in diagnosis LM with LC in univariate analyses. The “air-bronchogram sign”, which was defined as air within the bronchi or bronchioles passing through airless parenchyma visible as branching linear lucencies (28, 29). The air bronchograms within ILLs was significantly higher in LC than in LM in previous study (4). Perhaps because only 5 ILLs with air-bronchogram, the results significant in univariate analysis, while multivariate analysis was not statistically significant. ILLs contain a GGO component commonly seen in primary lung adenocarcinomas. Only a few reports have described cases of pulmonary metastases (30, 31). Typical LM appear as solid round nodules. In our study, all of GGOs was observed in LC, which was statistically significant in univariate analysis. Thus, GGO density of ILLs may be used to support the diagnosis of LC rather than LM.
The resolution for most PET scanners is 5–6 mm. Therefore, PET is less likely to obtain additional helpful information for smaller lung lesions compared with CT (32, 33). SUV is a semi-quantitative measurement of radiopharmaceutical uptake at the region of interest, which is influenced by many factors (timing of uptake, blood sugar level, capacity effect and statistical noise, etc.). We exclude patients with low SUVmax (<0.5) and small tumor size (<0.8cm) to avoid underestimate of SUVmax. Previous studies have demonstrated the usefulness of 18F-FDG PET/CT in the diagnosis of lung metastases for various tumors (32, 34, 35). SUVmax were not found to be significant in our study. The results are consistent with previous reports (8, 27). To further analyze the role of SUVmax, we divided ILLs into higher than mediastinal and lower than mediastinal according to previous literature reports (27). Unfortunately, the SUVmax was not a significant discriminating factor. In addition to the differential diagnosis of ILLS, the capacity of 18F-FDG PET/CT to detect extra abdominal disease represents an advantage compared to conventional imaging techniques, allowing better determination of the different M1 categories (36).
The number of ILLs is one of the most common prognostic factors found in previous studies (37, 38). For the pathology was the gold standard in our study, some nodules without pathology were not included in the analysis. As we know, the larger the ILLs, the higher likelihood of malignancy for tumor patients; and that, larger nodules had more distinct morphological features. The diameter of the nodules is less than 1.0 cm, which makes it difficult to perform morphological analysis of such small lesions (39). It has been reported that the failure rate of 18F-FDG PET/CT for detecting sub centimeter lung nodules was nearly 50% (40, 41). For these reasons, we did not record and analyze all the nodules per patient.
In addition to 18F-FDG PET/CT features, we also analyzed the usefulness of clinical features in differentiating LM from LC. Unfortunately, except for age, other clinical features were not statistically significant in univariate analysis. It may be because the sample size was reduced after we divided patients into training cohort and validation cohort, which affected the statistical results.
Generally, young patients have been considered to have a more aggressive biological behavior and worst prognosis (42, 43). In our study, univariate analysis for age show an statistically significant result in training cohort, which result same with previous study (4). It means that older people are more likely to develop primary lung cancer. Smoking is not only a major risk factor for lung cancer, but also a a risk factor for pulmonary metastasis in CRC patients (44–46). This indicates that patients with CRC who smoke may develop lung cancer or lung metastases. There is no difference was observed in smoking status between LM and LC of CRCs in our study. DFI refers to the time from radical resection of CRC to diagnosis of lung metastasis, which considered a predictor of tumor biology and prognosis (47, 48). However, DFI cannot distinguish LM from LC in our results. This may be due to simultaneous multiple primary carcinoma or lung metastasis in 21 patients. Previous studies have shown that the higher the tumor stage for CRCs, the greater the chance of lung metastasis (49, 50).
Several limitations must be considered in this study. First of all, the retrospective nature of this study may have introduced potential selection and verification biases. Secondly, our sample size was relatively small due to we selected those lesions with histopathological results and larger than 8mm with high uptake for analysis, which limits the accuracy of the conclusions that can be drawn. Third, lymph node dissection was performed only in some patients, so the status of lymph node metastasis was not analyzed as an parameter in our study.
The pleural tags and contour obtained from 18F-FDG PET/CT might be more valuable than other parameters for discriminating LM from LC of CRC. The diagnostic model of ILLs could improve the diagnostic accuracy and contribute to determining an appropriate clinical option for CRCs.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding authors.
As the present analysis has merely a retrospective character, neither Institutional Review Board approval nor informed consent was required by national law.
RG SY, ZY, NL, and JY were involved in the conception and design of the study. RG, FW, HS and JY were involved in the collection and analysis of data. RG, NL, and JY wrote and revised the manuscript. All authors reviewed and approved the final manuscript. ZY, NL and JY were the principal investigator. All authors provided critical feedback and helped shape the research, analysis, and manuscript, and discussed the results. All authors contributed to the article and approved the submitted version.
This work was financially supported by the National Natural Science Foundation (No.81871387, 82171980) and Beijing Natural Science Foundation (No.7202027), Beijing Hospitals Authority Dengfeng Project (DFL20191102).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1017618/full#supplementary-material
1. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut (2017) 66(4):683–91. doi: 10.1136/gutjnl-2015-310912
2. Salah S, Watanabe K, Welter S, Park JS, Park JW, Zabaleta J, et al. Colorectal cancer pulmonary oligometastases: pooled analysis and construction of a clinical lung metastasectomy prognostic model. Ann Oncol (2012) 23(10):2649–55. doi: 10.1093/annonc/mds100
3. Jung EJ, Kim SR, Ryu CG, Paik JH, Yi JG, Hwang DY. Indeterminate pulmonary nodules in colorectal cancer. World J Gastroenterol (2015) 21(10):2967–72. doi: 10.3748/wjg.v21.i10.2967
4. Lee JE, Jeong WG, Kim YH. Differentiation of primary lung cancer from solitary lung metastasis in patients with colorectal cancer: a retrospective cohort study. World J Surg Oncol (2021) 19(1):28. doi: 10.1186/s12957-021-02131-7
5. Seo JB, Im JG, Goo JM, Chung MJ, Kim MY. Atypical pulmonary metastases: spectrum of radiologic findings. Radiographics (2001) 21(2):403–17. doi: 10.1148/radiographics.21.2.g01mr17403
6. Lee WS, Yun SH, Chun HK, Lee WY, Yun HR, Kim J, et al. Pulmonary resection for metastases from colorectal cancer: prognostic factors and survival. Int J Colorectal Dis (2007) 22(6):699–704. doi: 10.1007/s00384-006-0218-2
7. Li HC, Schmidt L, Greenson JK, Chang AC, Myers JL. Primary pulmonary adenocarcinoma with intestinal differentiation mimicking metastatic colorectal carcinoma: case report and review of literature. Am J Clin Pathol (2009) 131(1):129–33. doi: 10.1309/AJCPB04XWICTFERL
8. Ohtaki Y, Shimizu K, Nagashima T, Nakazawa S, Obayashi K, Azuma Y, et al. Clinical and radiological discrimination of solitary pulmonary lesions in colorectal cancer patients. World J Surg (2018) 42(4):1161–70. doi: 10.1007/s00268-017-4243-9
9. Gould MK, Maclean CC, Kuschner WG, Rydzak CE, Owens DK. Accuracy of positron emission tomography for diagnosis of pulmonary nodules and mass lesions: a meta-analysis. JAMA (2001) 285(7):914–24. doi: 10.1001/jama.285.7.914
10. Hellwig D, Baum RP, Kirsch C. FDG-PET, PET/CT and conventional nuclear medicine procedures in the evaluation of lung cancer: a systematic review. Nuklearmedizin (2009) 48(2):59–69.
11. Grgic A, Yuksel Y, Groschel A, Schafers HJ, Sybrecht GW, Kirsch CM, et al. Risk stratification of solitary pulmonary nodules by means of PET using (18)F-fluorodeoxyglucose and SUV quantification. Eur J Nucl Med Mol Imaging (2010) 37(6):1087–94. doi: 10.1007/s00259-010-1387-3
12. Briggs RH, Chowdhury FU, Lodge JP, Scarsbrook AF. Clinical impact of FDG PET-CT in patients with potentially operable metastatic colorectal cancer. Clin Radiol (2011) 66(12):1167–74. doi: 10.1016/j.crad.2011.07.046
13. Boellaard R. Standards for PET image acquisition and quantitative data analysis. J Nucl Med (2009) 50 Suppl 1:11S–20S. doi: 10.2967/jnumed.108.057182
14. Nahmias C, Wahl LM. Reproducibility of standardized uptake value measurements determined by 18F-FDG PET in malignant tumors. J Nucl Med (2008) 49(11):1804–8. doi: 10.2967/jnumed.108.054239
15. Kim SK, Allen-Auerbach M, Goldin J, Fueger BJ, Dahlbom M, Brown M, et al. Accuracy of PET/CT in characterization of solitary pulmonary lesions. J Nucl Med (2007) 48(2):214–20.
16. Goodgame B, Pillot GA, Yang Z, Shriki J, Meyers BF, Zoole J, et al. Prognostic value of preoperative positron emission tomography in resected stage I non-small cell lung cancer. J Thorac Oncol (2008) 3(2):130–4. doi: 10.1097/JTO.0b013e318160c122
17. Garcia-Velloso MJ, Bastarrika G, de-Torres JP, Lozano MD, Sanchez-Salcedo P, Sancho L, et al. Assessment of indeterminate pulmonary nodules detected in lung cancer screening: Diagnostic accuracy of FDG PET/CT. Lung Cancer (2016) 97:81–6. doi: 10.1016/j.lungcan.2016.04.025
18. Chuang SC, Scelo G, Tonita JM, Tamaro S, Jonasson JG, Kliewer EV, et al. Risk of second primary cancer among patients with head and neck cancers: A pooled analysis of 13 cancer registries. Int J Cancer (2008) 123(10):2390–6. doi: 10.1002/ijc.23798
19. Argiris A, Brockstein BE, Haraf DJ, Stenson KM, Mittal BB, Kies MS, et al. Competing causes of death and second primary tumors in patients with locoregionally advanced head and neck cancer treated with chemoradiotherapy. Clin Cancer Res (2004) 10(6):1956–62. doi: 10.1158/1078-0432.Ccr-03-1077
20. Shin JW, Lee SI, Moon HY. Significance of follow-up in detection of pulmonary metastasis of colorectal cancer. J Korean Soc Coloproctol (2010) 26(4):293–7. doi: 10.3393/jksc.2010.26.4.293
21. Lin BR, Chang TC, Lee YC, Lee PH, Chang KJ, Liang JT. Pulmonary resection for colorectal cancer metastases: duration between cancer onset and lung metastasis as an important prognostic factor. Ann Surg Oncol (2009) 16(4):1026–32. doi: 10.1245/s10434-008-0286-3
22. Suzuki H, Kiyoshima M, Kitahara M, Asato Y, Amemiya R. Long-term outcomes after surgical resection of pulmonary metastases from colorectal cancer. Ann Thorac Surg (2015) 99(2):435–40. doi: 10.1016/j.athoracsur.2014.09.027
23. Onaitis MW, Petersen RP, Haney JC, Saltz L, Park B, Flores R, et al. Prognostic factors for recurrence after pulmonary resection of colorectal cancer metastases. Ann Thorac Surg (2009) 87(6):1684–8. doi: 10.1016/j.athoracsur.2009.03.034
24. Kim CH, Huh JW, Kim HJ, Lim SW, Song SY, Kim HR, et al. Factors influencing oncological outcomes in patients who develop pulmonary metastases after curative resection of colorectal cancer. Dis Colon Rectum (2012) 55(4):459–64. doi: 10.1097/DCR.0b013e318246b08d
25. Kim CH, Huh JW, Kim HR, Kim YJ. Indeterminate pulmonary nodules in colorectal cancer: Follow-up guidelines based on a risk predictive model. Ann Surg (2015) 261(6):1145–52. doi: 10.1097/SLA.0000000000000853
26. Culverwell AD, Chowdhury FU, Scarsbrook AF. Optimizing the role of FDG PET-CT for potentially operable metastatic colorectal cancer. Abdom Imaging (2012) 37(6):1021–31. doi: 10.1007/s00261-012-9855-9
27. Bar-Shalom R, Kagna O, Israel O, Guralnik L. Noninvasive diagnosis of solitary pulmonary lesions in cancer patients based on 2-fluoro-2-deoxy-D-glucose avidity on positron emission tomography/computed tomography. Cancer (2008) 113(11):3213–21. doi: 10.1002/cncr.23928
28. Fleischner FG. The visible bronchial tree; a roentgen sign in pneumonic and other pulmonary consolidations. Radiology (1948) 50(2):184–9. doi: 10.1148/50.2.184
29. Soltesz I, Lingg GM. [Diagnostic imaging in rheumatology. I: Typical Roentgen Signs (Conventional)]. Fortschr Med (1998) 116(11):44–6.
30. Woodring JH, Bognar B. CT halo sign in pulmonary metastases from mucinous adenocarcinoma of the pancreas. South Med J (2001) 94(4):448–9. doi: 10.1097/00007611-200194040-00021
31. Miyake H, Matsumoto A, Terada A, Yoshida S, Takaki H, Mori H. Mucin-producing tumor of the lung: CT findings. J Thorac Imaging (1995) 10(2):96–8. doi: 10.1097/00005382-199521000-00003
32. Parnaby CN, Bailey W, Balasingam A, Beckert L, Eglinton T, Fife J, et al. Pulmonary staging in colorectal cancer: a review. Colorectal Dis (2012) 14(6):660–70. doi: 10.1111/j.1463-1318.2011.02601.x
33. Maithel SK, Ginsberg MS, D'Amico F, DeMatteo RP, Allen PJ, Fong Y, et al. Natural history of patients with subcentimeter pulmonary nodules undergoing hepatic resection for metastatic colorectal cancer. J Am Coll Surg (2010) 210(1):31–8. doi: 10.1016/j.jamcollsurg.2009.09.032
34. Selzner M, Hany TF, Wildbrett P, McCormack L, Kadry Z, Clavien PA. Does the novel PET/CT imaging modality impact on the treatment of patients with metastatic colorectal cancer of the liver? Ann Surg (2004) 240(6):1027–34. doi: 10.1097/01.sla.0000146145.69835.c5
35. Evangelista L, Panunzio A, Cervino AR, Vinante L, Al-Nahhas A, Rubello D, et al. Indeterminate pulmonary nodules on CT images in breast cancer patient: the additional value of 18F-FDG PET/CT. J Med Imaging Radiat Oncol (2012) 56(4):417–24. doi: 10.1111/j.1754-9485.2012.02408.x
36. Uchiyama S, Haruyama Y, Asada T, Hotokezaka M, Nagamachi S, Chijiiwa K. Role of the standardized uptake value of 18-fluorodeoxyglucose positron emission tomography-computed tomography in detecting the primary tumor and lymph node metastasis in colorectal cancers. Surg Today (2012) 42(10):956–61. doi: 10.1007/s00595-012-0225-6
37. Yedibela S, Klein P, Feuchter K, Hoffmann M, Meyer T, Papadopoulos T, et al. Surgical management of pulmonary metastases from colorectal cancer in 153 patients. Ann Surg Oncol (2006) 13(11):1538–44. doi: 10.1245/s10434-006-9100-2
38. Pastorino U, Buyse M, Friedel G, Ginsberg RJ, Girard P, Goldstraw P, et al. Long-term results of lung metastasectomy: prognostic analyses based on 5206 cases. J Thorac Cardiovasc Surg (1997) 113(1):37–49. doi: 10.1016/s0022-5223(97)70397-0
39. Quint LE, Park CH, Iannettoni MD. Solitary pulmonary nodules in patients with extrapulmonary neoplasms. Radiology (2000) 217(1):257–61. doi: 10.1148/radiology.217.1.r00oc20257
40. Metser U, You J, McSweeney S, Freeman M, Hendler A. Assessment of tumor recurrence in patients with colorectal cancer and elevated carcinoembryonic antigen level: FDG PET/CT versus contrast-enhanced 64-MDCT of the chest and abdomen. AJR Am J Roentgenol (2010) 194(3):766–71. doi: 10.2214/AJR.09.3205
41. Bryant AS, Cerfolio RJ. The maximum standardized uptake values on integrated FDG-PET/CT is useful in differentiating benign from malignant pulmonary nodules. Ann Thorac Surg (2006) 82(3):1016–20. doi: 10.1016/j.athoracsur.2006.03.095
42. da Fonseca LM, da Luz MM, Lacerda-Filho A, Cabral MM, da Silva RG. Colorectal carcinoma in different age groups : a histopathological analysis. Int J Colorectal Dis (2012) 27(2):249–55. doi: 10.1007/s00384-011-1299-0
43. Al-Barrak J, Gill S. Presentation and outcomes of patients aged 30 years and younger with colorectal cancer: a 20-year retrospective review. Med Oncol (2011) 28(4):1058–61. doi: 10.1007/s12032-010-9639-4
44. Yahagi M, Tsuruta M, Hasegawa H, Okabayashi K, Toyoda N, Iwama N, et al. Smoking is a risk factor for pulmonary metastasis in colorectal cancer. Colorectal Dis (2017) 19(9):O322–O8. doi: 10.1111/codi.13833
45. Makino A, Tsuruta M, Okabayashi K, Ishida T, Shigeta K, Seishima R, et al. The impact of smoking on pulmonary metastasis in colorectal cancer. Onco Targets Ther (2020) 13:9623–9. doi: 10.2147/OTT.S263250
46. National Center for Chronic Disease Prevention and Health Promotion (US) Office on Smoking and Health. The health consequences of smoking-50 years of progress: A report of the surgeon general. In: Reports of the surgeon general. Atlanta: GA.
47. Gonzalez M, Poncet A, Combescure C, Robert J, Ris HB, Gervaz P. Risk factors for survival after lung metastasectomy in colorectal cancer patients: a systematic review and meta-analysis. Ann Surg Oncol (2013) 20(2):572–9. doi: 10.1245/s10434-012-2726-3
48. Cho S, Song IH, Yang HC, Jheon S. Prognostic factors of pulmonary metastasis from colorectal carcinoma. Interact Cardiovasc Thorac Surg (2013) 17(2):303–7. doi: 10.1093/icvts/ivt164
49. Nordholm-Carstensen A, Wille-Jorgensen PA, Jorgensen LN, Harling H. Indeterminate pulmonary nodules at colorectal cancer staging: A systematic review of predictive parameters for malignancy. Ann Surg Oncol (2013) 20(12):4022–30. doi: 10.1245/s10434-013-3062-y
Keywords: PET/CT, lung lesion, primary lung cancer, lung metastasis, colorectal cancer
Citation: Guo R, Yan S, Wang F, Su H, Xie Q, Zhao W, Yang Z, Li N and Yu J (2022) A novel diagnostic model for differentiation of lung metastasis from primary lung cancer in patients with colorectal cancer. Front. Oncol. 12:1017618. doi: 10.3389/fonc.2022.1017618
Received: 12 August 2022; Accepted: 06 October 2022;
Published: 24 October 2022.
Edited by:
Punit Sharma, Apollo Gleneagles Hospitals, IndiaCopyright © 2022 Guo, Yan, Wang, Su, Xie, Zhao, Yang, Li and Yu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhi Yang, cGVreXpAMTYzLmNvbQ==; Nan Li, cmFpbmJvdzYyODNAc2luYS5jb20=; Jiangyuan Yu, eXVqaWFuZ3l1YW5AYWxpeXVuLmNvbQ==
†These authors have contributed equally to this work
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.