- 1Translational Research Laboratory, Tata Memorial Centre, Advanced Centre for Treatment, Research and Education in Cancer, Navi Mumbai, India
- 2Homi Bhabha National Institute, Mumbai, India
- 3Department of Head and Neck Surgery, Tata Memorial Centre, Tata Memorial Hospital, Mumbai, India
- 4Section of Biostatistics, Tata Memorial Centre, Centre for Cancer Epidemiology, Navi Mumbai, India
Background: Our earlier studies have shown that cell-free chromatin particles (cfChPs) that are released from dying cancer cells are readily internalised by bystander cells leading to activation of two hallmarks of cancer viz. genome instability and inflammation. These hallmarks could be down-regulated by deactivating cfChPs via medium of oxygen radicals generated upon admixing small quantities of the nutraceuticals resveratrol (R) and copper (Cu). In this exploratory study, we investigated whether oral administration of R and Cu (R-Cu) would down-regulate the hallmarks of cancer and immune checkpoints in advanced squamous cell carcinoma of oral cavity (OSCC).
Patients and methods: The study comprised of 25 patients divided into 5 equal groups. Five patients acted as controls; the remaining 20 were given R-Cu in four escalating doses. The lowest dose of R-Cu was 5.6mg and 560ng respectively, and the highest dose was 500mg and 5mg respectively. An initial biopsy was taken from patients at first presentation, and a second biopsy was taken 2 weeks later on the operating table. R-Cu was administered orally twice daily in the intervening period. Confocal microscopy was performed on tumour sections after fluorescent immuno-staining with anti-DNA and anti-histone antibodies to detect presence of cfChPs in the tumour micro-environment (TME). Immunofluorescence analysis was performed for 23 biomarkers representing the 10 Hallmarks of cancer, including 5 immune checkpoints, defined by Hanahan and Weinberg.
Results: Confocal microscopy detected copious presence of cfChPs in TME of OSCC, which were eradicated/deactivated following two-week treatment with R-Cu. Eradication of cfChPs from TME was associated with marked down-regulation of 21/23 biomarkers, including the five immune checkpoints. The lower two doses of R-Cu were more effective than the higher doses. No adverse effects attributable to R-Cu were observed.
Conclusion: These results suggest that cfChPs released into TME from dying cancer cells are global instigators for cancer hallmarks and immune checkpoints in surviving cancer cells. The ability of R-Cu to deactivate cfChPs raises the prospect of a novel and non-toxic form of cancer treatment which sans killing of cancer cells, and instead induces healing by down-regulating cancer hallmarks and immune check-points.
Clinical Trial Registration: http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=19801&EncHid=&userName=CTRI/2018/03/012459.
Introduction
Results of treatment of advanced squamous cell carcinoma of oral cavity (OSCC) continue to remain unsatisfactory, and is associated with considerable toxic side effects. Novel therapeutic approaches that are non-toxic are urgently needed. Our earlier studies have shown that cell-free chromatin particles (cfChPs) that are released from dying cancer cells can readily enter into surrounding bystander cells leading to activation of two critical hallmarks of cancer viz. dsDNA breaks (genome instability) and inflammation, which could lead to their oncogenic transformation (1). Activation of these cancer hallmarks could be abrogated by concurrent treatment with a combination of the nutraceuticals resveratrol (R) and copper (Cu) (1, 2). Fukuhara et al. were the first to demonstrate that oxygen radicals are generated upon admixing R and Cu (3). They showed that R acts as a catalyst to reduce Cu (II) to Cu (I) resulting in generation of oxygen radicals that are capable of cleaving plasmid pBR322 DNA (3, 4). We have extended these findings to show that oxygen radicals that are generated following admixture of R and Cu (R-Cu) can degrade genomic DNA and RNA (5), and can deactivate extra-cellular cfChPs by degrading their DNA component, both in vitro and in vivo (1, 2, 6–10).
Oxygen radicals are short lived molecular entities which contain an unpaired electron making them exceptionally reactive as they search for another electron to pair with, and in the process they can damage biomolecules such as DNA, proteins and lipids (11). Oxygen radical are normally generated by mitochondria (12); their over-production leads to oxidative stress which has several harmful effects on host cells (13). However, paradoxically, when oxygen radicals are artificially generated in the extracellular compartments of the body, such as by R-Cu, they can have wide ranging therapeutic effects in animal models and in human conditions that are associated with elevated levels of extracellular cfChPs (2, 6–10). These effects are mediated via the ability of oxygen radicals to deactivate extracellular cfChPs.
Oral administration of R-Cu leads to generation of oxygen radicals in the stomach, which are readily absorbed and have systemic effects in the form of deactivation of extracellular cfChPs. The cellular genomes are, however, protected from damage as the entry of oxygen radicals triggers the cells to activate their anti-oxidant defence mechanism which detoxifies the invading agents (8). Our pre-clinical studies have shown that oral administration of small quantities of R-Cu can have remarkable therapeutic effects in conditions associated with elevated levels of extracellular cfChPs (2, 6–8). For example, R-Cu administered orally can prevent toxic side effects of chemotherapy (6), and radiotherapy (2), and prevent fatality in mice following bacterial endotoxin induced sepsis (7). We have also shown that prolonged oral administration of R-Cu to ageing mice can down-regulate several biological hallmarks of ageing and neurodegeneration (8). Our early results in patients receiving high dose chemotherapy and bone marrow transplantation for multiple myeloma have shown that orally administered R-Cu can significantly reduce Grade III-IV mucositis (9). Blood levels of inflammatory cytokines were also found to be significantly reduced following R-Cu treatment in that study (9). We have also shown in an observational study that oral administration of R-Cu to patients with severe Covid-19 reduced mortality by nearly 50% (10). Significantly, we have discovered that cfChPs deactivating activity of R-Cu is retained even when the molar concentration of Cu is reduced 10,000 fold with respect to that of R (2, 5–10). Consequently, in our pre-clinical and clinical studies, the molar ratio of R to Cu was maintained at 1:10-4 (2, 6–10).
In the present exploratory study, we investigated whether oral administration of R-Cu would lead to down-regulation of hallmarks of cancer and immune checkpoints in patients with OSCC. We show that cfChPs that were released from dying cancer cells are abundantly present in the tumour microenvironment (TME), and that orally administered R-Cu dramatically reduced cfChPs in TME. Elimination of cfChPs from TME was associated with significant down-regulation of 21/23 biomarkers representing the 10 hallmarks of cancer, including 5 immune check-points, that have been defined by Hanahan and Weinberg. These exploratory findings suggest that prolonged treatment with R-Cu may have the potential to induce healing without having to kill cancer cells.
Patients and methods
Ethics approval
This study was approved by Institutional Ethics Committee (IEC) of Advanced Centre for Treatment, Research and Education in Cancer, Tata Memorial Centre. A written informed consent was obtained from all study participants as stipulated by IEC. The trial was registered under Clinical Trial Registry of India (http://ctri.nic.in/Clinicaltrials/pmaindet2.php?trialid=19801&EncHid=&userName=CTRI/2018/03/012459).
Patients and R-Cu treatment
The study comprised of 25 patients with advanced OSCC who were divided into 5 groups of 5 patients each. Demographic details, histopathological type and grade, and tumour stage distribution of the 25 patients are given in Supplementary Table 1. All patients who were deemed to be suitable for surgery by the attending surgeon were included in this study. This being as exploratory study, no formal sample size calculation was undertaken; 25 seemed to us to be a feasible sample size since this number of patients could be recruited in 1 year to give us an inkling as to whether R-Cu was effective at all. A representative image of OSCC and H&E section of the tumour is given in Supplementary Figure 1. The first 5 patients acted as controls; the remaining 20 were given R-Cu orally, twice daily, in four escalating doses (Supplementary Table 2). Resveratrol (trade name TRANSMAX™) was procured from Biotivia, USA https://www.biotivia.com/product/transmax/); Chelated Copper was procured from Calrson Laboratories, USA (https://carlsonlabs.com/chelated-copper/). The lowest dose of R-Cu (Dose level I) comprised of 5.6mg of R and 560ng of Cu (molar ratio 1:10-4). This dose was arrived at by direct conversion of dose of R and Cu that we have used in our pre-clinical studies (R = 1mg/Kg and that of Cu 0.1µg/Kg) using a standard conversion formula (14). Dose levels II and III were approximately 10 fold and 100 fold higher than dose level I; dose level IV comprised of doses of R and Cu that have been recommended by the respective vendors for use as health supplements. Toxic side effects related to R-Cu treatment was monitored using CTCAE v3.03 guidelines.
R is supplied by the vendor as capsules containing 500mg of trans-resveratrol powder, and Cu is supplied as 5 mg tablets. For the purposes of this study, R powder was removed from the capsules and weighed as per requirement. Cu tablets were crushed in a pestle and mortar and the powder was weighed as per requirement. R powder being insoluble in water, and unpalatable at higher doses, was administered using honey as a vehicle (~15 mL). Cu, being soluble in water was administered as water based solution (20mL). R and Cu were administered orally, one after the other, twice daily for 2 weeks. The control group received vehicles (honey and water) alone. All above administrations were made on an empty stomach.
A diagnostic punch biopsy was taken from patients under local anaesthesia at the time of their first presentation to Tata Memorial Hospital, and a second biopsy was taken 2 weeks later at the time of surgery on the operating table under anaesthesia. In the intervening two weeks, R-Cu was administered orally in four escalating doses as described above (Supplementary Table 2). The control group received vehicles alone (~15mL honey followed by 20 mL of water). No adverse side effects attributable to R-Cu were recorded.
Fluorescence immune-staining and confocal microscopy
Formalin fixed paraffin embedded (FFPE) sections of tumour tissues were stained with fluorescent anti-DNA and anti-histone antibodies and examined by confocal microscopy, as described by us earlier (7). This was done to investigate the presence of cfChPs (if any) in TME. Fluorescence intensity was quantified by randomly choosing five confocal fields (~ 50 cells per field), and calculating mean fluorescence intensity (MFI) which was expressed as mean ± S.E.M.
Immunofluorescence
Indirect immunofluorescence (IF) on FFPE sections of tumour tissues were performed according to the method described by us earlier (1). All IF analyses were performed in a blinded fashion such that the examiner was unaware of the identity of the slides being analysed. IF was used to assess the expression of 3 anti-oxidant enzymes viz. superoxide dismutase, catalase and glutathione peroxidase. Also examined by IF were 23 biomarkers representing 10 hallmarks of cancer, including 5 immune checkpoints (Supplementary Table 3). Source and catalogue numbers of antibodies used are given in (Supplementary Tables 4A–C). One thousand cells were analysed on each slide for all IF studies, and the number of positive cells was recorded. Results were expressed as mean number of positive cells (± SEM)/1000 cells.
Statistical analysis
Statistical comparison of pre versus post treatment values was performed in two ways: 1) combined comparison of all four treatment groups with the control group was performed applying two-way ANOVA using R software; 2) comparison of pre versus post treatment values within each treatment group was performed by paired Student’s t-test using Graphpad Prism 6.0 software.
Results
R-Cu treatment up-regulates anti-oxidant enzymes in tumour cells
As an initial step, we examined whether oxygen radicals generated following oral administration of R-Cu might have diffused into the tumour tissue? This was indirectly examined by IF analysis of the three antioxidant enzymes mentioned above. We observed that R-Cu treatment had led to increased expression of all three enzymes within tumour cells (Figure 1 upper panels). This indicated that oxygen-radicals had entered into the tumour cells, and that the latter in an attempt to detoxify the offending oxygen-radicals, had up-regulated anti-oxidant enzymes as a cellular defence mechanism. Combined statistical comparison of pre versus post treatment values of the four treatment groups with the control group using two-way ANOVA showed that R-Cu treatment had led to a highly significant up-regulation of all three antioxidant enzymes (Figure 1 lower panels). Individual comparison of pre and post treatment values within each treatment group using paired t test also showed significant increase in post R-Cu values for all three enzymes with respect to dose levels I – III. However, no significant difference between pre and post treatment values was seen in case of level IV for any of the three enzymes. No difference in antioxidant enzyme levels was seen between pre and post samples in the control group (Figure 1 lower panels).
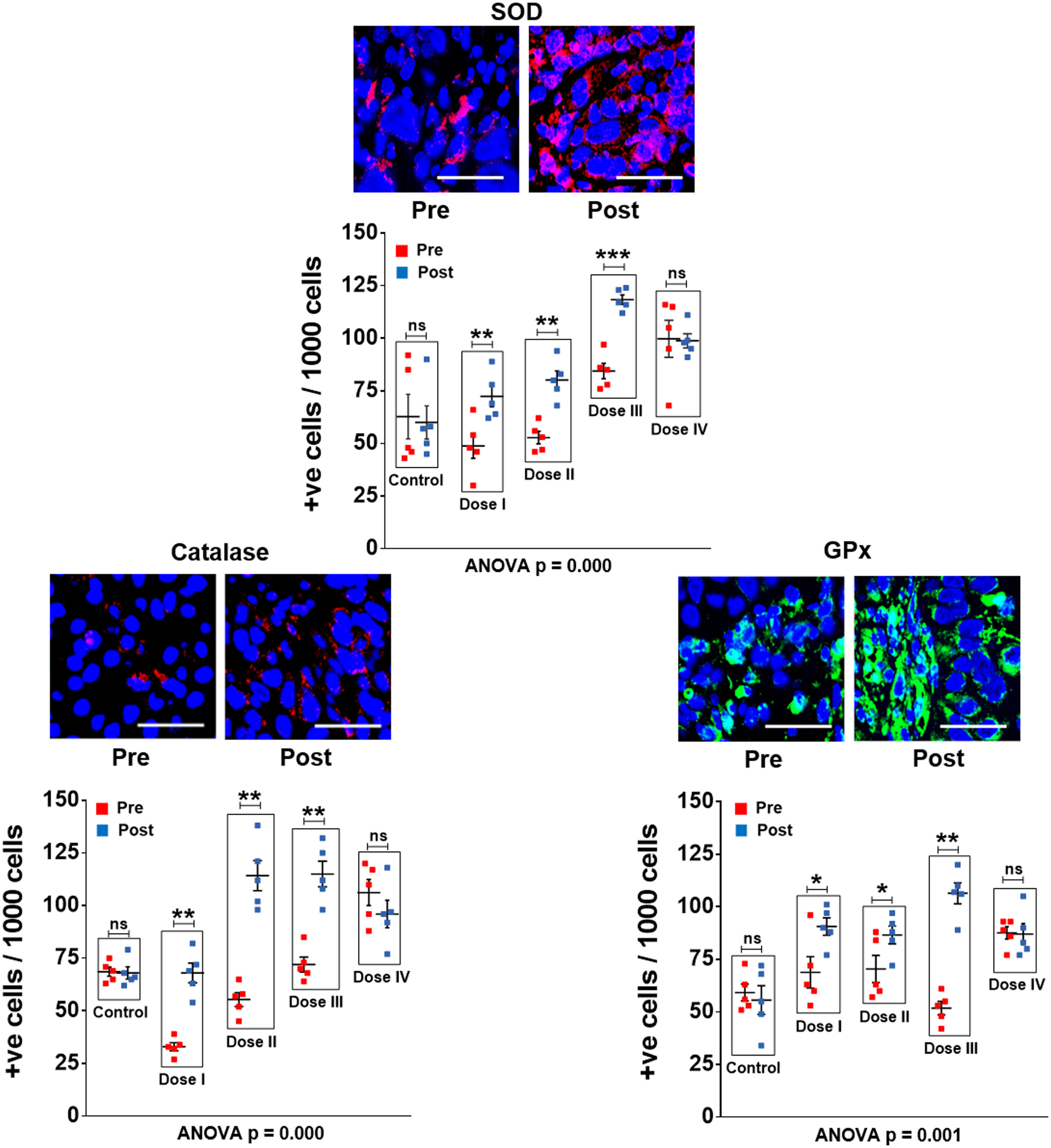
Figure 1 R-Cu treatment up-regulates anti-oxidant enzymes in OSCC tumour cells. FFPE sections of tumour tissues were stained with antibodies against superoxide dismutase (SOD), catalase and glutathione peroxidase (GPx), and examined under fluorescence microscopy. Upper panels provide representative images (scale bar 10µm); lower panels represent quantitative analysis of cells positive for respective anti-oxidant enzymes. One thousand cells were analysed in each case and the number of cells showing positive signals were recorded. Combined statistical comparison of pre versus post treatment values of all four treatment groups with the control group was done using two-way ANOVA; p values of which are given under each graph. Comparison of pre versus post treatment values within each treatment group was performed by paired Student’s t-test. Results of the latter are represented as mean ± SEM of 5 patients in each treatment group. *< p < 0.05; **< p < 0.01; ***< p < 0.001; ns, not significant.
R-Cu treatment eradicates/deactivates profusion of cfChPs present in TME
Confocal microscopy of FFPE sections of tumour tissues was performed after fluorescent immuno-staining with anti-DNA (red) and anti-histone (green) antibodies. Upon superimposing the fluorescent DNA and histone images, copious presence of yellow fluorescent signals, representing cfChPs, were detected in TME (Figure 2A). The yellow cfChPs signals were virtually eliminated following two-week treatment with R-Cu. Mean fluorescent intensity (MFI) of yellow fluorescent cfChPs signals was quantitatively estimated and statistical analysis of the results was performed. Combined statistical comparison of pre versus post treatment MFI values of the four treatment groups with the control group using two-way ANOVA showed that treatment with R-Cu had led to a highly significant reduction in cfChPs in TME (Figure 2B). A significant reduction in post treatment values was also seen within each treatment group when analysed by paired t test. No difference in pre and post treatment MFI values was seen in the control group (Figure 2B). It should be noted that some of the red (DNA) and green (histone) fluorescent signals had not strictly co-localised. This is likely to be due to unevenness of cut surfaces of FFPE sections which prevented the respective antibodies to have access to some of the DNA and Histone epitopes on cfChPs.
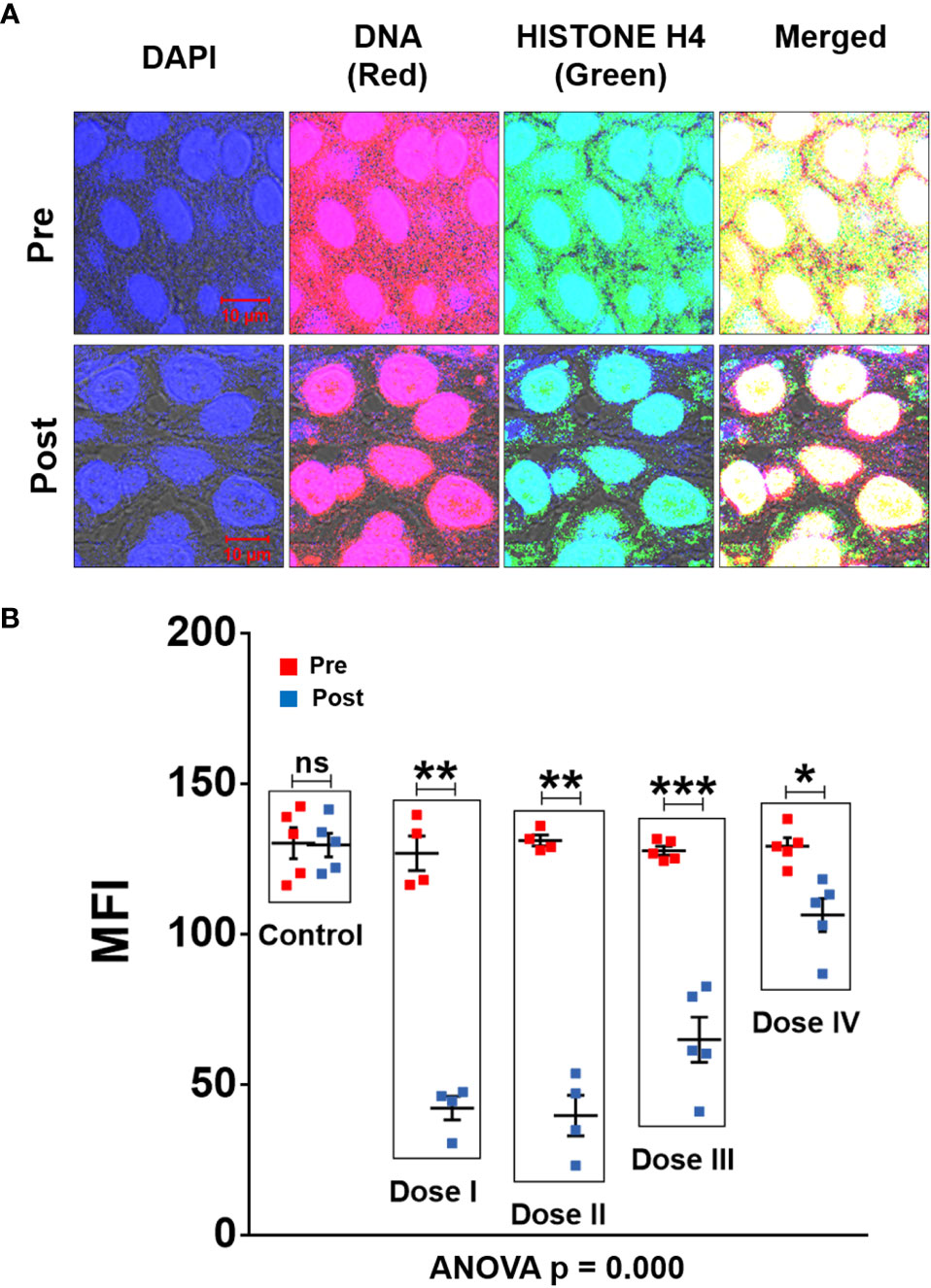
Figure 2 cfChPs are copiously present in TME of OSCC which are eradicated following treatment with R-Cu. (A) Representative confocal images of FFPE sections of tumour tissues immuno-stained with anti-DNA (red) and anti-histone (green) antibodies. Upon superimposing red and green fluorescence images, a profusion of cfCh particles in the form of yellow fluorescent signals is seen in TME. Yellow cfCh signals were markedly reduced following R-Cu treatment. (B) Quantitative analysis of MFI of yellow fluorescent signals representing C-cfChPs in five randomly chosen confocal fields (~50 cells per field). Combined statistical comparison of pre versus post treatment values of all four treatment groups with the control group was done using two-way ANOVA; p values of which are given under the graph. Comparison of pre versus post treatment values within each treatment group was performed by paired Student’s t-test. Results of the latter are represented as mean ± SEM of tissue sections of 5 patients in each treatment group, except for pre and post values of dose levels I and II, wherein n was = 4. *< p < 0.05; **< p < 0.01, ***< p < 0.001; ns, not significant).
R-Cu treatment down-regulates cancer hallmarks and immune checkpoints
Comparative results of IF analysis of pre and post R-Cu treatment values of the 10 hallmarks of cancer represented by 23 biomarkers, including 5 immune checkpoints, is given in Figure 3 (upper and lower panels). Combined statistical comparison of the four treatment groups with the control group using two-way ANOVA showed a highly significant reduction in biomarker expression in the post R-Cu treatment samples in 21/23 cases (Figure 3 lower panels). The exceptions were VEGFA (angiogenesis) and Glut1 (cellular energetics), where difference between pre and post values did not reach statistical significance. We conclude from these exploratory results that eradication of cfChPs from TME following R-Cu treatment is associated with down-regulation of 21/23 biomarkers representing 10 cancer hallmarks, and 5 immune checkpoints, that we examined.
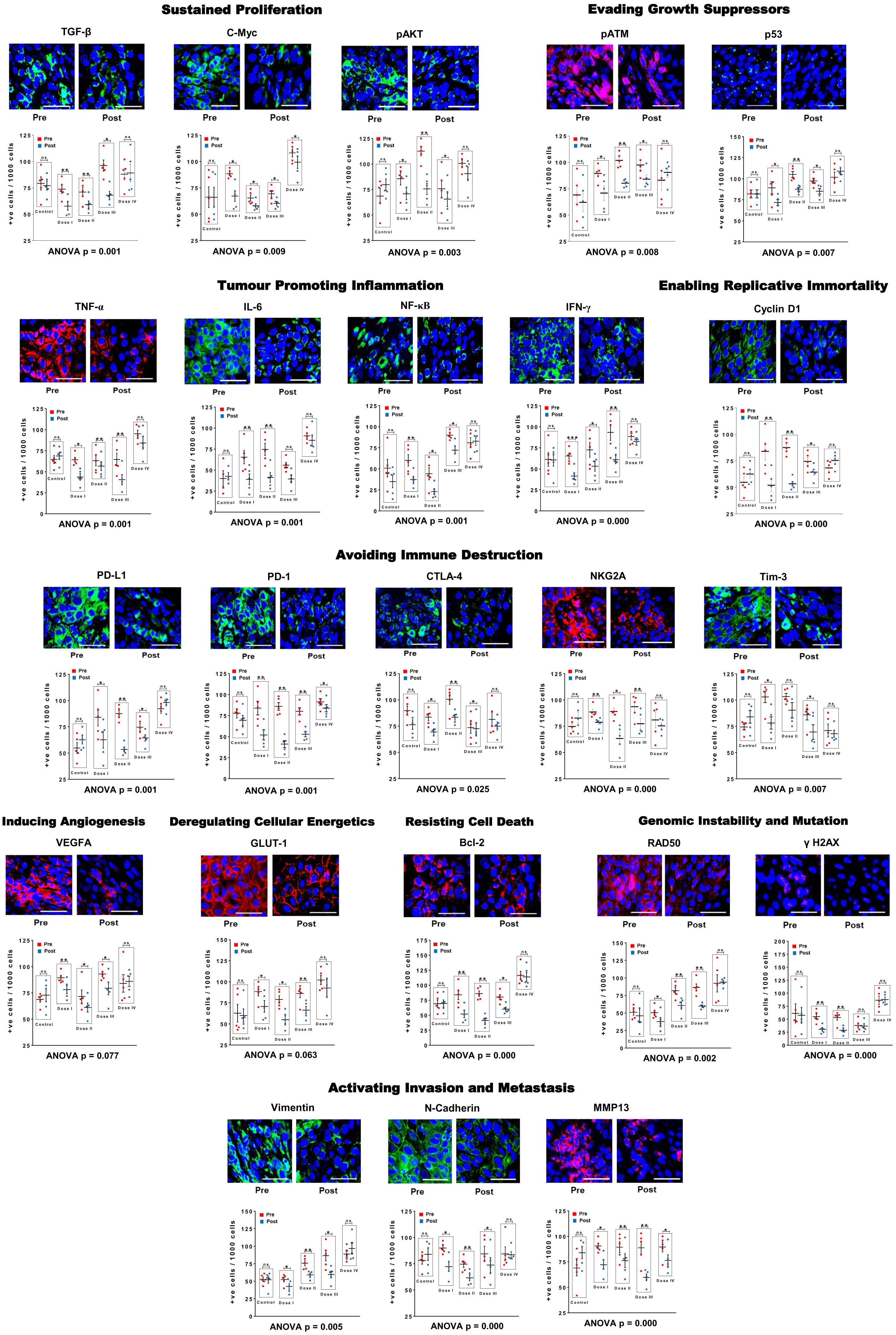
Figure 3 R-Cu treatment down-regulates cancer hallmark biomarkers, including five immune check-points, in OSCC. Upper panels are representative IF images (scale bar 10µm); lower panels represent results of quantitative analysis of various biomarkers. One thousand cells were analysed in each case and the number of cells showing positive fluorescent signals were recorded. Combined statistical comparison of pre versus post treatment values of all four treatment groups with the control group was done using two-way ANOVA; p values of which are given under each graph. Comparison of pre versus post treatment values within each treatment group was performed by paired Student’s t-test. Results are represented as mean ± SEM of tissue sections of 5 patients in each treatment group. *< p < 0.05; **< p < 0.01; ***< p < 0.001). ns, not significant.
Comparison of pre versus post treatment values within each treatment group using paired t-test showed that, on the whole, the two lower dose levels (I and II) were more effective. In these two treatment groups, a statistically significant reduction in biomarker expression was seen in the post treatment samples in all 23 biomarkers examined (23/23) (Figure 3 lower panels). In case of dose level III, 20/23 and biomarkers showed statistical significant reduction in the post treatment samples. Dose level IV was least effective, with only 3/23 biomarkers showing significantly reduced values in the post R-Cu samples (Figure 3 lower panels).
Immune checkpoint proteins are expressed by lymphocytes present in TME
We were curious to find out whether the immune checkpoints PD-1, CTLA-4, Tim3 and NKG2A were being expressed by tumour infiltrating lymphocytes (TIL) present in TME. We performed dual IF analyses on tumour sections in which each checkpoint protein was simultaneously immune-stained for CD4 and CD8 lymphocytes (Supplementary Figures 2A–D). We found that all four immune checkpoint proteins co-localised with both types of immune lymphocytes. This finding confirmed that cfChPs released from the dying tumour cells had induced TIL present in TME to activate immune checkpoint proteins.
Discussion
Results of this exploratory study need to be confirmed in a larger series of patients, and in other tumour types. Nonetheless, several observations that we made may be worthy of note. For example, that 1) oxygen radicals generated following oral administration of R-Cu were ostensibly absorbed from the stomach to enter into systemic circulation and reach the tumour cells; 2) this led the latter to up-regulate anti-oxidant enzymes as a cellular defence mechanism; 3) cfChPs are copiously present in TME having been derived from dying tumour cells; 4) oxygen radicals that diffuse into the extracellular spaces of the tumour eradicate/deactivate the profusion of cfChPs present in TME; 5) eradication of cfChPs from TME is associated with down-regulation of 21/23 biomarkers representing the 10 hallmarks of cancer, including 5 immune checkpoints (15). The mechanistic steps involved in oxygen radical induced down-regulation of cancer hallmarks are graphically illustrated in Figure 4.
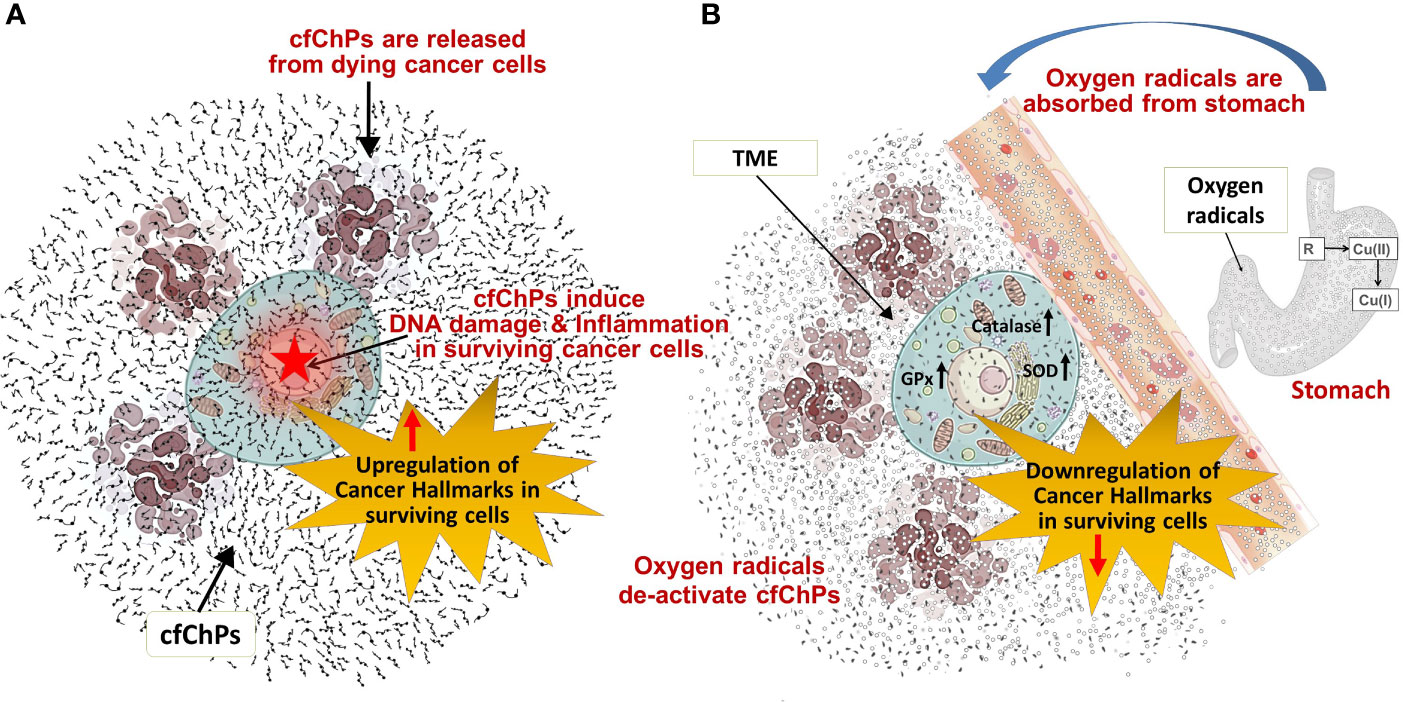
Figure 4 Graphical illustration of the mechanistic steps involved in oxygen radical induced down-regulation of cancer hallmarks. (A) cfChPs released from dying cancer cells induce DNA damage and inflammation, and up-regulate cancer hallmarks in surviving cancer cells. (B) Oxygen radicals generated upon oral ingestion of R-Cu are systemically absorbed from the stomach to reach TME leading to eradication of extra-cellular cfChPs and down-regulation of cancer hallmarks in surviving cells. Oxygen radicals also enter into the surviving cancer cells; but their cellular entry leads to activation of intracellular anti-oxidant enzymes which detoxify and eliminate the offending agents. SOD, superoxide dismutase; GPx, glutathione peroxidase; TME, tumour microenvironment.
In spite of the fact that R-Cu generated oxygen radicals had diffused into the tumour cells, we did not observe any gross damage to the tumour cell DNA following R-Cu treatment (Figure 2A). This suggested that the surviving tumour cells had protected themselves from damage by oxygen radical by up-regulating cellular anti-oxidant enzymes. No systemic toxic side effects were reported following R-Cu treatment for any of the dose levels administered; the up-regulated anti-oxidant enzymes had apparently protected all cells of the body from oxidative damage in a similar manner.
Results of our study suggest that cfChPs from dying cancer cells released into TME aggravate the oncogenic constitution of surviving cancer cells by activating cancer hallmarks in the tumour cells, and by activating immune checkpoint proteins in TIL. TME is known to play a critical role in all stages of tumour progression and metastases (16). It has been proposed that therapeutic targeting of constituents of TME could be a promising approach to controlling cancer (17). Our exploratory results presented herein suggest that treatment with R-Cu may be one such therapeutic approach which targets cfChPs present in TME via medium of oxygen radicals, and eradicates them to have potential therapeutic effects.
Our earlier results had shown, and which are confirmed in the current study, that cellular uptake of cfChPs leads to activation of two critical hallmarks of cancer viz. dsDNA breaks (genomic instability) and inflammation (1). These two hallmarks are also crucial sensors of cellular stress triggering widespread physiological and cellular responses, which also include cancer hallmarks (18, 19). This leads us to hypothesize that cfChPs from dying cancer cells trigger a stress response in surviving cells leading to global activation of cancer hallmarks and immune checkpoints.
We observed that the two lower dose levels of R-Cu were more effective than the higher ones. It is noteworthy that the amount of R present in the lowest dose (level I) was ~90 times less, and that of Cu ~4500 times less, than those that are recommended by the respective vendors for use as health supplements. Thus, small quantities of a combination of R and Cu can generate sufficient oxygen radicals to have profound effects in terms of down-regulation of cancer hallmarks and immune checkpoints by targeting a hitherto unknown constituent of TME.
Our analysis showed that R-Cu treatment led to down-regulation of all five immune check-points viz. PDL-1, PD-1, CTLA-4, TIM-3, and NKG2A that we examined. Targeted therapy of immune checkpoints has drawn much recent attention, and is beginning to show promising results in cancer treatment (20). However, what triggers activation of immune checkpoints has remained known. Our exploratory results suggest that cfChPs released from dying cancer cells into TME may be global activators of immune checkpoint proteins, and that they can be down-regulated by two-week treatment with small quantities of orally administered R-Cu. If the role of cfChPs in checkpoint activation is further confirmed, a new form of cancer immunotherapy might emerge whereby all known immune checkpoints are simultaneously down-regulated by small quantities of R-Cu.
Conclusion and future prospects
Our results suggest that cfChPs released from dying cancer cells are global instigators of cancer hallmarks and immune checkpoints. That cfChPs can be deactivated by small quantities of R-Cu raise the prospect of a novel and non-toxic form of cancer treatment which sans killing of cancer cells, and instead, induces healing by down-regulating hallmarks of cancer and immune checkpoint proteins. It has been argued that cancer is akin to a non-healing wound (21), and that hallmarks of cancer are also the hallmarks of wound healing (22). This being so, it is possible that cancer may be healed, like a wound, without having to be killed, and that oxygen radicals generated upon oral administration of R-Cu may prove to be a novel cancer healing agent of the future.
Data availability statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics statement
The studies involving human participants were reviewed and approved by Tata Memorial Center Institutional Ethics Committee. The patients/participants provided their written informed consent to participate in this study.
Author contributions
AP, SS, HT, and VJ: Investigation, Visualisation Methodology; HS, PC, and NK: Investigation, methodology, project administration and supervision; GR and KP: Data curation, formal analysis, investigation, software, supervision, validation, visualisation and writing of the original draft; AB: Statistical analysis; IM: Conceptualisation, funding acquisition, methodology, project administration, resources, overall supervision, writing original draft, writing –review and editing. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by Department of Atomic Energy, Government of India through its grant CTCTMC to Tata Memorial Centre awarded to IM. The study funders had no role in the conduct, design and interpretation of results in this study.
Acknowledgments
The authors sincerely thank Dr. Snehal Shabrish for preparing the infographic. Thanks are also due to Mr. Ashish Pawar and Mr. Roshan Shaikh for their help in preparing the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2022.1000957/full#supplementary-material
Supplementary Figure 1 | A representative photograph of advanced OSCC (A), and its squamous cell phenotype as seen on H&E section (B).
Supplementary Figure 2 | Representative images showing expression of various immune checkpoint proteins by CD4 and CD8 lymphocytes. FFPE sections of OSCC tumour tissues were simultaneously immune-stained with antibodies against various immune-checkpoint proteins and those against CD4 and CD8 lymphocytes (scale bar 10µm). Co-localisation of all four immune check-point proteins and CD4 and CD8 lymphocytes is clearly seen.
References
1. Mittra I, Samant U, Sharma S, Raghuram GV, Saha T, Tidke P, et al. Cell-free chromatin from dying cancer cells integrate into genomes of bystander healthy cells to induce DNA damage and inflammation. Cell Death Discov (2017) 3(1):1–14. doi: 10.1038/cddiscovery.2017.15
2. Kirolikar S, Prasannan P, Raghuram GV, Pancholi N, Saha T, Tidke P, et al. Prevention of radiation-induced bystander effects by agents that inactivate cell-free chromatin released from irradiated dying cells. Cell Death Dis (2018) 9(12):1–16. doi: 10.1038/s41419-018-1181-x
3. Fukuhara K, Miyata N. Resveratrol as a new type of DNA-cleaving agent. Bioorg Med Chem Lett (1998) 8(22):3187–92. doi: 10.1016/S0960-894X(98)00585-X
4. Fukuhara K, Nagakawa M, Nakanishi I, Ohkubo K, Imai K, Urano S, et al. Structural basis for DNA-cleaving activity of resveratrol in the presence of Cu(II). Bioorg Med Chem (2006) 14(5):1437–43. doi: 10.1016/j.bmc.2005.09.070
5. Subramaniam S, Vohra I, Iyer A, Nair NK, Mittra I. A paradoxical synergism between resveratrol and copper (II) with respect to degradation of DNA and RNA. F1000Research (2015) 4:1145. doi: 10.12688/f1000research.7202.1
6. Mittra I, Pal K, Pancholi N, Shaikh A, Rane B, Tidke P, et al. Prevention of chemotherapy toxicity by agents that neutralize or degrade cell-free chromatin. Ann Oncol (2017) 28(9):2119–27. doi: 10.1093/annonc/mdx318
7. Mittra I, Pal K, Pancholi N, Tidke P, Siddiqui S, Rane B, et al. Cell-free chromatin particles released from dying host cells are global instigators of endotoxin sepsis in mice. PloS One (2020) 15(3):1–22. doi: 10.1371/journal.pone.0229017
8. Pal K, Gorantla RV, D’souza J, Mehta S, Jadhav V, Shaikh A, et al. A pro-oxidant combination of resveratrol and copper down-regulates multiple biological hallmarks of ageing and neurodegeneration. bioRxiv (2022). doi: 10.1101/2022.05.04.489500
9. Agarwal A, Khandelwal A, Pal K, Khare NK, Jadhav V, Gurjar M, et al. A novel pro-oxidant combination of resveratrol and copper reduces transplant related toxicities in patients receiving high dose melphalan for multiple myeloma (RESCU 001). PloS One (2022) 17(2):1–9. doi: 10.1371/journal.pone.0262212
10. Mittra I, de Souza R, Bhadade R, Madke T, Shankpal PD, Joshi M, et al. Resveratrol and copper for treatment of severe COVID-19: an observational study (RESCU 002). medRxiv (2020) 6(165):1–13. doi: 10.1101/2020.07.21.20151423
11. Halliwell B, Gutteridge JMC. Role of free radicals and catalytic metal ions in human disease: An overview. Methods Enzymol (1990) 186:1–85. doi: 10.1016/0076-6879(90)86093-B
12. Ray PD, Huang BW, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal (2012) 24(5):981–90. doi: 10.1016/j.cellsig.2012.01.008
13. Pizzino G, Irrera N, Cucinotta M, Pallio G, Mannino F, Arcoraci V, et al. Oxidative stress: Harms and benefits for human health. Oxid Med Cell Longev (2017) 2017:1–13. doi: 10.1155/2017/8416763
14. Reagan-Shaw S, Nihal M, Ahmad N. Dose translation from animal to human studies revisited. FASEB J (2008) 22(3):659–61. doi: 10.1096/fj.07-9574LSF
15. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
16. Whiteside TL. The tumor microenvironment and its role in promoting tumor growth. Oncogene (2008) 27(45):5904–12. doi: 10.1038/onc.2008.271
17. Roma-Rodrigues C, Mendes R, Baptista PV, Fernandes AR. Targeting tumor microenvironment for cancer therapy. Int J Mol Sci (2019) 20(4):1–31. doi: 10.3390/ijms20040840
18. Sato H, Niimi A, Yasuhara T, Permata TBM, Hagiwara Y, Isono M, et al. DNA Double-strand break repair pathway regulates PD-L1 expression in cancer cells. Nat Commun (2017) 8(1):1–11. doi: 10.1038/s41467-017-01883-9
19. Liu YZ, Wang YX, Jiang CL. Inflammation: the common pathway of stress-related diseases. Front Hum Neurosci (2017) 11(316):1–11. doi: 10.3389/fnhum.2017.00316
20. Gong J, Chehrazi-Raffle A, Reddi S, Salgia R. Development of PD-1 and PD-L1 inhibitors as a form of cancer immunotherapy: A comprehensive review of registration trials and future considerations. J Immunother Cancer (2018) 6(1):1–18. doi: 10.1186/s40425-018-0316-z
21. Schäfer M, Werner S. Cancer as an overhealing wound: An old hypothesis revisited. Nat Rev Mol Cell Biol (2008) 9(8):628–38. doi: 10.1038/nrm2455
Keywords: tumour microenvironment, apoptosis, cell death, immune blockade, immunotherapy, stress
Citation: Pilankar A, Singhavi H, Raghuram GV, Siddiqui S, Khare NK, Jadhav V, Tandel H, Pal K, Bhattacharjee A, Chaturvedi P and Mittra I (2022) A pro-oxidant combination of resveratrol and copper down-regulates hallmarks of cancer and immune checkpoints in patients with advanced oral cancer: Results of an exploratory study (RESCU 004). Front. Oncol. 12:1000957. doi: 10.3389/fonc.2022.1000957
Received: 22 July 2022; Accepted: 31 August 2022;
Published: 16 September 2022.
Edited by:
Pranav Gupta, Albert Einstein College of Medicine, United StatesCopyright © 2022 Pilankar, Singhavi, Raghuram, Siddiqui, Khare, Jadhav, Tandel, Pal, Bhattacharjee, Chaturvedi and Mittra. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Indraneel Mittra, aW1pdHRyYUBhY3RyZWMuZ292Lmlu
†ORCID ID: Indraneel Mittra, orcid.org/0000-0002-5768-3821
 Aishwarya Pilankar1,2
Aishwarya Pilankar1,2 Atanu Bhattacharjee
Atanu Bhattacharjee Indraneel Mittra
Indraneel Mittra