- 1Division of Colorectal Surgery, Department of Surgery, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 2Division of General and Digestive Surgery, Department of Surgery, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 3Division of Colorectal Surgery, Department of Surgery, Chang Gung Memorial Hospital–Kaohsiung, Kaohsiung, Taiwan
- 4Division of Nutrition and Dietetics, Kaohsiung Medical University Hospital, Kaohsiung Medical University, Kaohsiung, Taiwan
- 5Division of Colon and Rectal Surgery, Department of Surgery, Chang Gung Memorial Hospital–Linkou, Taoyuan, Taiwan
- 6Division of Colon & Rectal Surgery, Department of Surgery, Taipei Veterans General Hospital, Taipei, Taiwan
- 7Division of Nutrition, Chang Gung Memorial Hospital–Linkou, Taoyuan, Taiwan
- 8Division of Nutrition, Taichung Veterans General Hospital, Taichung, Taiwan
- 9Division of Colorectal Surgery, Department of Surgery, Taichung Veterans General Hospital, Taichung, Taiwan
- 10Department of Surgery, Faculty of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
- 11Graduate Institute of Clinical Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
- 12Graduate Institute of Medicine, College of Medicine, Kaohsiung Medical University, Kaohsiung, Taiwan
- 13Center for Cancer Research, Kaohsiung Medical University, Kaohsiung, Taiwan
- 14Center for Liquid Biopsy and Cohort Research, Kaohsiung Medical University, Kaohsiung, Taiwan
- 15Clinical Pharmacogenomics and Pharmacoproteinomics, College of Pharmacy, Taipei Medical University, Taipei, Taiwan
- 16Pingtung Hospital, Ministry of Health and Welfare, Pingtung, Taiwan
Malnutrition and systemic inflammatory response (SIR) frequently occur in patients with colorectal cancer (CRC) and are associated with poor prognosis. Anti-inflammatory nutritional intervention is not only a way to restore the malnourished status but also modulate SIR. Nine experts, including colorectal surgeons, physicians and dieticians from 5 hospitals geographically distributed in Taiwan, attended the consensus meeting in Taiwan Society of Colon and Rectum Surgeons for a 3-round discussion and achieved the consensus based on a systematic literature review of clinical studies and published guidelines. The consensus recommends that assessment of nutritional risk and SIR should be performed before and after CRC treatment and appropriate nutritional and/or anti-inflammatory intervention should be adapted and provided accordingly.
Introduction
Malnutrition is frequently encountered in patients with cancer and varied from 34% to 71% (1–5). Cancer is a condition that increases metabolism, which raises the body’s needs for energy and protein. Moreover, symptoms such as anorexia, nausea, vomiting, early satiety, pain, bleeding, and bowel obstruction that result from cancer itself reduce food intake, and this phenomenon is especially observed in gastrointestinal cancer. Toxicities of chemotherapy and radiotherapy (e.g., mucositis, fatigue, constipation and diarrhea) as well as surgical outcomes further impair oral feeding. In addition, mental reactions to disease and treatments, which include depression, psychological stress and decreased physical activities also influence the appetite. A long-term lack of enteral stimulation by dietary intake results in the reduction of intestinal mucosal cell proliferation. Therefore, intestinal mucosal atrophy and collapse of intestinal mucosal barrier occur and cause malabsorption (6), which generates a vicious circle along with malnutrition.
During cancer progression, proinflammatory cytokines produced by cancer cells and host immune response to both tumors and cancer treatments result in systemic inflammatory response (SIR) (7, 8), which provokes metabolism. SIR affects the brain, muscle, liver, and fat function (9), leading to anorexia (10), muscle wasting (11), suppressed anticancer drug clearance (12), and depletion of fat (13), respectively. In turn, an imbalance between anabolism and catabolism worsens the nutritional status in cancer patients and results in a depletion of the body’s energy reserves. In the context of skeletal mass decline (i.e. sarcopenia), malnutrition has been associated with an increased risk of both surgery complications and chemotherapy adverse events (14–16). Consequently, dosage of anticancer drugs may be reduced, delayed or interrupted compared to the treatment recommendation, and all of these are associated with poor survival (17–21). Moreover, sarcopenia further limits physical activity, which has adverse impacts on quality of life (QoL). Studies have demonstrated SIR, which is indicated by a high neutrophil-lymphocyte ratio (NLR) (22–26), an elevated C-reactive protein (CRP) (22, 23, 27–33) and Glasgow Prognostic Score (GPS) (i.e. the combination of elevated CRP and hypoalbuminemia) (34–40), is associated with a poor prognosis in cancer patients.
Methods
The literature in PubMed was searched for eligible studies published using the keywords “colorectal neoplasms” or “colorectal cancer,” “neoplasms” or “cancer,” “nutrition,” “malnutrition,” and “inflammation.” Clinical trials, non-controlled trials, and descriptive studies such as cohort studies, case series, and case reports published between January 1990 and December 2020 were retained. Published guidelines and consensus documents were also retrieved. The highest level of evidence available according to the systemic review of literature was assigned to each statement to categorize the quality of each statement to facilitate decision-making for panel members. Levels of evidence ranged from level 1 (randomized controlled trials) to level 5 (expert opinion) based on Oxford Centre for Evidence-Based Medicine.
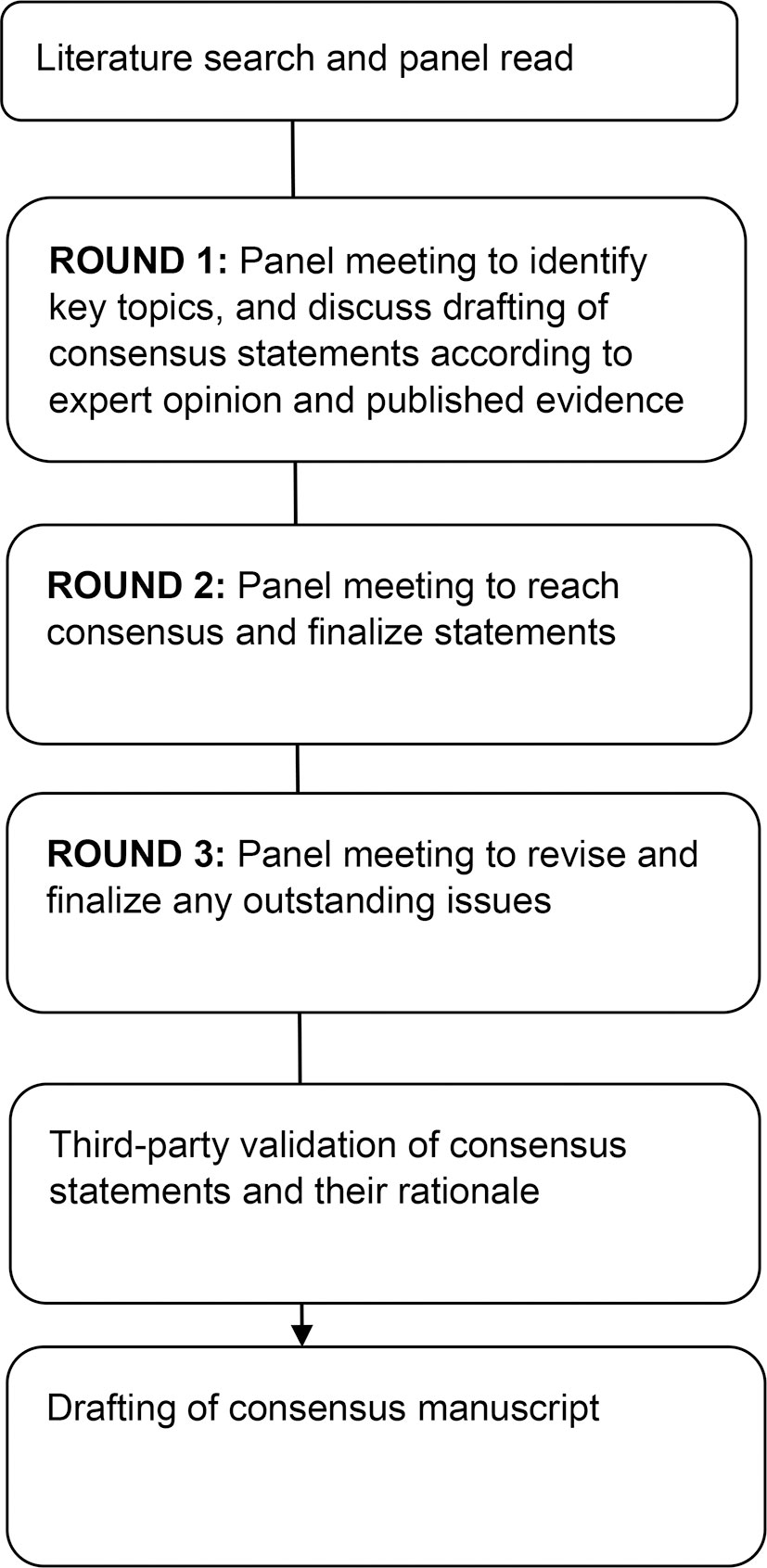
A modified Delphi process was adopted. The Taiwan Society of Colon and Rectum Surgeons (TSCRS) invited a group of 9 experts (colorectal surgeons, oncologists, and dieticians) from five hospitals geographically spread over Taiwan to review and discuss the literature face-to-face over three rounds (Figure 1). The panel members drafted consensus statements and the panel voted by showing hands for each statement and the consensus was formed if more than two-thirds of the panel members agreed.
Round 1
The panel first identified 3 key topics of inflammation in colorectal cancer (CRC) and further refined their search for, review of, and discussion of the evidence to address clinical questions focusing on (1): markers of inflammatory status in CRC (2); the prognostic value of inflammatory markers for CRC and NLR, CRP, and GPS were accepted; and (3) anti-inflammatory nutritional interventions in CRC and long-chain omega-3 polyunsaturated fatty acids (n-3 PUFAs) and glutamine were accepted.
Round 2
In round 2, discussion for specific inflammatory marker and anti-inflammatory nutritional intervention was launched. As the result, NLR and n-3 PUFAs were accepted into the final guideline document.
Round 3
Panel members were encouraged to discuss the statements in the final guideline document to determine the cut-off value of NLR and to revise and finalize any outstanding issues. In the end, the threshold for NLR ≥3 was accepted and post-treatment anti-inflammatory nutritional intervention was added.
The questions, statements, and their rationale were presented to a third-party panel composed of 2 colorectal surgeons, 2 oncologists and 2 dieticians for validation. Each question was discussed by the third party to eliminate bias and again, statements has to be voted and agreed with if more than two-thirds of the members of the third-party panel. The final statements were based on the level of evidence and the level of consensus. This document is an independent report of the panel and is not a policy statement of the TSCRS.
Prognostic Value of NLR in Patients with Cancer
NLR is one of the most evaluated inflammatory markers that are used as a predictor for outcomes of treatments in various cancer types. According to a review article published in 2018, which reviewed 36 trials containing data on 40,354 patients, CRC was the most common type with 10 trials containing data on 27,438 patients until January 2018, and NLR/derived NLR was assessed in 33 trials with data on 39,313 patients (41). NLR is calculated by dividing the neutrophil count by the lymphocyte count and can be used as an economic and simple inflammatory marker to measure SIR, since blood count is one of the initial routine examinations for patients in clinical practice (42). Neutrophils and lymphocytes are one of the first inflammatory cells that respond to SIR. Neutrophils, together with cancer cells, secret proinflammatory cytokines, chemokines and growth factors, where neutrophils are a source and a target at the same time. The secreted factors include interleukin (IL)-1, IL-6, IL-8, tumor necrosis factor-alpha (TNF-α), hepatocyte growth factor (HGF) and vascular endothelial growth factor (VEGF), which in turn promote not only tumor growth but also cancer metastasis in the tumor microenvironment (43). Moreover, neutrophils can inhibit the lymphocytes’ cytotoxic activities against cancer cells, resulting in immune escape of cancer cells (43). Therefore, high NLR is associated with poor oncological outcomes.
Prognostic Value of Pre-Treatment NLR in CRC
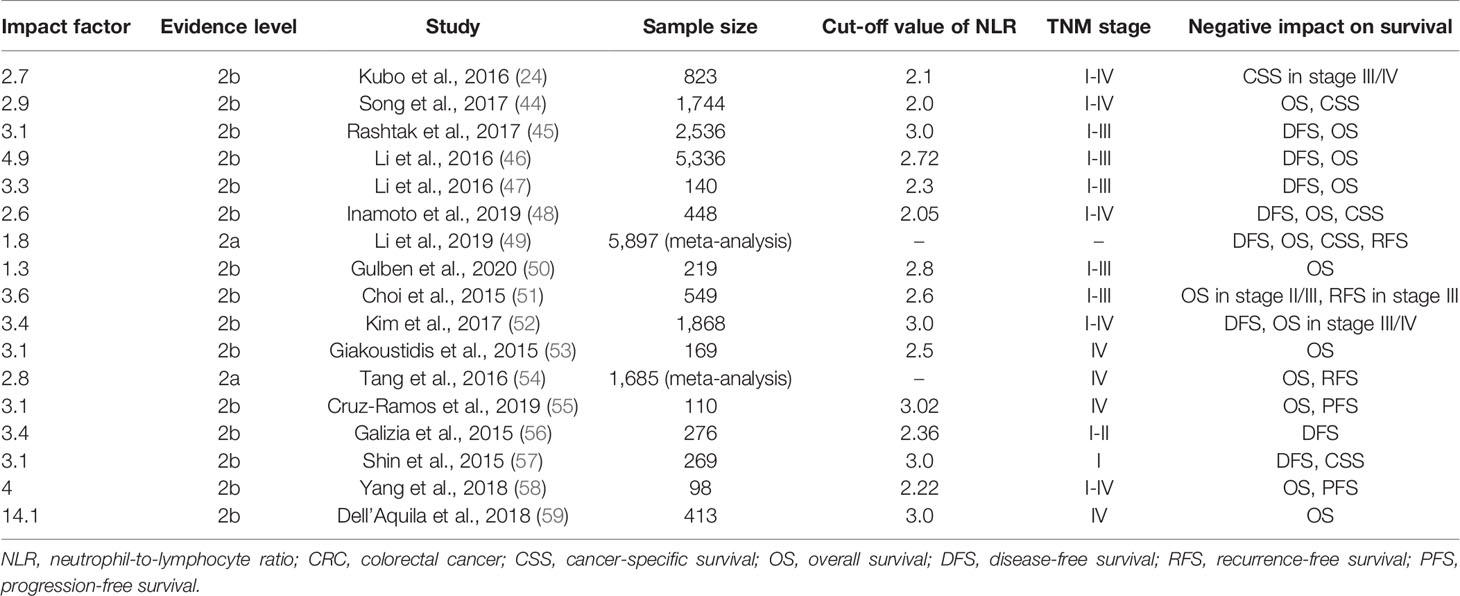
Studies of prognostic value of pre-treatment NLR in CRC are summarized in Table 1. It is revealed that not only higher NLR in CRC, adenomatous polyp and healthy individuals respectively (60–62), but also an elevated NLR positively correlates with larger tumor size, histological grade, and more advanced T, N, and TNM stage (24, 44, 45). As such, pre-operative high NLR was found significantly associated with shorter overall survival (OS), disease-free survival (DFS), and cancer-specific survival (CSS) (44–50), and this was more prominent in patients with diseases at stage III and IV (24, 51–55). In contrast, the association was controversial in early stage CRC (stage I and II). Some studies showed no significant association between NLR and stage I/II patients (24, 52), but other studies demonstrated that high pre-operative NLR significantly correlated with poor OS in stage II cancer (51), DFS in stage I/II cancer (56), or DFS and CSS in stage I cancer (57). For stage I/II diseases, the combination of NLR and other inflammatory markers may provide a more significant prognostic value. Inamoto et al. revealed that the combination of NLR and GPS stratified OS, CSS and DFS in stage I/II CRC, though this was not as pronounced as in stage III/IV CRC (48). In addition, high pre-treatment NLR predicted not only prognosis of operation, but also predicted decreased progression-free survival (PFS) and OS in CRC patients receiving neoadjuvant chemoradiotherapy (58), and OS in unresectable metastatic CRC (mCRC) patients receiving chemotherapy and biological agent (59).
Prognostic Value of Post-Treatment NLR in CRC
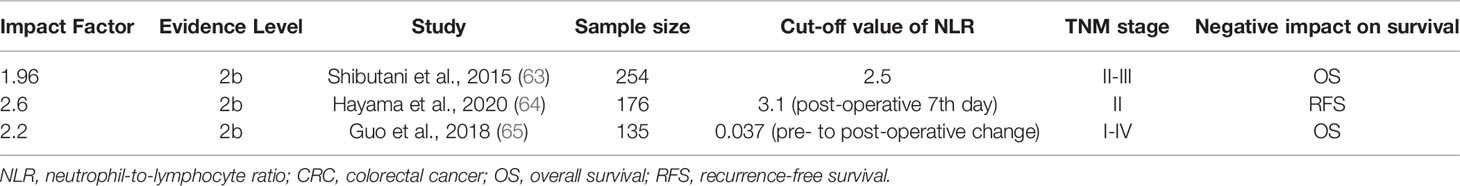
Compared to pre-treatment NLR, post-treatment NLR is much less investigated; however, the prognostic value of post-treatment NLR may be as important as that of pre-treatment NLR. Shibutani et al. demonstrated that increased post-operative NLR was an independent predictor for a poor OS in 254 patients with stage II/III CRC who underwent curative resection and oral fluorouracil monotherapy. Furthermore, patients with high post-operative NLR had a significantly shorter OS than those with low post-operative NLR in the low pre-operative NLR subgroup (63). Similarly, Hayama et al. reported that increased post-operative day 7 NLR was an independent prognostic factor of decreased recurrence-free survival (RFS) for stage II CRC in 176 individuals receiving curative surgery, and the study concluded that such patients may be candidates for adjuvant chemotherapy (64). Finally, Guo et al. showed that elevated pre-operative along with larger pre-operative to post-operative change in NLR had a significant correlation with poor OS but not DFS in 135 patients with stage I-IV CRC without significant correlation with DFS (65) (Table 2).
Anti-Inflammatory Nutritional Intervention
As mentioned above, cancer patients are at risk of SIR and malnutrition; hence, it is recommended to evaluate systemic inflammatory and nutritional status before cancer treatments. Oral nutritional supplements (ONS) or parenteral nutrition (PN) for those with inadequate enteral nutrition should be administered to meet the goal of 25–30 kcal/kg/day with 1.2–1.5 g protein/kg/day in patients at risk of malnutrition. Anti-inflammatory ingredients, such as n-3 PUFAs contained in fish oil, serve as a means of immune modulation for patients with cancer, who are at risk of SIR (9). Although other nutrients, including glutamine, arginine, curcumin, vitamin D3, vitamin B12, probiotics, selenium, zinc, and so on, also have anti-inflammatory properties, some of them have no effect on body weight and in order not to complicate the issue discussed, this review focused on n-3 PUFAs.
Apart from providing energy, n-3 PUFAs have an additional function in modulating lipid metabolism and SIR. N-3 PUFAs can be incorporated into phospholipids at cell membrane that may influence synthesis of secondary messengers and modulate the expression of certain adhesion molecules at the surface of lymphocytes, monocytes and endothelial cells (66, 67). Previously, n-3 PUFAs have been demonstrated to modify fluidity and permeability of cell membrane as well as to regulate cell membrane receptors and enzyme activities (68, 69). Additionally, n-3 PUFAs compete with n-6 PUFAs in the cyclooxygenase-2 (COX-2) pathway to produce anti-inflammatory leukotrienes, prostaglandins, and thromboxanes (70, 71), and thereby regulate the expression of pro- and anti-inflammatory cytokine genes (72). Thus, it appears to be a promising strategy in the context of conditions with inappropriate pro-inflammatory activity, such as major surgery (73–76), critical illness (77–79), and cancer (9).
The effects of n-3 PUFAs are dose and time dependent, and no correlation with clinical outcomes can be observed in a short period of perioperative use (80–82). Ranging from 8 weeks to 24 months according to individual studies, taking oral nutritional supplement (ONS) containing fish oil or n-3 PUFAs has been shown to lower inflammatory biomarkers and improve SIR in CRC patients (83–85). Moreover, taking ONS containing fish oil EPA or n-3 PUFAs was also associated with improved QoL through reducing chemotherapy related adverse events, such as fatigue, diarrhea, loss of appetite and neuropathy (84, 86), and therefore physical function can be improved (87, 88). Song et al. demonstrated that a higher intake of n-3 PUFAs (0.35 g/day) was associated with a lower risk of CRC with high FOXP3+ tumor infiltrating T cells, which regulate the development and function of regulatory T cells to shut down the immune response to cancer cells (89). Kansal et al. revealed fish oil suppressed cell growth and metastatic potential by regulating PTEN and NF-κB signaling in colorectal cancer (90). As a result, n-3 PUFAs are supposed to have a positive impact on cancer prognosis; however, the related clinical evidence is inconsistent according to the methodology, including duration and amount of intake of n-3 PUFAs, patients’ cancer stage, and nutritional and systemic inflammatory status as well. One cohort study enrolled 1659 patients with stage I-IV CRC showed that a higher n-3 PUFAs consumption by increased ≧ 0.15 g/day after diagnosis was associated with a better CSS compared with intake by increased < 0.02 g/day or no change. No association was found with OS (91). One study showed no differences in recurrence or survival in stage II CRC across the 24-month follow-up (87), and another randomized clinical trial showed that taking 2 g/day of fish oil- EPA containing 0.6 g/day of eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) during or after chemotherapy was associated with a longer PFS despite a lack of statistical significance in stage II-IV CRC (92). Notably, Shirai et al. demonstrated fish oil-enriched ONS containing 1.1 g of EPA and 0.5 g of DHA per day for 6 months was not only significantly associated with increased skeletal muscle and lean body mass and improved tolerance to chemotherapy, but also with a longer OS in gastrointestinal cancer patients with a modified GPS score of 1 or 2 (93). To take inflammatory and nutritional status into consideration, n-3 PUFAs may benefit selected cancer patients. Nevertheless, it is difficult to address the exact adequate amount of n-3 PUFAs supplement in cancer patients. According to Academy of Nutrition and Dietetics, which summarized nine studies (four randomized controlled trials, two non- randomized controlled trials, prospective cohort studies, and one time-series study), consumption 1.2 g to 2.2 g of EPA per day resulted in weight gain or weight stabilization in adult oncology patients with weight loss (94–102). In sum, it is reasonable to take at least 2.2 g of EPA per day for both inflammatory and nutritional purposes. More studies are needed to confirm the oncological outcomes of anti-inflammatory nutritional intervention of n-3 PUFAs.
Consensus Recommendations
Cut-Off Value of NLR
Studies had provided evidence of the prognostic and predictive value of elevated NLR in pre-treatment or post-treatment of mCRC. It is difficult to define a definite cut-off value of NLR prospectively, because all cut-off values reported were calculated retrospectively. Despite the threshold for NLR varied based on the statistical significance of cut-off value in each trial, the most common threshold for NLR was ≥3 (41), which is also the recommended value in this consensus [Grading of Recommendations, Assessment, Development, and Evaluation (GRADE): moderate].
Anti-Inflammatory Nutritional Intervention in Patients With CRC Before Treatment
Malnutrition has a negative impact on clinical outcomes in patients with cancer and was associated with more post-operative infections (103–106) and complications (107, 108), reduced QoL (109), increased toxicities of chemotherapy leading to reduced dose or delayed treatment (5, 110), a shorter survival (103, 105–107, 111, 112), as well as a longer length of hospital stay (2, 103, 107, 108, 112), and therefore increased health care costs (2, 113). As a result, malnutrition should be corrected before treatment to prevent the aforementioned negative effects. Additionally, SIR, indicated by NLR elevation in this consensus, also correlated with poor oncological outcomes, especially in patients with advanced colorectal cancer (24, 51–55). In order to employ an appropriate nutritional intervention for patients with CRC, assessment for SIR with measurement of NLR (GRADE: moderate) and evaluation for nutritional risk with body weight, body mass index, and additional screening tool(s), which may depend on what is adopted by the individual institute in TSCRS (GRADE: high), are recommended to be made before treatment for all patients. Treatment includes surgery, chemotherapy, targeted therapy, radiotherapy, immunotherapy, or any kind of therapy against CRC. For patients with a high risk of malnutrition, ONS or parenteral nutrition (PN) for those whose enteral intake is inadequate should be administered with a target range of 25–30 kcal/kg/day and 1.2–1.5 g protein/kg/day to maintain or restore lean body mass (GRADE: high). ONS containing n-3 PUFAs (e.g. EPA) or lipid emulsion in PN should be added for patients with NLR ≥3 (GRADE: very low) (Figure 2). Obesity is a potential source of inflammation, which also correlates with NLR (114–117), and obese patients with CRC may at risk of malnutrition, therefore, the recommendations are applied to this population as well.
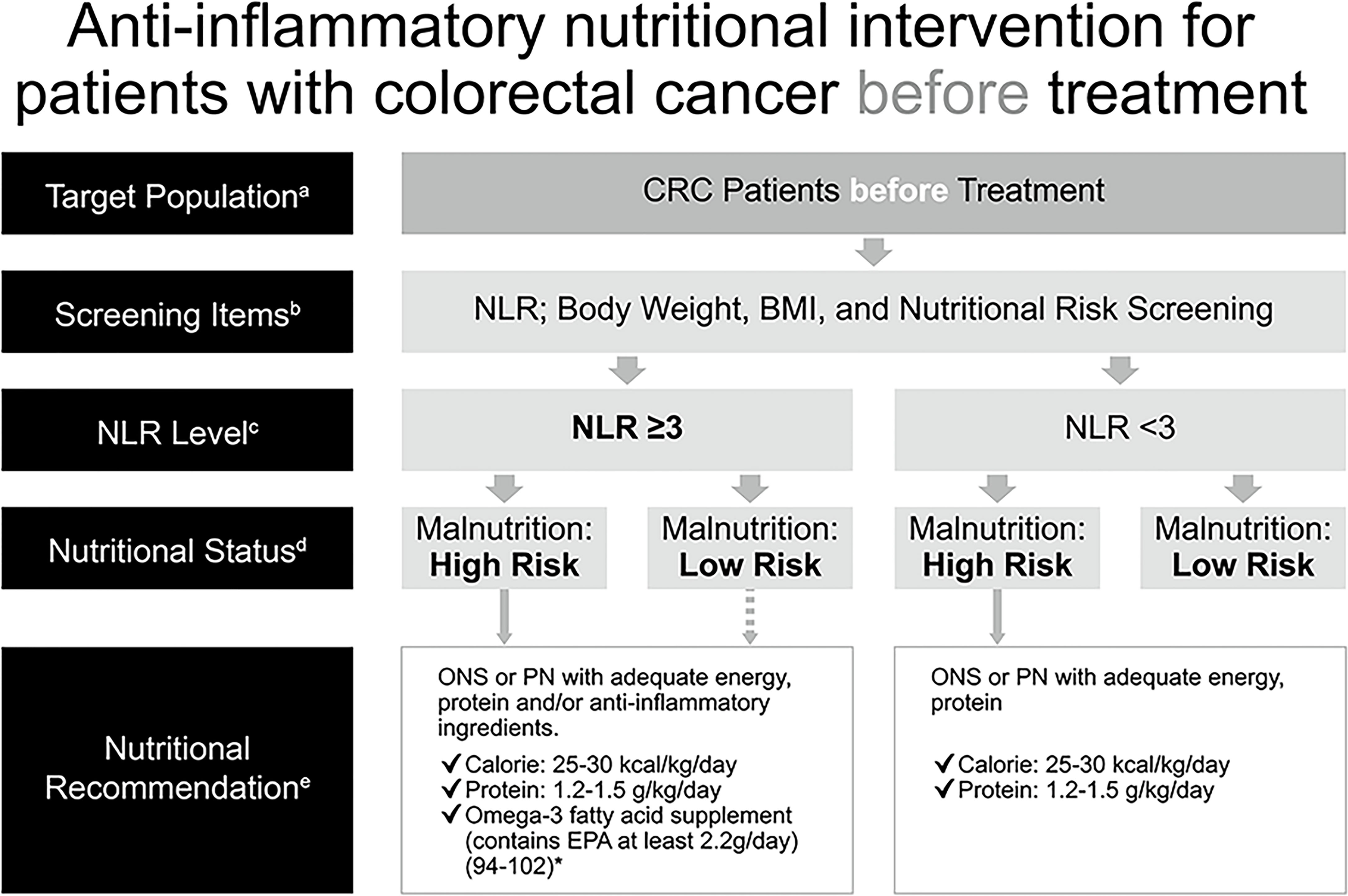
Figure 2 Anti-inflammatory nutritional intervention for patients with colorectal cancer before treatment. *(References 94–102) aCRC patients before treatment (cancer-related drug treatment, radiotherapy and surgical treatment). bCommonly used clinical nutrition screening/evaluation tools in Taiwan, which include PG-SGA, NRS 2002, MUST, MST. cThe NLR test is used to assess the risk of inflammation. dInterpretation based on the evaluation/screening tools used in the nutrition evaluation clinical practice routines of each hospital. eBased on the recommendations of ESPEN 2017, ASPEN, and the Academy of Nutrition and Dietetics, for cancer, diseases, and treatments that may cause inflammation, insufficient calorie intake, fatigue and low physical activity, omega-3 fatty acids (such as fish oil, EPA) should be consumed for their anti-inflammatory effects, in addition to ONS which provides additional calories and protein. Nutritional intervention can be performed if the patient has a high risk of malnutrition. There is no need to distinguish between precachexia and cachexia–directly start supplementing calories, protein, and omega-3 fatty acids for nutritional intervention, according to clinical guidelines, without further screening. The dotted line: If the patient is not at high risk of malnutrition but has an NLR ≥3, the follow-up decision depends on the physician, and the physician (or a supervisor) will then call the nutritionist. ASPEN, American Society for Parenteral and Enteral Nutrition; BMI, body mass index; CRC, colorectal cancer; EPA, eicosapentaenoic acid; ESPEN, European Society for Parenteral and Enteral Nutrition; MST, Malnutrition Screening Tool; MUST, Malnutrition Universal Screening Tool; NG, nasogastric; NLR, neutrophil-to-lymphocyte ratio; NRS, Nutritional Risk Screening; ONS, oral nutritional supplement; PG-SGA, Patient-Generated Subjective Global Assessment; PN, parenteral nutrition.
Anti-Inflammatory Nutritional Intervention in Patients With Colorectal Cancer After Treatment
Cancer patients may still be at risk of malnutrition after treatment at discharge because nutritional status is dynamic and may decline after treatment owing to additional SIR and stress metabolism induced by treatments for cancer. It was reported that 36.4% of cancer patients were at nutritional risk at discharge and only one-third of patients at risk of malnutrition had received any kind of nutritional intervention at discharge (2). Moreover, high post-treatment NLR was negatively associated with oncological survival (63–65). Of note, the immunomodulatory effects of n-3 PUFAs that reduce SIR are dose- and time-dependent and take at least 8 weeks to occur (83–85). Consequently, analyzing nutritional risk and SIR after treatment allows the implementation of appropriate nutrition therapy. Based on availability and convenience of clinical practice, the current consensus recommends checking NLR (GRADE: low) and nutritional risk with body weight, body mass index, and other appropriate screening tools at the first outpatient visit (GRADE: moderate). Likewise, ONS or PN of isocaloric 25–30 kcal/kg/day and isonitrogenous 1.2–1.5 g protein/kg/day is recommended to be administered as nutritional support (GRADE: high), and additional n-3 PUFAs should be employed for patients with NLR ≥3 (GRADE: very low) (Figure 3).
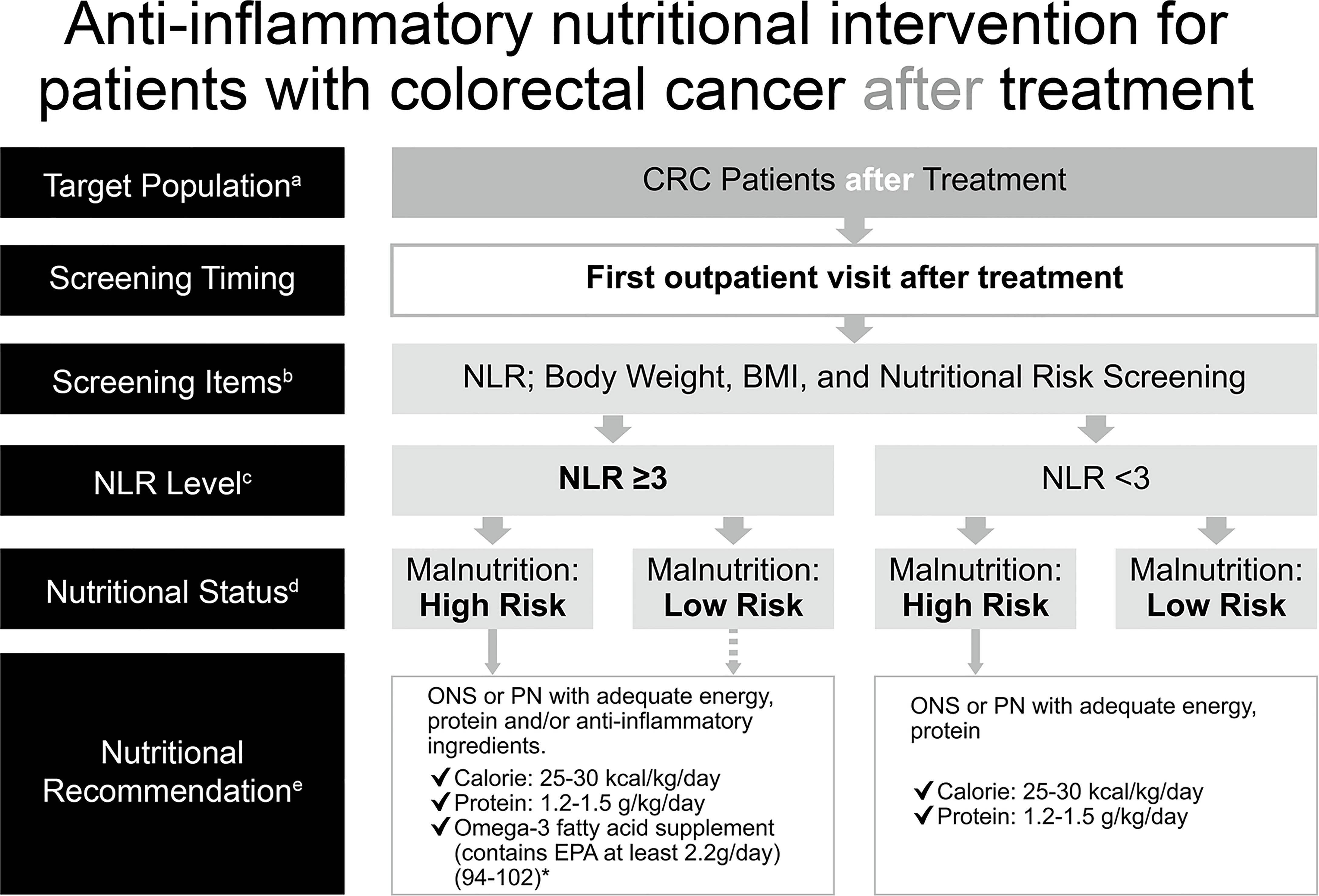
Figure 3 Anti-inflammatory nutritional intervention for patients with colorectal cancer after treatment. *(References 94–102). aCRC patients post-treatment (surgery, chemotherapy, including adjuvant chemotherapy or palliative chemotherapy). bCommonly used clinical nutrition screening/evaluation tools in Taiwan, which include PG-SGA, NRS 2002, MUST, MST. cFor patients with CRC after undergoing surgical treatment, the time point for postoperative NLR assessment is based on the clinical practice routines of blood sampling and tracking in various hospitals and on computer system settings. It is not strictly limited to 7 days; the first outpatient clinic after surgery is recommended. dInterpretation based on the evaluation/screening tools used in the nutrition evaluation clinical practice routines of each hospital. eBased on the recommendations of ESPEN 2017, ASPEN, and the Academy of Nutrition and Dietetics, for cancer, diseases, and treatments that may cause inflammation, insufficient calorie intake, fatigue and low physical activity, omega-3 fatty acids (such as fish oil, EPA) should be consumed for their anti-inflammatory effects, in addition to ONS which provides additional calories and protein. Nutritional intervention can be performed if the patient has a high risk of malnutrition. There is no need to distinguish between precachexia and cachexia–directly start supplementing calories, protein, and omega-3 fatty acids for nutritional intervention, according to clinical guidelines, without further screening. The dotted line: If the patient is not at high risk of malnutrition but has an NLR ≥3, the follow-up decision depends on the physician, and the physician (or a supervisor) will then call the nutritionist. ASPEN, American Society for Parenteral and Enteral Nutrition; BMI, body mass index; CRC, colorectal cancer; EPA, eicosapentaenoic acid; ESPEN, European Society for Parenteral and Enteral Nutrition; MST, Malnutrition Screening Tool; MUST, Malnutrition Universal Screening Tool; NLR, neutrophil-to-lymphocyte ratio; NRS, Nutritional Risk Screening; ONS, oral nutritional supplement; PG-SGA, Patient-Generated Subjective Global Assessment; PN, parenteral nutrition.
Conclusions
NLR is an economic and easily applicable inflammatory marker that predicts outcomes of cancers. Assessments for SIR and nutritional risk should be carried out before and after treatment. Accordingly, anti-inflammatory nutritional intervention not only provides energy but also modulates inflammatory response for patients at risk of malnutrition and SIR to facilitate optimal outcomes.
Limitations and Strengths of This Consensus
The current consensus is confined to the available resources, the available therapeutic options in the individual institute in TSCRS, and the associated rules and laws of Taiwan National Health Insurance Administration, Ministry of Health and Welfare specifying the current Taiwanese clinical practices. It is not a dogmatic management guideline but should be adapted according to local circumstances of the individual institution in TSCRS. The consensus may need updating; for example, the cut-off value of NLR may be adjusted with more real world evidence gathered and analyzed by TSCRS in the future. The strength of this consensus is that it provides a guide, rather a rule to manage CRC patients at nutritional risk with or without SIR. Lastly, the proposed recommendations are relevant to local clinical practice adopted in Taiwan.
Author Contributions
C-JM wrote the original draft. C-JM, M-CH, J-MC, P-SH, H-SW, C-LC, H-MH, C-CC, and J-YW reviewed and discussed the literature. J-YW reviewed and edited the manuscript. All authors have read and approved the submitted version of manuscript.
Funding
This work was supported by grants through funding from the Ministry of Science and Technology (MOST 109-2314-B-037-035, MOST 109-2314-B-037-040, MOST 109-2314-B-037-046-MY3, MOST110-2314-B-037-097) and the Ministry of Health and Welfare (MOHW109-TDU-B-212-134026, MOHW109-TDU-B-212-114006, MOHW110-TDU-B-212-1140026) and funded by the health and welfare surcharge of on tobacco products, and the Kaohsiung Medical University Hospital (KMUH110-0R37, KMUH110-0R38, KMUH110-0M34, KMUH110-0M35, KMUH110-0M36, KMUHSA11013, KMUH-DK(C)110010, KMUH-DK(B)110004-3) and KMU Center for Cancer Research (KMU-TC109A04-1) and KMU Center for Liquid Biopsy and Cohort Research Center Grant (KMU-TC109B05) and KMU Office for Industry-Academic Collaboration (S109036), Kaohsiung Medical University. In addition, this study was supported by the Grant of Taiwan Precision Medicine Initiative, Academia Sinica, Taiwan, R.O.C.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
This study was also partly supported by Abbott Nutrition Taiwan in data consolidation and logistic support without direct involvement in content development.
References
1. Hebuterne X, Lemarie E, Michallet M, de Montreuil CB, Schneider SM, Goldwasser F. Prevalence of Malnutrition and Current Use of Nutrition Support in Patients With Cancer. JPEN J Parenter Enteral Nutr (2014) 38:196–204. doi: 10.1177/0148607113502674
2. Planas M, Alvarez-Hernandez J, Leon-Sanz M, Celaya-Perez S, Araujo K, Garcia de Lorenzo A, et al. Prevalence of Hospital Malnutrition in Cancer Patients: A Sub-Analysis of the PREDyCES(R) Study. Supportive Care Cancer Off J Multinational Assoc Supportive Care Cancer (2016) 24:429–35. doi: 10.1007/s00520-015-2813-7
3. Attar A, Malka D, Sabate JM, Bonnetain F, Lecomte T, Aparicio T, et al. Malnutrition Is High and Underestimated During Chemotherapy in Gastrointestinal Cancer: An AGEO Prospective Cross-Sectional Multicenter Study. Nutr Cancer (2012) 64:535–42. doi: 10.1080/01635581.2012.670743
4. Silva FR, de Oliveira MG, Souza AS, Figueroa JN, Santos CS. Factors Associated With Malnutrition in Hospitalized Cancer Patients: A Croos-Sectional Study. Nutr J (2015) 14:123. doi: 10.1186/s12937-015-0113-1
5. Aaldriks AA, van der Geest LG, Giltay EJ, le Cessie S, Portielje JE, Tanis BC, et al. Frailty and Malnutrition Predictive of Mortality Risk in Older Patients With Advanced Colorectal Cancer Receiving Chemotherapy. J Geriatric Oncol (2013) 4:218–26. doi: 10.1016/j.jgo.2013.04.001
6. Altmann GG. Influence of Starvation and Refeeding on Mucosal Size and Epithelial Renewal in the Rat Small Intestine. Am J Anatomy (1972) 133:391–400. doi: 10.1002/aja.1001330403
7. Roxburgh CS, McMillan DC. Cancer and Systemic Inflammation: Treat the Tumour and Treat the Host. Br J Cancer (2014) 110:1409–12. doi: 10.1038/bjc.2014.90
8. Dantzer R. Cytokine-Induced Sickness Behaviour: A Neuroimmune Response to Activation of Innate Immunity. Eur J Pharmacol (2004) 500:399–411. doi: 10.1016/j.ejphar.2004.07.040
9. Arends J, Baracos V, Bertz H, Bozzetti F, Calder PC, Deutz NEP, et al. ESPEN Expert Group Recommendations for Action Against Cancer-Related Malnutrition. Clin Nutr (2017) 36:1187–96. doi: 10.1016/j.clnu.2017.06.017
10. Fearon K, Arends J, Baracos V. Understanding the Mechanisms and Treatment Options in Cancer Cachexia. Nat Rev Clin Oncol (2013) 10:90–9. doi: 10.1038/nrclinonc.2012.209
11. Argiles JM, Busquets S, Stemmler B, Lopez-Soriano FJ. Cancer Cachexia: Understanding the Molecular Basis. Nat Rev Cancer (2014) 14:754–62. doi: 10.1038/nrc3829
12. Tsoli M, Robertson G. Cancer Cachexia: Malignant Inflammation, Tumorkines, and Metabolic Mayhem. Trends Endocrinol Metab (2013) 24:174–83. doi: 10.1016/j.tem.2012.10.006
13. Bing C. Lipid Mobilization in Cachexia: Mechanisms and Mediators. Curr Opin Support Palliat Care (2011) 5:356–60. doi: 10.1097/SPC.0b013e32834bde0e
14. Prado CM, Baracos VE, McCargar LJ, Mourtzakis M, Mulder KE, Reiman T, et al. Body Composition as an Independent Determinant of 5-Fluorouracil-Based Chemotherapy Toxicity. Clin Cancer Res an Off J Am Assoc Cancer Res (2007) 13:3264–8. doi: 10.1158/1078-0432.CCR-06-3067
15. Palmela C, Velho S, Agostinho L, Branco F, Santos M, Santos MP, et al. Body Composition as a Prognostic Factor of Neoadjuvant Chemotherapy Toxicity and Outcome in Patients With Locally Advanced Gastric Cancer. J Gastric Cancer (2017) 17:74–87. doi: 10.5230/jgc.2017.17.e8
16. Shachar SS, Deal AM, Weinberg M, Williams GR, Nyrop KA, Popuri K, et al. Body Composition as a Predictor of Toxicity in Patients Receiving Anthracycline and Taxane-Based Chemotherapy for Early-Stage Breast Cancer. Clin Cancer Res an Off J Am Assoc Cancer Res (2017) 23:3537–43. doi: 10.1158/1078-0432.CCR-16-2266
17. Dolan RD, Almasaudi AS, Dieu LB, Horgan PG, McSorley ST, McMillan DC. The Relationship Between Computed Tomography-Derived Body Composition, Systemic Inflammatory Response, and Survival in Patients Undergoing Surgery for Colorectal Cancer. J Cachexia Sarcopenia Muscle (2019) 10:111–22. doi: 10.1002/jcsm.12357
18. Zhuang CL, Huang DD, Pang WY, Zhou CJ, Wang SL, Lou N, et al. Sarcopenia Is an Independent Predictor of Severe Postoperative Complications and Long-Term Survival After Radical Gastrectomy for Gastric Cancer: Analysis From a Large-Scale Cohort. Medicine (2016) 95:e3164. doi: 10.1097/MD.0000000000003164
19. da Silva JR Jr, Wiegert EVM, Oliveira L, Calixto-Lima L. Different Methods for Diagnosis of Sarcopenia and Its Association With Nutritional Status and Survival in Patients With Advanced Cancer in Palliative Care. Nutr (2019) 60:48–52. doi: 10.1016/j.nut.2018.09.003
20. Mintziras I, Miligkos M, Wachter S, Manoharan J, Maurer E, Bartsch DK. Sarcopenia and Sarcopenic Obesity Are Significantly Associated With Poorer Overall Survival in Patients With Pancreatic Cancer: Systematic Review and Meta-Analysis. Int J Surg (2018) 59:19–26. doi: 10.1016/j.ijsu.2018.09.014
21. Fattouh M, Chang GY, Ow TJ, Shifteh K, Rosenblatt G, Patel VM, et al. Association Between Pretreatment Obesity, Sarcopenia, and Survival in Patients With Head and Neck Cancer. Head Neck (2019) 41:707–14. doi: 10.1002/hed.25420
22. Malik HZ, Prasad KR, Halazun KJ, Aldoori A, Al-Mukhtar A, Gomez D, et al. Preoperative Prognostic Score for Predicting Survival After Hepatic Resection for Colorectal Liver Metastases. Ann Surg (2007) 246:806–14. doi: 10.1097/SLA.0b013e318142d964
23. Gomez D, Morris-Stiff G, Wyatt J, Toogood GJ, Lodge JP, Prasad KR. Surgical Technique and Systemic Inflammation Influences Long-Term Disease-Free Survival Following Hepatic Resection for Colorectal Metastasis. J Surg Oncol (2008) 98:371–6. doi: 10.1002/jso.21103
24. Kubo H, Murayama Y, Arita T, Kuriu Y, Nakanishi M, Otsuji E. The Prognostic Value of Preoperative Neutrophil-To-Lymphocyte Ratio in Colorectal Cancer. World J surg (2016) 40:2796–802. doi: 10.1007/s00268-016-3595-x
25. Haram A, Boland MR, Kelly ME, Bolger JC, Waldron RM, Kerin MJ. The Prognostic Value of Neutrophil-to-Lymphocyte Ratio in Colorectal Cancer: A Systematic Review. J Surg Oncol (2017) 115:470–9. doi: 10.1002/jso.24523
26. Renaud S, Seitlinger J, St-Pierre D, Garfinkle R, Al Lawati Y, Guerrera F, et al. Prognostic Value of Neutrophil to Lymphocyte Ratio in Lung Metastasectomy for Colorectal Cancer. Eur J Cardiothorac Surg (2019) 55:948–55. doi: 10.1093/ejcts/ezy388
27. Nielsen HJ, Christensen IJ, Sorensen S, Moesgaard F, Brunner N. Preoperative Plasma Plasminogen Activator Inhibitor Type-1 and Serum C-Reactive Protein Levels in Patients With Colorectal Cancer. The RANX05 Colorectal Cancer Study Group. Ann Surg Oncol (2000) 7:617–23. doi: 10.1007/BF02725342
28. Koike Y, Miki C, Okugawa Y, Yokoe T, Toiyama Y, Tanaka K, et al. Preoperative C-Reactive Protein as a Prognostic and Therapeutic Marker for Colorectal Cancer. J Surg Oncol (2008) 98:540–4. doi: 10.1002/jso.21154
29. Szturmowicz M, Rudzinski P, Kacprzak A, Langfort R, Bestry I, Broniarek-Samson B, et al. Prognostic Value of Serum C-Reactive Protein (CRP) and Cytokeratin 19 Fragments (Cyfra 21-1) But Not Carcinoembryonic Antigen (CEA) in Surgically Treated Patients With Non-Small Cell Lung Cancer. Pneumonol Alergol Pol (2014) 82:422–9. doi: 10.5603/PiAP.2014.0055
30. Artac M, Uysal M, Karaagac M, Korkmaz L, Er Z, Guler T, et al. Prognostic Impact of Neutrophil/Lymphocyte Ratio, Platelet Count, CRP, and Albumin Levels in Metastatic Colorectal Cancer Patients Treated With FOLFIRI-Bevacizumab. J Gastrointest Cancer (2017) 48:176–80. doi: 10.1007/s12029-016-9879-4
31. Miyamoto R, Oda T, Hashimoto S, Kurokawa T, Kohno K, Akashi Y, et al. Platelet X CRP Multiplier Value as an Indicator of Poor Prognosis in Patients With Resectable Pancreatic Cancer. Pancreas (2017) 46:35–41. doi: 10.1097/MPA.0000000000000697
32. Riedl JM, Barth DA, Brueckl WM, Zeitler G, Foris V, Mollnar S, et al. C-Reactive Protein (CRP) Levels in Immune Checkpoint Inhibitor Response and Progression in Advanced Non-Small Cell Lung Cancer: A Bi-Center Study. Cancers (Basel) (2020) 12:2319. doi: 10.3390/cancers12082319
33. Lu J, Xu BB, Xue Z, Xie JW, Zheng CH, Huang CM, et al. Perioperative CRP: A Novel Inflammation-Based Classification in Gastric Cancer for Recurrence and Chemotherapy Benefit. Cancer Med (2021) 10:34–44. doi: 10.1002/cam4.3514
34. Ishizuka M, Kita J, Shimoda M, Rokkaku K, Kato M, Sawada T, et al. Systemic Inflammatory Response Predicts Postoperative Outcome in Patients With Liver Metastases From Colorectal Cancer. J Surg Oncol (2009) 100:38–42. doi: 10.1002/jso.21294
35. Roxburgh CS, Wallace AM, Guthrie GK, Horgan PG, McMillan DC. Comparison of the Prognostic Value of Tumour- and Patient-Related Factors in Patients Undergoing Potentially Curative Surgery for Colon Cancer. Colorectal Dis Off J Assoc Coloproctol Great Br Irel (2010) 12:987–94. doi: 10.1111/j.1463-1318.2009.01961.x
36. McMillan DC. The Systemic Inflammation-Based Glasgow Prognostic Score: A Decade of Experience in Patients With Cancer. Cancer Treat Rev (2013) 39:534–40. doi: 10.1016/j.ctrv.2012.08.003
37. Shafique K, Proctor MJ, McMillan DC, Leung H, Smith K, Sloan B, et al. The Modified Glasgow Prognostic Score in Prostate Cancer: Results From a Retrospective Clinical Series of 744 Patients. BMC cancer (2013) 13:292. doi: 10.1186/1471-2407-13-292
38. Douglas E, McMillan DC. Towards a Simple Objective Framework for the Investigation and Treatment of Cancer Cachexia: The Glasgow Prognostic Score. Cancer Treat Rev (2014) 40:685–91. doi: 10.1016/j.ctrv.2013.11.007
39. Dolan RD, McMillan DC. The Prevalence of Cancer Associated Systemic Inflammation: Implications of Prognostic Studies Using the Glasgow Prognostic Score. Crit Rev Oncol Hematol (2020) 150:102962. doi: 10.1016/j.critrevonc.2020.102962
40. Wu D, Wang X, Shi G, Sun H, Ge G. Prognostic and Clinical Significance of Modified Glasgow Prognostic Score in Pancreatic Cancer: A Meta-Analysis of 4,629 Patients. Aging (Albany NY) (2021) 13:1410–21. doi: 10.18632/aging.202357
41. Dolan RD, Laird BJA, Horgan PG, McMillan DC. The Prognostic Value of the Systemic Inflammatory Response in Randomised Clinical Trials in Cancer: A Systematic Review. Crit Rev Oncol Hematol (2018) 132:130–7. doi: 10.1016/j.critrevonc.2018.09.016
42. Zahorec R. Ratio of Neutrophil to Lymphocyte Counts–Rapid and Simple Parameter of Systemic Inflammation and Stress in Critically Ill. Bratisl Lek Listy (2001) 102:5–14.
43. Templeton AJ, Ace O, McNamara MG, Al-Mubarak M, Vera-Badillo FE, Hermanns T, et al. Prognostic Role of Platelet to Lymphocyte Ratio in Solid Tumors: A Systematic Review and Meta-Analysis. Cancer Epidemiol Biomarkers Prev Publ Am Assoc Cancer Res cosponsored by Am Soc Prev Oncol (2014) 23:1204–12. doi: 10.1158/1055-9965.EPI-14-0146
44. Song Y, Yang Y, Gao P, Chen X, Yu D, Xu Y, et al. The Preoperative Neutrophil to Lymphocyte Ratio Is a Superior Indicator of Prognosis Compared With Other Inflammatory Biomarkers in Resectable Colorectal Cancer. BMC Cancer (2017) 17:744. doi: 10.1186/s12885-017-3752-0
45. Rashtak S, Ruan X, Druliner BR, Liu H, Therneau T, Mouchli M, et al. Peripheral Neutrophil to Lymphocyte Ratio Improves Prognostication in Colon Cancer. Clin Colorectal Cancer (2017) 16:115–23.e3. doi: 10.1016/j.clcc.2017.01.008
46. Li Y, Jia H, Yu W, Xu Y, Li X, Li Q, et al. Nomograms for Predicting Prognostic Value of Inflammatory Biomarkers in Colorectal Cancer Patients After Radical Resection. Int J Cancer (2016) 139:220–31. doi: 10.1002/ijc.30071
47. Li H, Song J, Cao M, Wang G, Li L, Zhang B, et al. Preoperative Neutrophil-to-Lymphocyte Ratio Is a More Valuable Prognostic Factor Than Platelet-to-Lymphocyte Ratio for Nonmetastatic Rectal Cancer. Int Immunopharmacol (2016) 40:327–31. doi: 10.1016/j.intimp.2016.09.014
48. Inamoto S, Kawada K, Okamura R, Hida K, Sakai Y. Prognostic Impact of the Combination of Neutrophil-to-Lymphocyte Ratio and Glasgow Prognostic Score in Colorectal Cancer: A Retrospective Cohort Study. Int J Colorectal Dis (2019) 34:1303–15. doi: 10.1007/s00384-019-03316-z
49. Li H, Zhao Y, Zheng F. Prognostic Significance of Elevated Preoperative Neutrophil-to-Lymphocyte Ratio for Patients With Colorectal Cancer Undergoing Curative Surgery: A Meta-Analysis. Med (2019) 98:e14126. doi: 10.1097/MD.0000000000014126
50. Gulben K, Berberoglu U, Ondes B, Uyar O, Guler OC, Turanli S. Preoperative Neutrophil-to-Lymphocyte Ratio as a Predictive Factor for Survival in Nonmetastatic Colorectal Cancer. J Cancer Res Ther (2020) 16:S189–S93. doi: 10.4103/jcrt.JCRT_489_18
51. Choi WJ, Cleghorn MC, Jiang H, Jackson TD, Okrainec A, Quereshy FA. Preoperative Neutrophil-To-Lymphocyte Ratio Is a Better Prognostic Serum Biomarker Than Platelet-To-Lymphocyte Ratio in Patients Undergoing Resection for Nonmetastatic Colorectal Cancer. Ann Surg Oncol (2015) 22 Suppl 3:S603–13. doi: 10.1245/s10434-015-4571-7
52. Kim JH, Lee JY, Kim HK, Lee JW, Jung SG, Jung K, et al. Prognostic Significance of the Neutrophil-to-Lymphocyte Ratio and Platelet-to-Lymphocyte Ratio in Patients With Stage III and IV Colorectal Cancer. World J Gastroenterol (2017) 23:505–15. doi: 10.3748/wjg.v23.i3.505
53. Giakoustidis A, Neofytou K, Khan AZ, Mudan S. Neutrophil to Lymphocyte Ratio Predicts Pattern of Recurrence in Patients Undergoing Liver Resection for Colorectal Liver Metastasis and Thus the Overall Survival. J Surg Oncol (2015) 111:445–50. doi: 10.1002/jso.23845
54. Tang H, Li B, Zhang A, Lu W, Xiang C, Dong J. Prognostic Significance of Neutrophil-To-Lymphocyte Ratio in Colorectal Liver Metastasis: A Systematic Review and Meta-Analysis. PloS One (2016) 11:e0159447. doi: 10.1371/journal.pone.0159447
55. Cruz-Ramos M, Del Puerto-Nevado L, Zheng B, Lopez-Bajo R, Cebrian A, Rodriguez-Remirez M, et al. Prognostic Significance of Neutrophil-to Lymphocyte Ratio and Platelet-to Lymphocyte Ratio in Older Patients With Metastatic Colorectal Cancer. J Geriatric Oncol (2019) 10:742–8. doi: 10.1016/j.jgo.2018.10.002
56. Galizia G, Lieto E, Zamboli A, De Vita F, Castellano P, Romano C, et al. Neutrophil to Lymphocyte Ratio Is a Strong Predictor of Tumor Recurrence in Early Colon Cancers: A Propensity Score-Matched Analysis. Surgery (2015) 158:112–20. doi: 10.1016/j.surg.2015.02.006
57. Shin JS, Suh KW, Oh SY. Preoperative Neutrophil to Lymphocyte Ratio Predicts Survival in Patients With T1-2N0 Colorectal Cancer. J Surg Oncol (2015) 112:654–7. doi: 10.1002/jso.24061
58. Yang J, Xu H, Guo X, Zhang J, Ye X, Yang Y, et al. Pretreatment Inflammatory Indexes as Prognostic Predictors for Survival in Colorectal Cancer Patients Receiving Neoadjuvant Chemoradiotherapy. Sci Rep (2018) 8:3044. doi: 10.1038/s41598-018-21093-7
59. Dell’Aquila E, Cremolini C, Zeppola T, Lonardi S, Bergamo F, Masi G, et al. Prognostic and Predictive Role of Neutrophil/Lymphocytes Ratio in Metastatic Colorectal Cancer: A Retrospective Analysis of the TRIBE Study by GONO. Ann Oncol Off J Eur Soc Med Oncol (2018) 29:924–30. doi: 10.1093/annonc/mdy004
60. Ucmak F, Tuncel ET. Relationship Between Lesions in Adenomatous Polyp-Dysplasia-Colorectal Cancer Sequence and Neutrophil-To-Lymphocyte Ratio. Med Sci Monit (2016) 22:4536–41. doi: 10.12659/msm.898879
61. Zhou WW, Chu YP, An GY. Significant Difference of Neutrophil-Lymphocyte Ratio Between Colorectal Cancer, Adenomatous Polyp and Healthy People. Eur Rev Med Pharmacol Sci (2017) 21:5386–91. doi: 10.26355/eurrev_201712_13924
62. Stojkovic Lalosevic M, Pavlovic Markovic A, Stankovic S, Stojkovic M, Dimitrijevic I, Radoman Vujacic I, et al. Combined Diagnostic Efficacy of Neutrophil-To-Lymphocyte Ratio (NLR), Platelet-To-Lymphocyte Ratio (PLR), and Mean Platelet Volume (MPV) as Biomarkers of Systemic Inflammation in the Diagnosis of Colorectal Cancer. Dis Markers (2019) 2019:6036979. doi: 10.1155/2019/6036979
63. Shibutani M, Maeda K, Nagahara H, Ohtani H, Iseki Y, Ikeya T, et al. The Prognostic Significance of a Postoperative Systemic Inflammatory Response in Patients With Colorectal Cancer. World J Surg Oncol (2015) 13:194. doi: 10.1186/s12957-015-0609-3
64. Hayama T, Hashiguchi Y, Okada Y, Ono K, Nemoto K, Shimada R, et al. Significance of the 7th Postoperative Day Neutrophil-to-Lymphocyte Ratio in Colorectal Cancer. Int J Colorectal Dis (2020) 35:119–24. doi: 10.1007/s00384-019-03463-3
65. Guo D, Han A, Jing W, Chen D, Jin F, Li M, et al. Preoperative to Postoperative Change in Neutrophil-to-Lymphocyte Ratio Predict Survival in Colorectal Cancer Patients. Future Oncol (2018) 14:1187–96. doi: 10.2217/fon-2017-0659
66. Wahle KW, Rotondo D. Fatty Acids and Endothelial Cell Function: Regulation of Adhesion Molecule and Redox Enzyme Expression. Curr Opin Clin Nutr Metab Care (1999) 2:109–15. doi: 10.1097/00075197-199903000-00003
67. Calder PC. N-3 Polyunsaturated Fatty Acids and Inflammation: From Molecular Biology to the Clinic. Lipids (2003) 38:343–52. doi: 10.1007/s11745-003-1068-y
68. Armstrong VT, Brzustowicz MR, Wassall SR, Jenski LJ, Stillwell W. Rapid Flip-Flop in Polyunsaturated (Docosahexaenoate) Phospholipid Membranes. Arch Biochem Biophys (2003) 414:74–82. doi: 10.1016/s0003-9861(03)00159-0
69. Senkal M, Geier B, Hannemann M, Deska T, Linseisen J, Wolfram G, et al. Supplementation of Omega-3 Fatty Acids in Parenteral Nutrition Beneficially Alters Phospholipid Fatty Acid Pattern. JPEN J Parenteral Enteral Nutr (2007) 31:12–7. doi: 10.1177/014860710703100112
70. Calder PC. Long-Chain N-3 Fatty Acids and Inflammation: Potential Application in Surgical and Trauma Patients. Braz J Med Biol Res (2003) 36:433–46. doi: 10.1590/s0100-879x2003000400004
71. Mayer K, Schaefer MB, Seeger W. Fish Oil in the Critically Ill: From Experimental to Clinical Data. Curr Opin Clin Nutr Metab Care (2006) 9:140–8. doi: 10.1097/01.mco.0000214573.75062.0a
72. Dupont IE. Peroxidation of Lipid Emulsions: Effects of Changes in Fatty Acid Pattern and Alpha-Tocopherol Content on the Sensitivity to Peroxidative Damage. Clin Nutr (1999) 18:113–6. doi: 10.1016/s0261-5614(99)80062-4
73. Berger MM, Tappy L, Revelly JP, Koletzko BV, Gepert J, Corpataux JM, et al. Fish Oil After Abdominal Aorta Aneurysm Surgery. Eur J Clin Nutr (2008) 62:1116–22. doi: 10.1038/sj.ejcn.1602817
74. Wichmann MW, Thul P, Czarnetzki HD, Morlion BJ, Kemen M, Jauch KW. Evaluation of Clinical Safety and Beneficial Effects of a Fish Oil Containing Lipid Emulsion (Lipoplus, MLF541): Data From a Prospective, Randomized, Multicenter Trial. Crit Care Med (2007) 35:700–6. doi: 10.1097/01.CCM.0000257465.60287.AC
75. Ma CJ, Sun LC, Chen FM, Lu CY, Shih YL, Tsai HL, et al. A Double-Blind Randomized Study Comparing the Efficacy and Safety of a Composite vs a Conventional Intravenous Fat Emulsion in Postsurgical Gastrointestinal Tumor Patients. Nutr Clin Pract Off Publ Am Soc Parenteral Enteral Nutr (2012) 27:410–5. doi: 10.1177/0884533611436115
76. Ma CJ, Wu JM, Tsai HL, Huang CW, Lu CY, Sun LC, et al. Prospective Double-Blind Randomized Study on the Efficacy and Safety of an N-3 Fatty Acid Enriched Intravenous Fat Emulsion in Postsurgical Gastric and Colorectal Cancer Patients. Nutr J (2015) 14:9. doi: 10.1186/1475-2891-14-9
77. Sabater J, Masclans JR, Sacanell J, Chacon P, Sabin P, Planas M. Effects on Hemodynamics and Gas Exchange of Omega-3 Fatty Acid-Enriched Lipid Emulsion in Acute Respiratory Distress Syndrome (ARDS): A Prospective, Randomized, Double-Blind, Parallel Group Study. Lipids Health Dis (2008) 7:39. doi: 10.1186/1476-511X-7-39
78. Sabater J, Masclans JR, Sacanell J, Chacon P, Sabin P, Planas M. Effects of an Omega-3 Fatty Acid-Enriched Lipid Emulsion on Eicosanoid Synthesis in Acute Respiratory Distress Syndrome (ARDS): A Prospective, Randomized, Double-Blind, Parallel Group Study. Nutr Metab (Lond) (2011) 8:22. doi: 10.1186/1743-7075-8-22
79. Hall TC, Bilku DK, Neal CP, Cooke J, Fisk HL, Calder PC, et al. The Impact of an Omega-3 Fatty Acid Rich Lipid Emulsion on Fatty Acid Profiles in Critically Ill Septic Patients. Prostaglandins Leukot Essent Fatty Acids (2016) 112:1–11. doi: 10.1016/j.plefa.2016.07.001
80. Liang B, Wang S, Ye YJ, Yang XD, Wang YL, Qu J, et al. Impact of Postoperative Omega-3 Fatty Acid-Supplemented Parenteral Nutrition on Clinical Outcomes and Immunomodulations in Colorectal Cancer Patients. World J Gastroenterol (2008) 14:2434–9. doi: 10.3748/wjg.14.2434
81. Sorensen LS, Thorlacius-Ussing O, Schmidt EB, Rasmussen HH, Lundbye-Christensen S, Calder PC, et al. Randomized Clinical Trial of Perioperative Omega-3 Fatty Acid Supplements in Elective Colorectal Cancer Surgery. Br J Surg (2014) 101:33–42. doi: 10.1002/bjs.9361
82. Sorensen LS, Rasmussen SL, Calder PC, Yilmaz MN, Schmidt EB, Thorlacius-Ussing O. Long-Term Outcomes After Perioperative Treatment With Omega-3 Fatty Acid Supplements in Colorectal Cancer. BJS Open (2020) 4:678–84. doi: 10.1002/bjs5.50295
83. Mocellin MC, Pastore e Silva Jde A, Camargo Cde Q, Fabre ME, Gevaerd S, Naliwaiko K, et al. Fish Oil Decreases C-Reactive Protein/Albumin Ratio Improving Nutritional Prognosis and Plasma Fatty Acid Profile in Colorectal Cancer Patients. Lipids (2013) 48:879–88. doi: 10.1007/s11745-013-3816-0
84. Golkhalkhali B, Rajandram R, Paliany AS, Ho GF, Wan Ishak WZ, Johari CS, et al. Strain-Specific Probiotic (Microbial Cell Preparation) and Omega-3 Fatty Acid in Modulating Quality of Life and Inflammatory Markers in Colorectal Cancer Patients: A Randomized Controlled Trial. Asia Pac J Clin Oncol (2018) 14:179–91. doi: 10.1111/ajco.12758
85. Haidari F, Abiri B, Iravani M, Ahmadi-Angali K, Vafa M. Randomized Study Design to Test Effects of Vitamin D and Omega-3 Fatty Acid Supplementation as Adjuvant Therapy in Colorectal Cancer Patients. Methods Mol Biol (2020) 2138:337–50. doi: 10.1007/978-1-0716-0471-7_24
86. Sanchez-Lara K, Turcott JG, Juarez-Hernandez E, Nunez-Valencia C, Villanueva G, Guevara P, et al. Effects of an Oral Nutritional Supplement Containing Eicosapentaenoic Acid on Nutritional and Clinical Outcomes in Patients With Advanced Non-Small Cell Lung Cancer: Randomised Trial. Clin Nutr (2014) 33:1017–23. doi: 10.1016/j.clnu.2014.03.006
87. Lewis C, Xun P, Fly AD, Luo J, He K. Fish Oil Supplementation and Quality of Life in Stage II Colorectal Cancer Patients: A 24-Month Follow-Up Study. Nutr Cancer (2015) 67:1239–46. doi: 10.1080/01635581.2015.1078900
88. van der Meij BS, Langius JA, Spreeuwenberg MD, Slootmaker SM, Paul MA, Smit EF, et al. Oral Nutritional Supplements Containing N-3 Polyunsaturated Fatty Acids Affect Quality of Life and Functional Status in Lung Cancer Patients During Multimodality Treatment: An RCT. Eur J Clin Nutr (2012) 66:399–404. doi: 10.1038/ejcn.2011.214
89. Song M, Nishihara R, Cao Y, Chun E, Qian ZR, Mima K, et al. Marine Omega-3 Polyunsaturated Fatty Acid Intake and Risk of Colorectal Cancer Characterized by Tumor-Infiltrating T Cells. JAMA Oncol (2016) 2:1197–206. doi: 10.1001/jamaoncol.2016.0605
90. Kansal S, Bhatnagar A, Agnihotri N. Fish Oil Suppresses Cell Growth and Metastatic Potential by Regulating PTEN and NF-KappaB Signaling in Colorectal Cancer. PloS One (2014) 9:e84627. doi: 10.1371/journal.pone.0084627
91. Song M, Zhang X, Meyerhardt JA, Giovannucci EL, Ogino S, Fuchs CS, et al. Marine Omega-3 Polyunsaturated Fatty Acid Intake and Survival After Colorectal Cancer Diagnosis. Gut (2017) 66:1790–6. doi: 10.1136/gutjnl-2016-311990
92. Camargo Cde Q, Mocellin MC, Pastore Silva Jde A, Fabre ME, Nunes EA, Trindade EB. Fish Oil Supplementation During Chemotherapy Increases Posterior Time to Tumor Progression in Colorectal Cancer. Nutr Cancer (2016) 68:70–6. doi: 10.1080/01635581.2016.1115097
93. Shirai Y, Okugawa Y, Hishida A, Ogawa A, Okamoto K, Shintani M, et al. Fish Oil-Enriched Nutrition Combined With Systemic Chemotherapy for Gastrointestinal Cancer Patients With Cancer Cachexia. Sci Rep (2017) 7:4826. doi: 10.1038/s41598-017-05278-0
94. Barber MD, Ross JA, Voss AC, Tisdale MJ, Fearon KC. The Effect of an Oral Nutritional Supplement Enriched With Fish Oil on Weight-Loss in Patients With Pancreatic Cancer. Br J Cancer (1999) 81:80–6. doi: 10.1038/sj.bjc.6690654
95. Barber MD, McMillan DC, Preston T, Ross JA, Fearon KC. Metabolic Response to Feeding in Weight-Losing Pancreatic Cancer Patients and Its Modulation by a Fish-Oil-Enriched Nutritional Supplement. Clin Sci (2000) 98:389–99. doi: 10.1042/CS19990273
96. Bauer J, Capra S, Battistutta D, Davidson W, Ash S, Cancer Cachexia Study G. Compliance With Nutrition Prescription Improves Outcomes in Patients With Unresectable Pancreatic Cancer. Clin Nutr (2005) 24:998–1004. doi: 10.1016/j.clnu.2005.07.002
97. de Luis DA, Izaola O, Aller R, Cuellar L, Terroba MC. A Randomized Clinical Trial With Oral Immunonutrition (Omega3-Enhanced Formula vs. Arginine-Enhanced Formula) in Ambulatory Head and Neck Cancer Patients. Ann Nutr Metab (2005) 49:95–9. doi: 10.1159/000084742
98. Guarcello M RS, Buosi R, d’Andrea F. EPA-Enriched Oral Nutritional Support in Patients With Lung Cancer: Effects on Nutritional Status and Quality of Life. Nutr Ther Metab (2007) 25:25–30.
99. Read JA, Beale PJ, Volker DH, Smith N, Childs A, Clarke SJ. Nutrition Intervention Using an Eicosapentaenoic Acid (EPA)-Containing Supplement in Patients With Advanced Colorectal Cancer. Effects on Nutritional and Inflammatory Status: A Phase II Trial. Supportive Care Cancer Off J Multinational Assoc Supportive Care Cancer (2007) 15:301–7. doi: 10.1007/s00520-006-0153-3
100. de Luis DA, Izaola O, Aller R, Cuellar L, Terroba MC, Martin T, et al. A Randomized Clinical Trial With Two Enteral Diabetes-Specific Supplements in Patients With Diabetes Mellitus Type 2: Metabolic Effects. Eur Rev Med Pharmacol Sci (2008) 12:261–6.
101. van der Meij BS, Langius JA, Smit EF, Spreeuwenberg MD, von Blomberg BM, Heijboer AC, et al. Oral Nutritional Supplements Containing (N-3) Polyunsaturated Fatty Acids Affect the Nutritional Status of Patients With Stage III Non-Small Cell Lung Cancer During Multimodality Treatment. J Nutr (2010) 140:1774–80. doi: 10.3945/jn.110.121202
102. Weed HG, Ferguson ML, Gaff RL, Hustead DS, Nelson JL, Voss AC. Lean Body Mass Gain in Patients With Head and Neck Squamous Cell Cancer Treated Perioperatively With a Protein- and Energy-Dense Nutritional Supplement Containing Eicosapentaenoic Acid. Head Neck (2011) 33:1027–33. doi: 10.1002/hed.21580
103. Pressoir M, Desne S, Berchery D, Rossignol G, Poiree B, Meslier M, et al. Prevalence, Risk Factors and Clinical Implications of Malnutrition in French Comprehensive Cancer Centres. Br J Cancer (2010) 102:966–71. doi: 10.1038/sj.bjc.6605578
104. Fukuda Y, Yamamoto K, Hirao M, Nishikawa K, Maeda S, Haraguchi N, et al. Prevalence of Malnutrition Among Gastric Cancer Patients Undergoing Gastrectomy and Optimal Preoperative Nutritional Support for Preventing Surgical Site Infections. Ann Surg Oncol (2015) 22(Suppl 3):S778–85. doi: 10.1245/s10434-015-4820-9
105. Loeffen EA, Brinksma A, Miedema KG, de Bock GH, Tissing WJ. Clinical Implications of Malnutrition in Childhood Cancer Patients–Infections and Mortality. Supportive Care Cancer Off J Multinational Assoc Supportive Care Cancer (2015) 23:143–50. doi: 10.1007/s00520-014-2350-9
106. Triarico S, Rinninella E, Cintoni M, Capozza MA, Mastrangelo S, Mele MC, et al. Impact of Malnutrition on Survival and Infections Among Pediatric Patients With Cancer: A Retrospective Study. Eur Rev Med Pharmacol Sci (2019) 23:1165–75. doi: 10.26355/eurrev_201901_17009
107. Leandro-Merhi VA, de Aquino JL. Determinants of Malnutrition and Post-Operative Complications in Hospitalized Surgical Patients. J Health Popul Nutr (2014) 32:400–10.
108. Thomas MN, Kufeldt J, Kisser U, Hornung HM, Hoffmann J, Andraschko M, et al. Effects of Malnutrition on Complication Rates, Length of Hospital Stay, and Revenue in Elective Surgical Patients in the G-DRG-System. Nutr (2016) 32:249–54. doi: 10.1016/j.nut.2015.08.021
109. Gellrich NC, Handschel J, Holtmann H, Kruskemper G. Oral Cancer Malnutrition Impacts Weight and Quality of Life. Nutrients (2015) 7:2145–60. doi: 10.3390/nu7042145
110. Tan CS, Read JA, Phan VH, Beale PJ, Peat JK, Clarke SJ. The Relationship Between Nutritional Status, Inflammatory Markers and Survival in Patients With Advanced Cancer: A Prospective Cohort Study. Supportive Care Cancer Off J Multinational Assoc Supportive Care Cancer (2015) 23:385–91. doi: 10.1007/s00520-014-2385-y
111. Martin L, Senesse P, Gioulbasanis I, Antoun S, Bozzetti F, Deans C, et al. Diagnostic Criteria for the Classification of Cancer-Associated Weight Loss. J Clin Oncol (2015) 33:90–9. doi: 10.1200/JCO.2014.56.1894
112. Maasberg S, Knappe-Drzikova B, Vonderbeck D, Jann H, Weylandt KH, Grieser C, et al. Malnutrition Predicts Clinical Outcome in Patients With Neuroendocrine Neoplasia. Neuroendocrinol (2017) 104:11–25. doi: 10.1159/000442983
113. Freijer K, Tan SS, Koopmanschap MA, Meijers JM, Halfens RJ, Nuijten MJ. The Economic Costs of Disease Related Malnutrition. Clin Nutr (2013) 32:136–41. doi: 10.1016/j.clnu.2012.06.009
114. Yilmaz H, Ucan B, Sayki M, Unsal I, Sahin M, Ozbek M, et al. Usefulness of the Neutrophil-to-Lymphocyte Ratio to Prediction of Type 2 Diabetes Mellitus in Morbid Obesity. Diabetes Metab Syndr (2015) 9:299–304. doi: 10.1016/j.dsx.2014.04.009
115. Bozkus F, Dikmen N, Samur A, Bilal N, Atilla N, Arpag H. Does the Neutrophil-to-Lymphocyte Ratio Have Any Importance Between Subjects With Obstructive Sleep Apnea Syndrome With Obesity and Without Obesity? Tuberk Toraks (2018) 66:8–15. doi: 10.5578/tt.66535
116. Suarez-Cuenca JA, Ruiz-Hernandez AS, Mendoza-Castaneda AA, Dominguez-Perez GA, Hernandez-Patricio A, Vera-Gomez E, et al. Neutrophil-To-Lymphocyte Ratio and Its Relation With Pro-Inflammatory Mediators, Visceral Adiposity and Carotid Intima-Media Thickness in Population With Obesity. Eur J Clin Invest (2019) 49:e13085. doi: 10.1111/eci.13085
Keywords: anti-inflammation, systemic inflammatory response, neutrophil-to-lymphocyte ratio, malnutrition, colorectal cancer
Citation: Ma C-J, Hu W-H, Huang M-C, Chiang J-M, Hsieh P-S, Wang H-S, Chiang C-L, Hsieh H-M, Chen C-C and Wang J-Y (2022) Taiwan Society of Colon and Rectum Surgeons (TSCRS) Consensus for Anti-Inflammatory Nutritional Intervention in Colorectal Cancer. Front. Oncol. 11:819742. doi: 10.3389/fonc.2021.819742
Received: 22 November 2021; Accepted: 22 December 2021;
Published: 17 January 2022.
Edited by:
Dario Baratti, Fondazione IRCCS Istituto Nazionale Tumori, ItalyReviewed by:
Emiliano Tognoli, National Cancer Institute Foundation (IRCCS), ItalySerena Della Valle, National Cancer Institute Foundation (IRCCS), Italy
Copyright © 2022 Ma, Hu, Huang, Chiang, Hsieh, Wang, Chiang, Hsieh, Chen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jaw-Yuan Wang, Y3k2MTQxMTJAbXMxNC5oaW5ldC5uZXQ=; SmF3eXVhbndhbmdAZ21haWwuY29t
 Cheng-Jen Ma1,2
Cheng-Jen Ma1,2 Wan-Hsiang Hu
Wan-Hsiang Hu Chien-Ling Chiang
Chien-Ling Chiang Jaw-Yuan Wang
Jaw-Yuan Wang