- 1Department of Pharmacy, West China Second University Hospital, Sichuan University, Chengdu, Sichuan, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, Sichuan, China
- 3Department of Pharmacy, The People’s Hospital of Langzhong, Langzhong, Sichuan, China
- 4University-Town Hospital of Chongqing Medical University, Chongqing, China
- 5Department of Pharmacy, Sichuan Provincial People’s Hospital Jinniu Hospital, Chengdu, Sichuan, China
- 6The First People’s Hospital of Bijie City, Guizhou, China
Background: With the widespread application of platinum drugs in antitumor therapy, the incidence of platinum drug adverse events (ADEs) is always severe. This study aimed to explore the adverse event signals of Cisplatin, Carboplatin and Oxaliplatin, three widely used platinum-containing drugs, and to provide a reference for rational individualized clinical drug use.
Methods: The adverse event report data of the three platinum drugs from the first quarter of 2017 to the fourth quarter of 2021 were extracted from the FAERS database, and the data mining and risk factors for the relevant reports were carried out using the reporting odds ratio (ROR) method the proportional reporting ratio (PRR)and the comprehensive criteria (MHRA) method.
Results: A total of 1853 effective adverse event signals were obtained for the three platinum agents, including 558 effective signals for Cisplatin, 896 effective signals for Carboplatin, and 399 effective signals for Oxaliplatin. The signals involve 23 effective different system organs (SOCs). The adverse events of Cisplatin are mainly fixed on blood and lymphatic system diseases, gastrointestinal diseases, systemic diseases and various reactions at the administration site. The adverse events of Carboplatin are mainly focused on blood and lymphatic system diseases, respiratory system, thoracic and mediastinal diseases, while the adverse events of Oxaliplatin are mainly concentrated in respiratory system, thoracic and mediastinal diseases, various nervous system diseases, and gastrointestinal system diseases.
Conclusion: It was found that the main systems involved in common adverse events of platinum drugs are different, and the correlation strength of platinum drugs with the certain adverse events of each system is different.
Introduction
Since cisplatin was available in the United States in 1978 (1), platinum drugs such as carboplatin (2), nedaplatin, oxaliplatin (3), and lobaplatin have played an extremely important role in the treatment of tumors (4). Adverse drug events (ADEs), such as myelosuppression, nephrotoxicity, allergic reactions, etc., are even life threatening (5, 6). The other one important aspect of platinum resistance is that oxaliplatin is the only platinum drug recommended for platinum resistance current (7).The aim is to choose the appropriate platinum drugs used for chemotherapy, which could help to reduce adverse drug reactions in important target organs and reduce the occurrence of platinum resistance while exerting curative effects (8).
The FDA adverse event reporting system (FAERS), as a global authoritative open-source database for spontaneous reporting of ADEs (9), can make up for shortcomings such as the lag, uncertainty, and incompleteness of the instructions. According to the investigation, the literature was rarely reported based on the FAERS database. The ADEs related to platinum drugs remain unclear in the real world. The authors mine and analyze the ADE signals of platinum drugs in the real world of the last 5 years to explore the differences in ADEs of platinum drugs, to provide a reference for the selection and safe use of platinum drugs and to provide ideas (10) for the development of new drugs.
Materials and methods
Data sources and processing
FAERS updates drug ADEs quarterly. This study extracted 20 quarterly data points from the first quarter of 2017 to the fourth quarter of 2021 in the FAERS. To overcome problems with data quality, we manually corrected mistakes in the data entities and deleted duplicates according to FDA’s recommended method, after deleting duplicate reports, screening was performed, and reports with cisplatin, carboplatin, and oxaliplatin as the primary suspected drugs were obtained. ADEs are coded by preferred terms (PTs) in the Medical Dictionary for Regulatory Activities terminology (11, 12). The system organ class (SOC) was also used to classify its ADEs (13).
Data mining analysis
In pharmacovigilance research, data mining algorithms have been developed for mining adverse reaction signals. The international mainstream signal detection methods include proportional reporting ratio (PRR), reporting odds ratio (ROR) (14, 15), medicine and health products regulatory agency (MHRA), Bayesian confidence propagation neural network (BCPNN) and multiitem gamma pass shrink (MGPS) the empirical Bayes geometric mean (EBGM), etc. The formulas of the ROR method and PRR method in the frequency method are simple, easy to calculate and understand. This study will also use this method for research and analysis. Signal PTs were screened out through the threshold setting, the events in which the lower limit of 95% CI >1 and PTs >3 in the ROR method, and the events with PRR≥2, χ2≥4 in which PTs >3 in the MHRA method (12, 16).
Results
Basic data of ADE reports
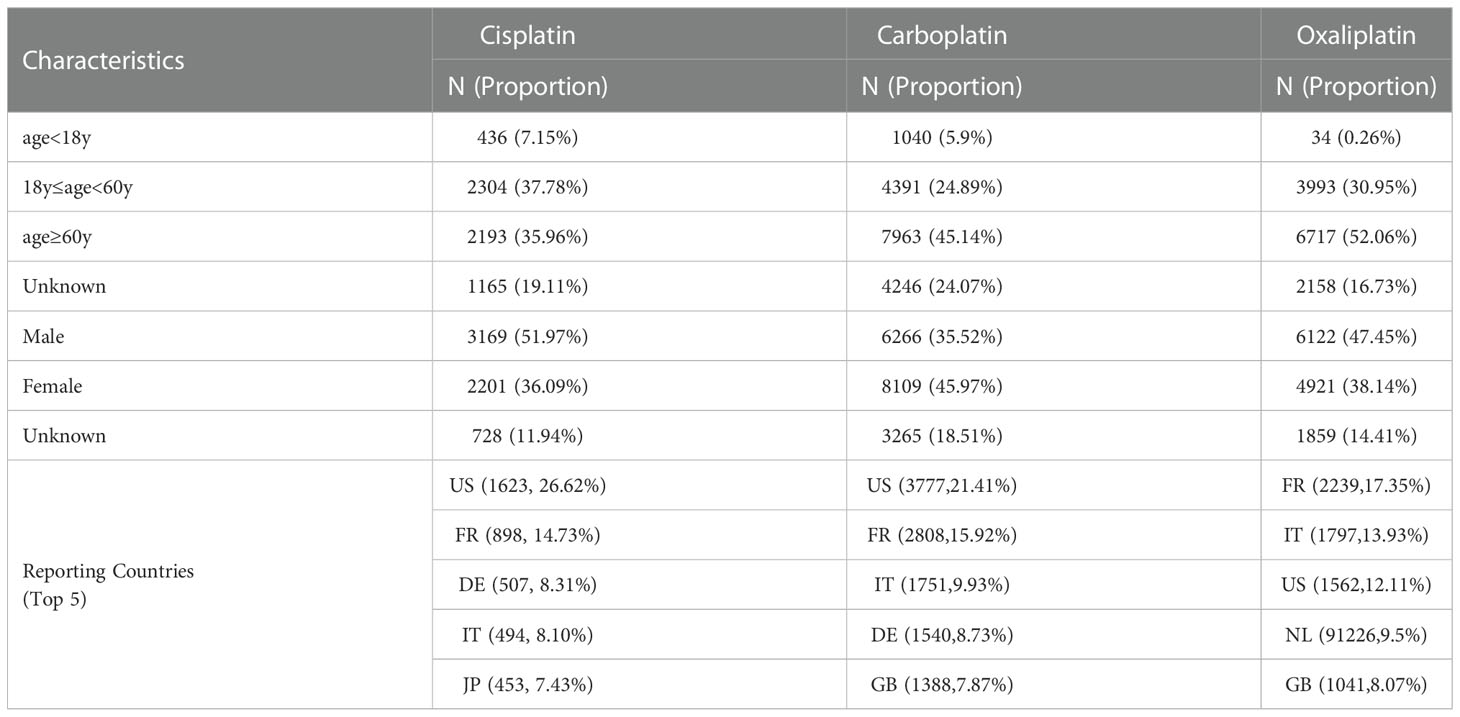
As shown in Table 1, the number of reports with cisplatin as the primary suspected drug was 6098, the number of reports with carboplatin as the primary suspected drug was 17640, and the number of reports with oxaliplatin as the primary suspected drug was 12902. Among the patients of known ages, patients over 60 years old accounted for the largest proportion, followed by patients between 18 and 60 years old. Among patients of known gender, adverse events of cisplatin were 51.97% for males and 31.69% for females, carboplatin was 35.52% for males and 45.97% for females, and oxaliplatin was 47.45% for males and 38.14% for females. The top reporting countries are mainly the United States, France, Germany and other European countries.
Comparative analysis
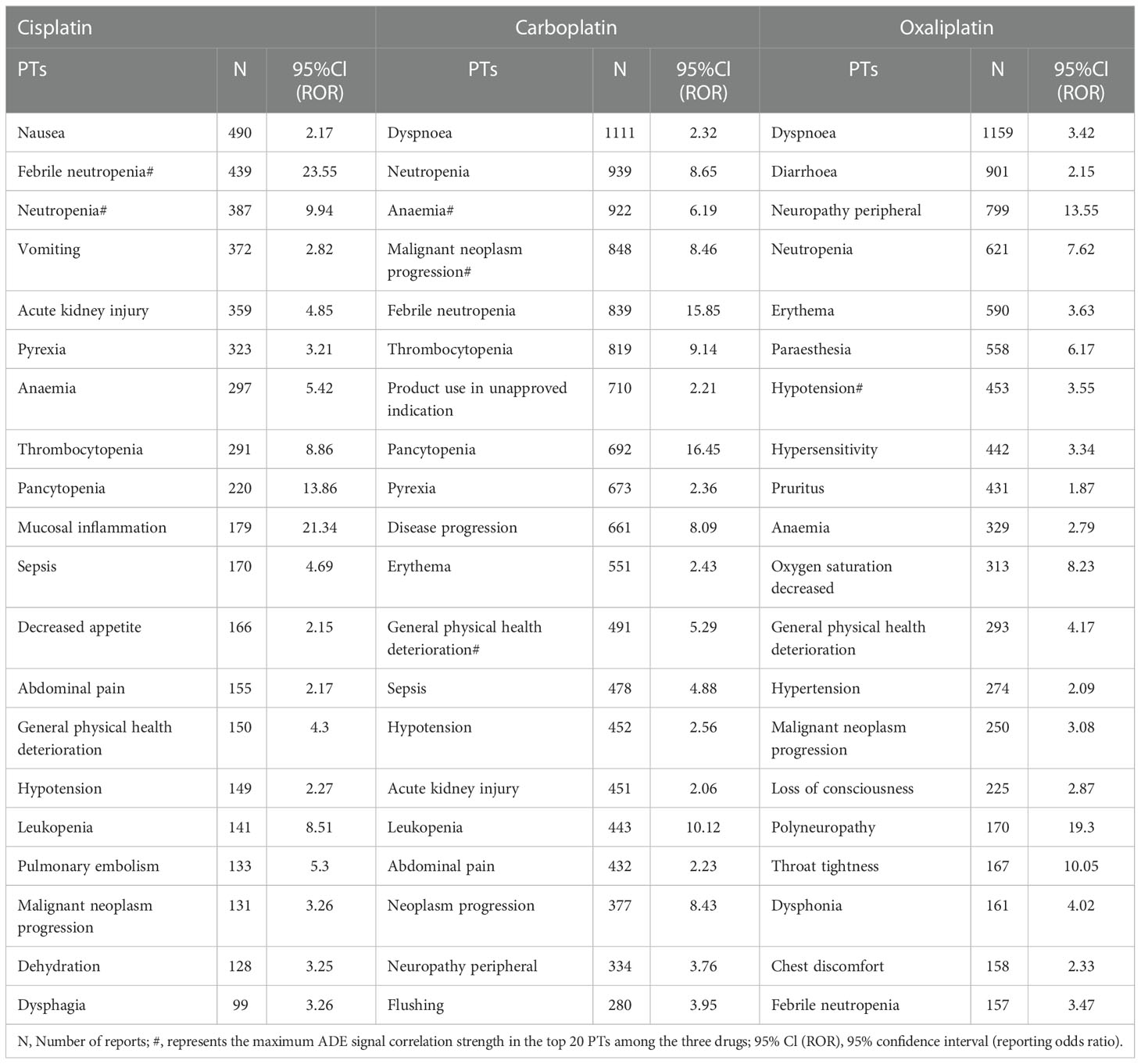
Table 2 shows that the main ADE symptoms of cisplatin are nausea, febrile neutropenia, and neutropenia; those of carboplatin are dyspnea, neutropenia, and anemia; and those of oxaliplatin are dyspnea, diarrhea, and peripheral neuropathy. The larger the ROR and PRR values, the stronger the signal, and the stronger the correlation between the drug and the adverse event.
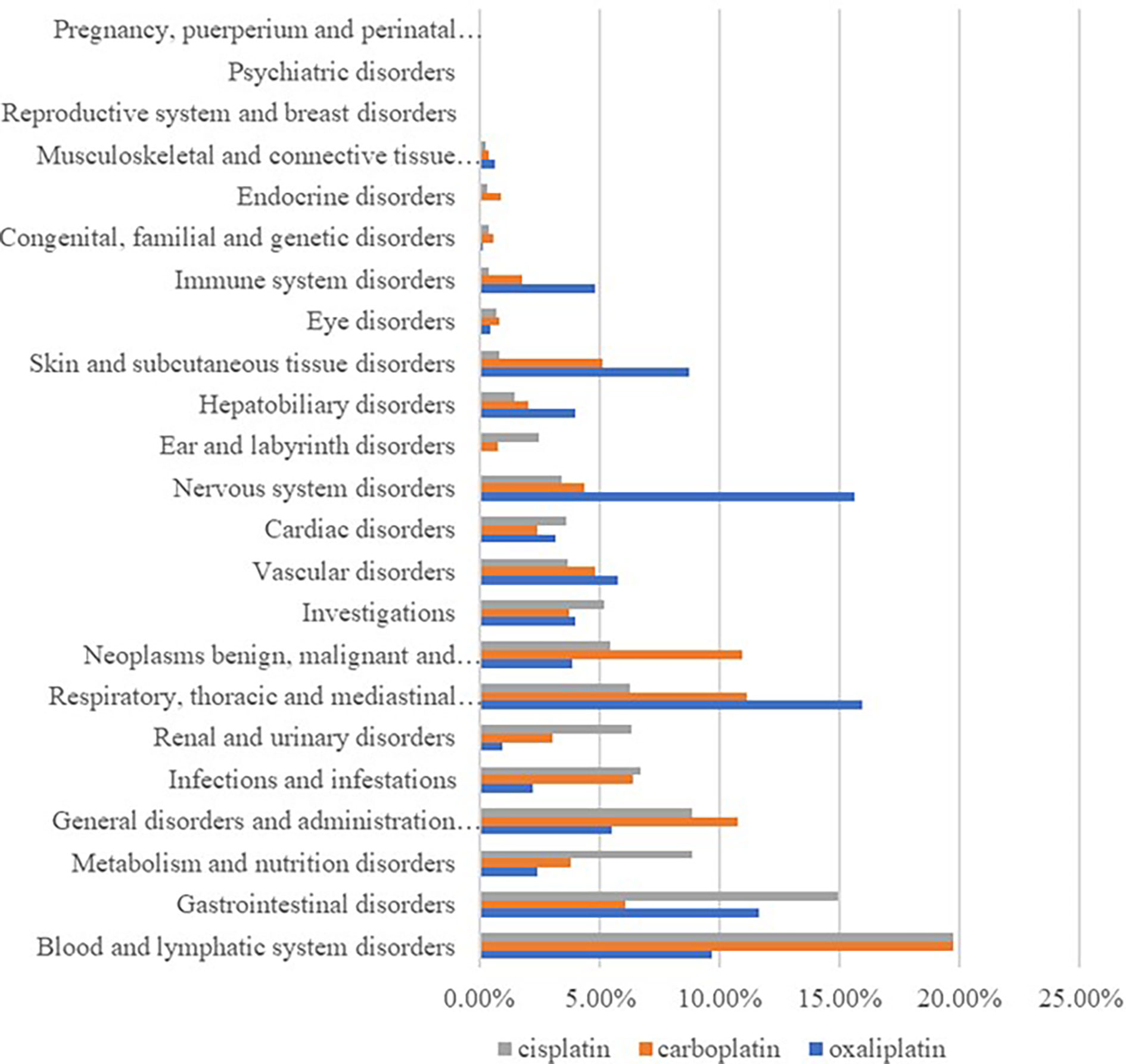
By classifying signaled PTs by SOC, excluding signals unrelated to adverse drug reactions such as product problems, social environment, various injuries, poisoning and operational complications, and various surgical and medical operations, 23 SOCs of all the ADE signals were identified. Among them, cisplatin ADEs involved 21 SOCs, carboplatin ADEs involved 23 SOCs, and oxaliplatin ADEs involved 22 SOCs, and differences in the proportion of reported cases involving platinum-based drugs in systemic ADE are shown in Figure 1. Cisplatin ADE reports mainly involve: Blood and lymphatic system disorders, Gastrointestinal disorders, Renal and urinary disorders, Carboplatin ADE reports mainly involve Blood and lymphatic system disorders, Respiratory, thoracic and mediastinal disorders, Oxaliplatin ADE reports mainly involve Nervous system disorders, Respiratory, thoracic and mediastinal disorders, Gastrointestinal disorders.
Analysis of SOC with prominent signals of platinum drugs
Through data mining, the major adverse events and signal intensity of platinum drugs are quite different.
This result (Table 3) suggested that cisplatin had a higher reported rate and the highest correlation with Nephropathy toxic (PRR: 20.52,χ2: 1132.37) and acute kidney injury (PRR: 5.14,χ2: 1206.79). Table 4 confirms Oxaliplatin is strongly associated with peripheral neuropathy (PRR: 2.74,χ2: 85.33) and paresthesia (PRR: 6.47, χ2: 2570.85). Carboplatin had the highest correlation with polyneuropathy (PRR: 12.31, χ2: 1312.47). Table 5 confirms that blood and lymphatic system diseases are the most common adverse events caused by platinum drugs, and the incidence rate ranks first among all adverse events. This result suggested that cisplatin had a strong correlation with febrile neutropenia (PRR: 24.17, χ2: 9586.96), which was significantly higher than that of carboplatin and cisplatin. Table 6 shows that cisplatin has a high reporting rate in nausea and vomiting, and no effective signal was detected by carboplatin and oxaliplatin. There are more reports with carboplatin on abdominal pain and oxaliplatin on diarrhea. Respiratory, thoracic and mediastinal disorder-related adverse events were the most important adverse events of oxaliplatin (Table 7). There was a significant difference in the proportion of reported cases among the three drugs. In terms of correlation, cisplatin has a strong correlation with pulmonary embolism (PRR: 6.18, χ2: 577.11), and oxaliplatin has a strong correlation with throat tightness (PRR: 11.59, χ2: 1585.02).
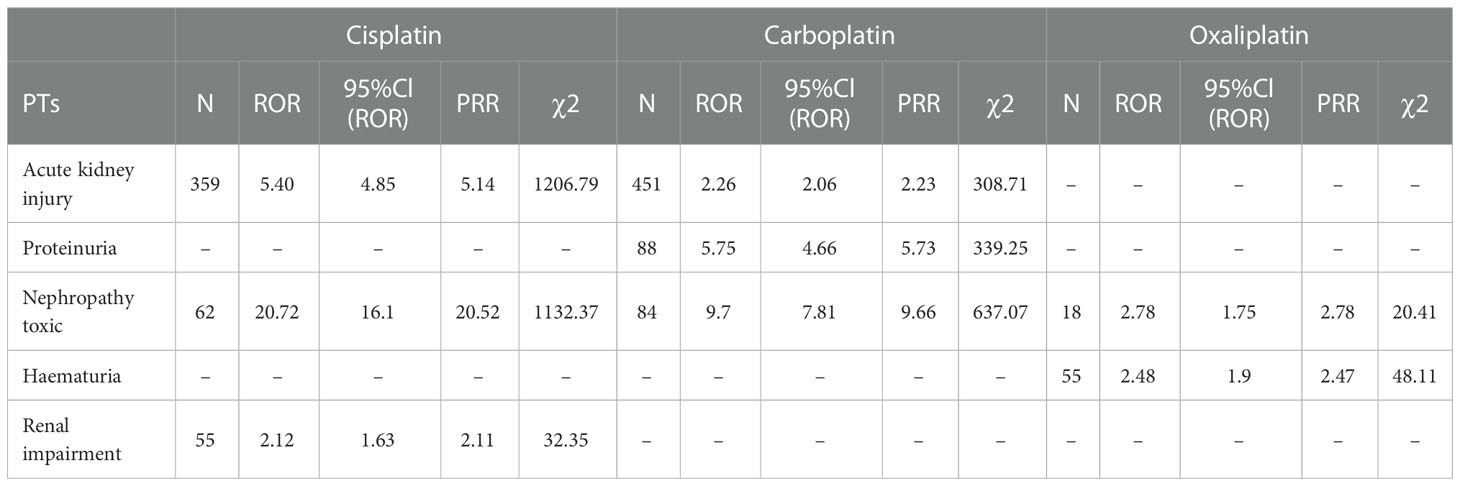
Table 3 Kidney and urinary system disorder-related adverse events of Cisplatin, Carboplatin and Oxaliplatin.
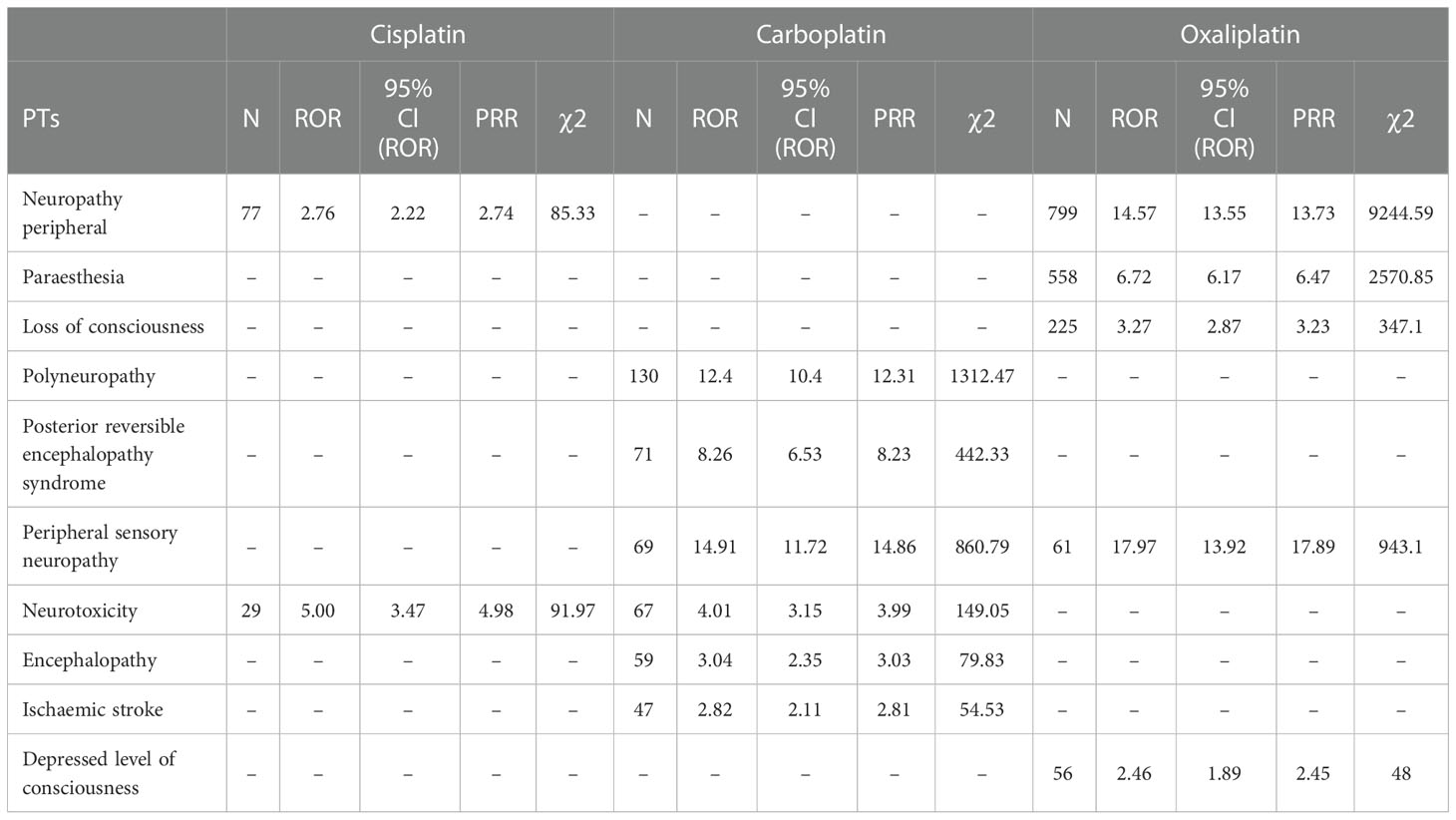
Table 4 Various types of nervous system disorder-related adverse events of Cisplatin, Carboplatin and Oxaliplatin.
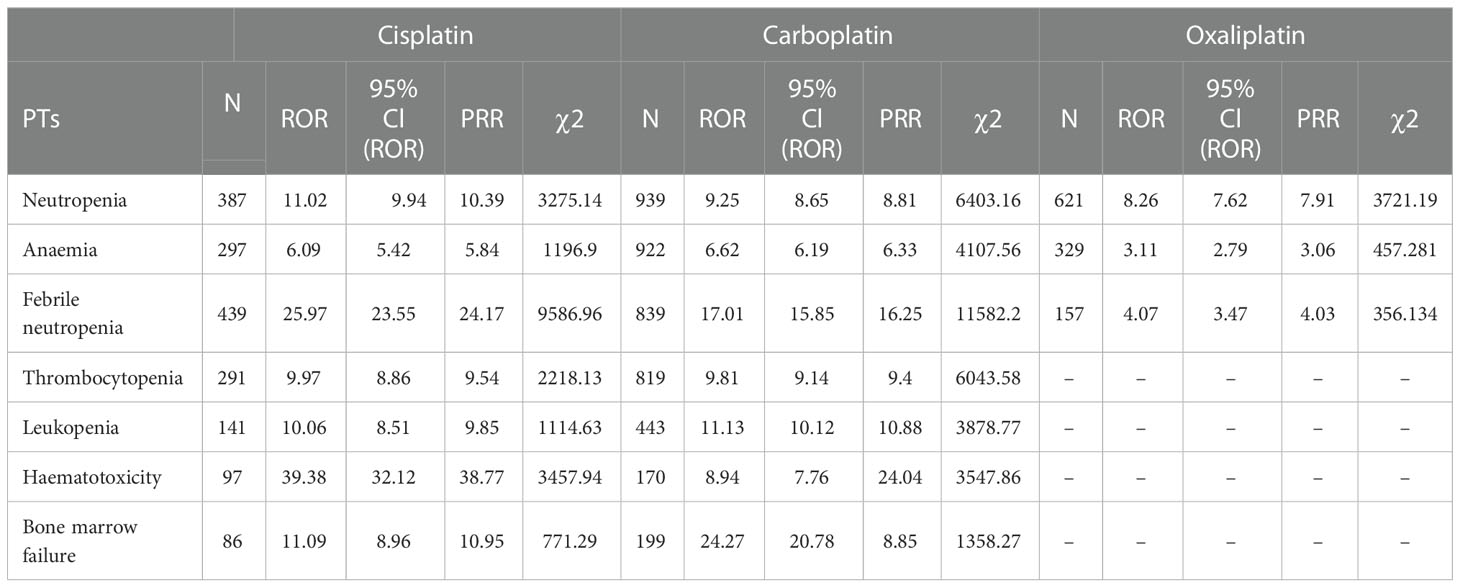
Table 5 Blood and lymphatic system disorder-related adverse events of Cisplatin, Carboplatin and Oxaliplatin.
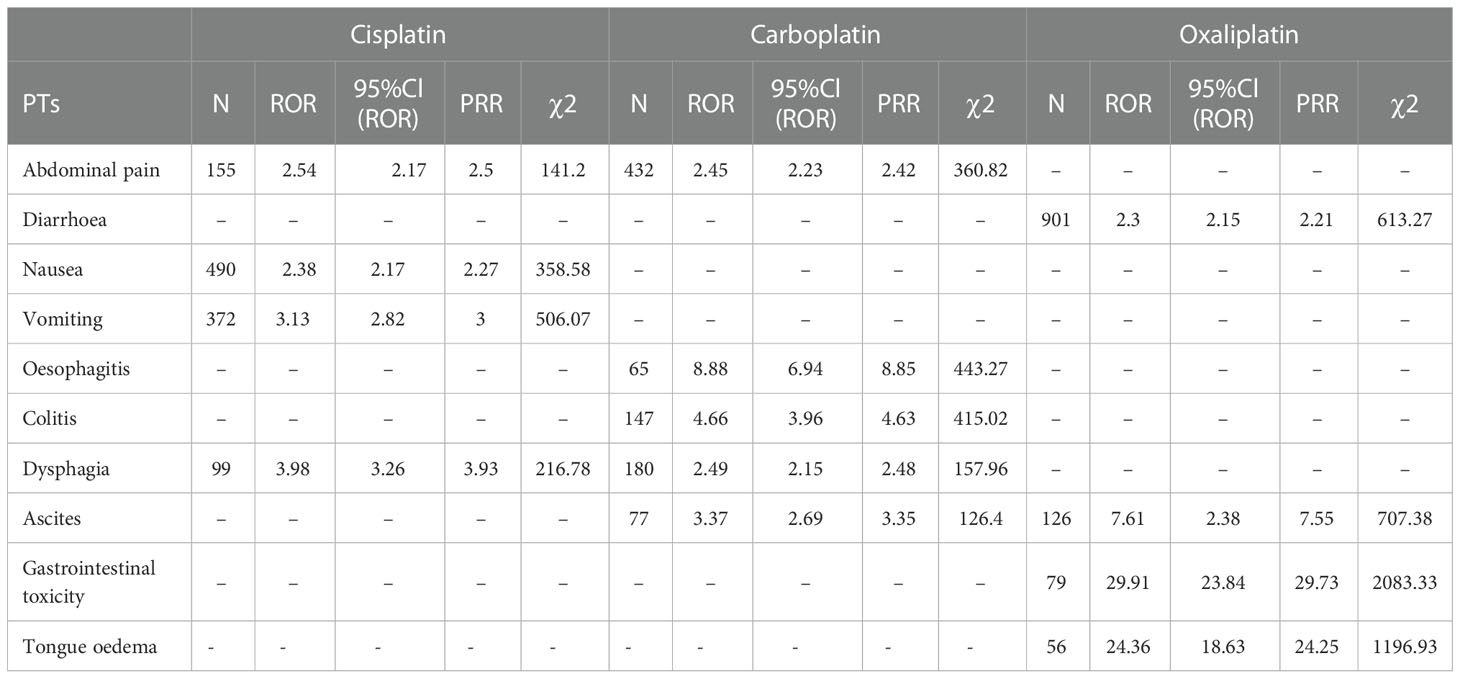
Table 6 Gastrointestinal system disorder-related adverse events of Cisplatin, Carboplatin and Oxaliplatin.
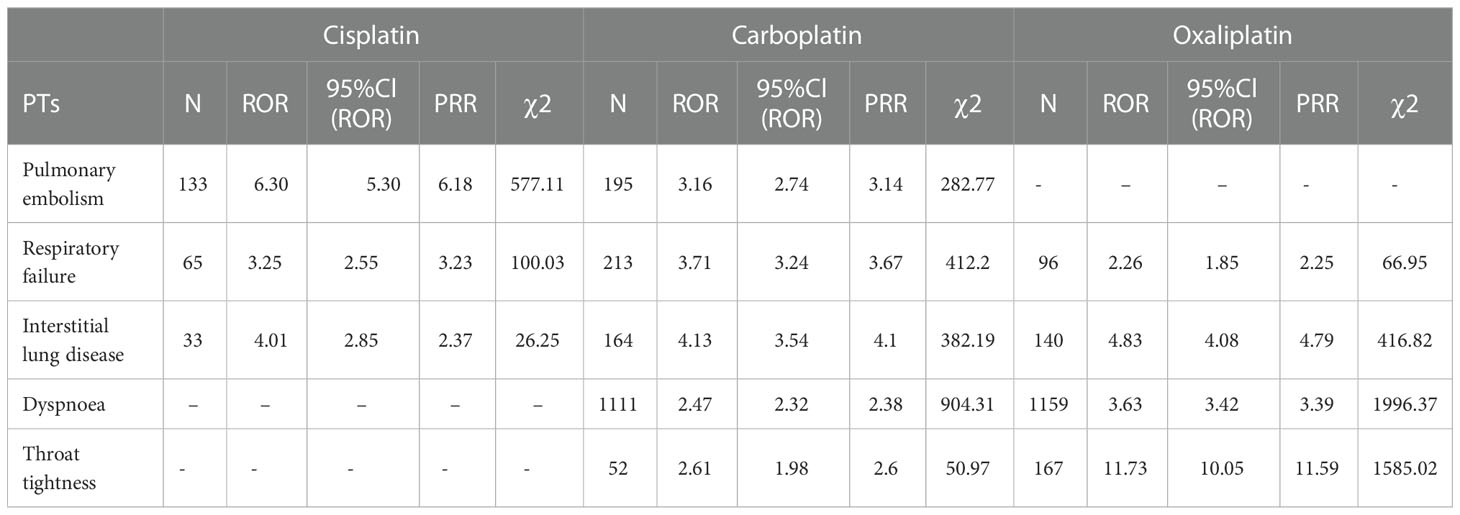
Table 7 Respiratory, thoracic and mediastinal disorder-related adverse events of Cisplatin, Carboplatin and Oxaliplatin.
Oxaliplatin had a significantly higher incidence of ADEs in immune system disorders than cisplatin and carboplatin (Table 8), and oxaliplatin had a strong correlation with type I hypersensitivity (PRR: 49.51, χ2: 4760.50).
Cisplatin showed an extremely high correlation in neurosensory hypoacusis (PRR: 416.69, χ2: 3989.40) and mixed deafness (PRR: 169.42, χ2: 439.51). The correlation in Ototoxicity was significantly different for platinum drugs (Table 9).
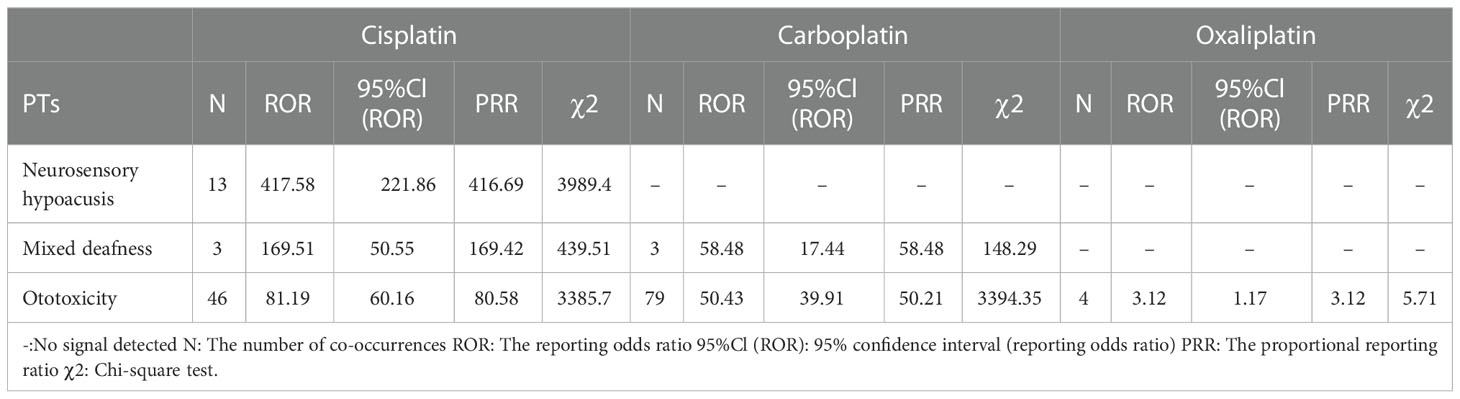
Table 9 Ear and labyrinth disorder-related adverse events of Cisplatin, Carboplatin and Oxaliplatin.
Discussion
As shown in Table 1, the number of reports with cisplatin as the primary suspected drug was 6098, the number of reports with carboplatin as the primary suspected drug was 17640, and the number of reports with oxaliplatin as the primary suspected drug was 12902. The top reporting countries are mainly the United States, France, Germany and other European countries. The number of reported cases in Asia, Africa and other populous countries is seriously underestimated for different reasons. Among the patients of known ages, patients over 60 years old accounted for the largest proportion, followed by patients between 18 and 60 years old, which is consistent with the report that tumors occur in the elderly population. Among patients of known sex, carboplatin had a slightly higher number of adverse events reported in female patients than in male patients, and the opposite was true for cisplatin and oxaliplatin, but no gender-related differences were reported. The adverse event signals shown in the data are basically consistent with the instructions, indicating that this study has certain credibility (17).
Table 3 suggests that cisplatin had the highest correlation with toxic nephropathy and acute kidney injury, followed by carboplatin. Oxaliplatin had the least number of reports on renal and urinary system diseases and the lowest correlation strength, which was consistent with the drug instructions and reports. Therefore, when using these three platinum drugs in patients with primary disease or risk factors for renal disease, cisplatin should be more alert to possible renal injury (8, 18).
The data confirm that neurotoxic is the most report among platinum drugs, with oxaliplatin being more neurotoxic (19). Therefore, more attention should be given to the adverse effects of oxaliplatin on neurological diseases (20), and various neurological diseases during use should be treated in a timely manner (21–23).
Blood and lymphatic system diseases are the most common adverse events of platinum drugs (24); however, effective signals such as thrombocytopenia and leukopenia hematotoxicity have not been detected when using oxaliplatin. Oxaliplatin did not detect an effective signal in febrile neutropenia, Haematotoxicity, Thrombocytopenia, Leukopenia and Bone marrow failure, suggesting that oxaliplatin may be less myelosuppressive than the other two drugs. Oxaliplatin may more suitable for patients with myelosuppression intolerance.
According to the analysis in Figure 1 and Table 6, diseases of the gastrointestinal system are also the main system for the occurrence of adverse events of platinum drugs. Of the three drugs, oxaliplatin was more strongly associated with gastrointestinal toxicity and diarrhea. Cisplatin was more strongly associated with nausea, vomiting, abdominal pain, and dysphagia. As shown in Table 6, the association of PTs, such as nausea and vomiting, with carboplatin and oxaliplatin was low (no signal detected), but this does not mean that the incidence of adverse events was low [14]. Carboplatin can be used instead of cisplatin or oxaliplatin when formulating chemotherapy regimens, thereby reducing the risk of gastrointestinal reactions in patients [15, 21, 29].
For ADEs such as dyspnea, throat tightness, skin and subcutaneous tissue system diseases, the incidence and correlation of oxaliplatin were significantly higher than those of carboplatin and cisplatin. Some scholars have proposed initiating cisplatin in patients with proven immediate hypersensitivity to carboplatin or oxaliplatin to ensure safety (25–27). However, during the use of any platinum drug, drug monitoring should be strengthened to prevent allergies and timely symptomatic treatment (28).
Oxaliplatin was more likely to cause allergic reactions (29), and the hypersensitivity and Type I hypersensitivity are particularly evident from the data in this paper. Some scholars have proposed Type II hypersensitivity reactions after oxaliplatin rechallenge can be life threatening (5). A desensitization protocol without premedication may be considered in those patients with a history of oxaliplatin hypersensitivity reactions with avoidance of the cumulative exposure to pretreatment medications (30). Our data suggest that carboplatin and cisplatin may be safer in allergy-prone patients.
A well described and prevalent consequence of cisplatin chemotherapy is ototoxicity, which results from the death of cochlear outer hair cells. Cisplatin-induced ototoxicity is permanent and progressive (31). The data also confirmed the strong correlation of cisplatin in neurosensory hypoacusis. Only 2 effective signals and 11 reports were detected for oxaliplatin, suggesting that the ototoxicity of oxaliplatin is significantly lower than that of cisplatin and carboplatin, and it is safer for children and adolescents (32–34). In many cases oxaliplatin is simply not as effective a drug as cisplatin. The choice has to be a combination of activity and side effects. Such as the effectiveness of cisplatin and carboplatin is higher than oxaliplatin in juvenile trophoblastic tumors and ovarian cancer and they are more widely used. In other words, the effectiveness of the drug is also very important (2022).
This study did not find strong correlation between platinum drugs and cardiac disorders, for all that the cardiotoxicity of platinum-based drugs is of great concern. The mechanisms of cardiotoxicity induced by cisplatin and oxaliplatin include cytotoxicity, oxidative stress and inflammation, cardiomyocyte apoptosis, drug-induced mitochondrial dysfunction and cardiotoxicity (35) and NLRP3 - dependent release of IL - 1 beta induces immune cells, primarily CD4 T cells, to express and release IL - 22 (36).
The well-known Dexrazoxane, A CardioProtective Agent Specific for Anthracy cline Chemotherapy, in addition to Continuous Cardiac monitoring, baseline and regular Electrocardiographic and echocardiographic studies, Carvedilol or Nebivolol, ACE inhibitor, ARB (Sacubitril/Valsartan) or Spironolactone, Statins, Dexrazoxane (37) and Butyric acid can be used to treat or prevent cardiotoxicity caused by chemotherapy drugs (38, 39).
Studies have shown that adiposity is associated with increased cardiometabolic risk after cisplatin-based chemotherapy and higher body mass index were more likely to develop paclitaxel- or oxaliplatin-induced CIPN posttreatment (40, 41), relevant mechanisms need to be further studied.
This study has counted the adverse event outcomes of platinum drugs in the latest five-year. We provide data support for clinical and readers from the perspective of drug safety. The effectiveness and economics of drugs are also important considerations. Which is better to choose oxaliplatin or cisplatin for treatment? The final chemotherapy plan is determined based on many factors, we will investigate in the future work.
Factors such as the level of medical technology, the occupation and professional level of the reporting person, the lack of data such as gender, age, and dosage in the report, the total number of people without drug use, and the randomness of reporting all have a certain impact on the results. Although the ROR method and the MHRA method can reduce the bias caused by the selection of the control group, the obtained results are consistent, and this study increases the accuracy by increasing the threshold of signal detection; it still cannot completely exclude false positive signals. Signals may also be missed. The adverse event signal identified in this study only indicates that there is a statistical correlation between platinum drugs and this signal, but further clinical trials are still needed when choosing drugs in clinical practice.
Conclusion
In this study, the ROR method and the PRR method were used to perform data mining on the related reports of cisplatin, carboplatin and oxaliplatin. Three drugs have ADE involving the main systems: cisplatin mainly focuses on blood and lymphatic system diseases and gastrointestinal system diseases; carboplatin is mainly concentrated in blood and lymphatic system diseases and respiratory thoracic and mediastinal diseases; oxaliplatin is mainly concentrated in respiratory system, thoracic and mediastinal diseases, various neurological diseases, and gastrointestinal system diseases. Among them, cisplatin has a strong correlation with toxic nephropathy, febrile neutropenia, neurosensory hypoacusis and mixed deafness, carboplatin has a strong correlation with polyneuropathy, and oxaliplatin has a strong correlation with peripheral neuropathy, paresthesia, and type I hypersensitivity. Through data mining and risk factor analysis, it was found that each platinum drug has its own specific ADE, which suggests that oxaliplatin in some diseases may suitable for juvenile patients and is not recommended for patients with allergic constitution. Cisplatin has the greatest nephrotoxicity and ototoxicity. Carboplatin can be used instead of cisplatin or oxaliplatin when formulating chemotherapy regimens, thereby reducing the risk of gastrointestinal reactions in patients. The clinical selection of platinum drugs should be combined with the patient’s relevant medical history and individualized administration and pay more attention to drug effectiveness to reduce the occurrence of adverse events in patients and promote rational clinical drug use.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
GF, XZ, JC, and DL designed the study. GF, DL, and XZ performed the data analysis. JC managed and checked all the data. All authors contributed equally to the manuscript writing. All authors read, checked, and approved the final manuscript.
Funding
This work was supported by grants from the National Natural Science Foundation of China (82073921).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1012093/full#supplementary-material
References
1. Rottenberg S, Disler C, Perego P. The rediscovery of platinum-based cancer therapy. Nat Rev Cancer (2021) 21(1):37–50. doi: 10.1038/s41568-020-00308-y
2. Ho GY, Woodward N, Coward JI. Cisplatin versus carboplatin: comparative review of therapeutic management in solid malignancies. Crit Rev Oncol Hematol (2016) 102(6):37–46. doi: 10.1016/j.critrevonc.2016.03.014
3. Hector S, Bolanowska-Higdon W, Zdanowicz J, Hitt S, Pendyala L. In vitro studies on the mechanisms of oxaliplatin resistance. Cancer Chemother Pharmacol (2001) 48(5):398–406. doi: 10.1007/s002800100363
4. Wang K, Zhu C, He Y, Zhang Z, Zhou W, Muhammad N, et al. Restraining cancer cells by dual metabolic inhibition with a mitochondrion-targeted platinum (II) complex. Angew. Chem Int Ed. Engl (2019) 58(14):4638–43. doi: 10.1002/anie.201900387
5. Vyskocil J, Tucek S, Kiss I, Fedorova L, Nevrlka J, Zdrazilova-Dubska L. Type II hypersensitivity reactions after oxaliplatin rechallenge can be life threatening. Int Immunopharmacol (2019) 74(9):105728. doi: 10.1016/j.intimp.2019.105728
6. Makovec T. Cisplatin and beyond: molecular mechanisms of action and drug resistance development in cancer chemotherapy. Radiol Oncol (2019) 53(2):148–58. doi: 10.2478/raon-2019-0018
7. Dieras V, Bougnoux P, Petit T, Chollet P, Beuzeboc P, Borel C, et al. Multicentre phase II study of oxaliplatin as a single-agent in cisplatin/carboplatin +/- taxane-pretreated ovarian cancer patients. Ann Oncol (2002) 13(2):258–66. doi: 10.1093/annonc/mdf018
8. McKeage MJ. Comparative adverse effect profiles of platinum drugs. Drug Saf (1995) 13(4):228–44. doi: 10.2165/00002018-199513040-00003
9. Wichelmann TA, Abdulmujeeb S, Ehrenpreis ED. Bevacizumab and gastrointestinal perforations: a review from the FDA adverse event reporting system (FAERS) database. Aliment. Pharmacol Ther (2021) 54(10):1290–7. doi: 10.1111/apt.16601
10. Douglas CC. An open framework for dynamic big-data-driven application systems (DBDDAS) development. Proc Comput Sci (2014) 29:1246–55. doi: 10.1016/j.procs.2014.05.112
11. Schaeffer C, Booton L, Halleck J, Studeny J, Coustasse A. Big data management in US hospitals: Benefits and barriers. Health Care Manag. (2017) 36(1):87–95. doi: 10.1097/HCM.0000000000000139
12. Shu Y, Ding Y, Dai B, Zhang Q. A real-world pharmacovigilance study of axitinib: data mining of the public version of FDA adverse event reporting system. Expert Opin Drug Saf (2022) 21(4):563–72. doi: 10.1080/14740338.2022.2016696
13. Trotti A, Colevas AD, Setser A, Basch E. Patient-reported outcomes and the evolution of adverse event reporting in oncology. J Clin Oncol (2007) 25(2):5121–7. doi: 10.1200/JCO.2007.12.4784
14. Kadoyama K, Sakaeda T, Tamon A, Okuno Y. Adverse event profile of tigecycline: data mining of the public version of the U.S. food and drug administration adverse event reporting system. Biol Pharm Bull (2012) 35(6):967–70. doi: 10.1248/bpb.35.967
15. Sakaeda T, Tamon A, Kadoyama K, Okuno Y. Data mining of the public version of the FDA adverse event reporting system. Int J Med Sci (2013) 10(7):796–803. doi: 10.7150/ijms.6048
16. Hu W, Chen L, Li H, Liu J. Eye disorders associated with newer antiepileptic drugs: A real-world disproportionality analysis of FDA adverse event reporting system. Seizure (2022) 96(3):66–73. doi: 10.1016/j.seizure.2022.01.011
17. Sakaeda T, Kadoyama K, Okuno Y. Adverse event profiles of platinum agents: Data mining of the public ver- sion of the FDA adverse event reporting system, AERS, and reproducibility of clinical observations. Int J Med Sci (2011) 6(8):487–91. doi: 10.7150/ijms.8.487
18. Pasteur J, Favier L, Pernot C, Guerriaud M, Bernigaud C, Lepage C, et al. Low cross-reactivity between cisplatin and other platinum salts. J Allergy Clin Immunol Pract (2019) 7(6):1894–900. doi: 10.1016/j.jaip.2019.01.057
19. Staff NP, Cavaletti G, Islam B, Lustberg M, Psimaras D, Tamburin S. Platinum-induced peripheral neurotoxicity: From pathogenesis to treatment. J Peripher. Nerv. Syst (2019) 24 Suppl 2:S26–39. doi: 10.1111/jns.12335
20. Pasetto LM, D'andrea MR, Rossi E, Monfardini S. Oxaliplatin-related neurotoxicity: How and why? Crit Rev Oncol Hematol (2006) 59(2):159–68. doi: 10.1016/j.critrevonc.2006.01.001
21. Gomez-Ruiz S, Maksimovic-Ivanic D, Mijatovic S, Kaluderovic GN. On the discovery, biological effects, and use of cisplatin and metallocenes in anticancer chemotherapy. Bioinorg Chem Appl (2012) 2012(7):140284. doi: 10.1155/2012/140284
22. Grolleau F, Gamelin L, Boisdron-Celle M, Lapied B, Pelhate M, Gamelin E. A possible explanation for a neurotoxic effect of the anticancer agent oxaliplatin on neuronal voltage-gated sodium channels. J Neurophysiol (2001) 85(5):2293–7. doi: 10.1152/jn.2001.85.5.2293
23. Calls A, Carozzi V, Navarro X, Monza L, Bruna J. Pathogenesis of platinum-induced peripheral neurotoxicity: Insights from preclinical studies. Exp Neurol (2020) 325(3):113141. doi: 10.1016/j.expneurol.2019.113141
24. Hanada K, Suda M, Kanai N, Ogata H. Pharmacokinetics and toxicodynamics of oxaliplatin in rats: application of a toxicity factor to explain differences in the nephrotoxicity and myelosuppression induced by oxaliplatin and the other platinum antitumor derivatives. Pharm Res (2010) 27(9):1893–9. doi: 10.1007/s11095-010-0189-4
25. Jardim DL, Rodrigues CA, Novis Y, Rocha VG, Hoff PM. Oxaliplatin-related thrombocytopenia. Ann Oncol (2012) 23(8):1937–42. doi: 10.1093/annonc/mds074
26. Tsao LR, Young FD, Otani IM, Castells MC. Hypersensitivity reactions to platinum agents and taxanes. Clin Rev Allergy Immunol (2021) 62(3):432–448. doi: 10.1007/s12016-021-08877-y
27. Zhang Y, Zheng J, Jiang Y, Huang X, Fang L. Neglected, drug-induced platinum accumulation causes immune toxicity. Front Pharmacol (2020) 111166. doi: 10.3389/fphar.2020.01166
28. Rose PG, Metz C, Link N. Desensitization with oxaliplatin in patients intolerant of carboplatin desensitization. Int J Gynecol. Cancer (2014) 24(9):1603–6. doi: 10.1097/IGC.0000000000000295
29. Zhu L, Li H, Du Q, Ye X, Yu S, Luo X, et al. Meta-analysis of risk factors associated with oxaliplatin hypersensitivity reactions in cancer patients. Int J Clin Oncol (2021) 26(12):2194–204. doi: 10.1007/s10147-021-02034-3
30. Park HJ, Lee JH, Kim SR, Kim SH, Park KH, Lee CK, et al. A new practical desensitization protocol for oxaliplatin-induced immediate hypersensitivity reactions: A necessary and useful approach. J Investig Allergol Clin Immunol (2016) 26(3):168–76. doi: 10.18176/jiaci.0038
31. Freyer DR, Brock PR, Chang KW, Dupuis LL, Epelman S, Knight K, et al. Prevention of cisplatin-induced ototoxicity in children and adolescents with cancer a clinical practice guideline. (2020) 2(4):141–50. doi: 10.1016/S2352-4642(19)30336-0
32. Surnar B, Kolishetti N, Basu U, Ahmad A, Goka E, Marples B, et al. Reduction of cisplatin-induced ototoxicity without compromising its antitumor activity. Biochemistry (2018) 57(46):6500–13. doi: 10.1021/acs.biochem.8b00712
33. Gersten BK, Fitzgerald TS, Fernandez KA, Cunningham LL. Ototoxicity and platinum uptake following cyclic administration of platinum-based chemotherapeutic agents. J Assoc Res Otolaryngol (2020) 21(4):303–21. doi: 10.1007/s10162-020-00759-y
34. Waissbluth S, Chuang A, Del VA, Cordova M. Long term platinum-induced ototoxicity in pediatric patients. Int J Pediatr Otorhinolaryngol. (2018) 107(4):75–79. doi: 10.1016/j.ijporl.2018.01.028
35. Ma H, Jones KR, Guo R, Xu P, Shen Y, Ren J. Cisplatin compromises myocardial contractile function and mitochondrial ultrastructure: role of endoplasmic reticulum stress. Clin Exp Pharmacol Physiol (2010) 37(4):460–5. doi: 10.1111/j.1440-1681.2009.05323.x
36. Voigt C, May P, Gottschlich A, Markota A, Wenk D, Gerlach I, et al. Cancer cells induce interleukin-22 production from memory CD4 (+) T cells via interleukin-1 to promote tumor growth. Proc Natl Acad Sci U.S.A. (2017) 114(49):12994–9. doi: 10.1073/pnas.1705165114
37. Frey MK, Arfsten H, Pavo N, Han E, Kastl S, Hulsmann M, et al. Sacubitril/valsartan is well tolerated in patients with longstanding heart failure and history of cancer and improves ventricular function: real-world data. Cardiooncology (2021) 7(1):35. doi: 10.1186/s40959-021-00121-y
38. Quagliariello V, Masarone M, Armenia E, Giudice A, Barbarisi M, Caraglia M, et al. Chitosan-coated liposomes loaded with butyric acid demonstrate anticancer and anti-inflammatory activity in human hepatoma HepG2 cells. Oncol Rep (2019) 41(3):1476–86. doi: 10.3892/OR.2018.6932
39. Herrmann J. Adverse cardiac effects of cancer therapies: cardiotoxicity and arrhythmia. Nat Rev Cardiol (2020) 17(8):474–502. doi: 10.1038/s41569-020-0348-1
40. Wibmer AG, Dinh PC, Travis LB, Chen C, Bromberg M, Zheng J, et al. Associations of body fat distribution and cardiometabolic risk of testicular cancer survivors after cisplatin-based chemotherapy. JNCI Cancer Spectr (2022) 6(4):pkac030. doi: 10.1093/jncics/pkac030
Keywords: platinum drugs, FAERS, adverse event, date mining, ADE signals
Citation: Feng G, Zhou X, Chen J, Li D and Chen L (2023) Platinum drugs-related safety profile: The latest five-year analysis from FDA adverse event reporting system data. Front. Oncol. 12:1012093. doi: 10.3389/fonc.2022.1012093
Received: 05 August 2022; Accepted: 12 December 2022;
Published: 11 January 2023.
Edited by:
Dana Kristjansson, Norwegian Institute of Public Health (NIPH), NorwayReviewed by:
Vincenzo Quagliariello, G. Pascale National Cancer Institute Foundation (IRCCS), ItalyNicholas Patrick Farrell, Virginia Commonwealth University, United States
Copyright © 2023 Feng, Zhou, Chen, Li and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Li Chen, Y2hlbmxfaHhleUBzY3UuZWR1LmNu
 Guowen Feng
Guowen Feng Xiaodan Zhou
Xiaodan Zhou Jia Chen
Jia Chen Dan Li1,2,6
Dan Li1,2,6 Li Chen
Li Chen