- Cancer Center, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
Background: Pituitary metastasis accounts for a very low percentage of cases of brain metastasis from lung cancer, and there are uncertainties and challenges in diagnosis and treatment. We hope to shed some light on the diagnosis and treatment by reporting a case of ALK fusion mutation-positive lung cancer pituitary metastasis.
Case presentation: We report a 48-year-old female patient with an initial diagnosis of stage IVB lung adenocarcinoma with ALK fusion. The patient developed headache, dizziness, hypopituitarism and hyperprolactinemia one year after treatment with crizotinib. Later, the patient underwent neurosurgical resection of the pituitary tumor and then symptomatic relief. Postoperative pathology suggested pituitary metastasis, and the next-generation gene sequencing conducted on the pituitary metastasis indicated that secondary drug resistance mutation ALK-I1171s occurred after the ALK fusion gene.
Conclusion: In this article, we present a patient with suspected pituitary metastases with lung cancer. The progression to pituitary mass resection and next-generation gene sequencing of the pituitary metastasis are suggestive for further diagnosis and treatment.
Introduction
According to available reports, pituitary metastases from lung cancer are very rare, especially when compared with metastases to other endocrine glands, such as the adrenal gland and thyroid gland (1). Lung cancer (24.2%) is the second most common malignant tumor for pituitary metastases after breast cancer (37.2%), followed by prostate cancer (5%), kidney cancer (5%) and lymphoma (2). Some studies have found that the average overall survival of patients with pituitary metastases is 10-13 months (3, 4). Patients with pituitary metastases from lung cancer are usually asymptomatic at first. However, as the tumor grows to a certain size, the mass will cause central nervous system and endocrine system symptoms. The most common symptoms are visual impairment, followed by hypopituitarism, hyperprolactinemia, and diabetes insipidus (5). Clinical symptoms and imaging examination cannot effectively differentiate pituitary metastases from benign pituitary tumors. Chemotherapy, radiation therapy, hormonal therapy and surgery are the available treatment options, but there is no conclusive evidence that these treatments can prolong the survival of patients (6). This paper reports an ALK fusion mutation-positive lung adenocarcinoma patient with pituitary metastasis. After surgical treatment, the symptoms of dizziness, headache, hypopituitarism and hyperprolactinemia were significantly relieved. Meanwhile, our team also conducted next-generation gene sequencing of the pituitary metastasis, and the results indicated that secondary drug resistance mutation ALK-I1171s occurred in the ALK fusion gene.
Case presentation
A 48-year-old Chinese woman with no smoking history presented to the Union Hospital of Tongji Medical College of Huazhong University of Science and Technology in October 2020 for chest tightness and chest pain. The 18F-fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) revealed a soft tissue density shadow in the right lower lobe with a size of 3.4*2.4 cm; enlarged bilateral supraclavicular and mediastinal lymph nodes, thickened bilateral pleura and increased pericardial effusion were metabolism increased; the metabolism in thoracic vertebrae T4, lumbar vertebrae L1, L5, and right ilium were also increased (Figure 1). Baseline lung enhanced CT images are shown in Figure 2A. Brain MRI showed no abnormalities (Figure 2F). In October 2020, the patient underwent ultrasound-guided pericardiocentesis of pericardial effusion. Upon pathological examination of the pericardial effusion, cancer cells were detected, which was consistent with lung adenocarcinoma (Figure 3). Immunohistochemistry yielded the following: PCK (+), TTF-1(+), i+), CK5/6 (–), EGFR (–), C-met (–), Ros-1 (–), ALK (+). Next-generation genetic sequencing (whole-exome sequencing by company of gloriousmed) suggested the ALK EML4 (7, 8) - ALK (9, 10) fusion. Variant allele frequency (VAF) was 3.81%. Another driver mutation was CUX1 (mutation region: chr7: 101821924, 11 exon c.1004delA, p. Asn335fsTer20, VAF 1:00%), and others were somatic mutations of no clinical value, such as INPP4A, MED12, CDC73, CIC, SACS, NOTCH3, ARID2, RPS2, IRS2, INHA. Therefore, the patient was initially diagnosed with lung adenocarcinoma stage cT2N3M1c IVB with EML4-ALK gene fusion (AJCC 8th Edition). She started treatment with crizotinib (p.o. 250 mg bid) in October 2020 for financial reasons. In January 2021, the patient’s lung CT showed that the lesion in the lower lobe of the right lung was 2.2*1.7 cm, and the pericardial effusion was significantly reduced (Figure 2B. According to the criteria of resist1.1, the therapeutic effect was evaluated as partial response (PR). The patient started to have headache and dizziness in September 2021, and brain MRI showed that the pituitary gland was full in shape on September 7, 2021, approximately 1.4*1.4*1.3 cm in size, and the lung lesion was stable (Figures 2C, G). Then, a lumbar puncture was performed, and no significant abnormalities were seen in the cerebrospinal fluid panel, cerebrospinal fluid biochemistry or cerebrospinal fluid cytology. ACTH (0 am): 5.83 pg/ml, ACTH (8 am): 10.20 pg/ml, ACTH (4 pm): 7.03 pg/ml (normal value: 7-64 pg/ml); cortisol (0 am):7.0μg/L, cortisol (8 am):3.0μg/L, cortisol (4 pm): 1.0μg/L (normal values: 37.0-194 μg/L); prolactin: 113.35 ng/ml (normal values: 1.2-29.9 ng/ml). No abnormalities were seen in sex hormones, thyroid hormone, growth hormone, or insulin-like growth factor. Afterward, our team conducted a multidisciplinary consultation with neurology, neurosurgery, and endocrinology and recommended a follow-up MRI after 1 month of supplemental hormone therapy. However, one month later, the patient’s symptoms of headache and dizziness worsened, and the brain MRI re-examination showed that the pituitary gland was enlarged with a size of approximately 1.8*1.4*1.6 cm. On November 15, 2021 (Figure 2H), the patient underwent sphenoid sinus exploration and nasal endoscopic saddle area tumor excision in our neurosurgery department. During the operation, the neurosurgery team found an occupying lesion in the sellar area with high dural tension, approximately 2*1.5 cm in size, pink in color, and tightly adherent to the surrounding tissues. The postoperative pathology showed that it was consistent with metastatic adenocarcinoma with neuroendocrine marker expression in some areas of pulmonary origin. Immunohistochemistry yielded the following: PCK (+), TTF-1 (+), Napsin A (+), ACTH (–), GH (–), PRL (–), FSH (–), LH (–), TSH (–), Ki67 (Li: 5%) (Figure 4). The next-generation sequencing (whole-exome sequencing by company of gloriousmed) conducted on the pituitary metastasis suggested EML4 (6)-ALK (10) fusion with VAF of 2.63%. Interestingly, the ALK gene then revealed a missense mutation (mutation region: chr2: 29445213, 22 exon p. Ile1171Ser, VAF: 29.2%). Four non-clinically relevant somatic gene variants (missense mutations) were also detected, namely INPP4A, MAGI2, AMER1, MED12.The patient’s postoperative headache and dizziness were significantly relieved compared to before. The patient’s hormone levels were reviewed on November 29, 2021, and most of the abnormal hormone levels returned to normal. On December 01, 2021, the pituitary gland MRI was reviewed and showed that the pituitary tumor was significantly smaller than before. Based on the patient’s gene sequencing results, we recommended that the patient replace crizotinib with ceritinib. On February 7, 2022, the patient’s lung CT showed that the lesion in the lower lobe of the right lung had shrunk further with a size of 1.8*1.4 cm, and the brain MRI re-examination showed no abnormalities (Figures 2D, I). The patient was reexamined every two months by brain MRI and lung CT. The latest reexamination was on August 7, 2022, and no progression was observed (Figures 2E, J).
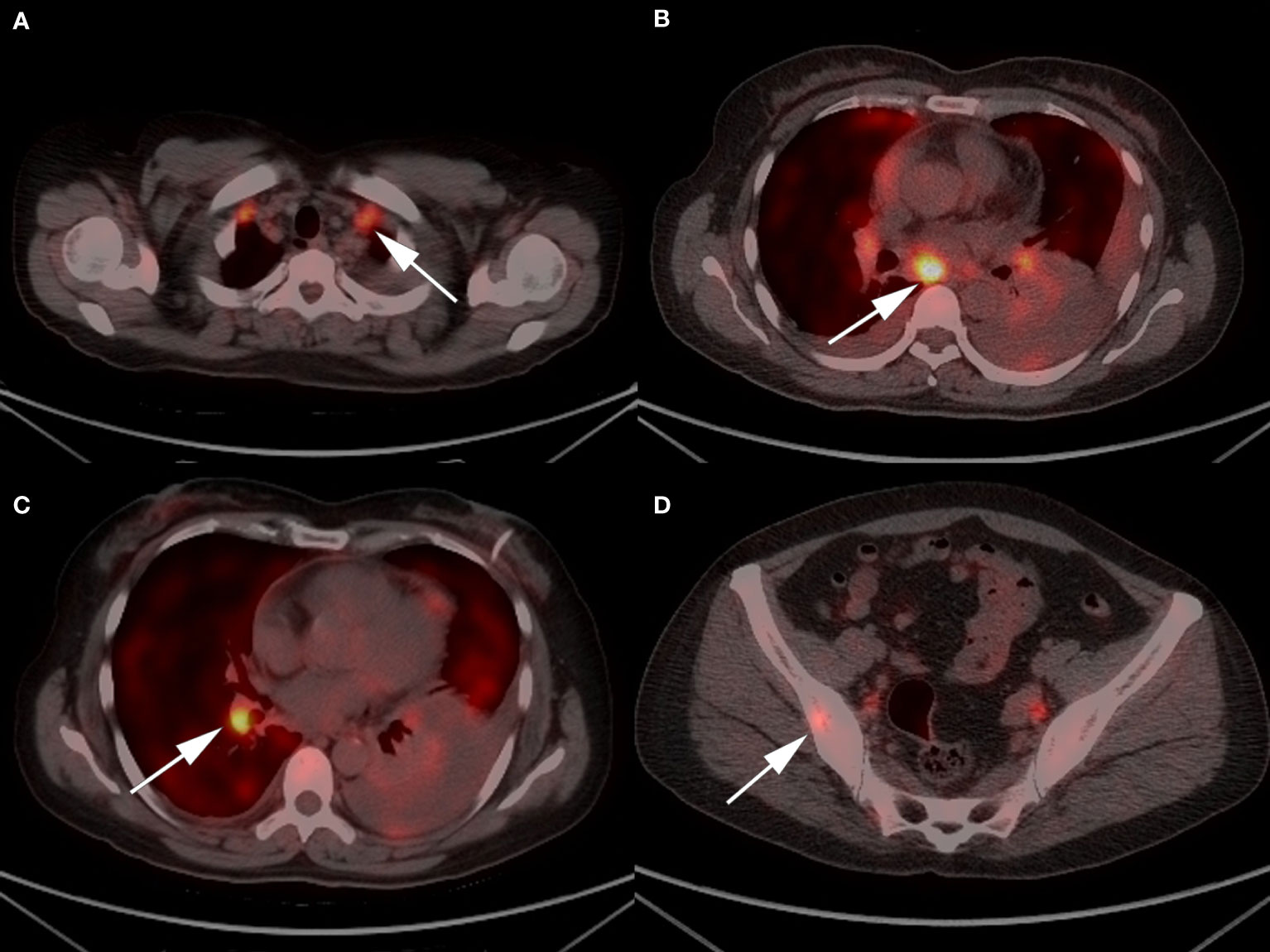
Figure 1 Representative 18F/FDG PET-CT images of the patient: (A) enlarged bilateral supraclavicular and (B) mediastinal lymph nodes; (C) a 3.4*2.4 cm soft tissue density mass in the right lower lobe; and (D) increased metabolism in right ilium.
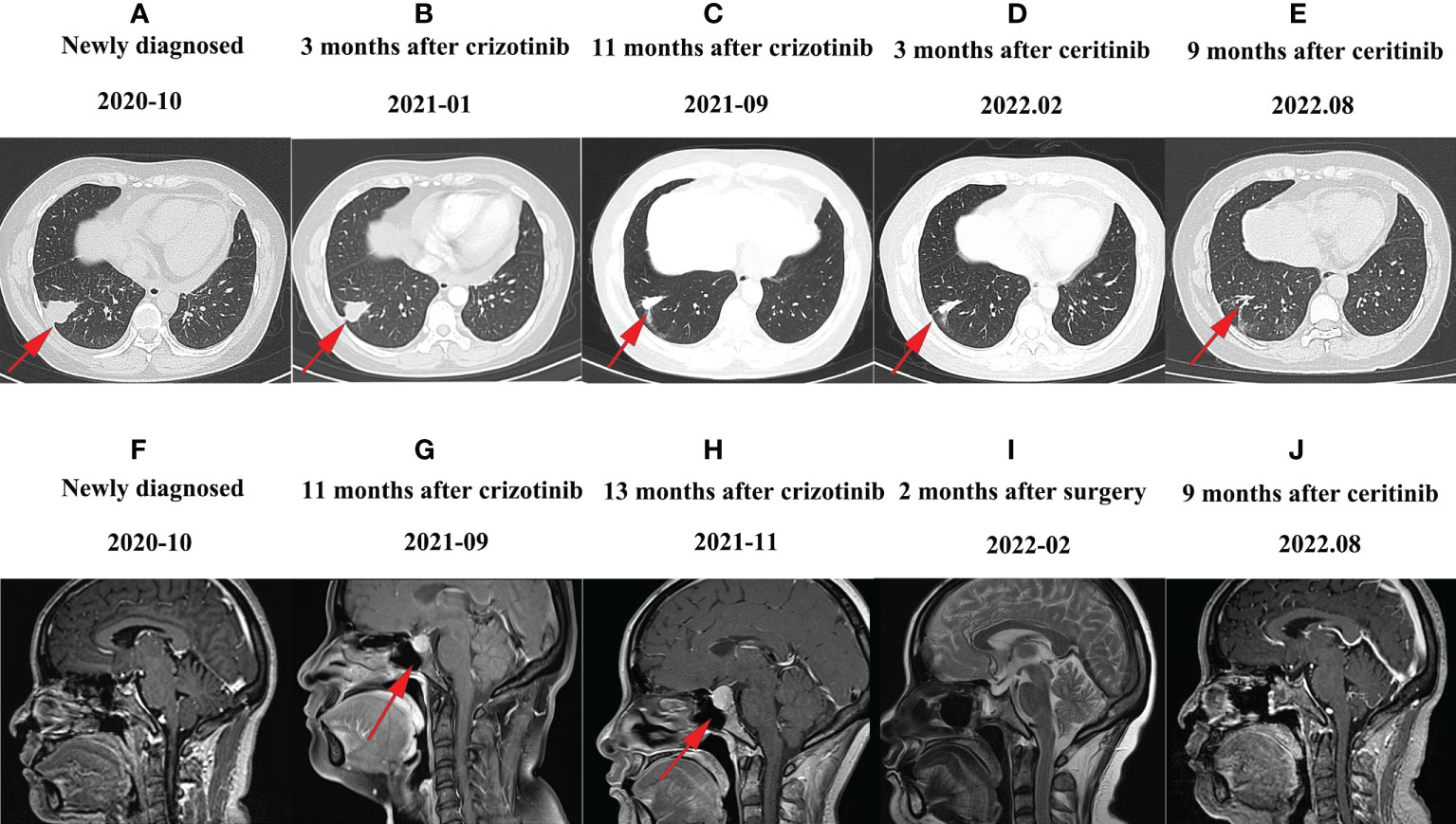
Figure 2 Representative CT or MRI images of the patient: (A) CT image of October 2020 before the treatment. (B) CT image of January 2021 after treatment with crizotinib. The therapeutic effect of the patients was evaluated as partial response. (C) CT image of September 2021 after pituitary metastases; the lung lesion was stable. (D) CT image of February 2022 after treatment with ceritinib; the lung lesions shrank further. (E) CT image of August 2022 after treatment with ceritinib; the lung lesion is still shrinking. (F) MRI image obtained in October 2020 before treatment; brain MRI showed no abnormalities. (G) MRI image of September 2021 after finding pituitary metastases. (H) MRI image after 1 month of supplemental hormone therapy. The pituitary gland was enlarged. (I) MRI image after sphenoid sinus exploration and nasal endoscopic saddle area tumor excision, and the brain MRI showed no mass. (J) Follow-up MRI images 9 months after tumor resection and 9 months after ceritinib administration.
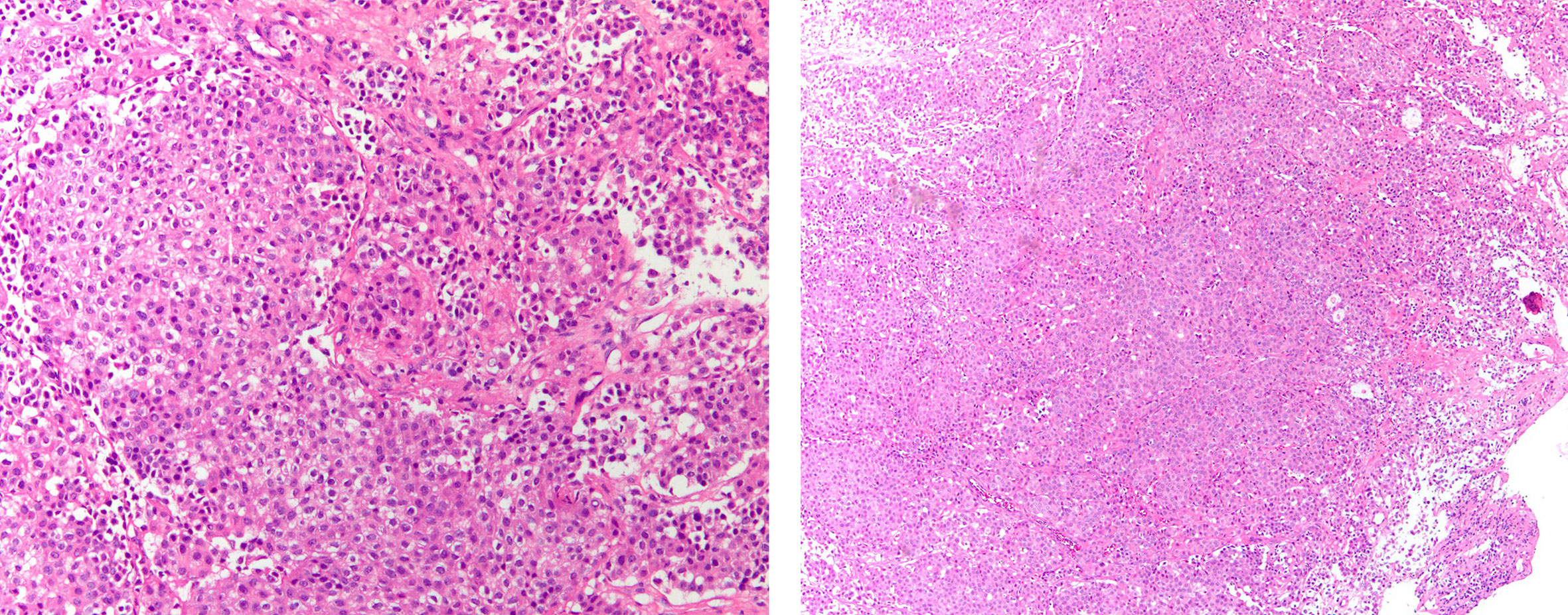
Figure 3 Histopathological findings of pericardial effusion by paraffin embedding of cell sediment. IHC yielded the following: PCK (+), Claudin-4(+), TTF-1(+), NapsinA(+), Calretinin (–), CK5/6 (–), P40 (–), EGFR (–), C-met (–), Ros-1 (–), ALK(+).
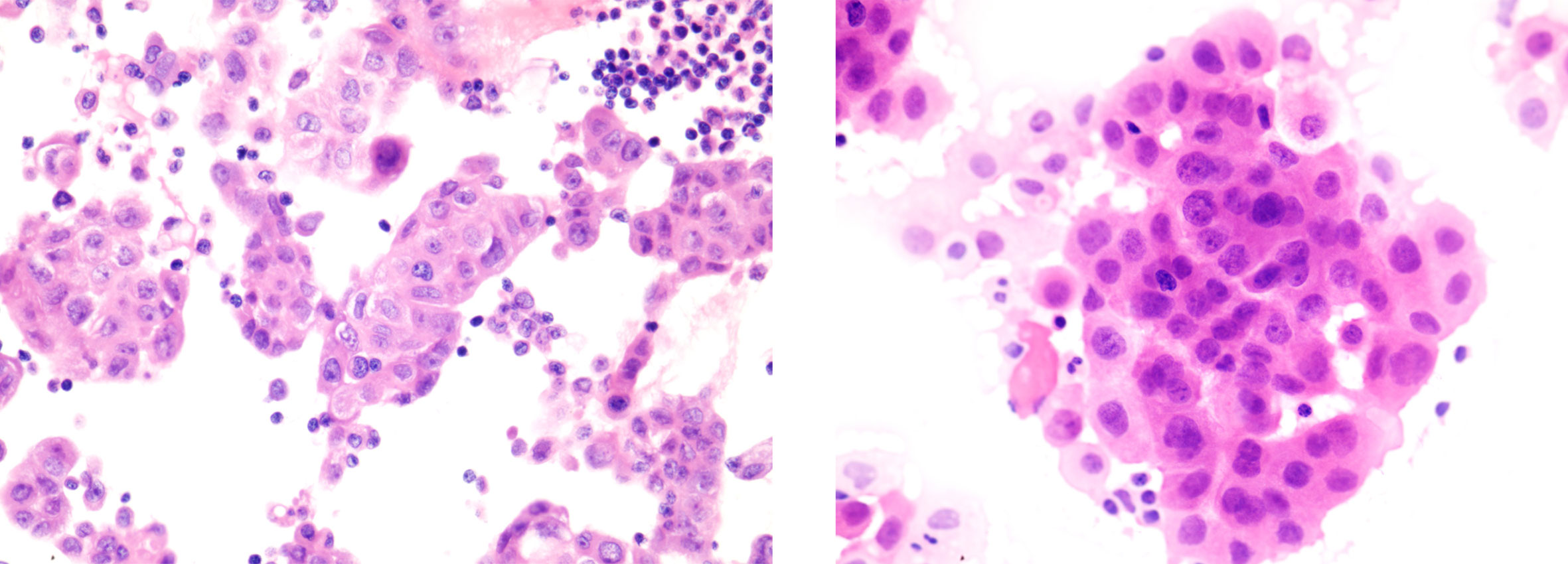
Figure 4 Histopathological findings of pituitary metastases. IHC yielded the following: PCK (+), TTF-1 (+), NapsinA (+), EMA (+), Syn (partial +), CgA (focal +), GFAP (–), S1001 (-), CD68 (-), CD34 (-), ACTH (-), GH (-), PRL (-), FSH (-), LH (-), TSH (-), Ki67 (Li:5%).
Discussion
The incidence of pituitary metastases accounts for 1% of the total incidence of pituitary lesions (7), making clinical differentiation of pituitary tumors from pituitary metastases very challenging. Patients with pituitary metastases from lung cancer are often asymptomatic at the initial stage, and many patients are found to have pituitary occupancy at the time of MRI examination (8). Several studies have confirmed that visual impairment, hypopituitarism, and high prolactin levels are the most common clinical manifestations of pituitary metastases (5, 11). Some studies have shown no significant difference in the overall survival of patients between surgical and nonsurgical treatment of pituitary metastases from lung cancer (12). Aida Javanbakht et al. surveyed 289 patients with pituitary metastases at Dewart Medical Center from 1984-2018 and found that 178 (61.6%) patients opted for surgery for treatment (4). The main surgical option currently available is transsphenoidal surgery, but surrounding tissue adhesions and abundant blood supply often make surgery difficult, and the performance of pituitary metastasectomy requires multidisciplinary cooperation and an experienced neurosurgical team (13). Patients undergoing surgery can suffer from secondary hypopituitarism, which requires lifelong hormone replacement therapy (14). In this case, the patient had a remarkable clinical presentation and underwent surgery after multidisciplinary collaboration between the medical oncology team and the neurosurgery team.
The ALK gene has been identified in a variety of solid tumors and is a potent oncogenic driver gene (15). ALK protein is located on the cell membrane with an extracellular receptor region and an intracellular kinase region. Under normal conditions, two ALK proteins are coupled by extracellular ligands to activate signaling pathways and promote cell growth (16). There are three types of mutations in the ALK gene: 1. ALK fusion mutations are the most common, especially EML4-ALK fusion mutations, and the protein can be activated without ligand; 2. Point mutations are less common and occur mainly in the intracellular kinase region; and after the mutation, they continue downstream conveyance of cell growth signals; 3. Amplification mutations are even less common (17). ALK fusion gene mutation frequency accounts for 2-5% of all non-small cell lung cancers worldwide (16, 17). Patients with ALK fusion mutation non-small cell lung cancer can benefit from crizotinib with an objective response rate of 60% and progression-free survival of 8-10 months, but drug resistance occurs within 1-2 years, with central nervous system relapse being the most common reversal (18, 19). However, alectinib has been shown to have longer PFS than crizotinib in multiple phase III clinical trials, and alectinib is now widely used as a first-line therapy, especially in cases of brain metastasis (20). Since the patient had stable lesions elsewhere, our team decided to perform pituitary occupancy resection to clarify the nature of the lesions and to perform next-generation sequencing if the pathology suggested metastatic adenocarcinoma. Thirty-seven percent of crizotinib resistance cases occur due to ALK secondary resistance mutations, which can be classified as ALK kinase region mutations (28%) and ALK fusion gene copy number amplification (9%) (9). In addition, mechanisms associated with crizotinib resistance also include driver gene conversion as well as tumor heterogeneity (10). This patient showed ALK missense mutations after the development of pituitary metastases, and pituitary tissue genetic sequencing suggested the presence of ALK-I1171S. I1171S refers to the conversion of the second nucleotide (3512 nucleotides of full-length ALK) encoding isoleucine ATC to AGC (serine [S]). Similarly, I1171 includes I1171N (isoleucine ATC converts to AAC (asparagine [N])) and I1171T (isoleucine ATC converts to ACC threonine [T]), etc. Currently, it is unclear why this mutation lead to resistance to crizotinib. Further basic and clinical analyses are required to investigate the biology and clinical significance of the I1171 mutation. However, the I1171 mutation was more frequently reported in secondary resistance mutations after alectinib failure than with crizotinib (21–23). In 2014, Ou SH et al. reported that two NSCLC patients with the EML4-ALK variant initially responded to crizotinib and then alectinib but developed acquired resistance with the presence of a mutation in amino acid residue 1171 (I1171N and I1171S, respectively) located in the hydrophobic regulatory spine of ALK kinase (24). I1171 mutations are believed to decrease the stability of the inhibitor through autophosphorylation (25). Gainor et al. found that ceritinib was effective when patients developed the secondary ALK-1171S mutation (26). Importantly, many researchers found that ALK-I1171 mutations mediate resistance to alectinib while generating sensitivity to ceritinib. ALK-I1171 mutations were found to be the second-most common resistance mutations in post-alectinib therapy (12%). Based on the above evidence, we recommended that our patient switch to ceritinib instead of alectinib, and to date, progression-free survival has been 8 months (22, 26).
Conclusion
Pituitary metastasis from lung cancer is very rare, and surgical treatment could be a positive option. Although crizotinib was chosen for financial reasons in this patient, alectinib has been shown to have longer PFS than crizotinib in multiple phase III clinical trials, and alectinib is now widely used as first-line therapy, especially in brain metastasis. This patient developed pituitary metastasis after crizotinib resistance, and next-generation sequencing suggested the presence of the ALK I1171S mutation. The ALK I1171S mutation has been shown to result in secondary resistance to crizotinib, and ceritinib has proved effective when patients develop the secondary ALK 1171S mutation.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Ethics statement
Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author contributions
SF and DH had the original idea for the article and guided the treatment and management of the patient. DH and KZ wrote the article incorporating the comments from LZ. All authors reviewed and approved the final draft of the article. All authors contributed to the article and approved the submitted version.
Funding
This work was funded by the National Natural Science Foundation of China (82002825 to DH).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bailey D, Mau C, Zacharia B. Pituitary metastasis from urothelial carcinoma: A case report and review of the diagnosis and treatment of pituitary metastases. Cureus (2021) 13(8):e17574. doi: 10.7759/cureus.17574
2. Komninos J, Vlassopoulou V, Protopapa D, Korfias S, Kontogeorgos G, Sakas DE, et al. Tumors metastatic to the pituitary gland: Case report and literature review. J Clin Endocrinol Metab (2004) 89(2):574–80. doi: 10.1210/jc.2003-030395
3. Henry A, Nugent A, Wallace IR, Oladipo B, Sheehy O, Johnston PC. Pituitary metastasis: a clinical overview. Ulster Med J (2021) 90(3):146–50.
4. Javanbakht A, D’Apuzzo M, Badie B, Salehian B. Pituitary metastasis: a rare condition. Endocrine connections. (2018) 7(10):1049–57. doi: 10.1530/ec-18-0338
5. Al-Aridi R, El Sibai K, Fu P, Khan M, Selman WR, Arafah BM. Clinical and biochemical characteristic features of metastatic cancer to the sella turcica: an analytical review. Pituitary (2014) 17(6):575–87. doi: 10.1007/s11102-013-0542-9
6. Lithgow K, Siqueira I, Senthil L, Chew HS, Chavda SV, Ayuk J, et al. Pituitary metastases: presentation and outcomes from a pituitary center over the last decade. Pituitary (2020) 23(3):258–65. doi: 10.1007/s11102-020-01034-2
7. He W, Chen F, Dalm B, Kirby PA, Greenlee JD. Metastatic involvement of the pituitary gland: a systematic review with pooled individual patient data analysis. Pituitary (2015) 18(1):159–68. doi: 10.1007/s11102-014-0552-2
8. Hwang JM, Kim YH, Kim TM, Park SH. Differential diagnosis and management of a pituitary mass with renal cell carcinoma. J Korean Neurosurgical Society. (2013) 54(2):132–5. doi: 10.3340/jkns.2013.54.2.132
9. Recondo G, Facchinetti F, Olaussen KA, Besse B, Friboulet L. Making the first move in EGFR-driven or ALK-driven NSCLC: first-generation or next-generation TKI? Nat Rev Clin Oncol (2018) 15(11):694–708. doi: 10.1038/s41571-018-0081-4
10. Pan Y, Deng C, Qiu Z, Cao C, Wu F. The resistance mechanisms and treatment strategies for ALK-rearranged non-small cell lung cancer. Front Oncol (2021) 11:713530. doi: 10.3389/fonc.2021.713530
11. Vasilev V, Rostomyan L, Daly AF, Potorac I, Zacharieva S, Bonneville JF, et al. MANAGEMENT OF ENDOCRINE DISEASE: Pituitary ‘incidentaloma’: neuroradiological assessment and differential diagnosis. Eur J Endocrinol (2016) 175(4):R171–84. doi: 10.1530/eje-15-1272
12. Zoli M, Mazzatenta D, Faustini-Fustini M, Pasquini E, Frank G. Pituitary metastases: role of surgery. World Neurosurg (2013) 79(2):327–30. doi: 10.1016/j.wneu.2012.03.018
13. Habu M, Tokimura H, Hirano H, Yasuda S, Nagatomo Y, Iwai Y, et al. Pituitary metastases: Current practice in Japan. J Neurosurg (2015) 123(4):998–1007. doi: 10.3171/2014.12.Jns14870
14. Shah KK, Anderson RJ. Acute secondary adrenal insufficiency as the presenting manifestation of small-cell lung carcinoma. BMJ Case Rep (2014) 2014. doi: 10.1136/bcr-2013-203224
15. Altorki NK, Markowitz GJ, Gao D, Port JL, Saxena A, Stiles B, et al. The lung microenvironment: An important regulator of tumour growth and metastasis. Nat Rev Cancer (2019) 19(1):9–31. doi: 10.1038/s41568-018-0081-9
16. Wu J, Savooji J, Liu D. Second- and third-generation ALK inhibitors for non-small cell lung cancer. J Hematol Oncol (2016) 9:19. doi: 10.1186/s13045-016-0251-8
17. Du X, Shao Y, Qin HF, Tai YH, Gao HJ. ALK-rearrangement in non-small-cell lung cancer (NSCLC). Thorac Cancer (2018) 9(4):423–30. doi: 10.1111/1759-7714.12613
18. Nishio M, Kim DW, Wu YL, Nakagawa K, Solomon BJ, Shaw AT, et al. Crizotinib versus chemotherapy in Asian patients with ALK-positive advanced non-small cell lung cancer. Cancer Res Treat (2018) 50(3):691–700. doi: 10.4143/crt.2017.280
19. Yoshida T, Oya Y, Tanaka K, Shimizu J, Horio Y, Kuroda H, et al. Differential crizotinib response duration among ALK fusion variants in ALK-positive non-Small-Cell lung cancer. J Clin oncology: Off J Am Soc Clin Oncol (2016) 34(28):3383–9. doi: 10.1200/jco.2015.65.8732
20. Hida T, Nokihara H, Kondo M, Kim YH, Azuma K, Seto T, et al. Alectinib versus crizotinib in patients with ALK-positive non-small-cell lung cancer (J-ALEX): an open-label, randomised phase 3 trial. Lancet (London England) (2017) 390(10089):29–39. doi: 10.1016/s0140-6736(17)30565-2
21. Noé J, Lovejoy A, Ou SI, Yaung SJ, Bordogna W, Klass DM, et al. ALK mutation status before and after alectinib treatment in locally advanced or metastatic ALK-positive NSCLC: Pooled analysis of two prospective trials. J Thorac Oncol: Off Publ Int Assoc Study Lung Cancer (2020) 15(4):601–8. doi: 10.1016/j.jtho.2019.10.015
22. Ou SH, Milliken JC, Azada MC, Miller VA, Ali SM, Klempner SJ. ALK F1174V mutation confers sensitivity while ALK I1171 mutation confers resistance to alectinib. importance serial biopsy post progression. Lung Cancer (Amsterdam Netherlands) (2016) 91:70–2. doi: 10.1016/j.lungcan.2015.09.006
23. Ou SH, Greenbowe J, Khan ZU, Azada MC, Ross JS, Stevens PJ, et al. I1171 missense mutation (particularly I1171N) is a common resistance mutation in ALK-positive NSCLC patients who have progressive disease while on alectinib and is sensitive to ceritinib. Lung Cancer (Amsterdam Netherlands) (2015) 88(2):231–4. doi: 10.1016/j.lungcan.2015.02.005
24. Ou SH, Klempner SJ, Greenbowe JR, Azada M, Schrock AB, Ali SM, et al. Identification of a novel HIP1-ALK fusion variant in non-Small-Cell lung cancer (NSCLC) and discovery of ALK I1171 (I1171N/S) mutations in two ALK-rearranged NSCLC patients with resistance to alectinib. J Thorac oncology: Off Publ Int Assoc Study Lung Cancer (2014) 9(12):1821–5. doi: 10.1097/jto.0000000000000368
25. Bresler SC, Weiser DA, Huwe PJ, Park JH, Krytska K, Ryles H, et al. ALK mutations confer differential oncogenic activation and sensitivity to ALK inhibition therapy in neuroblastoma. Cancer Cell (2014) 26(5):682–94. doi: 10.1016/j.ccell.2014.09.019
Keywords: pituitary metastasis, crizotinib, drug resistance, ALK, lung adenocarcinoma
Citation: Han D, Zhao K, Yang Q, Zhang L and Fei S (2022) Secondary mutant ALK-I1171s in pituitary metastases from a patient with ALK fusion-positive advanced lung adenocarcinoma: A case report and literature review. Front. Oncol. 12:1016320. doi: 10.3389/fonc.2022.1016320
Received: 10 August 2022; Accepted: 29 September 2022;
Published: 17 October 2022.
Edited by:
Mln Yu, West China Hospital, Sichuan University, ChinaReviewed by:
Karam Khaddour, University of Illinois at Chicago, United StatesSong Xu, Tianjin Medical University General Hospital, China
Copyright © 2022 Han, Zhao, Yang, Zhang and Fei. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shihong Fei, MzUzOTc3MzAzQHFxLmNvbQ==; Liling Zhang, bGlseS0xMjI4QGhvdG1haWwuY29t
†These authors have contributed equally to this work
 Dan Han†
Dan Han† Kewei Zhao
Kewei Zhao Liling Zhang
Liling Zhang