
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CASE REPORT article
Front. Oncol. , 10 November 2022
Sec. Pediatric Oncology
Volume 12 - 2022 | https://doi.org/10.3389/fonc.2022.1000099
This article is part of the Research Topic Advances in the Medical Management of Infantile Hemangioma View all 6 articles
A correction has been applied to this article in:
Corrigendum: Case report: deterioration of infantile hemangioma related to oral or nebulized administration of β2-AR agonist: three cases reports
Infantile hemangioma (IH) is a benign vascular tumor, characterized by a unique sequence of non-linear growth and spontaneous involution. Some hemangiomas require intensive treatment to avoid functional and aesthetic insufficiency. Although β-adrenergic receptor (β-AR) antagonists have been increasingly used as the first-line treatment since 2008, the IH rebound still exists with uncertain mechanism. Here, we report three cases of abrupt IH deteriorations that are mainly related to β2-AR agonist administration. Potential IH proliferation induced by β2-AR agonists, especially from oral or nebulized approaches, should be recognized more widely by healthcare providers. Additionally, it is necessary to carry out large sample studies to analyze the influence of β2-AR agonist administration on the deterioration of IH.
Infantile hemangioma (IH) occurs in approximately 5%–10% of infants (1, 2). The potential risk factors for IH are prematurity, low birth weight, female gender, Caucasian, multiple pregnancy, progesterone therapy, and family heredity. Characterized by proliferative phase, plateau, and regression phase, the growth cycle of IH is non-linear but usually rapid during the first 3 months (3). Even though 90% of IH eventually involutes within 1–10 years, it still causes alarm about affecting aesthetic appearance and psychological development, as well as developing life-threatening complications if associated with vital functions (4–7). Oral propranolol therapy (OPT) is widely recognized for its efficacy and safety. Several mechanisms may involve in the treatment of IH, including vasoconstriction (8), angiogenesis inhibition (9, 10), and induction of apoptosis in endothelial cells (8, 11). However, rebound growth after propranolol discontinuation has been noted in 6%–25% of patients, and the exact mechanism remains unclear, which may be interfering with the age of discontinuation, deep IH component, and female gender (12–14).
In this study, we report three cases of increased size and redness in regressed and almost regressed IHs, which were mainly related to oral or nebulized administration of β2-adrenergic receptor (β2-AR) agonists. To the best of our knowledge, it is rarely reported and not widely recognized by healthcare providers (15). Clinical findings and laboratory evidence are articulated to emphasize the significance of clarifying the relationship between β2-AR agonists and IH deterioration in its management.
A 5-month-old boy was diagnosed with IH and admitted to the Department of Dermatology at Children’ Hospital of Chongqing Medical University for primary OPT. He was a premature infant at 30 weeks gestation with a birth weight of 1.35 kg. The IH had been rapidly growing on his left chest wall since birth, measuring 3.0 × 1.5cm at maximum and 0.6 × 0.4cm at minimum with bright red color (Figure 1A). With close monitoring of vital signs, propranolol was initiated at 1.0 mg/kg per day and maintained at 2.0 mg/kg per day (increase by 0.5 mg/kg every 2 days). After discharge, propranolol was maintained at 2.0 mg/kg per day and had significantly improved the IH until this admission for pneumonia (Figure 1B).
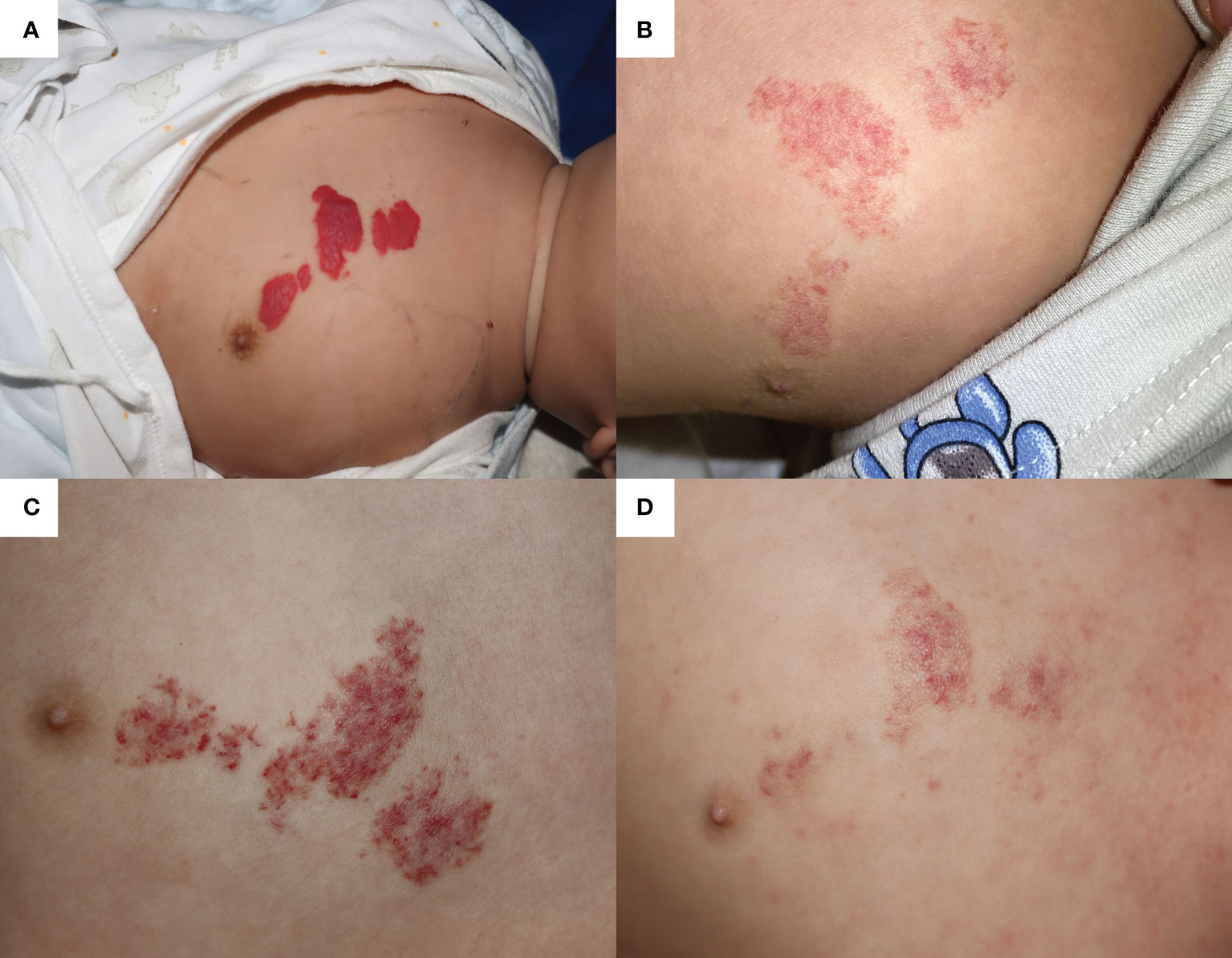
Figure 1 The infantile hemangioma in patient 1 at different periods. (A) Proliferating infantile hemangioma before oral propranolol therapy. (B) Involuted infantile hemangioma when the patient was admitted for pneumonia at the age of 13 months. (C) Infantile hemangioma worsened after nearly 1 day of oral procaterol therapy. (D) Involuted hemangioma after 1 month of oral propranolol therapy and one laser therapy.
At the age of 13 months, the patient suffered from severe pneumonia and received 3 days of non-invasive ventilation, antibiotics, and antitussive–expectorant nebulization inhalation (budesonide, ipratropium bromide, and ambroxol), during which OPT was discontinued to avoid bronchospasm. OPT was restarted as respiratory symptoms improved. Unfortunately, because of worsening bronchitis, OPT only lasted for 6 days and was replaced by topical timolol. Additionally, prednisone acetate tablets (7.5 mg twice a day) and the procaterol hydrochloride oral solution (2.5 ml twice a day) were added to relieve bronchospasm symptoms. After nearly 1 day of comprehensive treatment, the color of the IH progressed into deep red and showed a trend of proliferation (Figure 1C). However, because of the current prioritization of bronchitis treatment, more aggressive IH management could not be implemented. Finally, the vasodilation of IH was in significant remission in 1 month of OPT and a single 585-nm pulsed dye laser (PDL; Cynergy™, Cynosure®) therapy after discharge (Figure 1D).
A 4-month-old boy was diagnosed with IH (located on the medial side of the right shoulder joint) and admitted for OPT (Figure 2A). With close monitoring of heart rate and blood pressure, propranolol was initiated at 1.0 mg/kg per day and gradually increased to 2.0 mg/kg per day during the 4 days of hospitalization (increase by 0.5 mg/kg every 2 days). After discharge, in addition to maintenance OPT at a dosage of 2.0 mg/kg per day, the patient also received five times of 585-nm PDL therapies, which helped to achieve significant and nearly complete involution of the IH (Figure 2B).
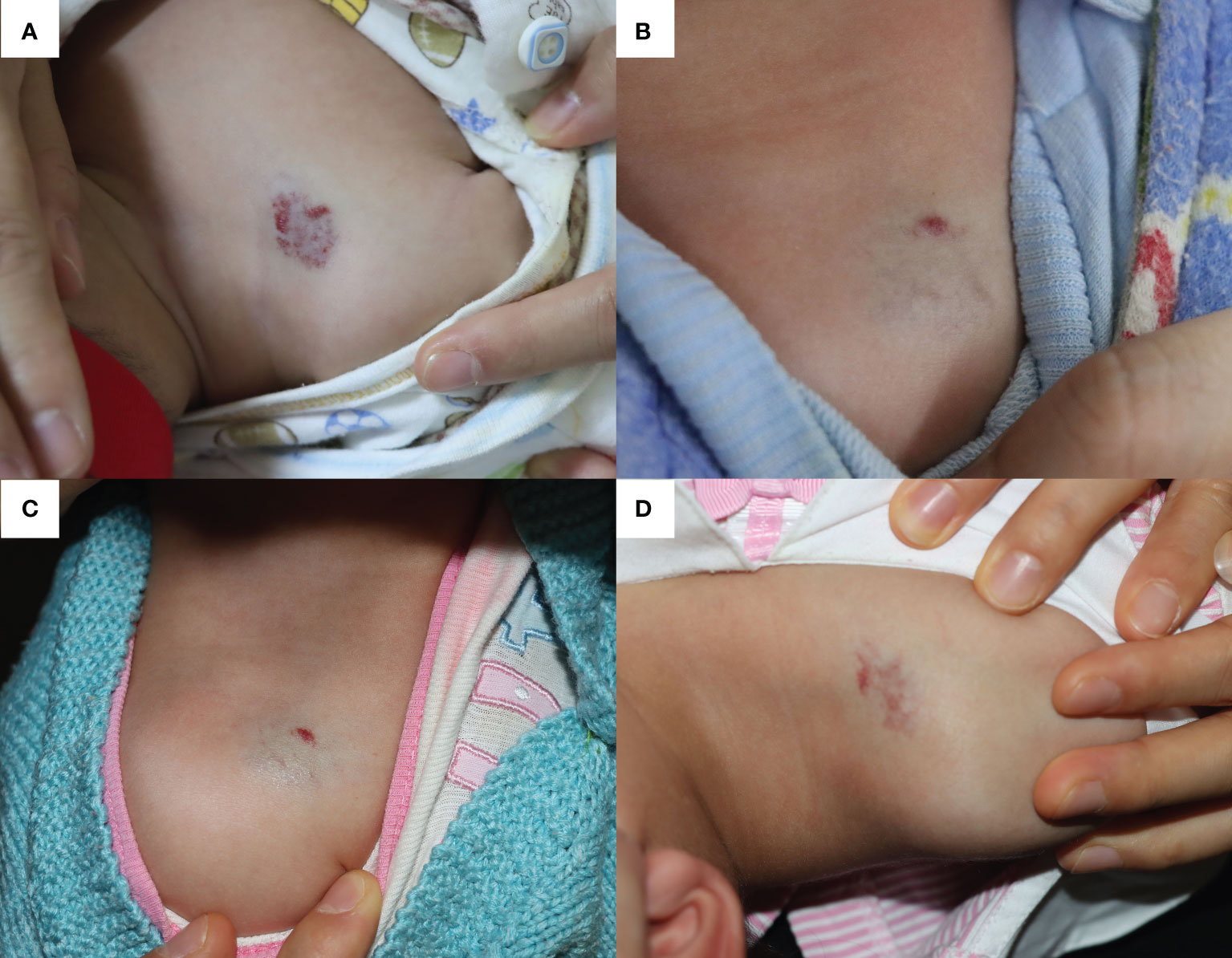
Figure 2 The infantile hemangioma in patient 2 at different periods. (A) Proliferating infantile hemangioma before oral propranolol therapy and laser therapy. (B) Involuted infantile hemangioma when the patient was admitted for acute bronchitis at the age of 15 months. (C) Infantile hemangioma showed a proliferation trend after 7 days of nebulized salbutamol treatment. (D) Infantile hemangioma worsened after receiving another 3 days of nebulized salbutamol therapy in the outpatient.
At 15 months old, he presented with acute bronchitis and started to receive nebulized inhalation treatment (salbutamol 2.5 mg twice a day and budesonide 1.0 mg twice a day). Meanwhile, OPT was replaced with topical timolol to avoid its bronchospasmodic effects. However, after 7 days of respiratory management, the IH showed a trend of proliferation (Figure 2C). As the respiratory symptoms did not completely resolve even after discharge, he received another 3 days of nebulized therapy in outpatient clinic, and the IH size further expanded (Figure 2D).
A 4-month-old boy was diagnosed with IH, which was located on the right periauricular area (Figure 3A). Ten months of OPT (2.0 mg/kg per day) and nine times of 585-nm PDL therapies significantly reduced its size and faded its color into pale pink when he was 14 months old (Figure 3B). After evaluation, propranolol and 585-nm PDL therapy were discontinued as the residuum was simply left to fade over time. Fourteen months after OPT discontinuation, he suffered from acute bronchopneumonia and received antibiotics, clenbuterol hydrochloride oral solution (7.5 ml bid), and antitussive–expectorant nebulization inhalation (ipratropium bromide, budesonide, salbutamol) at a local clinic. After 6 days of respiratory management, increased redness and macules were found on the original IH (Figure 3C). Therefore, laser therapy and OPT were restarted. These newly developed hemangiomas faded rapidly after 1 month of comprehensive treatments (Figure 3D).
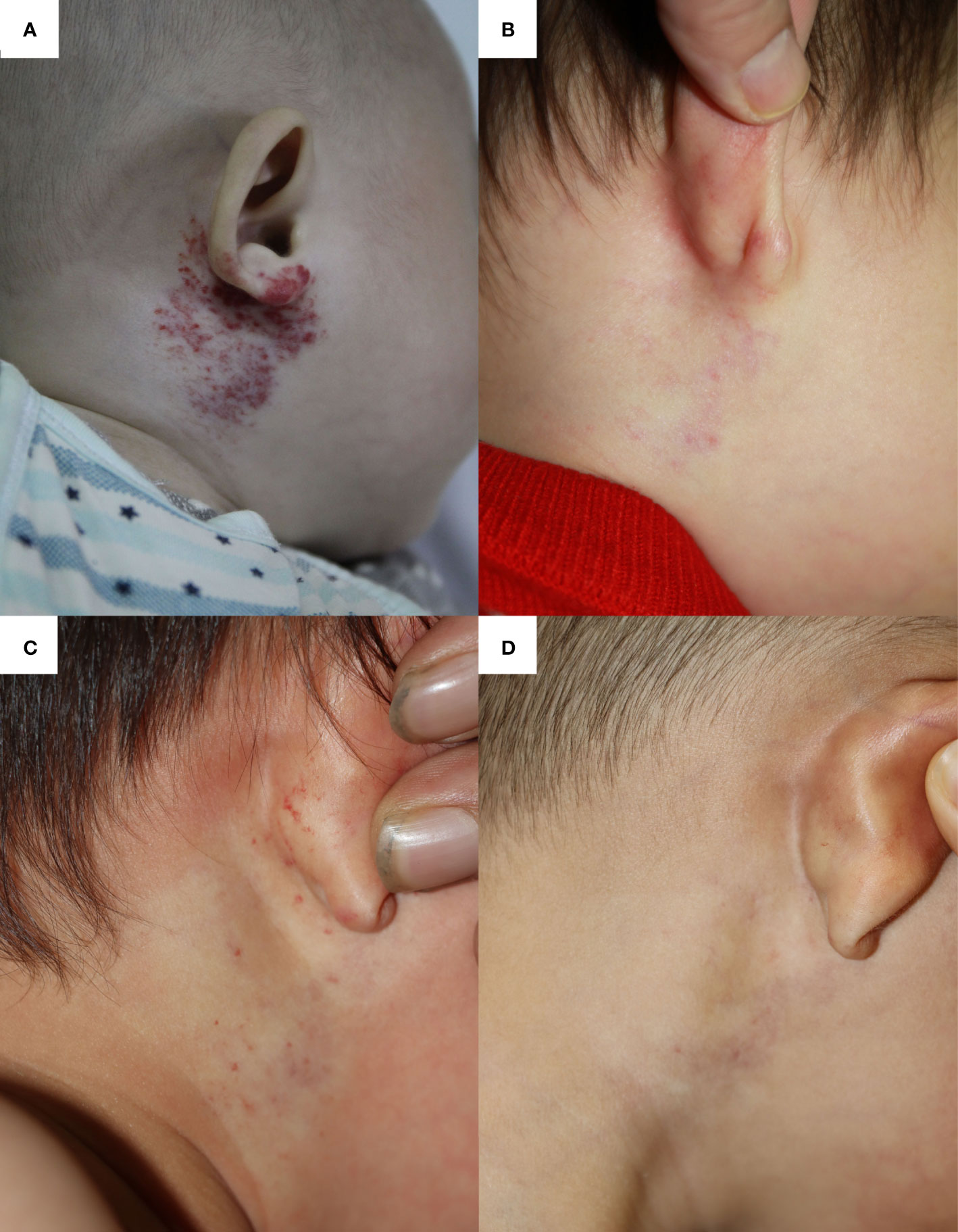
Figure 3 The infantile hemangioma in patient 3 at different periods. (A) Proliferating infantile hemangioma before oral propranolol therapy and laser therapy. (B) Involuted infantile hemangioma when the patient had completed the 10-month oral propranolol therapy and nine times of laser therapy at the age of 14 months. (C) Deterioration of infantile hemangioma after receiving 6 days of oral clenbuterol and nebulized salbutamol. (D) Involuted hemangioma after 1 month of oral propranolol therapy and one laser treatment.
OPT is currently the first-line treatment for IH because of its high response rate and safety (16–20). It usually lasts for 6–12 months or longer to overcome the rapid growth during the proliferative phase (21). In most cases, a rapid effect can be observed on color and texture after several days of OPT at a dose of 2–3 mg/kg per day. Complete or nearly complete regression can be observed in 60% of cases after 6 months of treatment (16, 22). The mechanism of action may be associated with endothelial cells and pericytes (8, 23). As a non-selective β-AR antagonist, propranolol can inhibit the NO release induced by norepinephrine through the PI3K/Akt/eNOS/NO pathway, thereby inhibiting vasodilation (24). Propranolol can also enhance the expression of α-smooth muscle actin in pericytes by blocking the β2-AR pathway, which results in vasoconstriction (23, 25).
According to published literature, rebound growth was recorded in 6%–25% of IH cases after discontinuation of propranolol (12–14). In this study, we report dramatic IH involution after 8–10 months of OPT (discontinued or not) and abrupt deterioration during the treatment of bronchitis. For case 1, IH reproliferation only occurred 1 day within the second propranolol withdrawal but did not occur within the 3-day period of the first withdrawal. It suggested that propranolol withdrawal might not be the main cause of IH deterioration in this patient. Then, what if it was because of the inhalation of budesonide, ipratropium bromide, and ambroxol during the first withdrawal period? Budesonide is a locally acting glucocorticoid used mainly to reduce the contractile response of bronchial smooth muscle. Ipratropium bromide is a muscarinic receptor antagonist that has not been reported to be associated with IH or its pathway. Ambroxol is a typical expectorant medication that mainly reduces the viscosity of sputum. Additionally, even though there are potential pathways for these medications to cause IH deterioration, the symptoms should have appeared earlier. Therefore, we excluded the speculation of these medications and turned our attention to the specific course of orally taken procaterol hydrochloride, which showed a close sequence with deterioration and might suggest a causal relationship. For case 2, the nearly involuted IH showed progressive vasodilatation during a total of 10 days of propranolol withdrawal and nebulized salbutamol therapy. Because of the lack of further examinations, there was no direct evidence to definitively determine whether propranolol withdrawal or nebulized salbutamol therapy caused the deterioration in this case. Current reports of IH deterioration after propranolol discontinuation or β2-AR agonist administration suggested that it was not a common occurrence and the interval time varied widely from 1 to 8 months (12, 26). A variety of factors can play a role in IH deterioration, and the exact mechanism remains unclear. By analyzing the unique characteristics of each patient’s disease course, we may be able to identify some evidence to help determine the most reasonable interpretation. Shinji et al. (26) reported three cases of IH rebound in 1 month after completing the treatments, in multiple times. Nicole et al. (15) reported a case with a single course of IH rebound directly associated with intravenous salbutamol. Two important differences between these two studies were the frequency of IH rebound (multiple or single) and the external intervention such as the intravenous β2-AR agonists. Therefore, these two studies proposed interpretations for the recurrence in different considerations, the gene mutation of CYP2D6 (a cytochrome P450 isozymes) or the reaction of β2-AR agonists. For our patient, the single deterioration course and a clear history of salbutamol inhalation supported the use of β2-AR agonists as the culprit in a particularly high probability. For case 3, after 14 months of propranolol discontinuation, the involuted IH showed proliferation after 6 days of oral clenbuterol and nebulized salbutamol inhalation. The discussion above for patient 2 applies equally to this patient, and because the involuted IH had stopped growing for more than 1 year, the probability of relapse caused by propranolol withdrawal was extremely low at this point, and it would be more reasonable (than in the previous two cases) to believe that the deterioration was due to β2-AR agonist intake. Salbutamol, procaterol, and clenbuterol are common selective β2-AR agonists that produce opposite effects to β2-AR antagonist via the coincident intracellular β2-AR–driven proangiogenic pathway, such as promoting norepinephrine-induced NO release and inhibiting the α-smooth muscle actin expression in pericytes. Their combined effects enhance the vasodilation of IHs, which results in increased size and redness in clinical observations. Furthermore, a recent study showed no significant difference in β2-AR expression among proliferative, involutional, and propranolol-responsive hemangiomas, which may explain the role of β2-AR agonists in the involutional phase (12–14, 27). Therefore, we speculated that the aggravation of IH in these three patients was mainly due to β2-AR agonist administration.
Even though it is not the first report on IH deterioration associated with β2-AR agonist, it is still not taken seriously by most clinicians. Nicole et al. (15) reported a patient who developed overt IH rebound within 24 h of continuous intravenous salbutamol (2.0 mg/kg per min). It rebounded more quickly and profoundly than ours, possibly because the continuous intravenous administration achieved higher blood concentration of β2-AR agonist than our cases with oral or nebulized approach. This work complements the research on IH deterioration associated with the administration of β2-AR agonists and calls for special attention to their oral or nebulized approaches, as they are frequently prescribed in the treatment of pediatric respiratory diseases. Large-sample-size studies should be carried out to analyze the influence of β2-AR agonists on IH deterioration/rebound. Although whether β2-AR agonist is a risk factor still needs further evidence, current reports and evidence urge the healthcare providers to be more prudent when administrating β2-AR agonists to pediatric patients with IH. If β2-AR agonist cannot be avoided in some cases, patients and/or their legal guardians should be warned of the possible deterioration of IH.
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
The studies involving human participants were reviewed and approved by Ethics Committee of Children’s Hospital of Chongqing Medica University. Written informed consent from the participants’ legal guardian/next of kin was not required to participate in this study in accordance with the national legislation and the institutional requirements.
Conception and design: QC, YZ, and SN. Administrative support: SN and HW. Provision of study materials or patients: QC, LL, XL, and HW. Collection and assembly of data: YZ and CS. Data analysis and interpretation: YZ, QC, and CS. Native English polish and provided critical opinion: CS. Manuscript writing: All authors. Final approval of manuscript: All authors.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Munden A, Butschek R, Tom WL, Marshall JS, Poeltler DM, Krohne SE, et al. Prospective study of infantile haemangiomas: incidence, clinical characteristics and association with placental anomalies. Br J Dermatol (2014) 170:90713. doi: 10.1111/bjd.12804
2. Drolet BA, Esterly NB, Frieden IJ. Hemangiomas in children. N Engl J Med (1999) 341:173–81. doi: 10.1056/NEJM199907153410307
3. Tollefson MM, Frieden IJ. Early growth of infantile hemangiomas: what parents' photographs tell us. Pediatrics (2012) 130:e314–20. doi: 10.1542/peds.2011-3683
4. Darrow DH, Greene AK, Mancini AJ, Nopper AJ. Section on dermatology, section on otolaryngology–head and neck surgery, and section on plastic surgery. diagnosis and management of infantile hemangioma. Pediatrics (2015) 136(4):e1060–104. doi: 10.1542/peds.2015-2485
5. Pandey A, Gangopadhyay AN, Upadhyay VD. Evaluation and management of infantile hemangioma: an overview. Ostomy Wound Manage (2008) 54:1629.
6. Bauland CG, Lüning TH, Smit JM, Zeebregts CJ, Spauwen PHM. Untreated hemangiomas: growth pattern and residual lesions. Plast Reconstr Surg (2011) 127:1643–8. doi: 10.1097/PRS.0b013e318208d2ac
7. Macca L, Altavilla D, Di Bartolomeo L, Irrera N, Borgia F, Pomi F, et al. Update on treatment of infantile hemangiomas: What's new in the last five years? Front Pharmacol (2022) 13:879602. doi: 10.3389/fphar.2022.879602
8. Storch CH, Hoeger PH. Propranolol for infantile haemangiomas: insights into the molecular mechanisms of action. Br J Dermatol (2010) 163:269–74. doi: 10.1111/j.1365-2133.2010.09848.x
9. Chim H, Armijo BS, Miller E, Gliniak C, Serret MA, Gosain AK. Propranolol induces regression of hemangioma cells through HIF-1α-mediated inhibition of VEGF-a. Ann Surg (2012) 256:146–56. doi: 10.1097/SLA.0b013e318254ce7a
10. Sasaki M, North PE, Elsey J, Bubley J, Rao S, Jung Y, et al. Propranolol exhibits activity against hemangiomas independent of beta blockade. NPJ Precis Oncol (2019) 3:27. doi: 10.1038/s41698-019-0099-9
11. Sommers Smith SK, Smith DM. Beta blockade induces apoptosis in cultured capillary endothelial cells. In vitro. Cell Dev Biol Anim (2002) 38:298–304. doi: 10.1290/1071-2690(2002)038<0298:BBIAIC>2.0.CO;2
12. Shah SD, Baselga E, McCuaig C, Pope E, Coulie J, Boon L, et al. Rebound growth of infantile hemangiomas after propranolol therapy. Pediatrics (2016) 137:e20151754. doi: 10.1542/peds.2015-1754
13. Xiao Q, Li Q, Zhang B, Yu W. Propranolol therapy of infantile hemangiomas: efficacy, adverse effects, and recurrence. Pediatr Surg Int (2013) 29:575–81. doi: 10.1007/s00383-013-3283-y
14. Bagazgoitia L, Hernández-Martín Á, Torrelo A. Recurrence of infantile hemangiomas treated with propranolol. Pediatr Dermatol (2011) 28:658–62. doi: 10.1111/j.1525-1470.2011.01644.x
15. Knöpfel N, Oesch V, Theiler M, Szello P, Weibel L. Rebound of involuted infantile hemangioma after administration of salbutamol. Pediatrics (2020) 145:e20191942. doi: 10.1542/peds.2019-1942
16. Léauté-Labrèze C, Dumas de la Roque E, Hubiche T, Boralevi F, Thambo JB, Taïeb A. Propranolol for severe hemangiomas of infancy. N Engl J Med (2008) 358:2649–51. doi: 10.1056/NEJMc0708819
17. Léauté-Labrèze C, Hoeger P, Mazereeuw-Hautier J, Guibaud L, Baselga E, Posiunas G, et al. A randomized, controlled trial of oral propranolol in infantile hemangioma. N Engl J Med (2015) 372:735–46. doi: 10.1056/NEJMoa1404710
18. Malik MA, Menon P, Rao KL, Samujh R. Effect of propranolol vs prednisolone vs propranolol with prednisolone in the management of infantile hemangioma: a randomized controlled study. J Pediatr Surg (2013) 48:2453–9. doi: 10.1016/j.jpedsurg.2013.08.020
19. Bauman NM, McCarter RJ, Guzzetta PC, Shin JJ, Oh AK, Preciado DA, et al. Propranolol vs prednisolone for symptomatic proliferating infantile hemangiomas: a randomized clinical trial. JAMA Otolaryngol Head Neck Surg (2014) 140:323–30. doi: 10.1001/jamaoto.2013.6723
20. Adams DM, Ricci KW. Infantile hemangiomas in the head and neck region. Otolaryngol Clin North Am (2018) 51:77–87. doi: 10.1016/j.otc.2017.09.009
21. Johansen ML, Mahendran G, Lawley LP. Is prolonged monitoring necessary? an updated approach to infantile hemangioma treatment with oral propranolol. Pediatr Dermatol (2021) 38:800–5. doi: 10.1111/pde.14651
22. Léauté-Labrèze C, Harper JI, Hoeger PH. Infantile haemangioma. Lancet (2017) 390:85–94. doi: 10.1016/S0140-6736(16)00645-0
23. Lee D, Boscolo E, Durham JT, Mulliken JB, Herman IM, Bischoff J. Propranolol targets the contractility of infantile haemangioma-derived pericytes. Br J Dermatol (2014) 171:1129–37. doi: 10.1111/bjd.13048
24. Pan WK, Li P, Guo ZT, Huang Q, Gao Y. Propranolol induces regression of hemangioma cells via the down-regulation of the PI3K/Akt/eNOS/VEGF pathway. Pediatr Blood Cancer (2015) 62:1414–20. doi: 10.1002/pbc.25453
25. Alarcon-Martinez L, Yilmaz-Ozcan S, Yemisci M, Schallek J, Kılıç K, Can A, et al. Capillary pericytes express α-smooth muscle actin, which requires prevention of filamentous-actin depolymerization for detection. Elife (2018) 7:e34861. doi: 10.7554/eLife.34861
26. Kagami S, Kaneko M, Kishi A, Katori T. Prolonged growth of infantile hemangioma after pulsed dye laser and oral propranolol treatment. J Dermatol (2018) 45:1109–12. doi: 10.1111/1346-8138.14517
Keywords: infantile hemangioma, recurrence, propranolol, β2-adrenergic receptor, salbutamol, procaterol
Citation: Chen Q, Zhang Y, Sun C, Liu L, Luo X, Wang H and Ni S (2022) Case report: Deterioration of infantile hemangioma related to oral or nebulized administration of β2-AR agonist: Three cases reports. Front. Oncol. 12:1000099. doi: 10.3389/fonc.2022.1000099
Received: 21 July 2022; Accepted: 20 October 2022;
Published: 10 November 2022.
Edited by:
Jia Wei Zheng, Shanghai Jiao Tong University, ChinaReviewed by:
Shinji Kagami, Kanto Central Hospital of the Mutual Aid Association of Public School Teachers, JapanCopyright © 2022 Chen, Zhang, Sun, Liu, Luo, Wang and Ni. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sili Ni, Mzg5NjIxNDlAcXEuY29t
†These authors share first authorship
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.