- 1Department of Neurosurgery, First Hospital of Jilin University, Changchun, China
- 2Department of Geriatrics, Second Affiliated Hospital of Harbin Medical University, Harbin, China
- 3Department of Emergency Medicine, Binzhou People’s Hospital, Binzhou, China
- 4Department of Clinical Laboratory, Second Hospital of Jilin University, Changchun, China
Microfibrillar-associated protein 2 (MFAP2), a component of the extracellular matrix, is important in controlling growth factor signal transduction. Recent studies have shown that MFAP2, an effective prognostic molecule for various tumors, is associated with tumor occurrence and development and may be involved in remodeling the extracellular matrix and regulating proliferation, apoptosis, invasion, tumor cell metastasis, and tumor angiogenesis. However, MFAP2’s specific mechanism in these tumor processes remains unclear. This article reviewed the possible mechanism of MFAP2 in tumorigenesis and progression and provided a reference for the clinical prognosis of patients with cancer and new therapeutic target discovery.
Introduction
The extracellular matrix (ECM) is a highly dynamic, non-cellular network composed of collagen, proteoglycans, glycosaminoglycans, elastin, fibronectin, laminins, and other glycoproteins (1, 2). ECM components are produced intracellularly by cells and released into the ECM through extracellular secretion. In normal and tumor tissues, ECM components and cell adhesion receptors bind to one another to form complex cell scaffolds for cell residence. In addition, ECM is the reservoir and binding site of bioactive molecules. Cell surface receptors transmit signals from the ECM to cells, regulating various cellular functions, including survival, growth, migration, differentiation, and immunity, for maintaining normal homeostasis (3–5). The ECM promotes cancer cell growth, survival, and invasion and modifies fibroblast and immune cell behavior to drive metastasis and impair treatment (6, 7). One study showed that metastatic liver cells promote mesenchymal cell transformation to the epithelium by regulating extracellular matrix citrullination and promoting liver metastasis progression (8). Due to its pleiotropism, the ECM can influence the fate of cells through various mechanisms (9, 10). Cells can dynamically regulate ECM remodeling in several ways, including chemical modification, composition, degradation, and reorganization (11, 12). Therefore, tumor cells interact with ECM to regulate tumor occurrence and reversal.
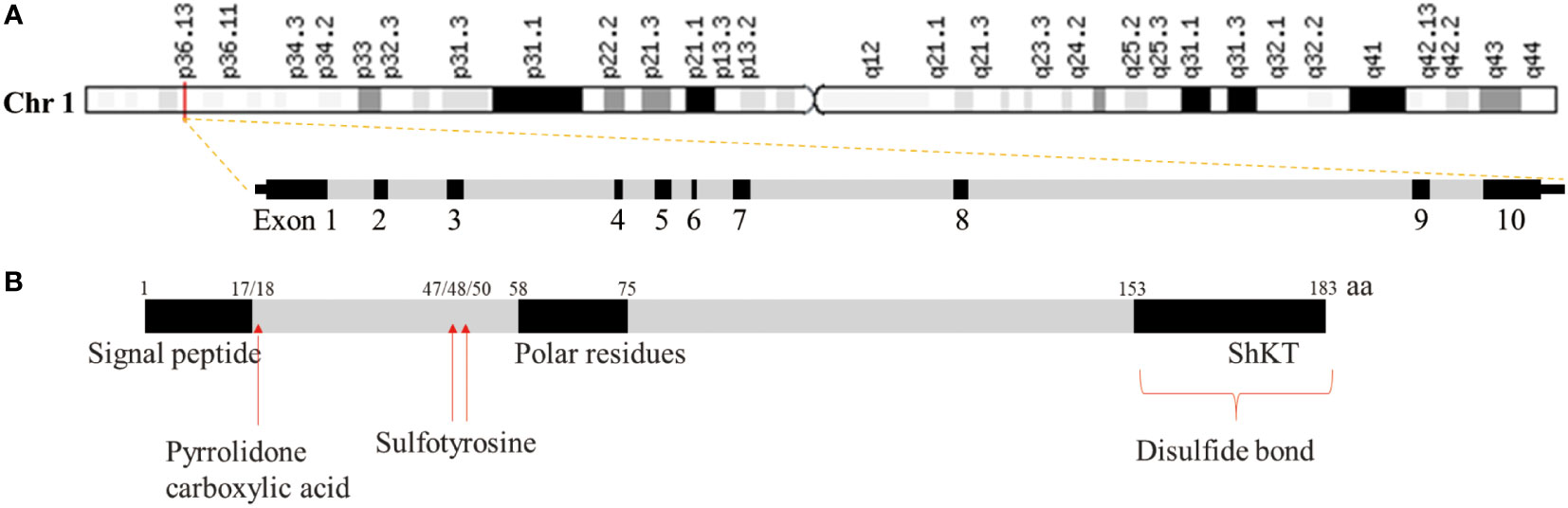
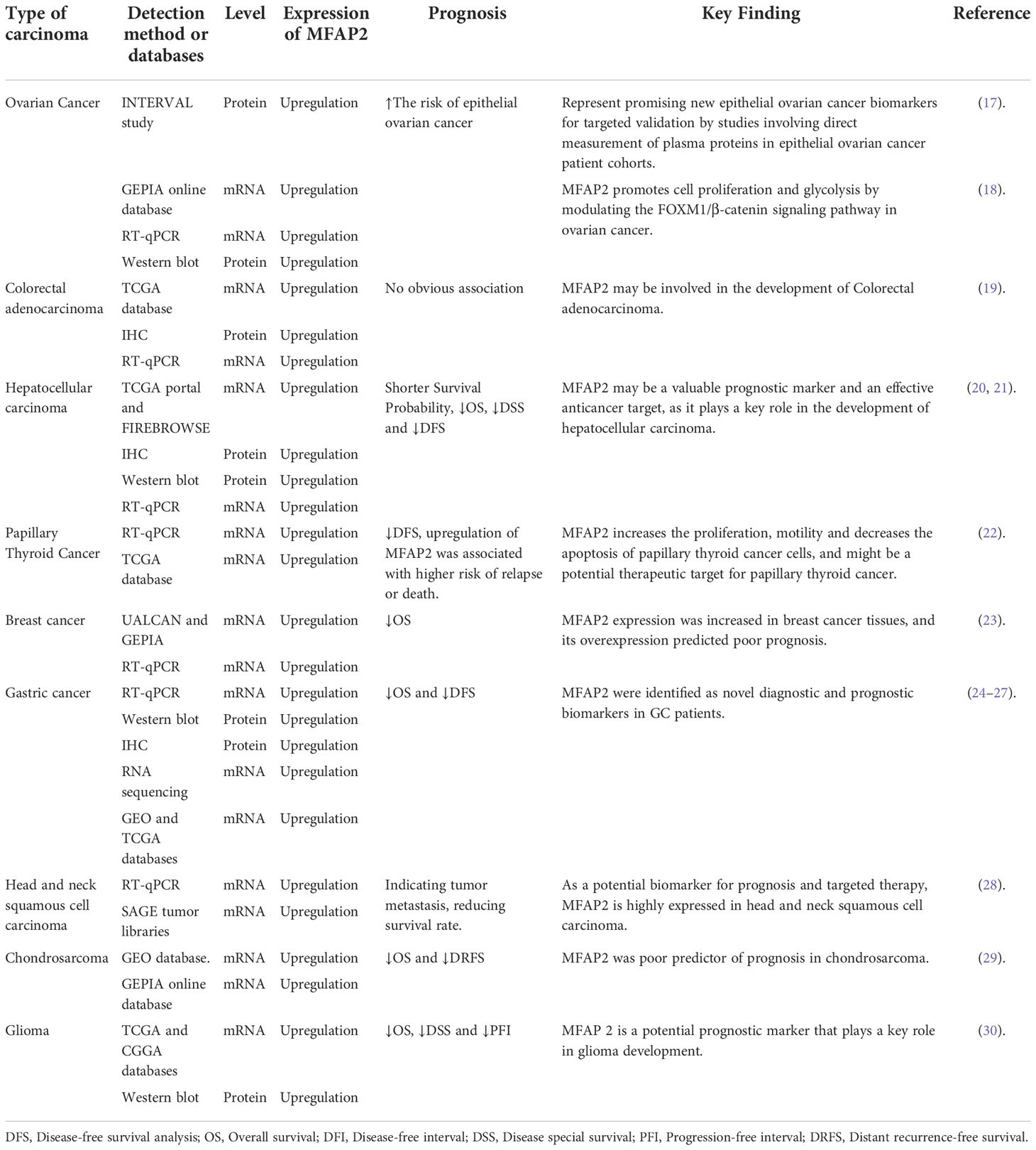
Microfiber-associated proteins (MFAPs) are an extracellular matrix glycoprotein group, which are extracellular matrix microfibril components involved in microfiber-assembly elastin production and tissue environmental stability (13). MFAPs include five subfamily members (MFAP1–5), among which microfibrillar-associated protein 2 (MFAP2) was the first to be characterized, playing an important role in controlling growth factor signal transduction (14). The human MFAP2 gene (GenBank: GC01M016974) is located in chr1:16974502-16981583 (GRCh38/HG38), containing 10 exons with 7,082 base length and a minus-strand orientation (Figure 1A). Through MFAP2 transcription regulation study in muscle cells, it was found that MFAP2 was transcribed from the main transcription start site embedded in CpG islands. A 5’flanking sequence region between nucleotides -339 and -109 is the basic MFAP2 promoter. The -256/-270 KLF sequence motifs, an E-box at -222/-229, and a GC-box at -117/-125 are crucial for promoter function. KLF motifs mediate GKLF/KLF4 binding, whereas E-box is the target of upstream stimulatory factors 1 and 2, with the GC box forming complexes with Sp1 and Sp3 (15). The MFAP2 protein molecular structure analysis showed a signal sequence at 1–17 amino acid residues, a polar residue at 58–75, and an Shkt domain at 153–183 (Figure 1B), indicating that it had potential channel regulatory activity. There is a high affinity between the growth factor binding region near the MFAP2 N-terminus and the transforming growth factor-β (TGF-β) superfamily members; the mutual effect between MFAP2 and the active TGF-β form has biological consequences (16). Numerous studies have shown abnormal MFAP2 levels and its effects on prognosis in different cancers (Table 1), highlighting its significance in tumor progression, particularly during epithelial-mesenchymal transformation (EMT), because of its association with TGF-β (24, 31, 32). Changes in the MFAP2 level regulate ECM remodeling, playing an important role in tumorigenesis (32). In addition, MFAP2 is important in tumor cell apoptosis, proliferation, angiogenesis, invasion, and prognosis (18, 20, 23, 24).
Studies on the MFAP2 mechanism of action are expected to provide new strategies and novel therapeutic markers for cancer treatment. This review aimed to summarize MFAP2 expression in tumor cells and its role in tumor cell proliferation, apoptosis, invasion, and metastasis and discuss its possible signaling pathways to provide new ideas for tumor therapy.
The expression of MFAP2 in tumors
Qiu et al. (33) analyzed MFAP2 mRNA levels in different tumors and corresponding normal tissues using oncomine database. The results showed that compared with normal tissues, the expression of mfap2 was higher in bladder cancer, brain and central nervous system cancer, breast cancer, colorectal cancer, esophageal cancer, gastric cancer, head and neck cancer, lymphoma, melanoma, myeloma, ovarian cancer, pancreatic cancer and sarcoma. In order to further evaluate the expression of MFAP2 in human cancer, the integration of transcriptome data of all tumors in TCGA and GTEX showed that MFAP2 of Bladder urothelial carcinoma, Breast invasive carcinoma, Cholangiocarcinoma, Colon adenocarcinoma, Esophageal carcinoma, Head and neck squamous cell carcinoma, Liver hepatocellular carcinoma, Lung adenocarcinoma, Lung squamous cell carcinoma, Rectum adenocarcinoma, Stomach adenocarcinoma, Thyroid carcinoma and Uterine corpus endometrial carcinoma was significantly higher than that of adjacent normal tissues, and MFAP2 in Kidney chromophobe, Kidney renal clear cell carcinoma, Kidney renal papillary cell carcinoma, Prostate adenocarcinoma was significantly lower than that of adjacent normal tissues. There was no difference in MFAP2 expression in Pancreatic adenocarcinoma. In addition, the expression of MFAP2 protein increased in breast cancer, colon cancer, and lung adenocarcinoma, and decreased in clear cell renal cell carcinoma.
In addition to the MFAP2 expression database in various tumors, extensive in vivo and in vitro experimental data have further confirmed MFAP2 expression at the gene and protein levels in tumors. MFAP2 expression in Bladder Urothelial Carcinoma, Breast invasive carcinoma, and Head and neck squamous cell carcinoma was detected using quantitative polymerase chain reaction (qPCR). It was found that its expression in tumor tissues was significantly higher than that in normal tissues (33). The MFAP2 mRNA and protein levels in all ovarian cancer cell lines were much higher than those in non-tumor IOSE80 cell lines (18). In colorectal adenocarcinoma, the MFAP2 mRNA expression in tumor tissues was significantly higher than that in the adjacent non-tumor tissues. Immunohistochemical staining has shown that the MFAP2 protein expression in tumor tissues is significantly higher than that in normal tissues (19). In hepatocellular carcinoma, quantitative real-time PCR (RT-qPCR) analysis confirmed that MFAP2 mRNA expression was significantly upregulated in tumor tissues. Immunohistochemistry and western blotting results showed that MFAP2 protein expression in tumor tissues was significantly higher than that in adjacent normal tissues (20). Moreover, Zhu et al. (21) confirmed that upregulating MFAP2 mRNA levels in hepatocellular carcinoma was positively correlated with the TNM stage and tumor size using RT-qPCR. In obesity-associated colon cancer, the circulating concentration of MFAP2 and its gene expression in visceral adipose tissue decreased (32). Circulating (plasma) protein levels predicted by the MFAP2 gene were positively associated with the risk of all invasive epithelial ovarian cancers (17). In papillary thyroid carcinoma, whole-transcriptome sequencing was performed on tumor tissues and corresponding normal tissues adjacent to the carcinoma; the sequencing data were verified using RT-qPCR, confirming that MFAP2 was highly expressed in the tumor tissue (22). In breast cancer, Gong et al. (23) found that MFAP2 was upregulated in tumor tissues compared with that in normal tissues through bioinformatics analysis using online tools (UALCAN and GEPIA) further confirming the upregulation of MFAP2 expression in tumor tissues and cell lines using RT-qPCR. In gastric cancer, RT-qPCR, western blotting, and immunohistochemistry analysis confirmed that MFAP2 mRNA and protein expression levels in tumor tissues were significantly higher than those in the adjacent tissues. MFAP2 expression level in different gastric cancer cell lines is higher than that in gastric epithelial-derived cell lines (24, 25). In head and neck squamous cell carcinoma, the MFAP2 marker is expressed at higher levels (at least 13 times or more) in tumors than in normal tissues, as verified using RT-qPCR (28). In glioma, Our previous research shows that MFAP2 mRNA was upregulated in tumor tissues compared with that in normal tissues through bioinformatics analysis using TCGA and CGGA databases, and western blotting analysis confirmed that MFAP2 protein expression levels in tumor tissues were significantly higher than those in the adjacent tissues (30). The above data showed that MFAP2 was upregulated in many tumor tissues, it favors specific tumor type. However, the specific mechanism needs further study.
The carcinogenic effect of MFAP2 and its mechanisms
The roles of MFAP2 in tumor cell proliferation
Strict proliferation regulation is critical for development; unregulated cell proliferation is an essential hallmark of cancer (34–36). A study on hepatocellular carcinoma cells showed that MFAP2 was significantly overexpressed in hepatocellular carcinoma cells, correlating with the cancer stage. Regarding mechanism, MFAP2 was mainly involved in ATP formation; TP53 mutation interacted with MFAP2 to participate in the hepatocellular carcinoma cell occurrence, and MFAP2 knockdown inhibited hepatocellular carcinoma cell proliferation (21). MFAP2 modulates gastric cancer cell proliferation through integrin-stimulated focal adhesion kinase activation (25). A previous study showed that MFAP2 knockdown could inhibit breast cancer cell proliferation; MFAP2 restoration could significantly reverse the lcpat1 knockdown effect, suggesting that LCPAT1/MFAP2 signaling pathway may be involved in breast cancer progression (23). A papillary thyroid carcinoma study showed that MFAP2 downregulation inhibited papillary thyroid carcinoma cell proliferation (22). MFAP2 is a direct mir-423-5p target. mir-423-5p overexpression can downregulate MFAP2 protein expression and inhibit colon cancer cell proliferation (19). MFAP2 overexpression significantly increased ovarian cancer cell clones’ viability and number, whereas MFAP2 knockdown produced opposite results. Similarly, MFAP2 overexpression reduced G1 phase cell proportion and increased those of S and G2/M phase cells in ovarian cancer, suggesting that MFAP2 could stimulate G1-S phase cell transition. In contrast, MFAP2 knockdown induced significant arrest in the G0/G1 phase and decreased the S and G2/M phase cell proportion, indicating that cell cycle processes are affected (18). These data showed that MFAP2 promotes tumor cell proliferation through numerous mechanisms. The inhibition of MFAP2 may inhibit the proliferation of tumor cells, and then inhibit the progress of tumor, which plays an important role in tumor treatment.
MFAP2 is involved in tumor cell invasion and metastasis
Activating invasion and metastasis are the main cancer characteristics (34). Invasion is an initial and key step in metastasis. For invasion, tumor cells change their shape and connection with other cells and the ECM through epithelial-mesenchymal transformation (EMT). EMT is a dynamic process related to motility, invasion status acquisition, and cancer stem cell emergence (37, 38). Through EMT, tumor cells can be separated from the main tumor and invade the ECM, blood vessels, or lymphatic vessels as single cells. Therefore, EMT is involved in most tumor invasion and metastasis steps by imparting the ability to invade and spread to tumor cells. However, invasion and metastasis mechanisms vary depending on the cancer type. In addition to participating in tumor cell proliferation, MFAP2 plays an important role in tumor invasion and metastasis through EMT in tumor cells. For example, hepatocellular carcinoma cell migration and invasion ability were reduced by inhibiting EMT-related protein expression in MHCC97H cells after MFAP2-knockdown. Furthermore, MFAP2 inhibition by si-MFAP2 in YY-8103 and HuH-7 cells showed time-dependent low relative mobility (20, 21). In vitro colorectal adenocarcinoma studies found that MFAP2 silencing inhibited the migration of SW480 and HCT116 cells (19). In thyroid papillary carcinoma, MFAP2 downregulation inhibits BCPAP and TPC-1 cell migration and invasion; MFAP2 is associated with lymph node metastasis (22). MFAP2 plays a role as an oncogene in breast cancer. After it was silenced, the MCF-7 and MDA-MB-231 cell migration and invasion abilities were impaired; after MFAP2 was restored, the migration and invasion abilities were enhanced (23). Although in TCGA and GTEX database, there was no significant difference in the MFAP2 expression between Skin cutaneous melanoma tumors and normal tissue, cells with MFAP2 inhibition inhibited B16 cell invasion and migration in vitro. The downregulated MFAP2 cell’s ability to form tumors in the lung was significantly reduced in the nude mouse lung metastasis model established by B16 cells. These results suggest that downregulating MFAP2 expression limits melanoma cell migration and invasion in vitro and in vivo. Moreover, MFAP2 regulates epithelial-mesenchymal transformation to achieve this effect (39). MFAP2 expression was upregulated in gastric cancer organization and cell lines, and its downregulation inhibited AGS and HGC-27 cell wound healing, migration, and invasion. In vivo, mice injected with MFAP2 knockout cells had significantly fewer metastatic nodules on the lung and liver surfaces than those injected with control cells. Furthermore, MFAP2-knockdown BGC823 and MKN-45 cells significantly decreased migration and invasion by interfering with EMT (24, 25). The above studies show that MFAP2 plays a role in tumor invasion and metastasis by promoting tumor cell EMT. Since MFAP2 is an important component of extracellular matrix, more and more in-depth studies are needed to confirm whether MFAP2 can inhibit the EMT of tumor cells and inhibit the invasion and metastasis of tumor cells in all tumors.
MFAP2 inhibits tumor cell apoptosis
As a complex regulator, apoptosis plays an important role in maintaining development and homeostasis (40). The apoptosis mechanism imbalance is associated with various diseases; the most concerning is carcinogenesis and malignant tumor occurrence and development (34). Apoptosis is a distinguishing cancer feature, and its deregulation often leads to chemoresistance (41). Therefore, the therapeutic method of specifically inducing cancer cell apoptosis has undeniable value in the fight against this type of disease. Significant efforts have also been made in developing and researching related drugs (42, 43). Dong et al. showed that MFAP2, an oncogene in thyroid papillary carcinoma, was overexpressed in thyroid papillary carcinoma compared to normal tissues. The MFAP2 expression level was significantly correlated with lymph node metastasis, tissue type, and tumor focus type, and its downregulation induced BCPAP and TPC-1 cell apoptosis. Therefore, MFAP2 downregulation promotes apoptosis in thyroid papillary carcinoma cells (22). As an oncogene in breast cancer, MFAP2 knockout can significantly increase MCF-7 and MDA-MB-231 cell apoptosis. However, restoring MFAP2 expression can reduce apoptosis. Moreover, lncRNA LCPAT1 interacts with RBBP4 and recruits it to the MFAP2 promoter, promoting breast cancer progression by activating MFAP2 transcription (23). In colon adenocarcinoma, silencing MFAP2 promoted SW480 and HCT116 cell apoptosis. The increased apoptosis induced by si-kcnq1ot1 and mir-423-5p simulated transfection could be neutralized by transferring the MFAP2 overexpression plasmid into SW480 and HCT116 cells. The results showed that MFAP2 inhibits tumor cell apoptosis in colon adenocarcinoma (19). The above results indicate that MFAP2 can inhibit the apoptosis of tumor cells and promote the survival of tumor cells through its high expression in tumor cells. Targeting MFAP2 may play an important role in tumor therapy.
The roles of MFAP2 in tumor angiogenesis
The circulatory system is essential for delivering nutrients and chemicals to tissues, cleaning waste, and maintaining homeostasis. Malignant tumor cells require oxygen and nutrients for survival and reproduction (44, 45). Continuous angiogenesis is a hallmark of cancer. In addition to providing oxygen and nutrition, newly formed blood vessels also produce paracrine factors to support the cancer microenvironment and promote tumor growth, progression, and metastasis (46–50). However, although the current anti-angiogenic drugs initially had a breakthrough, they encountered major difficulties in follow-up trials and failed to be used in treating most malignant solid tumors, possibly due to low clearance efficiency or insufficient inhibition of tumor-related endothelial cell functions (51–53). During blood vessel development, endothelial cells exit their quiescent state, migrate, and proliferate into the surrounding and previously degraded matrix in response to angiogenic stimuli to form new functional blood vessels (54–56). Therefore, developing new therapies that inhibit endothelial cells is essential in cancer treatment (57, 58). Epidermal growth factor-like protein-7 (EGFL7) is involved in blood vessel development, and vascular lumen formation drives tumor angiogenesis, contributing to the pathological tumor vascular phenotype (59, 60); these EGFL7 features contribute to blood vessel wall instability and promote vascular leakage, characteristic of tumor endothelial cells (61). EGFL7 promotes glioma growth and stimulates tumor vascularization by generating mature blood vessels covered by pericytes and smooth muscle cells (62). High EGFL7 levels are associated with higher tumor grade and poor prognosis as a potential cancer target. However, it was found that MFAP2 is necessary for depositing EGFL7 into microfibrils, which is crucial for vascular development, showing that MFAP2 is important in tumor angiogenesis (63). An in vitro human umbilical vein endothelial cells (HUVEC) tube-forming experiment showed that vascular endothelial growth factor A decreased significantly after MFAP2 knockdown in hepatocellular carcinoma. The MFAP2 knockout MHCC97H cell supernatant reduced HUVEC’s average tube length, network, and branch number. These results showed that silencing MFAP2 could reduce angiogenesis (20). These findings emphasize the important role of MFAP2 in tumor hematoma formation. Considering the importance of neovascularization for tumor progression, targeted MFAP2 therapy may play an active role in tumor therapy, especially for vascular rich tumors. Therefore, further studies are needed to reveal MFAP2’s role and mechanism in tumor angiogenesis.
Related signal pathways of MFAP2 in tumors
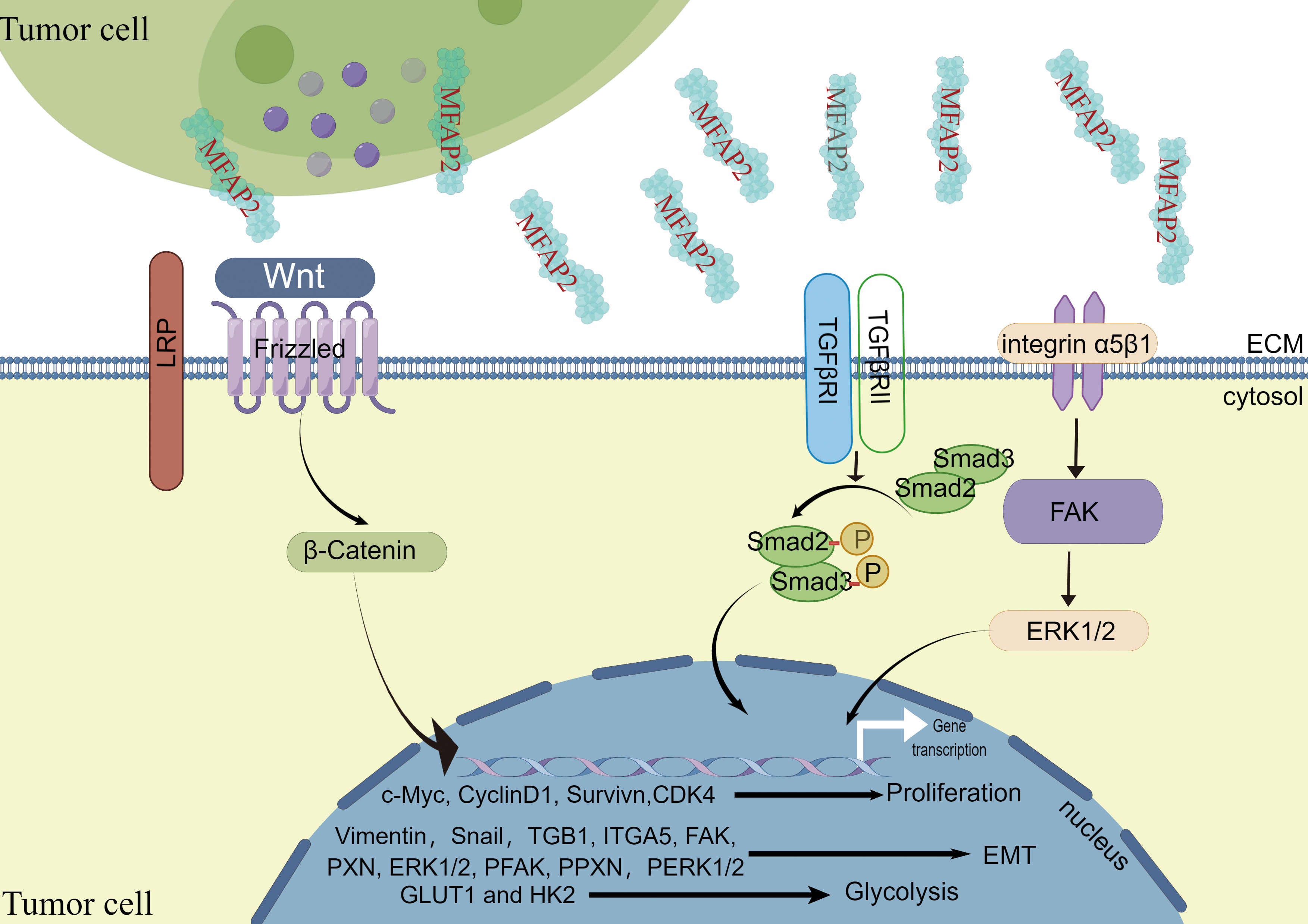
Cancer is characterized by genetic changes that affect signaling pathways controlling cell cycle progression, apoptosis, and cell growth; however, these changes’ extent, mechanisms, and co-occurrence vary (64). Various authors have shown that MFAP2 overexpression promotes tumor progression by activating cellular signaling pathways (Figure 2).
Wnt signaling pathway. Although the mechanism of the Wnt signaling pathway varies in different physiological processes, its signal transduction dysregulation can contribute to human diseases (65). Current methods for Wnt signaling mainly target cancer interventions. The abnormal Wnt signaling pathway is involved in various tumors, including pancreatic, lung, breast, ovarian, and colorectal cancers (66, 67). Wnt/β-catenin is one of the main signaling pathways contributing to EMT in various cancer growth and progression (68, 69). In melanoma, MFAP2 disruption influenced Wnt/β-catenin linked protein levels, and β-catenin neutralized MFAP2’s effect on EMT, as evidenced by the altered EMT molecular markers’ protein levels. This study showed that MFAP2 activates the Wnt/β-catenin signaling pathway and enhances EMT during melanoma metastasis (39).Forkhead box M1 (FoxM1) belongs to the forkhead box transcription factor family. It is abnormally expressed in various human cancers and plays a role in tumor initiation, progression, invasion, metastasis, and angiogenesis (70). Studies have shown that FOXM1 strongly enhances cancer cellβ-catenin nuclear translocation (71, 72). Signaling activation relies on β-catenin nuclear localization, and FOXM1 promotes it in different cancer cells, which is highly correlated with tumor metastasis (71, 73, 74). In contrast, cancer cell energy requirements are increased, and metabolic reprogramming, a cancer cell marker, is critical for tumor growth and metastasis (75). In ovarian cancer, MFAP2 promotes β-catenin transfer from the cytoplasm to the nucleus via the FoxM1/β-Catenin signal pathway, increasing the glycolysis-related gene (GLUT1 and HK2) expression levels in A2780 and SKOV3 cells (18).
Integrin/FAK/ERK1/2 signaling pathway. Increased integrin expression leads to focal adhesion kinase (FAK) activation, activating survival pathways, including PI3K/Akt, and promoting cell migration and invasion (76–78). It has been reported that in gastric cancer, MFAP2 plays an important role in regulating the integrin signaling pathway in tumor cell ECM interaction as an integrin/FAK/ERK1/2 signaling activator during gastric cancer progression (25).
TGF-β signaling pathway. The TGF-β signaling pathway is involved in oncogenesis in multiple ways (79, 80). Moreover, TGF-β acts as a tumor inhibitor in the early oncogenesis phases; however, it may also enhance tumor progression in later phases (81). The classic SMAD pathway involves binding TGF-β receptors TβRI and TβRII to their respective ligands, forming a tetramer compound containing phosphorylated Smad2 and Smad3. After translocating to the nucleus, phosphorylated Smad2 and Smad3 interact with Smad4 to regulate epithelial-mesenchymal transition. Epithelial cells acquire mesenchymal characteristics because of epithelial-mesenchymal transition, resulting in increased migration, invasiveness, and resistance to therapies (82, 83). It has been reported in gastric cancer that MFAP2 knockdown significantly reduced TGF-β expression and activity, as assessed by Smad-2 and Smad-3 phosphorylation. However, TGF-β treatment reversed MFAP2 knockdown gastric cancer cell damaged migration and invasion without changing MFAP2 expression. Therefore, MFAP2 acts as an upstream regulator by activating TGF-β/Smad2/3 signaling, promoting proliferation, migration, invasion, and EMT phenotype (24). Similarly, the biological analysis showed that MFAP2 combines with TGF-β and BMP through its highly acidic sequence near the N-terminus. MFAP2 binds to active TGF‐β1 but not to latent TGF‐β1. MFAP2 deletion increases the total TGF-β stored in cultured cells’ ECM because the active TGF‐β1 does not bind to fibrillin, indicating that MFAP2 plays an active role in TGFβ signaling in the ECM (31).
The above research results show that MFAP2 participates in multiple signal pathways and plays an important role in tumor invasion, metastasis, metabolism and resistance to therapies in tumors, emphasizing that MFAP2 may be used as a major target of anti-tumor therapy to develop relevant drugs.
Prognostic value of MFAP2 in tumors
Identifying the molecular mechanisms responsible for tumor development and maintenance is essential in developing targeted cancer therapies (84). This can be achieved by compiling genetic alterations across multiple tumor types in large-scale cross-sectional molecular studies for cancer, including The Cancer Genome Atlas (TCGA) and the International Cancer Genome Consortium (85, 86). Some authors have shown that MFAP2 can be a prognostic indicator for various tumors. Through the pan cancer analysis of MFAP2, Qiu et al. (33) showed that high expression of MFAP2 was associated with poor DFI for Adrenocortical carcinoma, Breast invasive carcinoma, Cervical squamous cell carcinoma and endocervical adenocarcinoma, Cholangiocarcinoma, Ovarian serous cystadenocarcinoma, and Pancreatic adenocarcinoma, with poor DSS for Adrenocortical carcinoma, Breast invasive carcinoma, Cervical squamous cell carcinoma and endocervical adenocarcinoma, Cholangiocarcinoma, Kidney renal clear cell carcinoma, Brain lower grade glioma, Liver hepatocellular carcinoma, and Sarcoma, with poor OS for Adrenocortical carcinoma, Breast invasive carcinoma, Cervical squamous cell carcinoma and endocervical adenocarcinoma, Kidney renal clear cell carcinoma, Brain lower grade glioma, Liver hepatocellular carcinoma, and Sarcoma, and poor PFI for Adrenocortical carcinoma, Bladder urothelial carcinoma, Breast invasive carcinoma, Cervical squamous cell carcinoma and endocervical adenocarcinoma, Kidney chromophobe, Kidney renal clear cell carcinoma, Brain lower grade glioma, and Sarcoma. In the specific analysis of each cancer, as a pivotal gene related to tumorigenesis, MFAP2 is a biomarker for the diagnosis and prognosis of gastric cancer. It contributes to personalized treatment and is an independent prognostic factor for the overall survival of patients with gastric cancer. And in gastric adenocarcinoma, high MFAP2 expression was significantly correlated with the first progression and post-progressive survival shortening; the overall and disease-free survivals were shorter in patients with increased MFAP2 expression (26, 27). Studies have shown that MFAP2 is significantly upregulated in hepatocellular carcinoma and is associated with tumor progression and prognosis. The higher the MFAP2 expression, the worse the prognosis and the lower the survival rate. MFAP2 promotes hepatocellular carcinoma cell proliferation, migration, and invasion. MFAP2 may also be a promising prognostic biomarker with important clinical significance as a potential immunotherapeutic target for hepatocellular carcinoma patients (21). MFAP2 is a poor prognosis marker in chondrosarcoma malignant transformation; its high expression predicts poor prognosis (29). The predicted circulating MFAP2 protein level is associated with epithelial ovarian cancer risk. The regional genome map confirms the genetic association signal overlap between the plasma protein level and the risk of epithelial ovarian cancer (17). Studies have shown that MFAP2 expression can distinguish papillary thyroid cancer tissues from normal ones. Upregulated MFAP2 expression in papillary thyroid cancer is associated with an increased recurrence or death risk. Therefore, MFAP2 overexpression predicts a poor prognosis in papillary thyroid cancer (22). As a potential biomarker of head and neck squamous cell carcinoma tumorigenesis, MFAP2 is significantly overexpressed in SAGE tumor libraries (28). In addition, MFAP2 is a prognostic marker that correlates with the immune microenvironment in glioma (30). MFAP2 is important in the prognosis of the above tumor types; however, the specific mechanism and prognostic value of MFAP2 in other cancers with low expression of MFAP2, such as Kidney chromophore, Kidney renal clear cell carcinoma, Kidney renal papillary cell carcinoma, and Prostate adenocarcinoma, need to be further explored.
Relationship between MFAP2 expression level and immune infiltration in tumors
The interaction of various factors constituting the tumor microenvironment forms the characteristics of cancer and has a significant impact on the antitumor immune response (34). These factors include cell components, surrounding extracellular matrix and interstitial fluid. Among them, the cellular components include tumor cells themselves and stromal cells such as fibroblasts, endothelial cells and infiltrating immune cells. As part of the immune response against cancer, infiltrating immune cells play a crucial role (87). Zhu X et al. investigated the relationship between the expression of MFAP2 and immune factors in Hepatocellular carcinoma and got the fact that some immunostimulators, immunoinhibitors, and Regulatory T cells for which expression was significantly correlated with MFAP2 expression by filtering (21). Qiu Z et al. analyzed the interaction of MFAP2 with various immune cell infiltration, and found that the expression of MFAP2 was significantly positively correlated with the infiltration level of B cells, CD4+T cells, CD8+T cells, dendritic cells, macrophages and neutrophils in bladder urothelial carcinoma, breast invasive carcinoma and low-grade glioma. In addition, MFAP2 might regulate macrophage polarization in Bladder Urothelial Carcinoma, Colon adenocarcinoma, Esophageal carcinoma, Kidney Chromophobe, Brain Lower Grade Glioma, Liver hepatocellular carcinoma, Pancreatic adenocarcinoma, Prostate adenocarcinoma, Rectum adenocarcinoma, Stomach adenocarcinoma, Head and Neck squamous cell carcinoma-HPV-, Thyroid carcinoma, and Thymoma (33). In our previous research, the expression level of MFAP2 in glioma was positively correlated with Th2 cells, macrophages, eosinophils, neutrophils and T cells. Additionally, MFAP2 expression level in glioma was positively correlated with key markers of T-cell exhaustion (30). These results suggest that the high expression of MFAP2 in different types of tumors can form tumor immune infiltration, and then regulate tumor microenvironment, which plays an important role in immunotherapy of a variety of tumors, deserve further studies for their potential clinical implications.
Discussion
MFAP2 is known for its function in microfibril assembly (88). It has been shown that MFAP2(−/−) mice exhibit obesity, metabolic dysfunction, adipocyte hypertrophy, and reduced heat generation. TGF-β activity increased in MFAP2(−/−) adipose tissue, and treating MFAP2(−/−) mice with a TGF-β-neutralizing antibody improved their body temperature and prevented the increased obesity (89). In addition, MFAP2 is a bone remodeling regulator; its deficient mice show progressive osteopenia, accompanied by increased osteoclasts and NF- κB ligand-receptor activator expression (90, 91). MFAP2 expression is diverse at different developmental stages, highest in fetuses and newborns and lowest in adults (92). In addition, by summarizing MFAP2 research, it was found that its protein is not necessary for normal development; however, mutations in the MFAP2 gene cause defects in multiple organ systems (14). Recently, it was found that MFAP2 affects different tissue tropisms, playing an important role in regulating steady tissue state, cell survival, and tumor progression (13). Bioinformatics and computational biology play critical roles in bioscience and biomedical research (93). Therefore, MFAP2 expression in tumor tissues and its prognostic relevance were determined. Targeting MFAP2 is important in inhibiting tumor cell proliferation, migration, invasion, angiogenesis, and promoting tumor cell apoptosis. Similar conclusions were verified in in vivo and in vitro experiments. Regarding the mechanism, MFAP2 exerts oncogenic function through an alternative mechanism because it is associated with microfibrils in the ECM and induces other matrix remodeling gene expressions, including Versican (94). MFAP2 dysregulation greatly changes the ECM status in the cancer microenvironment, modulating the cancer cell phenotypes (25). Therefore, MFAP2-targeted therapy may play an important role in adult tumor therapy with minimal collateral damage. Zhu et al. (13) provided us with a comprehensive understanding of microfibril related proteins in diseases by summarizing the molecular structure and function of microfibril related proteins in bone, metabolic disorders, and cancer. In light of novel data on microfibril-related proteins, including the role and mechanism of MFAP2 in tumors, we focused on the role and mechanism of MFAP2 in cancer to provide an updated review. However, insights have been restricted to studies employing siRNA-mediated knockdown of expression or the generation of knockout lines of cell and mice until recently. Despite these approaches, the specific functions of MFAP2 in cancer remain uncertain, and this possibility must be addressed experimentally with specific pharmacological tools. Additionally, the study on the regulation mechanism upstream of MFAP2 shows that the expression of MFAP2 is regulated by lncRNAs, such as lncRNA LCPAT1 (23) and lncRNA KCNQ1OT1 (19). To compare MFAP2 expression and degradation in tumor versus healthy cells, and aid anti-tumor drug development, it is necessary to conduct an in-depth study on the regulation mechanism of MFAP2 in tumors.
In conclusion, the MFAP2 mechanism in various cancers requires further exploration. Identifying and utilizing MFAP2 involvement in cancer will help develop new diagnostic, therapeutic, and prognostic methods for patients with malignant tumors.
Author contributions
WX, MW and YB designed and analyzed the research. WX and MW drafted the manuscript. LZ and YL participated in the critical revision of the manuscript. XM, YC and ZY participated in the data collection and literal modification of the manuscript. All authors read and approved the final manuscript.
Funding
This work was supported by National Nature and Science Foundation of China (81672505), the S&T Development Planning Program of Jilin Province (20200404101YY and 20200201613JC), Jilin Province Medical and Health Talent Project (JLSWSRCZX2021-052) and Health and Wellness Technology Enhancement Project of Jilin Province (2021LC007).
Acknowledgments
Figure 2 was drawn by Figdraw; and we would like to thank Editage (www.editage.cn) for English language editing.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Theocharis AD, Skandalis SS, Gialeli C, Karamanos NK. Extracellular matrix structure. Adv Drug Delivery Rev (2016) 97:4–27. doi: 10.1016/j.addr.2015.11.001
2. Karamanos NK, Theocharis AD, Piperigkou Z, Manou D, Passi A, Skandalis SS, et al. A guide to the composition and functions of the extracellular matrix. FEBS J (2021) 288(24):6850–912. doi: 10.1111/febs.15776
3. Brown NH. Extracellular matrix in development: Insights from mechanisms conserved between invertebrates and vertebrates. Cold Spring Harb Perspect Biol (2011) 3(12):a005082. doi: 10.1101/cshperspect.a005082
4. Humphrey JD, Dufresne ER, Schwartz MA. Mechanotransduction and extracellular matrix homeostasis. Nat Rev Mol Cell Biol (2014) 15(12):802–12. doi: 10.1038/nrm3896
5. Merino-Casallo F, Gomez-Benito MJ, Hervas-Raluy S, Garcia-Aznar JM. Unravelling cell migration: Defining movement from the cell surface. Cell Adh Migr (2022) 16(1):25–64. doi: 10.1080/19336918.2022.2055520
6. Kai F, Drain AP, Weaver VM. The extracellular matrix modulates the metastatic journey. Dev Cell (2019) 49(3):332–46. doi: 10.1016/j.devcel.2019.03.026
7. Yuzhalin AE. Parallels between the extracellular matrix roles in developmental biology and cancer biology. Semin Cell Dev Biol (2022) 128:90–102. doi: 10.1016/j.semcdb.2021.09.010
8. Yuzhalin AE, Gordon-Weeks AN, Tognoli ML, Jones K, Markelc B, Konietzny R, et al. Colorectal cancer liver metastatic growth depends on Pad4-driven citrullination of the extracellular matrix. Nat Commun (2018) 9(1):4783. doi: 10.1038/s41467-018-07306-7
9. Oskarsson T. Extracellular matrix components in breast cancer progression and metastasis. Breast (2013) 22 Suppl 2:S66–72. doi: 10.1016/j.breast.2013.07.012
10. Garantziotis S, Savani RC. Proteoglycans in toll-like receptor responses and innate immunity. Am J Physiol Cell Physiol (2022) 323(1):C202–14. doi: 10.1152/ajpcell.00088.2022
11. Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol (2011) 3(12):a005058. doi: 10.1101/cshperspect.a005058
12. Lodge W, Zavortink M, Golenkina S, Froldi F, Dark C, Cheung S, et al. Tumor-derived mmps regulate cachexia in a drosophila cancer model. Dev Cell (2021) 56(18):2664–80.e6. doi: 10.1016/j.devcel.2021.08.008
13. Zhu S, Ye L, Bennett S, Xu H, He D, Xu J. Molecular structure and function of microfibrillar-associated proteins in skeletal and metabolic disorders and cancers. J Cell Physiol (2021) 236(1):41–8. doi: 10.1002/jcp.29893
14. Craft CS, Broekelmann TJ, Mecham RP. Microfibril-associated glycoproteins magp-1 and magp-2 in disease. Matrix Biol (2018) 71-72:100–11. doi: 10.1016/j.matbio.2018.03.006
15. Segade F, Mecham RP. Regulatory elements of microfibril-associated glycoprotein-1 gene expression in muscle cells. Biochim Biophys Acta (2005) 1731(3):215–24. doi: 10.1016/j.bbaexp.2005.10.007
16. Craft CS. Magp1, the extracellular matrix, and metabolism. Adipocyte (2015) 4(1):60–4. doi: 10.4161/adip.32209
17. Considine DPC, Jia G, Shu X, Schildkraut JM, Pharoah PDP, Zheng W, et al. Genetically predicted circulating protein biomarkers and ovarian cancer risk. Gynecol Oncol (2021) 160(2):506–13. doi: 10.1016/j.ygyno.2020.11.016
18. Zhao LQ, Sun W, Zhang P, Gao W, Fang CY, Zheng AW. Mfap2 aggravates tumor progression through activating Foxm1/Beta-Catenin-Mediated glycolysis in ovarian cancer. Kaohsiung J Med Sci (2022) 38(8):772–80. doi: 10.1002/kjm2.12546
19. Yin X, Jiang A, Ma Z, Lu X, Li D, Chen Y. Long non-coding rna-Kcnq1ot1 mediates mir-423-5p/Microfibril-Associated protein 2 axis in colon adenocarcinoma. Histol Histopathol (2021) 36(10):1099–110. doi: 10.14670/HH-18-386
20. Zhang N, Shao F, Jia W. Upregulation of microfibrillar-associated protein 2 is closely associated with tumor angiogenesis and poor prognosis in hepatocellular carcinoma. Oncol Lett (2021) 22(4):739. doi: 10.3892/ol.2021.13000
21. Zhu X, Cheng Y, Wu F, Sun H, Zheng W, Jiang W, et al. Mfap2 promotes the proliferation of cancer cells and is associated with a poor prognosis in hepatocellular carcinoma. Technol Cancer Res Treat (2020) 19:1533033820977524. doi: 10.1177/1533033820977524
22. Dong SY, Chen H, Lin LZ, Jin L, Chen DX, Wang OC, et al. Mfap2 is a potential diagnostic and prognostic biomarker that correlates with the progression of papillary thyroid cancer. Cancer Manag Res (2020) 12:12557–67. doi: 10.2147/CMAR.S274986
23. Gong X, Dong T, Niu M, Liang X, Sun S, Zhang Y, et al. Lncrna Lcpat1 upregulation promotes breast cancer progression Via enhancing Mfap2 transcription. Mol Ther Nucleic Acids (2020) 21:804–13. doi: 10.1016/j.omtn.2020.07.015
24. Wang JK, Wang WJ, Cai HY, Du BB, Mai P, Zhang LJ, et al. Mfap2 promotes epithelial-mesenchymal transition in gastric cancer cells by activating tgf-Beta/Smad2/3 signaling pathway. Onco Targets Ther (2018) 11:4001–17. doi: 10.2147/OTT.S160831
25. Yao LW, Wu LL, Zhang LH, Zhou W, Wu L, He K, et al. Mfap2 is overexpressed in gastric cancer and promotes motility Via the Mfap2/Integrin Alpha5beta1/Fak/Erk pathway. Oncogenesis (2020) 9(2):17. doi: 10.1038/s41389-020-0198-z
26. Sun T, Wang D, Ping Y, Sang Y, Dai Y, Wang Y, et al. Integrated profiling identifies Slc5a6 and Mfap2 as novel diagnostic and prognostic biomarkers in gastric cancer patients. Int J Oncol (2020) 56(2):460–9. doi: 10.3892/ijo.2019.4944
27. Tang F, Hu C, Long Z, Liu Y, Li G. Molecular characteristics and prognostic role of Mfap2 in stomach adenocarcinoma. J Healthc Eng (2022) 2022:1417238. doi: 10.1155/2022/1417238
28. Silveira NJ, Varuzza L, Machado-Lima A, Lauretto MS, Pinheiro DG, Rodrigues RV, et al. Searching for molecular markers in head and neck squamous cell carcinomas (Hnscc) by statistical and bioinformatic analysis of larynx-derived sage libraries. BMC Med Genomics (2008) 1:56. doi: 10.1186/1755-8794-1-56
29. Wu J, Huang Y, Yu C, Li X, Wang L, Hong J, et al. The key gene expression patterns and prognostic factors in malignant transformation from enchondroma to chondrosarcoma. Front Oncol (2021) 11:693034. doi: 10.3389/fonc.2021.693034
30. Xu W, Geng R, Zhao Y, Ma X, Bai Y, Jiang Y, et al. Microfibrillar-associated protein 2 is a prognostic marker that correlates with the immune microenvironment in glioma. Front Genet (2022) 13:989521. doi: 10.3389/fgene.2022.989521
31. Broekelmann TJ, Bodmer NK, Mecham RP. Identification of the growth factor-binding sequence in the extracellular matrix protein magp-1. J Biol Chem (2020) 295(9):2687–97. doi: 10.1074/jbc.RA119.010540
32. Gomez de Segura I, Ahechu P, Gomez-Ambrosi J, Rodriguez A, Ramirez B, Becerril S, et al. Decreased levels of microfibril-associated glycoprotein (Magp)-1 in patients with colon cancer and obesity are associated with changes in extracellular matrix remodelling. Int J Mol Sci (2021) 22(16):8485. doi: 10.3390/ijms22168485
33. Qiu Z, Xin M, Wang C, Zhu Y, Kong Q, Liu Z. Pan-cancer analysis of microfibrillar-associated protein 2 (Mfap2) based on bioinformatics and qpcr verification. J Oncol (2022) 2022:8423173. doi: 10.1155/2022/8423173
34. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
35. Riddell M, Nakayama A, Hikita T, Mirzapourshafiyi F, Kawamura T, Pasha A, et al. Apkc controls endothelial growth by modulating c-myc Via Foxo1 DNA-binding ability. Nat Commun (2018) 9(1):5357. doi: 10.1038/s41467-018-07739-0
36. Sack LM, Davoli T, Li MZ, Li Y, Xu Q, Naxerova K, et al. Profound tissue specificity in proliferation control underlies cancer drivers and aneuploidy patterns. Cell (2018) 173(2):499–514 e23. doi: 10.1016/j.cell.2018.02.037
37. Schiano Lomoriello I, Giangreco G, Iavarone C, Tordonato C, Caldieri G, Serio G, et al. A self-sustaining endocytic-based loop promotes breast cancer plasticity leading to aggressiveness and pro-metastatic behavior. Nat Commun (2020) 11(1):3020. doi: 10.1038/s41467-020-16836-y
38. Dongre A, Weinberg RA. New insights into the mechanisms of epithelial-mesenchymal transition and implications for cancer. Nat Rev Mol Cell Biol (2019) 20(2):69–84. doi: 10.1038/s41580-018-0080-4
39. Chen Z, Lv Y, Cao D, Li X, Li Y. Microfibril-associated protein 2 (Mfap2) potentiates invasion and migration of melanoma by emt and Wnt/Beta-catenin pathway. Med Sci Monit (2020) 26:e923808. doi: 10.12659/MSM.923808
40. Wolf P, Schoeniger A, Edlich F. Pro-apoptotic complexes of bax and bak on the outer mitochondrial membrane. Biochim Biophys Acta Mol Cell Res (2022) 1869(10):119317. doi: 10.1016/j.bbamcr.2022.119317
41. Frank T, Tuppi M, Hugle M, Dotsch V, van Wijk SJL, Fulda S. Cell cycle arrest in mitosis promotes interferon-induced necroptosis. Cell Death Differ (2019) 26(10):2046–60. doi: 10.1038/s41418-019-0298-5
42. Pfeffer CM, Singh ATK. Apoptosis: A target for anticancer therapy. Int J Mol Sci (2018) 19(2):448. doi: 10.3390/ijms19020448
43. Montico B, Nigro A, Casolaro V, Dal Col J. Immunogenic apoptosis as a novel tool for anticancer vaccine development. Int J Mol Sci (2018) 19(2):594. doi: 10.3390/ijms19020594
44. Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell Mol Life Sci (2020) 77(9):1745–70. doi: 10.1007/s00018-019-03351-7
45. Geindreau M, Bruchard M, Vegran F. Role of cytokines and chemokines in angiogenesis in a tumor context. Cancers (Basel) (2022) 14(10):2446. doi: 10.3390/cancers14102446
46. Adams RH, Alitalo K. Molecular regulation of angiogenesis and lymphangiogenesis. Nat Rev Mol Cell Biol (2007) 8(6):464–78. doi: 10.1038/nrm2183
47. Ferrara N, Kerbel RS. Angiogenesis as a therapeutic target. Nature (2005) 438(7070):967–74. doi: 10.1038/nature04483
48. Carmeliet P. Angiogenesis in life, disease and medicine. Nature (2005) 438(7070):932–6. doi: 10.1038/nature04478
49. Jain RK. Antiangiogenesis strategies revisited: From starving tumors to alleviating hypoxia. Cancer Cell (2014) 26(5):605–22. doi: 10.1016/j.ccell.2014.10.006
50. Sobierajska K, Ciszewski WM, Sacewicz-Hofman I, Niewiarowska J. Endothelial cells in the tumor microenvironment. Adv Exp Med Biol (2020) 1234:71–86. doi: 10.1007/978-3-030-37184-5_6
51. Bergers G, Hanahan D. Modes of resistance to anti-angiogenic therapy. Nat Rev Cancer (2008) 8(8):592–603. doi: 10.1038/nrc2442
52. Fan Y. Vascular detransformation for cancer therapy. Trends Cancer (2019) 5(8):460–3. doi: 10.1016/j.trecan.2019.05.007
53. Nakai H, Matsumura N. The roles and limitations of bevacizumab in the treatment of ovarian cancer. Int J Clin Oncol (2022) 27(7):1120–6. doi: 10.1007/s10147-022-02169-x
54. Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell (2011) 146(6):873–87. doi: 10.1016/j.cell.2011.08.039
55. Globig P, Madurawala R, Willumeit-Romer R, Martini F, Mazzoni E, Luthringer-Feyerabend BJC. Mg-based materials diminish tumor spreading and cancer metastases. Bioact Mater (2023) 19:594–610. doi: 10.1016/j.bioactmat.2022.05.002
56. Li Z, Ning F, Wang C, Yu H, Ma Q, Sun Y. Normalization of the tumor microvasculature based on targeting and modulation of the tumor microenvironment. Nanoscale (2021) 13(41):17254–71. doi: 10.1039/d1nr03387e
57. Zhang H, Feng L, de Andrade Mello P, Mao C, Near R, Csizmadia E, et al. Glycoengineered anti-Cd39 promotes anticancer responses by depleting suppressive cells and inhibiting angiogenesis in tumor models. J Clin Invest (2022) 132(13):e157431. doi: 10.1172/JCI157431
58. Augustin HG, Koh GY. Antiangiogenesis: Vessel regression, vessel normalization, or both? Cancer Res (2022) 82(1):15–7. doi: 10.1158/0008-5472.CAN-21-3515
59. Usuba R, Pauty J, Soncin F, Matsunaga YT. Egfl7 regulates sprouting angiogenesis and endothelial integrity in a human blood vessel model. Biomaterials (2019) 197:305–16. doi: 10.1016/j.biomaterials.2019.01.022
60. Charpentier MS, Taylor JM, Conlon FL. The Casz1/Egfl7 transcriptional pathway is required for rhoa expression in vascular endothelial cells. Small GTPases (2013) 4(4):231–5. doi: 10.4161/sgtp.26849
61. Heissig B, Salama Y, Takahashi S, Okumura K, Hattori K. The multifaceted roles of Egfl7 in cancer and drug resistance. Cancers (Basel) (2021) 13(5):1014. doi: 10.3390/cancers13051014
62. Dudvarski Stankovic N, Bicker F, Keller S, Jones DT, Harter PN, Kienzle A, et al. Egfl7 enhances surface expression of integrin Alpha5beta1 to promote angiogenesis in malignant brain tumors. EMBO Mol Med (2018) 10(9):e8420. doi: 10.15252/emmm.201708420
63. Villain G, Lelievre E, Broekelmann T, Gayet O, Havet C, Werkmeister E, et al. Magp-1 and fibronectin control Egfl7 functions by driving its deposition into distinct endothelial extracellular matrix locations. FEBS J (2018) 285(23):4394–412. doi: 10.1111/febs.14680
64. Sanchez-Vega F, Mina M, Armenia J, Chatila WK, Luna A, La KC, et al. Oncogenic signaling pathways in the cancer genome atlas. Cell (2018) 173(2):321–37 e10. doi: 10.1016/j.cell.2018.03.035
65. Rim EY, Clevers H, Nusse R. The wnt pathway: From signaling mechanisms to synthetic modulators. Annu Rev Biochem (2022) 91:571–98. doi: 10.1146/annurev-biochem-040320-103615
66. Hayat R, Manzoor M, Hussain A. Wnt signaling pathway: A comprehensive review. Cell Biol Int (2022) 46(6):863–77. doi: 10.1002/cbin.11797
67. Hall DCN, Benndorf RA. Aspirin sensitivity of Pik3ca-mutated colorectal cancer: Potential mechanisms revisited. Cell Mol Life Sci (2022) 79(7):393. doi: 10.1007/s00018-022-04430-y
68. Jayachandran J, Srinivasan H, Mani KP. Molecular mechanism involved in epithelial to mesenchymal transition. Arch Biochem Biophys (2021) 710:108984. doi: 10.1016/j.abb.2021.108984
69. Balaji S, Kim U, Muthukkaruppan V, Vanniarajan A. Emerging role of tumor microenvironment derived exosomes in therapeutic resistance and metastasis through epithelial-to-Mesenchymal transition. Life Sci (2021) 280:119750. doi: 10.1016/j.lfs.2021.119750
70. Ros S, Wright AJ, D'Santos P, Hu DE, Hesketh RL, Lubling Y, et al. Metabolic imaging detects resistance to Pi3kalpha inhibition mediated by persistent Foxm1 expression in er(+) breast cancer. Cancer Cell (2020) 38(4):516–33 e9. doi: 10.1016/j.ccell.2020.08.016
71. Zhang N, Wei P, Gong A, Chiu WT, Lee HT, Colman H, et al. Foxm1 promotes beta-catenin nuclear localization and controls wnt target-gene expression and glioma tumorigenesis. Cancer Cell (2011) 20(4):427–42. doi: 10.1016/j.ccr.2011.08.016
72. Aoki T, Nishida N, Kudo M. Clinical significance of the duality of Wnt/Beta-catenin signaling in human hepatocellular carcinoma. Cancers (Basel) (2022) 14(2):444. doi: 10.3390/cancers14020444
73. Raychaudhuri P, Park HJ. Foxm1: A master regulator of tumor metastasis. Cancer Res (2011) 71(13):4329–33. doi: 10.1158/0008-5472.CAN-11-0640
74. Bowman A, Nusse R. Location, location, location: Foxm1 mediates beta-catenin nuclear translocation and promotes glioma tumorigenesis. Cancer Cell (2011) 20(4):415–6. doi: 10.1016/j.ccr.2011.10.003
75. Rao TN, Hansen N, Hilfiker J, Rai S, Majewska JM, Lekovic D, et al. Jak2-mutant hematopoietic cells display metabolic alterations that can be targeted to treat myeloproliferative neoplasms. Blood (2019) 134(21):1832–46. doi: 10.1182/blood.2019000162
76. Luo M, Guan JL. Focal adhesion kinase: A prominent determinant in breast cancer initiation, progression and metastasis. Cancer Lett (2010) 289(2):127–39. doi: 10.1016/j.canlet.2009.07.005
77. Paoli P, Giannoni E, Chiarugi P. Anoikis molecular pathways and its role in cancer progression. Biochim Biophys Acta (2013) 1833(12):3481–98. doi: 10.1016/j.bbamcr.2013.06.026
78. Wang Q, Jiang D, Ye Q, Zhou W, Ma J, Wang C, et al. A widely expressed free immunoglobulin kappa chain with a unique Vkappa4-1/Jkappa3 pattern promotes colon cancer invasion and metastasis by activating the integrin Beta1/Fak pathway. Cancer Lett (2022) 540:215720. doi: 10.1016/j.canlet.2022.215720
79. Chen B, Mu C, Zhang Z, He X, Liu X. The love-hate relationship between tgf-beta signaling and the immune system during development and tumorigenesis. Front Immunol (2022) 13:891268. doi: 10.3389/fimmu.2022.891268
80. Peng D, Fu M, Wang M, Wei Y, Wei X. Targeting tgf-beta signal transduction for fibrosis and cancer therapy. Mol Cancer (2022) 21(1):104. doi: 10.1186/s12943-022-01569-x
81. Wang J, Xu Z, Wang Z, Du G, Lun L. Tgf-beta signaling in cancer radiotherapy. Cytokine (2021) 148:155709. doi: 10.1016/j.cyto.2021.155709
82. Su J, Morgani SM, David CJ, Wang Q, Er EE, Huang YH, et al. Tgf-beta orchestrates fibrogenic and developmental emts Via the ras effector Rreb1. Nature (2020) 577(7791):566–71. doi: 10.1038/s41586-019-1897-5
83. Dai Y, Wang H, Sun R, Diao J, Ma Y, Shao M, et al. Modified shenlingbaizhu decoction represses the pluripotency of colorectal cancer stem cells by inhibiting tgf-beta mediated emt program. Phytomedicine (2022) 103:154234. doi: 10.1016/j.phymed.2022.154234
84. Rabadan R, Mohamedi Y, Rubin U, Chu T, Alghalith AN, Elliott O, et al. Identification of relevant genetic alterations in cancer using topological data analysis. Nat Commun (2020) 11(1):3808. doi: 10.1038/s41467-020-17659-7
85. Garraway LA, Lander ES. Lessons from the cancer genome. Cell (2013) 153(1):17–37. doi: 10.1016/j.cell.2013.03.002
86. Vogelstein B, Papadopoulos N, Velculescu VE, Zhou S, Diaz LA Jr., Kinzler KW. Cancer genome landscapes. Science (2013) 339(6127):1546–58. doi: 10.1126/science.1235122
87. Wu AA, Drake V, Huang HS, Chiu S, Zheng L. Reprogramming the tumor microenvironment: Tumor-induced immunosuppressive factors paralyze T cells. Oncoimmunology (2015) 4(7):e1016700. doi: 10.1080/2162402X.2015.1016700
88. De Maria A, Wilmarth PA, David LL, Bassnett S. Proteomic analysis of the bovine and human ciliary zonule. Invest Ophthalmol Vis Sci (2017) 58(1):573–85. doi: 10.1167/iovs.16-20866
89. Craft CS, Pietka TA, Schappe T, Coleman T, Combs MD, Klein S, et al. The extracellular matrix protein Magp1 supports thermogenesis and protects against obesity and diabetes through regulation of tgf-beta. Diabetes (2014) 63(6):1920–32. doi: 10.2337/db13-1604
90. Craft CS, Zou W, Watkins M, Grimston S, Brodt MD, Broekelmann TJ, et al. Microfibril-associated glycoprotein-1, an extracellular matrix regulator of bone remodeling. J Biol Chem (2010) 285(31):23858–67. doi: 10.1074/jbc.M110.113019
91. Craft CS, Broekelmann TJ, Zou W, Chappel JC, Teitelbaum SL, Mecham RP. Oophorectomy-induced bone loss is attenuated in Magp1-deficient mice. J Cell Biochem (2012) 113(1):93–9. doi: 10.1002/jcb.23331
92. Mecham RP, Gibson MA. The microfibril-associated glycoproteins (Magps) and the microfibrillar niche. Matrix Biol (2015) 47:13–33. doi: 10.1016/j.matbio.2015.05.003
93. Gnimpieba EZ, VanDiermen MS, Gustafson SM, Conn B, Lushbough CM. Bio-tds: Bioscience query tool discovery system. Nucleic Acids Res (2017) 45(D1):D1117–D22. doi: 10.1093/nar/gkw940
Keywords: MFAP2, proliferation, invasion, metastasis, extracellular matrix (ECM), apoptosis, angiogenesis, prognosis
Citation: Xu W, Wang M, Bai Y, Chen Y, Ma X, Yang Z, Zhao L and Li Y (2022) The role of microfibrillar‐associated protein 2 in cancer. Front. Oncol. 12:1002036. doi: 10.3389/fonc.2022.1002036
Received: 24 July 2022; Accepted: 03 November 2022;
Published: 30 November 2022.
Edited by:
Giuseppe Giaccone, Cornell University, United StatesReviewed by:
Esmaeel Babaeenezhad, Shahid Beheshti University of Medical Sciences, IranXuyu Gu, Southeast University, China
Xinyan Lu, University of Pittsburgh, United States
Copyright © 2022 Xu, Wang, Bai, Chen, Ma, Yang, Zhao and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Liyan Zhao, emhhb2xpeUBqbHUuZWR1LmNu; Yunqian Li, eXVucWlhbkBqbHUuZWR1LmNu
†These authors have contributed equally to this work and share first authorship
 Wanzhen Xu
Wanzhen Xu Manfeng Wang2†
Manfeng Wang2† Yong Chen
Yong Chen Xiaoshan Ma
Xiaoshan Ma