- 1Division of Quality of Life and Palliative Care, Department of Oncology, St. Jude Children’s Research Hospital, Memphis, TN, United States
- 2Pediatric Oncology Education Program, St. Jude Children’s Research Hospital, Memphis, TN, United States
- 3Division of Critical Care Medicine, Departments of Pediatric Medicine and Bone Marrow Transplantation and Cellular Therapy, St. Jude Children’s Research Hospital, Memphis, TN, United States
- 4Department of Biostatistics, St. Jude Children’s Research Hospital, Memphis, TN, United States
- 5Division of Anesthesiology, Department of Pediatric Medicine, St. Jude Children’s Research Hospital, Memphis, TN, United States
Context: Approximately 40%-60% of deaths in the pediatric intensive care unit (PICU) are in the context of de-escalation of life-sustaining treatments (LSTs), including compassionate extubation, withdrawal of vasopressors, or other LSTs. Suffering at the end of life (EOL) is often undertreated and underrecognized. Pain and poor quality of life are common concerns amongst parents and providers at a child’s EOL. Integration of palliative care (PC) may decrease suffering and improve symptom management in many clinical situations; however, few studies have described medical management and symptom burden in children with cancer in the pediatric intensive care unit (PICU) undergoing de-escalation of LSTs.
Methods: A retrospective chart review was completed for deceased pediatric oncology patients who experienced compassionate extubation and/or withdrawal of vasopressor support at EOL in the PICU. Demographics, EOL characteristics, and medication use for symptom management were abstracted. Descriptive analyses were applied.
Results: Charts of 43 patients treated over a 10-year period were reviewed. Most patients (69.8%) were white males who had undergone hematopoietic stem cell transplantation and experienced compassionate extubation (67.4%) and/or withdrawal of vasopressor support (44.2%). The majority (88.3%) had a physician order for scope of treatment (POST – DNaR) in place an average of 13.9 days before death. PC was consulted for all but one patient; however, in 18.6% of cases, consultations occurred on the day of death. During EOL, many patients received medications to treat or prevent respiratory distress, pain, and agitation/anxiety. Sedative medications were utilized, specifically propofol (14%), dexmedetomidine (12%), or both (44%), often with opioids and benzodiazepines.
Conclusions: Pediatric oncology patients undergoing de-escalation of LSTs experience symptoms of pain, anxiety, and respiratory distress during EOL. Dexmedetomidine and propofol may help prevent and/or relieve suffering during compassionate de-escalation of LSTs. Further efforts to optimize institutional policies, education, and collaborations between pediatric intensivists and PC teams are needed.
Introduction
Each year in the United States, approximately 20,000 children die, and beyond the first year of life, the majority of those deaths are due to accidental trauma, congenital anomalies, malignancy, or intentional injury (1, 2). Although the distribution of the causes of pediatric deaths has not changed significantly over several decades (2), the events leading up to death have. The advancement of medical treatments and evolution of pediatric critical care has altered the progression of several pediatric disorders and increased invasive interventions during the end-of-life (EOL) period (1, 3).
Life-sustaining treatments (LSTs), such as mechanical ventilation and vasoactive support, play a substantial role in supporting patients during EOL; nevertheless, they may contribute to symptom burden and suffering (4, 5). Palliative care (PC) as a medical subspecialty focuses on improving quality of life (QOL) and decreasing suffering through symptom management, psychosocial support, and advanced-care planning (5–7). Integration of PC throughout the disease trajectory and within the pediatric intensive care unit (PICU) may improve outcomes and has become increasingly accepted, yet such services remain underutilized (8, 9).
In pediatric oncology, advancements in hematopoietic cell transplantation (HCT) and immunotherapy have increased overall survival and critical care needs in this population have risen accordingly (10). In fact, nearly 40% of pediatric oncology patients are admitted to the PICU at some point during - therapy, and more than half of these patients require multiple PICU admissions (3, 11). Additionally, mortality rates for pediatric oncology patients admitted to the PICU are notably 4-fold greater than those for the general pediatric population who require intensive care (12, 13). Patients undergoing HCT can have further increases in mortality risk that are associated with respiratory failure requiring mechanical ventilation and prolonged PICU stays (>15 days) (10, 14).
However, regardless of diagnosis, prognosticating in the PICU is difficult and comes with substantial uncertainty (15). When faced with a terminal prognosis, families can find themselves having to make difficult decisions regarding LSTs (16). In this context, involvement of PC specialists during PICU admissions can improve shared medical decision making, decrease parental regret, and assist with bereavement (8, 17). Nearly 40%-60% of pediatric deaths in the PICU occur after a decision is made to withdraw LSTs (1, 18–20); however, little is known about how parents and families arrive at that decision. One study suggests that, in most cases, medical professionals initiate conversations about compassionate de-escalation of LSTs, and consensus is reached between medical teams and families after 1-2 meetings (21). The role of PC during this time remains poorly defined, with the potential for missed opportunities to provide improved QOL, support decision making, and give psychosocial support to these families.
In general, literature on compassionate de-escalation of LSTs is limited and it is rarely specific to pediatric oncology. To address this gap in the literature, we conducted a retrospective review of deceased pediatric hematology/oncology patients treated at an academic hospital over a 10-year period, with the goal of describing this patient population and their EOL experiences.
Methods
An Institutional Review Board–exempt, retrospective review was performed of pediatric hematology/oncology patients treated at St. Jude Children’s Research Hospital between April 1, 2011, and January 1, 2021. This date range defines a period when patients would have all pertinent data for this study placed into the electronic medical record system.
For the purposes of this study, the term LSTs was defined as patients who required mechanical ventilation and/or vasoactive support. Patients requiring non-invasive respiratory support eventually progressed to intubation and mechanical ventilation and thus are also represented in this definition. Inclusion criteria consisted of age <25 years, a confirmed hematologic or oncologic diagnosis, death occurring in the PICU, and patients who had de-escalation of LSTs, specifically compassionate extubation, withdrawal of vasopressor support, or both. Other LST withdrawal was not assessed due to the complexity of recognizing the rationale for withdrawal in our electronic medical record.
Two study members (AC and MP) performed data extraction in a systematic fashion using a data dictionary to ensure consistency. Data collected included demographics [age at diagnosis, sex, race/ethnicity, religious affiliation, date of diagnosis, date of death, age at time of death (TOD)], disease characteristics (primary oncology or hematology diagnosis, stage of disease, presence of relapse or recurrence, history of hematopoietic cell transplantation, type of transplant, cancer-directed treatment within the last month and week of life, infectious complications during last admission), EOL care characteristics (date of PC first contact, goal of care at the time of PC consultation, number of PC visits, hospice enrollment and date if applicable, date of first pain service consultation, number of pain service visits, intubation status at TOD, withdrawal of LSTs, cardiopulmonary resuscitation on day of death (DOD), Do Not Resuscitate status) and medications used for symptom control within 24 hours of death. Of note, the presence of symptoms (i.e., pain, anxiety, nausea, etc.) was ascertained through daily progress note documentation. Discrepancies were reviewed by both study members until a consensus was reached.
Descriptive statistics of the data included frequency (percent), mean ± standard deviation (SD), and median [Max, Min]. SAS (version 9.4, SAS Inc.) was used for all analyses.
Results
Patient demographics
A total of 721 patients died during the study period: 244 deaths occurred in the inpatient setting, and 107 occurred in the PICU. Of those patients who died in the PICU, 43 (40.2%) had withdrawal of LSTs and thus met the study’s criteria. The majority (58.1%) were male, primarily white (69.8%), and their mean age at diagnosis was 5.54 years (median [Min, Max] = 3 [0.02, 18]) (Table 1).

Table 1 Demographics of 43 pediatric hematology/oncology patients who received compassionate de-escalation of life-sustaining treatments.
Disease characteristics
Sixteen (37.2%) patients had acute lymphoblastic leukemia, and 11 (25.6%) had acute myelogenous leukemia. Evidence of relapsed disease was found in 24 (55.8%) patients, and 23 (53.5%) had undergone allogeneic HCT (Table 2).

Table 2 Disease characteristics of 43 pediatric hematology/oncology patients who received compassionate de-escalation of life-sustaining treatments.
End-of-life care
Of the 43 patients included in the study, 25 (58.1%) received cancer-directed therapy during their last month of life, and 20 (46.5%) received it during their last week (Table 3). Among the patients requiring compassionate de-escalation of LSTs, 24 (55.8%) underwent compassionate extubation, 14 (32.6%) had withdrawal of vasoactive support, and 5 (11.6%) experienced both (Table 4).

Table 3 End-of-life characteristics of 43 pediatric patients undergoing compassionate de-escalation of life-sustaining treatments.
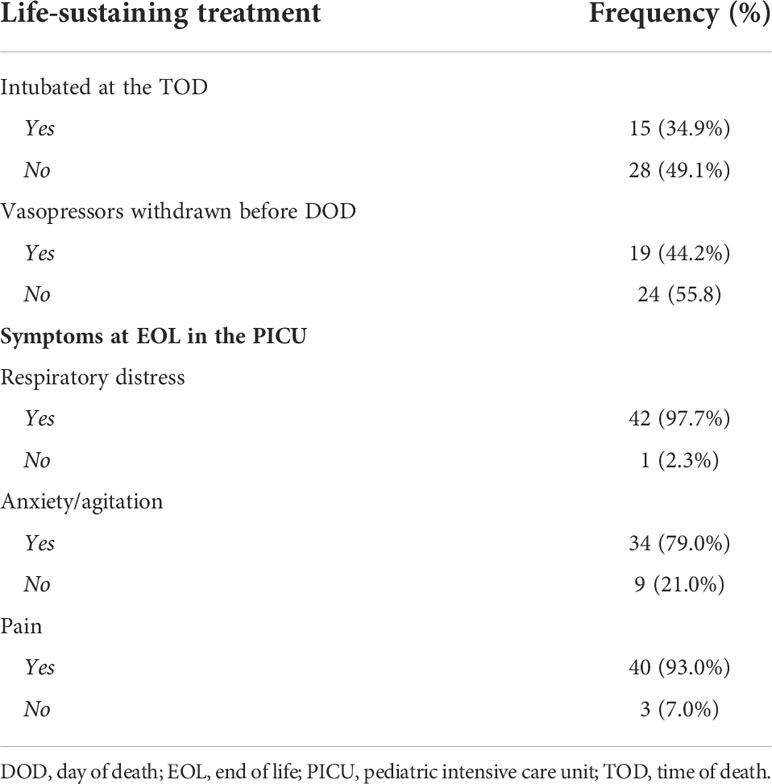
Table 4 Frequency of life-sustaining treatments withdrawn at the end of life and symptoms experienced.
Regarding EOL characteristics, 38 (88.3%) patients had a physician order for scope of treatment (POST - DNaR) in place before death, and only 1 (2.3%) patient received cardiopulmonary resuscitation on the DOD (Table 3). POSTs were completed an average of 13.9 days (median [Min, Max] = 1 [0, 373]) before DOD (Table 3).
Palliative care consultation
PC was consulted for 42 (97.6%) patients, and the average number of PC visits was 14.2 (median [Min, Max] = 9 [0, 61]) (Table 3). Of note, 8 (18.6%) patients received PC consultation on the DOD; however, for the remainder, PC was involved approximately 2 weeks before the DOD. For many patients and families, the goal of care remained cure, despite PC involvement, and only 5 (11.6%) patients enrolled in hospice.
Pain management consultation
Only 6 (14.2%) patients received pain service consultation, and the average number of pain service visits was 6.9 (median [Min, Max] = 0 [0, 118]) (Table 3). Those who received pain service consultation did so on average 4.3 months before the DOD and the consult was for pharmacological pain management. None of the patients received interventional pain modalities for pain management at the end of life.
Medication management
Complex medication regimens addresses the symptom burden at the EOL. Respiratory distress (97.6%, n=42), pain (93.0%, n=40), and anxiety/agitation (79.1%, n=34) were the most commonly reported symptoms experienced at the EOL (Table 4).
All patients received one or more opioid medication to manage their symptoms. Midazolam (79.1%, n=34) and lorazepam (62.8%, n=27) were the two benzodiazepines most often prescribed (Figure 1A). For vasopressor and inotropic support, norepinephrine and dopamine were most commonly prescribed (46.5%, n=20), followed by epinephrine (44.2%, n=19) and vasopressin (32.6%, n=14) (Figure 1B). Sedative agents, such as propofol (14.0%, n=6), dexmedetomidine (11.6%, n=5), or both (44.2%, n=19), were administered (Figure 1C).
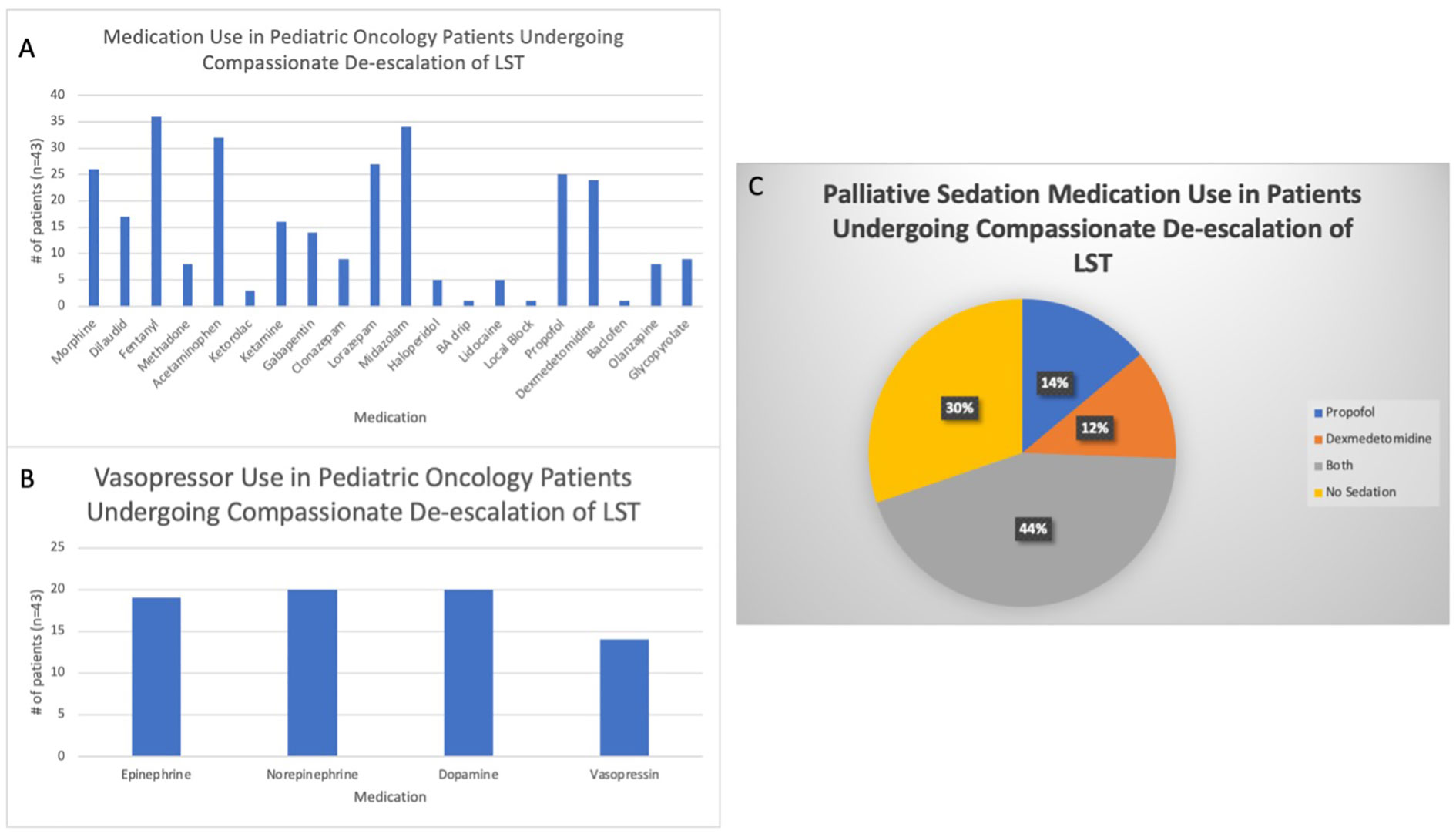
Figure 1 Medications used to treat pediatric oncology patients during compassionate de-escalation of life-sustaining treatments (LSTs). (A) All medications prescribed to the study population to treat symptoms at the end of life. BA= Benadryl/Ativan. (B) Vasopressor medications used to treat hypotension in the study cohort. (C) Use of palliative sedation therapy, specifically dexmedetomidine and propofol, during the end of life.
Discussion
Our study examined de-escalation of LSTs for pediatric hematology/oncology patients at the EOL in the PICU setting, a topic that has not been summarized in more than a decade and is often generalized for all pediatric patients (13). The need for intensive care, and by default implementation of LSTs, such as mechanical ventilation and vasopressor or inotropic support, for pediatric hematology/oncology patients is associated with increased mortality risk (13, 22–25), especially for patients undergoing HCT (10, 14). Additional factors associated with poor overall survival of patients admitted to the PICU include multisystem organ dysfunction (24, 26–28) and sepsis (13). Despite advances in the treatment of childhood cancers, the risk for therapeutic toxicities, including death, and the prevalence of suffering at the EOL remain prominent (29).
We found that across all deaths that occurred in the PICU during the study period, 40.2% of patients experienced compassionate de-escalation of LSTs, specifically either removal of vasopressor/inotropic support, or compassionate extubation, or both. This is consistent with the literature, which suggests that approximately 40%-60% of all PICU deaths occur after removal of LSTs (18–20). The majority of patients had leukemia, which was not surprising, as leukemia is the most common oncologic diagnosis in children (30). Additionally, HCT is performed primarily for hematologic malignancies at the study institution, as noticed in over half of the study cohort, and is associated with significant risk for treatment-related toxicity, including death (31–34). Despite medical advances in the treatment of childhood cancer, 1 in 5 patients will still succumb to their disease (35), and it is well documented that the EOL period can be complicated by physical, psychosocial, emotional, and spiritual suffering (5, 31, 35–37).
From the onset of diagnosis, oncologists facilitate important conversations surrounding disease status and therapeutic options and partner with pediatric intensivists to care for patients when intensive care is required and potentially during the EOL period (38). One way to help minimize the suffering experienced by patients during the EOL in the PICU setting and improve QOL may be through early engagement and collaboration with PC teams. Our study showed that early collaboration between intensivists and PC teams is feasible and it is supported by the literature. Evidence throughout the literature suggests several opportunities for interfacing and collaboration between intensivists and PC team, to improve the EOL experience of patients, their families, and even medical teams, through assistance with advanced-care planning, shared medical decision making, particularly when faced with decisions about de-escalation of care and symptom management, which may include palliative sedation therapy (PST) (3, 7, 32, 39). PST is defined as, “the use of sedative medications to relieve intolerable and refractory distress by the reduction in patient consciousness” (40–44). Over the last several years, acceptance of early integration of PC in the realm of pediatric oncology and PICU care has grown with institutions using the PICU admission as a trigger for PC consultation (38, 45–49).
All but one patient in our study engaged with the PC team. The PC team was involved an average of 2 weeks before the DOD, allowing for time to build rapport and trust with families. This is important for the patient and family, as well as all medical teams, as this time enables providers to gain a better understanding of what is important to a patient and family and what their goals may be. For example, most of the families reported a goal of cure for their child, which may explain the finding that approximately half of our study population received cancer-directed treatment during the last month and/or week of life. By extension, this information helps the medical team provide support and guidance to patients and families through shared medical decision making. Although the literature is rich on the topic of shared medical decision making, little is known about how families come to the decision to forgo LSTs (50–53). One prospective study found that parents initiate a conversation about compassionate de-escalation of care in about 25% of cases, and that consensus can be reached between medical teams and families after one meeting in about 50% of cases (21). Future exploration of the timing of decision making, withdrawal of LSTs, use of PST, and barriers to family consensus are recommended.
In addition to augmenting discussions on de-escalation of LSTs, the PC team can work with the medical teams to facilitate conversations of advanced-care planning, specifically in providing an extra layer of support for families and medical staff. In some instances, these conversation may include facilitating death in the home setting and coordinating hospice services, and for many patients in an acute ICU setting these conversations simply revolve around creating a calm, loving EOL period with family members at the bedside and forgoing cardiopulmonary resuscitation that may incur suffering (1, 54, 55). More than 80% of our cohort had a Do Not Resuscitate order in place before death, and only 1 patient received cardiopulmonary resuscitation. Additionally, the average time between Do Not Resuscitate decisions and death was approximately 14 days, which we hypothesized allowed families time to discuss all options and make a well-informed, goal-centric decision for their child and family. In contrast, approximately 20% of the patients in our cohort met the PC team on the DOD. We believe that unexpected acute changes to signify a high likelihood of death and consultation occurring as the team was preparing to remove LSTs most likely contributed to this delay and poses a potential opportunity for practice improvement. As PC encompasses a holistic approach to caring for patients and families, this concept becomes increasingly important during points of high patient acuity in one’s care journey, especially when PICU care is required.
Previous research suggests the PICU admission as a time point for consideration in engaging PC teams (56, 57) and optimizing PC integration in the PICU setting. One way this may be accomplished is through an embedded model in which PICU staff members are identified and trained to be PC champions; a model that currently exists with success in some PICUs, as well as pediatric critical care fellowship programs across the nation (48). Training would include PC course work and subspecialty clinical rotation experience, with the goal of increasing awareness and education of ICU staff about PC services available to patients and families (48). Prospective studies and qualitative data would help determine the most effective way for PC teams to support patients and families during EOL in a PICU.
When death becomes inevitable, many families and caregivers begin to hope for a “good death” for their child (58, 59). The concept of a good death looks different to each family unit, and in some instances, compassionate de-escalation of LSTs has been requested to occur in the home, an option that may not be considered by healthcare professionals (4, 60–62). Many parents describe a high symptom burden at the EOL and state this as a major contributor to their child’s suffering (29). Specifically, pain, dyspnea, fatigue, and anxiety are commonly reported by patients and noted as a source of suffering by parents (7, 29, 36). Our finding of pain, anxiety/agitation, and respiratory distress being present for nearly all patients in our study further supports this. Traditional symptom management, with medications and psychological coping behaviors, are often enough to alleviate suffering, but what happens when they are not? In some cases, advanced adjuvant medications for pain can be employed (lidocaine or ketamine infusions), or interventional strategies (e.g., nerve blocks or neuraxial blocks), and even implants, such as epidural catheters, can be employed (63–67).
In rare cases, suffering persists despite all interventions, and PST is an effective tool for refractory suffering at the EOL (40–44). PST practices are variable in pediatric oncology, and medication choices for implementation of PST are evolving (40, 68, 69). Propofol and dexmedetomidine are utilized more commonly for this intervention (68–71).
Dexmedetomidine and propofol are commonly used in the PICU setting (72). However, we took a closer look at what medications were used within 24 hours of the TOD to decipher how often patients received medication regimens like those used in PST practices. Many patients were actively receiving propofol, dexmedetomidine, or both at the TOD. We hypothesize that these medications are often used for sedation while the patient receives mechanical ventilation and/or for symptom control before de-escalation of care and were continued to avoid withdrawal and ensure adequate symptom management until the TOD. An observational study of death in the PICU completed more than 2 decades ago demonstrated similar findings of sedative and analgesic use after de-escalation of LSTs in the PICU (1). It also noted 13% of medical professionals were dissatisfied with the EOL care provided and felt that the level of medication administration was inadequate for symptom management (1). However, the patient population in that study encompassed all of pediatrics and was not focused on pediatric hematology/oncology. Propofol or dexmedetomidine administration in our study may not have been intended for PST, but it remains a fascinating finding and offers an opportunity for increased education, awareness, and implementation of PST practices in the PICU. Of the 13 patients who did not receive propofol or dexmedetomidine, four had one formal documented brain death exam and died prior to a second, confirmatory exam, and one patient had documented compression of the brainstem. It is also important to note that medications, such as opioids, benzodiazepines, antiemetics, muscle relaxants, and gabapentinoids, still have an important role in symptom management at the EOL and many patients in this study required these medications as part of their symptom management.
Pain medicine specialists and anesthesiologists are an additional resource for patients, families, and medical teams mediating EOL symptoms. Our study showed that after compassionate de-escalation of LSTs, most pediatric oncology patients who died in the PICU did not involve the pain service in their care. Collaboration between the PC team and pain specialists can help optimize traditional strategies, incorporate interventional tactics (e.g., nerve blocks), and initiate PST if warranted (40).
Overall, this study builds on the limited literature on pediatric oncology patients facing de-escalation of LSTs at the EOL. We highlight opportunities for further PC integration to help optimize shared decision making, advanced-care planning, and symptom management at the EOL in the PICU. Future studies characterizing how families decide to compassionately de-escalate LSTs and prospective studies analyzing symptom burden, relief, and interventions specifically surrounding PST practices and drugs like propofol and dexmedetomidine, would be most informative.
This study had several limitations. First, it represents the experience of a single institution that sees a focused patient population with a higher level of patient acuity and death. Second, retrospective data collection relies on precise documentation; therefore, information, such as timing of discontinuation of a medication or LST, and specifics around decision making are not always easily identified. As such, this study design limits our ability to draw conclusions on causality or intent of medication use (i.e., propofol for PST) and necessitates the need for future prospective investigations.
Conclusion
Pediatric hematology/oncology patients admitted to the PICU have increased risk of mortality, and especially when LSTs are necessary, early integration of PC may be beneficial. It remains unclear how families decide to compassionately de-escalate LSTs, how this decision may affect suffering at the EOL, and how medication practices in the PICU may incorporate concepts of PST. Collaborations between oncologists, intensivists, and PC specialists may help optimize QOL and minimize suffering. Furthermore, prospective and qualitative studies in this realm and increased educational awareness of EOL interventions, including the use of PST, are needed.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
This work was funded by the American Lebanese Syrian Associated Charities (ALSAC). As a participant in the Pediatric Oncology Education Program at St. Jude Children’s Research Hospital, MP was supported, in part, by R25 CA23944 from the National Cancer Institute. The content of this paper is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Acknowledgments
We would like to acknowledge the support of the pediatric palliative care and pain management experts for their care to our patients, as well as the patients who participated in our study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
DOD, day of death; EOL, end of life; HCT, hematopoietic cell transplantation; LST, life-sustaining treatment; PC, palliative care; PICU, pediatric intensive care unit; POST, physician order for scope of treatment; PST, palliative sedation therapy; QOL, quality of life; TOD, time of death.
References
1. Burns JP, Rushton CH. End-of-life care in the pediatric intensive care unit: Research review and recommendations. Crit Care Clin (2004) 20(3):467–85. doi: 10.1016/j.ccc.2004.03.004
2. Cunningham RM, Walton MA, Carter PM. The major causes of death in children and adolescents in the united states. N Engl J Med (2018) 379(25):2468–75. doi: 10.1056/NEJMsr1804754
3. Kaye EC, Gushue CA, DeMarsh S, Jerkins J, Sykes A, Lu Z, et al. Illness and end-of-life experiences of children with cancer who receive palliative care. Pediatr Blood Cancer (2018) 65(4): p. 1550–6. doi: 10.1002/pbc.26895
4. Gupta N, Harrop E, Lapwood S, Shefler A. Journey from pediatric intensive care to palliative care. J Palliat Med (2013) 16(4):397–401. doi: 10.1089/jpm.2012.0448
5. Snaman JM, Kaye E.C, Lu JJ, Sykes A, Baker JN, et al. Palliative care involvement is associated with less intensive end-of-Life care in adolescent and young adult oncology patients. J Palliat Med (2017) 20(5):509–16. doi: 10.1089/jpm.2016.0451
6. Wolfe J, Friebert S, Hilden J. Caring for children with advanced cancer: integrating palliative care. Pediatr Clinics (2002) 49(5):1043–62.
7. Wolfe J, Hammel JF, Edwards KE, Duncan J, Comeau M, Breyer J, et al. Easing of suffering in children with cancer at the end of life: is care changing? J Clin Oncol (2008) 26(10):1717–23. doi: 10.1200/JCO.2007.14.0277
8. Suttle ML, Jenkins TL, Tamburro RF. End-of-Life and bereavement care in pediatric intensive care units. Pediatr Clin North Am (2017) 64(5):1167–83. doi: 10.1016/j.pcl.2017.06.012
9. Weaver MS, Heinze KE, Bell CJ, Wiener L, Garee AM, Kelly KP, et al. Establishing psychosocial palliative care standards for children and adolescents with cancer and their families: An integrative review. Palliat Med (2016) 30(3):212–23. doi: 10.1177/0269216315583446
10. Tamburro RF, Barfield RC, Shaffer ML, Rajasekaran S, Woodard P, Morrison RR, et al. Changes in outcomes (1996-2004) for pediatric oncology and hematopoietic stem cell transplant patients requiring invasive mechanical ventilation. Pediatr Crit Care Med (2008) 9(3):270–7. doi: 10.1097/PCC.0b013e31816c7260
11. Dalton HJ, Slonim AD, Pollack MM. MultiCenter outcome of pediatric oncology patients requiring intensive care. Pediatr Hematol Oncol (2009) 20(8):643–9.
12. Van Veen A, Karstens A, Hoek Der Van A, Tibboel D, Hählen K, Voort der van E, et al. The prognosis of oncologic patients in the pediatric intensive care unit. Intensive Care Med (1996) 22(3):237–41. doi: 10.1007/BF01712243
13. Dursun O, Hazar V, Karasu GT, Uygun V, Tosun O, Yesilipek A, et al. Prognostic factors in pediatric cancer patients admitted to the pediatric intensive care unit. J Pediatr Hematol/Oncol (2009) 31(7):481–4. doi: 10.1097/MPH.0b013e3181a330ef
14. Rowan CM, Gertz SJ, McArthur J, Fitzgerald JC, Nitu ME, Loomis A, et al. Invasive mechanical ventilation and mortality in pediatric hematopoietic stem cell transplantation: A multicenter study. Pediatr Crit Care Med (2016) 17(4):294–302. doi: 10.1097/PCC.0000000000000673
15. Feudtner C. Collaborative communication in pediatric palliative care: A foundation for problem-solving and decision-making. Pediatr Clinics North Am (2007) 54(5):583–607. doi: 10.1016/j.pcl.2007.07.008
16. Postier A, Catrine K, Remke S. Interdisciplinary pediatric palliative care team involvement in compassionate extubation at home: From shared decision-making to bereavement. Children (Basel) (2018) 5(3): p. 37. doi: 10.3390/children5030037
17. Levine D, Cohen K, Wendler D. Shared medical decision-making: considering what options to present based on an ethical analysis of the treatment of brain tumors in very young children. Pediatr Blood Cancer (2012) 59(2):216–20. doi: 10.1002/pbc.24189
18. Mink RB, Pollack MM. Resuscitation and withdrawal of therapy in pediatric intensive care. Pediatrics (1992) 89(5):961–3. doi: 10.1542/peds.89.5.961
19. Vernon DD, Dean JM, Timmons OD, Banner W Jr, Allen-Webb EM. Modes of death in the pediatric intensive care unit: withdrawal and limitation of supportive care. Crit Care Med (1993) 21(11):1798–802. doi: 10.1097/00003246-199311000-00035
20. Lantos JD, Berger AC, Zucker AR. Do-not-resuscitate orders in a children’s hospital. Crit Care Med (1993) 21(1):52–5. doi: 10.1097/00003246-199301000-00012
21. Garros D, Rosychuk RJ, Cox PN. Circumstances surrounding end of life in a pediatric intensive care unit. Pediatrics (2003) 112(5):e371. doi: 10.1542/peds.112.5.e371
22. Butt W, Barker G, Walker C, Gillis J, Kilham H, Stevens M, et al. Outcome of children with hematologic malignancy who are admitted to an intensive care unit. Crit Care Med (1988) 16(8):761–4. doi: 10.1097/00003246-198808000-00005
23. Hallahan AR, Shaw PJ, Rowell G, O’Connell A, Schell D, Gillis J. Improved outcomes of children with malignancy admitted to a pediatric intensive care unit. Crit Care Med (2000) 28(11):3718–21. doi: 10.1097/00003246-200011000-00030
24. Abraham RB, Toren A, Ono N, Weinbroum AA, Vardi A, Barzilay Z, et al. Predictors of outcome in the pediatric intensive care units of children with malignancies. J Pediatr Hematol/Oncol (2002) 24(1):23–6. doi: 10.1097/00043426-200201000-00007
25. Sivan Y, Schwartz P, Schonfeld T, Cohen I, Newth C, et al. Outcome of oncology patients in the pediatric intensive care unit. Intensive Care Med (1991) 17(1):11–5. doi: 10.1007/BF01708402
26. Heying R, Schneider DT, Körholz D, Stannigel H, Lemburg P, Göbel U, et al. Efficacy and outcome of intensive care in pediatric oncologic patients. Crit Care Med (2001) 29(12):2276–80. doi: 10.1097/00003246-200112000-00007
27. Meyer S, Gottschling S, Biran T, Georg T, Ehlayil K, Graf N, et al. Assessing the risk of mortality in paediatric cancer patients admitted to the paediatric intensive care unit: a novel risk score? Eur J Pediatr (2005) 164(9):563–7. doi: 10.1007/s00431-005-1695-y
28. Haase R, Mathony U, Lieser U, Nagel F, Sitka U, Burdach S, et al. Oncology patients in a pediatric intensive care unit–a 7-year experience. Klinische Padiatrie (2003) 215(4):234–40.
29. Wolfe J, Grier H.E, Klar N, Levin S.B, Ellenbogen J.M, Salem-Schatz S, et al. Symptoms and suffering at the end of life in children with cancer. New Engl J Med (2000) 342(5):326–33. doi: 10.1056/NEJM200002033420506
30. Poplack DG, Pizzo PA. Childhood cancer: Incidence, survival and mortality. In: Principles and practice of pediatric oncology, 7th ed. Philadelphia, PA: Lippincott Williams & Wilkins (2016).
31. Ullrich CK, Dussel V, Hilden JM, Sheaffer JW, Lehmann L, Wolfe J. End-of-life experience of children undergoing stem cell transplantation for malignancy: parent and provider perspectives and patterns of care. Blood J Am Soc Hematol (2010) 115(19):3879–85.
32. Ullrich CK, Lehmann L, London W.B, Guo D, Sridharan M, Koch R, et al. End-of-Life care patterns associated with pediatric palliative care among children who underwent hematopoietic stem cell transplant. Biol Blood Marrow Transplant (2016) 22(6):1049–55. doi: 10.1016/j.bbmt.2016.02.012
33. Munchel A, Chen A, Symons H. Emergent complications in the pediatric hematopoietic stem cell transplant patient. Clin Pediatr Emergency Med (2011) 12(3):233–44. doi: 10.1016/j.cpem.2011.07.005
34. Snaman JM, Talleur AC, Lu J, Levine DR, Kaye EC, Sykes A, et al. Treatment intensity and symptom burden in hospitalized adolescent and young adult hematopoietic cell transplant recipients at the end of life. Bone marrow Transplant (2018) 53(1):84–90. doi: 10.1038/bmt.2017.187
35. Ward E, DeSantis C, Robbins A, Kohler B, Jemal A. Childhood and adolescent cancer statistics, 2014. CA: Cancer J Clin (2014) 64(2):83–103. doi: 10.3322/caac.21219
36. Wolfe J, Orellana L, Ullrich C, Cook EF, Kang TI, Rosenberg A, et al. Symptoms and distress in children with advanced cancer: Prospective patient-reported outcomes from the PediQUEST study. J Clin Oncol (2015) 33(17):1928–35. doi: 10.1200/JCO.2014.59.1222
37. Bona K, London WB, Guo D, Abel G, Lehmann L, Wolfe J. Prevalence and impact of financial hardship among new England pediatric stem cell transplantation families. Biol Blood Marrow Transplant (2015) 21(2):312–8. doi: 10.1016/j.bbmt.2014.10.016
38. Cuviello A, Raisanen J, Donohue PK, Wiener L, Boss RD. Defining the boundaries of palliative care in pediatric oncology. J Pain Symptom Manage (2019):1033–42. doi: 10.1016/j.jpainsymman.2019.11.022
39. Blais S, Cohen-Gogo S, Gouache E, Guerrini-Rousseau L, Brethon B, Rahal I, et al. End-of-life care in children and adolescents with cancer: perspectives from a French pediatric oncology care network. Tumori J (2021) p. doi: 10.1177/03008916211013384
40. Anghelescu DL, Knapp E, Johnson LM, Baker JN. The role of the pediatric anesthesiologist in relieving suffering at the end of life: when is palliative sedation appropriate in pediatrics? Paediatr Anaesth (2017) 27(4):443–4. doi: 10.1111/pan.13103
41. Johnson LM, Frader J, Wolfe J, Baker JN, Anghelescu DL, Lantos JD, et al. Palliative sedation with propofol for an adolescent with a DNR order. Pediatrics (2017) 140(2). doi: 10.1542/peds.2017-0487
42. de Graeff A, Dean M. Palliative sedation therapy in the last weeks of life: a literature review and recommendations for standards. J Palliat Med (2007) 10(1):67–85. doi: 10.1089/jpm.2006.0139
43. Cherny NI, Portenoy RK. Sedation in the management of refractory symptoms: guidelines for evaluation and treatment. J Palliative Care (1994) 10(2):31–8. doi: 10.1177/082585979401000207
44. Lo B, Rubenfeld G. Palliative sedation in dying patients:“we turn to it when everything else hasn’t worked”. Jama (2005) 294(14):1810–6.
45. Dalberg T, McNinch NL, Friebert S. Perceptions of barriers and facilitators to early integration of pediatric palliative care: A national survey of pediatric oncology providers. Pediatr Blood Cancer (2018) 65(6):e26996. doi: 10.1002/pbc.26996
46. Levine DR, Mandrell BN, Sykes A, Pritchard M, Gibson D, Symons HJ, et al. Patients’ and parents’ needs, attitudes, and perceptions about early palliative care integration in pediatric oncology. JAMA Oncol (2017) 3(9):1214–20. doi: 10.1001/jamaoncol.2017.0368
47. Mack JW, Wolfe J. Early integration of pediatric palliative care: for some children, palliative care starts at diagnosis. Curr Opin Pediatr (2006) 18(1):10–4. doi: 10.1097/01.mop.0000193266.86129.47
48. Moynihan KM, Snaman JM, Kaye EC, Morrison WE, DeWitt AG, Sacks LD, et al. Integration of pediatric palliative care into cardiac intensive care: A champion-based model. Pediatrics (2019) 144(2). doi: 10.1542/peds.2019-0160
49. Boss R, Nelson J, Weissman D, Campbell M, Curtis R, Frontera J, et al. Integrating palliative care into the PICU: a report from the improving palliative care in the ICU advisory board. Pediatr Crit Care Med (2014) 15(8):762–7. doi: 10.1097/PCC.0000000000000209
50. Levetown M, Pollack MM, Cuerdon TT, Ruttimann UE, Glover JJ. Limitations and withdrawals of medical intervention in pediatric critical care. JAMA (1994) 272(16):1271–5. doi: 10.1001/jama.1994.03520160055043
51. Wall SN, Partridge JC. Death in the intensive care nursery: physician practice of withdrawing and withholding life support. Pediatrics (1997) 99(1):64–70. doi: 10.1542/peds.99.1.64
52. Devictor D, Nguyen D. The groupe francophone de réanimation et dUrgences pédiatriques. forgoing lifesustaining treatments: how the decision is made in French pediatric intensive care units. Crit Care Med (2001) 29(7):1356–9.
53. Keenan SP, Busche KD, Chen LM, McCarthy L, Inman KJ, Sibbald WJ, et al. A retrospective review of a large cohort of patients undergoing the process of withholding or withdrawal of life support. Crit Care Med (1997) 25(8):1324–31. doi: 10.1097/00003246-199708000-00019
54. Burns JP, Mitchell C, Outwater KM, Geller M, Griffith JL, Todres ID, et al. End-of-life care in the pediatric intensive care unit after the forgoing of life-sustaining treatment. Crit Care Med (2000) 28(8):3060–6. doi: 10.1097/00003246-200008000-00064
55. Kaye EC, DeMarsh S, Gushue CA, Jerkins J, Sykes A, Lu Z, et al. Predictors of location of death for children with cancer enrolled on a palliative care service. Oncol. (2018) 23(12):1525–32. doi: 10.1634/theoncologist.2017-0650
56. Cuviello A, Raisanen JC, Donohue PK, Wiener L, Boss RD. Initiating palliative care referrals in pediatric oncology. J Pain Symptom Manage (2020) 1:81–9.
57. Cuviello A, Yip C, Battles H, Wiener L, Boss R. Triggers for palliative care referral in pediatric oncology. Cancers (2021) 13(6):1419. doi: 10.3390/cancers13061419
58. Broden EG, Hinds PS, Werner-Lin A, Quinn R, Asaro LA, Curley MA, et al. Nursing care at end of life in pediatric intensive care unit patients requiring mechanical ventilation. Am J Crit Care (2022) 31(3):230–9. doi: 10.4037/ajcc2022294
59. Broden EG, Hinds PS, Werner-Lin AV, Curley MA, Investigators RS. I Didn’t want my baby to pass, but I didn’t want him suffering either: Comparing bereaved parents’ narratives with nursing end-of-Life assessments in the pediatric intensive care unit. J Hospice Palliative Nurs (2022) 10:1097.
60. Nelson H, Mott S, Kleinman ME, Goldstein RD. Parents’ experiences of pediatric palliative transports: A qualitative case series. J Pain Symptom Manage (2015) 50(3):375–80. doi: 10.1016/j.jpainsymman.2015.04.004
61. Raed M, Grossoehme DH, Brown M, Friebert S. Hospital to home transport at end of life: Survey of clinician experience. Palliative Med (2020) 34(3):424–9. doi: 10.1177/0269216319870641
62. Zwerdling T, Hamann KC, Kon AA. Home pediatric compassionate extubation: bridging intensive and palliative care. Am J Hospice Palliative Medicine® (2006) 23(3):224–8. doi: 10.1177/1049909106289085
63. Anghelescu DL, Faughnan LG, Baker JN, Yang J, Kane JR. Use of epidural and peripheral nerve blocks at the end of life in children and young adults with cancer: the collaboration between a pain service and a palliative care service. Paediatr Anaesth (2010) 20(12):1070–7. doi: 10.1111/j.1460-9592.2010.03449.x
64. Anghelescu DL, Guo A, Morgan KJ, Frett M, Prajapati H, Gold R, et al. Pain outcomes after celiac plexus block in children and young adults with cancer. J Adolesc Young Adult Oncol (2018) 7(6):666–72. doi: 10.1089/jayao.2018.0035
65. Anghelescu DL, Ryan S, Wu D, Morgan KJ, Patni T, Li Y, et al. Low-dose ketamine infusions reduce opioid use in pediatric and young adult oncology patients. Pediatr Blood Cancer (2022) 69(9):e29693. doi: 10.1002/pbc.29693
66. Anghelescu DL, Morgan KJ, Frett MJ, Wu D, Li Y, Han Y, et al. Lidocaine infusions and reduced opioid consumption-retrospective experience in pediatric hematology and oncology patients with refractory pain. Pediatr Blood Cancer (2021) 68(11):e29215. doi: 10.1002/pbc.29215
67. Hall EA, Sauer HE, Davis MS, Anghelescu DL. Lidocaine infusions for pain management in pediatrics. Paediatr Drugs (2021) 23(4):349–59. doi: 10.1007/s40272-021-00454-2
68. Anghelescu DL, Hamilton H, Faughnan LG, Johnson LM, Baker JN. Pediatric palliative sedation therapy with propofol: recommendations based on experience in children with terminal cancer. J Palliat. Med (2012) 15(10):1082–90. doi: 10.1089/jpm.2011.0500
69. Cuviello A, Anghelescu D, Johnson L-M, Baker J. Dexmedetomidine and propofol use for palliative sedation therapy (PST) in pediatric oncology: A ten-year review (GP750). J Pain Symptom Manage (2022) 63(6):1139–40. doi: 10.1016/j.jpainsymman.2022.04.141
70. Burns J, Jackson K, Sheehy KA, Finkel JC, Quezado ZM. The use of dexmedetomidine in pediatric palliative care: A preliminary study. J Palliat. Med (2017) 20(7):779–83. doi: 10.1089/jpm.2016.0419
71. Hooke MC, Grund E, Quammen H, Miller B, McCormick P, Bostrom B, et al. Propofol use in pediatric patients with severe cancer pain at the end of life. J Pediatr Oncol Nurs (2007) 24(1):29–34. doi: 10.1177/1043454206296026
Keywords: palliative care, palliative sedation therapy, dexmedetomidine, propofol, pediatric oncology, symptom management, end of life
Citation: Cuviello A, Pasli M, Hurley C, Bhatia S, Anghelescu DL and Baker JN (2022) Compassionate de-escalation of life-sustaining treatments in pediatric oncology: An opportunity for palliative care and intensive care collaboration. Front. Oncol. 12:1017272. doi: 10.3389/fonc.2022.1017272
Received: 11 August 2022; Accepted: 30 September 2022;
Published: 13 October 2022.
Edited by:
Kris Michael Mahadeo, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Jerelyn Roberson Moffet, Duke University, United StatesPaulo Roberto Carvalho, Federal University of Rio Grande do Sul, Brazil
Shira Gertz, Saint Barnabas Medical Center, United States
Copyright © 2022 Cuviello, Pasli, Hurley, Bhatia, Anghelescu and Baker. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Andrea Cuviello, YWpjdXZpZWxsb0BnbWFpbC5jb20=
†Present address: Melisa Pasli, Office of Medical Education, Brody School of Medicine, East Carolina University, Greenville, NC, United States
 Andrea Cuviello
Andrea Cuviello Melisa Pasli
Melisa Pasli Caitlin Hurley
Caitlin Hurley Shalini Bhatia4
Shalini Bhatia4 Doralina L. Anghelescu
Doralina L. Anghelescu