
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 09 November 2022
Sec. Breast Cancer
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1002360
This article is part of the Research Topic Reviews in Breast Cancer View all 20 articles
Carriers with BRCA1/2 germline pathogenic variants are associated with a high risk of breast and ovarian cancers (also pancreatic and prostate cancers). While the spectrum on germline BRCA mutations among the Chinese population shows ethnic specificity, the identification of carriers with germline BRCA mutation before cancer onset is the most effective approach to protect them. This review focused on the current status of BRCA1/2 screening, the surveillance and prevention measures, and discussed the issues and potential impact of BRCA1/2 population screening in China. We conducted literature research on databases PubMed and Google Scholar, as well as Chinese databases CNKI and Wangfang Med Online database (up to 31 March 2022). Latest publications on germline BRCA1/2 prevalence, spectrum, genetic screening as well as carrier counseling, surveillance and prevention were captured where available. While overall 15,256 records were retrieved, 72 publications using germline BRCA1/2 testing were finally retained for further analyses. Germline BRCA1/2 mutations are common in Chinese patients with hereditary breast, ovarian, prostate and pancreatic cancers. Within previous studies, a unique BRCA mutation spectrum in China was revealed. Next-generation sequencing panel was considered as the most common method for BRCA1/2 screening. Regular surveillance and preventive surgeries were tailored to carriers with mutated-BRCA1/2. We recommend that all Chinese diagnosed with breast, ovarian, pancreatic or prostate cancers and also healthy family members, shall undergo BRCA1/2 gene test to provide risk assessment. Subsequently, timely preventive measures for mutation carriers are recommended after authentic genetic counseling.
Breast cancer genes BRCA1 and BRCA2 are tumor suppressor genes that function in DNA double-strand break repair in the homologous recombination pathway. Mutated BRCA1/2 genes can cause BRCA1/2 protein deficiency and genome instability (1). Since the identification of BRCA1 and BRCA2 genes in the 1990s as the landmarks of hereditary breast and ovarian cancer, human beings enter the era of cancer genetic testing. Female BRCA mutation carriers have 60-80% of lifetime risk of developing breast cancer and 20-40% of risk of ovarian cancer (2). Mutation in BRCA is also associated with an increased risk of prostate and pancreatic cancers (3). In addition, BRCA pathogenic mutation carriers are significantly associated with increased disease risk for three additional cancers, including biliary tract cancer, gastric cancer, and esophageal cancer (4). Notably, BRCA1 pathogenic variants carriers have a 4.30, 2.36 and 2.17-fold elevated lifetime risk of the male breast, pancreatic and stomach cancers compared to non-carriers. BRCA2 pathogenic variants carriers have 44.0, 3.69, 3.34 and 2.22-fold elevated lifetime risk of the male breast, stomach, pancreatic and prostate cancers compared to non-carriers, respectively (5).
Early detection and prevention have been proven to reduce cancer incidence and mortality (while increasing cancer survival) in mutation carriers (3, 6). Therefore, identifying BRCA mutation carriers is important to reduce cancer risk. In this review, we conducted literature research on PubMed, Google Scholar and Chinese databases about germline BRCA1/2 mutation in the Chinese populations included literature published up to 31 March 2022. A total of 15,256 publications were obtained: PubMed (n=856), Google Scholar (n=6,153), CNKI (n=4,935) and Wangfang Med Online database (n=3,312). After removing duplicates, selecting the title and the abstract and carefully reading the whole paper, 72 publications related to germline BRCA1/2 testing were finally included. Based on the comprehensive literature review, we discuss population screening approaches for comprehensive identification of the BRCA mutation carriers in the Chinese population and propose the ideal procedure for achieving the goals in China (Figure 1) (7–11).
In the general Chinese population, the prevalence of pathogenic BRCA1/2 variation has been reported to range from 0.29 to 1.10% (0.02 to 0.34% for BRCA1 and 0.11 to 0.27% for BRCA2) (12–15).
The prevalence of BRCA1/2 in the general population varies by country and ethnicity (16, 17). It was 0.18% in a Malaysian group of 2,809 individuals, 0.26% among 22,731 Japanese, 0.38% in a Mexican population of 3,985 individuals, 0.53% in 50,726 US people and 2.17% in the Ashkenazi Jewish population, which is the highest (18–22). The prevalence of BRCA1/2 mutation in the general Chinese population is intermediate.
The spectrum of BRCA variation in Chinese is rather different from those in non-Chinese populations (15, 17). It was reported that approximately 38-41.4% of BRCA variants were only present in the Chinese population (23, 24). Even when compared to neighboring India, only 4.1% and 0.4% of shared BRCA1 and BRCA2 variants were found in both populations (24).
In a large-scale cohort with 1,245 pathogenic variants identified, 48 most common pathogenic BRCA1/2 variants (39.86% of total) were not reported as common variants in Caucasians (15). The pathogenic variant BRCA1 c.5470_5477del was determined as a founder mutation in the Chinese Han population (25, 26). Interestingly, another systematic review with 2,128 BRCA1/2 variants derived from 35,178 Chinese individuals from 23 provinces also reported that c.5470_5477del ranked as the highest frequency of all BRCA1 variants identified while the c.3109C>T ranks highest in BRCA2 (12). Further, BRCA1 c.3770_3771delAG was the most common variant in Chinese ovarian cancer patients (27). The proportions of frameshift, nonsense, splice and missense mutations in Chinese ovarian cancer patients were determined as 51.2%, 39.3%, 7.1% and 2.4%, respectively (28). But the founder mutations in other ethnic populations, such as BRCA1 c.66_67delAG, BRCA2 c.5946delT in Ashkenazi Jewish, BRCA 1 c.303T>G, c.1623dupG in African, BRCA1 c.390C>A in Japanese and Korean and many other founder mutations in different non-Chinese populations, were absent or at low prevalence in Chinese population (24).
We summarized the prevalence of BRCA1/2 germline mutation in different populations from large-scale cohort studies published within five years (Table 1). A total of 41 studies were included for further analysis (13–15, 27, 29–35, 64).
In the Chinese cancer patients, a study showed that the prevalence rate was 5.53% for BRCA1/2 (43.7% in BRCA1 and 56.3% in BRCA2) in unselected breast cancer patients (15). In comparison, a higher prevalence of 9.06-19.54% for BRCA1/2 mutation was observed in familial breast cancer patients (29, 31, 33). 60% of breast cancer patients carrying BRCA1 deleterious mutation were classified as triple-negative breast cancer, while only 10 to 20% were triple-negative breast cancer in unselected cancer patients (33, 34). Patients with BRCA1/2 mutated breast cancer generally show an earlier age of onset, on average 5 to 8 years earlier than patients with sporadic breast cancer (33, 35). BRCA1/2 pathogenic variants are also enriched in bilateral breast cancer and patients with family history of breast or other cancers (33, 34). In unselected ovarian cancer patients, BRCA1 pathogenic variants were more common compared to BRCA2 (20.07% vs. 6.19%) (27). Pathogenic mutations in BRCA1 genes were more related to a younger diagnosis age, serous ovarian carcinoma and hereditary breast and ovarian cancer syndrome (HBOC) (27). Among prostate cancer patients carrying germline mutations, BRCA2 is the most common mutated gene among DNA damage repair pathway genes. The prevalence of BRCA1 and BRCA2 pathogenic variants was 0.38% and 4.30%, respectively, in prostate cancer patients (55). BRCA2 was also reported as the most frequent gene in the germline in pancreatic cancer patients, with a prevalence rate of 1.9%; the frequency of BRCA1 variants was 0.5% (57). However, there is a lack of multicenter studies on BRCA mutations in pancreatic cancer. It is worth noting that the actual prevalence may be higher than what is now predicted because the data for pathogenic variants interpretation are mainly from non-Chinese populations. In addition, most of the studies summarized in the Table 1 examined only single nucleotide variants and indels and did not detect mutations of large genomic rearrangements (LGRs). It is possible that many unknown pathogenic variants have not been identified.
Despite increasing data from large-scale and multicenter BRCA studies having been reported, no BRCA data is reported for the Chinese living in many remote areas (12). Most BRCA1/2 prevalence studies were from cities with relatively developed economies and medical care, such as Beijing, Shanghai, Hong Kong, Guangdong, Zhejiang and Sichuan. Possibly because genetic testing is not yet covered by basic medical insurance, patients in economically developed regions are more likely to afford expensive genetic testing. Meanwhile, economically and medically developed regions have more medical resources, such as genetic testing facilities and genetic counseling services (65). The bias is also because these regions have more investigators and research funds and are more likely to conduct clinical studies. However, considering the regional and ethnic specificity of BRCA gene variation, substantial efforts are needed to generate a comprehensive BRCA variation map for the Chinese population.
In the mid-1990s, the identification of the relationship between BRCA1/2 mutation and cancer risk heralded the era of genetic testing for susceptibility to cancer. Subsequently, germline BRCA1 and BRCA2 mutations were extensively studied in the Caucasian populations, and associations with breast and ovarian cancers were established (66). Sanger sequencing has been widely used in BRCA variant identification since the 1990s, but the development of next-generation sequencing (NGS) revolutionized the detection strategy due to its affordability and efficiency.
NGS, including whole-genome sequencing (WGS), whole-exome sequencing (WES) and panel sequencing, have facilitated BRCA mutation research (67). Also because of the policy support in 2015, large-scale BRCA studies in China have increased rapidly since then (12, 42). Due to the lack of hotspot variation, NGS is currently the optimal option for BRCA1/2 genetic testing in the Chinese population. NGS panel test is widely implemented for clinical BRCA test in China in recent years. The two-gene panel is a more preferred option for the general population, breast cancer and ovarian cancer patients, while pancreatic, prostate and other cancer patients tend to be suggested with the multi-gene panel in China (Tables 1, 2).
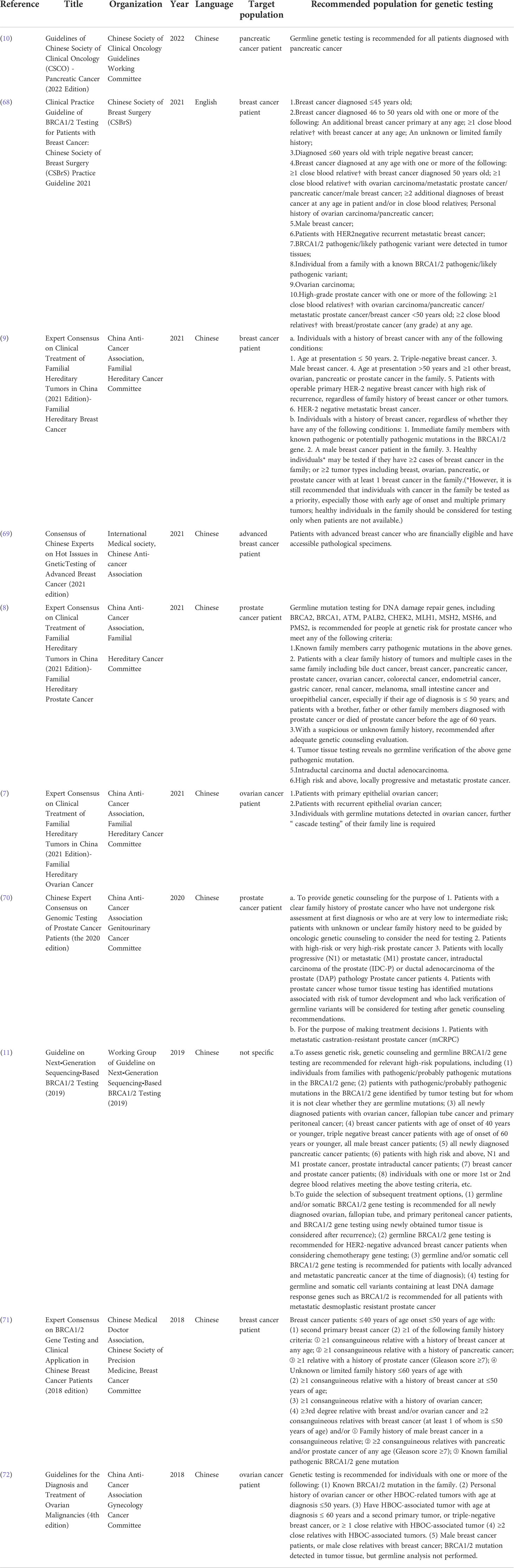
Table 2 Summary of guidelines and consensus about BRCA1/2 genetic testing in recent 5 years in China.
Because of its accuracy, Sanger sequencing remains to be a gold standard for detecting BRCA variants and validating NGS-detected BRCA variants and can be used in confirming the findings (67). Practical test- and laboratory-specific criteria have been proposed for confirmation strategy to facilitate timely delivery of clinical accuracy (73).
Many studies involving different populations have shown that LGRs in BRCA1/2 can be identified in HBOC (74–76). Multiplex ligation-dependent probe amplification (MLPA) is a cheap, sensitive and reliable method for detecting gene rearrangements (77). In the eastern Chinese population, 2.9% of HBOC patients without detectable BRCA1/2 small pathogenic variants were identified harboring LGRs in BRCA (78). The data are similar to those from the Myriad data set with high-risk patients, most of whom were diagnosed with early-onset ovary cancer or male breast cancer. The study reported an overall BRCA1/2 mutation rate of 23.8%, of which 9.9% were LGRs. Thus, large genomic rearrangement testing is recommended if the NGS result is negative for high-risk populations to avoid the missed diagnosis of BRCA1/2 mutation carriers (71).
NGS panel, the recommended method for BRCA1/2 testing in clinical practice, still has some problems. First, variants of uncertain significance (VUS) increase with testing a larger panel or increasing genome sequencing length, making BRCA1 and BRCA2 interpretation more complex (79). For example, 24.7% of variants reported in the general population and 43.8% reported in breast cancer were identified as VUS, respectively (13, 31). Classification of VUS as pathogenic or benign variants has important clinical implications for cancer diagnosis and treatment (80). The methods to identify VUS as pathogenic or benign need to become more efficient and accurate, considering the huge abundance of VUS. BRCA1/2 variants interpretation mainly follows the Chinese expert consensus on BRCA1/2 variant interpretation (2021 version) (81) and the American College of Medical Genetics and Genomics and the Association for Molecular Pathology (ACMG/AMP) guideline (82) in China.
Nevertheless, the population, disease-specific and sequence databases commonly used for interpretation contain few Chinese or Asian data. Lacking Chinese ethnic-specific data makes variant interpretation highly reliant on the peer-reviewed literature, which is also limited. This challenging context prevents many pathogenic variants from being identified and the VUS increases even more (83). Suggesting a new classification system for Chinese is needed, including but not limited to the databases based on Chinese populations and biological function identification of Chinese specific variants. Chinese Familial & Hereditary Cancer Susceptibility Gene Mutation Database (CFCSG-database) is one of the biggest cancer susceptibility gene mutation databases based on Chinese population. But the amount of BRCA1 and BRCA2 variants is still limited in it. More large-scale population studies and function studies of BRCA1/2 mutation in Chinese are needed to obtain more evidence to optimize the mutation interpretation.
As new shreds of evidence accumulate, the variant classification could be change over time. A study of 21,216 Breast cancer patients and 6,434 healthy controls performed VUS reclassification in the cohort. After the reclassification, 7 VUS were re-grouped into benign, which reduced the VUS ratio in both patient and healthy control (from 9.8 to 7.9% and from 6.9 to 5.3%) (15), indicating that the evidence should frequently be updated for VUS reclassification, and emphasizing the VUS carriers should be followed up.
Another notable issue concerns the price of BRCA mutation testing (84). Currently the price of BRCA mutation testing for a single sample in China is roughly 300 dollars ($), which is only paid by the patient side and not covered by the government side through basic medical insurance (85). Actually, the price of a single BRCA mutation testing is too high for the majority of ordinary Chinese. Therefore, financial investment from the Chinese government side is necessary to promote the widespread of BRCA mutation testing across China, e. g., Chinese government could offer reimbursement through Chinese basic medical insurance system for the high-risk population who took BRCA mutation testing. Additionally, evidence shows population-based BRCA mutation screening is also cost-effective for Chinese data with an incremental cost-effectiveness ratio of $18,066 from a societal perspective and $23,485 from a payer perspective per quality-adjusted life year (86).
In fact, while these issues are prominent in China, they also exist in many other countries and need to be addressed through collective efforts.
Currently, the principles of BRCA mutation detection in China mainly refers to the guideline on next-generation sequencing-based BRCA1/2 testing (2019) (11), the US National Comprehensive Cancer Network (NCCN) guidelines (87) and the European Society of Medical Oncology (ESMO) guidelines (88), as well as other Chinese expert consensus on specific cancers or genetic testing (summarized in Table 2). We summarized the criteria proposed in 10 different guidelines and consensuses for Chinese population BRCA screening in recent 5 years (7–11, 68–72).
Genetic counseling is essential in pre- and post-sequencing stage for the test individuals. The purpose is to accurately estimate the probability of cancer susceptibility gene mutations (89) and offer early prevention advice and medical management such as regular surveillance, chemoprevention or surgical prevention for BRCA mutation carriers (27, 28). In a study with 839 breast cancer patients and 510 relatives, who are considered high-risk populations, 86.4% and 63.8% cases showed a strong willingness to accept genetic counseling and genetic testing, respectively (90). For those high-risk populations who are willing to do the genetic testing of BRCA1/2, the mutation rate was 19.9%. Despite the high willingness, most of the high-risk individuals lacked knowledge of cancer inheritance (90). We are glad to find out that another study exhibited that 79% of germline mutation carriers were aware of the risk and the importance of surveillance, while 56% accepted preventive interferences after genetic counseling on gynecologic tumors (91).
However, the development of cancer genetic counseling in China is in its beginning. Unlike some developed countries where specialized and certified genetics health professionals are available (92), cancer genetic counseling relies heavily on clinicians. Setting up standardized workflows and training eligible counselors is pivotal for promoting genetic counseling in China. Although the “oncologist-led BRCA consultation” mode has improved access to cancer genetic testing in developing countries (93), specialized cancer genetic counselors are urgently needed. Organizations like the Chinese Board of Genetic Counseling and others are now dedicated to training genetic counselors in more than 15 provinces across China (65). Meanwhile, the Chinese Anti-Cancer Association is urging hospitals nationwide to set up cancer genetic counseling clinics to accommodate the increased demand for counseling. Still, the training projects and qualified counselors are minimal and lack statistics.
After genetic testing, the frequency of regular surveillance for female mutation carriers was significantly higher compared to non-carriers, according to the report on high-risk southern Chinese females (94).
Early-stage breast cancer lacks apparent signs and symptoms. Possible symptoms of breast cancer can be skin dimpling, red or thickening, nipple retraction and lymph nodes swelling. But a painless hard lump with irregular edges discovered accidentally by patients themselves is the most common early sign. Ninety-one percent of Chinese breast cancer patients had dense gland (95), which significantly affected the quality and effectiveness of palpation examination. For the surveillance of high-risk female carriers, in addition to regular breast self-examination and clinical breast examination, X-ray combined with ultrasound and magnetic resonance imaging (MRI) are usually selected as the methods recommended for women aged >40 years to detect early signs of breast cancer in China (96). Given that Chinese women have dense breasts and many younger patients with BRCA1/2 mutated breast cancer, mammography screening has a lower sensitivity. A prospective study comparing different screening methods for patients with BRCA1/2 mutations found the sensitivity of 77% with MRI compared with 36% with mammography and 33% with ultrasound (97–99).
Regular pelvic examination, tumor marker CA125 detection and transvaginal ultrasound are the methods recommended for detecting early signs of ovarian cancer (88). Annual prostate-specific antigen (PSA) testing and digital rectal examination are recommended for prostate cancer screening and surveillance, especially for BRCA1 carriers (8). A study showed that multiparameter MRI has high diagnostic efficacy for BRCA1 or BRCA2 mutated prostate cancer patients. As soon as PSA elevation is detected, multiparameter MRI is recommended for BRCA1 or BRCA2 mutation carriers aged >55 years for further diagnosis (100). Besides, annual imaging examinations can be considered to prevent pancreatic cancer for BRCA2 carriers, although the efficacy of this approach remains to be validated (88). The recommended starting age for monitoring breast cancer, ovarian cancer, prostate cancer and pancreatic cancer is 25, 30, 40 and 50 years, respectively, or ten years earlier than the earliest confirmed case in the family (81, 88, 94, 101).
Many studies confirmed that for BRCA mutation carriers, chemoprevention or surgical prevention play an important role in reducing the occurrence of HBOC (102–104). In high-risk women, prophylactic mastectomy can reduce the incidence of breast cancer by 90% and the mortality rate by 81% (103). A study showed that 23.8% and 32% of patients chose prophylactic mastectomy and prophylactic salpingo-oophorectomy; more than 17% of healthy carriers also had prophylactic surgery in Hongkong, China (102). In mainland China, however, healthy carriers and surgeons are more cautious about choosing prophylactic surgery. Only one study reported that three healthy carriers with deleterious BRCA1/2 variant underwent prophylactic nipple-sparing mastectomy (105). Breast cancer patients carrying BRCA1/2 deleterious variants had a 4.52-fold and 5.54-fold increased risk of contra-lateral breast cancer, respectively, compared to non-carriers (106). Preventive contra-lateral prophylactic mastectomy can be an optimal selection for BRCA1/2 mutated breast cancer patients in China (9). Risk-reducing salpingo-oophorectomy, which can significantly reduce the risk of breast, ovarian, and fallopian tube cancers, is recommended for high-risk women after childbirth to prevent ovarian cancer (7, 107).
Studies found that BRCA1/2-mutated patients are more likely to benefit from platinum-based chemotherapy (108–110). Since DNA damage caused by platinum-based drugs requires DNA homologous recombination for repair, the functional defects caused by mutations in the BRCA1/2 gene make tumor cells more sensitive to platinum-based drugs. The TNT phase III trial compared the efficacy between carboplatin and docetaxel in unselected advanced TNBC. In the germline BRCA1/2-mutated subgroup, the objective response rate with carboplatin was 2-fold higher than it with docetaxel (68% vs. 33%) (110). Recently, cancer patients with BRCA mutation could be benefited from poly (ADP-ribose) polymerase (PARP) targeted therapy due to the increased sensitivity to PARP inhibitors (62, 111). PARP inhibitor specifically causes the death of cancer cells with BRCA1/2 mutations through the “synthetic lethal effect” (112). The OlympiA trial has confirmed the efficacy of PARP inhibitors in the adjuvant treatment of early-stage BRCA1/2-mutated breast cancer (113), while the OlympiAD trial, as well as many other phase III clinical trials, have proved the role of PARP inhibitors in advanced BRCA1/2-mutated breast cancer (114, 115). PARP inhibitors are widely used for BRCA1/2-mutated ovarian cancer patients as maintenance therapy in China based on the results of several phase III trials, including SOLO-1, SOLO-2, PAOLA-1, PRIMA and NOVA (116–120). PARP inhibitor olaparib is recommended for metastatic castration-resistant prostate cancer patients based on the PROfound trial. The phase III PROfound study showed a more prolonged imaging-based progression-free survival in the olaparib group compared with the control group (median, 7.4 months vs. 3.6 months) (121). PARP inhibitors are increasingly used to treat BRCA-mutated patients, but whether they can be used for prevention needs further investigation.
Chemoprevention for cancer-free BRCA1/2 carriers remains controversial. Only a small retrospective study has shown that tamoxifen, a selective estrogen receptor modulator, reduces the risk of breast cancer in healthy carriers of BRCA2 mutations by 62%. But it is unclear whether it has a preventive effect in BRCA1-mutated healthy carriers (122). The evidence is not enough to support tamoxifen as a prevention strategy for healthy BRCA1/2 mutated carriers (9). Oral contraceptives have proven preventive efficacy for ovarian cancer with a family history. However, it is controversial whether oral contraceptives increase the risk of breast cancer in BRCA1/2 mutation carriers (123).
Taken together, germline BRCA1/2 mutations are common in Chinese patients with hereditary breast, ovarian, prostate and pancreatic cancers. Because of its ethnic specificity, the unique features in the spectrum of BRCA mutations have already been revealed but the extension of the sequencing efforts to the whole Chinese population remains yet to be achieved. Many Chinese consensuses today recommend BRCA1/2 genetic testing for cancer patients only. Regarding the prevalence in healthy populations, approximately one in every 300 healthy Chinese is a BRCA1/2 mutation carrier (12, 15). BRCA mutation-related cancer is one of the most preventable cancers. Whether or not to perform population screening should not solely be based on cost-effectiveness but should also consider more non-cost factors such as social, political, public interest and patients’ benefits. Under the current political and economic conditions in China, to achieve early prevention of BRCA mutation carriers, we recommend that the criteria be relaxed and all Chinese diagnosed with breast, ovarian, pancreatic or prostate cancer, as well as healthy individuals with a clear family history, should undergo BRCA1/2 genetic testing to provide a risk assessment. Subsequently, preventive measures such as regular surveillance, chemoprevention or surgical prevention for mutation carriers are recommended after authentic genetic counseling.
Evidence had shown that relying on personal and family history may not be sufficient to determine the risk for BRCA1/2 variants (20). Population BRCA screening is considered the trend in the near future (124, 125). Thus, a growing number of healthy individuals harboring pathogenic mutations can be identified for cancer prevention. Population screening for carriers with BRCA germline mutations in the Chinese population is highly warranted to promote prevention, early detection, early diagnosis, and timely treatment of BRCA mutation-related cancers, which may increase 5-year survival for BRCA mutation-related cancer patients. Also, the ethical, psychological and legal issues cannot be ignored.
TC were responsible for the study concept and design. HL, MZ, LZ, KH, X-jW and TC drafted the manuscript, and all authors revised it for important intellectual content. The work reported in the paper has been performed by the authors, unless clearly specified in the text. All authors contributed to the article and approved the submitted version.
This work was supported by grants from National Key Research-Development Program of China (2019YFE0198800), Key Research-Development Program of Zhejiang Province (2017C03013), Ten-Thousand Talents Plan of Zhejiang Province (2021R52020), and Start-up Funds for Recruited Talents in Zhejiang Cancer Hospital. The funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
CFCSG-database, Chinese Familial & Hereditary Cancer Susceptibility Gene Mutation database; ESMO, the European Society of Medical Oncology; HBOC, hereditary breast and ovarian cancer syndrome; LGRs, large genomic rearrangements; MLPA, multiplex ligation-dependent probe amplification; MRI, magnetic resonance imaging; NCCN, National Comprehensive Cancer Network; NGS, next-generation sequencing; PARP, poly (ADP-ribose) polymerase.
PSA, prostate-specific antigen; SGO, the Society of Gynecologic Oncology; VUS, variants of uncertain significance; WES, whole-exome sequencing; WGS, whole-genome sequencing.
1. Sun S, Brazhnik K, Lee M, Maslov AY, Zhang Y, Huang Z, et al. Single-cell analysis of somatic mutation burden in mammary epithelial cells of pathogenic Brca1/2 mutation carriers. J Clin Invest (2022) 132(5):e148113. doi: 10.1172/JCI148113
2. Kobayashi H, Ohno S, Sasaki Y, Matsuura M. Hereditary breast and ovarian cancer susceptibility genes (Review). Oncol Rep (2013) 30(3):1019–29. doi: 10.3892/or.2013.2541
3. Collins JM, Isaacs C. Management of breast cancer risk in Brca1/2 mutation carriers who are unaffected with cancer. Breast J (2020) 26(8):1520–7. doi: 10.1111/tbj.13970
4. Momozawa Y, Sasai R, Usui Y, Shiraishi K, Iwasaki Y, Taniyama Y, et al. Expansion of cancer risk profile for Brca1 and Brca2 pathogenic variants. JAMA Oncol (2022) 8(6):871–8. doi: 10.1001/jamaoncol.2022.0476
5. Li S, Silvestri V, Leslie G, Rebbeck TR, Neuhausen SL, Hopper JL, et al. Cancer risks associated with Brca1 and Brca2 pathogenic variants. J Clin Oncol (2022) 40(14):1529–41. doi: 10.1200/JCO.21.02112
6. Lubinski J. Breast cancer genetics: 20 years later. Clin Genet (2014) 85(1):5–6. doi: 10.1111/cge.12293
7. Familial Hereditary Cancer Committee CA-CA. Expert consensus on clinical treatment of familial hereditary tumors in China (2021 edition)-familial hereditary ovarian cancer. Chin J Clin Oncol (2022) 48(24):5. doi: 10.12354/j.issn.1000-8179.2021.20211800
8. Familial Hereditary Cancer Committee CA-CA. Expert consensus on clinical treatment of familial hereditary tumors in China (2021 edition)-familial hereditary prostate cancer. Chin J Clin Oncol (2022) 49(2):5. doi: 10.12354/j.issn.1000-8179.2022.20211805
9. Familial Hereditary Cancer Committee CA-CA. Expert consensus on clinical treatment of familial hereditary tumors in China (2021 Edition)(1) -familial hereditary breast cancer. Chin J Clin Oncol (2022) 48(23):1189–95. doi: 10.12354/j.issn.1000-8179.2021.20211553
10. Chinese Society of Clinical Oncology GWC. Guidelines of Chinese society of clinical oncology (Csco) - pancreatic cancer (2022 edition) Beijing: People’s Medical Publishing House. (2022), 107.
11. Chen G, Chen G, Chen M, Cheng W, Cui W, Ding W, et al. Working group of guideline on Next⁃Generation Sequencing⁃Based BRCA1/2 testing. [Guideline on Next⁃Generation Sequencing⁃Based Brca1/2 testing (2019)]. Chin J Pathol (2019) 48(9):8. doi: 10.3760/cma.j.issn.0529⁃5807.2019.09.002
12. Gao X, Nan X, Liu Y, Liu R, Zang W, Shan G, et al. Comprehensive profiling of Brca1 and Brca2 variants in breast and ovarian cancer in Chinese patients. Hum Mutat (2020) 41(3):696–708. doi: 10.1002/humu.23965
13. Dong H, Chandratre K, Qin Y, Zhang J, Tian X, Rong C, et al. Prevalence of Brca1/Brca2 pathogenic variation in Chinese han population. J Med Genet (2021) 58(8):565–9. doi: 10.1136/jmedgenet-2020-106970
14. Qin Z, Kuok CN, Dong H, Jiang L, Zhang L, Guo M, et al. Can population brca screening be applied in non-ashkenazi Jewish populations? experience in Macau population. J Med Genet (2021) 58(9):587–91. doi: 10.1136/jmedgenet-2020-107181
15. Liu Y, Wang H, Wang X, Liu J, Li J, Wang X, et al. Prevalence and reclassification of Brca1 and Brca2 variants in a Large, unselected Chinese han breast cancer cohort. J Hematol Oncol (2021) 14(1):18. doi: 10.1186/s13045-020-01010-0
16. Sekine M, Nishino K, Enomoto T. Differences in ovarian and other cancers risks by population and brca mutation location. Genes (Basel) (2021) 12(7):1050. doi: 10.3390/genes12071050
17. Bhaskaran SP, Huang T, Rajendran BK, Guo M, Luo J, Qin Z, et al. Ethnic-specific Brca1/2 variation within Asia population: Evidence from over 78 000 cancer and 40 000 non-cancer cases of Indian, Chinese, Korean and Japanese populations. J Med Genet (2021) 58(11):752–9. doi: 10.1136/jmedgenet-2020-107299
18. Fernandez-Lopez JC, Romero-Cordoba S, Rebollar-Vega R, Alfaro-Ruiz LA, Jimenez-Morales S, Beltran-Anaya F, et al. Population and breast cancer patients' analysis reveals the diversity of genomic variation of the brca genes in the Mexican population. Hum Genomics (2019) 13(1):3. doi: 10.1186/s40246-018-0188-9
19. Gabai-Kapara E, Lahad A, Kaufman B, Friedman E, Segev S, Renbaum P, et al. Population-based screening for breast and ovarian cancer risk due to Brca1 and Brca2. Proc Natl Acad Sci U.S.A. (2014) 111(39):14205–10. doi: 10.1073/pnas.1415979111
20. Manickam K, Buchanan AH, Schwartz MLB, Hallquist MLG, Williams JL, Rahm AK, et al. Exome sequencing-based screening for Brca1/2 expected pathogenic variants among adult biobank participants. JAMA Netw Open (2018) 1(5):e182140. doi: 10.1001/jamanetworkopen.2018.2140
21. Momozawa Y, Iwasaki Y, Parsons MT, Kamatani Y, Takahashi A, Tamura C, et al. Germline pathogenic variants of 11 breast cancer genes in 7,051 Japanese patients and 11,241 controls. Nat Commun (2018) 9(1):4083. doi: 10.1038/s41467-018-06581-8
22. Maxwell KN, Domchek SM, Nathanson KL, Robson ME. Population frequency of germline Brca1/2 mutations. J Clin Oncol (2016) 34(34):4183–5. doi: 10.1200/JCO.2016.67.0554
23. Zhang J, Sun J, Chen J, Yao L, Ouyang T, Li J, et al. Comprehensive analysis of Brca1 and Brca2 germline mutations in a Large cohort of 5931 Chinese women with breast cancer. Breast Cancer Res Treat (2016) 158(3):455–62. doi: 10.1007/s10549-016-3902-0
24. Bhaskaran SP, Chandratre K, Gupta H, Zhang L, Wang X, Cui J, et al. Germline variation in Brca1/2 is highly ethnic-specific: Evidence from over 30,000 Chinese hereditary breast and ovarian cancer patients. Int J Cancer (2019) 145(4):962–73. doi: 10.1002/ijc.32176
25. Meng H, Yao L, Yuan H, Xu Y, Ouyang T, Li J, et al. Brca1 C.5470_5477del, a founder mutation in Chinese han breast cancer patients. Int J Cancer (2020) 146(11):3044–52. doi: 10.1002/ijc.32877
26. Li J, Han S, Zhang C, Luo Y, Wang L, Wang P, et al. Identification of Brca1:C.5470_5477del as a founder mutation in Chinese ovarian cancer patients. Front Oncol (2021) 11:655709. doi: 10.3389/fonc.2021.655709
27. Li A, Xie R, Zhi Q, Deng Y, Wu Y, Li W, et al. Brca germline mutations in an unselected nationwide cohort of Chinese patients with ovarian cancer and healthy controls. Gynecol Oncol (2018) 151(1):145–52. doi: 10.1016/j.ygyno.2018.07.024
28. Bu H, Chen J, Li Q, Hou J, Wei Y, Yang X, et al. Brca mutation frequency and clinical features of ovarian cancer patients: A report from a Chinese study group. J Obstet Gynaecol Res (2019) 45(11):2267–74. doi: 10.1111/jog.14090
29. Lang GT, Shi JX, Hu X, Zhang CH, Shan L, Song CG, et al. The spectrum of brca mutations and characteristics of brca-associated breast cancers in China: Screening of 2,991 patients and 1,043 controls by next-generation sequencing. Int J Cancer (2017) 141(1):129–42. doi: 10.1002/ijc.30692
30. Shao D, Cheng S, Guo F, Zhu C, Yuan Y, Hu K, et al. Prevalence of hereditary breast and ovarian cancer (Hboc) predisposition gene mutations among 882 hboc high-risk Chinese individuals. Cancer Sci (2020) 111(2):647–57. doi: 10.1111/cas.14242
31. Dong L, Zhang H, Zhang H, Ye Y, Cheng Y, Li L, et al. The mutation landscape of multiple cancer predisposition genes in Chinese Familial/Hereditary breast cancer families. Cancer Biol Med (2021) 19(6):850–70. doi: 10.20892/j.issn.2095-3941.2021.0011
32. Cao WM, Gao Y, Yang HJ, Xie SN, Ding XW, Pan ZW, et al. Novel germline mutations and unclassified variants of Brca1 and Brca2 genes in Chinese women with familial Breast/Ovarian cancer. BMC Cancer (2016) 16:64. doi: 10.1186/s12885-016-2107-6
33. Sun J, Meng H, Yao L, Lv M, Bai J, Zhang J, et al. Germline mutations in cancer susceptibility genes in a Large series of unselected breast cancer patients. Clin Cancer Res (2017) 23(20):6113–9. doi: 10.1158/1078-0432.CCR-16-3227
34. Deng M, Chen HH, Zhu X, Luo M, Zhang K, Xu CJ, et al. Prevalence and clinical outcomes of germline mutations in Brca1/2 and Palb2 genes in 2769 unselected breast cancer patients in China. Int J Cancer (2019) 145(6):1517–28. doi: 10.1002/ijc.32184
35. Chen B, Zhang G, Li X, Ren C, Wang Y, Li K, et al. Comparison of brca versus non-brca germline mutations and associated somatic mutation profiles in patients with unselected breast cancer. Aging (Albany NY) (2020) 12(4):3140–55. doi: 10.18632/aging.102783
36. Wang Q, Wu H, Lan Y, Zhang J, Wu J, Zhang Y, et al. Changing patterns in clinicopathological characteristics of breast cancer and prevalence of brca mutations: Analysis in a rural area of southern China. Int J Gen Med (2021) 14:7371–80. doi: 10.2147/IJGM.S333858
37. Li G, Guo X, Tang L, Chen M, Luo X, Peng L, et al. Analysis of Brca1/2 mutation spectrum and prevalence in unselected Chinese breast cancer patients by next-generation sequencing. J Cancer Res Clin Oncol (2017) 143(10):2011–24. doi: 10.1007/s00432-017-2465-8
38. Wang J, Li W, Shi Y, Huang Y, Sun T, Tang L, et al. Germline mutation landscape of Chinese patients with familial Breast/Ovarian cancer in a panel of 22 susceptibility genes. Cancer Med (2019) 8(5):2074–84. doi: 10.1002/cam4.2093
39. Li JY, Jing R, Wei H, Wang M, Xiaowei Q, Liu H, et al. Germline mutations in 40 cancer susceptibility genes among Chinese patients with high hereditary risk breast cancer. Int J Cancer (2019) 144(2):281–9. doi: 10.1002/ijc.31601
40. Wang YA, Jian JW, Hung CF, Peng HP, Yang CF, Cheng HS, et al. Germline breast cancer susceptibility gene mutations and breast cancer outcomes. BMC Cancer (2018) 18(1):315. doi: 10.1186/s12885-018-4229-5
41. Kwong A, Ho JCW, Shin VY, Kurian AW, Tai E, Esserman LJ, et al. Rapid detection of Brca1/2 recurrent mutations in Chinese breast and ovarian cancer patients with multiplex snapshot genotyping panels. Oncotarget (2018) 9(8):7832–43. doi: 10.18632/oncotarget.23471
42. Wei H, Wang M, Ou J, Jiang W, Tian F, Sheng Y, et al. Multicenter cross-sectional screening of the brca gene for Chinese high hereditary risk breast cancer populations. Oncol Lett (2018) 15(6):9420–8. doi: 10.3892/ol.2018.8538
43. Ye F, He M, Huang L, Lang G, Hu X, Shao Z, et al. Insights into the impacts of brca mutations on clinicopathology and management of early-onset triple-negative breast cancer. Front Oncol (2020) 10:574813. doi: 10.3389/fonc.2020.574813
44. Chen L, Fu F, Huang M, Lv J, Zhang W, Wang C. The spectrum of Brca1 and Brca2 mutations and clinicopathological characteristics in Chinese women with early-onset breast cancer. Breast Cancer Res Treat (2020) 180(3):759–66. doi: 10.1007/s10549-020-05573-x
45. Shen M, Yang L, Lei T, Xiao L, Li L, Zhang P, et al. Brca1/2 mutation spectrum in Chinese early-onset breast cancer. Transl Cancer Res (2019) 8(2):483–90. doi: 10.21037/tcr.2019.03.02
46. Ma D, Chen SY, Ren JX, Pei YC, Jiang CW, Zhao S, et al. Molecular features and functional implications of germline variants in triple-negative breast cancer. J Natl Cancer Inst (2021) 113(7):884–92. doi: 10.1093/jnci/djaa175
47. Ji G, Bao L, Yao Q, Zhang J, Zhu X, Bai Q, et al. Germline and tumor Brca1/2 pathogenic variants in Chinese triple-negative breast carcinomas. J Cancer Res Clin Oncol (2021) 147(10):2935–44. doi: 10.1007/s00432-021-03696-2
48. Liang Y, Yang X, Li H, Zhu A, Guo Z, Li M. Prevalence and spectrum of Brca1/2 germline mutations in women with breast cancer in China based on next-generation sequencing. Med Sci Monit (2018) 24:2465–75. doi: 10.12659/msm.905812
49. Li W, Shao D, Li L, Wu M, Ma S, Tan X, et al. Germline and somatic mutations of multi-gene panel in Chinese patients with epithelial ovarian cancer: A prospective cohort study. J Ovarian Res (2019) 12(1):80. doi: 10.1186/s13048-019-0560-y
50. Wu X, Wu L, Kong B, Liu J, Yin R, Wen H, et al. The first nationwide multicenter prevalence study of germline Brca1 and Brca2 mutations in Chinese ovarian cancer patients. Int J Gynecol Cancer (2017) 27(8):1650–7. doi: 10.1097/IGC.0000000000001065
51. You Y, Li L, Lu J, Wu H, Wang J, Gao J, et al. Germline and somatic Brca1/2 mutations in 172 Chinese women with epithelial ovarian cancer. Front Oncol (2020) 10:295. doi: 10.3389/fonc.2020.00295
52. Luo Y, Wu H, Huang Q, Rao H, Yu Z, Zhong Z. The features of Brca1 and Brca2 germline mutations in hakka ovarian cancer patients: Brca1 C.536 a>T maybe a founder mutation in this population. Int J Gen Med (2022) 15:2773–86. doi: 10.2147/IJGM.S355755
53. Ji G, Yao Q, Bao L, Zhang J, Bai Q, Zhu X, et al. Germline and tumor Brca1/2 mutations in Chinese high grade serous ovarian cancer patients. Ann Transl Med (2021) 9(6):453. doi: 10.21037/atm-20-6827
54. Shi T, Wang P, Xie C, Yin S, Shi D, Wei C, et al. Brca1 and Brca2 mutations in ovarian cancer patients from China: Ethnic-related mutations in Brca1 associated with an increased risk of ovarian cancer. Int J Cancer (2017) 140(9):2051–9. doi: 10.1002/ijc.30633
55. Wu X, Chen Z, Ren P, Zhao X, Tang D, Geng H, et al. Identifying sequence variants of 18 hereditary ovarian cancer-associated genes in Chinese epithelial ovarian cancer patients. BioMed Res Int (2021) 2021:5579543. doi: 10.1155/2021/5579543
56. Zhao Q, Yang J, Li L, Cao D, Yu M, Shen K, et al. Germline and somatic mutations in homologous recombination genes among Chinese ovarian cancer patients detected using next-generation sequencing. J Gynecol Oncol (2017) 28(4):e39. doi: 10.3802/jgo.2017.28.e39
57. Zhang X, Mao T, Zhang B, Xu H, Cui J, Jiao F, et al. Characterization of DNA damage response deficiency in pancreatic cancer patients from China. Cancer Commun (Lond) (2022) 42(1):70–4. doi: 10.1002/cac2.12238
58. Zhan Q, Wen C, Zhao Y, Fang L, Jin Y, Zhang Z, et al. Identification of copy number variation-driven molecular subtypes informative for prognosis and treatment in pancreatic adenocarcinoma of a Chinese cohort. EBioMedicine (2021) 74:103716. doi: 10.1016/j.ebiom.2021.103716
59. Shui L, Li X, Peng Y, Tian J, Li S, He D, et al. The Germline/Somatic DNA damage repair gene mutations modulate the therapeutic response in Chinese patients with advanced pancreatic ductal adenocarcinoma. J Transl Med (2021) 19(1):301. doi: 10.1186/s12967-021-02972-6
60. Zhu Y, Wei Y, Zeng H, Li Y, Ng CF, Zhou F, et al. Inherited mutations in Chinese men with prostate cancer. J Natl Compr Canc Netw (2021) 20(1):54–62. doi: 10.6004/jnccn.2021.7010
61. Wei Y, Wu J, Gu W, Qin X, Dai B, Lin G, et al. Germline DNA repair gene mutation landscape in Chinese prostate cancer patients. Eur Urol (2019) 76(3):280–3. doi: 10.1016/j.eururo.2019.06.004
62. Wu J, Wei Y, Pan J, Jin S, Gu W, Gan H, et al. Prevalence of comprehensive DNA damage repair gene germline mutations in Chinese prostate cancer patients. Int J Cancer (2021) 148(3):673–81. doi: 10.1002/ijc.33324
63. Liao H, Cai S, Bai Y, Zhang B, Sheng Y, Tong S, et al. Prevalence and spectrum of germline cancer susceptibility gene variants and somatic second hits in colorectal cancer. Am J Cancer Res (2021) 11(11):5571–80.
64. Lin J, Shi J, Guo H, Yang X, Jiang Y, Long J, et al. Alterations in DNA damage repair genes in primary liver cancer. Clin Cancer Res (2019) 25(15):4701–11. doi: 10.1158/1078-0432.CCR-19-0127
65. Sun L, Liang B, Zhu L, Shen Y, He L. The rise of the genetic counseling profession in China. Am J Med Genet C Semin Med Genet (2019) 181(2):170–6. doi: 10.1002/ajmg.c.31693
66. Mavaddat N, Peock S, Frost D, Ellis S, Platte R, Fineberg E, et al. Cancer risks for Brca1 and Brca2 mutation carriers: Results from prospective analysis of embrace. J Natl Cancer Inst (2013) 105(11):812–22. doi: 10.1093/jnci/djt095
67. Rehder C, Bean LJH, Bick D, Chao E, Chung W, Das S, et al. Next-generation sequencing for constitutional variants in the clinical laboratory, 2021 revision: A technical standard of the American college of medical genetics and genomics (Acmg). Genet Med (2021) 23(8):1399–415. doi: 10.1038/s41436-021-01139-4
68. Xie F, Wang S. Clinical practice guideline of Brca1/2 testing for patients with breast cancer: Chinese society of breast surgery (Csbrs) practice guideline 2021. Chin Med J (Engl) (2021) 134(13):1516–8. doi: 10.1097/CM9.0000000000001587
69. International Medical society CA-cA. [Consensus of Chinese experts on hot isssues in gnetictesting of advanced breast cancer (2021 edition)]. Chin J Oncol (2021) 44(1):8. doi: 10.3760/cma.j.cn112152-20211111-00837
70. Genitourinary Cancer Committee CA-CA. [Chinese expert consensus on genomic testing of prostate cancer patients (the 2020 edition)]. China Oncol (2020) 30(7):10. doi: 10.19401/j.cnki.1007-3639.2020.07.011
71. Cao X, Zeng X, Chen C, Chen J, Cheng J, Cui S, et al. Chinese Medical doctor association CSoPM, breast cancer committee. [Expert consensus on Brca1/2 gene testing and clinical application in Chinese breast cancer patients (2018 edition)]. China Oncol (2018) 28(10):14. doi: 10.19401/j.cnki.1007-3639.2018.10.011
72. China Anti-Cancer Association GCC. [Guidelines for the diagnosis and treatment of ovarian malignancies (4th edition)]. ZHONGGUO SHIYONG FUKE YU CHANKE ZAZHI (2018) 34(7):11. doi: 10.19538/j.fk2018070110
73. Lincoln SE, Truty R, Lin CF, Zook JM, Paul J, Ramey VH, et al. A rigorous interlaboratory examination of the need to confirm next-generation sequencing-detected variants with an orthogonal method in clinical genetic testing. J Mol Diagn (2019) 21(2):318–29. doi: 10.1016/j.jmoldx.2018.10.009
74. Kang P, Mariapun S, Phuah SY, Lim LS, Liu J, Yoon SY, et al. Large Brca1 and Brca2 genomic rearrangements in Malaysian high risk breast-ovarian cancer families. Breast Cancer Res Treat (2010) 124(2):579–84. doi: 10.1007/s10549-010-1018-5
75. Bozsik A, Pocza T, Papp J, Vaszko T, Butz H, Patocs A, et al. Complex characterization of germline Large genomic rearrangements of the Brca1 and Brca2 genes in high-risk breast cancer patients-novel variants from a Large national center. Int J Mol Sci (2020) 21(13):4650. doi: 10.3390/ijms21134650
76. van der Merwe NC, Oosthuizen J, Theron M, Chong G, Foulkes WD. The contribution of Large genomic rearrangements in Brca1 and Brca2 to south African familial breast cancer. BMC Cancer (2020) 20(1):391. doi: 10.1186/s12885-020-06917-y
77. Hogervorst FB, Nederlof PM, Gille JJ, McElgunn CJ, Grippeling M, Pruntel R, et al. Large Genomic deletions and duplications in the Brca1 gene identified by a novel quantitative method. Cancer Res (2003) 63(7):1449–53.
78. Cao WM, Zheng YB, Gao Y, Ding XW, Sun Y, Huang Y, et al. Comprehensive mutation detection of Brca1/2 genes reveals Large genomic rearrangements contribute to hereditary breast and ovarian cancer in Chinese women. BMC Cancer (2019) 19(1):551. doi: 10.1186/s12885-019-5765-3
79. Wong RSJ, Lee SC. Brca sequencing of tumors: Understanding its implications in the oncology community. Chin Clin Oncol (2020) 9(5):66. doi: 10.21037/cco-19-198
80. Li D, Shi Y, Li A, Cao D, Su H, Yang H, et al. Retrospective reinterpretation and reclassification of Brca1/2 variants from Chinese population. Breast Cancer (2020) 27(6):1158–67. doi: 10.1007/s12282-020-01119-7
81. Zhang B, Wang Z, Chen G, Zhou X, Wu H, Meng H, et al. Chinese Society of pathology CPQCC. [Chinese expert consensus on Brca1/2 variant Interpretation(2021 version)]. Zhonghua Bing Li Xue Za Zhi (2021) 50(6):565–71. doi: 10.3760/cma.j.cn112151-20201027-00809
82. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, et al. Standards and guidelines for the interpretation of sequence variants: A joint consensus recommendation of the American college of medical genetics and genomics and the association for molecular pathology. Genet Med (2015) 17(5):405–24. doi: 10.1038/gim.2015.30
83. Qu S, Chen Q, Yi Y, Shao K, Zhang W, Wang Y, et al. A reference system for brca mutation detection based on next-generation sequencing in the Chinese population. J Mol Diagn (2019) 21(4):677–86. doi: 10.1016/j.jmoldx.2019.03.003
84. Hemminki K, Sundquist K, Sundquist J, Forsti A, Hemminki A, Li X. Familial risks and proportions describing population landscape of familial cancer. Cancers (Basel) (2021) 13(17):4385. doi: 10.3390/cancers13174385
85. Sun L, Cui B, Wei X, Sadique Z, Yang L, Manchanda R, et al. Cost-effectiveness of genetic testing for all women diagnosed with breast cancer in China. Cancers (Basel) (2022) 14(7):1839. doi: 10.3390/cancers14071839
86. Manchanda R, Sun L, Patel S, Evans O, Wilschut J, De Freitas Lopes AC, et al. Economic evaluation of population-based Brca1/Brca2 mutation testing across multiple countries and health systems. Cancers (Basel) (2020) 12(7):1929. doi: 10.3390/cancers12071929
87. Daly MB, Pal T, Berry MP, Buys SS, Dickson P, Domchek SM, et al. Genetic/Familial high-risk assessment: Breast, ovarian, and pancreatic, version 2.2021, nccn clinical practice guidelines in oncology. J Natl Compr Canc Netw (2021) 19(1):77–102. doi: 10.6004/jnccn.2021.0001
88. Paluch-Shimon S, Cardoso F, Sessa C, Balmana J, Cardoso MJ, Gilbert F, et al. Prevention and screening in brca mutation carriers and other Breast/Ovarian hereditary cancer syndromes: Esmo clinical practice guidelines for cancer prevention and screening. Ann Oncol (2016) 27(suppl 5):v103–v10. doi: 10.1093/annonc/mdw327
89. Hung FH, Wang YA, Jian JW, Peng HP, Hsieh LL, Hung CF, et al. Evaluating brca mutation risk predictive models in a Chinese cohort in Taiwan. Sci Rep (2019) 9(1):10229. doi: 10.1038/s41598-019-46707-6
90. Cheng X, Gu Z, Sun X, Zhuang Z. Study on the differences of opinions and choices of high-risk breast cancer populations in China before and after genetic testing. Transl Cancer Res (2019) 8(8):2893–905. doi: 10.21037/tcr.2019.11.43
91. Xue Y, Shi Y, Xu Y, Xu Z, Chen X, Wang C. Questionnaire survey on status quo of genetic counseling on gynecologic tumors. Acad J Second Military Med Univ (2021) 42(6):10. doi: 10.16781/j.0258-879x.2021.06.0641
92. Reid S, Spalluto LB, Lang K, Weidner A, Pal T. An overview of genetic services delivery for hereditary breast cancer. Breast Cancer Res Treat (2022) 191(3):491–500. doi: 10.1007/s10549-021-06478-z
93. Yoon SY, Wong SW, Lim J, Ahmad S, Mariapun S, Padmanabhan H, et al. Oncologist-led brca counselling improves access to cancer genetic testing in middle-income Asian country, with no significant impact on psychosocial outcomes. J Med Genet (2022) 59(3):220–9. doi: 10.1136/jmedgenet-2020-107416
94. Kwong A, Chu AT, Wu CT, Tse DM. Attitudes and compliance of clinical management after genetic testing for hereditary breast and ovarian cancer among high-risk southern Chinese females with breast cancer history. Fam Cancer (2014) 13(3):423–30. doi: 10.1007/s10689-014-9706-7
95. Wang Y, Li Y, Song Y, Chen C, Wang Z, Li L, et al. Comparison of ultrasound and mammography for early diagnosis of breast cancer among Chinese women with suspected breast lesions: A prospective trial. Thorac Cancer (2022). doi: 10.1111/1759-7714.14666
96. Bao W, Chen W, Du L, Gu F, Guo L, Han J, et al. Consulting group of China guideline for the screening and early diagnosis and treatment of female breast cancer. [China guideline for the screening and early detection of female breast cancer (2021,Beijing)]. China Cancer (2021) 30(3):31. doi: 10.11735/j.issn.1004-0242.2021.03.A001
97. Sardanelli F, Podo F, D'Agnolo G, Verdecchia A, Santaquilani M, Musumeci R, et al. Multicenter comparative multimodality surveillance of women at genetic-familial high risk for breast cancer (Hibcrit study): Interim results. Radiology (2007) 242(3):698–715. doi: 10.1148/radiol.2423051965
98. Riedl CC, Ponhold L, Flory D, Weber M, Kroiss R, Wagner T, et al. Magnetic resonance imaging of the breast improves detection of invasive cancer, preinvasive cancer, and premalignant lesions during surveillance of women at high risk for breast cancer. Clin Cancer Res (2007) 13(20):6144–52. doi: 10.1158/1078-0432.CCR-07-1270
99. Kuhl CK, Schrading S, Leutner CC, Morakkabati-Spitz N, Wardelmann E, Fimmers R, et al. Mammography, breast ultrasound, and magnetic resonance imaging for surveillance of women at high familial risk for breast cancer. J Clin Oncol (2005) 23(33):8469–76. doi: 10.1200/JCO.2004.00.4960
100. Segal N, Ber Y, Benjaminov O, Tamir S, Yakimov M, Kedar I, et al. Imaging-based prostate cancer screening among brca mutation carriers-results from the first round of screening. Ann Oncol (2020) 31(11):1545–52. doi: 10.1016/j.annonc.2020.06.025
101. Kim YC, Zhao L, Zhang H, Huang Y, Cui J, Xiao F, et al. Prevalence and spectrum of brca germline variants in mainland Chinese familial breast and ovarian cancer patients. Oncotarget (2016) 7(8):9600–12. doi: 10.18632/oncotarget.7144
102. Kwong A, Wong CH, Shea C, Suen DT, Choi CL. Choice of management of southern Chinese brca mutation carriers. World J Surg (2010) 34(7):1416–26. doi: 10.1007/s00268-010-0477-5
103. Cao A, Huang L, Shao Z. The preventive intervention of hereditary breast cancer. Adv Exp Med Biol (2017) 1026:41–57. doi: 10.1007/978-981-10-6020-5_3
104. Cheng A, Li L, Wu M, Lang J. Pathological findings following risk-reducing salpingo-oophorectomy in brca mutation carriers: A systematic review and meta-analysis. Eur J Surg Oncol (2020) 46(1):139–47. doi: 10.1016/j.ejso.2019.09.002
105. Zhang D, Fu F, Xie L, Chu F, Wan Q, Xie Y. Clinical practice of prophylactic nipple- sparing mastectomy and immediate recon struction in Chinese healthy women with Brca1/Brca2 germline mutation. Chin J Clin Oncol (2020) 47(1):5. doi: 10.3969/j.issn.1000-8179.2020.01.429
106. Su L, Xu Y, Ouyang T, Li J, Wang T, Fan Z, et al. Contralateral breast cancer risk in Brca1 and Brca2 mutation carriers in a Large cohort of unselected Chinese breast cancer patients. Int J Cancer (2020) 146(12):3335–42. doi: 10.1002/ijc.32918
107. Rebbeck TR, Kauff ND, Domchek SM. Meta-analysis of risk reduction estimates associated with risk-reducing salpingo-oophorectomy in Brca1 or Brca2 mutation carriers. J Natl Cancer Inst (2009) 101(2):80–7. doi: 10.1093/jnci/djn442
108. Wattenberg MM, Asch D, Yu S, O'Dwyer PJ, Domchek SM, Nathanson KL, et al. Platinum response characteristics of patients with pancreatic ductal adenocarcinoma and a germline Brca1, Brca2 or Palb2 mutation. Br J Cancer (2020) 122(3):333–9. doi: 10.1038/s41416-019-0582-7
109. Byrski T, Dent R, Blecharz P, Foszczynska-Kloda M, Gronwald J, Huzarski T, et al. Results of a phase ii open-label, non-randomized trial of cisplatin chemotherapy in patients with Brca1-positive metastatic breast cancer. Breast Cancer Res (2012) 14(4):R110. doi: 10.1186/bcr3231
110. Tutt A, Tovey H, Cheang MCU, Kernaghan S, Kilburn L, Gazinska P, et al. Carboplatin in Brca1/2-mutated and triple-negative breast cancer brcaness subgroups: The tnt trial. Nat Med (2018) 24(5):628–37. doi: 10.1038/s41591-018-0009-7
111. Tung NM, Zakalik D, Somerfield MR. Hereditary breast cancer guideline expert p. adjuvant parp inhibitors in patients with high-risk early-stage Her2-negative breast cancer and germline brca mutations: Asco hereditary breast cancer guideline rapid recommendation update. J Clin Oncol (2021) 39(26):2959–61. doi: 10.1200/JCO.21.01532
112. Curtin NJ, Szabo C. Poly(Adp-ribose) polymerase inhibition: Past, present and future. Nat Rev Drug Discovery (2020) 19(10):711–36. doi: 10.1038/s41573-020-0076-6
113. Tutt ANJ, Garber JE, Kaufman B, Viale G, Fumagalli D, Rastogi P, et al. Adjuvant olaparib for patients with Brca1- or Brca2-mutated breast cancer. N Engl J Med (2021) 384(25):2394–405. doi: 10.1056/NEJMoa2105215
114. Robson M, Im SA, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for metastatic breast cancer in patients with a germline brca mutation. N Engl J Med (2017) 377(6):523–33. doi: 10.1056/NEJMoa1706450
115. Litton JK, Hurvitz SA, Mina LA, Rugo HS, Lee KH, Goncalves A, et al. Talazoparib versus chemotherapy in patients with germline Brca1/2-mutated Her2-negative advanced breast cancer: Final overall survival results from the embraca trial. Ann Oncol (2020) 31(11):1526–35. doi: 10.1016/j.annonc.2020.08.2098
116. Pujade-Lauraine E, Ledermann JA, Selle F, Gebski V, Penson RT, Oza AM, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a Brca1/2 mutation (Solo2/Engot-Ov21): A double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol (2017) 18(9):1274–84. doi: 10.1016/S1470-2045(17)30469-2
117. Moore K, Colombo N, Scambia G, Kim BG, Oaknin A, Friedlander M, et al. Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med (2018) 379(26):2495–505. doi: 10.1056/NEJMoa1810858
118. Ray-Coquard I, Pautier P, Pignata S, Perol D, Gonzalez-Martin A, Berger R, et al. Olaparib plus bevacizumab as first-line maintenance in ovarian cancer. N Engl J Med (2019) 381(25):2416–28. doi: 10.1056/NEJMoa1911361
119. Gonzalez-Martin A, Pothuri B, Vergote I, DePont Christensen R, Graybill W, Mirza MR, et al. Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med (2019) 381(25):2391–402. doi: 10.1056/NEJMoa1910962
120. Del Campo JM, Matulonis UA, Malander S, Provencher D, Mahner S, Follana P, et al. Niraparib maintenance therapy in patients with recurrent ovarian cancer after a partial response to the last platinum-based chemotherapy in the engot-Ov16/Nova trial. J Clin Oncol (2019) 37(32):2968–73. doi: 10.1200/JCO.18.02238
121. de Bono J, Mateo J, Fizazi K, Saad F, Shore N, Sandhu S, et al. Olaparib for metastatic castration-resistant prostate cancer. N Engl J Med (2020) 382(22):2091–102. doi: 10.1056/NEJMoa1911440
122. Smith SG, Sestak I, Howell A, Forbes J. Cuzick j. participant-reported symptoms and their effect on long-term adherence in the international breast cancer intervention study I (Ibis I). J Clin Oncol (2017) 35(23):2666–73. doi: 10.1200/JCO.2016.71.7439
123. Park J, Huang D, Chang YJ, Lim MC, Myung SK. Oral contraceptives and risk of breast cancer and ovarian cancer in women with a Brca1 or Brca2 mutation: A meta-analysis of observational studies. Carcinogenesis (2022) 43(3):231–42. doi: 10.1093/carcin/bgab107
124. King MC, Levy-Lahad E, Lahad A. Population-based screening for Brca1 and Brca2: 2014 lasker award. JAMA (2014) 312(11):1091–2. doi: 10.1001/jama.2014.12483
Keywords: population screening, BRCA, germline mutation, China, familial risk
Citation: Lei H, Zhang M, Zhang L, Hemminki K, Wang X-j and Chen T (2022) Overview on population screening for carriers with germline BRCA mutation in China. Front. Oncol. 12:1002360. doi: 10.3389/fonc.2022.1002360
Received: 25 July 2022; Accepted: 24 October 2022;
Published: 09 November 2022.
Edited by:
Maria Rosaria De Miglio, University of Sassari, ItalyReviewed by:
Muhammad Asif, Balochistan University of Information Technology, Engineering and Management Sciences, PakistanCopyright © 2022 Lei, Zhang, Zhang, Hemminki, Wang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tianhui Chen, Y2hlbnRoQHpqY2Mub3JnLmNu; Xiao-jia Wang, d2FuZ3hqQHpqY2Mub3JnLmNu
†These authors have contributed equally to this work and share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.