- 1Department of General Surgery, Grand Hospital of Shuozhou, Shuozhou, China
- 2Department of Clinical Pharmacy, Shenyang Pharmaceutical University, Shenyang, China
Purpose: Glypican-3 (GPC-3) expression is abnormal in the occurrence and development of hepatocellular carcinoma (HCC). To explore whether GPC-3 has diagnostic accuracy and prognostic significance of HCC, we did a systematic review and meta-analysis.
Method: PubMed, Embase, Cochrane Library, and China National Knowledge Infrastructure were searched with keywords “GPC-3” and “HCC” and their MeSH terms from inception to July 2022. We applied the hierarchical summary receiver operating characteristic model and evaluated the diagnostic value of GPC-3 alone and combination, and the correlation between high and low GPC-3 expression on clinicopathological features and survival data in prognosis.
Results: Forty-one original publications with 6,305 participants were included, with 25 of them providing data for diagnostic value and 18 records were eligible for providing prognostic value of GPC-3. GPC-3 alone got good diagnostic value in patients with HCC when compared with healthy control and moderate diagnostic value when compared with patients with cirrhosis. In addition, combination of GPC-3 + AFP and GPC-3 + GP73 got great diagnostic value in HCC versus cirrhosis groups; the combination of GPC-3 can also improve the diagnostic accuracy of biomarkers. Moreover, we discovered that overexpression of GPC-3 was more likely found in HBV infection, late tumor stage, and microvascular invasion groups and causes shorter overall survival and disease free survival, which means poor prognosis.
Conclusion: GCP-3 could be used as a biomarker in HCC diagnosis and prognosis, especially in evaluated diagnostic value in combination with AFP or GP73, and in forecasting worse survival data of overexpression GPC-3
Systematic Review Registration: https://www.crd.york.ac.uk/PROSPERO/, identifier [CRD42022351566].
Introduction
Hepatocellular carcinoma (HCC) is a kind of high-degree malignancy, with incidence rate and mortality rising year over year, and ranking sixth and fourth respectively in the world (1). HCC onset was hidden in the early stage, which can only be found in imaging scans (2–4). In addition, the malignancy develops rapidly in the middle to late stage, leading to the poor prognosis of patients with HCC. Moreover, for patients with cirrhosis, the risk of progression to HCC is high, so early diagnosis of HCC, especially in patients with cirrhosis, is particularly important (5–9). At present, alpha-fetoprotein (AFP), the most commonly used tumor marker in HCC, has a poor diagnostic performance (10, 11). Hence, it is particularly important to improve the diagnostic value of early HCC, especially HCC in cirrhosis. Recent studies have found that Glypican-3 (GPC-3) expression is abnormal in the occurrence and development of HCC (12, 13), which may be related to the diagnosis and prognosis of HCC.
GPC-3 protein is a heparan sulfate glycoprotein on the cell membrane surface, which is connected to the cell membrane surface through glycosylphosphatidylinositol anchor (14). GPC-3 anchored to the cell surface is a key glycoprotein that interacts with cells and extracellular matrix components. It has a strong potential to bind to functional biological macromolecules such as proteins and sugars, especially to a variety of cell growth factors, including vascular endothelial growth factor, epidermal growth factor, hepatocyte growth factor, fibroblast growth factor, and transforming growth factor–β. GPC-3 binds to a variety of growth factors to form receptor signal transduction complexes, which activate the tyrosine kinase activity of growth factor receptors, thereby regulating cell growth, differentiation, adhesion, proliferation, and migration, which may be related to the occurrence and progression of HCC (15–18).
However, the diagnostic accuracy and prognostic significance of GPC-3 have not yet been determined. The aim of our meta-analysis was to explore the role of GPC-3 in the diagnostic accuracy and prognostic significance of HCC versus controls. None of the published meta-analyses (19–21) provide a comprehensive overview of the diagnostic and prognostic roles of GPC-3 in tumorigenesis.
Methods
The selected publications and synthesis program followed the Predesigned Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement for diagnostic test accuracy and prognostic test significance (22, 23). In addition, the protocol of our study were pre-designed and registered with the PROSPERO website (No. CRD42022351566) (24).
Search strategy and selection criteria
Four online electronic databases (PubMed, Embase, Cochrane Library, and China National Knowledge Infrastructure) were searched using keywords “Glypican-3”, “GPC-3”, “Hepatocellular Carcinoma”, “Liver cancer”, and “HCC” and their MeSH terms from their inception to July 2022. Two researchers (DJ and YZ) independently screened titles, abstracts, and full text. Moreover, the disagreements were discussed with the sophisticated reviewer (WW).
Eligible studies should meet the following criteria: research studies should focus on the diagnostic test accuracy and/or prognostic test significance of GPC-3, and patients should be diagnosed with HCC, with useful data available. For diagnostic type study, both data from HCC group and non-HCC group were considered to evaluate the diagnostic value of GPC-3. For prognostic type study, the data of correlation between high and low GPC-3 expression were considered. No language restriction was set, and non-English articles were translated. Reviews and original studies with no control group and with a study population being patients with HCC recurrence were excluded. Moreover, the reference list of previously published articles was also checked to avoid omissions of potential articles.
Data extraction and quality assessment
Baseline characteristic and clinical diagnostic and prognostic data were extracted from every potential included research studies and were categorized into pre-set forms by two independent researchers (DJ and YZ). For studies that reported data of GPC-3 diagnostic accuracy, baseline characteristic of the first author, publication year, region, sample type, control type, detection method, cutoff value of GPC-3, sample size, gender, age, hepatitis B virus (HBV) infection (present/absent), hepatitis C virus (HCV) infection (present/absent), cirrhosis (present/absent), Child–Pugh score (B–C vs. A), and diagnostic value data of true positive (TP), false negative (FN), true negative (TN), and false positive (FP) were also extracted for assessment.
For studies that reported data of GPC-3 prognostic significance, baseline characteristic of the first author, publication year, region, detection method, cutoff value of GPC-3, sample size, gender, age, clinicopathological features of tumor size (small vs. big), HBV (present/absent), HCV (present/absent), cirrhosis (present/absent), AFP(<20 ng/ml/>20 ng/ml), tumor grade (I–II/III–IV), microvascular invasion (present/absent), Child–Pugh score (A vs. B–C), BCLC grade (A–B vs. C–D), differentiation (Well-moderate vs. high), and survival data of overall survival (OS) rate (high expression vs. low expression) and disease-free survival (DFS, high expression vs. low expression) in high versus low expression of GPC-3 were also extracted for assessment.
In diagnostic studies for the quality assessment process, modified Quality Assessment of Diagnostic Accuracy Studies 2 (QUADAS-2) tool was used (25). QUADAS-2 consists of four domains: patient selection, index test, reference standard, and flow of patients through the study, which could be included without high-risk options. None of the above domains could contain high-risk options; otherwise, the original research will not be allowed to be included in this meta-analysis. In prognostic studies for the quality assessment process, the Newcastle–Ottawa Scale (NOS) scale (26) was used. If a study got NOS score of less than 4 (max 10), the observational study then cannot be included in this meta-analysis.
In addition, the quality of evidence for both types of research was assessed on the basis of the GRADE system. For diagnostic outcomes, grades for recommendations, assessments, developments, and evaluations were estimated (27). In addition, for prognostic study, the risk of bias, imprecision, inconsistency, indirectness of the every outcome, and publication bias were evaluated (28). Moreover, quality assessment programs were also independently assessed by two independent researchers.
Outcomes and data synthesis
To pool outcomes from diagnostic research, we applied the hierarchical summary receiver operating characteristic (ROC) model (29) and evaluated the diagnostic value of overexpression GPC-3, GPC-3 + AFP, GPC-3 + GP73, and GPC-3 + GP73 + AFP as biomarkers under investigation. Moreover, sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio, and their 95% confidence intervals (CIs) as well as the area under the ROC curves were obtained from diagnostic models of overall participants. Subgroup analysis and meta-regression for each subtype of control include cirrhosis and hepatitis, cirrhosis only, and healthy control. A P-value less than 0.05 from the meta-regression indicates that this grouping method had a great impact on the overall results. Moreover, Deeks’ asymmetry test was used to determine potential publication bias.
To merge outcomes from prognostic research, random-effects models were applied to consider significant difference from odds ratios (ORs), hazard ratio (HR), or standardized mean differences (SMDs) with their 95% CIs in both clinicopathological features and survival data. I2 > 50% or P-value < 0.05 indicates high heterogeneity. Moreover, Begg’s and Egger’s tests were used to determine the publication bias among the included studies (30, 31). MetaDisc (version 1.4) and STATAMP (version 14.0) software were used to perform this meta-analysis.Results
Results
Study characteristics and quality assessment
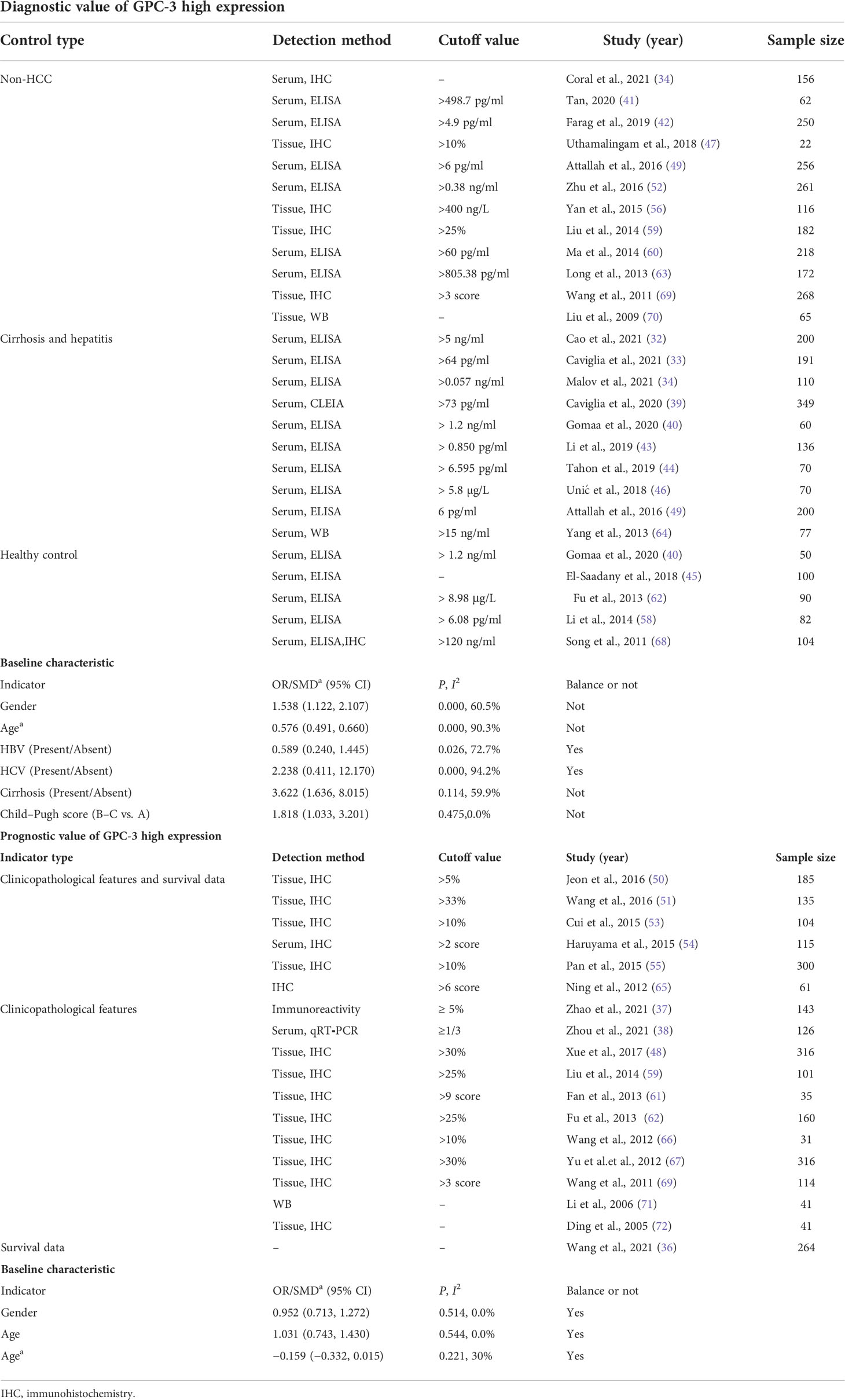
From the four online electronic databases, 931 non-repetitive publications have been screened. After checking titles and abstracts, 89 full-text articles have been assessed for eligibility. After careful screening, 40 articles (31–72) could be included in this meta-analysis, 25 of them (3,717 participants) reported data of GPC-3 diagnostic accuracy and 18 of them (2,588 patients with HCC) reported data of GPC-3 prognostic significance (Figure 1). The sample size of our included studies was 22–261, and most of them were published in Asia. We also summarized the control type, indicator type, detection method, and cutoff value; the cutoff value varied widely among the included studies that could have great influence on the overall results (Table 1; see details in Tables S1, S2). Moreover, we also meta-analyzed baseline indicator to determine whether the baseline is balance. We could notice that from diagnostic type baseline, there are more male patients with HCC, older, and more patients with HCC with liver cirrhosis and worse liver function grades (Child–Pugh score B–C). Moreover, baseline characteristic was balanced in data from prognostic significance studies (Table 1). Moreover, the quality assessment of the included studies is presented in Tables S3, S4; all of them got acceptable score.
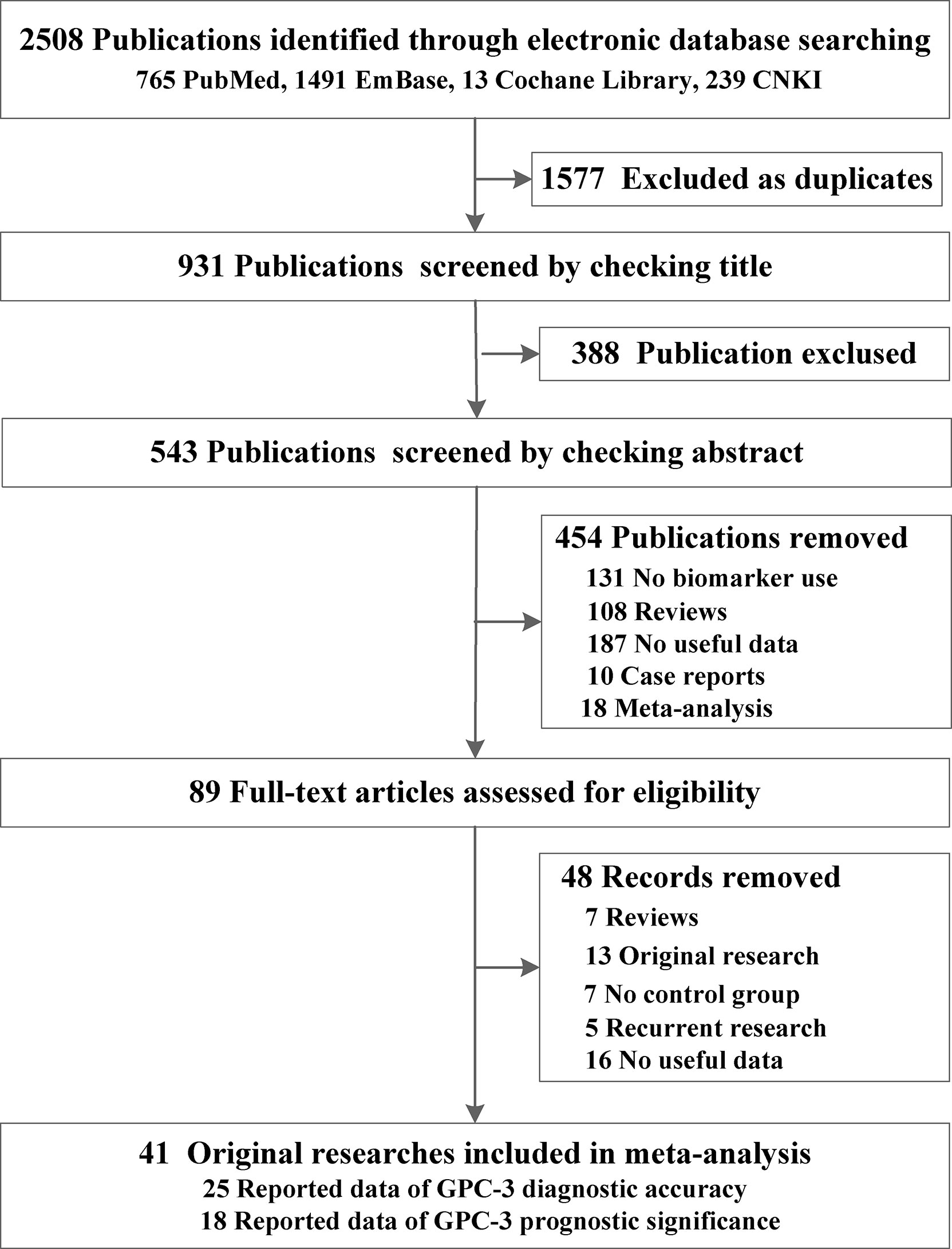
Figure 1 Preferred reporting items for systematic reviews and meta-analyses flowchart of the identification of eligible research studies.
Diagnostic value of GPC-3
First, we meta-analyzed data for the diagnostic value of GPC-3 alone in HCC. We can notice that the diagnostic value of GPC-3 alone in HCC vs. all control type is good with AUC of 0.8006 (Figure 2A), and the good diagnostic value could also be found in HCC vs. healthy control subgroup (AUC = 0.8835; Figure 2C). Nonetheless, the diagnostic value of HCC vs. cirrhosis and hepatitis/cirrhosis alone was ordinary, with AUC = 0.7203 and 0.7326 (Figure 2B). Meta-regression did not declare the main source of the difference. No publication bias was found from Deeks’ asymmetry test in the meta-analysis of GPC-3’s diagnostic value, and the quality of evidence was low to moderate. These results demonstrate that GPC-3 alone has good diagnostic efficacy in patients with HCC compared with healthy people (Table 2; Figure S1). However, for patients with liver cirrhosis progressing to HCC, its diagnostic efficiency is not high, so improving the diagnostic efficiency in this population has more clinical value.
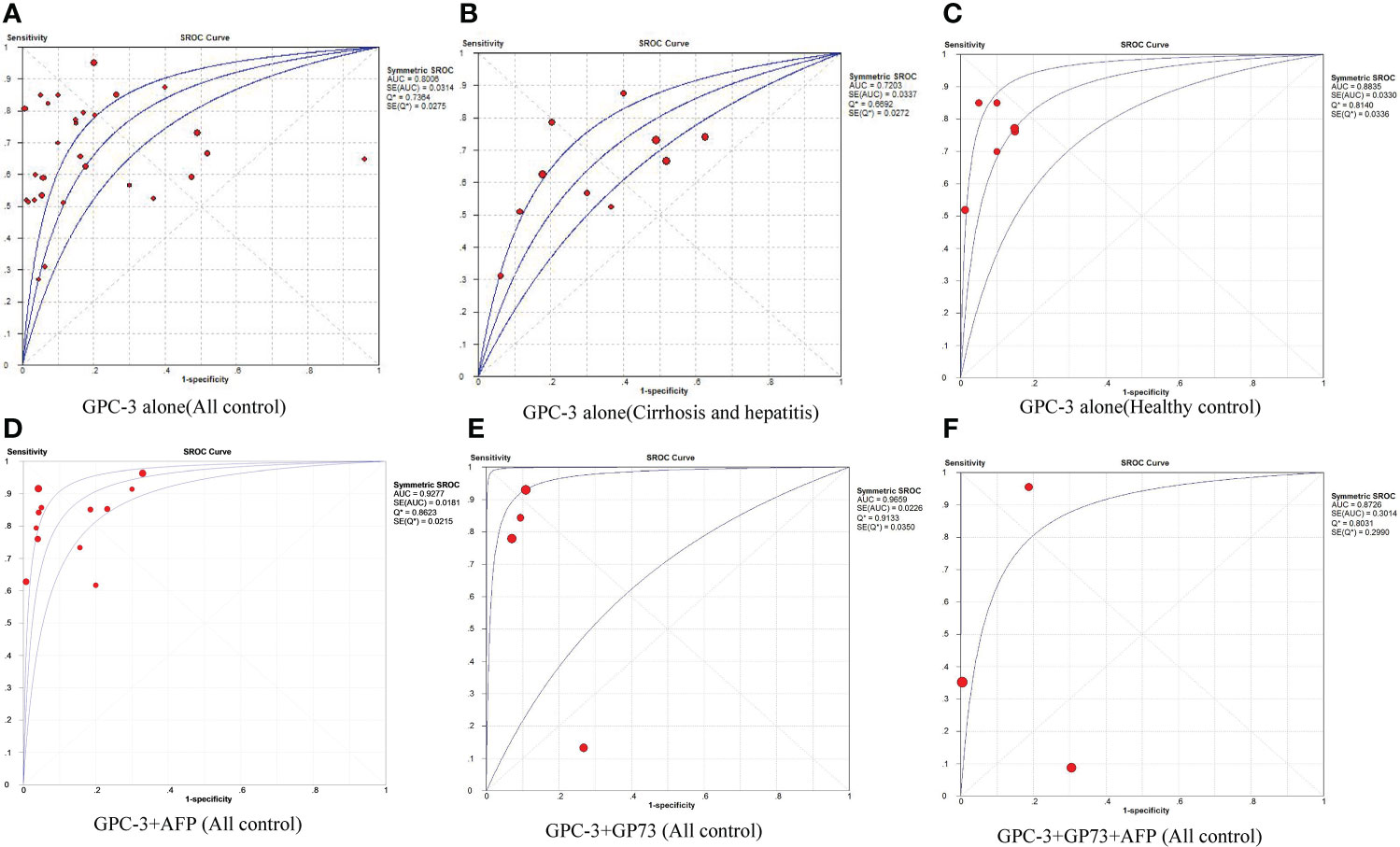
Figure 2 The pooled diagnostic accuracy of overexpression of GPC-3 alone in diagnosing HCC vs. all controls (A), HCC vs. cirrhosis and hepatitis (B), HCC vs. healthy control (C), and combination diagnostic accuracy of GPC-3 + AFP (D), GPC-3 + GP73 (E), and GPC-3 + GP73 + AFP (F) in diagnosing HCC vs. all controls. AUC, area under the curve; GPC-3, Glypican-3; HCC, hepatocellular carcinoma; SROC, summary receiver operating characteristic curves.
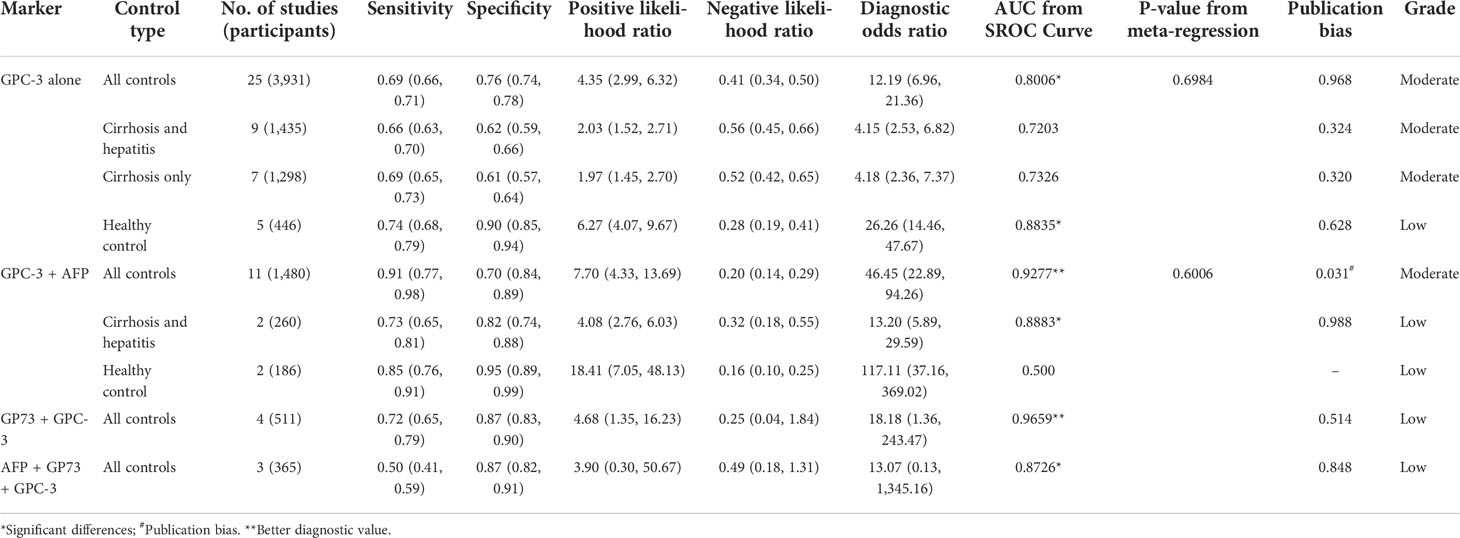
Table 2 Summarized of pooled sensitivity, specificity, positive likelihood ratio, negative likelihood ratio, diagnostic odds ratio, and SROC curve of GPC-3 in diagnosing hepatocellular carcinoma patients from controls.
Then, to improve the diagnostic value of HCC compared with liver cirrhosis, we studied the combined diagnostic value of GCP-3 combination with AFP and GP73. In combination diagnostics value of GPC-3 + AFP, great diagnostics value could be found in HCC vs. all control type of AUC of 0.9277 (Figure 2D). However, publication bias could also be found in this group of 0.031 and moderate GRADE. In addition, great diagnostics value could be found in HCC vs. cirrhosis and hepatitis, with AUC of 0.8883 with low grade; the value of HCC vs. healthy control is low due to small sample size. Subsequently, great diagnostics value could also be found in HCC vs. all control group in GPC-3 + GP73 (AUC = 0.9659; Figure 2E) and GPC-3 + GP73 + AFP (AUC = 0.8726; Figure 2F) diagnostic groups (Table 2; Figure S1). In general, the diagnostic efficacy of GPC-3 combined with AFP or GP73 has been improved; especially, GPC-3+ AFP group can improve the diagnostic value of HCC in patients with liver cirrhosis.
Prognostic value of GPC-3
First, we summarized the clinicopathological features of high GPC-3 vs. low GPC-3 expression in patients with HCC. We could notice that significant differences could be found in HBV (present/absent) group (OR = 1.340; 95% CI: 1.015 to 1.768) with low heterogeneity (P = 0.465, I2 = 0.0%), tumor grade (III–IV/I–II) group (1.653, 1.201 to 2.276) with low heterogeneity (1.653, 1.201 to 2.276), and microvascular invasion (present/absent) group (1.830, 1.009 to 3.318) with substantial heterogeneity (0.011, 59.7%). No publication bias was found in all clinicopathological features with low to moderate grade. These results indicate that high GPC-3 expression is not good for the prognosis of patients with HCC, especially patients with HCC with HBV infection, late tumor stage, and microvascular invasion.
Second, in evaluating survival data of high expression vs. low expression of GPC-3 in HCC, OS and DFS were taken into account. In terms of OS, seven research studies have reported ordinary data, and the merged outcome was (HR = 1.57; 95% CI: 1.11 to 2.03) with low heterogeneity (P = 0.510, I2 = 0.0%). When considering DFS, only four studies provide data, and the outcome from meta-analysis was (1.75, 1.14 to 2.35; 0.760, 0.0%) (Figure 3). The above data prove that the high expression of GPC-3 has a poor prognosis for patients with HCC.
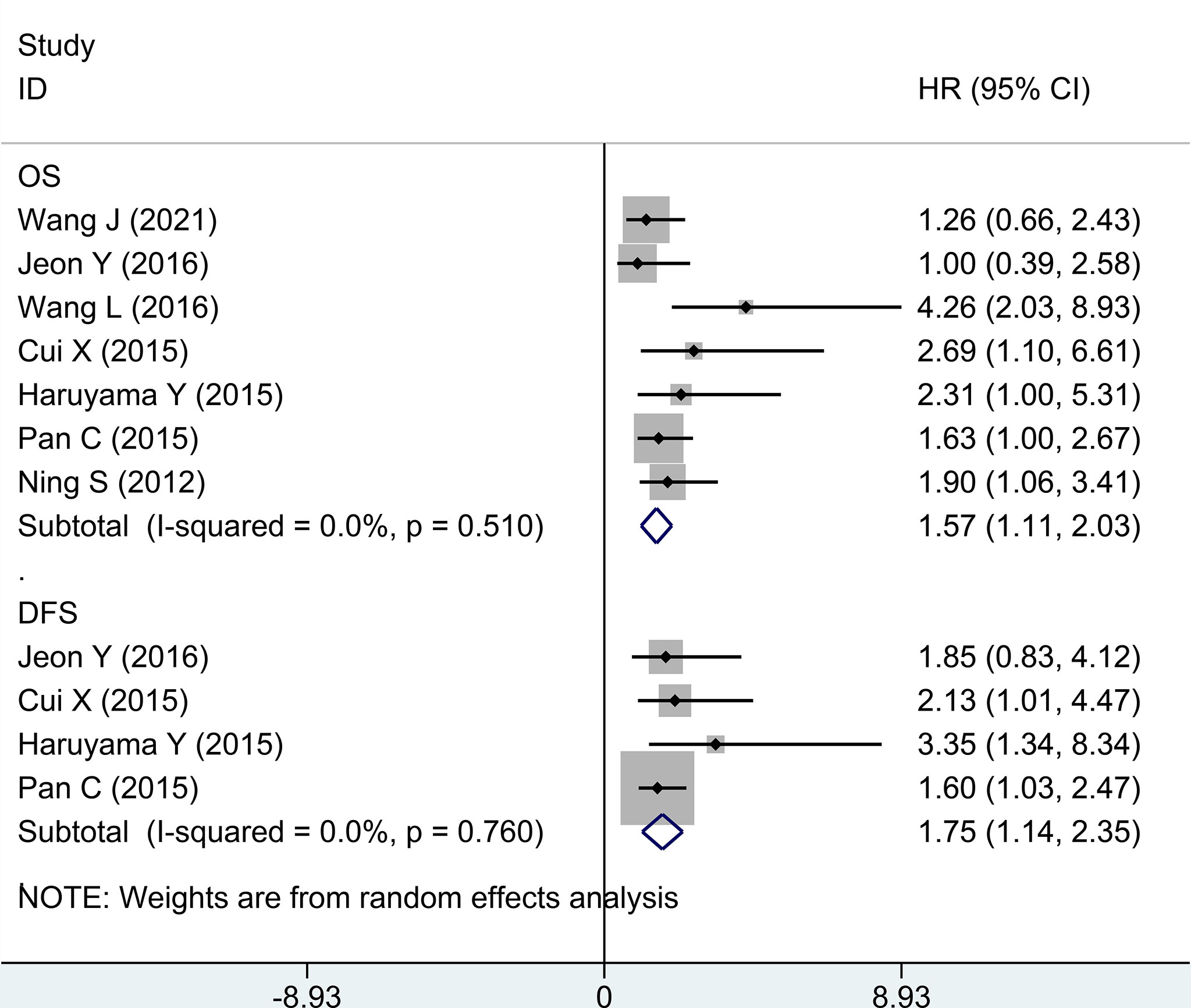
Figure 3 Forest plot for high GPC-3 expression versus low GPC-3 expression for overall survival and disease-free survival in patients with HCC. GPC-3, Glypican-3.
Discussion
This work focuses on the diagnostic accuracy and prognostic significance of GPC-3 as a biomarker of HCC, with 41 original publications and 6,315 participants included. First, we did a meta-analysis to explore whether the baseline level is balanced or not. The baseline indicators were not balanced in gender, age, liver cirrhosis numbers, and Child–Pugh liver function in diagnostic accuracy research studies (Figure 1; Table 1). Second, we did a diagnostic meta-analysis to evaluate the diagnostic value of GPC-3 alone. We determine that, compared with healthy control, the diagnostic value of GPC-3 alone is relatively great. However, in comparison with liver cirrhosis, the diagnostic value of GPC-3 alone is moderate. As a result, combination diagnostic value of GPC-3 was researched, and we detected that combination use of GPC-3 + AFP and GPC-3 + GP73 group got great diagnostic value of AUC > 0.9 in HCC versus cirrhosis groups (Figure 2; Table 2). These results demonstrate that the combination of GPC-3 can also improve the diagnostic accuracy of tumor biomarkers that have already been used in clinical practice (e.g., AFP). Third, the clinicopathological features and survival data in prognosis of HCC were meta-analyzed. We discovered that overexpression of GPC-3 was more likely found in HBV infection, late tumor stage, and microvascular invasion groups and causes shorter OS and DFS, which means poor prognosis (Figure 3; Table 3).
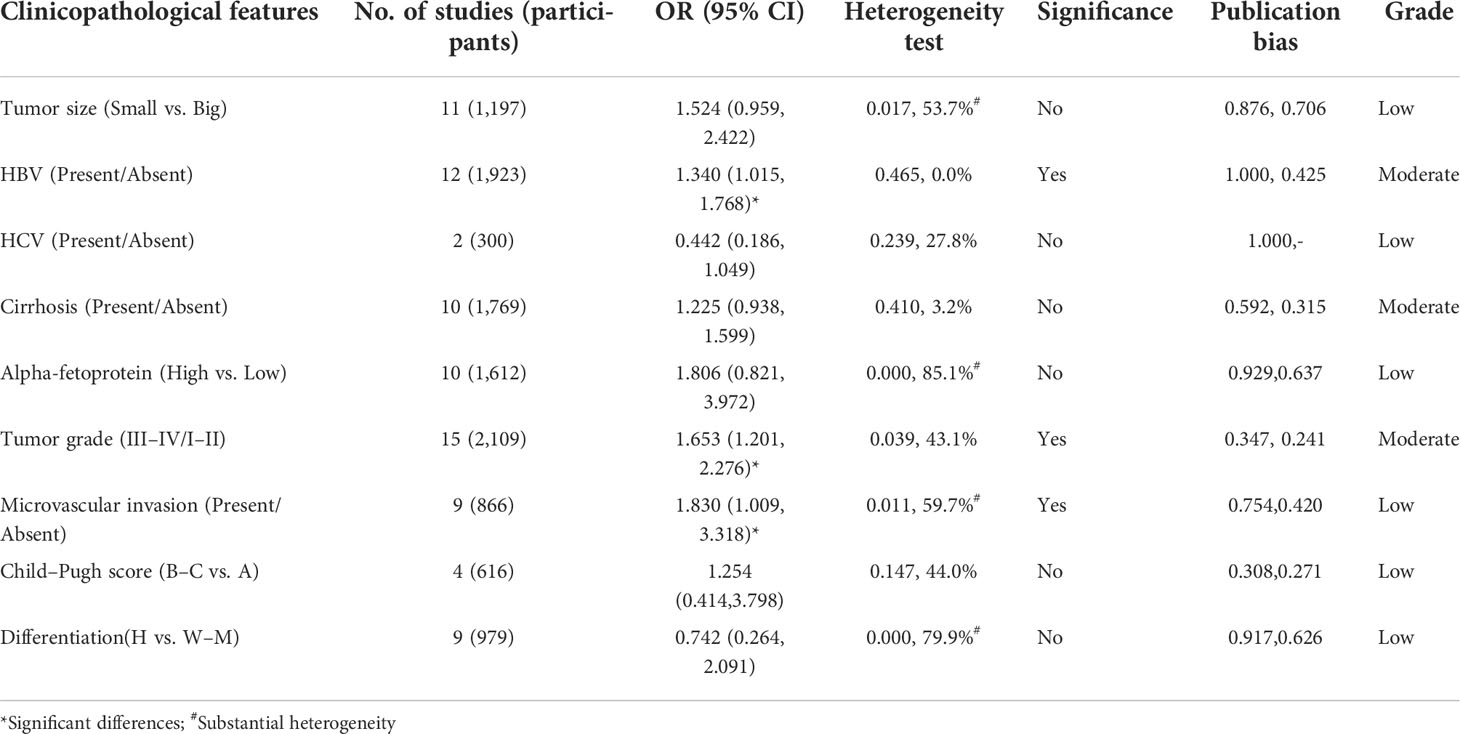
Table 3 Summarized of association between high GPC-3 expression and clinicopathological features in prognosis of patients with hepatocellular carcinoma from low GPC-3 expression.
Our systematic review and meta-analysis followed PRISMA checklist (22, 23) and was registered with PROSPERO website (24). The review by Yu et al. presented an idea that GPC-3 is a new HCC biomarker discovered after AFP, which is expressed not only in tissue but also in serum. GPC-3 has high sensitivity and specificity in the diagnosis of HCC, especially in the early stage of tumorigenesis. It has great clinical application value and brings hope for the diagnosis of early HCC (73). In addition, GPC-3 combined with AFP could use as a novel risk scoring model for predicting early recurrence of HCC after curative resection, which were shown to be effective at predicting early recurrence of HCC after curative resection (74). The research study by Zhou et al. showed that diagnosis of sarcomatoid HCC is rare and has a relatively poor prognosis. A panel of markers HSP70, GS, and GPC-3 served as an independent prognostic factor for sarcomatoid HCC (75). The research study by Kaseb et al. showed that a greater GPC-3 expression is associated with a worse HCC prognosis and may be a promising prognostic marker (76). The results in the study by Miura et al. revealed that the preoperative GPC-3 levels in patients with recurrence were significantly higher than those in patients without recurrence, suggesting that GPC-3 could be a better predictive marker of risk of recurrence than AFP, and the validation of GPC-3 as a predictive marker of HCC recurrence in a larger population is warranted (77). The above studies support our results, both in terms of diagnosis and prognosis.
Our results have been confirmed not only in clinical research but also in basic experimental research. The experiment by Aydin et al. showed that the expression of p62 and GPC-3 was significantly increased in HCC tissues compared with adjacent cirrhotic liver, and GPC-3–positive exosomes can be used for HCC detection and prediction of treatment outcomes (78). Moreover, data from the study by Montalbano reveal new aspects of the role of GPC-3 in early hepatocyte transformation. In addition, we concluded that GPC-3 may serve as a new HCC immune-therapeutic target (79). All the above studies confirmed that GPC-3 should be a valuable tumor biomarker in HCC diagnosis and prognosis.
None of the previous publications related to this topic have pooled diagnostic and prognostic data (19–21). Therefore, our study shows that GPC-3 can be used as a marker for the diagnosis and prognosis of HCC more effectively. GPC-3 belongs to heparin sulfate protein polysaccharide family and anchors to cell surface by glycosylphosphatidylinositol. Glypicans interact with growth factors and play significant roles in cell proliferation, differentiation, and migration (80, 81). GPC-3 could be used as a biomarker, in which GPC-3 gene could be a potential target for promoting hepatoma cell apoptosis and inhibiting metastasis through the Wnt/β-catenin and Hedgehog signaling pathways (80–82). The expression of GPC-3 is related with tumor size of HCC, which suggests that GPC-3 may potentially become an early diagnostic biomarker of HCC. Mechanism research has suggested that the accuracy and sensitivity for early diagnosis of HCC by using combined serum GPC-3 and AFP were better than AFP alone. Therefore, the above mechanisms and in vivo studies confirm our results.
There are also some limitations among our included studies. First, most of our included original research studies were from China, which may cause geographic heterogeneity. Moreover, cutoff values of GPC-3 in all studies were inconsistent, which may also lead to the heterogeneity among our studies (Tables 1, S1, S2). Second, baseline indicators were not balanced in diagnostic accuracy research studies. The cause maybe that HCC itself has a higher incidence in men, is more likely to be accompanied by cirrhosis, and has a worse Child–Pugh grading of liver function than the control group (Table 1). Third, few research studies provide data of combination diagnostic value of GCP-3 + AFP/GP73. However, we still suppose that the results were credible. Interestingly, the combination of GCP-3 + AFP + GP73 got lower diagnostic value than the combination of the above two, probably because only two articles provided the original data (Figure 2; Table 2). Last but not least, there was no combined prognostic data of GCP-3 and AFP to evaluate its combined prognostic value (Figure 3; Table 3), which did not correspond with the diagnostic studies.
In conclusion, our results suggested that GCP-3 could be used as a useful biomarker in HCC diagnosis and prognosis, especially in evaluated diagnostic value in combination with AFP or GP73, and in forecasting worse survival data of overexpression GPC-3. Therefore, we recommend that patients with cirrhosis should frequently diagnose whether there is HCC with detection by GPC-3 + AFP. Furthermore, for patients with HCC, if there was a GPC-3 overexpression, then the tumor can easily invade and affect survival. However, the conclusion still needs to be confirmed in large-scale diagnostic and prognostic research studies of GPC-3.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Author contributions
DJ: Conceptualization, Methodology, Formal analysis, Data Curation, Resources, Writing- Original draft preparation. YZ: Data curation, Visualization, Investigation, Writing- Original draft preparation and Reviewing and Editing. YW: Data curation, Visualization, Investigation. FX: Software, Validation, Supervision. JL: Formal analysis, Software, Validation. WW: Conceptualization, Data curation, Methodology, Writing- Reviewing and Editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1012418/full#supplementary-material
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
2. Tian Z, Zhang W. Clinical efficacy of interventional chemotherapy embolization combined with monopolar radiofrequency ablation on patients with liver cancer. J Oncol (2022) 2022. doi: 10.1155/2022/2306451
3. Torres HA, Vauthey JN, Economides MP, Mahale P, Kaseb A. Hepatocellular carcinoma recurrence after treatment with direct-acting antivirals: First, do no harm by withdrawing treatment. J Hepatol (2016) 65(4):862–4. doi: 10.1016/j.jhep.2016.05.034
4. Memeo R, Pessaux P, Silvestris N, Brunetti O, Solimando AG, Gardini AC. Hepatocellular Cancer[M]//Practical medical oncology textbook. Cham: Springer (2021) p. 689–706.
5. Feng YQ, Li BA, Feng F, Chen YS, Ren YX, Zhang H, et al. Novel mTOR inhibitor enhances the sensitivity of hepatocellular carcinoma cells to molecular targeting agents. Onco Targets Ther (2020) 13:7165–76. doi: 10.2147/OTT.S244474
6. Ma DB, Liu XY, Jia H, Zhang Y, Jiang Q, Sun H, et al. A novel small-molecule inhibitor of SREBP-1 based on natural product monomers upregulates the sensitivity of lung squamous cell carcinoma cells to antitumor drugs. Front Pharmacol (2022) 13:895744. doi: 10.3389/fphar.2022.895744
7. Liu YY, Ding CZ, Chen JL, Wang ZS, Yang B, Wu XM. A novel small molecular inhibitor of DNMT1 enhances the antitumor effect of radiofrequency ablation in lung squamous cell carcinoma cells. Front Pharmacol (2022) 13:863339. doi: 10.3389/fphar.2022.863339
8. Du Y, Shi X, Ma W, Wen P, Yu P, Wang X, et al. Phthalates promote the invasion of hepatocellular carcinoma cells by enhancing the interaction between pregnane X receptor and E26 transformation specific sequence 1. Pharmacol Res (2021) 169:105648. doi: 10.1016/j.phrs.2021.105648
9. Du F, Sun H, Sun F, Yang S, Tan H, Li X, et al. Knockdown of TANK-binding kinase 1 enhances the sensitivity of hepatocellular carcinoma cells to molecular-targeted drugs. Front Pharmacol (2022) 13:924523. doi: 10.3389/fphar.2022.924523
10. Jiang X, Hui F, Qin X, Wu Y, Liu H, Gao J, et al. Diagnosis accuracy and prognostic significance of the dickkopf-1 protein in gastrointestinal carcinomas: Systematic review and network meta-analysis. J Cancer (2020) 11(24):7091–100. doi: 10.7150/jca.49970
11. Zhang Y, Gao J, Bao Y, Liu Y, Tong Y, Jin S, et al. Diagnostic accuracy and prognostic significance of osteopontin in liver cirrhosis and hepatocellular carcinoma: a meta-analysis. Biomarkers (2022) 27(1):13–21. doi: 10.1080/1354750X.2021.2008009
12. Zheng X, Liu X, Lei Y, Wang G, Liu M. Glypican-3: A novel and promising target for the treatment of hepatocellular carcinoma. Front Oncol (2022) 12:824208. doi: 10.3389/fonc.2022.824208
13. Shimizu Y, Suzuki T, Yoshikawa T, Endo I, Nakatsura T. Next-generation cancer immunotherapy targeting glypican-3. Front Oncol (2019) 9:248. doi: 10.3389/fonc.2019.00248
14. Guo M, Zhang H, Zheng J, Liu Y. Glypican-3: A new target for diagnosis and treatment of hepatocellular carcinoma. J Cancer (2020) 11(8):2008. doi: 10.7150/jca.39972
15. Hassan N, Greve B, Espinoza-Sánchez NA, Götte M. Cell-surface heparan sulfate proteoglycans as multifunctional integrators of signaling in cancer. Cell Signalling (2021) 77:109822. doi: 10.1016/j.cellsig.2020.109822
16. Elgundi Z, Papanicolaou M, Major G, Cox TR, Melrose J, Whitelock JM, et al. Cancer metastasis: the role of the extracellular matrix and the heparan sulfate proteoglycan perlecan. Front Oncol (2020) 9:1482. doi: 10.3389/fonc.2019.01482
17. Yang S, Cai C, Wang H, Ma X, Shao A, Sheng J, et al. Drug delivery strategy in hepatocellular carcinoma therapy. Cell Communication Signaling (2022) 20(1):1–14. doi: 10.1186/s12964-021-00796-x
18. Lepedda AJ, Nieddu G, Piperigkou Z, Kyriakopoulou K, Karamanos N, Formato M. Circulating heparan sulfate proteoglycans as biomarkers in health and disease[C]. Semin Thromb Hemostasis (2021) 47(03):295–307. doi: 10.1055/s-0041-1725063
19. Rapado-González Ó, Martínez-Reglero C, Salgado-Barreira Á, Takkouche B, López-López R, Suárez-Cunqueiro MM, et al. Salivary biomarkers for cancer diagnosis: A meta-analysis. Ann Med (2020) 52(3-4):131–44. doi: 10.1080/07853890.2020.1730431
20. Ge L, Pan B, Song F, et al. Comparing the diagnostic accuracy of five common tumour biomarkers and CA19-9 for pancreatic cancer: A protocol for a network meta-analysis of diagnostic test accuracy. BMJ Open (2017) 7(12):e018175. doi: 10.1136/bmjopen-2017-018175
21. Shao C, Yu Z, Xiao J, Ma J, Zeraatkar D, Zhou J, et al. Prognosis of pregnancy-associated breast cancer: A meta-analysis. BMC Cancer (2020) 20(1):746. doi: 10.1186/s12885-020-07248-8
22. Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ (2009) 339:b2700. doi: 10.1136/bmj.b2700
23. McInnes MDF, Moher D, Thombs BD, McGrath TA, Bossuyt PM, PRISMA-DTA Group, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: The PRISMA-DTA statement. JAMA (2018) 319(4):388–96. doi: 10.1001/jama.2017.19163
24. PROSPERO 2009. Systematic reviews: CRD’s guidance for undertaking reviews in health care. In: Centre for reviews and dissemination. University of York, York, England.
25. Whiting PF, Rutjes AW, Westwood ME, Mallett S, Deeks JJ, Reitsma JB, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med (2011) 155(8):529–36. doi: 10.7326/0003-4819-155-8-201110180-00009
26. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25:603–5. doi: 10.1007/s10654-010-9491-z
27. Brozek JL, Akl EA, Jaeschke R, Lang DM, Bossuyt P, Glasziou P, et al. Grading quality of evidence and strength of recommendations in clinical practice guidelines: Part 2 of 3. the GRADE approach to grading quality of evidence about diagnostic tests and strategies. Allergy (2009) 64(8):1109–16. doi: 10.1111/j.1398-9995.2009.02083.x
28. Balshem H, Helfand M, Schünemann HJ, Oxman AD, Kunz R, Brozek J, et al. GRADE guidelines: 3. rating the quality of evidence. J Clin Epidemiol (2011) 64(4):401–6. doi: 10.1016/j.jclinepi.2010.07.015
29. Rutter CM, Gatsonis CA. A hierarchical regression approach to meta-analysis of diagnostic test accuracy evaluations. Stat Med (2001) 20(19):2865–84. doi: 10.1002/sim.942
30. Yang C, Xu C, Li X, Zhang Y, Zhang S, Zhang T, et al. Could camrelizumab plus chemotherapy improve clinical outcomes in advanced malignancy? a systematic review and network meta-analysis. Front Oncol (2021) 11:700165. doi: 10.3389/fonc.2021.700165
31. Hui F, Xu C, Xu X, Chen J, Geng H, Yang C, et al. What is the most suitable agent combined with apatinib for transarterial chemoembolization treatment in advanced hepatocellular carcinoma patients? A systematic review and network meta-analysis. Front Oncol (2022) 12:887332. doi: 10.3389/fonc.2022.887332
32. Cao Y, Ma X, Zhang H, Tu Y. The relationship between the expression levels of GP73, glypican-3 and miR-335 in serum of patients with HCC and the diagnostic value of patients. Exp Lab Med (2021) 39(3):706–9.
33. Caviglia GP, Armandi A, Rosso C, Gaia S, Aneli S, Rolle E, et al. Biomarkers of oncogenesis, adipose tissue dysfunction and systemic inflammation for the detection of hepatocellular carcinoma in patients with nonalcoholic fatty liver disease. Cancers (Basel) (2021) 13(10):2305. doi: 10.3390/cancers13102305
34. Coral GP, Branco F, Meurer R, Marcon PDS, Fontes PRO, Mattos AA. Results of immunohistochemistry in the differential diagnosis of early hepatocellular carcinoma and nodules with high-grade dysplasia in patients with cirrhosis. Arq Gastroenterol (2021) 58(1):82–6. doi: 10.1590/S0004-2803.202100000-14
35. Malov SI, Malov IV, Kuvshinov AG, Marche PN, Decaens T, Macek-Jilkova Z, et al. Search for effective serum tumor markers for early diagnosis of hepatocellular carcinoma associated with hepatitis c. Sovrem Tekhnologii Med (2021) 13(1):27–33. doi: 10.17691/stm2021.13.1.03
36. Wang JY, Wang XK, Zhu GZ, Zhou X, Yao J, Ma XP, et al. Distinct diagnostic and prognostic values of glypicans gene expression in patients with hepatocellular carcinoma. BMC Cancer (2021) 21(1):462. doi: 10.1186/s12885-021-08104-z
37. Zhao J, Gao S, Sun W, Grimm R, Fu C, Han J, et al. Magnetic resonance imaging and diffusion-weighted imaging-based histogram analyses in predicting glypican 3-positive hepatocellular carcinoma. Eur J Radiol (2021) 139:109732. doi: 10.1016/j.ejrad.2021.109732
38. Zhou X, Lin B, Hou Y, Si S. Expression and clinical significance of miR-202 and its target gene glypican-3 in hepatocellular carcinoma. J Nanjing Med University (Natural Sciences) (2021) 41(3):349–54.
39. Caviglia GP, Ciruolo M, Abate ML, Grimm R, Fu C, Han J, et al. Alpha-fetoprotein, protein induced by vitamin K absence or antagonist II and glypican-3 for the detection and prediction of hepatocellular carcinoma in patients with cirrhosis of viral etiology. Cancers (Basel) (2020) 12(11):3218. doi: 10.3390/cancers12113218
40. Gomaa SH, Abaza MM, Elattar HA, Amin GA, Elshahawy DM. Soluble cluster of differentiation 26/soluble dipeptidyl peptidase-4 and glypican-3 are promising serum biomarkers for the early detection of hepatitis c virus related hepatocellular carcinoma in egyptians. Arab J Gastroenterol (2020) 21(4):224–32. doi: 10.1016/j.ajg.2020.04.016
41. Tan G. A dissertation submitted in partial fulfillment of the requirements for the degree of master of medicine. Wuhan: Huazhong University of Science and Technology (2020).
42. Farag RMA, Al Ayobi D, Alsaleh KA, Kwon HJ, EL-Ansary A, Dawoud EA. Studying the impact of golgi protein 73 serving as a candidate biomarker in early diagnosis for hepatocellular carcinoma among Saudi patients. Asian Pac J Cancer Prev (2019) 20(1):215–20. doi: 10.31557/APJCP.2019.20.1.215
43. Li J, Qiyu S, Wang T, Jin B, Li N. Improving the detection of hepatocellular carcinoma using serum AFP expression in combination with GPC-3 and micro-RNA MiR-122 expression. Open Life Sci (2019) 14:53–61. doi: 10.1515/biol-2019-0007
44. Tahon AM, El-Ghanam MZ, Zaky S, Emran TM, Bersy AM, El-Raey F, et al. Significance of glypican-3 in early detection of hepatocellular carcinoma in cirrhotic patients. J Gastrointest Cancer (2019) 50(3):434–41. doi: 10.1007/s12029-018-0095-2
45. El-Saadany S, El-Demerdash T, Helmy A, Mayah WW, El-Sayed Hussein B, Hassanien M, et al. Diagnostic value of glypican-3 for hepatocellular carcinomas. Asian Pac J Cancer Prev (2018) 19(3):811–7. doi: 10.22034/APJCP.2018.19.3.811
46. Unić A, Derek L, Duvnjak M, Patrlj L, Rakić M, Kujundžić M, et al. Diagnostic specificity and sensitivity of PIVKAII, GP3, CSTB, SCCA1 and HGF for the diagnosis of hepatocellular carcinoma in patients with alcoholic liver cirrhosis. Ann Clin Biochem (2018) 55(3):355–62.
47. Uthamalingam P, Das A, Behra A, Kalra N, Chawla Y. Diagnostic value of Glypican3, heat shock protein 70 and glutamine synthetase in hepatocellular carcinoma arising in cirrhotic and non-cirrhotic livers. J Clin Exp Hepatol (2018) 8(2):173–80. doi: 10.1016/j.jceh.2017.09.005
48. Xue R, Feng J, Meng Q, Lv F, Zhu Y, Yu H, et al. The significance of glypican-3 expression profiling in the tumor cellular origin theoretical system for hepatocellular carcinoma progression. J Gastroenterol Hepatol (2017) 32(8):1503–11. doi: 10.1111/jgh.13736
49. Attallah AM, El-Far M, Omran MM, Abdelrazek MA, Attallah AA, Saeed AM, et al. GPC-HCC model: A combination of glybican-3 with other routine parameters improves the diagnostic efficacy in hepatocellular carcinoma. Tumour Biol (2016) 37(9):12571–7. doi: 10.1007/s13277-016-5127-6
50. Jeon Y, Kim H, Jang ES, Hong S, Kim JW, Yoon YS, et al. Expression profile and prognostic value of glypican-3 in post-operative south Korean hepatocellular carcinoma patients. APMIS (2016) 124(3):208–15. doi: 10.1111/apm.12491
51. Wang L, Pan L, Yao M, Cai Y, Dong Z, Yao D. Expression of oncofetal antigen glypican-3 associates significantly with poor prognosis in HBV-related hepatocellular carcinoma. Oncotarget (2016) 7(27):42150–8. doi: 10.18632/oncotarget.9892
52. Zhu B, Xiao Y, Peng Y, Wang P, Zhang W, Fan J, et al. Values of combined detection of GPC-3 and AFP in diagnosis of primary liver cancer. Chin J Hepatol (2016) 8(2):110–2.
53. Cui X, Li Z, Gao PJ, Gao J, Zhu JY. Prognostic value of glypican-3 in patients with HBV-associated hepatocellular carcinoma after liver transplantation. Hepatobiliary Pancreat Dis Int (2015) 14(2):157–63. doi: 10.1016/s1499-3872(15)60349-6
54. Haruyama Y, Yorita K, Yamaguchi T, Kitajima S, Amano J, Ohtomo T, et al. High preoperative levels of serum glypican-3 containing n-terminal subunit are associated with poor prognosis in patients with hepatocellular carcinoma after partial hepatectomy. Int J Cancer (2015) 137(7):1643–51. doi: 10.1002/ijc.29518
55. Pan C, Wang X, Chen W, Tao C, Xu X, Jin L, et al. Reevaluation of glypican-3 as a prognostic marker in HCC using X-tile software. Med Oncol (2015) 32(1):359. doi: 10.1007/s12032-014-0359-z
56. Yan W, Xie M, Liu Y. The clinical value of GP73, AFP-L3 combined GPC-3 detection with in the diagnosis of primary hepatic carcinoma. Chin J Lab Diagnosis (2015) 19(10):1651–4.
57. Zhao Y, Wang M, Cui C, Zhang L, Liao F, Li H, et al. Significance of combined tests of serum golgi glycoprotein 73 and other biomarkers in diagnosis of small primary hepatocellular carcinoma. Cancer biomark (2015) 15(5):677–83. doi: 10.3233/CBM-150508
58. Li Z, Li X, Yang S, Qiu X. Performance of combined detection of serum glypican-3 and AFP for the diagnostic of hepatocellular carcinoma. Lab Med Clin (2014) 11(24):3424–5.
59. Liu M, Zeng X, Hou E, Qiu X, et al. Expressions and clinical significance of glypican-3, MMP-9 and MMP-14 in primary hepatocellular carcinoma. Chongqing Med J (2014) 43(2):173–6.
60. Ma Q, Huang Y, Tong H, Wang F, et al. Glypican-3, a novel tumor in diagnosis of human hepatocellular carcinoma. Clin J Cancer Prev Treat (2014) 21(2):127–30.
61. Fan G, Lin H, Li L, Jiang S, Ren Y, Cao J, et al. Expression and clinical significance of glypican-3 in primary hepatocellular carcinoma. Beijing Med J (2013) 35(6):423–6.
62. Fu SJ, Qi CY, Xiao WK, Li SQ, Peng BG, Liang LJ, et al, et al. SGlypican-3 is a potential prognostic biomarker for hepatocellular carcinoma after curative resection. Surgery (2013) 154(3):536–44. doi: 10.1016/j.surg.2013.02.014
63. Long L, Chen Z, Wang K, Tao Y, Yi B. Value of GPC-3, GP73, AFP-L3 and AFP detection in the diagnosis of primary hepatic carcinoma. China J Modern Med (2013) 23(28):46–50.
64. Yang H, Wang Y, Xu H, Sang X, Lu X, Xu Y, et al. Utility of alpha-fetoprotein, golgi protein 73 and glypican-3, alone or in combination, as biomarkers for hepatocellular carcinoma. Oncol Process (2013) 11(3):249–53.
65. Ning S, Bin C, Na H, Peng S, Yi D, Xiang-hua Y, et al. Glypican-3, a novel prognostic marker of hepatocellular cancer, is related with postoperative metastasis and recurrence in hepatocellular cancer patients. Mol Biol Rep (2012) 39(1):351–7. doi: 10.1007/s11033-011-0745-y
66. Wang YL, Zhu ZJ, Teng DH, et al. Glypican-3 expression and its relationship with recurrence of HCC after liver transplantation. World J Gastroenterol (2012) 18(19):2408–14. doi: 10.3748/wjg.v18.i19.2408
67. Xue R, Feng J, Meng Q, Yao Z, Gao W, Shen ZY. The significance of glypican-3 expression profiling in the tumor cellular origin theoretical system for hepatocellular carcinoma progression. J Gastroenterol Hepatol (2017) 32(8):1503–11. doi: 10.1111/jgh.13736
68. Song M, Yang Y, Wang D, You F, Chen L. The diagnostic role of combination of glypican-3 and AFP in primary hepatic carcinoma. J Clin Hepatobiliary Disease (2011) 27(4):391–9.
69. Wang T. The significance of glypican-3 in early diagnosis and differential diagnosis of hepatocellular carcinoma. Tianjin Med Univ (2011).
70. Liu X, Gong Y, Fang G. Glypican-3Protein expression in serum and its diagnostic value of joint detection with AFP in hepatocellular carcinoma. Pract Prev Med (2009) 16(2):562–4.
71. Li B, Zhao Q, Zhu Y, Zhang F, Dou K. Significance of phosphatidylinositol proteoglycan-3 mRNA expression in hepatocellular carcinoma tissues and peripheral blood cells. Clin J Surg (2006) 44(7):458–62.
72. Ding G, Wang H, Chen H, Wu M, Man X, Cong W, et al. Expression of the glypican-3 gene in α-fetoprotein-negative human hepatocellular carcinoma. Chinese-German J Clin Oncol (2005) 4(5):262–6. doi: 10.1007/s10330-005-0409-2
73. Yu J, Zhang Q, Xu W, Li M. Advances in the relationship between the detection of glypican-3 in serum and the diagnosis of primary hepatic carcinoma. J Mol Diagn Ther (2013) 5(3):189–93.
74. Bao S, Gu J, Gan K, Fang Y, Wang T, Lin J, et al. Glypican-3 and hepatocyte paraffin-1 combined with alpha-fetoprotein as a novel risk scoring model for predicting early recurrence of hepatocellular carcinoma after curative resection. Eur J Gastroenterol Hepatol (2021) 33(1S Suppl 1):e603–9. doi: 10.1097/MEG.0000000000002175
75. Zhou C, Zhang X, Zhou K, Hou Y, Chen F, Zhang X, et al. Long-term outcomes and prognosis for patients with sarcomatoid hepatocellular carcinoma. Ann Transl Med (2022) 10(7):394. doi: 10.21037/atm-21-4322
76. Kaseb AO, Hassan M, Lacin S, Abdel-Wahab R, Amin HM, Shalaby A, et al. Evaluating clinical and prognostic implications of glypican-3 in hepatocellular carcinoma. Oncotarget (2016) 7(43):69916–26. doi: 10.18632/oncotarget.12066
77. Miura M, Fujinami N, Shimizu Y, Mizuno S, Saito K, Suzuki T, et al. Usefulness of plasma full-length glypican-3 as a predictive marker of hepatocellular carcinoma recurrence after radial surgery. Oncol Lett (2020) 19(4):2657–66. doi: 10.3892/ol.2020.11371
78. Aydin Y, Koksal AR, Thevenot P, Chava S, Heidari Z, Lin D, et al. Experimental validation of novel glypican 3 exosomes for the detection of hepatocellular carcinoma in liver cirrhosis. J Hepatocell Carcinoma (2021) 8:1579–96. doi: 10.2147/JHC.S327339
79. Montalbano M, Rastellini C, McGuire JT, Prajapati J, Shirafkan A, Vento R, et al. Role of glypican-3 in the growth, migration and invasion of primary hepatocytes isolated from patients with hepatocellular carcinoma. Cell Oncol (Dordr) (2018) 41(2):169–84. doi: 10.1007/s13402-017-0364-2
80. Sun B, Huang Z, Wang B, Yu Y, Lin S, Luo L, et al. Significance of glypican-3 (GPC-3) expression in hepatocellular cancer diagnosis. Med Sci Monit (2017) 23:850–5. doi: 10.12659/msm.899198
81. Huang SL, Wang YM, Wang QY, Feng GG, Wu FQ, Yang LM, et al. Mechanisms and clinical trials of hepatocellular carcinoma immunotherapy. Front Genet (2021) 12:691391. doi: 10.3389/fgene.2021.691391
Keywords: Glypican-3, hepatocellular carcinoma, diagnosis, prognosis, meta-analysis
Citation: Jiang D, Zhang Y, Wang Y, Xu F, Liang J and Wang W (2022) Diagnostic accuracy and prognostic significance of Glypican-3 in hepatocellular carcinoma: A systematic review and meta-analysis. Front. Oncol. 12:1012418. doi: 10.3389/fonc.2022.1012418
Received: 05 August 2022; Accepted: 30 August 2022;
Published: 23 September 2022.
Edited by:
Fan Feng, The 302th Hospital of PLA, ChinaCopyright © 2022 Jiang, Zhang, Wang, Xu, Liang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Weining Wang, YmV5b25kNTA1ODhAMTYzLmNvbQ==
 Donglei Jiang1
Donglei Jiang1 Yingshi Zhang
Yingshi Zhang