- 1Department of Neurosurgery, Affiliated Hangzhou Chest Hospital, Zhejiang University School of Medicine, Hangzhou, China
- 2Department of Neurosurgery, The First Affiliated Hospital, Zhejiang University School of Medicine, Hangzhou, China
Purpose: The study aimed to identify clinical prognostic factors affecting overall survival (OS) in patients with central neurocytoma (CN) and to determine independent prognostic factors in the subgroups of different treatment modalities using a retrospective analysis based on the SEER database from 2003 to 2019.
Materials and methods: Data regarding patients with CN, including basic clinical characteristics, treatment measures, and prognosis follow-up, were extracted from the SEER database. The prognostic variables for all patients were assessed using log-rank test as well as univariate and multivariate analyses based on the Cox proportional hazards model. The same statistical methods were used for analysis in different subgroups of gross total resection (GTR), subtotal resection (STR), no surgery, radiotherapy (RT), and no RT.
Results: In total, 413 patients were enrolled in this study. Tumor size, primary site surgery, and RT were independent prognostic factors in all patients with CN. In subgroup analyses, RT was not an independent prognostic factor in patients with GTR. However, sex and race were independent prognostic factors in patients with STR. Additionally, tumor size was an independent prognostic factor in patients who did not undergo surgery. Furthermore, sex and primary site were independent prognostic factors in patients who received RT. Size and primary site surgery were independent prognostic factors in patients without RT.
Conclusion: In our study, patients with small tumors or GTR or those who did not receive RT showed a better prognosis. GTR was the preferred treatment for CN. RT was not recommended for patients after GTR. Men and African American showed certain advantages after STR surgery. Tumors with a size of >4 cm were recommended for active treatment. In the RT subgroup, patients with tumors outside the ventricle or women had a poorer prognosis than those with tumors within the ventricle or men, respectively. These findings will help clinicians and patients understand the treatment and prognosis of CN visually and intuitively.
1 Introduction
Central neurocytoma (CN) is a rare neoplasm of the central nervous system classified as a grade II tumor by the World Health Organization (WHO) (1). It typically affects people in their 30s, which is the most common age group for the onset of cancer. CN is usually found in the ventricle system (2), and few cases have been reported in previous case reports or literature reviews. However, prognostic factors for CN remain controversial. Currently, there are limited large-scale retrospective clinical prognostic studies on CN as well as subgroup analyses of various treatment modalities.
This study aimed to identify clinical prognostic factors influencing overall survival (OS) in patients with CN and to determine independent prognostic factors in different subgroups of gross total resection (GTR), subtotal resection (STR), no surgery, radiotherapy (RT), and no RT.
2 Materials and methods
2.1 Data collection
Data regarding patients with CN, including basic clinical characteristics, social factors, tumor characteristics, treatment measures, and prognosis follow-up, were extracted from the SEER Research Plus Data (17 Registries, Nov 2021) from 2000 to 2019 using SEER*Stat software (version 8.4.0).
The inclusion criteria were as follows: (1) patients with ICD-O-3 histologic codes of 9506/0 (CN, benign), 9506/1 (CN), or 9506/3 (CN, malignant); (2) those with clear vital status and OS; and (3) those with no significant data gaps.
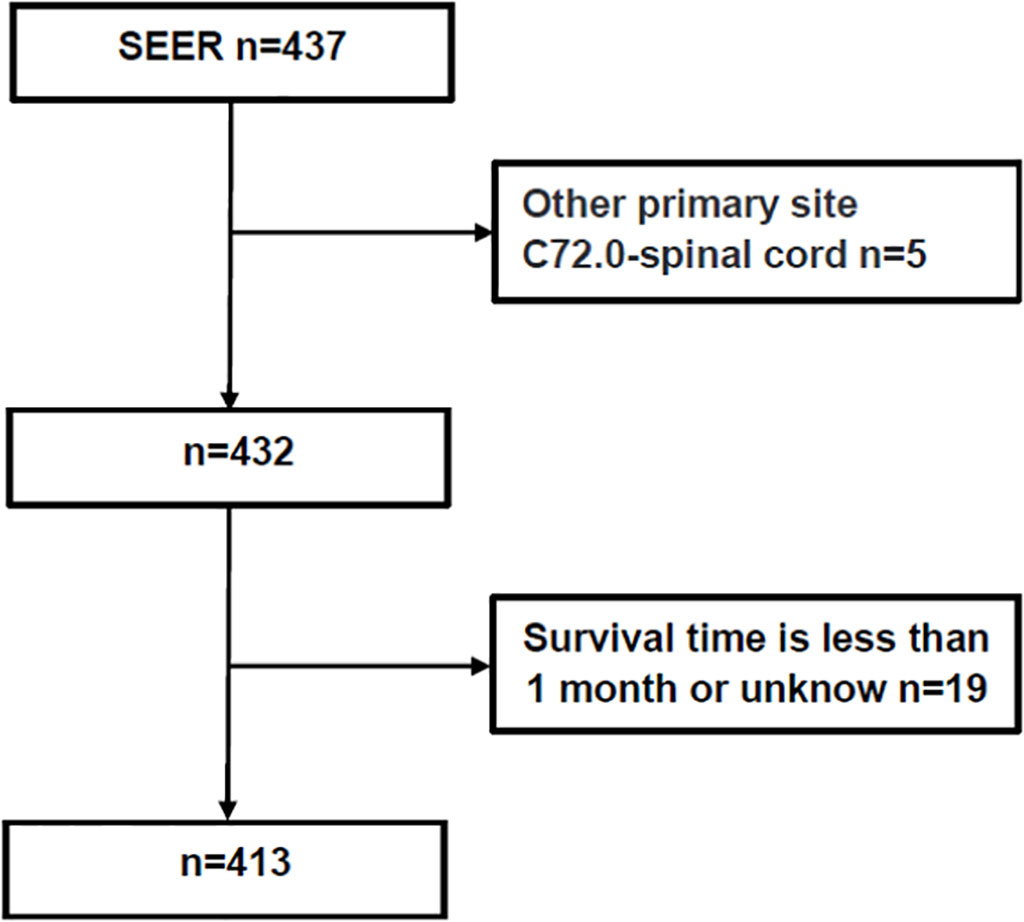
The exclusion criteria were as follows: (1) patients with no specific OS or OS of <1 month; (2) those with tumor locations involving other primary sites, such as the spinal cord (C72.0); or (3) those with significant data gaps or unknown mode of treatment.
The following patient data were retrieved: age, sex, race, year of diagnosis, reporting source, primary site (location), tumor size, pathology, laterality, primary site surgery (therapy), RT, chemotherapy, vital status, and OS. GTR was defined as gross total resection of the tumor under the naked eyes or the absence of residual tumor in early postoperative imaging examination, and STR was defined as subtotal total resection of the tumor or less than 10% residual tumor under the naked eyes. RT was defined as the application of radiation to destroy or treat the primary or metastases of local tumors. In this paper, RT included simple RT, preoperative RT, intraoperative RT or postoperative RT without specific dose. The methods for obtaining data from the SEER database are described in Figure 1.
2.2 Endpoints
As the primary endpoint, OS was defined as the time from diagnosis to death or the last investigation.
2.3 Statistical analysis
In all patients with CN, the prognostic factors were graphically assessed using log-rank test and Kaplan–Meier curves. The independent prognostic variables were identified using univariate and multivariate analyses based on the Cox proportional hazards model. Log-rank test as well as univariate and multivariate analyses were used to identify prognostic factors in different subgroups of GTR, STR, no surgery, RT, and no RT.
Statistical analysis was performed using Statistical Package for the Social Sciences (SPSS 26.0) and R software (R 4.1.2). Factors with P-values of <0.10 in the univariate analysis were included in the multivariate analysis. A two-tailed P-value of <0.05 was considered to indicate statistical significance.
3 Results
3.1 Total data analysis
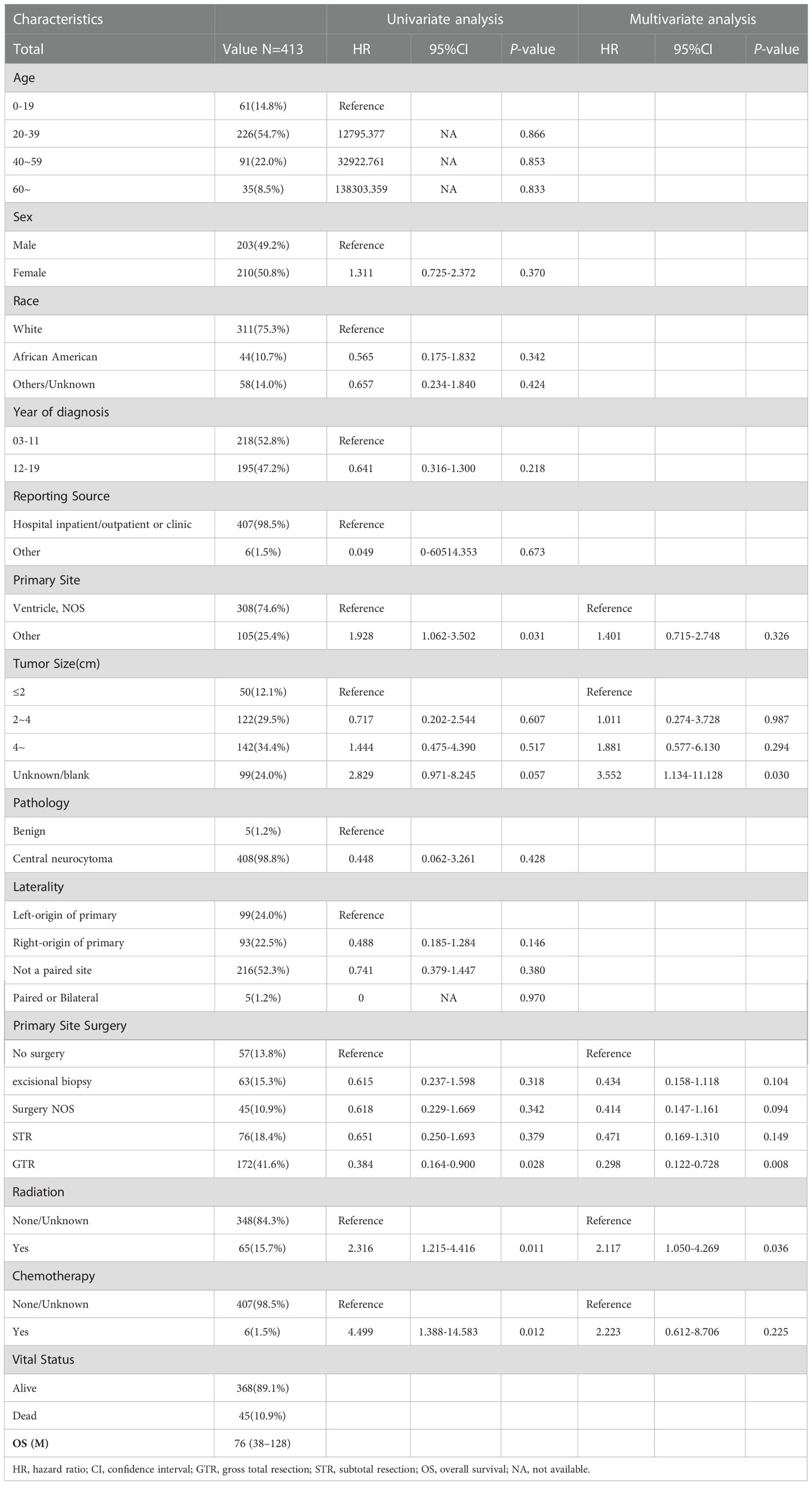
In total, 413 patients were included in this study (Figure 1), 203 male (49.2%) and 210 (50.8%) female. Median OS for all patients was 76 (interquartile range [IQR]: 38–128) months, 45 died (10.9%), and 368 (89.1%) survived (Table 1). As shown in Figure 2, the survival curves of tumor size (P = 0.0056; Figure 2A), primary site surgery (P = 0.024; Figure 2B), and RT (P = 0.0085; Figure 2C) were compared using log-rank test. As shown in Table 1, univariate analysis revealed that primary site (hazard ratio [HR]: 1.928, 95% confidence interval [CI]: 1.062–3.502, P = 0.031), tumor size (HR: 2.829, 95% CI: 0.971–8.245, P = 0.057), primary site surgery (HR: 0.384, 95% CI: 0.164–0.900, P = 0.028), RT (HR: 2.316, 95% CI: 1.215–4.416, P = 0.011), and chemotherapy (HR: 4.499, 95% CI: 1.388–14.583, P = 0.012) were statistically significant among the patients.

Figure 2 Overall survival (OS) in all patients with central neurocytoma. (A) OS among the different tumor size groups. (B) OS among the different primary site surgery (therapy) groups. (C) OS among the different radiation groups. OS, overall survival.
As shown in Table 1, multivariate analysis revealed that tumor size (HR: 3.552, 95% CI: 1.134–11.128, P = 0.030), primary site surgery (HR: 0.298, 95% CI: 0.122–0.728, P = 0.008), and RT (HR: 2.117, 95% CI: 1.050–4.269, P = 0.036) were independent prognostic factors in all patients with CN.
In our study, tumor size, primary site surgery, and RT were significant prognostic factors for CN. Patients with small tumors or GTR or those who did not receive RT showed a better prognosis.
3.2 Subgroup analysis
Overall, 172 patients with GTR were enrolled in subgroup analysis, 82 male (47.7%) and 90 female (52.3%). The median OS for patients with GTR was 81 (IQR: 40–128) months, 13 died (7.6%), and 159 (92.4%) survived (Table 2). As shown in Figure 3, the survival curves of RT (P = 0.15; Figure 3A) were compared using log-rank test. As shown in Table 2, univariate analysis revealed that neither RT (HR: 2.512, 95% CI: 0.689–9.165, P = 0.163) nor chemotherapy (HR: 0.046, 95% CI: 0–648364.957, P = 0.714) was statistically significant among the patients. A subgroup analysis revealed that RT did not significantly improve the prognosis of patients with GTR.
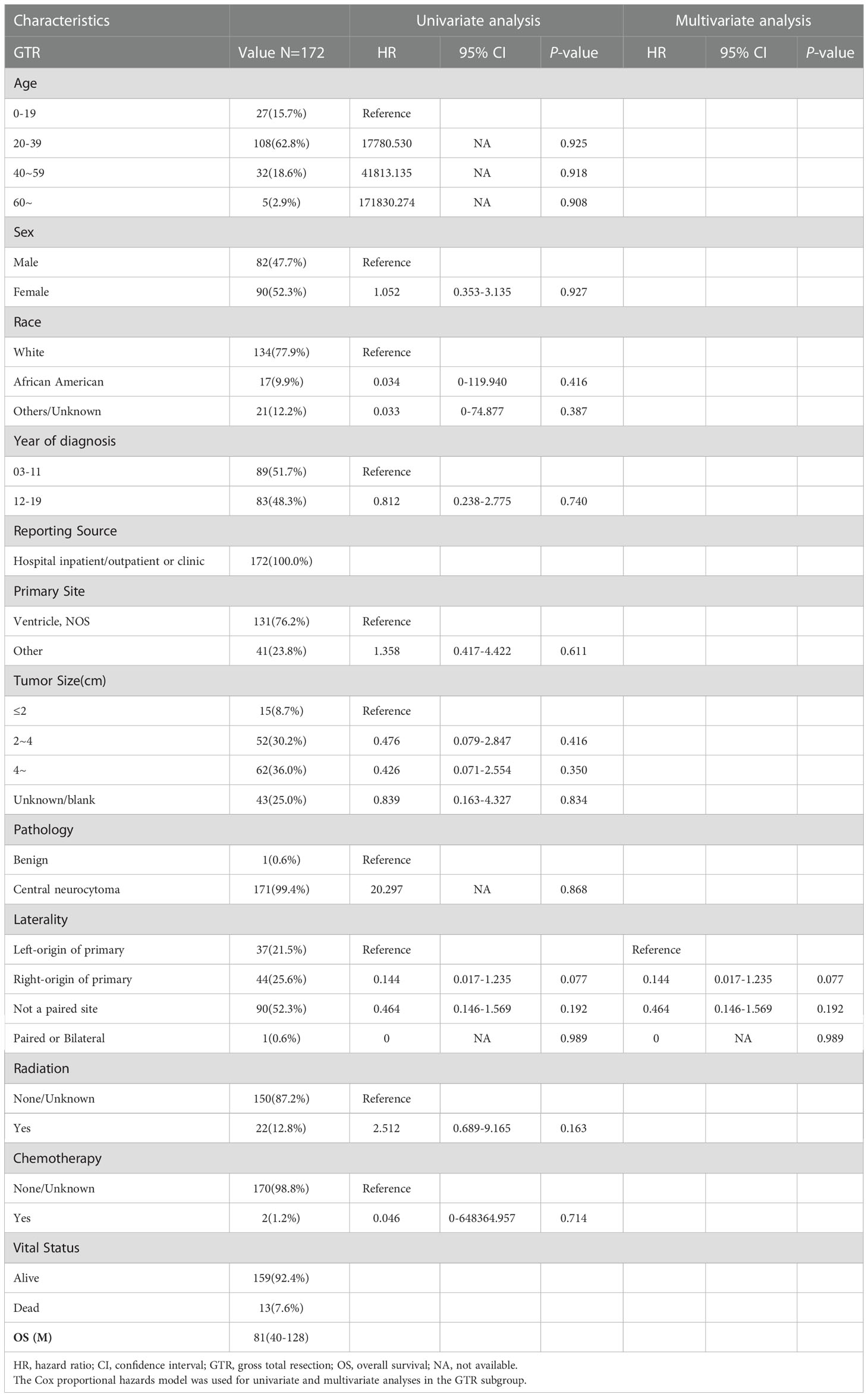
Table 2 The median overall survival (OS) of the gross total resection (GTR) was 81 (interquartile range (IQR): 40–128) months.
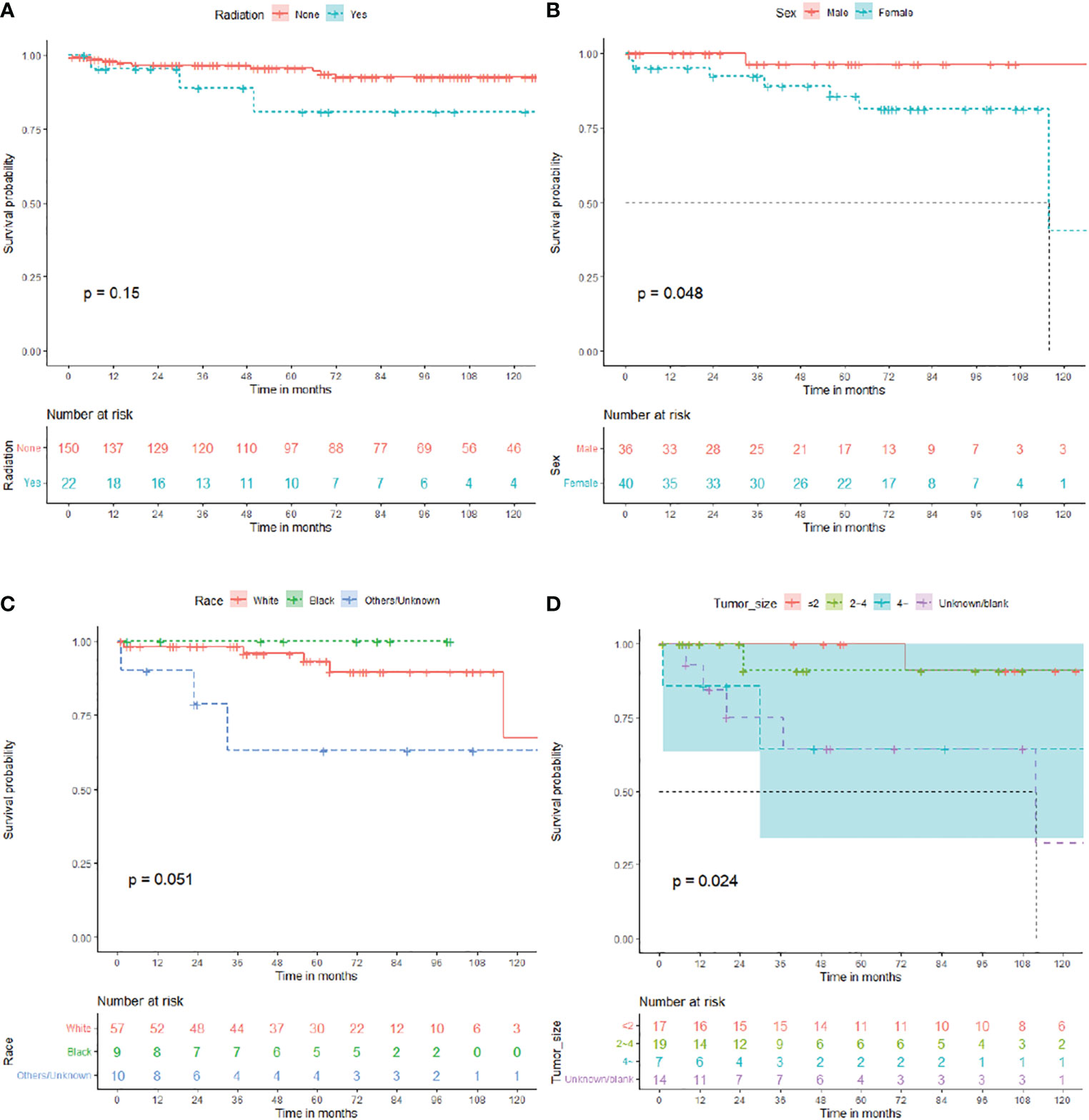
Figure 3 Overall survival (OS) for central neurocytoma in the gross total resection (GTR), subtotal resection (STR), and no surgery subgroups. (A) OS among the different radiotherapy groups in the GTR subgroup. (B) OS among the different sex groups in the STR subgroup. (C) OS among the different race groups in the STR subgroup. (D) OS among the different tumor size groups in the no surgery subgroup. OS, overall survival.
In total, 76 patients with STR were enrolled in subgroup analysis, 36 male (47.4%) and 40 female (52.6%). The median OS for patients with STR was 61.5 (IQR: 33.25–80) months, 8 died (10.5%), and 68 (89.5%) survived (Table 3). As shown in Figure 3, the survival curves of sex (P = 0.048; Figure 3B) and race (P = 0.051; Figure 3C) were compared using log-rank test. As presented in Table 3, univariate analysis revealed that sex (HR: 6.383, 95% CI: 0.780–52.215, P = 0.084), race (HR: 4.212, 95% CI: 0.991–17.904, P = 0.051), primary site (HR: 3.599, 95% CI: 0.801–16.167, P = 0.095), and chemotherapy (HR: 8.841, 95% CI: 0.981–79.670, P = 0.052) were statistically significant among the patients. As shown in Table 3, multivariate analysis revealed that sex (HR: 20.344, 95% CI: 1.589–260.418, P = 0.021) and race (HR: 13.637, 95% CI: 2.140–86.914, P = 0.006) were independent prognostic factors in patients with STR. In the STR subgroup, men and African American showed a better prognosis than women and other races, respectively.
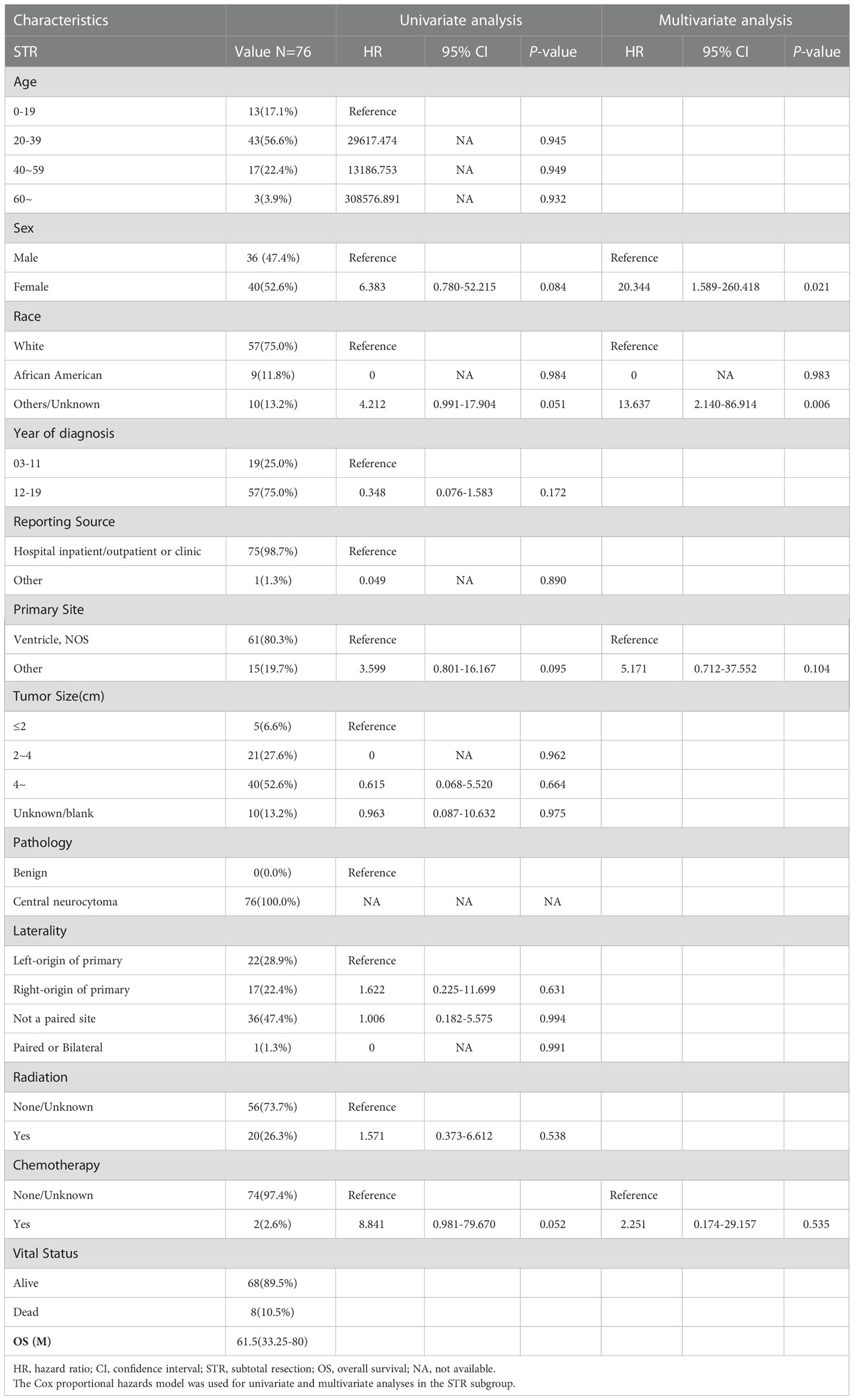
Table 3 The median overall survival (OS) of the subtotal resection (STR) was 61.5 (interquartile range (IQR): 33.25–80) months.
Furthermore, 57 patients who did not undergo surgery were enrolled in subgroup analysis, 27 male (47.4%) and 30 female (52.6%). The median OS for patients who did not undergo surgery was 46 (IQR: 16.5–108) months, 9 died (15.8%), and 48 (84.2%) survived (Table 4). As shown in Figure 3, the survival curves of tumor sizes (P = 0.024; Figure 3D) were compared using log-rank test. As presented in Table 4, univariate and multivariate analyses revealed that tumor size (HR: 10.604, 95% CI: 1.216–92.460, P = 0.033) was an independent prognostic factor in patients without surgery. Tumors with a size of >4 cm showed a worse prognosis in patients who did not undergo surgery.
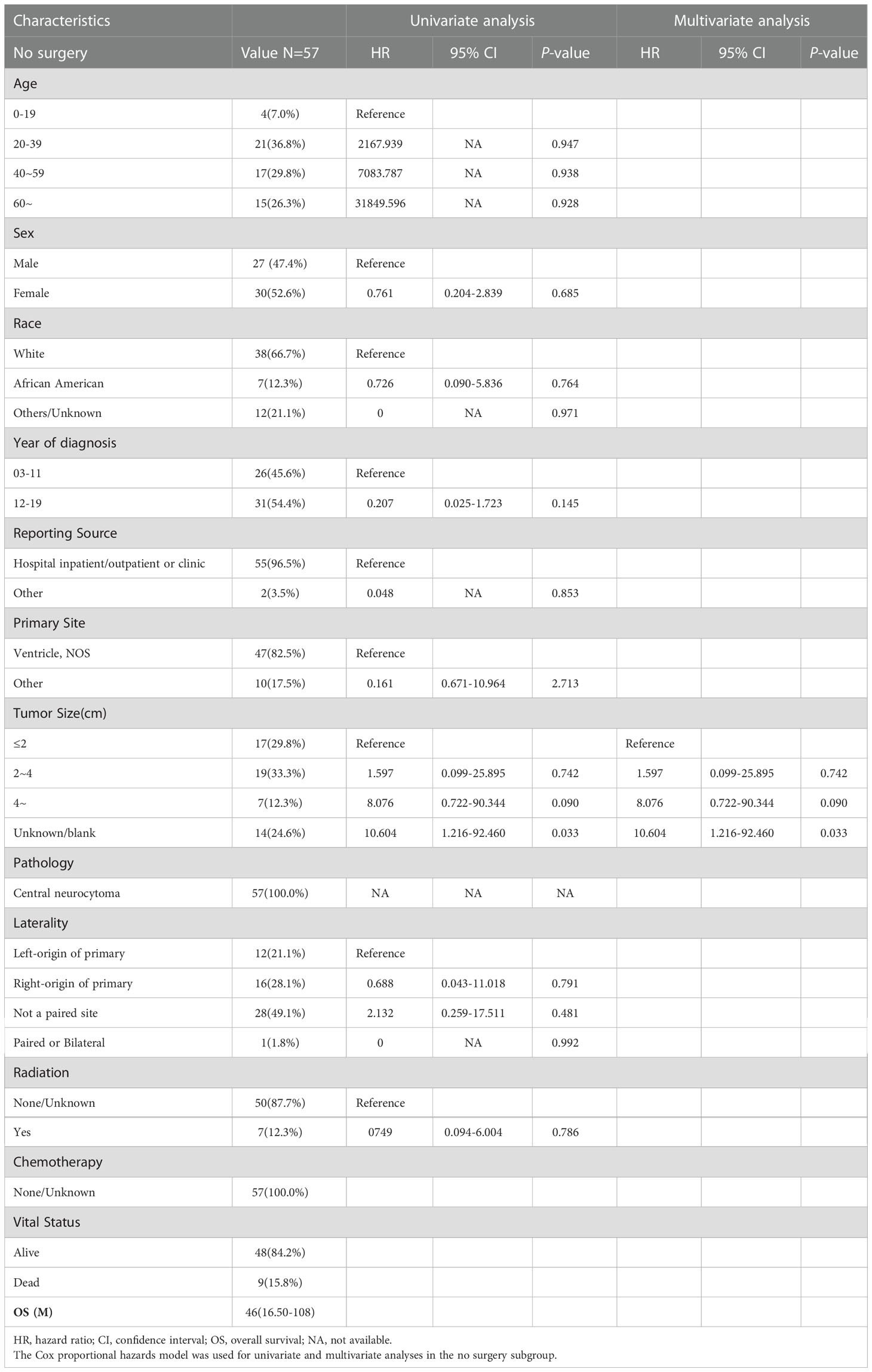
Table 4 The median overall survival (OS) of the no surgery subgroup was 46 (interquartile range (IQR): 16.5–108) months.
Overall, 65 patients who received RT were enrolled in subgroup analysis, 35 male (53.8%) and 30 female (46.2%). The median OS for patients who received RT was 67 (IQR: 30–115) months, 13 died (20.0%), and 52 (80.0%) survived (Table 5). As shown in Figure 4, the survival curves of sex (P = 0.033; Figure 4A) and primary site surgery (P = 0.0098; Figure 4B) were compared using log-rank test. As depicted in Table 5, univariate analysis revealed that sex (HR: 3.711, 95% CI: 1.018–13.535, P = 0.047), primary site (HR: 3.911, 95% CI: 1.278–11.970, P = 0.017), and pathology (HR: 0.141, 95% CI: 0.017–1.148, P = 0.067) were statistically significant among these patients. As shown in Table 5, multivariate analysis revealed that sex (HR: 5.330, 95% CI: 1.165–24.385, P = 0.031) and primary site (HR: 3.472, 95% CI: 1.098–10.983, P = 0.034) were independent prognostic factors in patients who received RT. In the RT subgroup, patients with tumors outside the ventricle or women had a poorer prognosis than those with tumors within the ventricle or men, respectively.
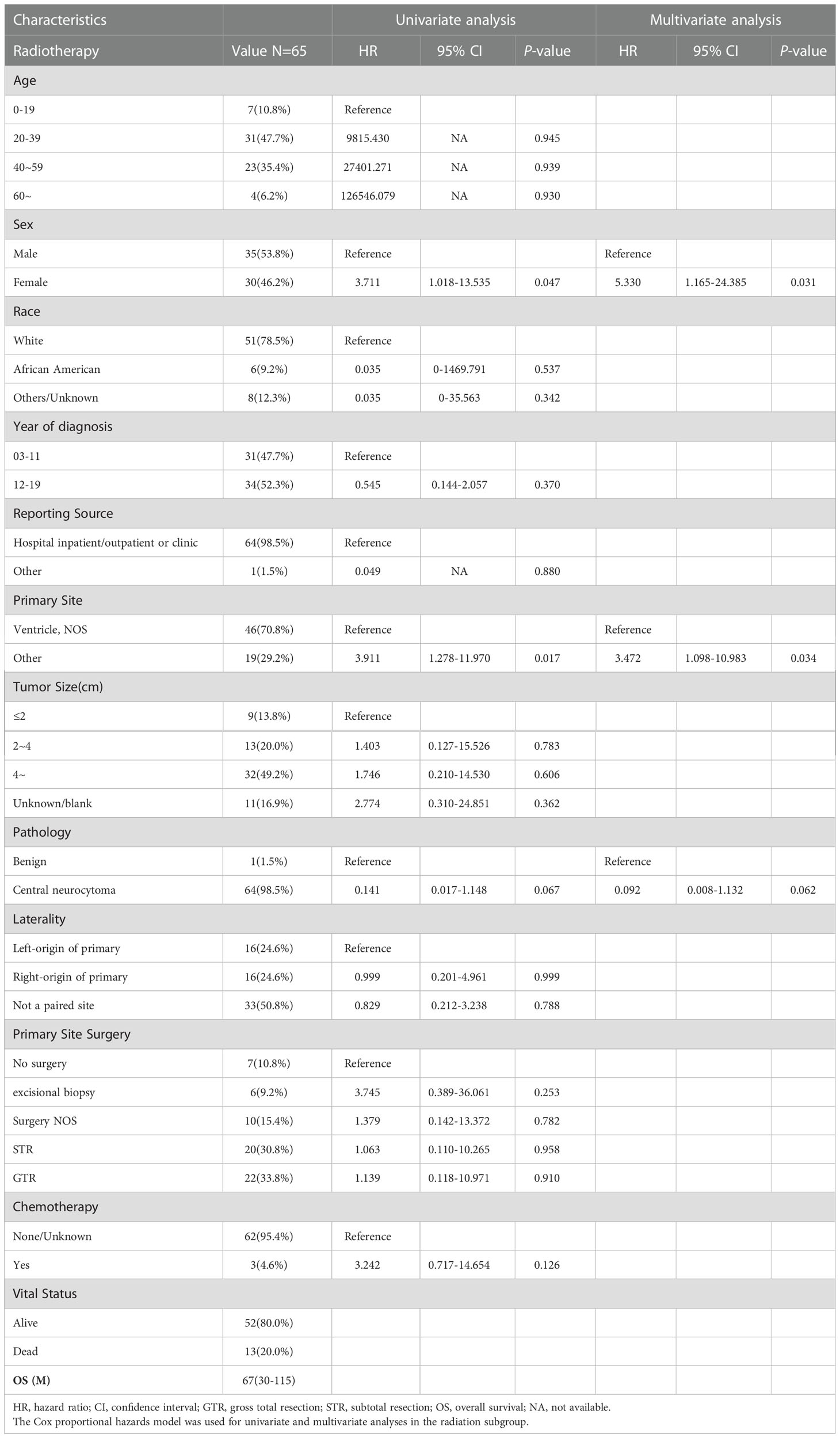
Table 5 The median overall survival (OS) of the radiotherapy subgroup was 67 (interquartile range (IQR): 30–115) months.
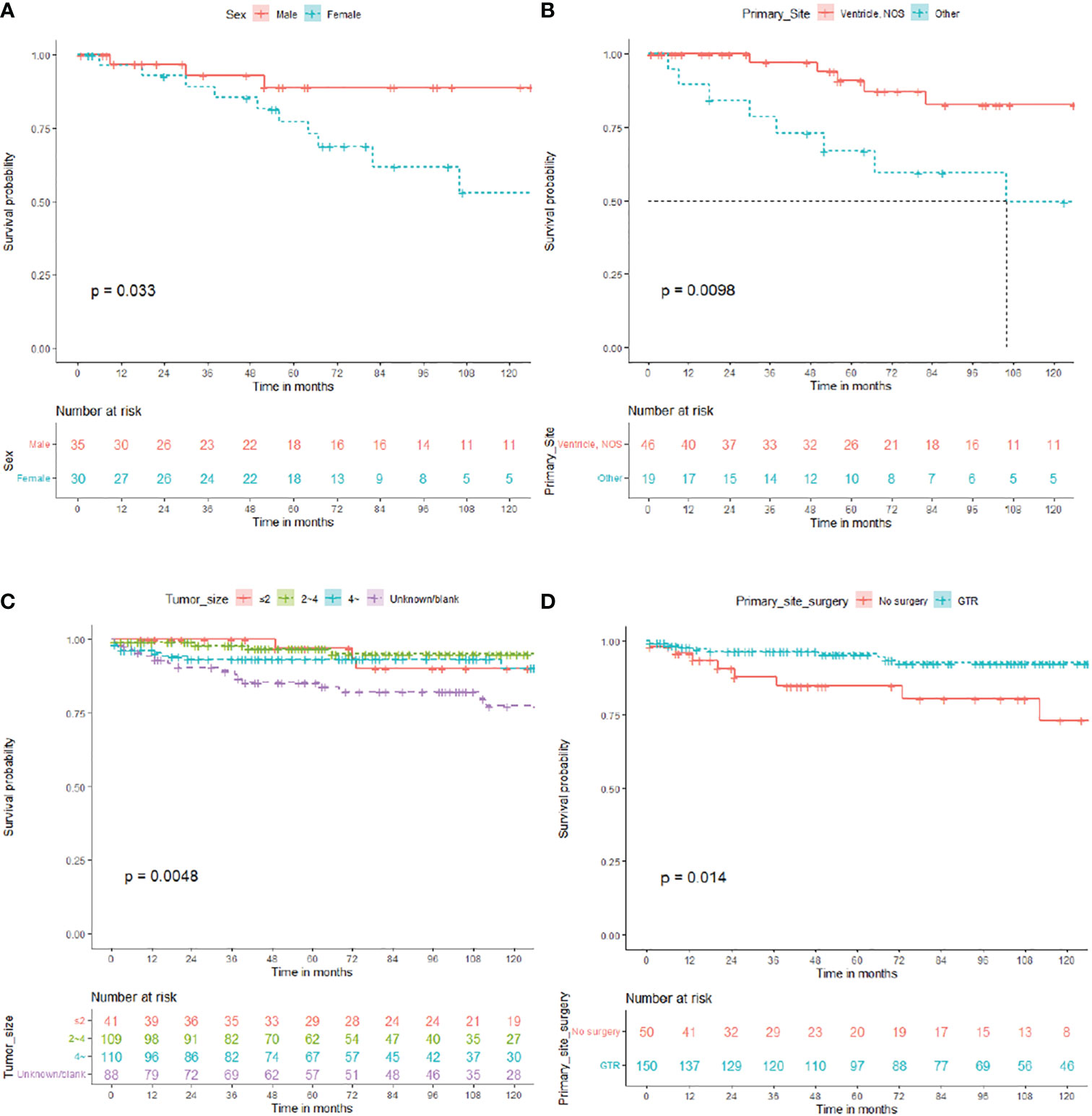
Figure 4 Overall survival (OS) for central neurocytoma in the radiotherapy and no radiotherapy. (A) OS among the different sex groups in the radiotherapy subgroup. (B) OS among the different primary site groups in the radiotherapy subgroup. (C) OS among the different tumor size groups in the no radiotherapy subgroup. (D) OS. among the different primary site surgery groups in the no radiotherapy subgroup. OS, overall survival.
In total, 348 patients who did not receive RT were enrolled in subgroup analysis, 168 male (48.3%) and 180 female (51.7%). The median OS for patients who did not receive RT was 79 (IQR: 38.25–132) months, 32 died (9.2%), and 316 (90.8%) survived (Table 6). As shown in Figure 4, the survival curves of tumor size (P = 0.0048; Figure 4C) and primary site surgery (P = 0.014; Figure 4D) were compared using log-rank test. As presented in Table 6, univariate analysis revealed that tumor size (HR: 2.922, 95% CI: 0.856–9.975, P = 0.087), laterality (HR: 0.999, 95% CI: 0.089–1.171, P = 0.085), and primary site surgery (HR: 0.329, 95% CI: 0.130–0.836, P = 0.019) were statistically significant among these patients. As shown in Table 6, multivariate analysis revealed that tumor size (HR: 3.918, 95% CI: 1.116–14.261, P = 0.034) and primary site surgery (HR: 0.275, 95% CI: 0.104–0.727, P = 0.009) were independent prognostic factors in patients without RT. In the no RT subgroup, patients with GTR showed a better prognosis.
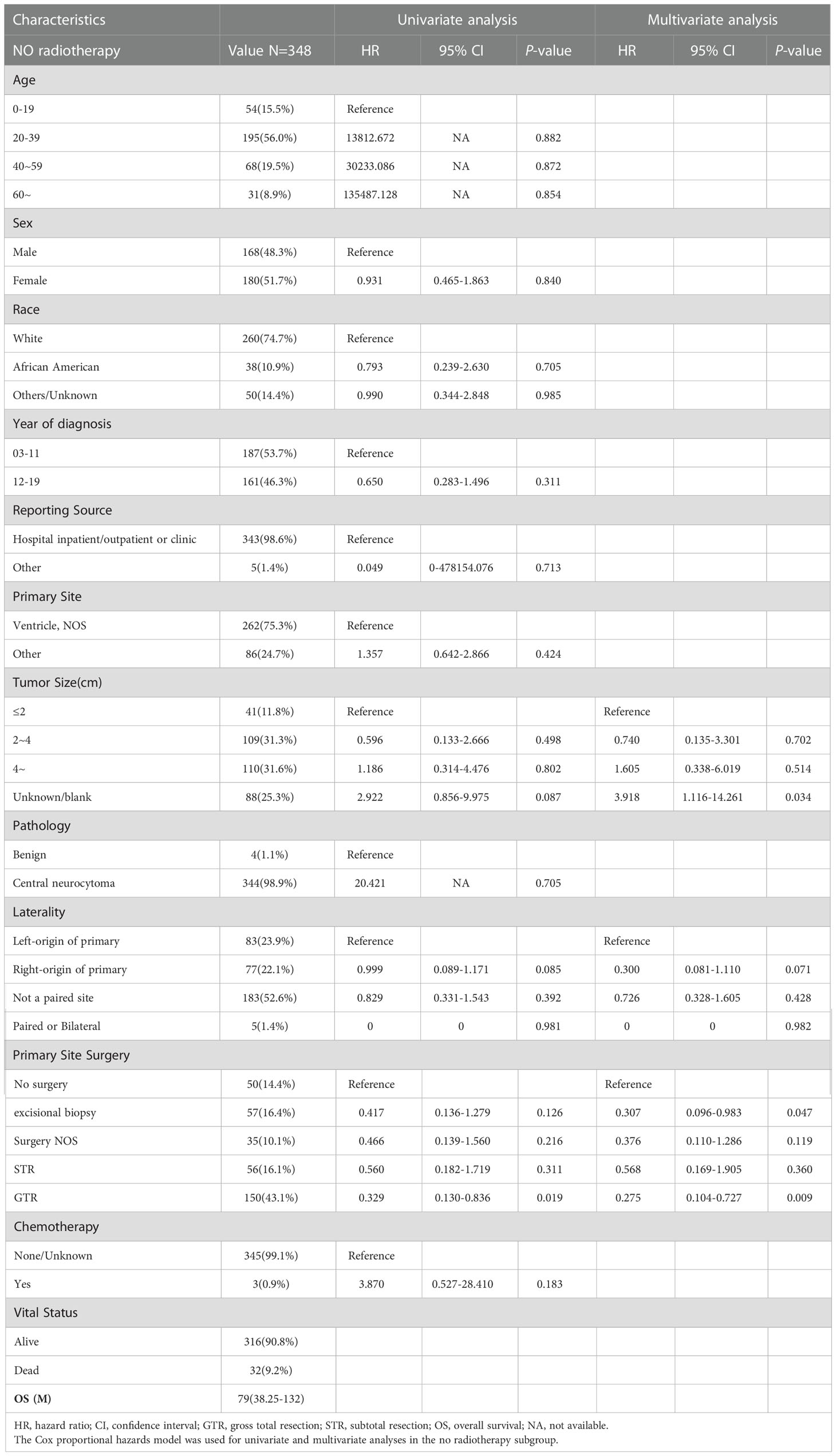
Table 6 The median overall survival (OS) of the no radiotherapy subgroup was 79 (interquartile range (IQR): 38.25–132) months.
4 Discussion
In 1982, Hassoun et al. identified two cases of tumors originating in the third ventricle and named them as CN (3). CN is a rare intracranial tumor that accounts for 0.1%–0.5% of all intracranial tumors and is classified as a grade II tumor by the World Health Organization in 2021 (1, 4, 5). CN commonly occurs in the lateral ventricle but is also found in the posterior fossa or other locations (6). Its pathogenesis is associated with various chromosomal aberrations (7). Mohammad et al. revealed that with no characteristic clinical symptoms of CN, a correct diagnosis can be made by radiographic imaging, histopathology assessment, and immunohistochemistry (8). Chang et al. analyzed 781 patients with cancer and revealed a 5-year OS rate of 87.2% (9). Gabriele et al. revealed that CN were consistent with a low-grade neuronal neoplasm of the central nervous system, especially extraventricular neurocytoma (EVN) (10).
To the best of our knowledge, only few studies on CN have been reported to date. Considering the rarity of this disease, we conducted a retrospective analysis of a relatively large sample size of patients with CN using the SEER database, which covered 30% of the US population. This study aimed to identify clinical prognostic factors affecting the OS in patients with CN and to determine independent prognostic factors in the subgroups of different treatment modalities.
4.1 Age, sex, and race
Approximately 25% of CN develops in adults in their 30s (5). The most common age of onset of CN and EVN is 20–34 years (11). In our study, patients ranged in age ranged from 0 to 85 years. Further, in the overall data, >50% of patients diagnosed with CN were aged 20–39 years.
Mattar et al. revealed that age was not a significant prognostic factor in 22 patients diagnosed with atypical CN between January 2009 and March 2018. After reviewing the literature, the previous study concluded that neither age nor sex had a significant effect on the median OS (12–15).
In our univariate and multivariate analyses of 413 patients, age was not a significant prognostic factor. Further, all subgroup analyses revealed that age was not a significant factor affecting prognosis, which is consistent with the results of previous reports. In the subgroup analysis of patients with STR and those who received RT, men showed better outcomes than women. The subgroup analysis of patients with STR revealed that African American had a better prognosis than other races.
4.2 Tumor size
In a retrospective analysis of 868 neurocytomas, Dutta et al. revealed that the median tumor size was 4–5 cm and that tumor size was not a determining factor. Even patients with a tumor size of >4 cm had a 5-year OS rate of 89%. Furthermore, patients with GTR had a 5-year OS rate of 96% (16).
Our study revealed that HR increased with tumor size. In the no surgery and no RT subgroups, patients with a tumor size of >4 cm had a higher HR than those with a tumor size of <2 cm, indicating that tumors with a size of >4 cm had a lower survival rate than smaller tumors. This is also consistent with the general tumor growth pattern. Larger tumors are more likely to invade surrounding brain tissues, nerves, and the vascular system. Further, larger tumors are more difficult to treat surgically and are more likely to have residual tumor tissues and recurrence after surgery.
4.3 Primary site (tumor location)
EVNs can occur in any brain tissue except the ventricle. They are broad-spectrum, more aggressive, and have a worse prognosis (5, 17, 18). Joonho et al. revealed that EVN may be a heterogenous disease entity and needed to be followed up for a long time (19). Shuran et al. revealed that an accurate diagnosis was difficult to be made preoperatively in 11 patients with EVNs. When the imaging findings are atypical, more aggressive treatment should be considered in patients (20). Treatment options and prognosis vary widely between CN and other ventricular tumors (21). According to our RT subgroup analysis, CN located outside the ventricle had a worse prognosis.
4.4 Primary site surgery (therapy)
Currently, surgery is considered the gold standard for treating CN. Han et al. conducted a single-center study involving 67 patients and found that complete tumor resection was the preferred treatment (22). In particular, patients with GTR have a favorable prognosis and a significantly lower risk of CN recurrence. In a study involving 310 patients with CN, the 5-year OS rate of patients with GTR was as high as 99% (5, 23, 24). Mattar et al. conducted a retrospective analysis of 22 cases and concluded that GTR was an independent prognostic factor for OS in patients with CN (12). Liang et al. revealed that surgery can benefit children and ensure relatively long-term progression-free survival in 14 patients with pediatric CN (25). Qiongxuan et al. revealed that use of GTR whenever possible and close imaging follow-up in 101 patients with CN (26).
Alqroom et al. used the transcortical and interhemispheric transcallosal approaches in 18 and 14 patients with CN, respectively, and found no difference in the scope of resection or protection of nerve function between the two surgical approaches (2, 27). Further, according to Sing et al., intraoperative neuroelectrophysiological monitoring is important for safe lesion resection (28).
According to a systematic review by Mahavadi et al., in cases of a high risk of GTR, maximal safe resection combined with adjunct RT can be used as a suboptimal treatment alternative for cancer (29).
However, in a retrospective analysis of 868 neurocytomas, Dutta et al. revealed that the extent of resection was not an independent prognostic factor for improved survival using multivariate analysis.
In our multivariate regression analysis, GTR (HR: 0.298, 95% CI: 0.122–0.728, P = 0.008; Table 2) was an independent prognostic factor for OS. We found that no surgery, biopsy, surgery, NOS, and STR subgroups were associated with a worse prognosis than the GTR subgroup. In the no RT subgroup, patients with GTR showed a better prognosis. The therapeutic effect of GTR on CN has been fully confirmed in previous studies. GTR should be performed while preserving as many important physiological structures as possible.
4.5 Radiotherapy
Adjunct RT, such as stereotactic radiosurgery (SRS) and fractionated RT, plays an important role in the treatment of CN (5, 30–32).
According to the findings of Han et al., RT is not recommended following complete tumor resection. After the complete excision of the atypical CNs, adjuvant RT was not recommended, and close radiographic follow-up was required (22). In patients with incomplete tumor resections, adjuvant RT should be advocated (27); moreover, postoperative RT can improve OS in these patients.
Nakamura et al. argued that SRS is an effective method for treating recurrent or residual CNs after STR. Meanwhile, Gamma knife surgery plays an essential role in the postoperative treatment of patients with CN (30). There are no specific SRS dosage guidelines for CN treatment. Lee et al. and Matsunaga et al. recommended that a minimum of 13 Gy is required for effective tumor control (5, 33). Bui et al. and Minniti et al. suggested that an RT dose between 13 and 18 Gy is relatively safe (31, 34). They examined 150 cases and found that RT had >90% local tumor control and that radiotoxicity was uncommon (31). In addition, RT is associated with delayed complications and radiation-induced toxicity, including leukoencephalopathy, radiation-induced malignancy, and radiation necrosis (14, 30, 32).
Dutta et al. conducted a retrospective analysis of 868 cases of CN and revealed that RT was not a vital prognostic factor using multivariate analyses (16, 35). Furthermore, Dutta et al. and Hussain et al. reported that adjuvant RT did not significantly improve the OS rate of patients with CN and that the effect of salvage RT was unknown (16, 36). Dan et al. revealed that postoperative RT also did not improve local control and survival in 43 patients with CN (37). By studying 68 patients with CN, Lei She et al. revealed that postoperative RT could improve Progression-free survival (PFS) in STR, but not in OS (38). Göktug et al. reported that use of RT as a primary or adjuvant treatment following surgical resection remained controversial in a study of 25 CNs (39).
In our study, the role of RT in treating patients was crucial. However, a multivariate analysis of all patient data revealed that RT may reduce the OS rate of patients. In a subgroup analysis, the RT did not significantly improve the prognosis of patients with GTR. RT was not recommended after complete tumor resection. In the RT subgroup, patients with tumors outside the ventricle or women have a poorer prognosis than those with tumors within the ventricle or men, respectively. This suggests that RT is recommended for men or those with tumors located within the ventricle.
This result may be attributed to the limitations of the SEER database and the insufficient sample size. Our findings suggested that the patient’s condition should be thoroughly assessed prior to RT. Physicians should consider RT toxicity and the harm caused by subsequent cognitive decline to patients (16). GTR or RT may impair important brain structures and functions, leading to a decline in quality of life. Extent of tumor resection and adjuvant treatments should always be balanced between prognosis improvement and maintenance/worsening of quality of life.
4.6 Chemotherapy
Currently, chemotherapy for treating patients with CN is controversial, with no corresponding treatment guidelines (40). According to Dutta et al., chemotherapy might be considered when patients are unable to complete surgery or RT. However, the most effective chemotherapy drugs are yet to be identified (16).
Johnson et al. conducted a retrospective analysis of 39 cases of CN treated with chemotherapy and concluded that there is significant heterogeneity in chemotherapy for CN. Furthermore, they emphasized that the benefits of temozolomide for treating CN are unclear and need further investigation (40). There are no prospective, multicenter, large-scale studies on chemotherapy for CN. In the multivariate regression analysis and the five treatments subgroup analysis, chemotherapy was not an independent prognostic factor for OS. Finally, only six patients completed chemotherapy, indicating that the efficacy of chemotherapy requires further investigation.
5 Limitations
Due to multiple changes in the diagnostic criteria for CN between 2000 and 2019, there was heterogeneity among patients included in the SEER database. In other words, there was a particular patient selection bias based on the SEER database. In our study, after data cleaning, there were no patients with malignant CN. The limitation of the article mentioned that our study lacked immunohistochemical data. In addition, the sample size is relatively small in this study. Longer follow-up and further multicenter studies with more sample sizes are needed.
6 Conclusion
In our study, patients with small tumors or GTR or those who did not receive RT showed a better prognosis. GTR was the preferred treatment for CN. RT was not recommended for patients after GTR. Men and African American showed certain advantages after STR surgery. Tumors with a size of >4 cm were recommended for active treatment. In the RT subgroup, patients with tumors outside the ventricle or women had a poorer prognosis than those with tumors within the ventricle or men, respectively. These findings will help clinicians and patients understand the treatment and prognosis of CN visually and intuitively.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/supplementary material.
Ethics statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s), and minor(s)’ legal guardian/next of kin, for the publication of any potentially identifiable images or data included in this article.
Author contributions
RZ and ZZ: conceived the design of the study. XJ and JY: perfected the researched idea and obtained detailed data resources. CZ, XP and YW: used and implemented statistical methods to data. QL, HC and ZZ: finished the preliminary writing of the paper. †: these authors contributed equally to this work. *: corresponding author. All authors: offered valuable advice and confirmed the final revision of the article. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Louis DN, Arie P, Pieter W, Brat DJ, Cree IA, Dominique FB, et al. The 2021 WHO classification of tumors of the central nervous system: A summary. Neuro-Oncology (2021) 23(8):1231–51. doi: 10.1093/neuonc/noab106
2. Alqroom RY. Delving inside the enigmatic central neurocytoma. J Am Coll Surg (2020) 231(4):e39. doi: 10.1016/j.jamcollsurg.2020.08.096
3. Hassoun J, Gambarelli D, Grisoli F, Pellet W, Salamon G, Pellissier JF, et al. Central neurocytoma. an electron-microscopic study of two cases. Acta Neuropathol (1982) 56(2):151–6. doi: 10.1007/BF00690587
4. Yang I, Ung N, Chung LK, Nagasawa DT, Thill K, Park J, et al. Clinical manifestations of central neurocytoma. Neurosurg Clinics North America (2015) 26(1):5–10. doi: 10.1016/j.nec.2014.09.011
5. Lee SJ, Bui TT, Chen C, Carlito L, Chung LK, Sabrin S, et al. Central neurocytoma: A review of clinical management and histopathologic features. Brain Tumor Res Treat (2016) 4(2):49–57. doi: 10.14791/btrt.2016.4.2.49
6. Rai P, Nayak R, Anand D, Menon G. Central neurocytoma in the posterior fossa. Case Rep (2019) 12(9):e231626. doi: 10.1136/bcr-2019-231626
7. Sander C, Wallenborn M, Brandt VP, Ahnert P, Reuschel V, Eisenlöffel C, et al. Central neurocytoma: SNP array analyses, subtel FISH, and review of the literature. Pathol - Res Pract (2019) 215(7):344–38. doi: 10.1016/j.prp.2019.03.025
8. Alsadiq MN, Al Sadah ZM, Butt S, Aldahmen AA. Central neurocytoma with hemorrhagic presentation case report and review of the literature. Case Rep Surg (2022) 2022:9731987. doi: 10.1155/2022/9731987
9. Su C, Giorgos K, Zhang PJ, Bai HX. Relative survival of patients with central neurocytoma. J Clin Neuroence (2018) 55:123–4. doi: 10.1016/j.jocn.2018.07.020
10. Gaggero G, Valle L, Ferro J, Taietti D, Spina B. Extraventricular neurocytoma. Autopsy Case Rep (2022) 12:e2021348. doi: 10.4322/acr.2021.348
11. Tish S, Habboub G, Jones J, Ostrom QT, Kruchko C, Barnholtz-Sloan JS, et al. The epidemiology of central and extraventricular neurocytoma in the united states between 2006 and 2014. J Neuro Oncol (2019) 143:123–127. doi: 10.1007/s11060-019-03144-9
12. Mattar M, Shebl AM, Toson EA. Atypical central neurocytoma: An investigation of prognostic factors. World Neurosurg (2020) 146(9):e184–e193. doi: 10.1016/j.wneu.2020.10.068
13. Byun J, Hong SHo, Yoon MJ, Kwon SM, Cho YH, Kim JH, et al. Prognosis and treatment outcomes of central neurocytomas: clinical interrogation based on a single center experience. J Neuro Oncol (2018) 140:669–677. doi: 10.1007/s11060-018-2997-z
14. Imber BS, Braunstein SE, Wu FY, Nabavizadeh N, Boehling N, Weinberg VK, et al. Clinical outcome and prognostic factors for central neurocytoma: twenty year institutional experience. J Neurooncol (2016) 126(1):193–200. doi: 10.1007/S11060-015-1959-Y
15. Mattar M, Badry AE. Clinical outcome and prognostic factors for central neurocytoma, a study of 14 cases. Roman Neurosurg (2018) 32(1):73–84. doi: 10.2478/romneu-2018-0009
16. Dutta SW, Kaleem TA, Muller DA, Peterson J, Harrell AC, Quinones-Hinojosa A, et al. Central neurocytoma: Clinical characteristics, patterns of care, and survival. J Clin Neurosci (2018) 53:106–111. doi: 10.1016/j.jocn.2018.04.015
17. Ahmad F, Rosenblum MK, Chamyan G, Sandberg DI. Infiltrative brainstem and cerebellar neurocytoma. J Neurosurg Pediatr (2012) 10(5):418. doi: 10.3171/2012.8.PEDS08286
18. Ogiwara H, Dubner S, Bigio E, Chandler J. Neurocytoma of the cerebellum. Surg Neurol Int (2011) 2(1):36. doi: 10.4103/2152-7806.78246
19. Byun J, Kim M, Song SW, Kim Y-H, Hong CK, Kim JH. Extraventricular neurocytoma: Clinical investigation of heterogenous prognosis. Brain Tumor Res Treat (2022) 10(1):22–8. doi: 10.14791/btrt.2022.10.e30
20. Huang S, Liu X, Zhu J, Sun Z, Chen Y, Yu H. MR finding of extraventricular neurocytoma. J Coll Phys Surgeons–pakistan (2022) 32(11):1478–82. doi: 10.29271/jcpsp.2022.11.1478
21. Steinsiepe VK, Frick H, Jochum W, Fournier JY. Differential diagnosis of central neurocytoma: Two cases. J Neurol Surg Part A: Cent Eur Neurosurg (2020) 82(06):599–603. doi: 10.1055/s-0040-1718693
22. Han S, Yang Z, Yang Y, Qi X, Yan C, Yu C. Individual treatment decisions for central neurocytoma. Front Neurol (2020) 11:834. doi: 10.3389/fneur.2020.00834
23. Rades D, Fehlauer F. Treatment options for central neurocytoma. Neurology (2002) 59(8):1268–70. doi: 10.1212/WNL.59.8.1268
24. Bertalanffy A, Roessler K, Koperek O, Gelpi E, Prayer D, Knosp E. Recurrent central neurocytomas. Cancer (2010) 104(1):135–42. doi: 10.1002/cncr.21109
25. Zhang L, Fang S, Li X. Central neurocytomas in children: Clinicopathologic features and long-term surgical outcomes. Child's Nervous System (2022) 1–10. doi: 10.1007/s00381-022-05663-9
26. Xie Q, Xie B, Ou L, Wang M, Tang Z, He Y, et al. Clinical outcomes and prognostic analysis of 101 patients of central neurocytoma: A 10-year treatment experience at a single institution. Front Oncol (2022) 12:881460. doi: 10.3389/fonc.2022.881460
27. Wang M, Zhou P, Zhang S, Liu X, Jiang S. Clinical features, treatment and long-term outcomes of central neurocytoma: A 20-year experience at a single center. World Neurosurg (2018) 109:e59–e66. doi: 10.1016/j.wneu.2017.09.103
28. Singh V, Borkar A, Moiyadi A, Shetty P. Tetraventricular atypical central neurocytoma - case report. World Neurosurg (2018) 122:454–7. doi: 10.1016/j.wneu.2018.10.233
29. Mahavadi AK, Patel PM, Kuchakulla M, Shah AH, Ivan ME. Central neurocytoma treatment modalities: A systematic review assessing the outcomes of combined maximal safe resection and radiotherapy with gross total resection. World Neurosurg (2020) 137:e176–e82. doi: 10.1016/j.wneu.2020.01.114
30. Nakamura A, Kano H, Niranjan A, Lunsford LD. Radiosurgery for central neurocytoma. Leksell Radiosurgery (2019) 34:232–7. doi: 10.1159/000493069
31. Bui TT, Carlito L, Chung LK, Stephen T, Percy L, Chin RK, et al. Systematic analysis of clinical outcomes following stereotactic radiosurgery for central neurocytoma. Brain Tumor Res Treat (2017) 5(1):10–. doi: 10.14791/btrt.2017.5.1.10
32. Yamanaka K, Iwai Y, Shuto T, Kida Y, Sato M, Hayashi M, et al. Treatment results of gamma knife radiosurgery for central neurocytoma: Report of a Japanese multi-institutional cooperative study. World Neurosurg (2016) 90:300–5. doi: 10.1016/j.wneu.2016.03.016
33. Matsunaga S, Shuto T, Suenaga J, Inomori S, Fujino H. Gamma knife radiosurgery for central neurocytomas. Neurol Med Chir (2010) 50(2):107–12. doi: 10.2176/nmc.50.107
34. Minniti G, Clarke E, Lanzetta G, Osti MF, Enrici RM. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol (London England) (2011) 6:48. doi: 10.1186/1748-717X-6-48
35. Richardson AM, Armstrong VL, Gernsback JE, Gultekin SH, Komotar RJ. Central neurocytoma: Rare presentation in fourth ventricle and review of literature. World Neurosurg (2019) 123:357–61. doi: 10.1016/j.wneu.2018.12.021
36. Hussain SA, Dollear FG. (P043) central neurocytoma: impact of resection extent and adjuvant radiotherapy on survival outcomes. Oncology (2015) 29(1):295–300. doi: 10.1007/BF02649312
37. Cao D, Chen Y, Guo Z, Ou Y, Chen J. Clinical outcome after microsurgical resection of central neurocytoma: A single-centre analysis of 15 years. Front Neurol (2021) 12:790641. doi: 10.3389/fneur.2021.790641
38. She L, Deng D, Su L, Liu C. Comparison of surgery with or without adjuvant radiotherapy in treating central neurocytoma: A single-center retrospective real-world study. J Neuro-Oncol (2022) 160:455–62. doi: 10.1007/s11060-022-04164-8
39. Akyoldas G, Samanci Y, Tugcu ES, Peker S. Gamma knife radiosurgery for the treatment of central neurocytoma: a single-institution experience of 25 patients. Neurosurg Rev (2021) 44:3427–35. doi: 10.1007/s10143-021-01518-0
Keywords: central neurocytoma, SEER, prognosis, subgroup analysis, clinical application
Citation: Zhang Z, Yu J, Zhang C, Pang X, Wei Y, Lv Q, Chen H, Jin X and Zhan R (2023) Clinical prognostic factors for central neurocytoma and subgroup analysis of different treatment measures: A SEER database-based retrospective analysis from 2003 to 2019. Front. Oncol. 12:1014506. doi: 10.3389/fonc.2022.1014506
Received: 08 August 2022; Accepted: 19 December 2022;
Published: 06 January 2023.
Edited by:
David D. Eisenstat, Royal Children’s Hospital, AustraliaReviewed by:
Angela Mastronuzzi, Bambino Gesù Children’s Hospital (IRCCS), ItalyPaolo Palmisciano, University of Cincinnati, United States
Copyright © 2023 Zhang, Yu, Zhang, Pang, Wei, Lv, Chen, Jin and Zhan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Renya Zhan, MTE5NjA1N0B6anUuZWR1LmNu; Xuhong Jin, amluZ3h1aG9uZzE5NzFAMTI2LmNvbQ==
†These authors have contributed equally to this work
 Zibin Zhang
Zibin Zhang Jianbo Yu
Jianbo Yu Chao Zhang
Chao Zhang Xiaojun Pang1
Xiaojun Pang1 Renya Zhan
Renya Zhan