
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 08 December 2022
Sec. Gastrointestinal Cancers: Colorectal Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1008394
This article is part of the Research Topic Advances in Genetics and Molecular Diagnosis in Colorectal Cancer. View all 12 articles
Background: Previous studies reported controversial results on the relationship between cholecystectomy (CHE) and colorectal cancer (CRC). We hypothesized that gallbladder disease (GBD), instead of cholecystectomy, increased the risk of CRC. We aimed to investigate the incidence of benign gallbladder disease (BGBD) and CHE in CRC patients and local adults undergoing annual health examination by analyzing large data from a tertiary hospital in southwest China.
Methods: A propensity score matching (PSM) analyzed, retrospective study from January 1, 2013, to August 31, 2020, including 7,471 pathologically confirmed CRC patients and 860,160 local annual health examination adults in the First Affiliated Hospital of Chongqing Medical University, was conducted. The prevalence of BGBD and the CHE rate were analyzed before and after a 1:1 PSM.
Results: Of the 7,471 CRC patients, 7,160 were eligible for the case group. In addition, 860,160 local health examination adults were included for comparison. The incidence of BGBD was higher in the CRC patients than in the local adults (19.2% vs. 11.3%, P < 0.001), but no significant difference in CHE rate existed between the case group and the control group (5.0% vs. 4.8%, P = 0.340). In the subgroup analysis, patients with BGBD had a higher risk of colon cancer than rectal cancer (20.4% vs. 18.2%, P = 0.024) and more significantly in the right colon (P = 0.037). A weakly positive correlation between CHE and right colon cancer was observed before PSM but no longer existed after PSM (P = 0.168).
Conclusions: Benign gallbladder disease was positively correlated with colorectal cancer, especially right colon cancer. Cholecystectomy did not increase the risk of colorectal cancer.
Colorectal cancer (CRC) ranks third among lethal cancers worldwide, accounting for nearly 10% of cancer-related deaths each year. It is the second most common cancer in women and the third most common cancer in men (1–4). In China, both the morbidity and mortality of CRC are fifth among cancers (5). Anatomically, based on tumor location, CRC can be divided into right colon cancer (also called proximal colon cancer, including the ascending colon and the front two-thirds of the transverse colon), left colon cancer (also called distal colon cancer, including the posterior third of the transverse colon, descending colon and sigmoid colon) and rectal cancer (4, 6). In general, distal colon cancer is more common than proximal colon cancer, and patients with distal colon cancer are younger than those with proximal colon cancer. In terms of sex distribution, men are more likely to develop CRC than women, possibly due to sex hormone effects. The disparity is more pronounced in older patients. However, there are more women diagnosed in the right colon than men, and the reverse is true in the left colon (6, 7). In 2017, the incidence of CRC was 46.9/100,000 in men and 35.6/100,000 in women in the United States, nearly double that in China (28.64/100,000 in men and 19.33/100,000 in women) (8). CRC patients are getting younger at diagnosis, with the median age of diagnosis dropping from 72years during 1988 and 1989 to 66years during 2015 and 2016. From 2012 to 2016, the prevalence increased by 9% to 10% annually among people 50years old or older, and by up to 24% annually among people under 50years old. From 2008 through 2017, the mortality rate for CRC patients over 65years declined by 35% per year, for those aged 50 years to 64years by 0.6% per year but increased by 1.3% per year for those aged under 50years (6, 9). With the incidence and mortality of CRC increasing annually and patients getting younger, it is crucial to identify the risk factors for CRC prevention and treatment.
Benign gallbladder disease (BGBD) is the most common cause of nonmalignant gastrointestinal death and can severely affect the quality of life (10–13). Clinically, common benign gallbladder diseases include gallstones, cholecystitis, and gallbladder polyps, of which gallstones are referred to as calculous diseases (CD), and cholecystitis and gallbladder polyps are collectively referred to as acalculous disease (ACD) (14). BGBD affects 10%~20% of the global population, 10%~30% in Western countries and 5.9%~21.9% in Asian countries. The prevalence of BGBD differs from 4.2% to 13.11% in different regions of China and varies from 10.45% to 11.64% in the Han population (10, 11, 15–17). In the general population, BGBD prevalence is higher in females than in males (10, 13, 14) and higher in older people than in younger people. The prevalence increases with age (14, 18). Studies by Shaffer (19) and Liu et al. (20) revealed that the incidence rate was 4–10 times and 3.02–3.11 times higher in those over 40 and over 50 than in those under 40 and under 50, respectively. Currently, cholecystectomy (CHE) is the standard treatment for symptomatic BGBD and BGBD with complications, especially gallstones and large gallbladder polyps (21). Because of high incidence of BGBD, cholecystectomy is one of the most performed procedures in surgery. There are approximately 300,000 cholecystectomies performed annually in the United States (22). Although lack of available data, more cholecystectomies may be performed in China, considering similar incidence and more population.
Studies on the relationship between BGBD or CHE and CRC can be traced back to 1978 (23, 24). Some of the current studies suggested a positive correlation with digestive system cancer (25–28), and some believed no correlation existed (29–31). Some studies have shown that the association varies by different tumor sites (32–34). Researchers who proposed a positive correlation believed in the following mechanisms. First, the two diseases shared the same risk factors (11, 33, 35). Risk factors for BGBD, including old age (15, 18), obesity (12), hypercholesterolemia (36, 37), smoking (35), diabetes (13, 38), low-fiber and high-fat diet, and low physical activity (39, 40), are also well-known risk factors for large bowel cancer. Ernst J. Kuipes et al. (4) revealed a 1 unit increased of body mass index (BMI) and a 2–3% increase in CRC risk. Sencond, alterations in bile flow, long-term inflammatory stimulation, and complications caused by BGBD can promote the occurrence of CRC (41–43). Third, Hill, MJ et al. (44) suggested that the gallbladder lost its storage function after CHE and increased secondary bile acids (SBAs), which continued to be secreted into the intestine without food dilution, induced carcinogenesis (45). Elevated levels of bile acids and derivatives have been found in stool from CRC patients and patients who accepted CHE (44). Some studies found that the correlation varied depending on sex and tumor site. A positive relationship in women has been confirmed by many studies, especially in the proximal colon. However, such a relationship has not been proven in men (33, 34). These views were also borne out in some Chinese studies (46). Although many studies suggested a positive correlation, studies that suggested no correlation were not uncommon (29, 31). Despite nearly half a century of exploration, the relationship remains a mystery. Given high frequency of BGBD and CHE worldwide, more evidence is needed to determine whether BGBD and CHE increase the risk of future CRC. A preliminary study in our data indicated there were much more CRC patients with BGBD than CRC patients with history of CHE. Based on a hypothesis that benign gallbladder disease, rather than cholecystectomy, can increase the risk of colorectal cancer, we carry out the analysis to provide more evidence to reveal relationship between benign gallbladder disease or loss of gall bladder and colorecatal cancer.
In this large, single-center, retrospective study, a total of 7,471 CRC patients admitted to the First Affiliated Hospital of Chongqing Medical University between January 1, 2013, and August 31, 2020, were screened, and 7,160 of them were eligible for the case group. A total of 860,160 people who visited the Medical Examination Center in the same period were included in the control group (Figure 1). For the case group, clinicopathological data, including sex, age, body mass index (BMI), tumor location, and time of CRC diagnosis, were collected. By reviewing abdominal ultrasound, computerized tomography (CT), magnetic resonance imaging (MRI), and electronic medical records (EMR), CRC patients with a history of BGBD were identified, including symptomatic and asymptomatic patients. The time of BGBD diagnosis, BGBD type, CHE or not, and time of CHE were included. For the control group, BGBD and CHE information was included through retrieval in the dedicated electronic system of the Medical Examination Center. In this study, BGBD types included gallstones, cholecystitis, and gallbladder polyps. The former subtype was called CD, and the latter two subtypes were collectively referred to as ACD.
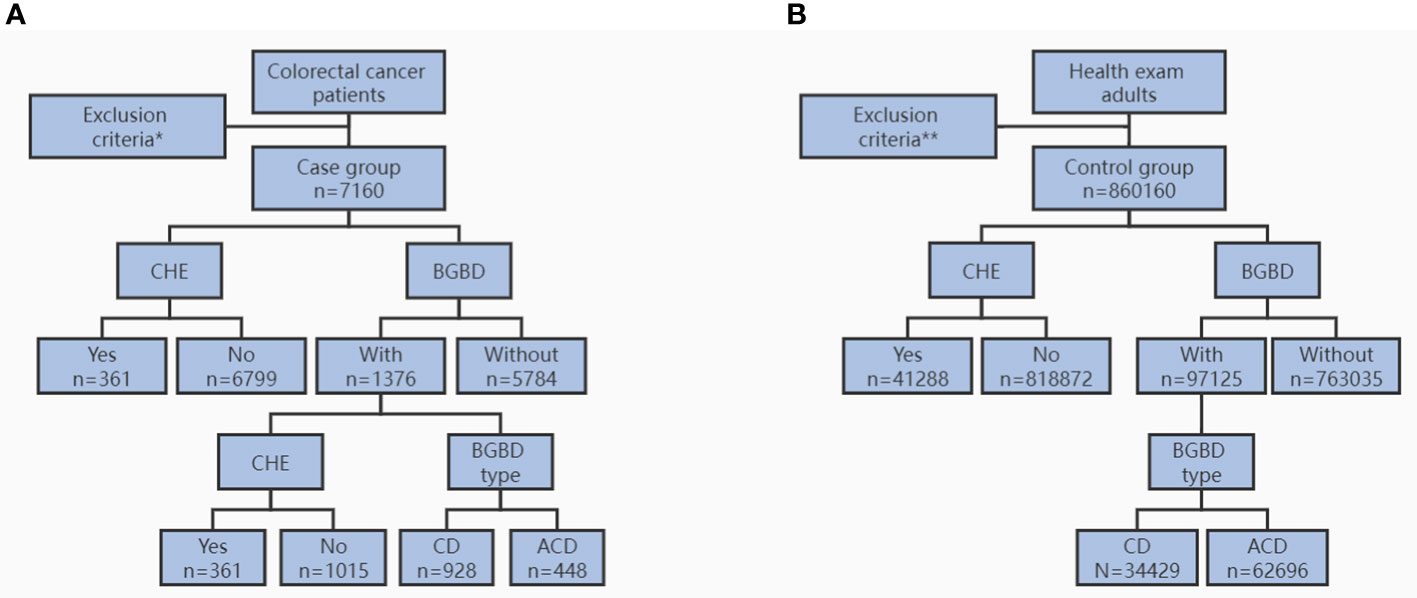
Figure 1 Flow diagram for study population of case group (A) and control group (B). CHE, cholecystectomy. BGBD, benign gallbladder disease. CD, calculous disease. ACD, acalculous disease. * age < 18 years, BMI < 15.0kg/m2, multifocal CRC, other cancer, and incomplete data. ** age < 18 years, BMI < 15.0kg/m2, any cancer, and incomplete data.
The inclusion criteria for the case group included: (1) age ≥ 18 years; (2) BMI ≥ 15.0kg/m2; (3) histologically or cytologically confirmed CRC. The exclusion criteria for the case group included: (1) age < 18 years; (2) BMI < 15kg/m2; (3) multifocal CRC; (4) other cancer; and (6) incomplete data.
The study population in the control group included an asymptomatic health examination population and a symptomatic physical check-up population during the same period as the case group. The inclusion criteria of the control group were as follows: (1) age ≥18 years and (2) BMI ≥15.0kg/m2. Exclusion criteria of the control group included (1) age < 18 years; (2) BMI < 15kg/m2; (3) any cancer; and (4) incomplete data.
This study was approved by the Clinical Research Ethics Review Committee of The First Affiliated Hospital of Chongqing Medical University (registration number: 2021-770).
To decrease the effect of selection bias and confounding factors and increase comparability between subgroups, we performed propensity score matching (PSM) analysis. PSM is a statistical method that can be used to balance interference factors between groups in observational studies, following the law of counterfactual reasoning (47). The PSM consists of the following steps: (1) Use the logistic regression model to calculate propensity scores. (2) Score matching is performed by nearest neighbor matching (NNM), radius matching, or kernel matching. (3) Evaluate the balance after matching. (4) Calculate the average intervention effect (ATT). (5) Conduct sensitivity analysis (48). All steps can be implemented in SPSS software. In our study, we calculated propensity scores by applying the sex, age, and BMI of patients in the case group to a logistic regression model and evaluated the goodness of fit with the caliper value level of 0.002. Finally, one-to-one PSM was achieved (without replacement). Then, we analyzed subgroups before and after 1:1 PSM. The process of PSM was implemented in Microsoft Office 2019 and SPSS® version 23.0 (IBM, Armonk, New York, USA).
All statistical analyses were performed in SPSS® version 23.0 (IBM, Armonk, New York, USA). The normality of continuous variables was tested using the P-P graph, histogram, and single-sample Kolmogorov−Smirnov test. PSM was used to match the patient’s sex, age, and BMI. Continuous variables are presented as the mean values with ranges, and categorical variables are presented as frequencies with percentages. P < 0.05 was used to denote a statistically significant difference.
The chi-square test was used to compare all categorical variables. Before PSM, an independent sample t test or one-way analysis of variance was employed to compare normally distributed data, and the Mann−Whitney U test was used to compare nonnormally distributed data. After PSM, the paired sample t test was used for normally distributed variables, and the Wilcoxon test or the Friedman test was used for nonnormally distributed data.
Of the 7,160 CRC patients in the case group, 1,376 (19.2%) had a history of BGBD, and 5,784 (80.8%) did not. Among them, 361 (5.0%) patients had previously undergone cholecystectomy, and 6,799 (95.0%) patients had not. A total of 860,160 local annual health examination adults were enrolled in the control group, of which 97,125 (11.3%) had BGBD and 763,035 (88.7%) did not. Among them, 41,288 (4.8%) accepted cholecystectomy, and 818,872 (95.2%) did not. The prevalence of BGBD in the case group was significantly higher than that in the control group (19.2% vs. 11.3%, P < 0.001, Table 1). However, there was no significant difference in the CHE rate between the case and the control groups (5.0% vs. 4.8%, P = 0.340, Table 1).
The characteristics of the subgroups based on tumor location showed that the features of right colon cancer were significantly different from those of left colon cancer and rectal cancer. Obvious differences were found in sex, age, and BMI distribution among the three subgroups (P < 0.001, Table 2). Generally, CRC was more common in men than women, but right colon cancer was more common in women than left colon cancer and rectal cancer (P < 0.001). On average, patients with rectal cancer and left colon cancer were younger than those with right colon cancer (62.6years vs. 63.6years vs. 64.5years, respectively, P < 0.001, Table 2). The right colon cancer had a lower BMI than the left colon cancer and rectal cancer (BMI were 21.9kg/m2 vs. 22.6kg/m2 vs. 22.5kg/m2, respectively, P < 0.001, Table 2), which was consistent with the clinical phenomenon—right colon cancer with mainly systemic symptoms and left colon cancer with mainly intestinal obstruction symptoms. As shown in Table 2, patients with previous BGBD were more likely to develop right colon cancer (P = 0.004), regardless of BGBD subtype (P = 0.074). Notably, no difference in CHE rate among the different locations of CRC existed (P = 0.074). However, the sex, age, and BMI of the three subgroups were not balanced at baseline, which made the above conclusions inconclusive and required further analysis by PSM.
Information was obtained from the comparison of colon cancer and rectal cancer (Table 3). Before PSM, an imbalance was found at baseline for sex, age, and BMI between the two groups. A higher proportion of BGBD was found in colon cancer patients than in rectal cancer patients (20.9% vs. 17.9%, P = 0.002), but no significant difference in CHE rate existed between the two groups (5.5% vs. 4.7%, P = 0.098). After PSM, factors including sex, age, and BMI, were reconciled. Statistically significant differences still existed in the prevalence of BGBD between colon cancer and rectal cancer (20.4% vs. 18.2%, P = 0.024). The CHE rate remained not significantly different (5.2% vs. 4.9%, P = 0.562).
Further analysis was performed before and after PSM 1:1 matching among right colon cancer, left colon cancer, and rectal cancer. As shown in Supplementary Tables 1-3, the three groups of patients were unbalanced at baseline. There was no difference in BGBD prevalence or CHE rate between right and left colon cancer before and after PSM analysis (Supplementary Table 1). Compared with rectal cancer, the prevalence of BGBD and CHE was higher in right colon cancer, but only the difference in BGBD remained after matching, and the difference in CHE disappeared (P = 0.037 and 0.168, respectively, Supplementary Table 2). There was no difference in the incidence of BGBD and CHE between left colon cancer patients and rectal cancer patients after matching (P = 0.126 and 0.523, respectively, Supplementary Table 3).
We had investigated association between benign gallbladder disease, cholecystectomy and colorectal cancer in a large sample, PSM-matched, case-control study. Our study revealed that benign gallbladder disease was positively associated with colorectal cancer, and this correlation was more pronounced in right colon cancer, which remained consistent before and after PSM analysis. However, our study did not found an increased risk of colorectal cancer caused by cholecystectomy. Before PSM, cholecystectomy was slightly positively correlated with right colon cancer, but this correlation no longer existed after matching the sex, age and BMI. Thus, cholecystectomy itself was not associated with the development of colorectal cancer. This might be explained by the reason that carcinogenic factors were formed as early as the occurrence of BGBD.
Previous studies, mainly case-control studies, cohort studies and inventory surveys, explored the relationship between BGBD, CHE and CRC but the results were controversial. Some studies revealed that both BGBD and CHE were risk factors of CRC and more closely related to proximal colon cancer (30, 49, 50), some studies revealed a positive correlation between BGBD and CRC but no correlation between CHE and CRC (51, 52), while some studies revealed an opposite result (27, 53). There were also studies revealed that neither BGBD nor CHE was associated with CRC (54, 55). Interestingly, Chen et al. (56) revealed a negative association between CHE and CRC through a long-term follow-up cohort study and believed that CHE was a protective factor for CRC. Our results favor that the BGBD, not the CHE, is a risk factor of CRC.
Studies supporting positive correlation between BGBD or CHE and CRC had further explored possible carcinogenic mechanisms.One widely accepted mechanism was that BGBD and CRC shared common risk factors, such as old age (15, 18), obesity (12), hypercholesterolemia (36, 37), smoking (35), diabetes (13, 38), low-fiber and high-fat diet (57), and low physical activity (39). In addition, studies by Almond, HR (45) and Adler et al. (58) revealed that shrinkage of the bile acid pool, changed bile lipid composition, increased secretion of secondary bile acids (SBAs), and increased enterohepatic circulation of bile acids were observed in patients with benign gallbladder disease, which might account for the development of colorectal cancer. It has been confirmed that SBAs in the stool of CRC patients (44), BGBD patients (59) and post-cholecystectomy patients (60) are significantly higher than those in normal people. SBA has been proved with strong carcinogenicity, its carcinogenic activities mainly occur through the following mechanisms. SBA inhibited peripheral blood lymphocytes and colonic mucosa lamina propria lymphocytes, reducing the secretion of secretory immunoglobulin A (SIgA) to weaken intestinal immune function, and the damaged intestinal mucosal barrier had increased permeability and susceptibility to carcinogens (61). SBA interfered with the detoxification of glutathione S-transferase (GST) against exogenous carcinogens (62). SBA promoted carcinogenesis and increased the invasive effect of cancer cells on blood vessels by activating AP21 through the protein kinase C (PKC) signaling pathway (63). SBA activated phosphatidylinositol 3 kinase (PI3K) through the epidermal growth factor receptor (EGFR) signaling pathway, regulating cell proliferation and apoptosis, and inducing carcinogenesis (64). SBA also destroyed the DNA stability of intestinal epithelial cells through oxidation, mutagenesis and transformation activities (65), resulting in biological toxicity. Due to an increase of highly carcinogenic SBAs after BGBD diagonsed or CHE performed and high concentration of SBAs in the stool of CRC patients, scholars had to speculate that BGBD or CHE might promote the occurrence of CRC through secondary bile acids.
However, Simmons (66) and Shaffer et al. (67) found that the bile lipid composition tended to normalize after cholecystectomy. They thought that carcinogenic factors were formed as early as BGBD occurred and cholecystectomy was only a treatment strategy after BGBD diagnosed. Cholecystectomy could not correct the carcinogenic effects of BGBD but itself was not a risk factor for CRC. Researches of Almond (45) and Metzger et al. (58) also revealed that bile acid pools, kinetics and diurnal variation of bile lipid composition did not significantly change before and after cholecystectomy. Thus, we suspected the opinion that cholecystectomy was a risk factor of colorectal cancer. Conversely, based on the bile lipid composition normalizing tendency, we hypothesized that cholecystectomy was a “correction” measure and protective factor for colorectal cancer. Finally, our study successfully provided convincing evidence that cholecystectomy was not a risk factor of colorectal cancer.
There were some obvious strengths of our study. First, this study had a large sample size in both the colorectal cancer group and the local annual health examination group, and the total number of participants was far larger than that in most previous studies. Second, we selected the population undergoing annual health examination as the control group, which could be representative of the local adult population. Some previous studies did not set up a control group, or the control group was not representative enough, such as stomach cancer patients. Inappropriate controls introduced confounding factors in addition to study variables, such as the presence or absence of gastric cancer. Both the lack of a control group and the weak representation of the control group could undermine the validity of their findings. Third, we used PSM analysis in subgroups, which could eliminate or reduce the selection bias or error brought by confounding factors. The BGBD in our study included clinically common subtypes: gallstones, cholecystitis, and gallbladder polyps. Most previous studies used cholelithiasis, cholecystitis, or cholecystectomy as a complete substitute for gallbladder disease and did not conduct PSM analysis.
Our research also had some shortcomings that need to be overcome. As a single-center retrospective study, selection bias and confounding factors could not be avoided, although large sample size and PSM analysis ameliorated part of the bias. In addition, due to lack of information on gender, age, BMI, and comorbidities of control group, we were unable to achieve PSM analysis between case and control groups and only performed PSM analysis in subgroups of case group. We failed to achieve a direct PSM analysis among three subgroups, which is an undisputed shortcoming that might reduce the validity of our results. However, methods supporting direct PSM analysis of the three subgroups were limited at present, and we tried repeatedly, but it was still difficult to achieve. Although the best PSM analysis was not achieved, we believed that results obtained from PSM analysis of the two groups were still convincing. We believe that with the maturity of PSM analysis, multisubgroup direct PSM analysis will eventually be realized. Furthermore, we could not identify whether patients with BGBD and CRC share the same oncogene mutation, because BGBD was not routinely tested for genes. Therefore, although BGBD occurred before CRC in our study, we could not completely rule out effect of causal inversion. To solve this disturbance, Mendelian randomization studies should be needed.
In conclusion, our study showed benign gallbladder disease was associated with increased risk of colorectal cancer, particularly with right colon cancer. Cholecystectomy was weakly positive with right colon cancer before PSM, but the association disappeared after PSM.
Thanks to Dr. Rong Luo’s team at the Medical Examination Center for providing the control group data for this study. Thanks to Dr. Shiqiao Luo for his guidance of this study.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1008394/full#supplementary-material
1. Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F, et al. Global patterns and trends in colorectal cancer incidence and mortality. Gut (2017) 66(4):683–91. doi: 10.1136/gutjnl-2015-310912
2. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: Cancer J Clin (2018) 68(6):394–424. doi: 10.3322/caac.21492
3. Fitzmaurice C, Abate D, Abbasi N, Abbastabar H, Abd-Allah F, Abdel-Rahman O, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2017: A systematic analysis for the global burden of disease study. JAMA Oncol (2019) 5(12):1749–68. doi: 10.1001/jamaoncol.2019.2996
4. Kuipers EJ, Grady WM, Lieberman D, Seufferlein T, Sung JJ, Boelens PG, et al. Colorectal cancer. Nat Rev Dis Primers (2015) 1:15065. doi: 10.1038/nrdp.2015.65
5. Chen W, Sun K, Zheng R, Zeng H, Zhang S, Xia C, et al. Report of cancer incidence and mortality in China, 2014. Chin J Oncol (2018) 40(1):5–13. doi: 10.3760/cma.j.issn.0253-3766.2018.01.002
6. Dekker E, Tanis PJ, Vleugels JLA, Kasi PM, Wallace MB. Colorectal cancer. Lancet (2019) - 1474-547X(Electronic):1467–80. doi: 10.1016/S0140-6736(19)32319-0
7. Chen H, Li N, Ren J, Feng X, Lyu Z, Wei L, et al. Participation and yield of a population-based colorectal cancer screening programme in China. Gut (2019) 68(8):1450–7. doi: 10.1136/gutjnl-2018-317124
8. Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA: Cancer J Clin (2017) 67(3):177–93. doi: 10.3322/caac.21395
9. Siegel RL, Miller KD, Sauer AG, Fedewa SA, Butterly LF, Anderson JC, et al. Colorectal cancer statistics, 2020. CA: Cancer J Clin (2020) 70(3):145–64. doi: 10.3322/caac.21601
10. Everhart JE, Khare M, Hill M, Maurer KR. Prevalence and ethnic differences in gallbladder disease in the united states. Gastroenterology (1999) 117(3):632–9. doi: 10.1016/S0016-5085(99)70456-7
11. Lammert F, Gurusamy K, Ko CW, Miquel J-F, Méndez-Sánchez N, Portincasa P, et al. Gallstones. Nat Rev Dis Primers (2016) 2:16024. doi: 10.1038/nrdp.2016.24
12. Aune D, Norat T, Vatten L. Body mass index, abdominal fatness and the risk of gallbladder disease. Eur J Epidemiol (2015) 30(9):1009–19. doi: 10.1007/s10654-015-0081-y
13. Ruhl C, Everhart J. Association of diabetes, serum insulin, and c-peptide with gallbladder disease. Hepatol (Baltimore Md.) (2000) 31(2):299–303. doi: 10.1002/hep.510310206
14. Einarsson K, Hellström K, Kallner M. Gallbladder disease in hyperlipoproteinaemia. Lancet (1975) 1(7905):484–7. doi: 10.1016/S0140-6736(75)92831-7
15. Zhu L, Aili A, Zhang C, Saiding A, Abudureyimu K. Prevalence of and risk factors for gallstones in uighur and han Chinese. World J Gastroenterol (2014) 20(40):14942–9. doi: 10.3748/wjg.v20.i40.14942
16. Aerts R, Penninckx F. The burden of gallstone disease in Europe. Alimentary Pharmacol Ther (2003) 18(Suppl 3):49–53. doi: 10.1046/j.0953-0673.2003.01721.x
17. Xu Q, Tao L-Y, Wu Q, Gao F, Zhang F-L, Yuan L, et al. Prevalences of and risk factors for biliary stones and gallbladder polyps in a large Chinese population. HPB Off J Int Hepato Pancreato Biliary Assoc (2012) 14(6):373–81. doi: 10.1111/j.1477-2574.2012.00457.x
18. Bateson M. Fortnightly review: gallbladder disease. BMJ (Clinical Res ed.) (1999) 318(7200):1745–8. doi: 10.1136/bmj.318.7200.1745
19. Shaffer E. Epidemiology and risk factors for gallstone disease: has the paradigm changed in the 21st century? Curr Gastroenterol Rep (2005) 7(2):132–40. doi: 10.1007/s11894-005-0051-8
20. Liu C-M, Tung T-H, Chou P, Chen VT-K, Hsu C-T, Chien W-S, et al. Clinical correlation of gallstone disease in a Chinese population in Taiwan: experience at Cheng hsin general hospital. World J Gastroenterol (2006) 12(8):1281–6. doi: 10.3748/wjg.v12.i8.1281
21. Kim SS, Donahue TR. Laparoscopic cholecystectomy. Jama (2018) 319(17):1834. doi: 10.1001/jama.2018.3438
22. Hassler KR, Collins JT, Philip K, Jones MW. Laparoscopic cholecystectomy. Treasure Island (FL: StatPearls Publishing (2022).
23. Capron JP, Delamarre J, Canarelli JP, Brousse N, Dupas JL. Does cholecystectomy predispose to colo-rectal cancer? Gastroenterol Clin Biol (1978) 2(4):383–9.
24. Hager J, Riedler L. Correlation between cholecystectomy and colonic carcinoma? ZFA (Stuttgart) (1978) 54(31):1607–9.
25. GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the global burden of disease study 2017. Lancet (London England) (2018) 392(10159):1736–88. doi: 10.1016/S0140-6736(18)32203-7
26. Zhou M, Wang H, Zeng X, Yin P, Zhu J, Chen W, et al. Mortality, morbidity, and risk factors in China and its provinces, 1990-2017: a systematic analysis for the global burden of disease study 2017. Lancet (London England) (2019) 394(10204):1145–58. doi: 10.1016/S0140-6736(19)30427-1
27. Jung YK, Yoon J, Lee KG, Kim HJ, Park B, Choi D, et al. De Novo cancer incidence after cholecystectomy in Korean population. J Clin Med (2021) 10(7):1445. doi: 10.3390/jcm10071445
28. Hindson J. Digestive Disease Week 2022. Nat Rev Gastroenterol Hepatol. (2022) 19(8):487. doi: 10.1038/s41575-022-00659-x
29. Maringhini A, Maringhini M. Gallstones and colon cancer: A result of a wrong study revived. Gastroenterology (2017) 153(5):1453–4. doi: 10.1053/j.gastro.2017.08.068
30. Nogueira L, Freedman ND, Engels EA, Warren JL, Castro F, Koshiol J, et al. Gallstones, cholecystectomy, and risk of digestive system cancers. Am J Epidemiol (2014) 179(6):731–9. doi: 10.1093/aje/kwt322
31. Maringhini A, Moreau JA, Melton LJ 3rd, Hench VS, Zinsmeister AR, DiMagno EP, et al. Gallstones, gallbladder cancer, and other gastrointestinal malignancies. an epidemiologic study in Rochester, Minnesota. Ann Internal Med (1987) 107(1):30–5. doi: 10.7326/0003-4819-107-1-30
32. Shabanzadeh D, Sørensen L, Jørgensen T. Association between screen-detected gallstone disease and cancer in a cohort study. Gastroenterology (2017) 152(8):1965–1974.e1. doi: 10.1053/j.gastro.2017.02.013
33. Ward HA, Murphy N, Weiderpass E, Leitzmann MF, Aglago E, Gunter MJ, et al. Gallstones and incident colorectal cancer in a large pan-European cohort study. Int J Cancer (2019) 145(6):1510–6. doi: 10.1002/ijc.32090
34. McFarlane M, Welch K. Gallstones, cholecystectomy, and colorectal cancer. Am J Gastroenterol (1993) 88(12):1994–9.
35. Lieberman DA, Prindiville S, Weiss DG, Willett W, VA Cooperative Study Group 380. Risk factors for advanced colonic neoplasia and hyperplastic polyps in asymptomatic individuals. JAMA (2003) 290(22):2959–67. doi: 10.1001/jama.290.22.2959
36. Neugut A. Relation between the frequency of colorectal adenoma and the serum cholesterol level. New Engl J Med (1987) 317(1):55–6. doi: 10.1056/nejm198707023170117
37. Törnberg SA, Holm LE, Carstensen JM, Eklund GA. Risks of cancer of the colon and rectum in relation to serum cholesterol and beta-lipoprotein. New Engl J Med (1986) 315(26):1629–33. doi: 10.1056/NEJM198612253152601
38. Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JPA. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ (Clinical Res ed.) (2015) 350:g7607. doi: 10.1136/bmj.g7607
39. Giovannucci E, Ascherio A, Rimm EB, Colditz GA, Stampfer MJ, Willett WC, et al. Physical activity, obesity, and risk for colon cancer and adenoma in men. Ann Internal Med (1995) 122(5):327–34. doi: 10.7326/0003-4819-122-5-199503010-00002
40. Lee I, Paffenbarger R. Quetelet's index and risk of colon cancer in college alumni. J Natl Cancer Institute (1992) 84(17):1326–31. doi: 10.1093/jnci/84.17.1326
41. Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature (2008) 454(7203):436–44. doi: 10.1038/nature07205
42. Grivennikov S, Greten F, Karin M. Immunity, inflammation, and cancer. Cell (2010) 140(6):883–99. doi: 10.1016/j.cell.2010.01.025
43. Aune D, Vatten L, Boffetta P. Tobacco smoking and the risk of gallbladder disease. Eur J Epidemiol (2016) 31(7):643–53. doi: 10.1007/s10654-016-0124-z
44. Hill MJ, Drasar BS, Williams RE, Meade TW, Cox AG, Simpson JE, et al. Faecal bile-acids and clostridia in patients with cancer of the large bowel. Lancet (London England) (1975) 1(7906):535–9. doi: 10.1016/S0140-6736(75)91556-1
45. Almond HR, Vlahcevic ZR, Bell CC Jr, Gregory DH, Swell L. Bile acid pools, kinetics and biliary lipid composition before and after cholecystectomy. New Engl J Med (1973) 289(23):1213–6. doi: 10.1056/NEJM197312062892302
46. Xu Y-K, Zhang F-L, Feng T, Li J, Wang Y-H. [Meta-analysis on the correlation of cholecystectomy or cholecystolithiasis to risk of colorectal cancer in Chinese population]. Ai zheng = Aizheng = Chin J Cancer (2009) 28(7):749–55. doi: 10.5732/cjc.008.10829
47. Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika (1983) 70:41–55. doi: 10.1093/biomet/70.1.41
48. Benedetto U, Head SJ, Angelini GD, Blackstone EH. Statistical primer: propensity score matching and its alternatives. Eur J Cardiothorac Surg (2018) 53(6):1112–7. doi: 10.1093/ejcts/ezy167
49. Pan Z, Lin Z, Fu S, Tian Y. Preliminary establishment of colorectal cancer screening model for patients with chronic enteritis in Zhoushan area. Laboratory Medicine (2021) 36(05):510–3. doi: 10.3969/j.issn.1673-8640.2021.05.010
50. Luo H, Yang Z. Study on the correlation between cholecystolithiasis, cholecystectomy and colorectal cancer[D]. Kunming Medical University (2019). doi: 10.27202/d.cnki.gkmyc.2019.000515
51. Li W, Chen S. The related risk factors and clinical analysis of colorectal cancer[D]. Fujian Medical University (2019). doi: 10.27020/d.cnki.gfjyu.2019.000574
52. Gosavi S, Mishra RR, Kumar VP. Study on the relation between colorectal cancer and gall bladder disease. J Clin Diagn Res (2017) 11(3):Oc25–oc27. doi: 10.7860/JCDR/2017/22954.9485
53. Mándi M, Keleti G, Juhász M. The role of appendectomy and cholecystectomy in the pathogenesis of colorectal carcinomas. Ann Med Surg (Lond) (2021) 72:102991. doi: 10.1016/j.amsu.2021.102991
54. Yan P, Yao P. Analysis of risk factors and establishment of risk scoring system for colorectal adenoma[D]. Xinjiang Medical University (2021). doi: 10.27433/d.cnki.gxyku.2021.000569.
55. Polychronidis G, Wang K, Lo C-H, Wang L, He M, Knudsen MD, et al. Gallstone disease and risk of conventional adenomas and serrated polyps: A prospective study. Cancer Epidemiol Biomarkers Prev (2021) 30(12):2346–9. doi: 10.1158/1055-9965.EPI-21-0515
56. Chen C, Lin C, Kao C. The effect of cholecystectomy on the risk of colorectal cancer in patients with gallbladder stones. Cancers (2020) 12(3):550. doi: 10.3390/cancers12030550
57. Song M, Chan AT, Sun J. Influence of the gut microbiome, diet, and environment on risk of colorectal cancer. Gastroenterology (2020) 158(2):322–40. doi: 10.1053/j.gastro.2019.06.048
58. Metzger AL, Adler R, Heymsfield S, Grundy SM. Diurnal variation in biliary lipid composition. possible role in cholesterol gallstone formation. New Engl J Med (1973) 288(7):333–6. doi: 10.1056/NEJM197302152880702
59. Hu H, Shao W, Liu Q, Liu N, Wang Q, Xu J, et al. Gut microbiota promotes cholesterol gallstone formation by modulating bile acid composition and biliary cholesterol secretion. Nat Commun (2022) 13(1):252. doi: 10.1038/s41467-021-27758-8
60. Shiha MG, Ashgar Z, Fraser EM, Kurien M, Aziz I. High prevalence of primary bile acid diarrhoea in patients with functional diarrhoea and irritable bowel syndrome-diarrhoea, based on Rome III and Rome IV criteria. EClinicalMedicine (2020) 25:100465. doi: 10.1016/j.eclinm.2020.100465
61. Rey J, Garin N, Spertini F, Corthésy B. Targeting of secretory IgA to peyer's patch dendritic and T cells after transport by intestinal m cells. J Immunol (Baltimore Md 1950) (2004) 172(5):3026–33. doi: 10.4049/jimmunol.172.5.3026
62. Baijal P, Fitzpatrick D, Bird R. Modulation of colonic xenobiotic metabolizing enzymes by feeding bile acids: comparative effects of cholic, deoxycholic, lithocholic and ursodeoxycholic acids. Food Chem Toxicol (1998) 36(7):601–7. doi: 10.1016/S0278-6915(98)00020-9
63. Tsujii M, DuBois R. Alterations in cellular adhesion and apoptosis in epithelial cells overexpressing prostaglandin endoperoxide synthase 2. Cell (1995) 83(3):493–501. doi: 10.1016/0092-8674(95)90127-2
64. Raufman J-P, Shant J, Guo CY, Roy S, Cheng K. Deoxycholyltaurine rescues human colon cancer cells from apoptosis by activating EGFR-dependent PI3K/Akt signaling. J Cell Physiol (2008) 215(2):538–49. doi: 10.1002/jcp.21332
65. Qiao D, Gaitonde SV, Qi W, Martinez JD. Deoxycholic acid suppresses p53 by stimulating proteasome-mediated p53 protein degradation. Carcinogenesis (2001) 22(6):957–64. doi: 10.1093/carcin/22.6.957
66. Simmons F, Ross A, Bouchier I. Alterations in hepatic bile composition after cholecystectomy. Gastroenterology (1972) 63(3):466–71. doi: 10.1016/S0016-5085(19)33295-0
Keywords: benign gallbladder disease, cholecystectomy, colorectal cancer, risk factor, propensity score matching analysis
Citation: Qin Q, Li W, Ren A, Luo R and Luo S (2022) Benign gallbladder disease is a risk factor for colorectal cancer, but cholecystectomy is not: A propensity score matching analysis. Front. Oncol. 12:1008394. doi: 10.3389/fonc.2022.1008394
Received: 31 July 2022; Accepted: 21 November 2022;
Published: 08 December 2022.
Edited by:
Farhadul Islam, Rajshahi University, BangladeshReviewed by:
Yen-Chun Peng, Taichung Veterans General Hospital, TaiwanCopyright © 2022 Qin, Li, Ren, Luo and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiqiao Luo, c2hpcWlhb2x1b0BxcS5jb20=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.