- 1Pediatric and Adolescent Medicine, Mayo Clinic, Rochester, MN, United States
- 2Division of Pediatrics, Stem Cell Transplantation and Cellular Therapy, The University of Texas MD Anderson Cancer Center, Houston, TX, United States
- 3Pediatric Hematology and Oncology, University of California San Francisco, San Francisco, CA, United States
- 4Pediatric Critical Care Medicine, University of California San Francisco, San Francisco, CA, United States
- 5Cancer and Blood Disorders Center, Boston Children’s Hospital, Boston, MA, United States
- 6Pediatric Critical Care, Massachusetts General Hospital, Boston, MA, United States
- 7Hematology-Oncology, Boston Children’s Hospital, Boston, MA, United States
- 8Pediatric Stem Cell Transplant, Dana Farber Cancer Institute/Boston Children’s Hospital, Boston, MA, United States
- 9Pediatric Hematology and Oncology, Mayo Clinic, Rochester, MN, United States
- 10Department of Quantitative Health Sciences, Mayo Clinic, Rochester, MN, United States
Endothelial dysfunction underlies many of the major complications following hematopoietic cell transplantation (HCT), including transplant-associated thrombotic microangiopathy (TA-TMA), veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS), and engraftment syndrome (ES). Emerging evidence similarly implicates endothelitis and microangiopathy in severe COVID-19-related multi-system organ dysfunction. Given the overlap in these two illness states, we hypothesize that prior COVID-19 infection may increase risk for HCT-related endotheliopathies. This retrospective, multicenter study included patients aged 0-25 years who underwent autologous or allogeneic HCT for any indication between January 1, 2020 and September 21, 2021, with close attention to those infected with COVID-19 in either the six months prior to transplant or twelve months following transplant. Incidences of TA-TMA, VOD/SOS, and ES were compared among patients with COVID-19 infection pre-HCT and post-HCT, as well as with historical controls who were never infected with SARS-CoV-2. Those who underwent HCT following COVID-19 infection displayed significantly increased rates of TA-TMA compared to those who were never infected. Additionally, our data suggests a similar trend for increased VOD/SOS and ES rates, although this did not reach statistical significance. Therefore, a history of COVID-19 infection prior to undergoing HCT may be a nonmodifiable risk factor for endothelial-related complications following HCT. Further studies are warranted to better clarify this relationship among larger cohorts and in the era of the Omicron SARS-CoV-2 variants.
1 Introduction
While scientific knowledge regarding the severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has expanded rapidly since the beginning of the coronavirus disease 2019 (COVID-19) pandemic, unanswered questions remain pertaining to the impact of this illness on medically unique populations. Pediatric patients undergoing hematopoietic cell transplantation (HCT) are a medically complex group in which the impact of SARS-CoV-2 infection is not well understood. While pediatric patients in general seem to be at lower risk for severe disease from COVID-19, adults undergoing HCT appear to be at increased risk for COVID-19-related severe disease and mortality (1–3).
Data characterizing the experience of this unique pediatric population is limited and predominately describes patients infected following HCT. Lucchini et al. recount the UK experience, in which the majority of their post-HCT pediatric patients who became infected with SARS-CoV-2 had mild illness, although one of the nine patients did develop severe COVID-19 infection with cytokine-release syndrome (4). A series of more severe cases was described by Barhoom et al., where they detailed four pediatric patients who contracted COVID-19 following HCT, three of whom required endotracheal intubation and ICU level care and two of whom died as a result of the infection (5). The largest study to date includes 62 children infected with SARS-CoV-2 following HCT, with 10% of those patients requiring ICU level care (6). COVID-19-specific mortality among this cohort was 5% (6). In total, most of the available literature highlights COVID-19 infection months following transplant, with little data available regarding patients who acquire SARS-CoV-2 prior to or immediately surrounding HCT. Additionally, no available literature describes the relationship between COVID-19 infection and HCT-specific complications or outcomes.
Mounting evidence suggests that severe disease from SARS-CoV-2 is largely mediated by systemic endothelial injury (7, 8). Analysis of biopsy samples from surviving and postmortem patients with severe COVID-19 infection reveals endothelial destruction in all major organ systems (9–11). Under normal conditions, endothelial cells regulate vascular tone, inflammatory cascades, vessel permeability, and the balance between prothrombic and antithrombic states. In the setting of SARS-CoV-2 infection, endothelial dysfunction likely arises from a combination of two factors – direct viral invasion of endothelial cells and cytokine hyperactivation initiated by pulmonary COVID-19 infection (10–12). Subsequently, diffuse vascular leakiness and interstitial edema, microvascular and macrovascular thromboembolic events, and overwhelming immune activation lead to multisystem organ dysfunction/failure (8, 10, 13).
The effect of COVID-19 infection on endothelial function may persist long after the acute infection has resolved. A study by Chioh et al. demonstrated that there were persistent cellular and biochemical markers of endothelial injury and cytokine hyperactivation among convalescing patients (14). Additionally, in prospective studies examining endothelial function as estimated by brachial artery flow-mediated dilation, multiple groups have demonstrated persistent endothelial dysfunction up to six months following COVID-19 infection (15, 16). These authors hypothesize that this continued endothelial dysfunction may predispose patients with a history of COVID-19 infection to thromboembolic events. Indeed, clinical data is accumulating that some adult patients may experience ongoing risk of vascular complications for weeks after acute SARS-CoV-2 infection (17, 18).
The systemic endothelial dysfunction seen in severe SARS-CoV-2 infection shares a similar pathophysiology with the endotheliopathies seen following HCT (19, 20). This collection of post-HCT complications include transplant associated thrombotic microangiopathy (TA-TMA), veno-occlusive disease/sinusoidal obstruction syndrome (VOD/SOS), engraftment syndrome (ES), graft-versus-host disease (GvHD), idiopathic pneumonia syndrome/diffuse alveolar hemorrhage (IPS/DAH), and thrombosis/coagulopathy. Factors that contribute to endothelial injury in HCT settings include high-intensity conditioning chemotherapy, total body irradiation, certain underlying oncologic diagnoses, degree of alloreactivity, and GvHD prophylactic agents such as calcineurin inhibitors (21). In each of these complications, systemic or organ-specific endothelial damage occurs, leading to increased vascular permeability, decreased regulation of vascular tone, activation of coagulation factors, and excess inflammatory/cytokine signaling (21).
Given these shared mechanisms, as well as the lingering nature of the endothelial dysfunction following COVID-19 infection, we hypothesize that patients with SARS-CoV-2 infection in the time period immediately pre- or post- HCT may be at increased risk of developing HCT-related complications mediated by endothelial dysfunction. Therefore, in this study we assessed the incidence of transplant-related endotheliopathies in patients with COVID-19 infection pre-HCT and post-HCT compared to historical controls without documented SARS-CoV-2.
2 Methods
This multicenter study evaluated patients across four geographically diverse academic pediatric centers. All patients aged 0-25 years who underwent autologous or allogeneic HCT for any indication from January 1, 2020 to September 21, 2021 were included. The study group was comprised of all patients with a laboratory confirmed case of COVID-19 in either the six months prior to or twelve months following HCT. Clinical variables of interest were collected via retrospective review of the medical record and stored on a de-identified, secure database.
Data was summarized using frequencies and percentages for categorical data and medians with either ranges or interquartile ranges for continuous data. Data was compared between groups (COVID-19 pre-HCT vs COVID-19 post-HCT) using a Fisher’s exact tests for categorical data, and a Wilcox rank sum test for continuous data. All tests were two-sided, and p-values ≤ 0.05 were considered statistically significant. All analyses were performed using R version 4.1.2 (R Core Team, R Foundation for Statistical Computing, Vienna, Austria).
This study was approved by the institutional review boards at each of the participating centers. Patient consent was not required given the de-identified and retrospective nature of the investigation.
3 Results
A total of 374 pediatric and young adult patients underwent HCT for any indication during the specified date range across the four sites. Ten patients contracted COVID-19 in the six months prior to HCT, and 13 contracted COVID-19 in the twelve months following HCT. Demographic characteristics of the infected patients are in Table 1A. No significant differences in age, sex, race, pre-transplant performance score, or underlying pre-transplant diagnosis were found between the COVID-19 Pre-HCT group and COVID-19 Post-HCT group.
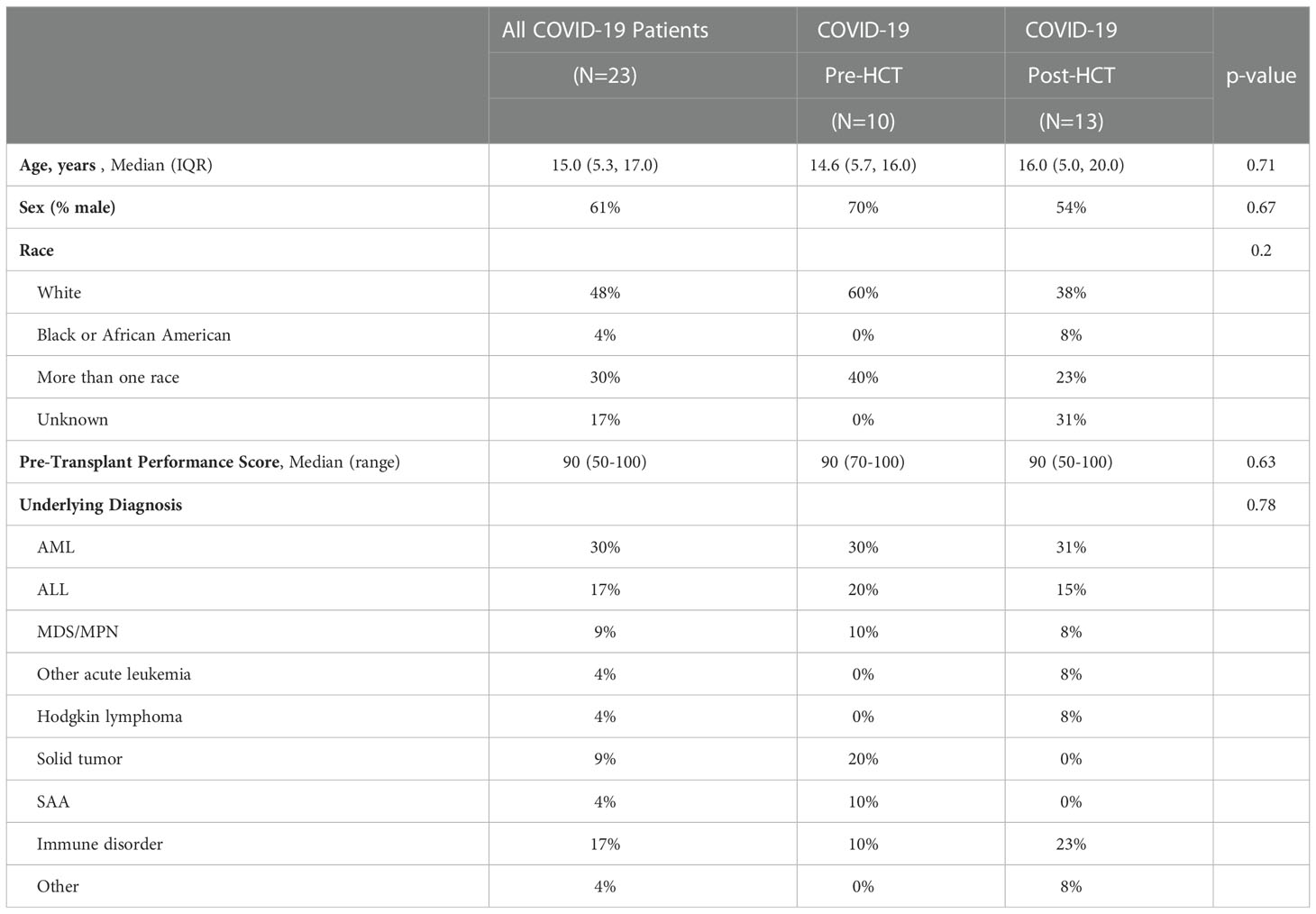
Table 1A Baseline demographic characteristic of HCT patients who contracted COVID-19 pre- and post-transplant.
Clinical data regarding HCT variables were compiled for patients who contracted COVID-19 (Table 1B). Transplant type, number of lifetime transplants, stem cell source, intensity of conditioning regimen, GVHD prophylactic agents, and exposure to serotherapy were similar between the groups. Patients in the COVID-19 Pre-HCT group were significantly more likely to have undergone haploidentical or mismatched transplantation, whereas patients in the COVID-19 Post-HCT group were more likely to have undergone matched transplantation.
COVID-19 infection characteristics were compiled (Table 2). Median date of COVID-19 diagnosis for the Pre-HCT group was day -107.5 (range: -73 to -205); the Post-HCT median infection date was day +206 (range: +54 to +345). Symptoms of COVID-19 infection were largely similar between groups, except for gastrointestinal symptoms, which were more likely to be reported in the Pre-HCT group. Rates of asymptomatic infection were similar between groups (20% vs. 31%, p = 0.66), as was need for intensive care (10% vs. 15%, p = 0.99).
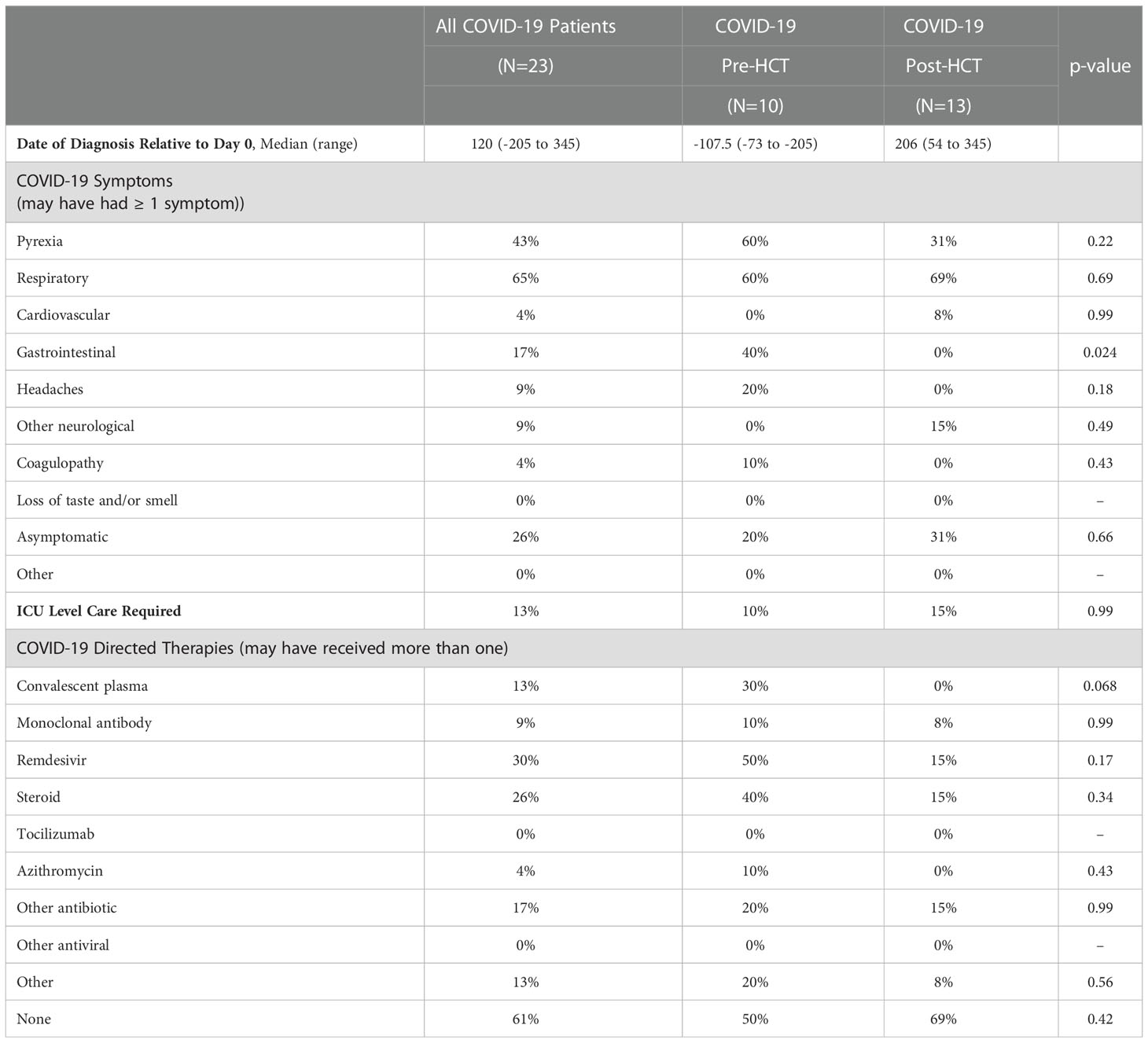
Table 2 SARS-Cov-2 infection details among HCT patients who contracted COVID-19 pre- and post-transplant.
Incidence of TA-TMA, VOD/SOS, and ES were compared between patients infected with COVID-19 Pre- and Post-HCT, as well as to the larger group of historical controls who were never infected with COVID-19 (Table 3). Patients in the Pre-HCT group were significantly more likely to experience TA-TMA as compared with patients who were never infected with COVID-19 (40% vs. 4.6%, p = 0.003). Several other non-statistically significant trends were noted as well. Incidence of TA-TMA may be higher among the Pre-HCT patients as compared to Post-HCT patients (40% vs. 8%, p = 0.20). VOD/SOS may occur more frequently among the Pre-HCT patients, as compared to the Post-HCT patients (30% vs. 8%, p = 0.42) and the non-COVID patients (30% vs. 14.8%, p = 0.42). ES displayed similar non-statistically significant trends. Incidence among the Pre-HCT patients may be increased as compared to the Post-HCT patients (20% vs. 0%, p = 0.35) and the non-COVID patients (20% vs. 8.8%, p = 0.35). Rates of VOD/SOS and ES were lowest in the Post-HCT patients (8%, 0%), which may be a reflection of small sample size.
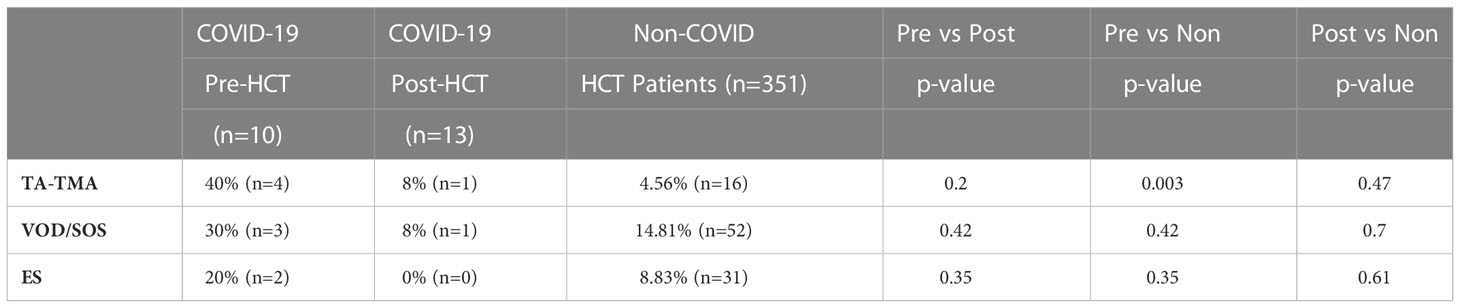
Table 3 Incidences of TA-TMA, VOD/SOS, and ES among HCT patients who contracted COVID-19 pre-transplant, post-transplant, or were never infected.
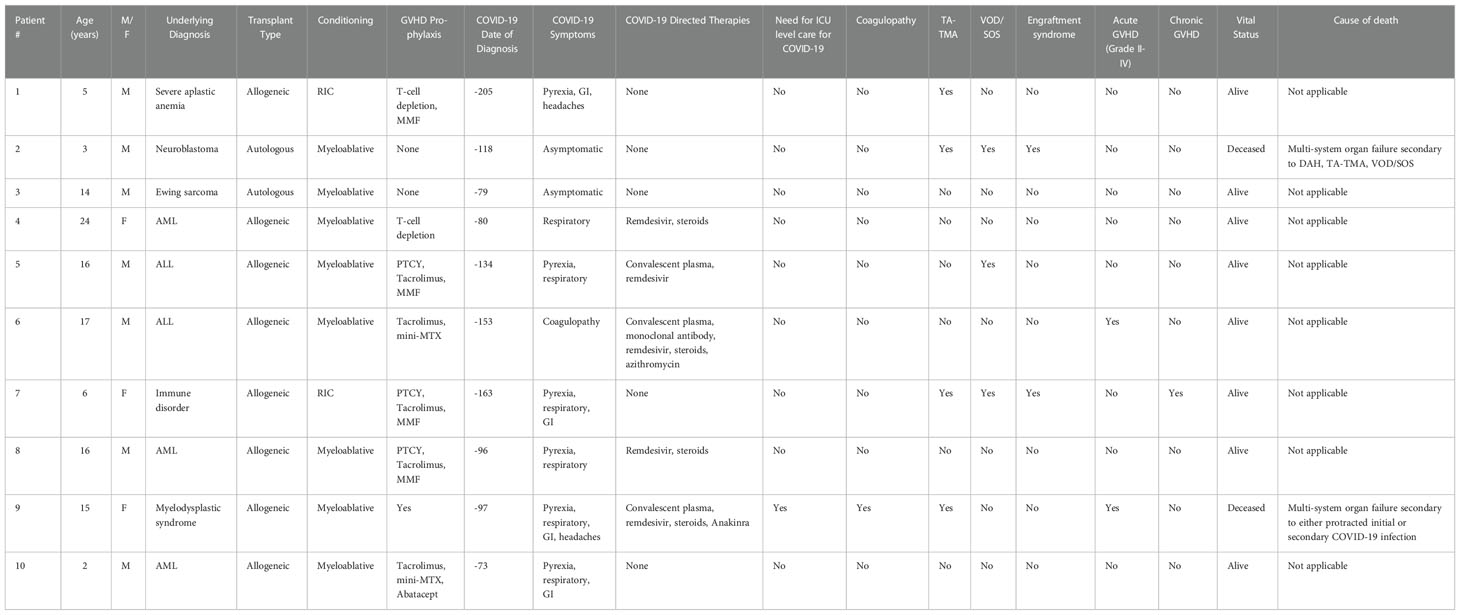
Individual case details for the 10 patients in the COVID-19 Pre-HCT group are outlined in Table 4. Two patients died (20%); one death was attributed to multi-system organ failure in the setting of multiple transplant-related endotheliopathies. The other death was due to multi-system organ failure from both COVID-19 infection and multiple transplant-related endotheliopathies. Among the patients who were in the COVID-19 Post-HCT group, two patients died (15.4%). Neither death was attributed to COVID-19 infection or transplant-related endotheliopathy.
4 Discussion
In this study, we sought to understand the impact of COVID-19 infection on pediatric HCT outcomes, particularly transplant-associated endotheliopathies. Among this small group of pediatric and young adult patients, those who underwent HCT following COVID-19 infection displayed significantly increased rates of TA-TMA compared to those who were infected after transplant or historical controls who were never infected. Additionally, our data suggests a similar trend for increased VOD/SOS and ES rates, although this did not reach statistical significance. We do acknowledge that rates of VOD/SOS and ES were lowest in our cohort of patients who contracted COVID-19 following HCT, however we suspect this is due to small sample size and low statistical power rather than a true protective effect. Finally, for both patients from the COVID-19 Pre-HCT cohort who died, multiple identified endotheliopathies contributed to mortality.
To our knowledge, no other studies have reported on HCT-associated outcomes in pediatric patients with a history of prior COVID-19 infection. However, there are a few cases in the literature that support the pathophysiologic link between transplant endothelitis and COVID-19 endothelial dysfunction. Among the patients described by Lucchini et al., one patient who had mild illness from COVID-19 infection on day +138 went on to develop TA-TMA, in the absence of other commonly accepted TA-TMA risk factors (4). The authors suggest that COVID-19 infection was the suspected trigger for endothelial derangement in this patient. In the same cohort, the only patient who experienced severe illness and cytokine release syndrome from COVID-19 infection had undergone HCT for sickle cell anemia, specifically due to cerebrovascular disease which may suggest pre-existing endothelial dysfunction (4). Additionally, one patient reported by Barhoom et al. who developed COVID-19 infection on day +27 of transplant course developed TA-TMA within days of the infection and ultimately died as a result of this complication (5).
Evidence to support the increased risk of TA-TMA among transplant recipients who experience COVID-19 infection reaches beyond the realm of HCT. Two cases of TMA concurrent with COVID-19 infection in renal transplant recipients have been reported. The first describes an adult patient who was nine years post-allograft receipt and developed TMA 11 days following COVID-19 diagnosis; other known causes of TMA were ruled out. His course was also characterized by multisystem organ dysfunction and myocardial infarction, suggestive of global endothelial dysfunction (22). The second patient contracted COVID-19 three months following renal transplantation; acute kidney injury, hemolytic anemia, and coagulopathy were the presenting features of her COVID-19 diagnosis. Renal biopsy confirmed the diagnosis of TMA, with SARS-CoV-2 infection named as the suspected cause (23).
The shared pathogenic mechanism between severe COVID-19 infection and post-HCT endotheliopathies may be rooted in excess complement activation. SARS-CoV-2 infections have been shown to cause aberrant complement activation via the classical, lectin, and alternative complement pathways (24, 25). Genetic differences in the individual components of the complement system have been proposed as a contributing factor to varying illness severity amongst patients with COVID-19. In particular, several polymorphisms of the MBL allele, which codes for the MBL protein of the lectin pathway, are known to cause excess complement activation and are associated with poor clinical outcomes in setting of severe infections (25). Similarly, excess complement activation is a contributing cause to post-HCT endothelial dysfunction, particularly TA-TMA. A number of pathogenic variants in a variety of complement factors have been identified in association with increased risk for TA-TMA (25–27). Further investigation is recommended to evaluate for unifying genetic predisposition for both conditions. Greater understanding of the genetic underpinnings for excess complement activation may eventually impact clinical guidance for increased screening, personalized transplant protocols, and therapeutic strategies for affected individuals.
Additionally, excess and prolonged cytokine release is likely a shared pathophysiologic mechanism that unifies severe COVID-19 illness and HCT-associated endotheliopathies. IL-6, IL-1β, and TNF-α have been named as key cytokines that become overexpressed in the setting of severe COVID-19 infection (28). Particularly for the pediatric population, these are key cytokines involved in the development of the multisystem inflammatory syndrome in children (MIS-C) that arises after COVID-19 infection. Specific therapeutic targets such as tocilizumab and anakinra are being investigated for their use in severe COVID-19 illness, due to their effects of modulating excess cytokine signaling. Likewise, IL-6 and TNF-α, in addition to a number of other cytokines, are excessively active in patients who have developed HCT-associated endothelial complications (21, 29). The use of targeted cytokine antagonists in preventing or treating HCT-associated endotheliopathies has been suggested in the literature, however to our knowledge has not been explored in a large-scale trial. Further investigation into cytokine signaling among patients undergoing HCT in close temporal relation to SARS-CoV-2 infection is warranted to better understand this relationship.
Two clinical applications of interest arise from these findings. First, our reported findings may suggest that prior COVID-19 infection could be a nonmodifiable risk factor for transplant-associated endotheliopathy. As such, institutions may consider implementing additional screening for affected patients throughout their transplant process. For example, we endorse the TA-TMA screening guidelines recommended by Dandoy et al., which includes twice weekly lactate dehydrogenase measurement and weekly urinalysis with urine protein creatinine ratio for the first 30 days post-HCT. For patients undergoing HCT for non-malignant and non-urgent indications who contract COVID-19 prior to HCT, consideration may be given to delaying HCT. The appropriate interval for delaying HCT is not yet defined, and further investigation is required prior to formalizing this recommendation (30). Second, recognition of the overlapping risks between HCT-related endotheliopathies and COVID-19 endothelial dysfunction raises the question of shared therapeutics between the two diseases. Prior researchers have called for studies investigating the use of agents for VOD/SOS and TA-TMA for severe COVID-19. Both defibrotide and eculizumab have demonstrated improved short- and long-term outcomes without significant adverse effects among patients with severe SARS-Cov-2 infection (31, 32).
Currently, the impact of SARS-CoV-2 infection on success of HCT engraftment and ultimate outcome is unknown. Key endothelial cell populations are thought to be involved in homing, engraftment, and restoration of bone marrow functioning following HCT (33, 34). Damage to crucial endothelial cells, such as by recent COVID-19 illness, may conceivably impair success of HCT engraftment. Further investigation into long term transplant outcomes amongst patients with recent SARS-CoV-2 infection who undergo HCT is imperative to understand this relationship.
The following limitations must be considered while interpreting the results of this study. First, although this study was conducted across four pediatric HCT centers, the frequency of patients infected with COVID-19 in the peri-HCT period was low. The sample size of our population of interest is small, which limits study power and ability to perform complete statistical analyses, including multivariate analysis. We strongly encourage ongoing investigation regarding this topic, as more patients will be available for study as the COVID-19 pandemic progresses. Second, he patients included in this research contracted COVID-19 prior to the emergence of the Omicron variants. The Omicron variant infected significantly more pediatric patients nationwide, however severe clinical outcomes were fewer as compared to pediatric patients infected with the Delta variant (35). Follow up studies are recommended to capture the impact of the Omicron strain, as well as future variants that may arise. Third, data regarding patient serologic status and evidence of prior COVID-19 infection was not available for our patient cohort, as these patients were studied prior to the widespread availability of serologic COVID-19 testing. Therefore, we do not have insight into whether these patients experienced a primary or subsequent COVID-19 infection and therefore cannot comment on the impact of subsequent infections on risk of developing endothelial complications. Fourth, we acknowledge a major potential confounder in our underlying HCT variables; patients who contracted COVID-19 prior to HCT were more likely to receive haploidentical grafts, as compared to patients who contracted COVID-19 following HCT. Increased degree of alloreactivity, as well as differences in conditioning and immunosuppression that coincide with haploidentical transplantation, are known risk factors for endothelial complications following HCT (36). Similar studies with larger cohorts are warranted to more effectively control for confounders.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by Mayo Clinic IRB, UCSF IRB, MD Anderson Cancer Center IRB, Dana Farber/Boston Children’s IRB. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Author contributions
SA, MK, KMM, MZ, LL designed the research. SA, DR, SK, GC, RB, GM, AF, MK collected the data. SA, KM, MK analyzed the data. SA, MK wrote the paper. All authors provided edits for the paper. SA and MK directly accessed and verified the underlying data reported in the manuscript. All authors should confirm that they had full access to all the data in the study and accept responsibility to submit for publication. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Tsankov B, Allaire J, Irvine M, Lopez AA, Sauvé LJ, Vallance BA, et al. Severe COVID-19 infection and pediatric comorbidities: A systematic review and meta-analysis. Int J Infect Dis (2021) 103:246–56. doi: 10.1016/j.ijid.2020.11.163
2. Xhaard A, Xhaard C, D’Aveni M, Salvator H, Chabi-Charvillat M-L, Coman T, et al. Risk factors for a severe form of COVID-19 after allogeneic haematopoietic stem cell transplantation: A société francophone de greffe de moelle et de thérapie cellulaire (SFGM-TC) multicentre cohort study. Br J Haematol (2021) 192(5):e121–4. doi: 10.1111/bjh.17260
3. Ljungman P, de la Camara R, Mikulska M, Tridello G, Aguado B, Zahrani Al M, et al. COVID-19 and stem cell transplantation; results from an EBMT and GETH multicenter prospective survey. Leukemia (2021) 35(10):2885–94. doi: 10.1038/s41375-021-01302-5
4. Lucchini G, Furness C, Lawson S, Gibson B, Wynn R, Slatter M, et al. UK And Ireland paediatric BMT group. COVID-19 infection paediatric recipients allogeneic Stem Cell transplantation: UK experience Br J Haematol (2021) 194(4):e74–7. doi: 10.1111/bjh.17547
5. Barhoom D, Mohseni R, Hamidieh A, Mohammadpour M, Sharifzadeh M, Navaeian A, et al. Clinical effects of COVID-19 on hematopoietic stem cell transplant outcomes in pediatric patients. Exp Clin Transplant (2021) 19(5):501–7. doi: 10.6002/ect.2020.0518
6. Averbuch D, de la Camara R, Corbacioglu S, Mikulska M, Sanchez Pinana JL, Tridello G, et al. COVID-19 in children following hematopoietic cell transplantation: A multinational study of the European bone marrow transplantation society (EBMT) and the Spanish group of hematopoietic stem cell transplantation (GETH). Blood (2021) 138(S1):2866. doi: 10.1182/blood-2021-146748
7. Sbirkov Y, Dzharov V, Todorova K, Hayrabedyan S, Sarafian V. Endothelial inflammation and dysfunction in COVID-19. Vasa (2022) 51(2):62–70. doi: 10.1024/0301-1526/a000991
8. Wang X, Sahu K, Cerny J. Coagulopathy, endothelial dysfunction, thrombotic microangiopathy and complement activation: Potential role of complement system inhibition in COVID-19. J Thromb Thrombolysis (2021) 51(3):657–62. doi: 10.1007/s11239-020-02297-z
9. Ackermann M, SE V, Kuehnel M, Haverich A, Welte T, Laengner F, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in covid-19. N Engl J Med (2020) 383(2):120–8. doi: 10.1056/NEJMoa2015432
10. Varga Z, Flammer A, Steiger P, Haberecker M, Andermatt R, Zinkernagel A, et al. Endothelial cell infection and endotheliitis in COVID-19. Lancet (2020) 395(10234):1417–8. doi: 10.1016/S0140-6736(20)30937-5
11. Dupont A, Rauch A, Staessens S, Moussa M, Rosa M, Corseaux D, et al. Vascular endothelial damage in the pathogenesis of organ injury in severe COVID-19. Arteriosclerosis Thrombosis Vasc Biol (2021) 41(5):1760–73. doi: 10.1161/ATVBAHA.120.315595
12. Maruhashi T, Higashi Y. Pathophysiological association of endothelial dysfunction with fatal outcome in COVID-19. Int J Mol Sci (2021) 22(10):5131. doi: 10.3390/ijms22105131
13. Bonaventura A, Vecchié A, Dagna L, Martinod K, Dixon D, Tassell Van B, et al. Endothelial dysfunction and immunothrombosis as key pathogenic mechanisms in COVID-19. Nat Rev Immunol (2021) 21(5):319–29. doi: 10.1038/s41577-021-00536-9
14. Chioh F, Fong S, Young B, Wu KX, Siau A, Krishnan S, et al. Convalescent COVID-19 patients are susceptible to endothelial dysfunction due to persistent immune activation. Elife (2021) 23(10):e64909. doi: 10.7554/eLife.64909.sa2
15. Oikonomou E, Souvaliotis N, Lampsas S, Siasos G, Poulakou G, Theofilis P, et al. Endothelial dysfunction in acute and long standing COVID-19: A prospective cohort study. Vascul Pharmacol (2022) 144:106975. doi: 10.1016/j.vph.2022.106975
16. Jud P, Gressenberger P, Muster V, Avian A, Meinitzer A, Strohmaier H, et al. Evaluation of endothelial dysfunction and inflammatory vasculopathy after SARS-CoV-2 infection-a cross-sectional study. Front Cardiovasc Med (2021) 8:750887–7. doi: 10.3389/fcvm.2021.750887
17. Vlachou M, Drebes A, Candilio L, Weeraman D, Mir N, Murch N, et al. Pulmonary thrombosis in covid-19: before, during and after hospital admission. J Thromb Thrombolysis (2021) 51(4):978–84. doi: 10.1007/s11239-020-02370-7
18. Katsoularis I, Fonseca-Rodriguez O, Farrington P, Lindmark K, Connolly A. Risk of acute myocardial infarction and ischaemic stroke following COVID-19 in Sweden: A self-controlled case series and matched cohort study. Lancet (2021) 398(10300):599–607. doi: 10.1016/S0140-6736(21)00896-5
19. Calabretta E, Moraleda J, Iacobelli M, Jara R, Vlodavsky I, O'Gorman P, et al. COVID-19-induced endotheliitis: Emerging evidence and possible therapeutic strategies. Br J Haematol (2021) 193(1):43–51. doi: 10.1111/bjh.17240
20. Cooke K, Jannin A, Ho V. The contribution of endothelial activation and injury to end-organ toxicity following allogeneic hematopoietic stem cell transplantation. Biol Blood Marrow Transplant (2008) 14(1):23–32. doi: 10.1016/j.bbmt.2007.10.008
21. Hildebrandt G, Chao N. Endothelial cell function and endothelial-related disorders following haematopoietic cell transplantation. Br J Haematol (2020) 190(4):508–19. doi: 10.1111/bjh.16621
22. Bascuñana A, Mijaylova A, Vega A, Macias N, Verde E, Rodriguez-Ferrero ML, et al. Thrombotic microangiopathy in a kidney transplant patient with COVID-19. Kidney Med (2021) 3(1):124–7. doi: 10.1016/j.xkme.2020.09.014
23. Jespersen Nizamic T, Huang Y, Alnimri M, Cheng M, Chen L, Jen K. COVID-19 manifesting as renal allograft dysfunction, acute pancreatitis, and thrombotic microangiopathy: A case report. Transplant Proc (2021) 53(4):1211–4. doi: 10.1016/j.transproceed.2020.10.048
24. Perico L, Benigni A, Casiraghi F, Ng L, Renia L, Remuzzi G, et al. Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol (2021) 17:46–64. doi: 10.1038/s41581-020-00357-4
25. Jodele S, Köhl J. Tackling COVID-19 infection through complement-targeted immunotherapy. Br J Pharmacol (2021) 178:2832–2848. doi: 10.1111/bph.15187
26. Jodele S, Licht C, Goebel J, Dixon BP, Zhang K, Sivakumaran TA, et al. Abnormalities in the alternative pathway of complement in children with hematopoietic stem cell transplant-associated thrombotic microangiopathy. Blood (2013) 122(12):2003–7. doi: 10.1182/blood-2013-05-501445
27. Jodele S, Zhang K, Zou F, Laskin B, Dandoy CE, Myers KC, et al. The genetic fingerprint of susceptibility for transplant-associated thrombotic microangiopathy. Blood (2016) 127(8):989–96. doi: 10.1182/blood-2015-08-663435
28. Que Y, Hu C, Wan K, Hu P, Wang R, Luo J, et al. Cytokine release syndrome in COVID-19: A major mechanism of morbidity and mortality. Int Rev Immunol (2022) 41(2):217–30. doi: 10.1080/08830185.2021.1884248
29. Schoettler M, Chonat S, Williams K, Lehmann L. Emerging therapeutic and preventive approaches to transplant-associated thrombotic microangiopathy. Curr Opin Hematol (2021) 28(6):408–16. doi: 10.1097/MOH.0000000000000687
30. Dandoy CE, Rotz S, Alonso PB, Klunk A, Desmond C, Huber J, et al. A pragmatic multi-institutional approach to understanding transplant-associated thrombotic microangiopathy after stem cell transplant. Blood Adv (2021) 5(1):1–11. doi: 10.1182/bloodadvances.2020003455
31. Ruggeri A, Voza A, Liberatore C, Catalono G, Corrado F, Filippo Di L, et al. Use of defibrotide in patients with COVID-19 pneumonia; results of the defi-VID19 phase 2 trial. Blood (2021) 138:672. doi: 10.1182/blood-2021-147784
32. Ruggenenti P, Di Marco F, Cortinovis M, Lorini L, Sala S, Novelli L, et al. Eculizumab in patients with severe coronavirus disease 2019 (COVID-19) requiring continuous positive airway pressure ventilator support: Retrospective cohort study. PloS One (2021) 16(12):e0261113. doi: 10.1371/journal.pone.0261113
33. Chen Q, Liu Y, HW J, Stehling M, VV D, Zhou B, et al. Apelin+ endothelial niche cells control hematopoiesis and mediate vascular regeneration after myeloablative injury. Cell Stem Cell (2019) 25(6):768–83. doi: 10.1016/j.stem.2019.10.006
34. Perlin JR, Sporrij A, Zon LI. Blood on the tracks: Hematopoietic stem cell-endothelial cell interactions in homing and engraftment. J Mol Med (2017) 95:809–819. doi: 10.1007/s00109-017-1559-8
35. Wang L, Berger N, Kaelber D, Davis P, Volkow N, Xu R, et al. Incidence rates and clinical outcomes of SARS-CoV-2 infection with the omicron and delta variants in children younger than 5 years in the US. JAMA Pediatr (2022) 176(8):811–3. doi: 10.1001/jamapediatrics.2022.0945
Keywords: COVID-19, hematopoietic cell transplant, TA-TMA, VOD/SOS, engraftment syndrome, endothelial dysfunction
Citation: Ariagno S, Ragoonanan D, Khazal S, Mahadeo KM, Cisneros GS, Zinter MS, Blacken RA, Mohan G, Lehmann LE, Ferdjallah A, Mara KC and Kohorst MA (2023) Prior COVID-19 infection may increase risk for developing endothelial dysfunction following hematopoietic cell transplantation. Front. Oncol. 12:1000215. doi: 10.3389/fonc.2022.1000215
Received: 21 July 2022; Accepted: 28 December 2022;
Published: 17 January 2023.
Edited by:
Jaume Mora, Sant Joan de Déu Hospital, SpainReviewed by:
Ioanna Sakellari, G. Papanikolaou General Hospital, GreeceSandra Castillo, Josep Carreras Leukaemia Research Institute (IJC), Spain
Copyright © 2023 Ariagno, Ragoonanan, Khazal, Mahadeo, Cisneros, Zinter, Blacken, Mohan, Lehmann, Ferdjallah, Mara and Kohorst. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sydney Ariagno, YXJpYWduby5zeWRuZXlAbWF5by5lZHU=
 Sydney Ariagno
Sydney Ariagno Dristhi Ragoonanan
Dristhi Ragoonanan Sajad Khazal
Sajad Khazal Kris M. Mahadeo
Kris M. Mahadeo Gabriel Salinas Cisneros3
Gabriel Salinas Cisneros3 Matt S. Zinter
Matt S. Zinter Gopi Mohan
Gopi Mohan Leslie E. Lehmann
Leslie E. Lehmann Asmaa Ferdjallah
Asmaa Ferdjallah Mira A. Kohorst
Mira A. Kohorst