- 1Department of Epidemiology and Biostatistics, School of Public Health, Xuzhou Medical University, Xuzhou, China
- 2Department of Hematology, Affiliated Hospital of Xuzhou Medical University, Xuzhou, China
- 3Department of Hematology, Qilu Hospital of Shandong University, Jinan, China
- 4Department of Hematology, The Affiliated Hospital of Jining Medical University, Jining, China
- 5Department of Hematology, Taian Central Hospital, Taian, China
- 6Department of Hematology, The First People’s Hospital of Changzhou, Changzhou, China
- 7Department of Hematology, Yancheng First People’s Hospital, Yancheng, China
- 8Department of Hematology, The First Affiliated Hospital of Anhui Medical University, Hefei, China
- 9Department of Nuclear Medicine, Affiliated Hospital of Xuzhou Medical University, Xuzhou, China
- 10Department of Hematology, The First Affiliated Hospital of Bengbu Medical College, Bengbu, China
- 11Department of Hematology, Huai’an First People’s Hospital, Huaian, China
- 12Department of Hematology, The General Hospital of Wanbei Coal-Electric Group, Suzhou, China
- 13Center for Medical Statistics and Data Analysis, School of Public Health, Xuzhou Medical University, Xuzhou, China
Diffuse large B-cell lymphoma (DLBCL) is a highly heterogeneous non-Hodgkin lymphoma, and the prognosis of DLBCL patients is widely affected by multivariables. Clinical-factors-based prognostic systems stratify the prognosis of DLBCL with certain limitations, and the value of ferritin on the prognosis of DLBCL is unclear. In this study, 225 cases were retrieved from 4 centers of Huaihai Lymphoma Working Group (HHLWG) as the derivation cohort, and 66 cases were from the other 6 centers of HHLWG as external validation cohort. X-Tile program divided ferritin into three groups when applying 175.00 and 391.90 μg/L as the optimal cutoff points. Based on multivariable analysis, ferritin appeared to be a stronger predictor. A total of three variables (ferritin, age, and lactate dehydrogenase) were included for the development of the nomogram. The C-indexes were 0.73 and 0.70 in the derivation and validation cohort, and the calibration curve showed the consistency between the nomogram prediction and the actual observation. In conclusion, Ferritin-based nomogram enhanced the prognostic value of IPI in DLBCL.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL) with high heterogeneity (1, 2). Cell origin, gene-based molecular stratification, immune markers, and other clinical variables pose great challenge to the DLBCL prognosis (3, 4). Clinical-characteristics-based International Prognostic Index (IPI) is widely used for DLBCL prognosis evaluation, but it failed to accurately classify variables such as age and lactate dehydrogenase (LDH). More importantly, with the addition of rituximab to anthracycline-containing therapy, its capacity to discriminate among risk groups was declined (5, 6). Several updated prognostic models have been developed and have greatly improved the prognostic accuracy of DLBCL (3, 6, 7). However, considering the high heterogeneity of DLBCL and advances of treatment, more variables such as nutritional markers, clinical factors, pathological characteristics, and genetic abnormalities are worthy of exploration to build more accurate prognostic system and to guide individualized treatment (8).
Nutritional markers are of great significance for the assessment of tumor prognosis. For example, obesity is associated with a 35%–40% increased risk of breast cancer recurrence and death (9). Ronald Wihal Oei et al. revealed that critical weight loss and low pretreatment hemoglobin were prognostic factors for survival in patients with nasopharyngeal carcinoma (10). Iron overload is usually associated with severe inflammation and pathological nutrition status. Some reports demonstrated that high serum ferritin level at diagnosis was a poor prognostic marker for some solid tumors including pancreatic cancer and head and neck cancer (11–13). In addition, Jun Zhou et al. found that high ferritin levels inversely correlate with survival for adult patients with hemophagocytic lymphohistiocytosis (HLH) (14). More specifically, a retrospective analysis showed that ferritin levels above 500 ng/ml could be an important marker for predicting poor survival outcomes in NHL (15). Da Jung Kim et al. revealed that high ferritin level (≥ 500 ng/ml) was an independent poor prognostic factor for progress-free survival (PFS) and overall survival (OS) in DLBCL patients with lower-risk IPI scores (16). However, few studies assessed the optimal cutoff point of ferritin level in the prognostic models for patients with DLBCL.
Different statistical methods have been used in predicting the outcomes of different diseases. Shaoxu Wu et al. made their prognosis for patients with bladder cancer by a nomogram (17, 18). The visual format of nomogram reflects a statistical prediction that can determine how many points are attributed for each variable value and the relative importance of predictors can be judged by the length of each line within the nomogram (19). X-Tile program can visualize the survival data and calculate the optimal cutoff point by dividing subgrouping. In addition, the associations can be determined by a variety of statistical tests, including the log-rank test for survival and means tests for associations between other marker data (20). In this study, continuous variables were processed by X-Tile program to determine the optimal threshold, and a nomogram was developed to stratify the prognosis for patients with DLBCL based on the levels of ferritin, other related nutritional markers, and baseline clinical factors.
Materials and Methods
Patients
From February 2015 to January 2021, a total of 291 patients with DLBCL and initial ferritin data were included in this study from HHLWG: 225 patients from 4 centers served as the derivation cohort, and 66 cases from the other 6 centers of HHLWG served as external validation cohort. All patients included in this study were with pathological diagnosis of DLBCL and treated with rituximab-based immunochemotherapy. Exclusion criteria were the following: (1) patients with other hematological malignancies; (2) special types of lymphoma (primary central nervous system lymphoma, primary mediastinal large B-cell lymphoma, and transformed DLBCL). The baseline characteristics included initial data of gender, age, extranodal involvement, performance status (21), presence of bulky disease (≥7.5cm), B symptoms, ferritin (Fer), lactate dehydrogenase (LDH), albumin (Alb), white blood cell count (WBC), hemoglobin (HGB), platelets (PLT), stage, cell of origin, and immunological markers. Follow-up was conducted through reviewing inpatient medical records and making phone calls. Overall survival (OS) was calculated as the interval between the time of diagnosis and death from any cause or the last follow-up. Study approval was obtained from the independent Ethics Committees of each center in HHLWG and met Helsinki Declaration.
Statistical Analysis
Baseline clinical characteristics were described by variable type using median and interquartile range. Outliers were verified by the hospital medical record system. All cases had complete clinical information to avoid unnecessary bias. The value of ferritin was used as a continuous variable in the Mann–Whitney U-test to explore the difference between groups in different pathological groups. Continuous variables of ferritin, albumin, Body Mass Index (BMI), and age were divided into groups using the X-Tile program based on survival time (20). Kaplan–Meier analysis was used to explore the effect of pathological factors combined with ferritin level on survival, and log-rank test was performed for the difference between groups. Cox proportional hazard model was used to analyze the univariate association between clinical features and prognosis, with p < 0.05. The best prediction variable set was obtained by both stepwise regression, and Akaike Information Criteria (AIC) was used to evaluate the model (22). The Harrell’s concordance index and calibration curve of the model were calculated by bootstrap method according to the regression results, and validation was carried out in parallel (21). The number of self-sampling B was 500. The closer the concordance index (C-index) to 1, the better the prediction performance. Statistical analysis was conducted with SPSS software [version 19.0 (IBM, NY, USA)] and R software (version 4.2.0; http://www.Rproject.org).
Results
Clinical Characteristics
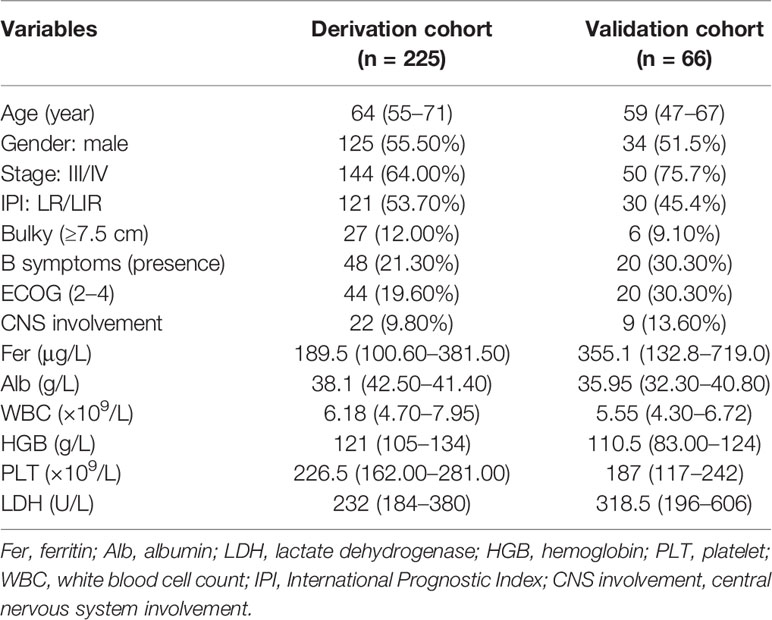
The characteristics of the patients are detailed in Table 1. The follow-up deadline was July 15, 2021, and the median follow-up time was 36.7 months in derivation cohort, and 23.1 months in validation cohort. At the end of follow-up, a total of 119 (40.90%) deaths occurred. The median age at diagnosis was 62 years, with 159 (54.60%) male and 168 (57.70%) patients were older than 60 years. Ann Arbor stage III/IV accounted for 66.60%.
The Cutoff Points of Ferritin and Other Continuous Variables Calculated by X-Tile
Based on the X-Tile program, the maximum chi-squared points 22.13 and 25.39 were reached when applying 175.00 and 391.90 μg/L as the optimal cutoff points (p < 0.0001, Figure 1). Therefore, the DLBCL patients were divided into three subgroups for further analyses by using these cutoff points. Similarly, the optimal cutoff points for age, Alb, WBC, HGB, PLT, and LDH were 75 years, 40.30 g/L, 8 ×1 09/L, 97 g/L, 205.50 × 109/L, and 248 U/L.
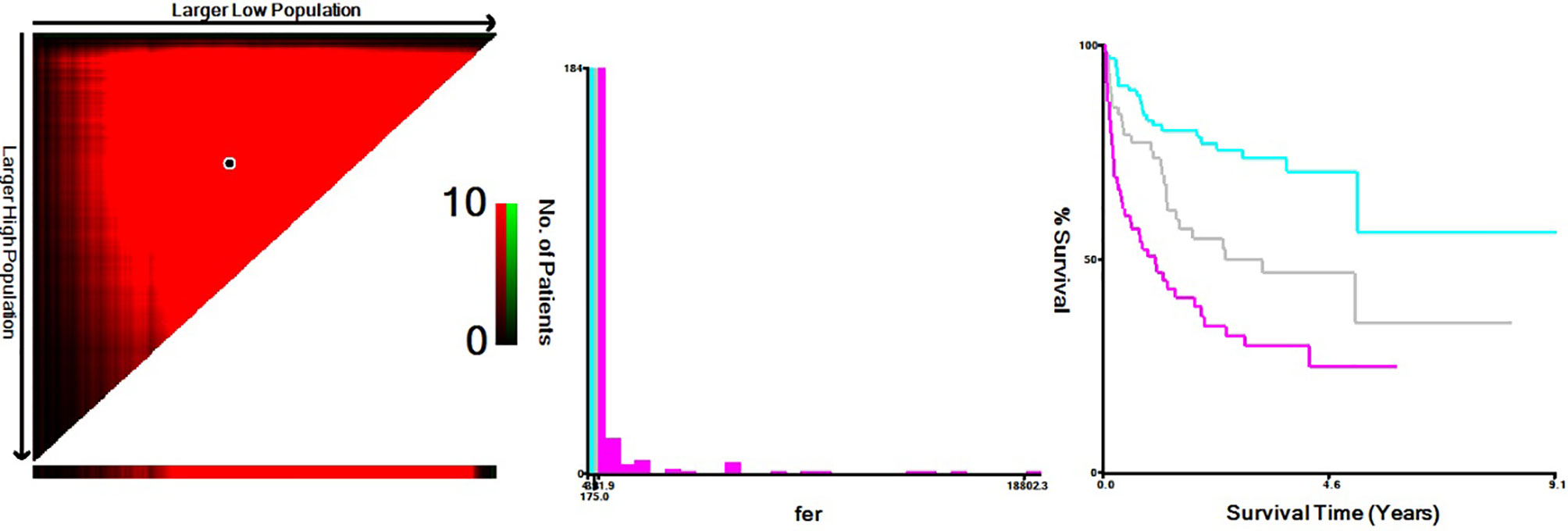
Figure 1 X-Tile data are displayed in a right-angled triangle grid, where each point represents a different cut point. The intensity of the color at each cutoff point indicates the strength of the association. X-Tile analysis of survival data from DLBCL patients reveals a continuous distribution based on ferritin. The plots show the c2 log-rank values produced when dividing the patients with two cut-points, producing high, middle, and low subsets.
Prognostic Value of Ferritin With Clinicopathological Patterns in DLBCL
Mann–Whitney U-test was conducted for exploring continuous ferritin values on different subtypes, and the results suggested that ferritin levels between Ann Arbor I/II, III/IV and IPI LR/LIR, HIR/HR groups were significantly different (p < 0.01), but no significant difference was found in BCL-2, BCL-6, and CD5 groups.
In the whole cohort, patients with high ferritin level (>391.90 μg/L) had poor survival with 5-year OS of only 28.2% (Figure 2A). Using cutoff values of 50% positive tumor cells for BCL-2 and BCL-6, 92 (31.60%) cases were positive for BCL-2, and 93 (31.90%) cases were positive for BCL-6. Thirty-six (12.4%) patients were with high Ki-67 score (≥ 0.9), and 81 (27.80%) were non-GCB. Kaplan–Meier analysis found that levels of ferritin could not re-stratify BCL-2, CD5, and Eastern Cooperative Oncology Group (ECOG) scores (≥2), and Ann Arbor Stage (I/II) (p > 0.05). However, the levels of ferritin could restratify the prognosis in BCL-2+, BCL-6, CD5+, cell of origin, ECOG score (<2), Ann Arbor Stage (III/VI), and IPI (p < 0.05, Figure 2).
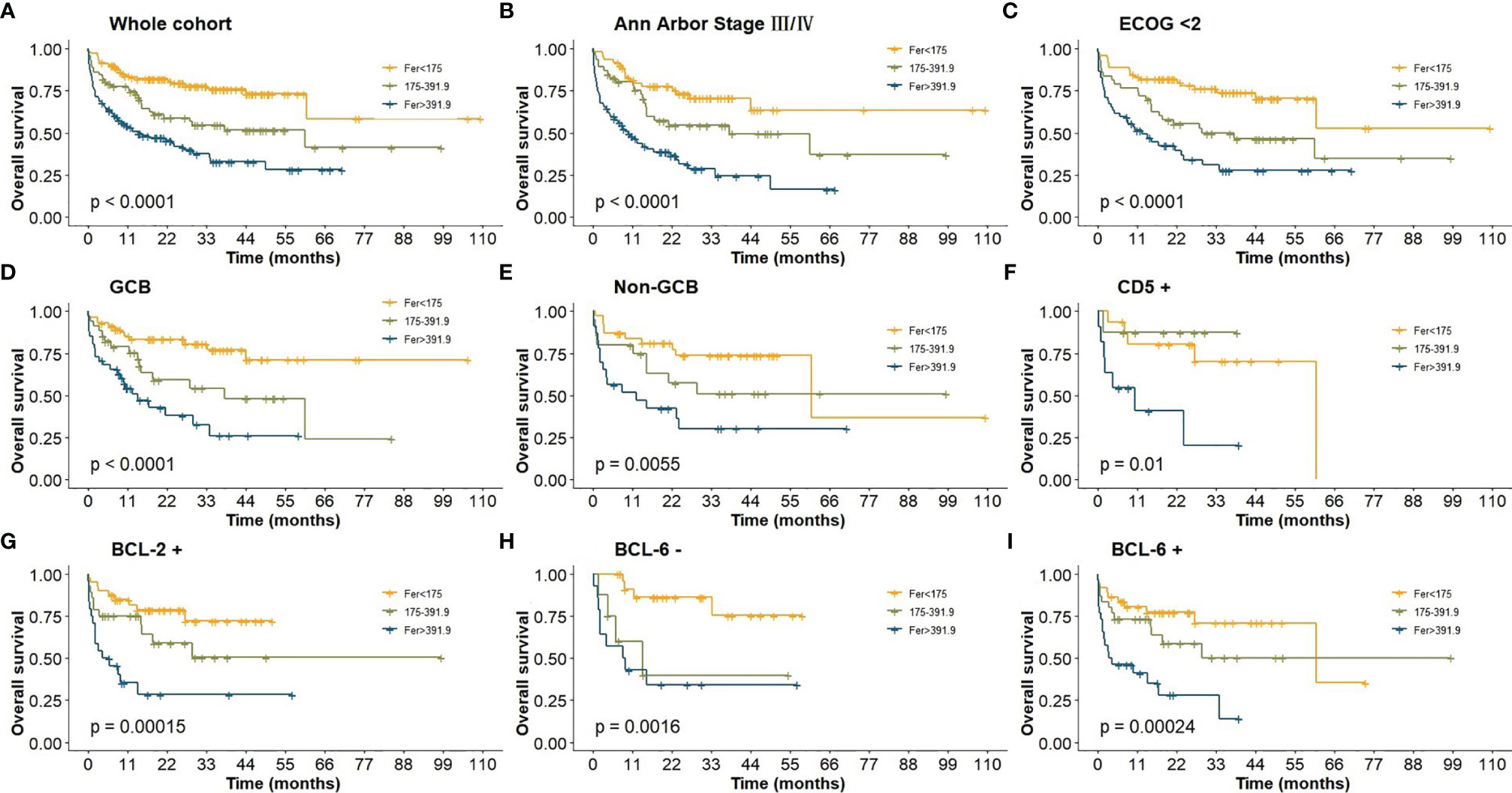
Figure 2 (A) OS of DLBCL patients in whole cohort; (B) OS of Ann Arbor Stage III/IV; (C) OS of ECOG (<2); (D–E) OS of cell-of-origin; (F) OS of CD5+; (G) OS of BCL-2+; (H–I) OS of BCL-6.
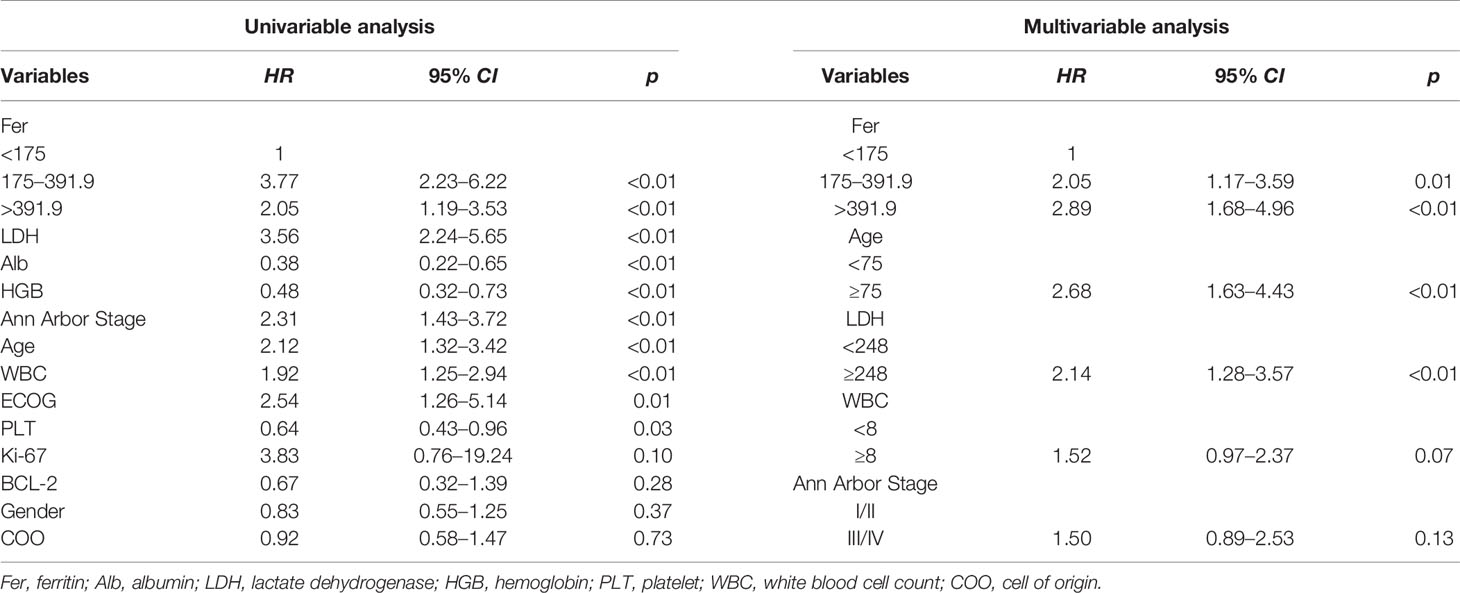
Univariable and Multivariable Analysis of DLBCL Patients
The effects of different clinical variables on OS were analyzed by univariable and multivariable analyses, and the results showed that the level of ferritin appeared to be a stronger predictor. Univariable analysis exhibited that Alb, HGB, PLT, and age were prognostic predictors (p < 0.05, Table 2). Following the model iterations in multivariable analysis, the final prognostic index consisted of three factors, as shown in Table 2. Ferritin was proved to be an adverse factor for the survival of DLBCL patients. Nevertheless, WBC in the current multivariable model was observed to be not predictive [p = 0.07, HR =1.52, 95% CI (0.97–2.37)].
Development of Ferritin-Based Prognostic Nomogram and Validation
Based on multivariable analysis, a prognostic nomogram was developed to predict 1-, 3-, and 5-year OS of DLBCL patients (Figure 3), and the Harrell’s concordance index and brier score (C-index = 0.73, Brier score = 0.17) were calculated between the predicted and the real outcome of the model for internal validation.
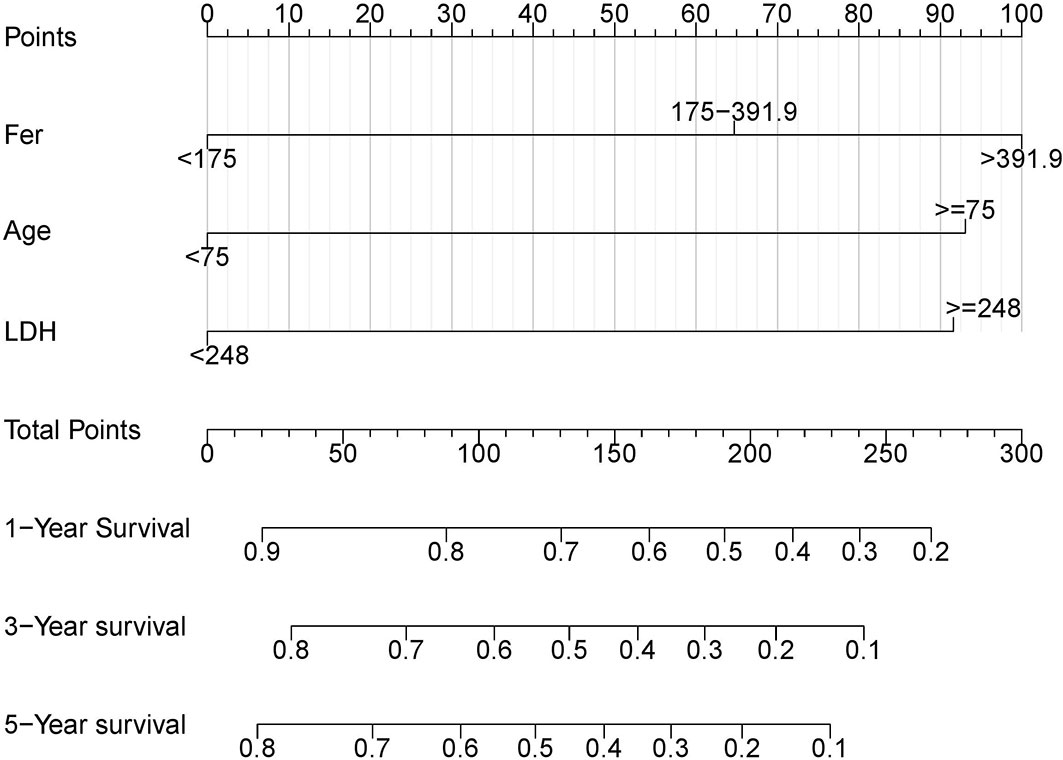
Figure 3 Nomogram for patients with DLBCL. The patient's clinical eigenvalues are placed on each variable axis and a line is drawn upward to determine the number of points gained for each variable value. The sum of these numbers lies on the total point axis, and then a line is drawn down on the survival axis to determine his overall survival probability.
We further validated this nomogram externally by the calibration curve and computed the C-index and Brier score in an independent validation cohort of 66 patients (C-index = 0.70, Brier score = 0.22) in the external validation. The calibration curves were close to the ideal curves, suggesting that the predicted result and the actual outcome had a good consistency (Figure 4).

Figure 4 The red solid line represents the performance of the nomograph, and the higher the fitting degree with diagonal dotted line, the better the prediction effect. (A) Derivation cohort; (B) Validation cohort.
Comparison of the Current Nomogram With IPI
In this study, all cases had complete data for all the variables required to calculate the IPI score. We analyzed the effect of ferritin levels on the prognosis in different IPI risk groups, and Kaplan–Meier analysis results are shown in Figure 5. Abnormal ferritin level was an adverse factor for patients in LIR/LR and HIR/HR groups in global comparisons (p <0.05, Figure 5). The 5-year OS for patients in different levels of ferritin in the LIR/LR group were 80.50%, 63.10%, and 51.20%, respectively (Figure 5A), and the 3-year OS in the HIR/HR group were 57.90%, 46.50%, and 21.50%, respectively (Figure 5B). Compared with IPI, the nomogram showed better accuracy in predicting survival of patients in both groups.
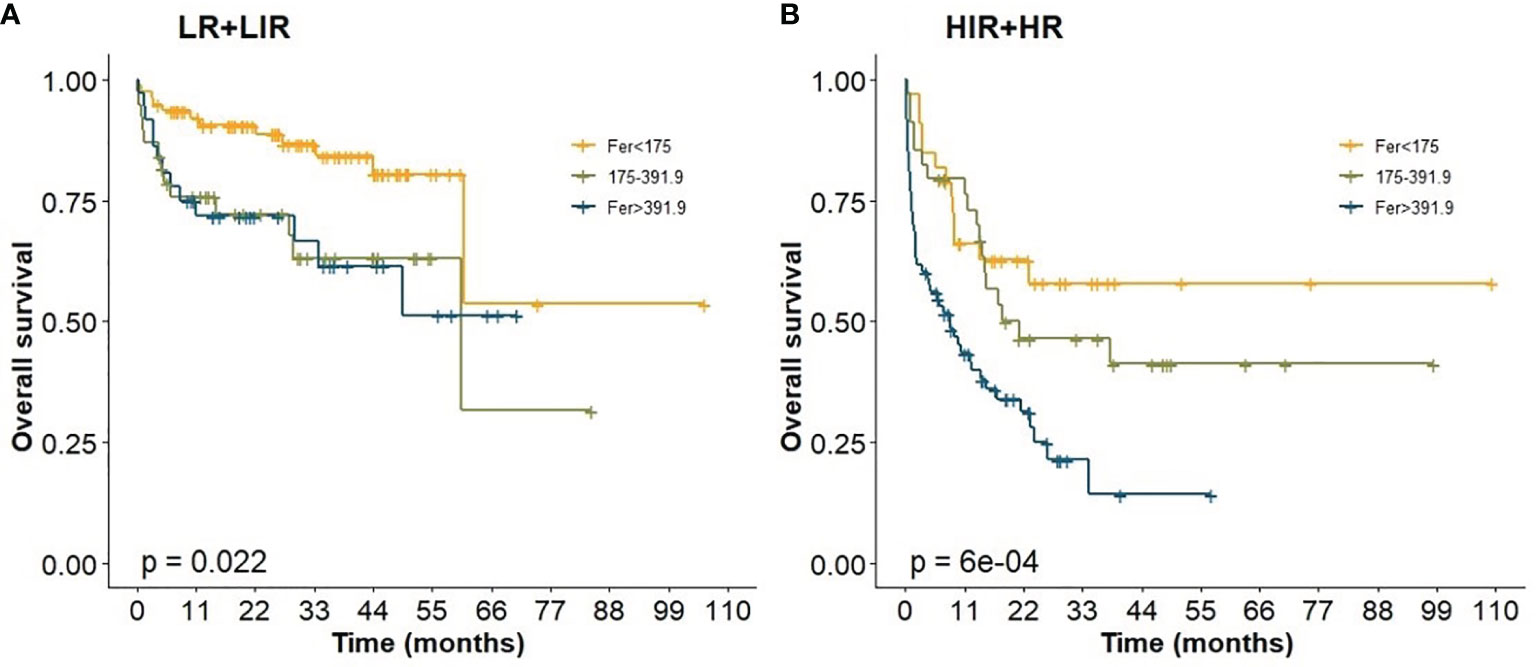
Figure 5 Prognostic impact of ferritin levels among patients in IPI. (A) Low risk/low intermediate risk group; (B) high intermediate risk/ high risk group.
Discussion
In this study, we assessed the level of ferritin to evaluate its prognostic value on DLBCL. Our research showed that patients with high level of ferritin had a poor prognosis. Based on model iteration, we established a prognosis nomogram for DLBCL patients, and the relative importance of the predictors could be determined by the length of the lines in the nomogram (23).
Nutritional markers affect the prognosis and survival of cancer patients. Indices such as the prognostic nutritional index (PNI) (24), albumin, BMI, and obesity have been well studied, and there is considerable evidence for their prognostic effects (25–29). Ferritin, an important indicator of metabolic level and nutrition, is associated with prognosis in end-stage liver disease, which can satisfactorily predict 11- and 90-day mortality (30).
A previous study in South Korea revealed that age, obesity, drinking habits, and glucose levels were significantly associated with women’s serum ferritin levels (31). Ferritin is detected at higher levels in the sera of many cancer patients, and the higher levels relate to aggressive disease and poor clinical outcome (32). In this study, we evaluated ferritin level using X-Tile program to seek more accurate cutoff point, and the results showed that the maximum chi-squared points of 22.13 and 25.39 were reached when applying 175.00 and 391.90 μg/L as the optimal cutoff points. Univariate Cox analysis showed that ferritin was a strong prognostic predictor of DLBCL. Elevated ferritin level (Fer ≥ 391.90 μg/L) was significantly correlated with prognosis. In addition, we used X-Tile program rather than the usual criteria to divide the age into two groups to achieve precise stratification. Multivariable analysis showed that when patients ≥75 years, the 3-year OS was only 28.10%.
R‐CHOP plus X regiments failed to improve OS in those patients with MYC and BCL‐2/BCL‐6 double expression/hit, activated B cells, and CD5-positive subtype. Previous studies have shown that different pathological immunophenotypes have an impact on outcomes (33, 34). In this study, ferritin level could restratify patients of CD5-positive group, and high levels of ferritin had a poor prognosis in both negative and positive BCL-6 groups (p < 0.05). High levels of ferritin in the GCB group also had a significant impact on the prognosis, but accurate stratification of ferritin in prognosis was not achieved in CD5- and BCL-2-negative groups.
The nomogram model demonstrated in this study included three variables: ferritin, age, and LDH. The specific DLBCL prognostic nomogram aimed to estimate the probability of 1-, 3-, and 5-year OS based on multivariable Cox proportional hazard models. Bootstrap resampling, C-index, and calibration curve were used to validate the model. The C-index was 0.73 between the predicted outcome and the real outcome of the model. The predicting clinical factors of the nomogram and original IPI were similar, with the former applying a refined categorization of age by X-Tile program. The C-index of the nomogram was higher than that of IPI, demonstrating a more obvious advantage than IPI. In conclusion, the addition of ferritin enhanced IPI for the prognosis of DLBCL. However, due to the inherent flaw of the retrospective design, further prospective studies need to be explored.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the independent Ethics Committees of each center in HHLWG and met Helsinki Declaration. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
WS and SH: designed this study. ZS and CH: analysis and interpretation. SZ, MZ, LH, QS, DY, JY, HZ, WG, YM, QL, CO, JZ, CW, and TZ: acquisition of data. CO provided the advices of this study. All authors contributed to the article and approved the submitted version.
Funding
This study was funded by the Natural Science Foundation of Jiangsu Province, Grant/Award Number BK20171181; Jiangsu Key Research and Development Project of Social Development, Grant/Award Number BE2019638; and Young Medical Talents of Jiangsu Science and Education Health Project, Grant/Award Number QNRC2016791.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
Thanks to the Huaihai Lymphoma Working Group (HHLWG) for its participation in this study.
References
1. Ma J, Yan Z, Zhang J, Zhou W, Yao Z, Wang H, et al. A Genetic Predictive Model for Precision Treatment of Diffuse Large B-Cell Lymphoma With Early Progression. Biomark Res (2020) 8:33. doi: 10.1186/s40364-020-00214-3
2. Sehn LH, Donaldson J, Chhanabhai M, Fitzgerald C, Gill K, Klasa R, et al. Introduction of Combined CHOP Plus Rituximab Therapy Dramatically Improved Outcome of Diffuse Large B-Cell Lymphoma in British Columbia. J Clin Oncol (2005) 23:5027–33. doi: 10.1200/JCO.2005.09.137
3. Sehn LH, Berry B, Chhanabhai M, Fitzgerald C, Gill K, Hoskins P, et al. The Revised International Prognostic Index (R-IPI) Is a Better Predictor of Outcome Than the Standard IPI for Patients With Diffuse Large B-Cell Lymphoma Treated With R-CHOP. Blood (2007) 109:1857–61. doi: 10.1182/blood-2006-08-038257
4. Gleeson M, Counsell N, Cunningham D, Lawrie A, Clifton-Hadley L, Hawkes E, et al. Prognostic Indices in Diffuse Large B-Cell Lymphoma in the Rituximab Era: An Analysis of the UK National Cancer Research Institute R-CHOP 14 Versus 21 Phase 3 Trial. Br J Haematol (2020) 192:1015–9. doi: 10.1111/bjh.16691.10.1111/bjh.16691
5. International Non-Hodgkin’s Lymphoma Prognostic Factors P. A Predictive Model for Aggressive Non-Hodgkin’s Lymphoma. N Engl J Med (1993) 329:987–94. doi: 10.1056/NEJM199309303291402
6. Zhou Z, Sehn LH, Rademaker AW, Gordon LI, Lacasce AS, Crosby-Thompson A, et al. An Enhanced International Prognostic Index (NCCN-IPI) for Patients With Diffuse Large B-Cell Lymphoma Treated in the Rituximab Era. Blood (2014) 123:837–42. doi: 10.1182/blood-2013-09-524108
7. Montalban C, Diaz-Lopez A, Dlouhy I, Rovira J, Lopez-Guillermo A, Alonso S, et al. Validation of the NCCN-IPI for Diffuse Large B-Cell Lymphoma (DLBCL): The Addition of Beta2 -Microglobulin Yields a More Accurate GELTAMO-IPI. Br J Haematol (2017) 176:918–28. doi: 10.1111/bjh.14489
8. Bentayeb H, Aitamer M, Petit B, Dubanet L, Elderwish S, Desaubry L, et al. Prohibitin (PHB) Expression Is Associated With Aggressiveness in DLBCL and Flavagline-Mediated Inhibition of Cytoplasmic PHB Functions Induces Anti-Tumor Effects. J Exp Clin Cancer Res (2019) 38:450. doi: 10.1186/s13046-019-1440-4
9. Jiralerspong S, Goodwin PJ. Obesity and Breast Cancer Prognosis: Evidence, Challenges, and Opportunities. J Clin Oncol (2016) 34:4203–16. doi: 10.1200/JCO.2016.68.4480
10. Oei RW, Ye L, Huang J, Kong F, Xu T, Shen C, et al. Prognostic Value of Nutritional Markers in Nasopharyngeal Carcinoma Patients Receiving Intensity-Modulated Radiotherapy: A Propensity Score Matching Study. Onco Targets Ther (2018) 11:4857–68. doi: 10.2147/OTT.S165133
11. Koyama S, Fujisawa S, Watanabe R, Itabashi M, Ishibashi D, Ishii Y, et al. Serum Ferritin Level Is a Prognostic Marker in Patients With Peripheral T-Cell Lymphoma. Int J Lab Hematol (2017) 39:112–7. doi: 10.1111/ijlh.12592
12. Kalousova M, Krechler T, Jachymova M, Kubena AA, Zak A, Zima T. Ferritin as an Independent Mortality Predictor in Patients With Pancreas Cancer Results Pilot Study. Tumour Biol (2012) 33:1695–700. doi: 10.1007/s13277-012-0426-z
13. Maxim PE, Veltri RW. Serum Ferritin as a Tumor Marker in Patients With Squamous Cell Carcinoma of the Head and Neck. Cancer (1986) 57:305–11. doi: 10.1002/1097-0142(19860115)57:2<305::aid-cncr2820570219>3.0.co;2-d
14. Zhou J, Zhou J, Wu ZQ, Goyal H, Xu HG. A Novel Prognostic Model for Adult Patients With Hemophagocytic Lymphohistiocytosis. Orphanet J Rare Dis (2020) 15:215. doi: 10.1186/s13023-020-01496-4
15. Yoh KA, Lee HS, Park LC, Lee EM, Shin SH, Park DJ, et al. The Prognostic Significance of Elevated Levels of Serum Ferritin Before Chemotherapy in Patients With Non-Hodgkin Lymphoma. Clin Lymphoma Myeloma Leuk (2014) 14:43–9. doi: 10.1016/j.clml.2013.09.008
16. Kim DJ, Kim T, Jeong JY, Jo JC, Lee WS, Shin HJ, et al. Poor Prognostic Impact of High Serum Ferritin Levels in Patients With a Lower Risk of Diffuse Large B Cell Lymphoma. Int J Hematol (2020) 111:559–66. doi: 10.1007/s12185-019-02816-6
17. Wu S, Zheng J, Li Y, Yu H, Shi S, Xie W, et al. A Radiomics Nomogram for the Preoperative Prediction of Lymph Node Metastasis in Bladder Cancer. Clin Cancer Res (2017) 23:6904–11. doi: 10.1158/1078-0432.CCR-17-1510
18. Chen S, Chen X, Nie R, Ou Yang L, Liu A, Li Y, et al. A Nomogram to Predict Prognosis for Gastric Cancer With Peritoneal Dissemination. Chin J Cancer Res (2018) 30:449–59. doi: 10.21147/j.issn.1000-9604.2018.04.08
19. Han Y, Yang J, Liu P, He X, Zhang C, Zhou S, et al. Prognostic Nomogram for Overall Survival in Patients With Diffuse Large B-Cell Lymphoma. Oncologist (2019) 24:e1251–61. doi: 10.1634/theoncologist.2018-0361
20. Camp RL, Dolled-Filhart M, Rimm DL. X-Tile: A New Bio-Informatics Tool for Biomarker Assessment and Outcome-Based Cut-Point Optimization. Clin Cancer Res (2004) 10:7252–9. doi: 10.1158/1078-0432.CCR-04-0713
21. Harrell FE Jr., Califf RM, Pryor DB, Lee KL, Rosati RA. Evaluating the Yield of Medical Tests. JAMA (1982) 247:2543–6. doi: 10.1001/jama.247.18.2543
22. Huang YQ, Liang CH, He L, Tian J, Liang CS, Chen X, et al. Development and Validation of a Radiomics Nomogram for Preoperative Prediction of Lymph Node Metastasis in Colorectal Cancer. J Clin Oncol (2016) 34:2157–64. doi: 10.1200/JCO.2015.65.9128
23. Bonnett LJ, Snell KIE, Collins GS, Riley RD. Guide to Presenting Clinical Prediction Models for Use in Clinical Settings. BMJ (2019) 365:l737. doi: 10.1136/bmj.l737
24. Onodera T, Goseki N, Kosaki G. Prognostic Nutritional Index in Gastrointestinal Surgery of Malnourished Cancer Patients. Nihon Geka Gakkai Zasshi (1984) 85:1001–5.
25. Bhaskaran K, Dos-Santos-Silva I, Leon DA, Douglas IJ, Smeeth L. Association of BMI With Overall and Cause-Specific Mortality: A Population-Based Cohort Study of 3.6 Million Adults in the UK. Lancet Diabetes Endocrinol (2018) 6:944–53. doi: 10.1016/S2213-8587(18)30288-2
26. Hansen JA, Stancel HH, Klesges LM, Tyc VL, Hinds PS, Wu S, et al. Eating Behavior and BMI in Adolescent Survivors of Brain Tumor and Acute Lymphoblastic Leukemia. J Pediatr Oncol Nurs (2014) 31:41–50. doi: 10.1177/1043454213515548
27. Mantzorou M, Koutelidakis A, Theocharis S, Giaginis C. Clinical Value of Nutritional Status in Cancer: What Is Its Impact and How It Affects Disease Progression and Prognosis? Nutr Cancer (2017) 69:1151–76. doi: 10.1080/01635581.2017.1367947
28. Pena-Romero AC, Navas-Carrillo D, Marin F, Orenes-Pinero E. The Future of Nutrition: Nutrigenomics and Nutrigenetics in Obesity and Cardiovascular Diseases. Crit Rev Food Sci Nutr (2018) 58:3030–41. doi: 10.1080/10408398.2017.1349731
29. Liu X, Wu Z, Lin E, Li W, Chen Y, Sun X, et al. Systemic Prognostic Score and Nomogram Based on Inflammatory, Nutritional and Tumor Markers Predict Cancer-Specific Survival in Stage II-III Gastric Cancer Patients With Adjuvant Chemotherapy. Clin Nutr (2019) 38:1853–60. doi: 10.1016/j.clnu.2018.07.015
30. Meier JA, Bokemeyer A, Cordes F, Fuhrmann V, Schmidt H, Husing-Kabar A, et al. Serum Levels of Ferritin and Transferrin Serve as Prognostic Factors for Mortality and Survival in Patients With End-Stage Liver Disease: A Propensity Score-Matched Cohort Study. United Eur Gastroenterol J (2020) 8:332–9. doi: 10.1177/2050640619891283
31. Ju SY, Ha AW. Dietary Factors Associated With High Serum Ferritin Levels in Postmenopausal Women With the Fifth Korea National Health and Nutrition Examination Survey (KNHANES V), 2010-2012. Nutr Res Pract (2016) 10:81–8. doi: 10.4162/nrp.2016.10.1.81
32. Alkhateeb AA, Connor JR. The Significance of Ferritin in Cancer: Anti-Oxidation, Inflammation and Tumorigenesis. Biochim Biophys Acta (2013) 1836:245–54. doi: 10.1016/j.bbcan.2013.07.002
33. Sang W, Shi M, Yang J, Cao J, Xu L, Yan D, et al. Phase II Trial of Co-Administration of CD19- and CD20-Targeted Chimeric Antigen Receptor T Cells for Relapsed and Refractory Diffuse Large B Cell Lymphoma. Cancer Med (2020) 9:5827–38. doi: 10.1002/cam4.3259.10.1002/cam4.3259
34. Staiger AM, Ziepert M, Horn H, Scott DW, Barth TFE, Bernd HW, et al. Clinical Impact of the Cell-Of-Origin Classification and the MYC/BCL2 Dual Expresser Status in Diffuse Large B-Cell Lymphoma Treated Within Prospective Clinical Trials of the German High-Grade Non-Hodgkin’s Lymphoma Study Group. J Clin Oncol (2017) 35:2515–26. doi: 10.1200/JCO.2016.70.3660
Keywords: ferritin, DLBCL, International Prognostic Index, prognosis, nomogram
Citation: Shen Z, Zhang S, Zhang M, Hu L, Sun Q, He C, Yan D, Ye J, Zhang H, Wang L, Gu W, Miao Y, Liu Q, Ouyang C, Zhu J, Wang C, Zhu T, Huang S and Sang W (2022) The Addition of Ferritin Enhanced the Prognostic Value of International Prognostic Index in Diffuse Large B‐Cell Lymphoma. Front. Oncol. 11:823079. doi: 10.3389/fonc.2021.823079
Received: 26 November 2021; Accepted: 27 December 2021;
Published: 19 January 2022.
Edited by:
Weili Zhao, Shanghai Jiao Tong University, ChinaReviewed by:
Caixia Li, The First Affiliated Hospital of Soochow University, ChinaHuilai Zhang, Tianjin Medical University, China
Copyright © 2022 Shen, Zhang, Zhang, Hu, Sun, He, Yan, Ye, Zhang, Wang, Gu, Miao, Liu, Ouyang, Zhu, Wang, Zhu, Huang and Sang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Sang, eHlmeWxibDUxNUB4emhtdS5lZHUuY24=
†These authors have contributed equally to this work and share first authorship
 Ziyuan Shen
Ziyuan Shen Shuo Zhang2†
Shuo Zhang2† Jingjing Ye
Jingjing Ye Hao Zhang
Hao Zhang Weiying Gu
Weiying Gu Shuiping Huang
Shuiping Huang Wei Sang
Wei Sang