- 1Department of Pharmacy, Union Hospital, Tongji Medical College, Huazhong University of Science and Technology, Wuhan, China
- 2Hubei Province Clinical Research Center for Precision Medicine for Critical Illness, Wuhan, China
- 3Glycation and Diabetes Group, Mater Research Institute - The University of Queensland, Translational Research Institute, Brisbane, QLD, Australia
Background: In China, thalidomide (THD) has been used to prevent chemotherapy-induced nausea and vomiting (CINV) following highly emetogenic chemotherapy (HEC); however, there is limited evidence on the efficacy and safety of THD in this setting. The aim of this study was to evaluate the efficacy, safety, and impact on quality of life (QoL) of THD on CINV following HEC.
Methods: Electronic databases were systematically searched for all randomized controlled trials (RCTs) in HEC using THD. The primary outcomes were complete response (CR) and no nausea, Secondary outcomes were the incidence of adverse events and QoL related indicators. We calculated risk ratios (RRs) and 95% confidence intervals (CIs) using a fixed-effects model. In the case of heterogeneity (I2≥50%), a random-effects model was performed.
Results: A total of 3168 patients were included from 34 RCTs. In terms of CR rate, THD plus 5-HT3 receptor antagonist (5-HT3RA) with or without dexamethasone (DEX) was significantly higher than 5-HT3RA with or without DEX in the acute phase (74.4% vs 67.4%; RR 1.10), delayed phase (70.6% vs 50.4%; RR 1.53), and overall phase (68.4% vs 53.4%; RR 1.28). In terms of no nausea rate, the THD group was also significantly higher than the control group in the acute phase (61.7% vs 55.5%; RR 1.12), delayed phase (50.5% vs 30.0%; RR 1.69), and overall phase (44.6% vs 29.9%; RR 1.50). There was no statistical difference in the incidence of fatigue, headache, diarrhea, rash, hepatorenal damage, and myelosuppression between those with and without THD. The incidence of increase in KPS scores, weight gain, appetite improvement, and sleep quality improvement were significantly higher with the addition of THD.
Conclusions: THD may be effective and safe for the prevention of CINV patients treated with HEC and may improve QoL.
1 Introduction
Chemotherapy-induced nausea and vomiting (CINV) is one of the most common disturbing adverse effects of anticancer chemotherapy, which can significantly impair the patient’s quality of life (QoL), adherence with future therapy, and nutritional status. American Society of Clinical Oncology (ASCO) guideline (2020) (1) classify chemotherapeutic agents according to their emetogenic potential (high, medium, low and minimal) and make recommendations based on their level of risk. For patients receiving highly emetogenic chemotherapy (HEC; CINV risk>90%), such as cisplatin- and anthracycline/cyclophosphamide (AC)-based regimens, National Comprehensive Cancer Network (NCCN) antiemesis guideline recommend a four-drug combination of a 5-HT3 receptor antagonist (5-HT3RA), a neurokinin-1 (NK1) RA, dexamethasone (DEX), and olanzapine (2). Even if CINV prevention is now dramatically improved, there is still a need to find more effective, safer and more economical drug regimens for better prevention because CINV remains a frequent and feared adverse effect.
The unintended teratogenic effect of thalidomide (THD), prescribed to treat morning sickness in pregnant women, is a historic tragedy, however with the approval of this drug for indications such as multiple myeloma. A randomized controlled double-blind phase III clinical study (3) in the Chinese population suggested that THD combined with palonosetron and DEX is efficacious and well-tolerated for the prevention of delayed CINV in anticancer chemotherapy-naive patients who undergo HEC. Rates of complete response and no nausea in the delayed phase were higher and adverse effects were mild to moderate in the THD group. Since pregnancy and childbirth are nearly impossible during anticancer chemotherapy in patients with malignant tumors, and THD prices are relatively low in China, there is some potential for THD to be useful in the management of CINV.
In China, there have been many controlled clinical trials using THD, in addition with antiemetic regimens, with results showing that THD can be used as a complementary and alternative medicine to prevent CINV following HEC. However, there is no systematic review or meta-analysis of its efficacy in the prevention of CINV, the incidence of adverse effects, and the improvement of QoL under HEC. Therefore, all controlled clinical trials using THD under HEC were systematically evaluated for efficacy in the prevention of CINV through multiple studies and large sample size.
2 Methods
The meta-analysis was pre-registered at PROSPERO (CRD42020158732).
2.1 Literature Search
This systematic review and meta-analysis was conducted and reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines (4). Relevant publications were searched in the Chinese National Knowledge Infrastructure (CNKI), the VIP Information Database, Wanfang Database, PubMed, EMBASE, and the Cochrane Library. The systematic review was performed in December 2019 and updated in August 2020.
The keywords for searching included: “chemotherapy-induced nausea and vomiting”, “CINV”, “vomit”, “emesis”, “thalidomide”, “highly emetogenic chemotherapy”, “CDDP”, “cisplatin”, or “anthracycline and cyclophosphamide”. References of the selected articles were also checked to identify further eligible trials.
2.2 Study Selection Criteria
Selecting studies that met the inclusion and exclusion criteria was independently performed by two authors(JX, CZ). Any disagreement between reviewers was resolved through public discussions until a consensus was reached.
Inclusion criteria: (a) randomized controlled trials (RCTs) in patients who received HEC (such as cisplatin-based treatment or AC regimen); (b) studies that reported either THD as an add-on treatment (5-HT2RA, with or without DEX) or THD monotherapy compared to standard treatment.
Exclusion criteria: (a) review articles or studies involving non-human subjects; (b) duplicate published articles; (c) studies where anticancer chemotherapy regimens and basic antiemetic regimens were inconsistent between experimental and control groups; (d) studies with a high risk of bias.
2.3 Outcomes
The primary outcomes: Complete response (CR) and no nausea. CR is defined as having no emetic episode and requiring no use of rescue medication. Nausea was categorized by using a 4-point Likert scale (0, no symptoms; 3, severe). CR and no nausea were measured in the acute phase (0-24 h), the delayed phase (24-120 h), and the overall phase (0-120 h). Secondary outcomes included the adverse events which was graded according to the common terminology criteria for adverse events (CTCAE) (5) and indicators related to QoL: Karnofsky performance scale (KPS) scores, weight, appetite, and sleep quality.
2.4 Quality Assessment
The quality of the included studies was assessed independently by two authors(SL, RD) based on the Cochrane Handbook for Systematic Review of Interventions (6). The Cochrane Collaboration’s tool for assessing the risk of bias for RCTs includes the following seven items: random sequence generation (selection bias), allocation concealment (selection bias), blinding of participants and personnel (performance bias), blinding of outcome assessments (detection bias), incomplete outcome data (attrition bias), selective outcome reporting (reporting bias), and other sources of bias. Each item was described as high risk of bias, low risk of bias, or unclear risk of bias. Disagreements were discussed and resolved by consensus between both reviewers or via consultation with a third reviewer (JX).
2.5 Statistical Analysis
Results were quantitatively synthesized by means of meta-analysis using the Review Manager (version 5.3; Cochrane Collaboration, Oxford, England). The Mantel-Haenszel method was used to estimate the pooled risk ratio (RR) for each dichotomous variable. I2 was used to evaluate heterogeneity across studies. When heterogeneity (I2≥50%) was detected, random-effects meta-analyses were performed. I2<50%, a fixed effect statistical model was used. Results obtained from the analyses were displayed by generating a forest plot. A p-value of < 0.05 was considered statistically significant.
3 Results
3.1 Study Selection and Trial Characteristics
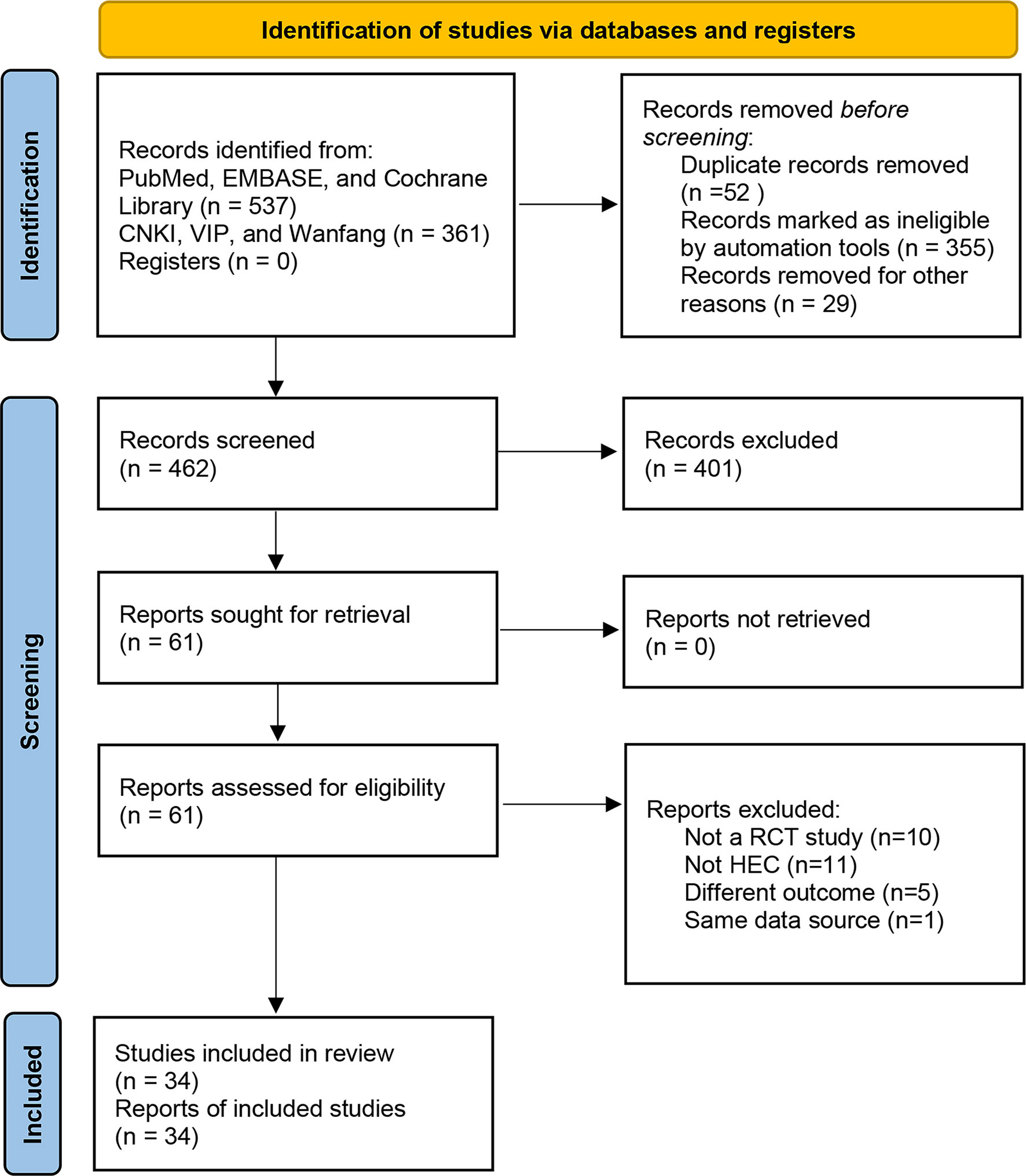
There were 898 records identified via database searching. 537 of the records were searched in PubMed, EMBASE, and the Cochrane library, 361 of the records were searched in the CNKI, VIP Information Database, and Wanfang Database (Figure 1).
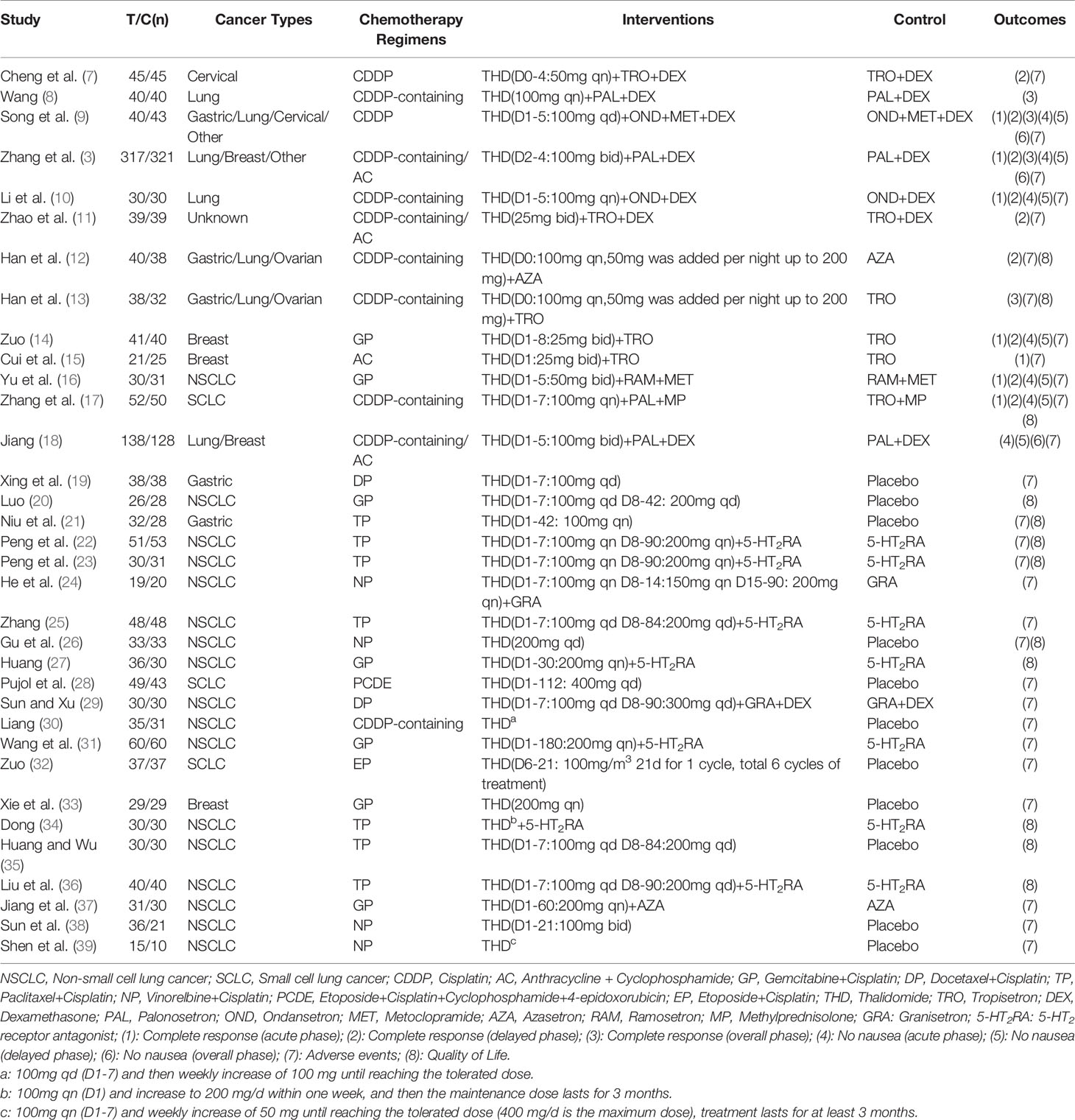
After removing the duplicates, there were 462 results. The titles and abstracts of 462 studies were screened, and the full text of 61 articles was reviewed. 27 studies were excluded for the following reasons: not a RCT study (n = 10), not HEC (n = 11), a different outcome (n = 5), and same data source (n = 1). Finally, 34 studies were assessed for eligibility and included in the quantitative synthesis. A total of 3168 patients were included. The characteristics of the included studies are shown in Table 1. All studies were RCTs. Patients’ tumor types include breast, gastric, non-small cell lung cancer (NSCLC), small cell lung cancer (SCLC), cervical, and others. All patients in these studies received HEC.
The included studies contained a total of 8 outcomes: CR (acute phase) (3, 9, 10, 14–17); CR (delayed phase) (3, 7, 9–12, 14, 16, 17); CR (overall phase) (3, 8, 9, 13); no nausea (acute phase) (3, 9, 10, 14, 16–18); no nausea (delayed phase) (3, 9, 10, 14, 16–18); no nausea (overall phase) (3, 9, 18); adverse events (3, 7, 9–19, 21–26, 28–33, 37–39); QoL (12, 13, 17, 20–23, 26, 27, 34–36).
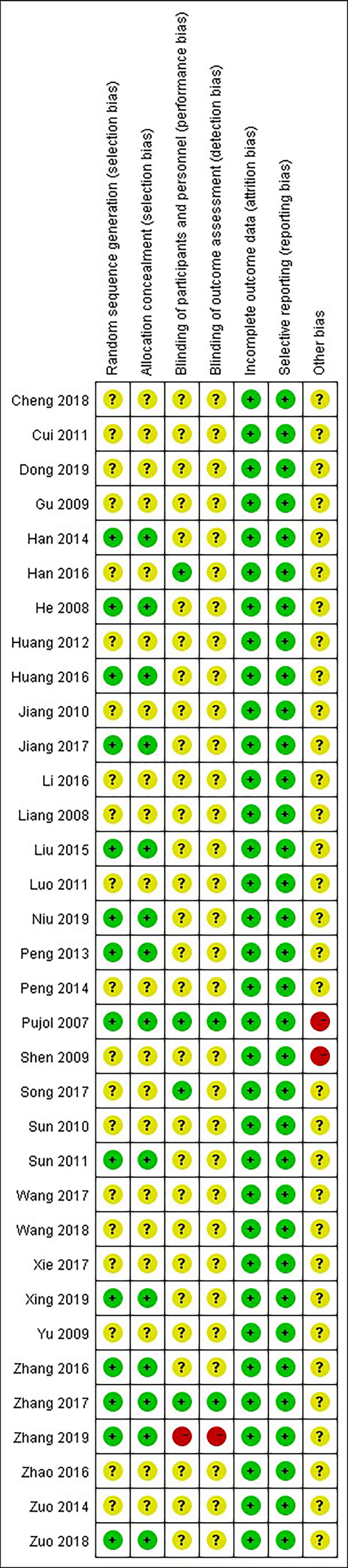
3.2 Risk of Bias and Quality Assessment
All of the included studies had a low risk of attrition bias and reporting bias. Only one study (25) had a high risk of performance bias and detection bias due to its single-blind method. Two of the included studies (28) and (39) had a high risk of other bias due to a possible conflict of interest or small sample size (Figure 2).
3.3 Primary Outcomes
3.3.1 CR in the Acute Phase
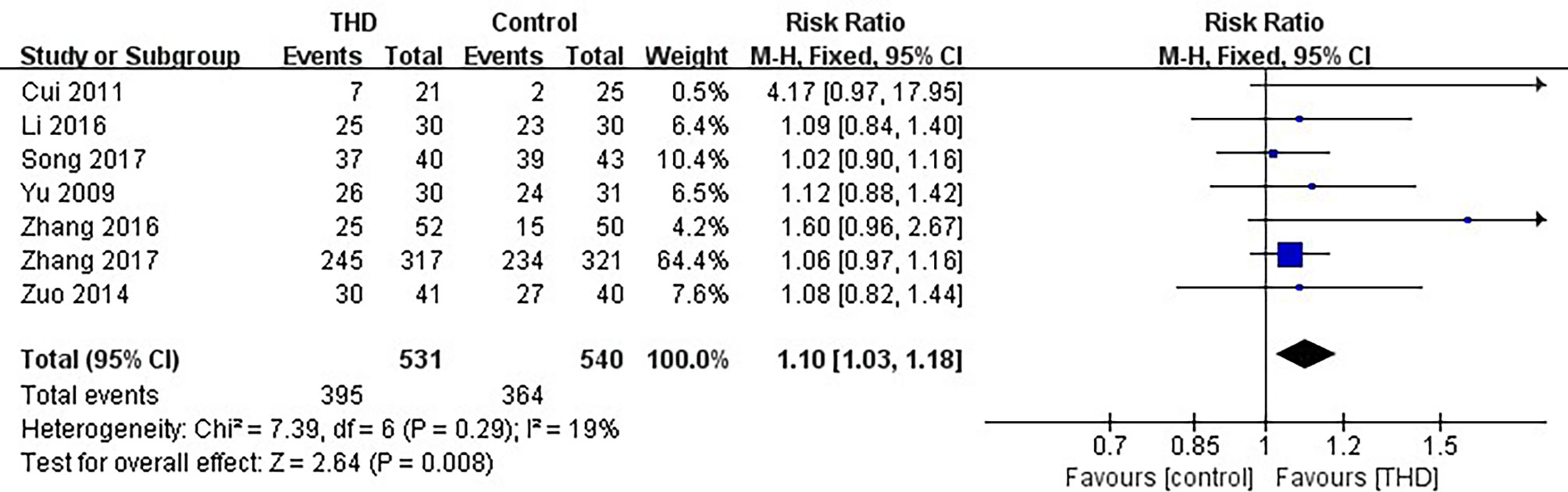
Data of CR in the acute phase were available in 7 studies, including 1071 patients: 531 patients in the experimental group were treated with THD added to the 5-HT3RA-based conventional antiemetic regimen, and 540 patients in the control group were treated with the 5-HT3RA-based conventional antiemetic regimen. The CR rate was significantly higher with the addition of THD in the acute phase: 74.4% vs 67.4% (RR 1.10, 95%CI 1.03-1.18, p=0.008), without significant heterogeneity among studies (I²=19%) (Figure 3).
3.3.2 CR in the Delayed Phase
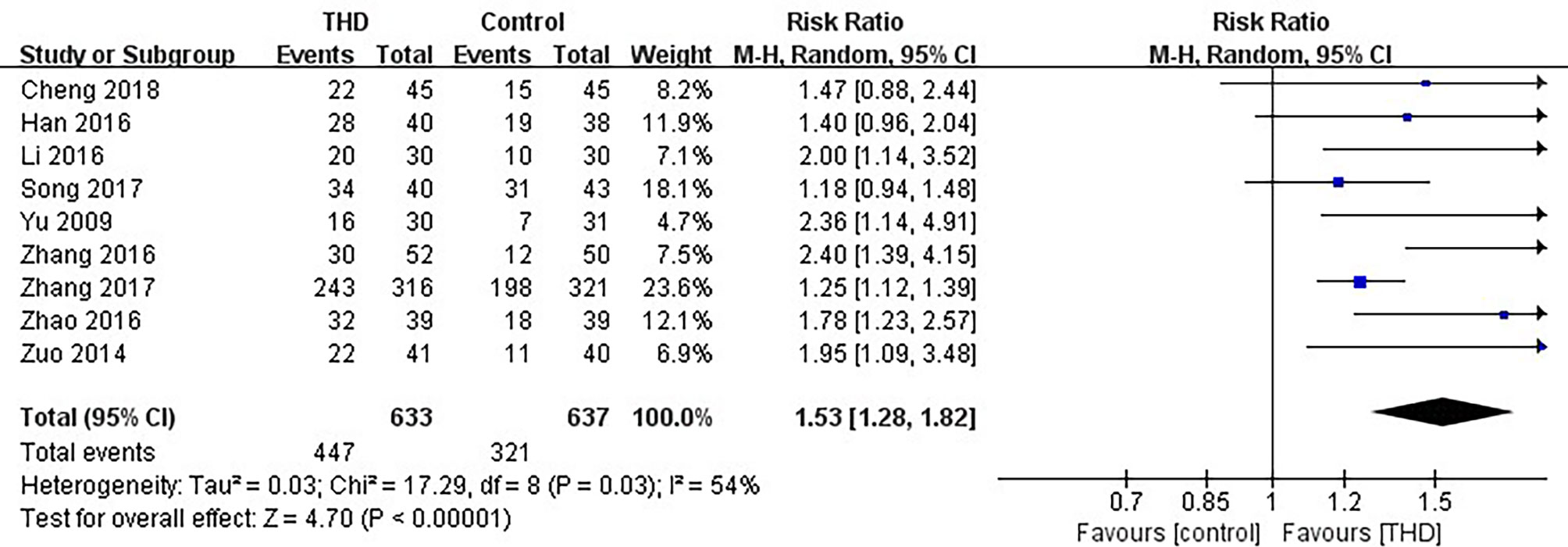
Data of CR in the delayed phase were available in 9 studies, including 1270 patients: 633 patients in the experimental group and 637 patients in the control group. The CR rate was significantly higher with the addition of THD in the delayed phase: 70.6% vs 50.4% (RR 1.53, 95%CI 1.28-1.82, p<0.00001), with significant heterogeneity among studies (I²=54%). Due to significant heterogeneity among the studies, a random-effects model was chosen for analysis (Figure 4).
3.3.3 CR in the Overall Phase
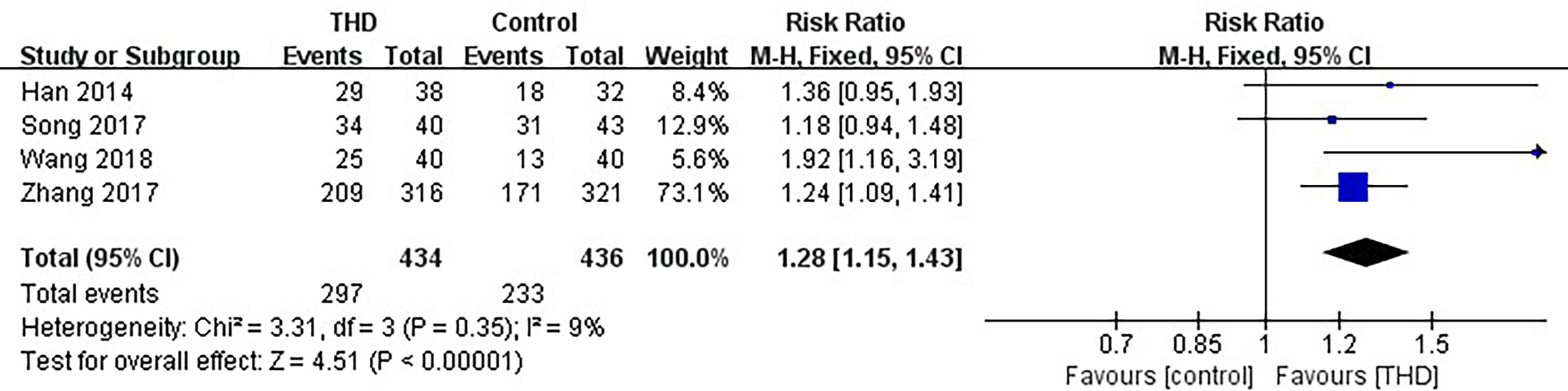
Data of CR in the overall phase were available in 4 studies, including 870 patients: 434 patients in the experimental group and 436 patients in the control group. The CR rate was significantly higher with the addition of THD in the overall phase: 68.4% vs 53.4% (RR 1.28, 95%CI 1.15-1.43, p<0.00001), without significant heterogeneity among studies (I²=9%) (Figure 5).
3.3.4 No Nausea in the Acute Phase
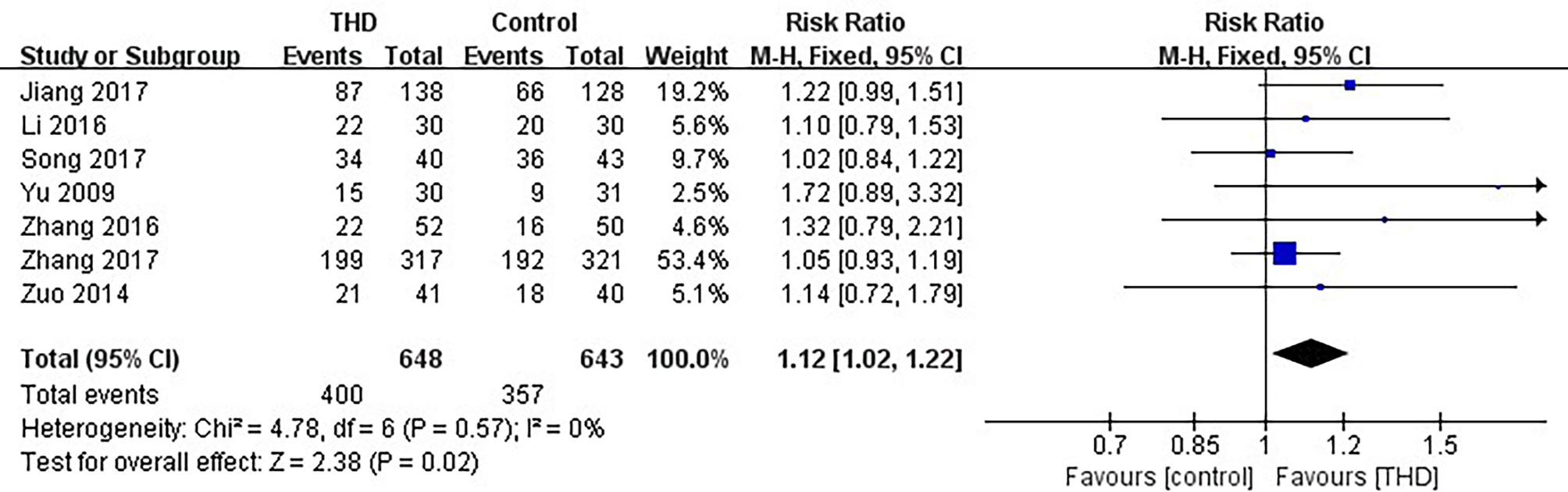
Data of no nausea in the acute phase were available in 7 studies, including 1291 patients: 648 patients in the experimental group and 643 patients in the control group. The no nausea rate was significantly higher with the addition of THD in the acute phase: 61.7% vs 55.5% (RR 1.12, 95%CI 1.02-1.22, p=0.02), without significant heterogeneity among studies (I²=0%) (Figure 6).
3.3.5 No Nausea in the Delayed Phase
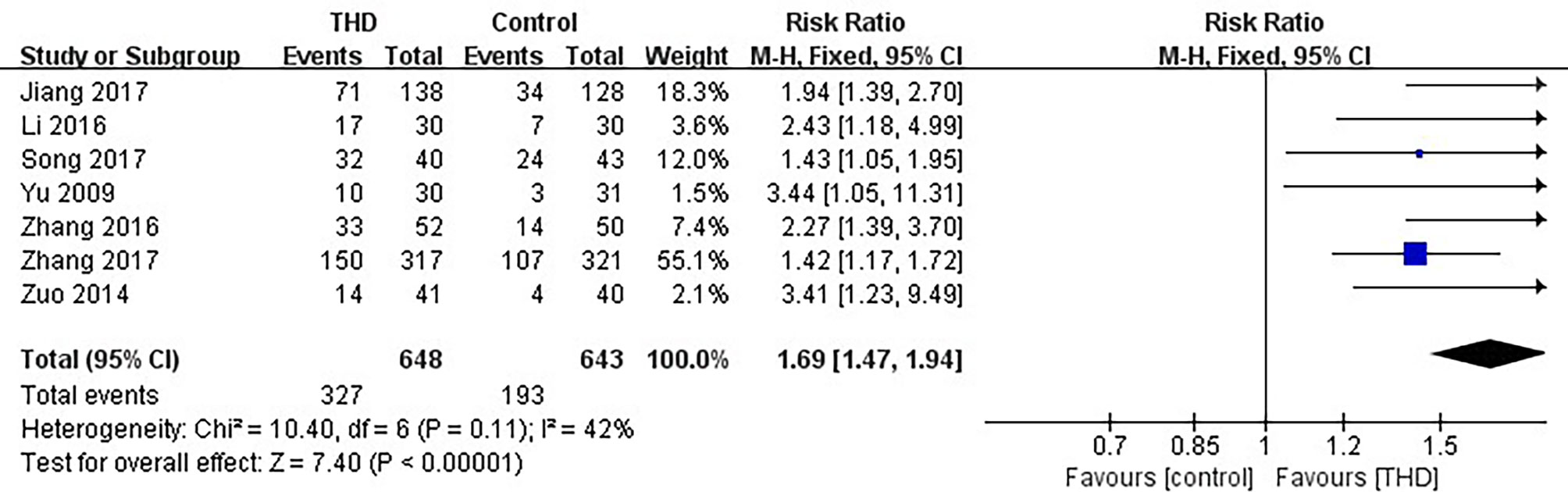
Data of no nausea in the delayed phase were available in 7 studies, including 1291 patients: 648 patients in the experimental group and 643 patients in the control group. The no nausea rate was significantly higher with the addition of THD in the delayed phase: 50.5% vs 30.0% (RR 1.69, 95%CI 1.47-1.94, p<0.00001), without significant heterogeneity among studies (I²=42%) (Figure 7).
3.3.6 No Nausea in the Overall Phase
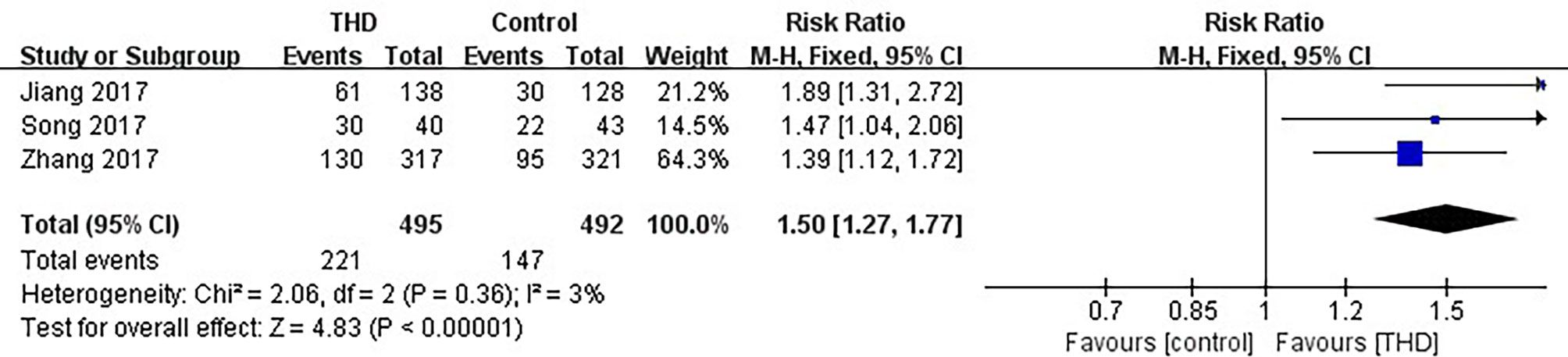
Data of no nausea in the overall phase were available in 3 studies, including 987 patients: 495 patients in the experimental group and 492 patients in the control group. The no nausea rate was significantly higher with the addition of THD in the overall phase: 44.6% vs 29.9% (RR 1.50, 95%CI 1.27-1.77, p<0.00001), without significant heterogeneity among studies (I²=3%) (Figure 8).
3.4 Secondary Outcomes
3.4.1 Adverse Events
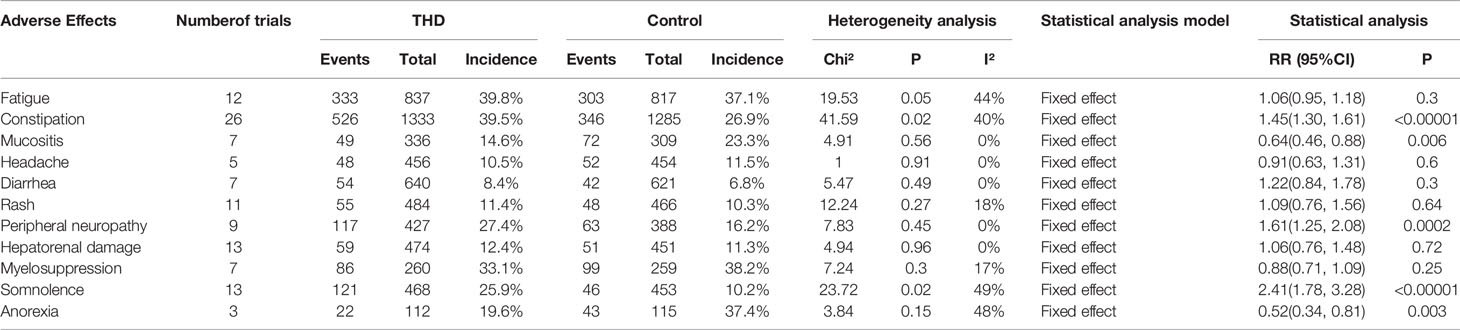
Data from 28 out of the 34 included articles involved safety studies of THD and 11 adverse events were included: fatigue (12 studies), constipation (26 studies), mucositis (7 studies), headache (5 studies), diarrhea (7 studies), rash (11 studies), peripheral neuropathy (9 studies), hepatorenal damage (13 studies), myelosuppression (7 studies), somnolence (13 studies), and anorexia (3 studies). There was no significant heterogeneity among all studies (I²<50%) and all analyses were performed using a fixed-effects model.
The incidence of mucositis and anorexia was significantly lower with the addition of THD: namely, 14.6% vs 23.3% (RR 0.64, 95%CI 0.46-0.88, p=0.006) of mucositis; and 19.6% vs 37.4% (RR 0.52, 95%CI 0.34–0.81, p=0.003) of anorexia.
The incidence of constipation, peripheral neuropathy, and somnolence was significantly higher with the addition of THD: namely, 39.5% vs 26.9% (RR 1.45, 95%CI 1.30-1.61, p<0.00001) of constipation; 27.4% vs 16.2% (RR 1.61, 95%CI 1.25-2.08, p=0.0002) of peripheral neuropathy; and 25.9% vs 10.2% (RR 2.41, 95%CI 1.78-3.28, p<0.00001) of somnolence.
There was no statistical difference in the incidence of fatigue, headache, diarrhea, rash, hepatorenal damage, and myelosuppression between those with and without THD (p>0.05) (Table 2).
3.4.2 QoL
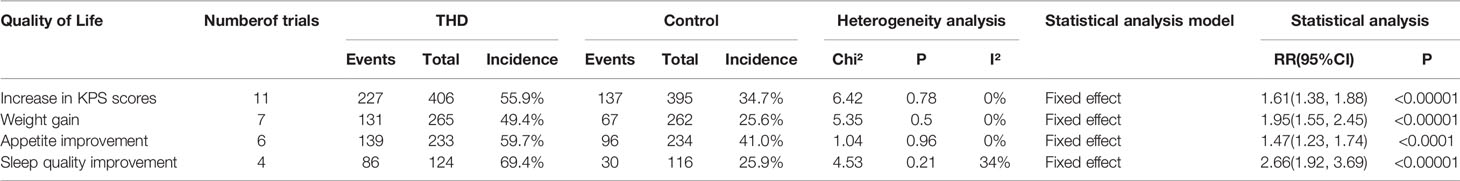
Data from 12 out of the 34 included articles examined the impact of THD on QoL and included 4 items: increase in the KPS scores (11 studies), weight gain (7 studies), appetite improvement (6 studies), and sleep quality improvement (4 studies). There was no significant heterogeneity among all studies (I²<50%) and all analyses were performed using a fixed-effects model.
The incidence of an increase in KPS scores, weight gain, appetite improvement, and sleep quality improvement was significantly higher with the addition of THD: namely, 55.9% vs 34.7% (RR 1.61, 95%CI 1.38-1.88, p<0.00001) of an increase in KPS; 49.4% vs 25.6% (RR 1.95, 95%CI 1.55-2.45, p<0.00001) of weight gain; 59.7% vs 41.0% (RR 1.47, 95%CI 1.23-1.74, p<0.00001) of appetite improvement; and 69.4% vs 25.9% (RR 2.66, 95%CI 1.92-3.69, p<0.00001) of sleep quality improvement (Table 3).
4 Discussion
There is evidence that THD should be considered as an effective additional antiemetic medication (40). This meta-analysis suggests that the addition of THD to 5-HT3RA treatment (with or without DEX) is beneficial. Our findings showed that the addition of THD prevents CINV following HEC during the acute, delayed, and overall phase. Among these phases, the THD group had the most significant improvement in CINV during the delayed phase (70.6% vs 50.4% and 50.5% vs 30.0% in CR and no nausea, respectively).
This meta-analysis also suggests a high safety profile for the use of THD in patients with tumors undergoing HEC. Although the THD group increased the incidence of constipation, peripheral neuropathy, and somnolence, the incidence was significantly lower in mucositis and anorexia. The addition of THD did not increase the incidence of many adverse events (fatigue, headache, diarrhea, rash, hepatorenal damage, and myelosuppression). Researchers speculate that THD protects the oral mucosa by inhibiting NF-κB and supporting epithelial repopulation (41). Chemotherapy-induced intestinal mucositis and delayed diarrhea are associated with AIM2 (absent in melanoma 2) inflammasome activation, while THD eliminates AIM2 signaling and significantly reduces the incidence of drug-induced diarrhea (42). This study shows that there is no statistical difference in the incidence of diarrhea between the THD group and the control group, which may require more rigorous clinical trials and a wider population.
As a complementary drug, THD has been shown to improve QoL in cancer patients in this meta-analysis.
THD significantly improves KPS scores, weight, sleep quality, and appetite in cancer patients receiving HEC (55.9% vs 34.7%, 49.4% vs 25.6%, 59.7% vs 41.0%, and 69.4% vs 25.9%, respectively). A Cochrane meta-analysis shows that there is insufficient evidence to refute or support the use of THD for the treatment of cachexia in patients with advanced cancer (43). THD combined with megestrol acetate was shown to be effective in terms of appetite, body weight, and QoL (44).
This study has several strengths. Firstly, we included 34 RCTs and 3168 cases, expanding the scope and number of THD studies and greatly improving sample size and test efficacy. Secondly, we compared the differences in the incidence of 11 adverse events between the THD and control groups to provide a reference for the safety study of THD use in cancer patients. Finally, we also analyzed the effect of THD in increasing KPS scores, increasing weight, improving sleep quality, and increasing appetite from the perspective of QoL of cancer patients.
This meta-analysis also has some limitations. First, although the search for this study was extensive and included both English and Chinese databases, the final population of the literature included in the study was Chinese, which is not representative of other regional populations and ethnicities. Second, many of the studies we included scored poorly on quality assessment, which to some extent affects the final results of the meta-analysis. Finally, the number of studies containing the same outcome was no more than 10, so a funnel plot was not used to test for publication bias.
5 Conclusion
According to this systematic review and meta-analysis, we conclude that THD is effective and safe for the prevention of CINV in patients being treated with HEC, and has a significant tendency to improve QoL. More high-quality RCTs with more participants are warranted to support our findings.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding authors.
Author Contributions
JX and CZ contributed to study design, literature search, data collection, data analysis, and manuscript drafting. SL and RD contributed to quality assessment and data collection. MS, BD, and QX contributed to critical revision. JW, CS, and YZ contributed to conception, design, supervision, and manuscript drafting. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China [82073402] and Key research and development program of Hubei Province [2020BCA060].
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank all the staff member for their invaluable efforts to this study.
References
1. Hesketh PJ, Kris MG, Basch E, Bohlke K, Barbour SY, Clark-Snow RA, et al. Antiemetics: ASCO Guideline Update. J Clin Oncol Off J Am Soc Clin Oncol (2020) 38(24):2782–97. doi: 10.1200/JCO.20.01296
2. Gilmore J, D'Amato S, Griffith N, Schwartzberg L. Recent Advances in Antiemetics: New Formulations of 5HT-Receptor Antagonists. Cancer Manag Res (2018) 10:1827–57. doi: 10.2147/CMAR.S166912
3. Zhang L, Qu X, Teng Y, Shi J, Yu P, Sun T, et al. Efficacy of Thalidomide in Preventing Delayed Nausea and Vomiting Induced by Highly Emetogenic Chemotherapy: A Randomized, Multicenter, Double-Blind, Placebo-Controlled Phase III Trial (CLOG1302 Study). J Clin Oncol Off J Am Soc Clin Oncol (2017) 35(31):3558–65. doi: 10.1200/JCO.2017.72.2538
4. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. BMJ (Clin Res ed) (2009) 339:b2535. doi: 10.1136/bmj.b2535
5. National Cancer Institute, National Institutes of Health, U.S. Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. Available at: https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_5.0/NCIt_CTCAE_5.0.xls (Accessed December 24, 2021).
6. Higgins JPT. Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Collaboration J Cochrane Database Systematic Rev (2011) 2011(14):753–4. doi: 10.2105/AJPH.2020.305609
7. Cheng Q, Chen Z, Wang N. The Clinical Efficacy of Thalidomide in Preventing Delayed Vomiting Caused by Cisplatin Chemotherapy. China Foreign Med Treat (2018) 37(18):92–4. doi: 10.16662/j.cnki.1674-0742.2018.18.092
8. Wang S. Efficacy of Thalidomide Combined With Palonosetron in the Prevention of Vomiting Induced by Cisplatin Chemotherapy in Lung Cancer Patients. Guide China Med (2018) 16(23):24–5. doi: 10.15912/j.cnki.gocm.2018.23.013
9. Song G, Wang N, Li FF, Wang NF. Thalidomide for Prevention of Chemotherapy-Induced Nausea and Vomiting Following Highly Emetogenic Chemotherapy. J Australas Med (2017) 10(3):192–8. doi: 10.21767/AMJ.2016.2826
10. Li M, Gao E, Cui F, Chen LQ, Zhang FL. Clinical Study of Thalidomide Combined With Ondansetron in the Prevention of Nausea and Vomiting in Lung Cancer Patients With Chemotherapy. J Mod Oncol (2016) 24(17):2719–23. doi: 10.3969/j.issn.1672-4992.2016.17.014
11. Zhao W, Yu P, Jin X, Qu LJ. Clinical Observation of Thalidomide in the Treatment of Delayed Vomiting Due to Chemotherapy. Chin J Convalescent Med (2016) 25(02):201–2. doi: 10.13517/j.cnki.ccm.2016.02.044
12. Han Z, Sun X, Jiang G, Du XP. Thalidomide for Control Delayed Vomiting in Cancer Patients Receiving Chemotherapy. J Coll Physicians Surg Pak (2016) 26(11):900.
13. Han Z, Sun X, Xu J, Li Y, Du X. Clinical Study of Treatment of Peri-Chemotherapy Nausea and Vomiting of Cancer Patients Using Thalidomide. Cancer Res Clin (2014) 26(10):667–9.
14. Zuo C. Effect of Thalidomide Combined With Tropisetron Hydrochloride on Nausea and Vomiting Induced by GP Chemotherapy for Metastatic Breast Cancer. Chin J Clin Res (2014) 27(12):1491–3. doi: 10.13429/j.cnki.cjcr.2014.12.019
15. Cui Y, Li J, Wang WY, Lu CX, Zhou Y, Liu MY. Clinical Observation of Thalidomide Combined With Tropisetron in the Treatment of Vomiting Associated With Adjuvant Chemotherapy for Breast Cancer. J Pract Med (2011) 27(19):3576–8.
16. Yu YL, Zhu ZT, Li JP, Ha MW, Liu XM, Wu Q, et al. The Effect of Thalidomide in Preventing Delayed Nausea and Vomiting Induced by GP Regimen of Chemotherapy for Non-Small Cell Lung Cancer. Chin J Oncol (2009) 31(12):937–40.
17. Zhang J, Kang H, Wang B, Shun GM. The Efficacy and Safety of Palonosetron With Thalidomide to Prevent Chemotherapy-Induced Nausea and Vomiting (CINV) During Chemotherapy in Small Cell Lung Cancer Patients. Chin J Clin Oncol Rehabil (2016) 23(08):943–6. doi: 10.13455/j.cnki.cjcor.2016.08.14
18. Jiang H. Thalidomide for Control Nausea in Cancer Patients Receiving Highly Emetogenic Chemotherapy. ShenYang: China Medical University (2017).
19. Xing A, Zhang N, Liu Y. Effect of Thalidomide Combination Chemotherapy on Serum MMP-9 and VGER Levels in Patients With Gastric Cancer. J Mudanjiang Med Univ (2019) 40(01):73–4+8. doi: 10.13799/j.cnki.mdjyxyxb.2019.01.024
20. Luo Q. Thalidomide Combined With GP Regimen for Advanced Non-Small Cell Lung Cancer. J Pract Med (2011) 27(06):1080–2. doi: 10.3969/j.issn.1006-5725.2011.06.069
21. Niu K, Deng W, Li N, Luo SX. Efficacy Observation of Thalidomide Combined With TP Regimen in Treatment of Advanced Gastric Cancer. Cancer Res Clin (2019) 31(09):610–3.
22. Peng Y, Wang M, Xie N, Li Y, Lu HY, Li BM. Paclitaxel and Cisplatin in Combination With Thalidomide in Advanced Non-Small Cell Lung Cancer: A Clinical Study. Chin J Clin Pharmacol Ther (2013) 18(03):317–21.
23. Peng Y, Zhang X, Xie N, Wang MQ, Li BM. Clinical Observation on Therapeutic Effect of Thalidomide in Combination With Paclitaxel Plus Cisplatin Regimen in the Treatment of Elderly Patients With Advanced Non-Small Cell Lung Cancer. Chin J Diffic Compl Cas (2014) 13(01):49–52. doi: 10.3969/j.issn.1671-6450.2014.01.018
24. He Q, Yi T, Luo B, Zhang XL. A Randomized Trial of NVB Plus DDP With Versus Without Thalidomide in the Treatment of Advanced Non-Small Cell Lung Cancer. Chin J Lung Cancer (2008) 11(02):264–7. doi: 10.3779/j.issn.1009-3419.2008.02.019
25. Zhang Z. Effect of Thalidomide Combined With TP Regimen Chemotherapy on Serum Tumor Markers in Patients With Advanced NSCLC. J Huaihai Med (2019) 37(04):338–40. doi: 10.14126/j.cnki.1008-7044.2019.04.003
26. Zhang X. Effect of Thalidomide Combined With TP Regimen Chemotherapy on Serum Tumor Markers in Patients With Advanced NSCLC. J Huaihai Med (2009) 37(04):338–40. doi: 10.14126/j.cnki.1008-7044.2019.04.003
27. Huang J. Effect of Thalidomide Combination With GP Regimen on Non-Small Cell Lung Cancer and Effects of Serum VEGF. J Mil Surg Southwest China (2012) 14(01):34–5. doi: 10.3969/j.issn.1672-7193.2012.01.017
28. Pujol JL, Breton JL, Gervais R, Tanguy ML, Quoix E, Janicot PD, et al. Phase III Double-Blind, Placebo-Controlled Study of Thalidomide in Extensive-Disease Small-Cell Lung Cancer After Response to Chemotherapy: An Intergroup Study FNCLCC Cleo04 IFCT 00-01. J Clin Oncol Off J Am Soc Clin Oncol (2007) 25(25):3945–51. doi: 10.1200/JCO.2007.11.8109
29. Sun X, Xu J. Clinical Observation of Thalidomide Combined With Docetaxel and Cisplatin in the Treatment of Advanced Non-Small Cell Lung Cancer. J China Tradit Chin Med Inf (2011) 03(03):159–61.
30. Liang D. Clinical Study on Thalidomide Combined With Chemotherapy in the Treatment of Advanced Non-Small Cell Lung Cancer. Guangxi: Guangxi Medical University (2008).
31. Wang J, Jia J, Wang Z, Wang XH. Effect of Thalidomide Combined With Targeted Therapy for Non-Small Cell Lung Cancer Patients on the Survival and Quality of Life. Chin J Med (2017) 52(05):37–40. doi: 10.3969/j.issn.1008-1070.2017.05.012
32. Zuo C. Clinical Observation of Thalidomide Combined With EP Chemotherapy in the Treatment of Extensive Stage Small Cell Lung Cancer. J Basic Clin Oncol (2018) 31(04):329–31. doi: 10.3969/j.issn.1673-5412.2018.04.015
33. Xie H, Liu H, Wang W. Curative Effect of the GP Combined With Thalidomide in the Treatment of Advanced Triple-Negative Breast Cancer. J Bengbu Med Coll (2017) 42(07):878–80+84. doi: 10.13898/j.cnki.issn.1000-2200.2017.07.012
34. Dong J. Effect of Thalidomide Combined With Paclitaxel in the Treatment of Advanced Non-Small Cell Lung Cancer in the Elderly. World Latest Med Inf (2019) 19(47):343+345. doi: 10.19613/j.cnki.1671-3141.2019.67.010
35. Huang Y, Wu Q. Clinical Study on Thalidomide Combined With TP Scheme in the Treatment of Advanced Non-Small Cell Lung Cancer. China Contin Med Educ (2016) 8(32):160–1. doi: 10.3969/j.issn.1674-9308.2016.32.089
36. Liu F, Li XY, Li FL, Chen DW. To Observe the Effect of Thalidomide in Combination With Paclitaxel Combined With TP Regimen in the Treatment of Elderly Patients With Advanced Non-Small Cell Lung Cancer. World Latest Med Inf (2015) 15(31):1–2. doi: 10.3969/j.issn.1671-3141.2015.31.001
37. Jiang W, Wang Y, Jiang H, Hu CX, Zhou Y. The Control Clinical Study on the Treatment of Advanced Non-Small Cell Lung Cancer by TGP Regimen and GP Regimen. Chin Clin Oncol (2010) 15(09):798–801.
38. Sun Y, Wang L, Chen W. Clinical Study of Thalidomide Combined With NP Regimen for Advanced Non-Small Cell Lung Cancer. Cancer Res Clin (2010) 22(01):38–40.
39. Shen Z, Wang C, Liang J, Bi Q, Yang RY. Clinical Research of Thalidomide in Corporation With NP in Treatment of III/IV Lung Cancer. Cancer Res Clin (2009) 21(10):663–5.
40. Gourd E. Thalidomide Reduces Chemotherapy-Induced Vomiting. Lancet Oncol (2017) 18(10):e570. doi: 10.1016/S1470-2045(17)30676-9
41. Frings K, Gruber S, Kuess P, Kleiter M, Dörr W. Modulation of Radiation-Induced Oral Mucositis by Thalidomide : Preclinical Studies. Strahlentherapie und Onkologie Organ der Deutschen Rontgengesellschaft [et al] (2016) 192(8):561–8. doi: 10.1007/s00066-016-0989-5
42. Lian QS, Xu J, Yan SS, Huang M, Ding HH, Sun XY. Chemotherapy-Induced Intestinal Inflammatory Responses Are Mediated by Exosome Secretion of Double-Strand DNA via AIM2 Inflammasome Activation. Cell Res (2017) 27(6):784–800. doi: 10.1038/cr.2017.54
43. Reid J, Mills M, Cantwell M, Cardwell CR, Murray LJ, Donnelly M. Thalidomide for Managing Cancer Cachexia. Cochrane Database Systematic Rev (2012) 2012(4):Cd008664. doi: 10.1002/14651858.CD008664.pub2
Keywords: chemotherapy-induced nausea and vomiting, thalidomide, safety, efficacy, highly emetogenic chemotherapy
Citation: Xie J, Zhang C, Li S, Dai R, Sullivan MA, Deng B, Xu Q, Wang J, Shi C and Zhang Y (2022) Efficacy and Safety of Thalidomide As a Pre-Medication of Chemotherapy-Induced Nausea and Vomiting (CINV) Following Highly Emetogenic Chemotherapy (HEC): A Systematic Review and Meta-Analysis. Front. Oncol. 11:818839. doi: 10.3389/fonc.2021.818839
Received: 20 November 2021; Accepted: 29 December 2021;
Published: 24 January 2022.
Edited by:
David A. Gewirtz, Virginia Commonwealth University, United StatesReviewed by:
Prasanth Ganesan, Jawaharlal Institute of Postgraduate Medical Education and Research (JIPMER), IndiaFlorian Slimano, Université de Reims Champagne-Ardenne, France
Copyright © 2022 Xie, Zhang, Li, Dai, Sullivan, Deng, Xu, Wang, Shi and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jinglin Wang, bGlubGludmMyMDJAMTYzLmNvbQ==; Chen Shi, MjkxMzY5MDlAcXEuY29t; Yu Zhang, emhhbmd3a3BAMTYzLmNvbQ==
†These authors have contributed equally to this work
 Jiyi Xie1,2†
Jiyi Xie1,2† Mitchell A. Sullivan
Mitchell A. Sullivan Bin Deng
Bin Deng Chen Shi
Chen Shi Yu Zhang
Yu Zhang