- 1Department of Perioperative Research Center of Chinese Medicine, The Second Affiliated Hospital of Guangzhou University of Chinese Medicine, Guangzhou, China
- 2Emergency Department, Dongguan People’s Hospital, Dongguan, China
- 3Department of Spine Surgery, The First Affiliated Hospital of Fujian Medical University, Fuzhou, Fujian, China
- 4Department of Spine Surgery, National Regional Medical Center, Binhai Campus of The Affiliated Hospital, Fujian Medical University, Fuzhou, China
- 5Department of General Surgery, Changhai Hospital, Naval Medical University, Shanghai, China
- 6Department of Colorectal Surgery, Changhai Hospital, Naval Medical University, Shanghai, China
- 7Department of Spine Surgery, The Second Affiliated Hospital, University of South China, Hengyang, Hunan, China
- 8Department of Anorectal Surgery, Shuguang Hospital Affiliated to Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 9Anorectal Disease Institute of Shuguang Hospital, Shanghai, China
- 10Department of General Surgery, Putuo District Central Hospital, Shanghai University of Traditional Chinese Medicine, Shanghai, China
Background: Metabolic changes may occur following gastric surgery, which has been reported to contribute to bone loss, osteoporosis and even bone fracture. However, the evidence regarding the relationship between gastric surgery for benign and malignant conditions and risk of fracture is controversial. This study was conducted with the aim to evaluate whether gastric surgery is associated with a high risk of fracture.
Methods: Major electronic databases were searched from inception through October 2021 for population-based cohort studies investigating the associations between gastric surgery (including bariatric gastric surgeries and surgeries for gastric benign and malignant gastric tumors) and risk of fracture compared with controls. Pooled relative risks (RRs) with 95% confidence intervals (CIs) were derived using the random-effects Mantel–Haenszel model. Multiple subgroup analyses and sensitivity analyses were carried out to test sources of heterogeneity stratified by various study characteristics and the robustness of the results.
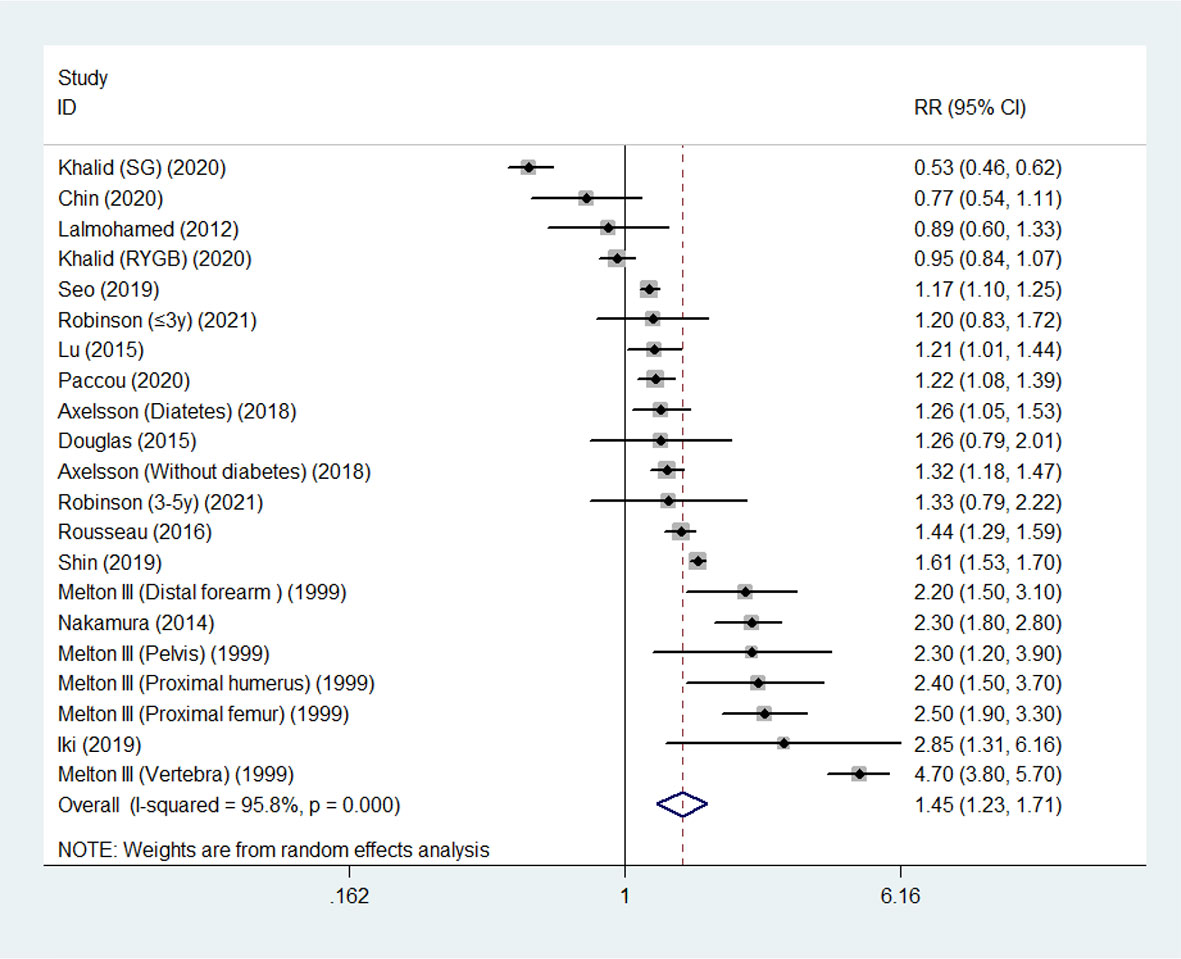
Results: A total of 14 studies comprising 693134 individuals were identified for analysis. The RR for the risk of fracture in people undergoing gastric surgery was 1.45 [95% confidence interval (CI) 1.23 - 1.72; I2 = 95.8%; P < 0.001] compared with that in control populations, among which the fracture sites of upper limb, spine, lower limb, pelvis and hip showed consistent significant results (all P < 0.05), whereas nonsignificant associations was noted for other fracture sites. Significant associations were also observed for patients having total or subtotal gastrectomy (RR 2.22, 95% CI 1.66 to 3.00), gastric bypass (RR 1.48, 95% CI 1.26 to 1.74), and a similar trend was observed for preserved passage procedures (including sleeve gastrectomy, gastric banding, vertical banded gastroplasty and other procedures that preserved the passage through the duodenum and proximal small bowel, in contrast to gastric bypass), though the difference did not reach statistically significant (RR 1.10, 95% CI 0.95 to 1.26). An evident increased risk in the age range from 40-59 years was observed (40-49 years: RR 1.36, 95% CI 1.19-1.55; 50-59 years: RR 2.48, 95% CI 1.58-3.90).
Conclusion: From this large pooled analysis of population-based cohort studies, evidence supports that fracture risk is increased in gastric surgery survivors compared with the control population. Early prevention and effective intervention strategies of bone fracture should be taken from clinicians and health policy makers.
Clinical Trial Registration: PROSPERO (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=291394), identifier CRD42021291394
Highlights
● People previously undergoing gastric surgery are subsequently at higher risk of fracture than control individuals.
● The highest fracture risk was seen after total or subtotal gastrectomy and gastric bypass.
● Increased risk for fractures was seen in the upper limb, spine, lower limb, pelvis and hip.
● Early prevention and effective intervention of bone fracture should be taken from clinicians in gastric surgery survivors.
Introduction
Bone fracture is a major public health problem worldwide, which causes a heavy economic burden and seriously affects the quality of life of the middle-aged and elderly adults. With the high rate of disability, fracture is also a major cause of premature death (1, 2). Although the global age-standardized incidence rate for fracture and low bone mineral density (BMD) decreased slightly from 1990 to 2019, the absolute burden still increased significantly (3, 4). Older age and women gender seem to be two risk factors for fracture and low BMD. Studies have found that the global incidence of fractures in women is higher than that in men over the age of 64 (3). In Western countries, 1 in 3 women and 1 in 5 men may have osteoporotic fractures after the age of 50 (5). In China, women are reported to have a higher risk of developing low BMD than men (4).
Gastric cancer, as one of the top burdensome cancers globally, represents the second commonest cause of cancer death globally. Surgical treatment remains the cornerstone of cancer cure and palliation (6). In addition, with the increase in overweight and obese people, bariatric surgery has also become one of the most commonly performed gastrointestinal surgeries globally (7). For these benign and malignant conditions, the two most common types of gastric surgery are gastrectomy for benign and malignant gastric lesions, and various weight loss operations. The reported incidence of bone fracture in these patients following gastric surgery ranged from 20-40 per 1000 person-years (8, 9). The possible mechanism of fracture following this kind of surgery is that these operations can lead to endocrine changes and weight loss, which contributes to bone loss (10, 11). Weight loss can also cause the decrease of bone mineral density (BMD), and consequently, the risk of bone fracture increases (12). A number of studies have reported that upper gastrointestinal surgery, such as gastrectomy for gastric tumors and bariatric surgery, is significantly associated with osteoporotic fractures (13–17). However, most of these studies were hospital-based cohorts, case-control and uncontrolled cross-sectional studies (18–20), lack of population-based longitudinal cohort studies and large sample prospective studies. Therefore, the evidence of whether gastric surgery leads to an increased risk of fracture is still insufficient.
The inconsistent results of these studies prompted us to comprehensively assess the associations between gastric surgery and subsequent fracture risk through a systematic review. Moreover, we tried to explore the moderators, including study design, sample size, geographical regions, patient age, control population, fracture site, risk of bias, measurement of association, adjusted variables, and surgery type.
Methods
This study is reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) (21) and Meta-analysis of Observational Studies in Epidemiology (MOOSE) guidelines (22), the protocol of which has been prospectively registered at PROSPERO (CRD42021291394) (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=291394).
Search strategy and selection criteria
We developed the search strategies for PubMed, EMBASE, and Cochrane Central Register of Controlled Trials without language restriction for original peer-reviewed articles published before October 31, 2021 investigating the associations between gastric surgery (including bariatric gastric surgeries and surgeries for gastric benign and malignant gastric tumors) and risk of fracture compared with controls. Terms related to the three primary concepts (gastric surgery, fracture, and cohort study) were searched both as MeSH (Pubmed/Cochrane) terms or Emtree (Embase) terms and as text words. Full details for the complete search strategies and the search terms are provided in Supplementary Methods. Cross-referencing the bibliographies of the selected references was also conducted to identify additional relevant publications. When multiple publications from the same cohort were identified, we included data from the most recent publication or summarized a set of most comprehensive and updated data from all relevant publications. After screening all titles and abstracts for the remaining citations, we obtained full-text citations to determine eligibility. The whole literature screening process was performed by two reviewers independently. Conflicts were resolved through group discussion until consensus was reached. If necessary, disagreements were resolved with consultation of a third reviewer.
Eligibility criteria
Studies were deemed appropriate for entry into the meta-analysis if they met the following inclusion or exclusion criteria: (1) study design: prospective/retrospective population-based cohort study; (2) participant: individuals previously underwent gastric surgery including bariatric surgery, total or subtotal gastrectomy for gastric lesions; (3) control: general populations having no history of gastric surgery matched or unmatched by demographic characteristics (4) the measure of association: studies reporting estimates including relative risk (RR), hazard ratio (HR), standardized incidence ratio (SIR) or incidence rate ratio (IRR) with corresponding 95% CIs that could be converted to the risk ratios. Cross-sectional studies, hospital-based or community-based observational studies and those providing inadequate data to generate precise estimates of the association between gastric cancer surgery and risk of fracture were all excluded. In addition, studies reported outcome of fracture resulting from metastatic cancer with bone localization, malnutrition/cachexia and bone loss due to the primary cancer were also excluded.
Study selection, data collection, and data extraction
Three authors compiled a piloted data extraction template and independently extracted data from each included study. In case of any discrepancies, discussion was initiated or the opinion of a senior author was requested. Several fields of general data were then extracted from each paper and entered into the data extraction template: first author of the study, publication year, study design, geographical region, study period, observation period, population sample size, participants’ mean or median age, control population, method of diagnosis of the cohort, outcome ascertainment, the main result of the study and measure of associations.
Quality assessment
For observational cohort studies, we used items from the Newcastle-Ottawa Scale (NOS) to evaluate methodological quality (23), with the primary aim to evaluate the representativeness of the population, selection of the cohorts and controls, ascertainment of exposure and outcomes and adequacy of follow-up. As was previously reported, an NOS score of 8-9 represented low risk of bias, and a score of 6-7 or less high risk of bias.
Statistical analysis
Statistical analyses were undertaken using STATA Statistical Software (version 14.0; Stata Corporation, College Station, TX, USA). The pooled RR of fracture for people in the gastric surgery survivors compared with those in the general population or nonsurgery controls was the primary outcome measure. To account for the anticipated heterogeneity across studies, we employed the DerSimonian and Laird random effects meta-analysis to synthesize results (RRs with their corresponding 95% CIs) (24). Because the absolute risk of fracture was relatively low, the RR in cohort studies mathematically approximated the OR and other risk esimates; therefore, we reported all results as RRs in our analysis (25). Generally, we selected the maximally adjusted RRs to pool the results when various risk estimates with several adjustments were reported in a study. If the included studies did not provide RR for the association between gastric surgery and risk of fracture, we would try to calculate indirectly based on the given information (data or curves) in the original study using the method as previously reported by Parmar et al. (26). We used Cochran’s Q-statistic to test for between-study heterogeneity, and the I2 statistic was used to quantify the amount of between-study heterogeneity (27). To further explore the sources of heterogeneity, we carried out multiple subgroup analysis when two or more datasets per subgroup were available for the given analysis in term of study design, study populations, comparisons exposures, outcome measurements and risk of bias in all included studies. Besides, publication bias was tested by funnel plot symmetry combined with Egger’s test to explore small study effects (28). If publication bias was found existence, we would apply a Duvall and Tweedle trim-and-fill method to adjust for risk estimates (29). Sensitivity analysis was performed to test the relative influence of individual cohort on the combined results. All statistical tests were 2-sided and P values of <0.05 were considered statistically significant.
Results
Literature search and study characteristics
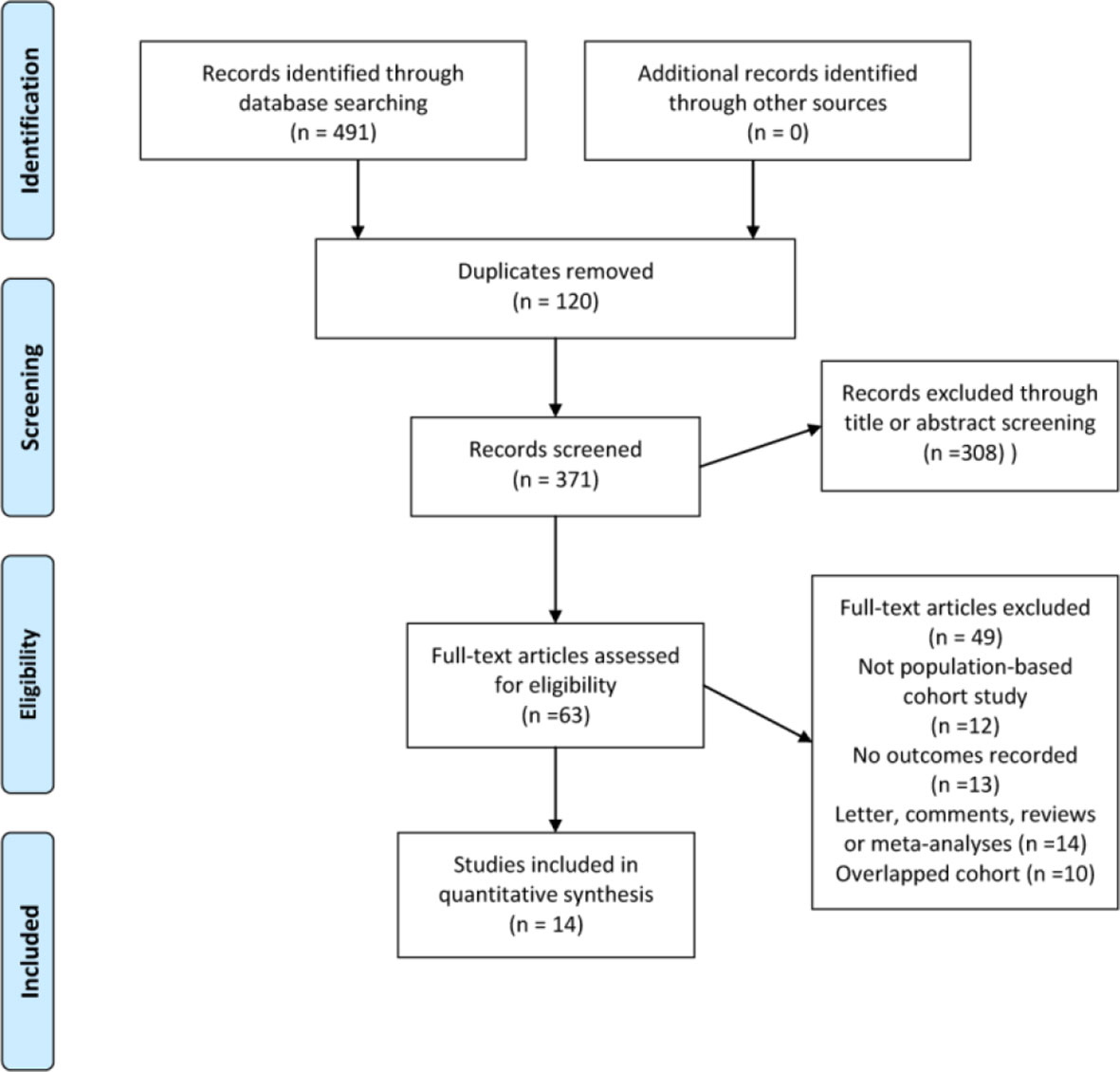
Our literature search identified 491 eligible citations, 120 of which were excluded due to duplication. A further 308 were subsequently excluded based on title and abstract review, yielding 63 citations for full-text review. Because of a lack of outcome data, non-population-based cohort study design, reviews and meta-analyses without original data, a total of 14 studies comprising 693134 individuals satisfied the inclusion criteria and were eligible to be included in the final meta-analysis and quality assessment (Figure 1). Table 1 presents the sociodemographic and clinical characteristics of the 14 included studies (13, 15–17, 30–39).
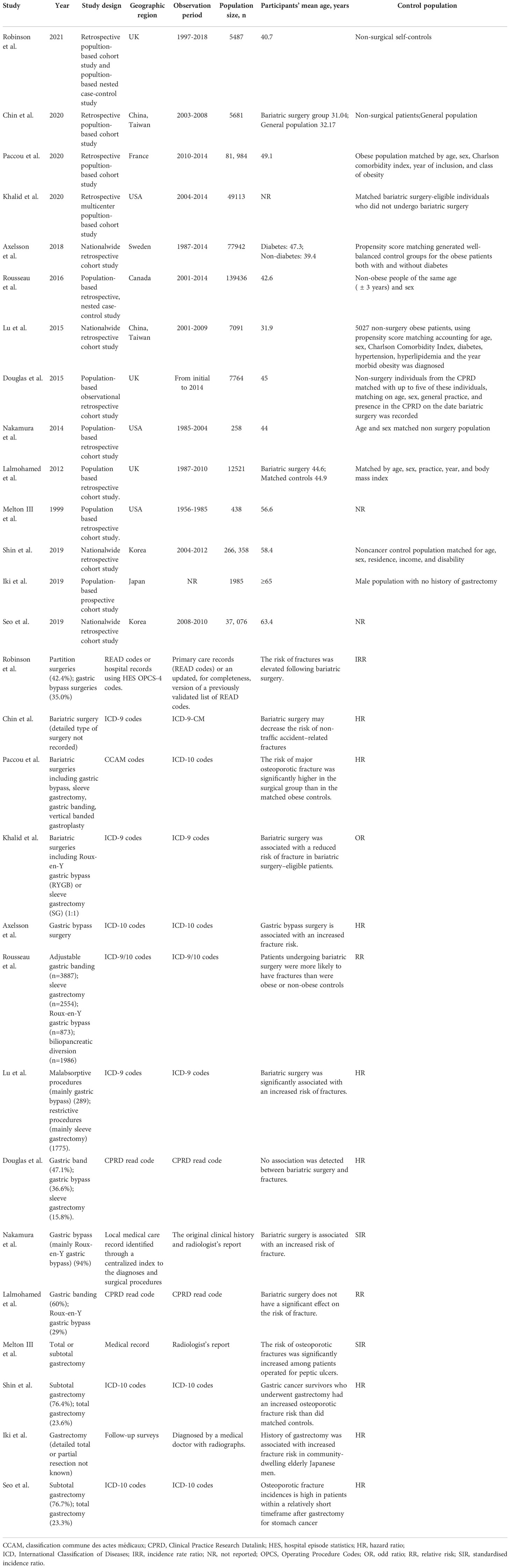
Table 1 Baseline characteristics of studies included in the analysis of associations of gastric surgery with subsequent fracture risk.
Of the studies published between 1999 and 2021, 4 studies were conducted in the United States (32, 34, 37, 39), 5 in Europe (13, 15, 30, 36, 38) and 5 in Asia (16, 17, 31, 33, 35). Most reports were retrospective cohort studies using population-based or national wide databases as data sources. Studies ranged in size from 258 to 266358 participants with a median sample size of 10143 (interquartile range, 5536-70735). The median follow-up duration was 4.5 years (range, 2.2-14.8 years). Nine studies enrolled individuals undergoing gastric surgery and control populations matched for at least five variables. Most studies ascertained the diagnosis and fracture outcome through medical records according to the ICD-9/10 codes.
Methodological quality (risk of bias)
The overall risk of bias was moderate to high for all studies based on the NOS tool. Bias was frequently seen in term of adequacy of follow-up followed by selection of control cohort and comparability of cohorts. We found that two studies (13, 35) was judged low risk of bias in all domains with an NOS score of 9. The detailed rationale for the risk of bias assessment is present in Table 2.
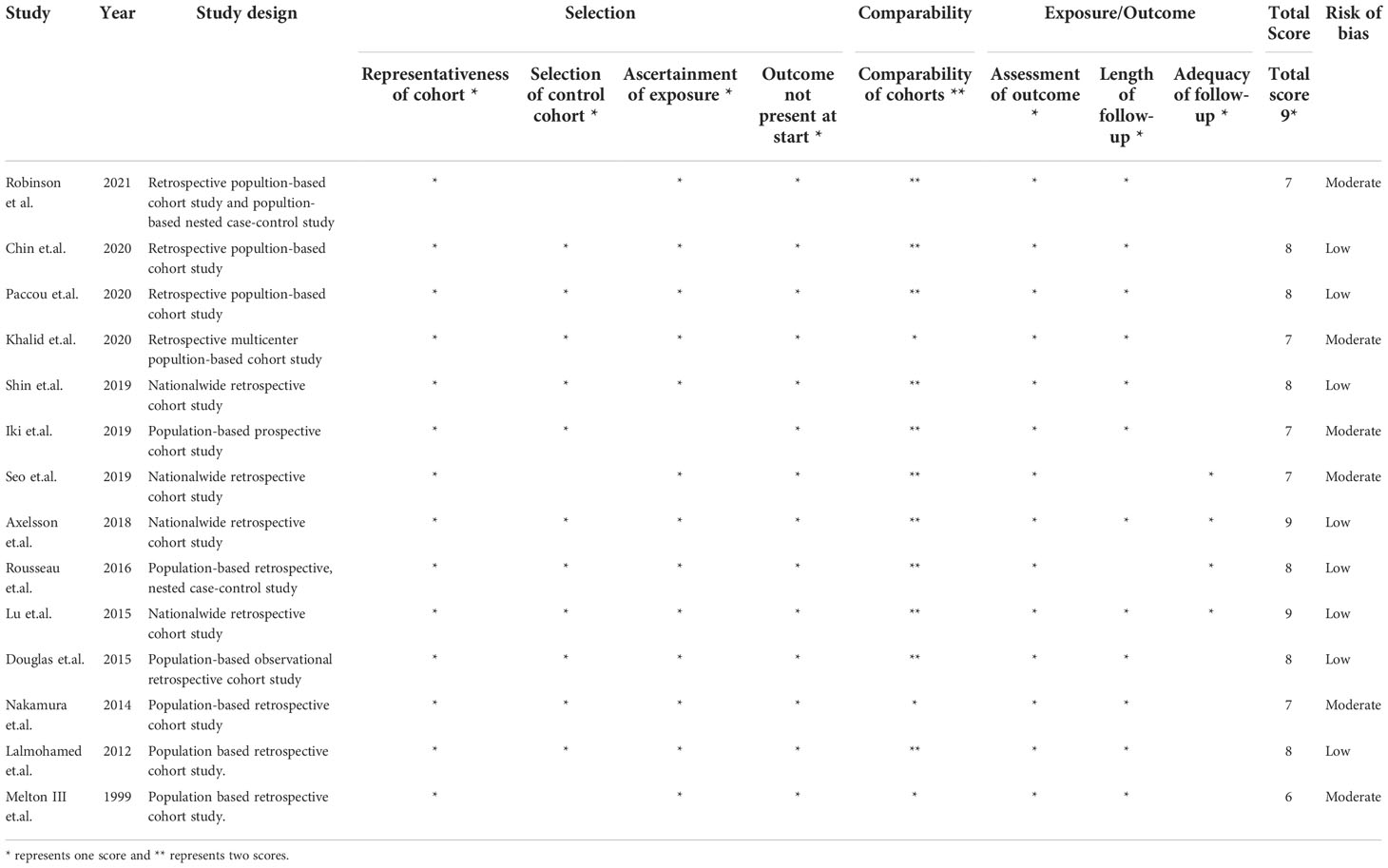
Table 2 Methodological quality score of the included studies based on the Newcastle–Ottawa scale (NOS) tool.
Associations between gastric surgery and the risk of fracture
Overall, random-effects meta-analysis of the 14 studies showed that the summary RR of fracture reached 1.45 (95% CI, 1.23 - 1.72) in survivors following gastric surgery compared with control populations. We noted significant inter-study heterogeneity (I2 = 95.8%; P< 0.001) (Figure 2).
Subgroup analysis
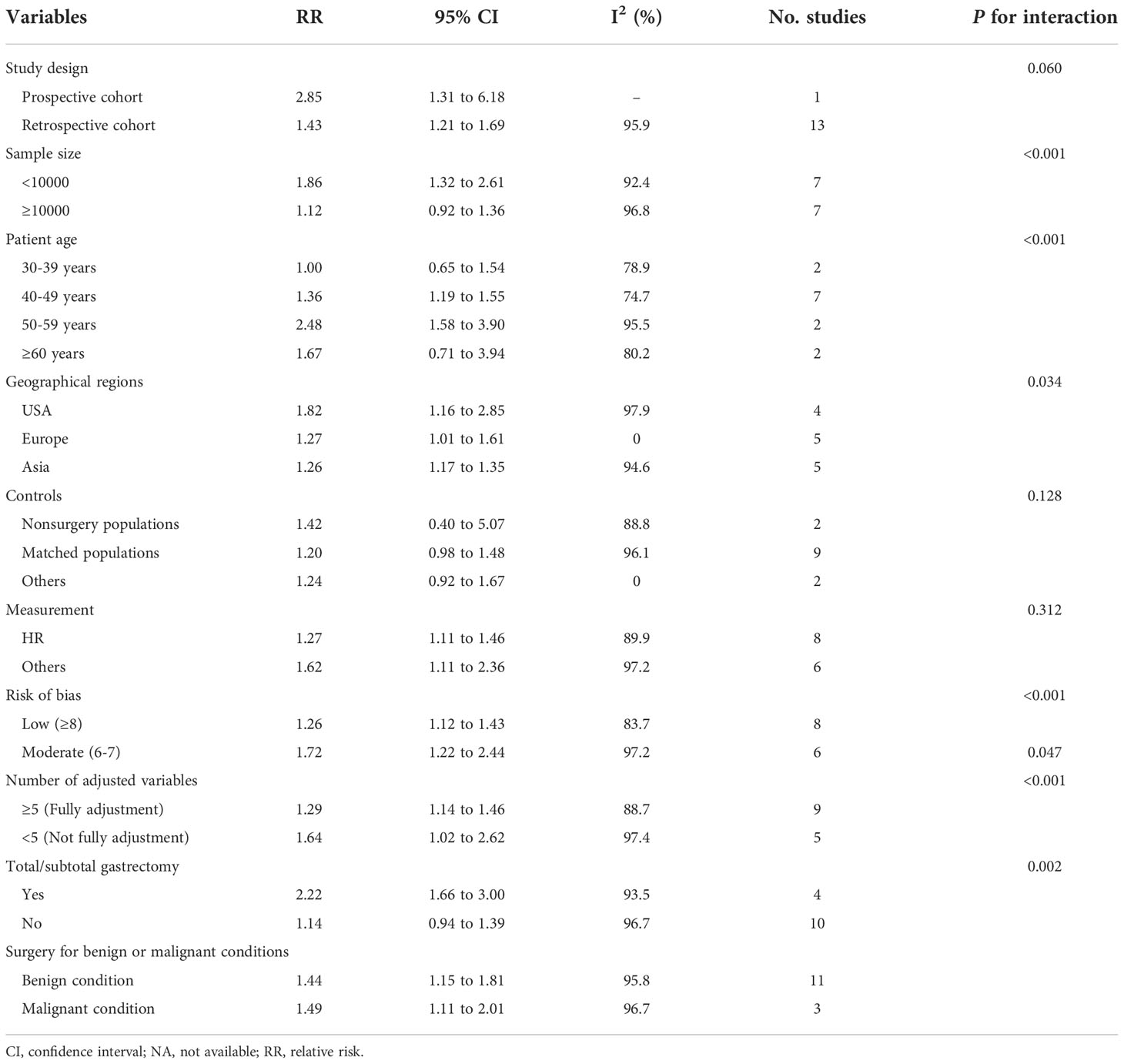
Subgroup analyses indicated that gastric surgery was associated with an increased risk of fracture among survivors with an age range from 40 to 59 years (40-49 years, RR 1.36, 95% CI 1.19-1.55; 50-59 years, RR 2.48, 95% CI 1.58-3.90), while not among survivors ≥ 65 years (RR 1.67, 95% CI 0.71-3.94) or with an age range from 30 to 39 years (RR 1.00, 95% CI 0.65-1.54) (Table 3). For specific investigated fracture sites, it was found that significant associations were noted for risks of upper limb (shoulder, humerus, elbow, forearm, and wrist) fracture (RR 1.33, 95% CI 1.09 -1.63), spine fracture (RR 1.34, 95% CI 1.05-1.71), pelvis and hip fracture (RR 1.89, 95% CI 1.48-2.42) and lower limb fracture (RR 1.53, 95% CI 1.10-2.11) (Table 4). Significant associations were also observed for patients having total or subtotal gastrectomy (RR 2.22, 95% CI 1.66 to 3.00), gastric bypass (RR 1.48, 95% CI 1.26 to 1.74) (Tables 3, 4), and a similar trend was observed for preserved passage procedures (including sleeve gastrectomy, gastric banding, vertical banded gastroplasty and other procedures that preserved the passage through the duodenum and proximal small bowel, in contrast to gastric bypass), though the difference did not reach statistically significant (RR 1.10, 95% CI 0.95 to 1.26). Furthermore, the increased fracture risk associated with gastric surgery was more evident among several other subgroups including in studies with different study design, sample size less than 10000, studies conducted in different geographical regions, with different measurement of associations, risk of bias and different degree of adjustment.
In addition, heterogeneity was high in the analysis of subgroups of studies conducted in the United States (I2 = 97.9%) and Asia (I2 = 94.6%), but was not detected in Europe (I2 = 0.0%). Similarly, heterogeneity was also significant in the subgroups of elder age (≥50 years) (I2 = 95.5% and 80.2%, respectively) and younger individuals (30-39 years) (I2 = 78.9%), fracture site of skull/face (I2 = 85.0%), upper limb (I2 = 88.8%) and lower limb(I2 = 86.2%), and surgery type of gastric bypass (I2 = 91.0%) and adjustable gastric banding (I2 = 84.1%), but was slight or not detected in the subgroups of fracture site of spine (I2 = 26.6%), pelvis and hip (I2 = 29.4%), the subgroup with surgery type of sleeve gastrectomy (38.1%). These analyses indicated that geographic region, age, surgery type, and fracture site could be potential sources of heterogeneity. Moreover, the residual heterogeneity could originate from other variation in demographic variables among the individuals of each included study.
Sensitivity analyses and publication bias
Using the leave-one-out sensitivity analyses, we further tested the stability of the result and indicated that no single study substantially altered the pooled risk estimates (lowest RR 1.35, 95% CI, 1.17-1.56; highest RR 1.53, 95% CI, 1.32-1.77) (Supplementary Table S1 and Supplementary Figure S1). Visual inspection of the contour enhanced funnel plot indicated asymmetry, which implied evidence of publication bias (Supplementary Figure S2). Publication bias test found no missing studies in the funnel plot region, suggesting that publication bias was unlikely to be the main cause of plot asymmetry. Both Begg’s test and Egger’s test for small study effects were insignificant (P= 0.381 for Begg’s test and P= 0.764 for Egger’s test).The trim and fill method used to adjusted for publication bias did not lead to imputation of any hypothetical missing studies, and the risk estimate remained the same.
Discussion
Principal findings
In this pooled analysis of 14 population-based cohort studies, we found statistically significant increase in the risk of fracture for gastric surgery survivors compared to that for nonsurgery individuals. The results remained largely unchanged after adjustment for potential publication bias. Moreover, our results indicate that gastric surgery contributes to the future development of fracture especially for individuals with an age range from 40 to 59 years, fracture sites including upper limb, lower limb, pelvis and hip, and the results is constant across different geographical regions and other study features.
Based on the results of subgroup analyses, we found that the fracture risk was significantly increased for different types of gastric surgery including gastric bypass and total or subtotal gastrectomy. These findings appear reasonable, because these surgeries either divert ingested nutrients or reduce the volume of the stomach, which will have a significant impact on nutrient absorption in the stomach and duodenum, affecting bone metabolism and increasing the risk of fracture. Despite the fact that the fracture risk for preserved passage procedures was not statistically significant, we propose that larger prospective cohort studies be conducted to demonstrate the associations. We also found that the risk of fracture was higher in patients over the age of 40, which indicated that the effect of gastric surgery on gastric absorptive compensation was more obvious in older patients than in younger patients.
The results of our study are similar to and support previously findings from other published systematic reviews and meta-analyses, which also demonstrated the association between gastric surgery and subsequent risk of fracture (40–43). However, those four review articles only focused on obese patients undergoing specific bariatric surgery. Our study further extended the participants including all individuals receiving gastric surgery for gastric tumor or ulcer removal, and weight loss (bariatric surgery). Moreover, these four meta-analyses mostly used non-representative cohorts with relatively high risk of selection bias. To the best of our knowledge, this pooled analysis is the first and most comprehensive one involving high representative populations to meta-analyze the associations between previous gastric surgery and subsequent fracture risk from less biased population-based cohorts.
An evident increased risk in the age range from 40-59 years was observed, indicated by the summary RR through subgroup analyses stratified by patient age (Table 3). Though the hypothesis of this finding is not clear, the result should be further confirmed by large prospective cohort studies as there were few studies included for analyses with limited statistical power.
Potential mechanisms
The potential mechanism underlying the gastric surgery-related increase in fracture risk is not so clearly demonstrated. Several possible theories have been proposed to explain this finding.
A significant metabolic change after gastric surgery is malabsorption of calcium and vitamin D (44). Due to low gastric acidity in the remnant stomach after gastrectomy or bariatric surgery, and there is no passage of nutrients through the duodenum in patients having had gastric bypass, calcium absorption will be reduced (45). Another possible cause of calcium deficiency is reduced food intake after gastric surgery (46). Other causes such as pancreatic exocrine dysfunction after gastric surgery or inactivation of lipase caused by bacterial overgrowth can also affect vitamin D absorption (17, 47). Secondly, inadequate dietary intake and changes in calcium and vitamin D metabolism can lead to secondary hyperparathyroidism (48, 49). Meanwhile, in order to maintain serum calcium levels, bone mass will decrease. Hyperparathyroidism can lead to adverse changes in the microstructure of cortical bone, which increases the risk of osteoporosis and fracture (50, 51).
Metabolism-related weight loss is the third potential cause of increased fracture risk in people after gastric surgery. Due to the changes in gastrointestinal anatomy after gastric surgery, gastrointestinal motility and function also change, resulting in irreversible functional changes such as dyspepsia and malabsorption (52, 53). Most of these patients will experience varying degrees of weight loss after operation (54). Weight loss can change the mechanical load bearing of human bones, which can increase the risk of fractures. In addition, bariatric surgery will lead to the reduction of a variety of hormones in the body, such as estrogen and insulin, thus affecting bone metabolism and aggravating bone loss (55, 56). It has been reported that weight loss after gastrectomy is the main factor aggravating bone loss (46, 57).
Strengths and limitations
Compared to previous ones, this study has several important strengths. Firstly, the current study included the first and the largest sample to date using high representative population for pooled analysis, providing a comprehensive summary of the evidence on the association between of gastric surgery and subsequent fracture risk. Secondly, we comprehensively searched the relevant databases using sensitive search strategies, facilitating retrieval of as many relevant studies as possible globally. Thirdly, we only selected studies of representative national wide or population-based cohort and excluded studies of hospital/community-based cohort studies. The high-quality evidence makes the results more credible. Fourthly, we explored the sources of heterogeneity and impact of publication bias through the use of multiple approaches including subgroup analyses, sensitivity analyses, trim and fill analyses. Our findings confirmed that the main results were robust.
Nevertheless, several limitations are evident in our study. Firstly, a high degree of inter-study heterogeneity was found. Though multiple subgroup analyses were conducted, there was still considerable moderate to high between-study heterogeneity. Even so, the results of the subgroup analysis and sensitivity analysis are mostly consistent with the main result. Therefore, we believe that heterogeneity does not substantially affect the main findings to a great extent. However, one major concern was that we could not assess the effect of participants’ treatment with vitamin D and calcium supplements on the result of our findings due to the unavailability of such information from the majority of the included studies. Secondly, our findings are mainly based on a retrospective cohort study (19, 20), in which the design of the study may be subject to a variety of confounding factors and bias. However, the results of 9 from those 14 studies were obtained from fully matched covariates (≥5 adjusted variables) and compared with the non-surgery or general population (Table 4), indicating that this association was consistent among different clinical scenarios. Thirdly, we performed the pooled analysis based on the study level evidence. Therefore, we could not carried out more subgroup analyses (e.g. fracture time point following surgery) due to the lack of access to the individual patient data. Fourthly, nonsignificant risk estimates obtained from a few subgroup analyses may be due to the low statistical power caused by insufficient sample size. We advocate that high quality prospective cohort studies on this aspect be carried out in the future. Finally, we only included three major databases (PubMed, Embase and Cochrane Library) without involving the unpublished grey literature for analysis though they covered more than 90% of all citations.
Implications
Despite all these limitations, the current study provides alarming clinical implication for risk of fracture in people undergoing gastric surgery, estimating a crude risk ratio for any fracture of 1.45 (95% CI, 1.23 - 1.72). Early prevention and timely interventions are of great clinical significance in the prevention and treatment of these high-risk individuals. Moreover, the increased risk of fracture should be also mentioned during the preoperative informed consent process. Additionally, there is need for better understanding of the pathophysiological mechanisms, basic research on hormonal and neuro-intestinal pathways responsible for decreased bone quality.
Conclusions
In conclusion, this meta-analysis showed that individuals who underwent gastric surgery may have an increased risk of fracture. Based on the subgroup analysis results stratified by most baseline variables, it is found that the results are still consistent and biologically plausible. However, before we get a high level of evidence based on prospective large cohort studies to prove this relationship, we should still interpret the results very carefully.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
Study concept and design: ZM, WZ, ZJ, ZC. Acquisition of data: QZ, CW, ZS, HW, ZM. Analysis and interpretation of data: CW, ZX, HW, ZM, WZ, ZJ, LC. Drafting of the manuscript: QZ, ZM. Critical revision of the manuscript for important intellectual content: All authors. Corresponding authors: ZM, WZ, ZJ, ZC.
Funding
This study was supported by the Department of Science and Technology of Guangdong Province (No.2021A151511077) and the Traditional Chinese Medicine Bureau of Guangdong Province (No.20225012), and the Special Project for Clinical Research of Guangdong Provincial Hospital of Chinese Medicine (YN10101902) , the Guangdong Administration for Market Regulation (Guangdong Intellectual Property Administration) (No.〔2022〕158), the National Regional Traditional Chinese Medicine (Specialist) Clinic Construction ((2018)205), Scientific Research Project from the Education Department of Fujian Province(No.JAT190205) and Joint Funds for the Innovation of Science and Technology, Fujian Province (No.2020Y9112).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1001662/full#supplementary-material
References
1. GBD 2019 Fracture Collaborators. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990-2019: a systematic analysis from the global burden of disease study 2019. Lancet Healthy Longevity (2021) 2(9):e580–e92. doi: 10.1016/s2666-7568(21)00172-0
2. Veronese N, Maggi S. Epidemiology and social costs of hip fracture. Injury (2018) 49(8):1458–60. doi: 10.1016/j.injury.2018.04.015
3. GBD 2019 Fracture Collaborators. Global, regional, and national burden of bone fractures in 204 countries and territories, 1990-2019: a systematic analysis from the global burden of disease study 2019. Lancet Healthy Longev (2021) 2(9):e580–92. doi: 10.1016/S2666-7568(21)00172-0
4. Chen Z, Wen Y, Qiu M, Fang L, Jin O, Gu J. The pattern and trends of disease burden due to low bone mineral density from 1990 to 2019 in China: findings from the global burden of disease study 2019. Arch Osteoporos. (2022) 17(1):39. doi: 10.1007/s11657-022-01079-9
5. Lorentzon M, Johansson H, Harvey NC, Liu E, Vandenput L, McCloskey EV, et al. Osteoporosis and fractures in women: the burden of disease. Climacteric. (2022) 25(1):4–10. doi: 10.1080/13697137.2021.1951206
6. Joshi SS, Badgwell BD. Current treatment and recent progress in gastric cancer. CA Cancer J Clin (2021) 71(3):264–79. doi: 10.3322/caac.21657
7. Welbourn R, Hollyman M. Bariatric-metabolic surgery utilisation in patients with and without diabetes: Data from the IFSO global registry 2015-2018. Obes Surg (2021) 31(6):2391–400. doi: 10.1007/s11695-021-05280-6
8. Ahlin S, Peltonen M, Sjöholm K, Anveden Å, Jacobson P, Andersson-Assarsson JC, et al. Fracture risk after three bariatric surgery procedures in Swedish obese subjects: up to 26 years follow-up of a controlled intervention study. J Intern Med (2020) 287(5):546–57. doi: 10.1111/joim.13020
9. Oh HJ, Lim CH, Yoon BH, Yoon SB, Baeg MK, Kim WC, et al. Fracture after gastrectomy for gastric cancer: A long-term follow-up observational study. Eur J Cancer. (2017) 72:28–36. doi: 10.1016/j.ejca.2016.11.023
10. Casimiro I, Sam S, Brady MJ. Endocrine implications of bariatric surgery: a review on the intersection between incretins, bone, and sex hormones. Physiol Rep (2019) 7(10):e14111–e11. doi: 10.14814/phy2.14111
11. Stein EM, Silverberg SJ. Bone loss after bariatric surgery: causes, consequences, and management. Lancet Diabetes Endocrinol (2014) 2(2):165–74. doi: 10.1016/S2213-8587(13)70183-9
12. Clarke BL, Khosla S. Physiology of bone loss. Radiologic Clinics (2010) 48(3):483–95. doi: 10.1016/j.rcl.2010.02.014
13. Axelsson KF, Werling M, Eliasson B, Szabo E, Näslund I, Wedel H, et al. Fracture risk after gastric bypass surgery: a retrospective cohort study. J Bone Miner Res (2018) 33(12):2122–31. doi: 10.1002/jbmr.3553
14. Oh HJ, Ryu KH, Park BJ, Yoon BH. Osteoporosis and osteoporotic fractures in gastrointestinal disease. J Bone Metab (2018) 25(4):213–17. doi: 10.11005/jbm.2018.25.4.213
15. Paccou J, Martignène N, Lespessailles E, Babykina E, Pattou F, Cortet B, et al. Gastric bypass but not sleeve gastrectomy increases risk of major osteoporotic fracture: French population-based cohort study. J Bone Miner Res (2020) 35(8):1415–23. doi: 10.1002/jbmr.4012
16. Seo GH, Kang HY, Choe EKJM. Osteoporosis and fracture after gastrectomy for stomach cancer: a nationwide claims study. (2018) 97(17):e0532. doi: 10.1097/MD.0000000000010532
17. Shin DW, Suh B, Lim H, Suh YS, Choi YJ, Jeong SM, et al. Increased risk of osteoporotic fracture in postgastrectomy gastric cancer survivors compared with matched controls: A nationwide cohort study in Korea. Am J Gastroenterol (2019) 114(11):1735–43. doi: 10.14309/ajg.0000000000000436
18. Alsaed OS, Al-Allaf AW, Elgenaied I, Jebril RA, Sasi S, Ahmed AO, et al. Increased fracture risk after bariatric surgery: a case-controlled study with a long-term follow-up. Obes Surg (2021) 31(11):4853–60. doi: 10.1007/s11695-021-05655-9
19. Elaine WY, Kim SC, Sturgeon DJ, Lindeman KG, Weissman JS. Fracture risk after roux-en-Y gastric bypass vs adjustable gastric banding among medicare beneficiaries. JAMA Surg (2019) 154(8):746–53. doi: 10.1001/jamasurg.2019.1157
20. Fashandi AZ, Mehaffey JH, Hawkins RB, Schirmer B, Hallowell PT. Bariatric surgery increases risk of bone fracture. Surg endoscopy (2018) 32(6):2650–55. doi: 10.1007/s00464-017-5628-4
21. Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. Int J Surg (2021) 88:105906. doi: 10.1016/j.ijsu.2021.105906
22. Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. meta-analysis of observational studies in epidemiology (MOOSE) group. Jama (2000) 283(15):2008–12. doi: 10.1001/jama.283.15.2008
23. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol (2010) 25(9):603–5. doi: 10.1007/s10654-010-9491-z
24. DerSimonian R, Laird N. Meta-analysis in clinical trials. Controlled Clin trials (1986) 7(3):177–88. doi: 10.1016/0197-2456(86)90046-2
25. Chaturvedi AK, Mbulaiteye SM, Engels EA. Underestimation of relative risks by standardized incidence ratios for AIDS-related cancers. Ann Epidemiol (2008) 18(3):230–4. doi: 10.1016/j.annepidem.2007.10.005
26. Parmar MK, Torri V, Stewart L. Extracting summary statistics to perform meta-analyses of the published literature for survival endpoints. Stat Med (1998) 17(24):2815–34. doi: 10.1002/(SICI)1097-0258(19981230)17:24<2815::AID-SIM110>3.0.CO;2-8
27. Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj (2003) 327(7414):557–60. doi: 10.1136/bmj.327.7414.557
28. Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj (1997) 315(7109):629–34. doi: 10.1136/bmj.315.7109.629
29. Duval S, Tweedie R. Trim and fill: A simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics (2000) 56(2):455–63. doi: 10.1111/j.0006-341X.2000.00455.x
30. Robinson DE, Douglas I, Tan GD, Delmestri A, Judge A, Cooper C, et al. Bariatric surgery increases the rate of major fracture: self controlled case series study in UK clinical practice research datalink. (2021) 36(11):2153–61. doi: 10.1002/jbmr.4405
31. Chin WL, Chi P-J, Hung W-C, Lin CW, Chen CY, Chen JH. Bariatric surgery decreases the risk of non-traffic accident–related fractures in patients with obesity: Real-world data from Taiwan. Obes Surg (2021) 31(5):2231–40. doi: 10.1007/s11695-021-05262-8
32. Khalid SI, Omotosho PA, Spagnoli A, Torquati A. Association of bariatric surgery with risk of fracture in patients with severe obesity. JAMA Network Open (2020) 3(6):e207419–e19. doi: 10.1001/jamanetworkopen.2020.7419
33. Iki M, Fujita Y, Kouda K, Yura A, Tachiki T, Tamaki J, et al. Increased risk of osteoporotic fracture in community-dwelling elderly men 20 or more years after gastrectomy: The fujiwara-kyo osteoporosis risk in men (FORMEN) cohort study. Bone (2019) 127:250–59. doi: 10.1016/j.bone.2019.06.014
34. Rousseau C, Jean S, Gamache P, Lebel S, Mac-Way F, Biertho L, et al. Change in fracture risk and fracture pattern after bariatric surgery: nested case-control study. (2016) 354:i3794. doi: 10.1136/bmj.i3794
35. Lu C-W, Chang Y-K, Chang H-H, Kuo CS, Huang CT, Hsu CC, et al. Fracture risk after bariatric surgery: a 12-year nationwide cohort study. (2015) 94(48):e2087. doi: 10.1097/MD.0000000000002087
36. Douglas IJ, Bhaskaran K, Batterham RL, Smeeth L. Bariatric surgery in the united kingdom: A cohort study of weight loss and clinical outcomes in routine clinical care. PloS Med (2015) 12(12):e1001925. doi: 10.1371/journal.pmed.1001925
37. Nakamura K, Haglind E, Clowes J, Achenbach SJ, Atkinson EJ, Melton LJ 3rd, et al. Fracture risk following bariatric surgery: a population-based study. Osteoporos Int (2014) 25(1):151–58. doi: 10.1007/s00198-013-2463-x
38. Lalmohamed A, de Vries F, Bazelier MT, Cooper A, van Staa TP, et al. Risk of fracture after bariatric surgery in the united kingdom: population based, retrospective cohort study. (2012) 345:e5085. doi: 10.1136/bmj.e5085
39. Melton IIIL, Crowson C, Khosla S, O'Fallon WM. Fracture risk after surgery for peptic ulcer disease: a population-based cohort study. Bone (1999) 25(1):61–7. doi: 10.1016/S8756-3282(99)00097-6
40. Chaves Pereira de Holanda N, de Lima Carlos I, Chaves de Holanda Limeira C, Cesarino de Sousa D, Serra de Lima Junior FA, Telis de Vilela Araújo A, et al. Fracture risk after bariatric surgery: A systematic literature review and meta-analysis. Endocrine Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinologists (2021) 28(1):58–69. doi: 10.1016/j.eprac.2021.09.007
41. Dargai F. Letter to the Editor concerning: Fractures in adults after weight loss from bariatric surgery and weight management programs for obesity: Systematic review and meta-analysis. Obes Surg (2019) 29(10):3363–64. doi: 10.1007/s11695-019-04121-x
42. Zhang Q, Chen Y, Li J, Chen D, Cheng Z, Xu S, et al. A meta-analysis of the effects of bariatric surgery on fracture risk. Obes Rev an Off J Int Assoc Study Obes (2018) 19(5):728–36. doi: 10.1111/obr.12665
43. Zhang Q, Dong J, Zhou D, Liu F. Comparative risk of fracture for bariatric procedures in patients with obesity: A systematic review and Bayesian network meta-analysis. Int J Surg (London England) (2020) 75:13–23. doi: 10.1016/j.ijsu.2020.01.018
44. Schafer AL. Vitamin d and intestinal calcium transport after bariatric surgery. J Steroid Biochem Mol Biol (2017) 173:202–10. doi: 10.1016/j.jsbmb.2016.12.012
45. Eom BW, Kim J, Kim DH, Kim YI, Yoon HM, Cho SJ, et al. Recovery of food intake after gastrectomy for gastric cancer: Based on a Large-scale gastric cancer cohort. Digestive Surg (2018) 35(3):220–29. doi: 10.1159/000477779
46. Baek KH, Jeon HM, Lee SS, Lim DJ, Oh KW, Lee WY, et al. Short-term changes in bone and mineral metabolism following gastrectomy in gastric cancer patients. Bone (2008) 42(1):61–7. doi: 10.1016/j.bone.2007.08.027
47. Straatman J, Wiegel J, van der Wielen N, Jansma EP, Cuesta MA, van der Peet DL. Systematic review of exocrine pancreatic insufficiency after gastrectomy for cancer. Digestive Surg (2017) 34(5):364–70. doi: 10.1159/000454958
48. Wei J-H, Lee W-J, Chong K, Lee YC, Chen SC, Huang PH, et al. High incidence of secondary hyperparathyroidism in bariatric patients: comparing different procedures. Obes Surg (2018) 28(3):798–804. doi: 10.1007/s11695-017-2932-y
49. Altawil E, Alkofide H, Alamri H, Alhassan N, Alsubaie H, Alqahtani A, et al. Secondary hyperparathyroidism in obese patients post sleeve gastrectomy. Diabetes Metab Syndrome Obesity: Targets Ther (2021) 14:4059. doi: 10.2147/DMSO.S325148
50. Silva BC, Bilezikian JP. Skeletal abnormalities in hypoparathyroidism and in primary hyperparathyroidism. Rev Endocrine Metab Disord (2020) 22(4):789–802. doi: 10.1007/s11154-020-09614-0
51. Ejlsmark-Svensson H, Rolighed L, Harsløf T, Rejnmark L. Risk of fractures in primary hyperparathyroidism: a systematic review and meta-analysis. Osteoporosis Int (2021) 32(6):1053–60. doi: 10.1007/s00198-021-05822-9
52. Sioka E, Tzovaras G, Perivoliotis K, Bakalis V, Zachari E, Magouliotis D, et al. Impact of laparoscopic sleeve gastrectomy on gastrointestinal motility. Gastroenterol Res Pract (2018) 2018:4135813. doi: 10.1155/2018/4135813
53. Park KB, Park JY, Lee SS, Kwon OK, Chung HY, et al. Impact of body mass index on the quality of life after total gastrectomy for gastric cancer. Cancer Res treatment: Off J Korean Cancer Assoc (2018) 50(3):852. doi: 10.4143/crt.2017.080
54. Davis JL, Ripley RT. Postgastrectomy syndromes and nutritional considerations following gastric surgery. Surg Clinics (2017) 97(2):277–93. doi: 10.1016/j.suc.2016.11.005
55. Sarwer DB, Spitzer JC, Wadden TA, Mitchell JE, Lancaster K, Courcoulas A, et al. Changes in sexual functioning and sex hormone levels in women following bariatric surgery. JAMA Surg (2014) 149(1):26–33. doi: 10.1001/jamasurg.2013.5022
56. Sarwer DB, Spitzer JC, Wadden TA, Rosen RC, Mitchell JE, Lancaster K, et al. Sexual functioning and sex hormones in men who underwent bariatric surgery. Surg Obes Related Dis (2015) 11(3):643–51. doi: 10.1016/j.soard.2014.12.014
Keywords: bone fracture, gastric cancer, surgery, cohort study, pooled analysis
Citation: Zou Q, Wei C, Shao Z, Wang H, Xiao Z, Cao L, Mei Z, Zhao W, Jiang Z and Chen Z (2022) Risk of fracture following gastric surgery for benign and malignant conditions: A study level pooled analysis of population-based cohort studies. Front. Oncol. 12:1001662. doi: 10.3389/fonc.2022.1001662
Received: 23 July 2022; Accepted: 01 November 2022;
Published: 21 November 2022.
Edited by:
Jinqiu Jacky Yuan, Seventh Affiliated Hospital, Sun Yat-sen University, ChinaReviewed by:
Sina Azadnajafabad, Tehran University of Medical Sciences, IranMagnus Sundbom, Uppsala University, Sweden
Copyright © 2022 Zou, Wei, Shao, Wang, Xiao, Cao, Mei, Zhao, Jiang and Chen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zubing Mei, aGVycm1heW9yQDEyNi5jb20=; aGVycm1heW9yQHNodXRjbS5lZHUuY24=; Wei Zhao, emhhb3dlaTc1QHNpbmEuY29t; Zhiqiang Chen, d3NzcUBnenVjbS5lZHUuY24=; Zhi Jiang, MjAwMGppYW5nemhpQDE2My5jb20=
†These authors have contributed equally to this work and share senior authorship
 Qiuping Zou1,2†
Qiuping Zou1,2† Zhuo Shao
Zhuo Shao Hao Wang
Hao Wang Lixing Cao
Lixing Cao Zubing Mei
Zubing Mei Zhi Jiang
Zhi Jiang