- 1Neurosurgical Clinic, AOUP “Paolo Giaccone”, Post Graduate Residency Program in Neurologic Surgery, Department of Biomedicine Neurosciences and Advanced Diagnostics, School of Medicine, University of Palermo, Palermo, Italy
- 2Department of Neurosurgery, Cannizzaro Hospital Trauma Center Gamma Knife Center, Catania, Italy
- 3Department of Neurosurgery, Highly Specialized Hospital and of National Importance “Garibaldi”, Catania, Italy
- 4Institute of Neurosurgery, Catholic University School of Medicine, Policlinico “A. Gemelli”, Rome, Italy
Spine is a frequent site of bone metastases, with a 8.5 months median survival time after diagnosis. In most cases treatment is only palliative. Several advanced techniques can ensure a better Quality of Life (QoL) and increase life expectancy. Radiofrequency ablation (RFA) uses alternating current to produce local heating and necrosis of the spinal lesion, preserving the healthy bone. RFA is supported by vertebral reinforcement through kyphoplasty and vertebroplasty in order to stabilize the fracture with polymethylmethacrylate (PMMA) injection, restoring vertebral body height and reducing the weakness of healthy bone. The aim of this study is to demonstrate the efficacy and advantages of RFA plus vertebral reinforcement through PMMA vertebroplasty and fixation in patients affected by bone spinal metastases. We retrospectively analyzed 54 patients with thoraco-lumbar metastatic vertebral fractures admitted to our Unit between January 2014 and June 2020. Each patient underwent RFA followed by PMMA vertebroplasty and transpedicle fixation. We evaluated pain relief through the Visual Analogue Scale (VAS) Score and PMMA vertebral filling based on the mean Saliou filling score. Analysis of variance (ANOVA) was used to test pain relief with statistical significance for p<0.05. A total of 54 patients (median age 63,44 years; range 34-86 years), with a total of 63 infiltrated vertebrae, were treated with RFA, PMMA vertebroplasty and transpedicular screw fixation; average operative time was 60.4 min (range 51–72). The preoperative average VAS score decreased significantly from 7.81 to 2.50 (p < 0.05) after 12 months. Based on Saliou filling score, filling was satisfactory (12–18) in 20 vertebrae (31,7%), mediocre (6–11) in 33 vertebrae (52,4%), inadequate (0–5) in 10 vertebrae (15,9%). A consistent PMMA filling of vertebral bodies was successfully achieved with significant pain relief. Concomitant RFA, PMMA vertebroplasty and pedicle screw fixation represent a safe and effective technique for the management of spinal metastases, improving clinical outcome and pain control.
Introduction
Spine is a frequent site of bone metastases. The high vascularity related to the Batson’s venous plexus and arterial spreads may contribute to metastatic localization (1, 2). In US, this is the second cause of death and the median overall survival after surgery is 8.5 months in patients with colon, breast, prostate, thyroid, renal cell, lung, and skin cancers. Until recent times, palliation is the aim of most treatments; nevertheless, several advanced techniques can ensure a better quality of life and increase life expectancy in patients with spinal metastases (3–7).
Radiofrequency ablation (RFA) uses alternating current to produce local heating and necrosis of the spinal lesion, while preserving the healthy bone. In 1931, RFA was used for the first time by Krischner to treat trigeminal neuralgia through thermocoagulation of gasserian ganglion. In late 1950s, Cosman and Aronow fine-tuned the first radiofrequency machine. Finally, RFA has been used by Rosenthal since 1992 as palliative treatment of bone metastases (8–11).
In several cases of metastatic lesion, pain can be caused by a combination of fractures and related vertebral instability, periosteal involvement, and pro-inflammatory cytokines, and pain relief is probably achieved by reduction of osteoclast activity and destruction of periosteal nerve endings (7, 12–15).
Percutaneous techniques, such as vertebroplasty, kyphoplasty and RFA achieve a key role in management of vertebral metastatic lesions (16–20).
This technique has been already performed to treat colon cancer, liver, and kidney tumors. However, vertebral RFA alone may increase the risk of fracture since it can weaken healthy bone. For this reason, this technique is usually supported by vertebral reinforcement through kyphoplasty and vertebroplasty, in order to stabilize the fracture with injection of bone cement (i.e. polymethylmethacrylate, PMMA), thus restoring vertebral height (1, 7, 21–23). The aim of this study is to demonstrate the efficacy and advantages of RFA plus vertebral fixation and a reinforcement through PMMA vertebroplasty in patients affected by spinal metastases and to demonstrate the relationship between vertebral restoration and pain relief.
Methods
From January 2014 to June 2020, a total of 54 patients (29 Males and 25 females) affected by thoraco-lumbar vertebral body metastases were admitted to the Department of Neurosurgery, BIND, University of Palermo (Italy) and they were retrospectively analyzed. All the patients underwent RFA plus vertebral body reinforcement for the treatment of vertebral metastases. In addition, pedicle screw fixation of the adjacent vertebrae was performed to restore stability.
Inclusion criteria were: Karnofsky score >= 60, unremitting thoraco-lumbar pain (VAS score >=5), osteolytic lesion on neuroimaging, unresecable tumors (according to Tokuhashi score), intractable pain with chemotherapy, radiation therapy and refractory to analgesic drugs. Exclusion criteria were: Karnofsky score <60, mild thoraco-lumbar pain (VAS <5), osteoblastic tumors on neuroimaging, general contraindications for surgery (infection, allergy, bleeding diseases), intradural and intramedullary tumors and neurological impairments caused by spinal metastasis itself. Patients’ demographics and relative comorbidities are summarized in Table 1.
Before the procedure, written informed consent was obtained from all patients. Each patient underwent radiofrequency ablation followed by PMMA vertebroplasty and subsequent open or percutaneous transpedicle screw fixation with or without posterior decompression during the same surgical procedure. RFA was performed with the STAR Tumor Ablation System (consisting of the SpineSTAR ablation instrument and the MetaSTAR generator; DFine, San Jose, CA, USA). The SpineSTAR is an articulated navigational bipolar radiofrequency electrode containing a pair of thermocouples positioned along the length of the electrode, 10 and 15 mm from the center of the ablation zone. This area is reached via a transpedicular approach, in order to avoid vascular injuries. The ablation zone has 3: 2 length-to-width aspect ratio, with a maximum ablated zone of 3 cm × 2 cm when the proximal thermocouple reaches 50 degrees Celsius. This system has been used to treat pedicles and anterior/posterior vertebral bodies metastasis especially thanks to the STAR probe that can be curved to reach different vertebral portions through the same entry point. The MetaSTAR generator continuously displays the temperature from the two thermocouples, in order to allow real-time monitoring of the peripheral edge of the ablation zone. After RFA, cement reinforcement (StabiliT Vertebral Augmentation System; DFine) was performed in all cases through the same working cannula. The entire procedure was performed under fluoroscopic guidance to evaluate cement distribution and screw trajectories.
In the first post-operative day, each patient performed a thin-slice CT scan. PMMA vertebral filling was evaluated using the mean Saliou filling score. Each vertebral body was divided into 18 3D equal portions (nine portions in AP projection, nine portions in LL projection). Each portion was considered filled if cement was visible inside. Filling score ranged from one to eighteen. According to Saliou et al., we considered the filling satisfactory if the score was more than twelve, mediocre when the score ranged from six to twelve, inadequate if the score was less than six (24).
We evaluated use of analgesic drugs (NSAIDS, Steroids, Opioids) and pain relief through Visual Analogic Scale score (0 to 10 ranged). The questionnaire was administered before surgery, one week, one, three, six months after surgical procedure.
Results
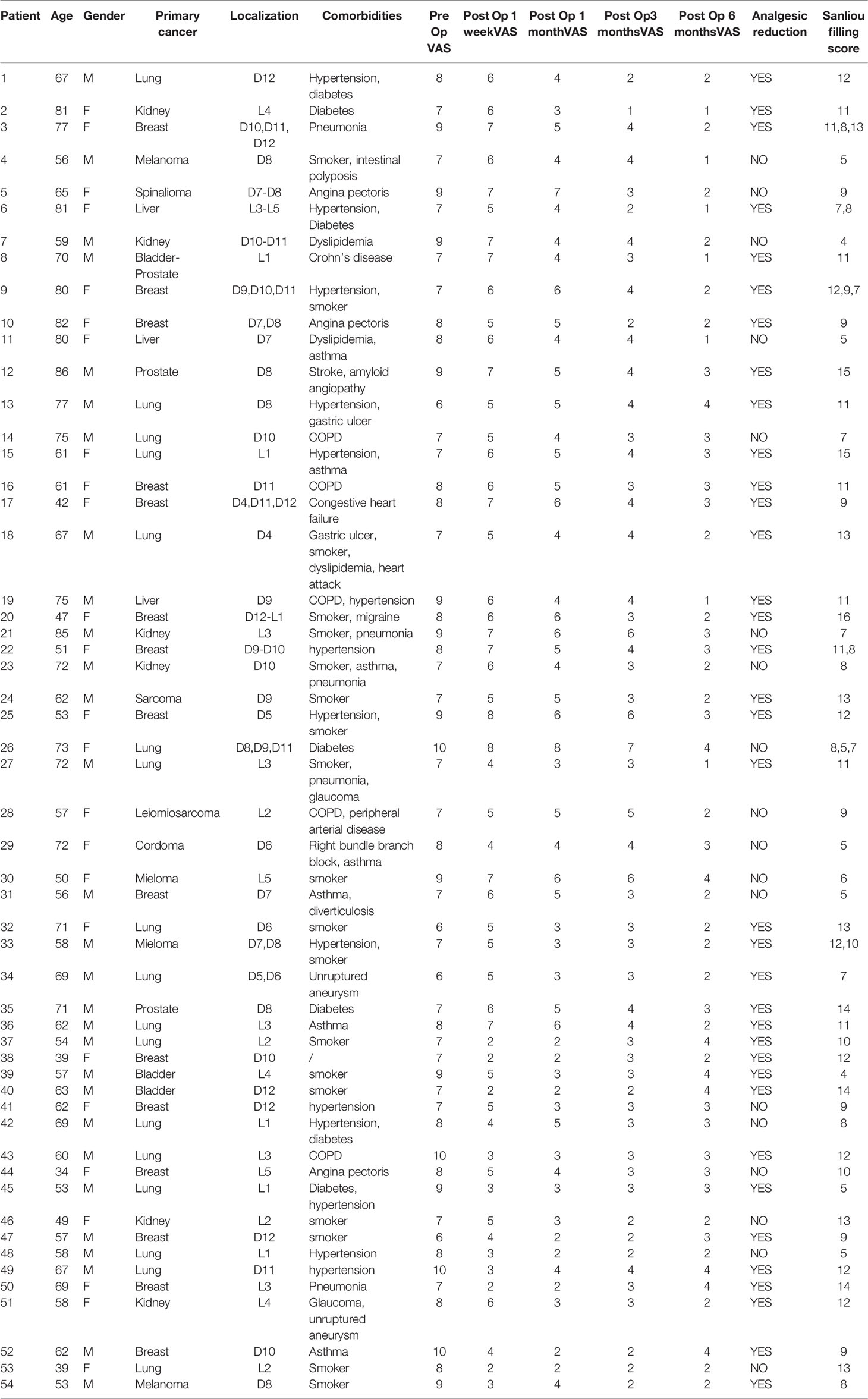
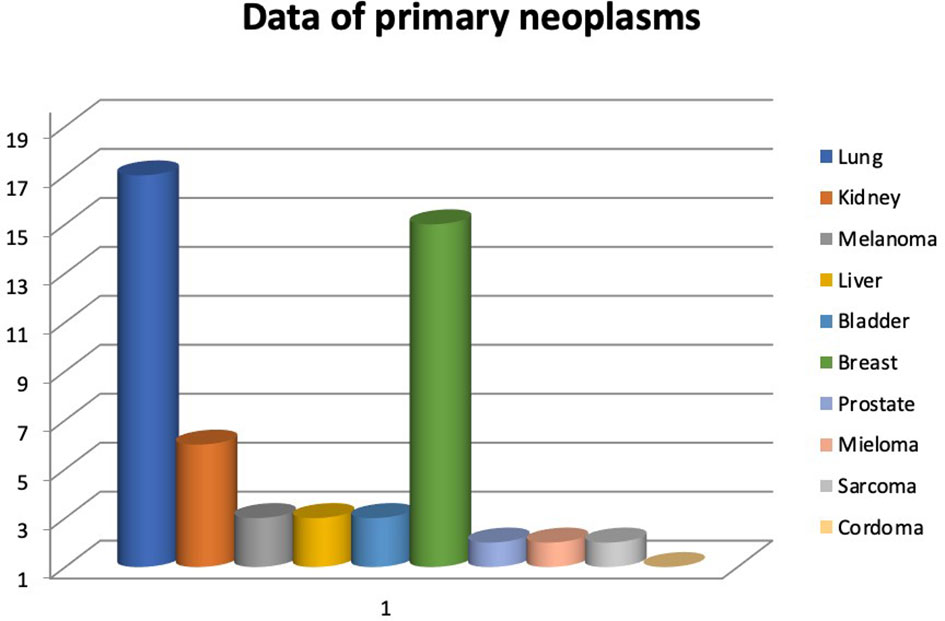
During the abovementioned time frame, 54 patients for a total of 63 vertebrae were treated. Median age was 63,44 years (range 34-86 years). The primary neoplasms sites associated to vertebral metastasis in order by frequency were lung, breast, kidney, melanoma, liver, bladder, prostate, multiple myeloma, sarcoma, chordoma (Figure 1). Levels of metastatic vertebrae were showed in Figure 2. The combination of radiofrequency ablation followed by vertebroplasty and subsequent open or percutaneous transpedicular screw fixation and posterior decompression was performed in 23 out of 54 patients (42.6%).
The most common patients’ comorbidities are hypertension (14%), diabetes (12,72%), smoking (34,54%), asthma(12,72%), pneumonia (9,09%), glaucoma (3,63%), Crohn’s disease (1,81%), abdominal unruptured aneurysm, Chronic obstructive pulmonary disease (COPD) (9,09%), gastric ulcer (3,63%), dyslipidemia (5,45%), cardiovascular disorders (12,72%), bowel disease (3,63%), amyloid angiopathy (1,81%), stroke (1,81%), migraine (1,81%). (Table 1)
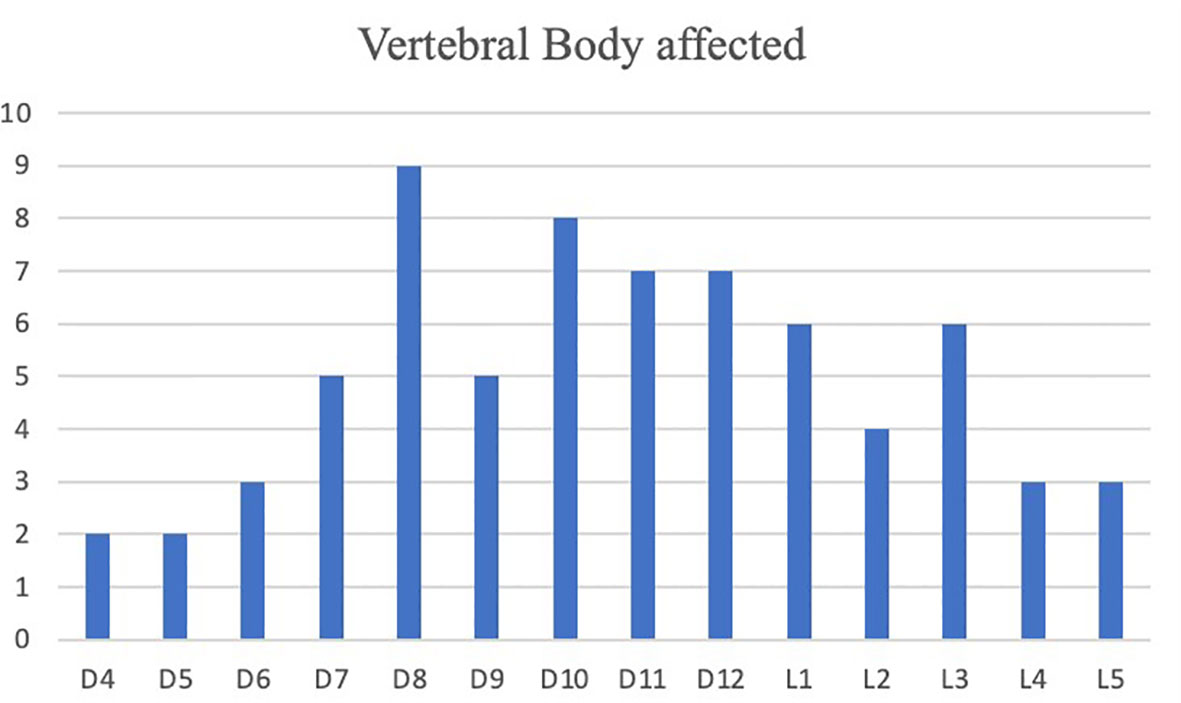
Surgical treatment had a significant impact on pain relief. Mean preoperative VAS score was 7.81/10 (range 6-10). A significant reduction of overall subjective pain perception was registered, with a persistent reduction in VAS score 1 week (5,16/10), 1 month (4,11/10), 3 months (3,35/10) and 6 months (2,50/10) respective after surgery. (Figure 3) These results, compared to preoperative data, suggest that our combined treatment had a significant impact on pain relief (p < 0.05), and in QoL as consequence. Intraoperative neuromonitoring (IONM) was performed during the entire procedure in order to avoid neurological complications. PMMA injection within the ablated cavity was monitored by continuous fluoroscopic guidance. After surgery, a thin-slice CT scan was performed, in order to evaluate cement distribution. According to the previously described radiological evaluation, Saliou filling score was satisfactory (12–18) in 20 vertebrae (31,7%), mediocre (6–11) in 33 vertebrae (52,4%), inadequate (0–5) in 10 vertebrae (15,9%). (Figure 4)
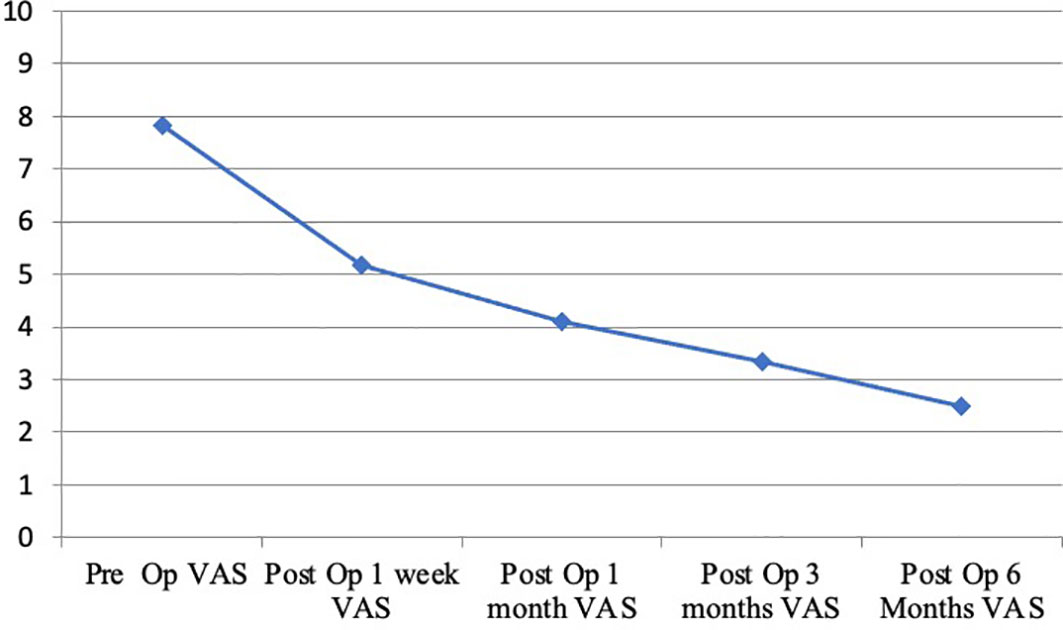
Figure 3 Trend of the visual analogue scale (VAS, 0 to 10 ranged) in patients with back pain during a six month follow-up.

Figure 4 Filling grade of treated vertebrae according to Saliou filling score (percentage of treated vertebrae).
A small amount of cement leakage into perivertebral tissues was found in 7 vertebrae (11,1%). However, no neurovascular structure was affected and no vascular or neurological injuries were recorded. Peri-operative mortality related to the surgical procedure was 0%.
Case Illustration
A 70-year-old male patient with a previous history of a prostatic carcinoma, was admitted to our department complaining intense low back pain (VAS score 7/10), which worsened two weeks before admission. A lumbar CT scan showed an osteolytic L1 vertebral body lesion. (Figures 5, 6) Therefore, he underwent surgery with L1 laminectomy and neural decompression, biopsy, RFA of L1 lesion with STAR Tumor Ablation System (DFine, San Jose, CA, USA) and T11-T12-L2 posterior thoraco-lumbar fixation. The post-operative course was uneventful, with a significant pain relief (VAS 7/10 1 week after procedure, 4/10 after 1 month, 3/10 after three months, 1/10 after six months). A post-operative thoracolumbar CT scan showed an average cement filling (11 Based on Saliou filling score) and correct screw placement. (Figures 7, 8).
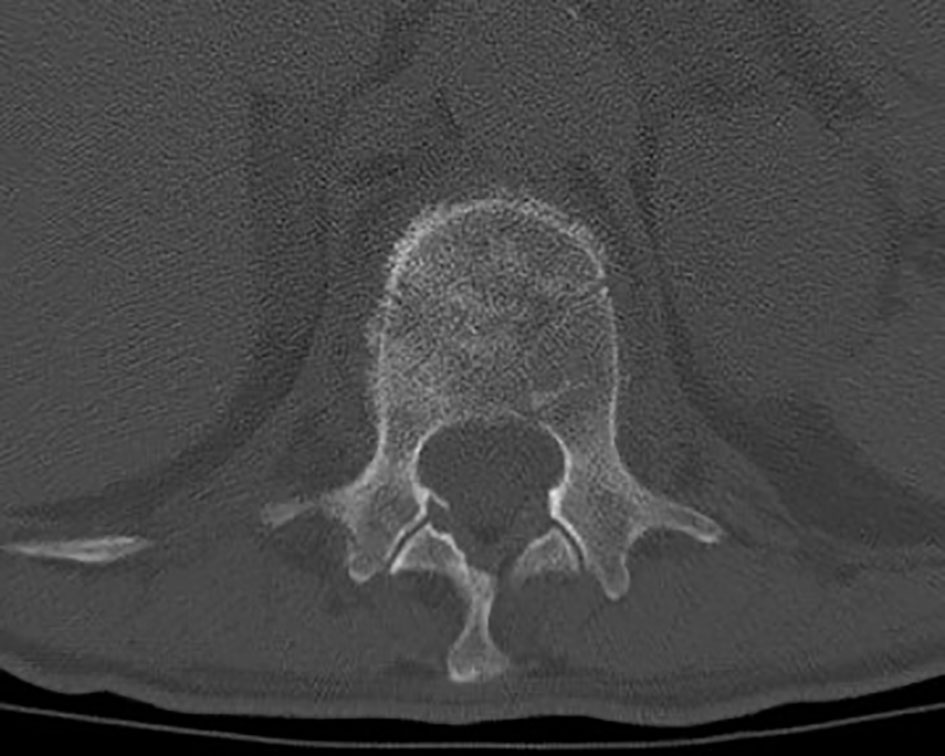
Figure 5 Axial thoraco-lumbar CT scan showing L1 metastatic lesion with altered bone density and osteolytic areas.
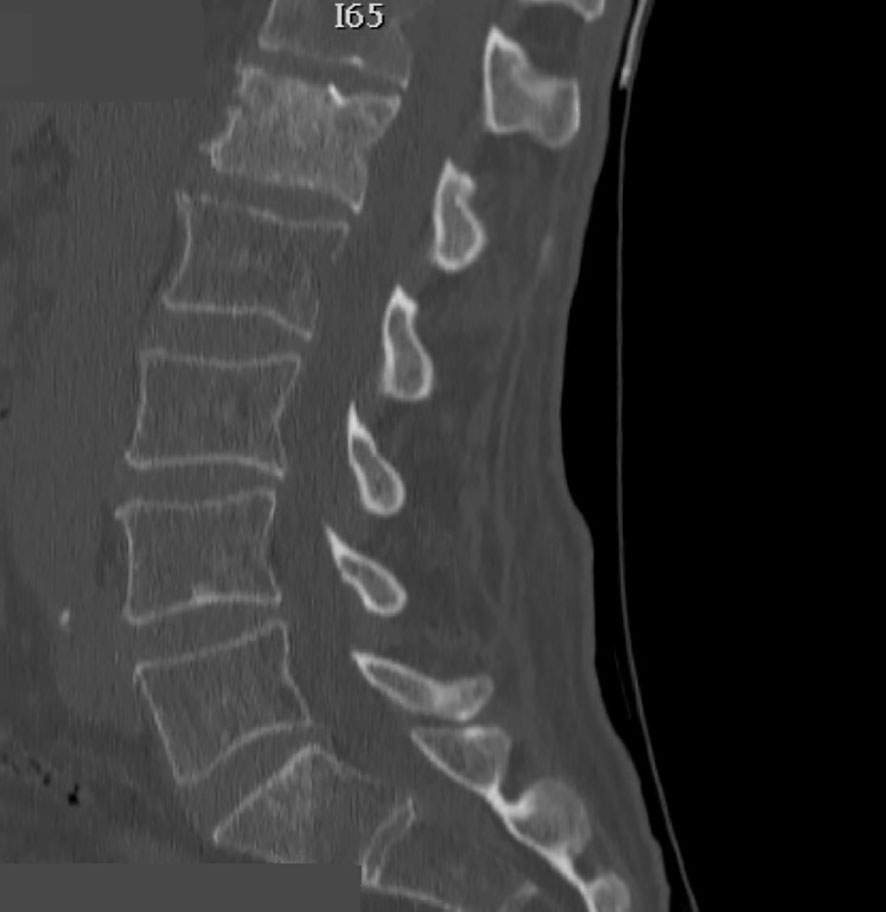
Figure 6 Sagittal thoraco-lumbar CT scan showing L1 metastatic lesion with altered bone density and osteolytic areas.
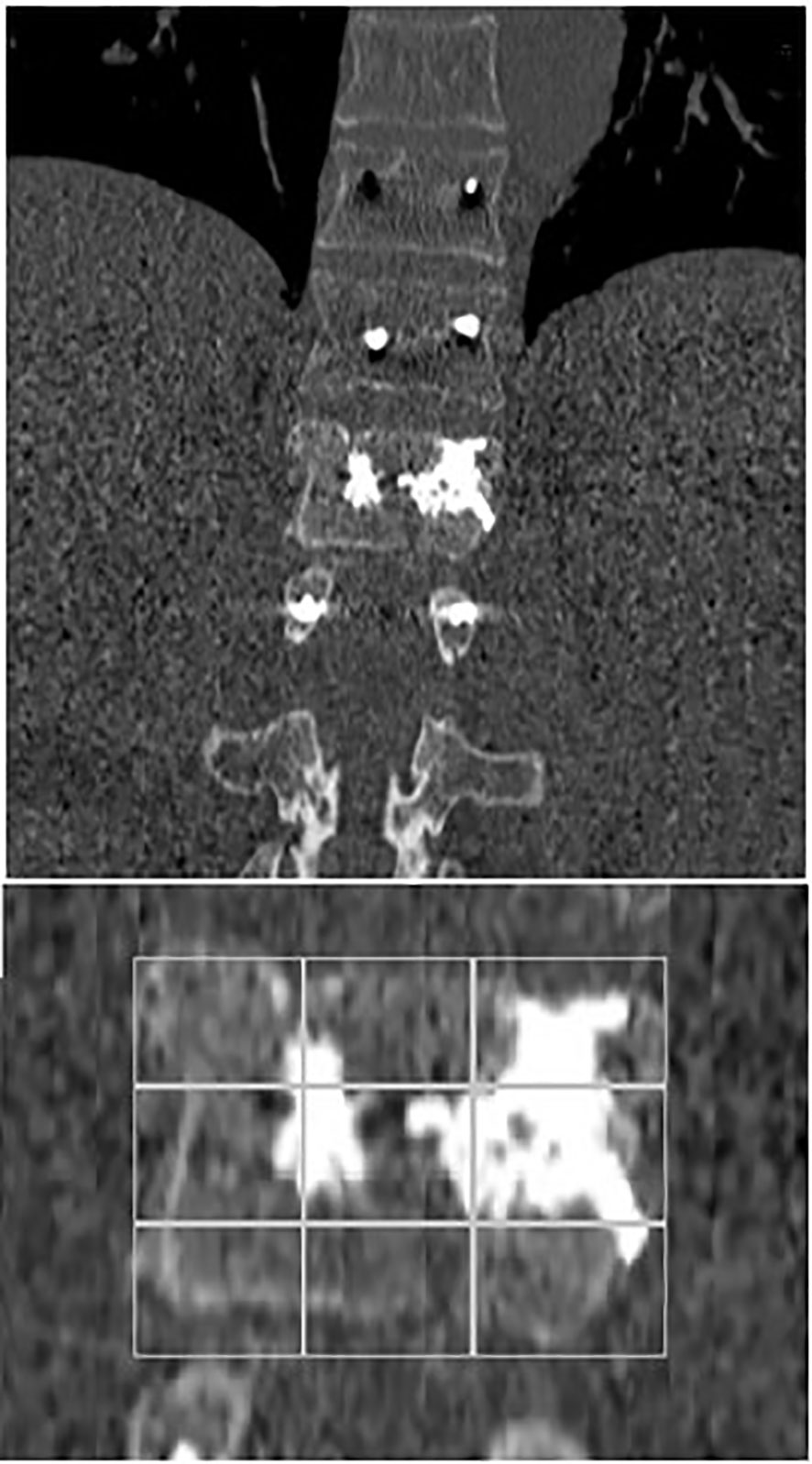
Figure 7 Coronal early post-operative CT scan showing an L1 average cement filling based on Saliou filling score 11/18 (6 + 5) and a T11-T12-L2 vertebral fixation.
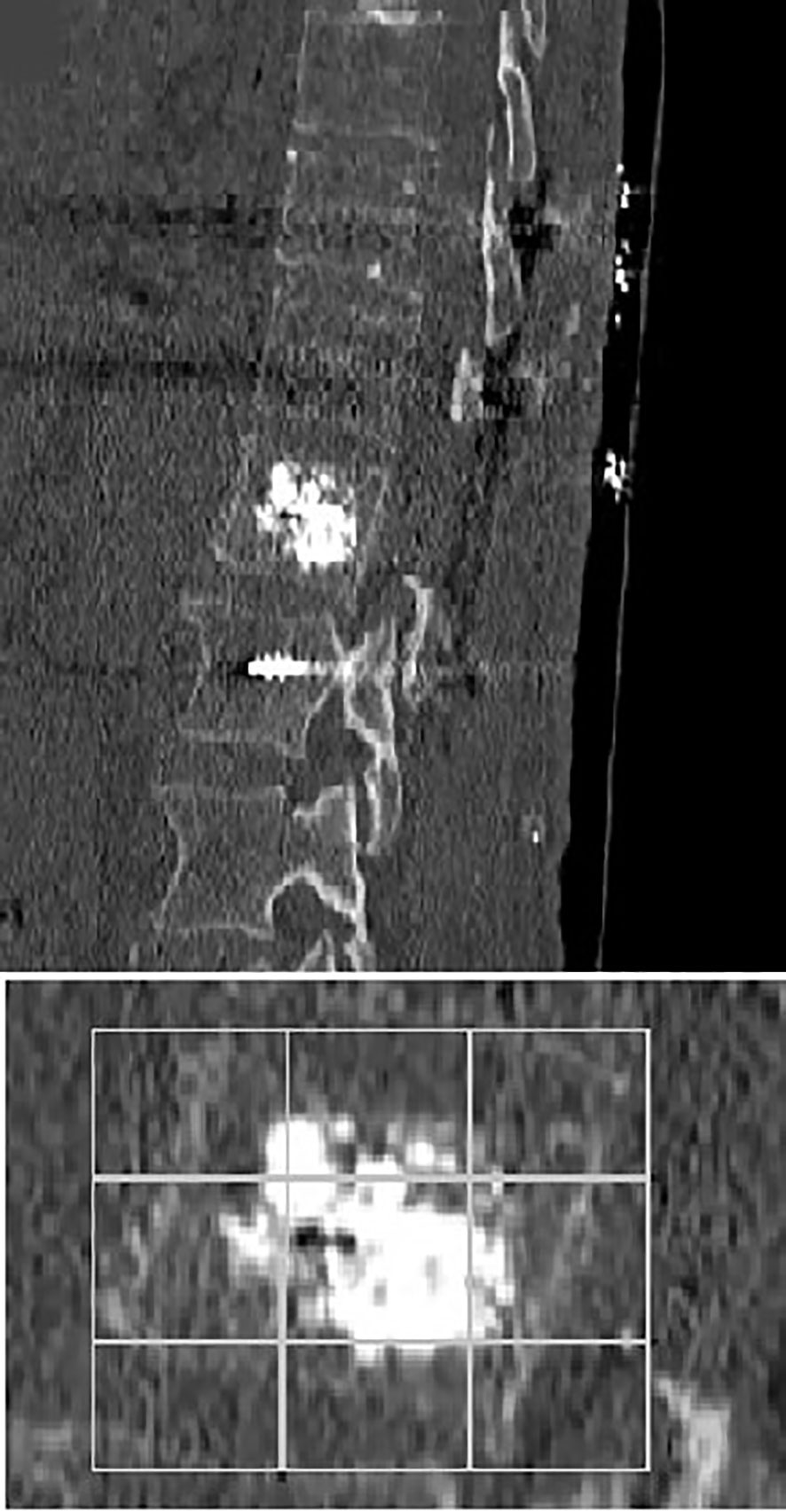
Figure 8 Sagittal early post-operative CT scan showing an L1 average cement filling based on Saliou filling score 11/18 (6 + 5) and a T11-T12-L2 vertebral fixation.
Discussion
RFA is currently used as one of the most safe and effective technique for bone metastases. This technique, based on the use of alternating current with a bipolar or monopolar device, provokes a coagulative necrosis of the tumoral lesion. Nevertheless, as a main consequence, this technique can weaken surrounding healthy bone. For this reason, the concomitant use of Polymethylmethacrylate (PMMA) guarantees a good result in terms of pain and stability. PMMA is placed with a needle in the affected vertebra and it acts as a “glue”: it fills the vertebra and then it is fixed through an exothermic reaction (25–27).
Pain relief is obtained through the techniques previously mentioned, with a lesser use of painkillers during follow up. Nerve fibers transmission, tumor cells and their microenvironment enriched by proinflammatory cytokines, osteoclast activity may together participate to the pathophysiology of metastatic pain. Since RFA interferes with these mechanisms, it has a positive effect on pain. In our cohort we obtained a harmless ablation through the use of an articulated electrode with two thermocouples and a continuous monitoring of local temperature and tissue impedance. In particular, mean VAS scores were at baseline (7,81/10), at 1 week (5,16/10), and at months 1(4,11/10), 3 (3,35/10), and 6 months (2,50/10) respectively; with an optimum decrease in post-operative course. As regards recent literature, our series was compared to the largest case series in order to evaluate the positive impact of RFA in relieving pain. (Table 2).
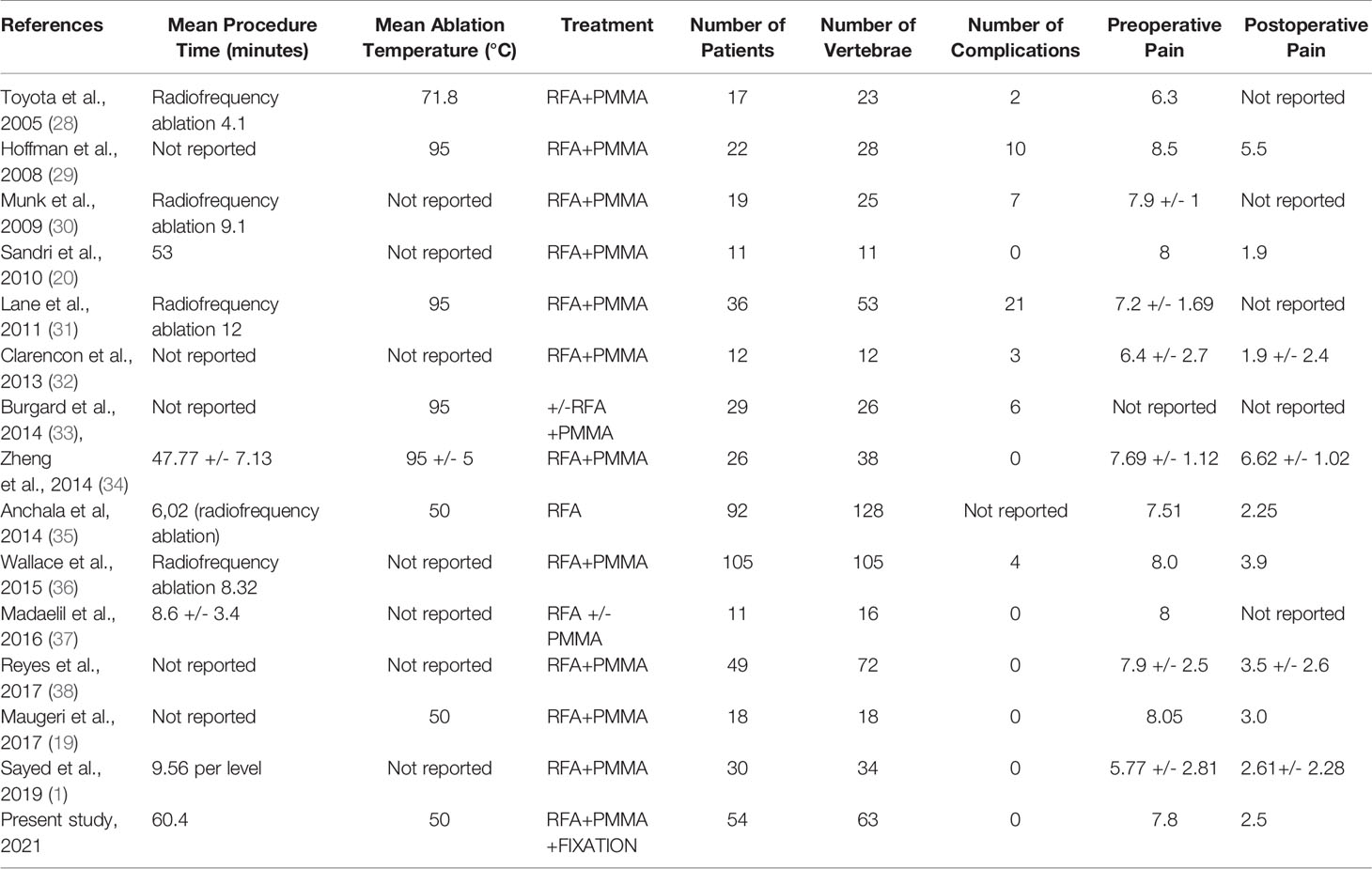
Table 2 Literature review of the largest reported clinical series of patients treated by RFA and PMMA vertebral reinforcement.
Indeed, Sandri et al. in their systematic review reported that 11 patients treated with RFA and vertebroplasty presented a significant pain relief (1, 20). Furthermore, in 2014, Anchala et al. reported a remarkable VAS score improvement in 92 patients with a total of 128 lesions treated using RFA with or without vertebral cement reinforcement (1, 35, 39). Schaefer et al. showed a case of a patient with an osteolytic lesion at L3 who was treated successfully with RFA and vertebroplasty in one step (19, 40–43).
Moreover, PMMA neurotoxicity could contribute to RFA painkiller effect since it could destroy nerves terminations and it gives a better stability to the spinal column at the same time (19, 20, 34, 44–46). As regard vertebral stability, our study analyzed PMMA filling of affected vertebrae based on Saliou filling score. Filling was considered satisfactory if the score was more than 12 (2/3 of the vertebra), mediocre when the score ranged from 6 to 12, and inadequate when the score was less than 6. After surgery, in order to evaluate if the procedure was performed correctly and with the proper amount of PMMA, a thin-slice CT scan was mandatory. Based on Saliou filling score was satisfactory (12–18) in 20 vertebrae (31,7%), mediocre (6–11) in 33 vertebrae (52,4%), inadequate (0–5) in 10 vertebrae (15,9%).
To our knowledge this is the first reported experience on combined RFA and vertebral reinforcement followed by transpedicular screw fixation. Cianfoni et al. reported a percutaneous technique called SAIF (stent screw–assisted internal fixation) following RFA and vertebral reinforcement that, through a minimal invasive procedure, ensures stability and height restoration in pathological and osteoporotic vertebral bodies. This is the unique study provided by the literature review that looks up to our same goal. Nevertheless, although this is an interesting and innovative procedure, transpedicular screw fixation represents a faster and more accessible technique to reach the same results (47–49).
In our single center experience, we noticed that, according to literature data about RFA plus vertebral reinforcement (Table 2), these three combined approaches guarantee a significant and more noticeable pain relief thus representing a good therapeutic compromise for patients’ treatment with metastatic spinal lesions. Moreover, a transpedicular screw fixation can be significant useful when spinal instability, as the patients included in our study, occurs (according to the spinal instability neoplastic score), guarantying not only a considerable pain relief but also a vertebral body reconstruction and subsequent vertebral stability.
This study has certainly some limitations. This is a retrospective analysis of a single medical center, without control group. Nevertheless, it shows an heterogenous group of patients with different vertebral locations and different clinic features. Further prospective studies with larger patient’s cohorts are mandatory to better evaluate the specific role of RFA procedure combined with vertebroplasty and followed by spinal fixation in the management of vertebral metastatic disease.
Conclusion
The combination of RFA, PMMA vertebroplasty and transpedicle screw fixation represents a safe and effective technique for the management of spinal metastases guarantying a good and long-lasting clinical outcome in terms of pain relief.
Data Availability Statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceptualization, GG, RC, and RM. Methodology, GG. Investigation, RC. Resources, UB, MP, and FP. Data curation, LBr, LBa, and CG. Writing—original draft preparation, RC and MAP. Writing—review and editing, GG, PP, and GS. Visualization, GU, DM, FG, and RG. Supervision, DI, MM, and RM. Project administration, DI. All authors have read and agreed to the published version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Sayed D, Jacobs D, Sowder T, Haines D, Orr W. Spinal Radiofrequency Ablation Combined With Cement Augmentation for Painful Spinal Vertebral Metastasis: A Single-Center Prospective Study. Pain Physician (2019) 22(5):E441–9. doi: 10.36076/ppj/2019.22.E441
2. Batson OV. The Function of the Vertebral Veins and Their Role in the Spread of Metastases. Ann Surg (1940) 112(1):138–49. doi: 10.1097/00000658-194007000-00016
3. Rothrock RJ, Barzilai O, Reiner AS, Lis E, Schmitt AM, Higginson DS, et al. Survival Trends After Surgery for Spinal Metastatic Tumors: 20-Year Cancer Center Experience. Neurosurg (2021) 88(2):402–12. doi: 10.1093/neuros/nyaa380
4. Lee SK, Weiss B, Yanamadala V, Brook A. Percutaneous Interventional Management of Spinal Metastasis. Semin Intervent Radiol (2019) 36(3):249–54. doi: 10.1055/s-0039-1694698
5. Wibmer C, Leithner A, Hofmann G, Clar H, Kapitan M, Berghold A, et al. Survival Analysis of 254 Patients After Manifestation of Spinal Metastases: Evaluation of Seven Preoperative Scoring Systems. Spine (Phila Pa 1976) (2011) 36(23):1977–86. doi: 10.1097/BRS.0b013e3182011f84
6. Tharmalingam S, Chow E, Harris K, Hird A, Sinclair E. Quality of Life Measurement in Bone Metastases: A Literature Review. J Pain Res (2008) 1:49–58. doi: 10.2147/jpr.s4572
7. Greif DN, Ghasem A, Butler A, Rivera S, Al Maaieh M, Conway SA. Multidisciplinary Management of Spinal Metastasis and Vertebral Instability: A Systematic Review. World Neurosurg (2019) 128:e944–55. doi: 10.1016/j.wneu.2019.05.042
8. Warfield C, Bajwa Z. Principles and Practice of Pain Medicine. New York (USA): Mcgraw-Hill (2004) p. 938.
10. Aronow S. The Use of Radio-Frequency Power in Making Lesions in the Brain. J Neurosurg (1960) 17:431–8. doi: 10.3171/jns.1960.17.3.0431
11. Rosenthal DI, Alexander A, Rosenberg AE, Springfield D. Ablation of Osteoid Osteomas With a Percutaneously Placed Electrode: A New Procedure. Radiol (1992) 183(1):29–33. doi: 10.1148/radiology.183.1.1549690
12. Stephenson MB, Glaenzer B, Malamis A. Percutaneous Minimally Invasive Techniques in the Treatment of Spinal Metastases. Curr Treat Options Oncol (2016) 17(11):56. doi: 10.1007/s11864-016-0433-1
13. Mannion RJ, Woolf CJ. Pain Mechanisms and Management: A Central Perspective. Clin J Pain (2000) 16(3 Suppl):S144–56. doi: 10.1097/00002508-200009001-00006
14. Goetz MP, Callstrom MR, Charboneau JW, Farrell MA, Maus TP, Welch TJ, et al. Percutaneous Image-Guided Radiofrequency Ablation of Painful Metastases Involving Bone: A Multicenter Study. J Clin Oncol (2004) 22(2):300–6. doi: 10.1200/JCO.2004.03.097
15. Kurup AN, Schmit GD, Morris JM, Atwell TD, Schmitz JJ, Weisbrod AJ, et al. Avoiding Complications in Bone and Soft Tissue Ablation. Cardiovasc Intervent Radiol (2017) 40(2):166–76. doi: 10.1007/s00270-016-1487-y
16. Maugeri R, Giugno A, Giammalva RG, Gulì C, Basile L, Graziano F, et al. A Thoracic Vertebral Localization of a Metastasized Cutaneous Merkel Cell Carcinoma: Case Report and Review of Literature. Surg Neurol Int (2017) 8:190. doi: 10.4103/sni.sni_70_17
17. Ponzo G, Umana GE, Giuffrida M, Furnari M, Nicoletti GF, Scalia G. Intramedullary Craniovertebral Junction Metastasis Leading to the Diagnosis of Underlying Renal Cell Carcinoma. Surg Neurol Int (2020) 11:152. doi: 10.25259/SNI_259_2020
18. Ponzo G, Umana GE, Valastro M, Giuffrida M, Tranchina MG, Nicoletti GF, et al. Cervical Radiculopathy as First Presentation of CD3-Positive Diffuse Large B-Cell Lymphoma of the Cervico-Thoracic Junction. Br J Neurosurg (2020) 5:1–4. doi: 10.1080/02688697.2020.1828278
19. Maugeri R, Graziano F, Basile L, Gulì C, Giugno A, Giammalva GR, et al. Reconstruction of Vertebral Body After Radiofrequency Ablation and Augmentation in Dorsolumbar Metastatic Vertebral Fracture: Analysis of Clinical and Radiological Outcome in a Clinical Series of 18 Patients. Acta Neurochir Suppl (2017) 124:81–6. doi: 10.1007/978-3-319-39546-3_13
20. Sandri A, Carbognin G, Regis D, Gaspari D, Calciolari C, Girardi V, et al. Combined Radiofrequency and Kyphoplasty in Painful Osteolytic Metastases to Vertebral Bodies. Radiol Med (2010) 115(2):261–71. doi: 10.1007/s11547-009-0431-5
21. Feng S, Wang L, Xiao Z, Maharjan R, Chuanxing L, Fujun Z, et al. 125i Seed Implant Brachytherapy for Painful Bone Metastases After Failure of External Beam Radiation Therapy. Med (Baltimore) (2015) 94(31):e1253. doi: 10.1097/MD.0000000000001253
22. Jakobs TF, Hoffmann RT, Trumm C, Reiser MF, Helmberger TK. Radiofrequency Ablation of Colorectal Liver Metastases: Mid-Term Results in 68 Patients. Anticancer Res (2006) 26(1B):671–80.
23. Lencioni R, Della Pina C, Bartolozzi C. Percutaneous Image-Guided Radiofrequency Ablation in the Therapeutic Management of Hepatocellular Carcinoma. Abdom Imaging (2005) 30(4):401–8. doi: 10.1007/s00261-004-0254-8
24. Saliou G, Kocheida el M, Lehmann P, Depriester C, Paradot G, Le Gars D, et al. Percutaneous Vertebroplasty for Pain Management in Malignant Fractures of the Spine With Epidural Involvement. Radiol (2010) 254(3):882–90. doi: 10.1148/radiol.09081698
25. Maugeri R, Giammalva GR, Graziano F, Iacopino DG. May Autologue Fibrin Glue Alone Enhance Ossification? An Unexpected Spinal Fusion. World Neurosurg (2016) 95:611–2. doi: 10.1016/j.wneu.2016.06.128
26. Saravana-Bawan S, David E, Sahgal A, Chow E. Palliation of Bone Metastases-Exploring Options Beyond Radiotherapy. Ann Palliat Med (2019) 8(2):168–77. doi: 10.21037/apm.2018.12.04
27. Weill A, Chiras J, Simon JM, Rose M, Sola-Martinez T, Enkaoua E. Spinal Metastases: Indications for and Results of Percutaneous Injection of Acrylic Surgical Cement. Radiol (1996) 199(1):241–7. doi: 10.1148/radiology.199.1.8633152
28. Toyota N, Naito A, Kakizawa H, Hieda M, Hirai N, Tachikake T, et al. Radiofrequency Ablation Therapy Combined With Cementoplasty for Painful Bone Metastases: Initial Experience. Cardiovasc Intervent Radiol (2005) 28(5):578–83. doi: 10.1007/s00270-004-0208-0
29. Hoffmann RT, Jakobs TF, Trumm C, Weber C, Helmberger TK, Reiser MF. Radiofrequency Ablation in Combination With Osteoplasty in the Treatment of Painful Metastatic Bone Disease. J Vasc Interv Radiol (2008) 19(3):419–25. doi: 10.1016/j.jvir.2007.09.016
30. Munk PL, Rashid F, Heran MK, Papirny M, Liu DM, Malfair D, et al. Combined Cementoplasty and Radiofrequency Ablation in the Treatment of Painful Neoplastic Lesions of Bone. J Vasc Interv Radiol (2009) 20(7):903–11. doi: 10.1016/j.jvir.2009.03.035
31. Lane MD, Le HB, Lee S, Young C, Heran MK, Badii M, et al. Combination Radiofrequency Ablation and Cementoplasty for Palliative Treatment of Painful Neoplastic Bone Metastasis: Experience With 53 Treated Lesions in 36 Patients. Skeletal Radiol (2011) 40(1):25–32. doi: 10.1007/s00256-010-1010-5
32. Clarençon F, Jean B, Pham HP, Cormier E, Bensimon G, Rose M, et al. Value of Percutaneous Radiofrequency Ablation With or Without Percutaneous Vertebroplasty for Pain Relief and Functional Recovery in Painful Bone Metastases. Skeletal Radiol (2013) 42(1):25–36. doi: 10.1007/s00256-011-1294-0
33. Burgard CA, Dinkel J, Strobl F, Paprottka PM, Schramm N, Reiser M, et al. CT Fluoroscopy-Guided Percutaneous Osteoplasty With or Without Radiofrequency Ablation in the Treatment of Painful Extraspinal and Spinal Bone Metastases: Technical Outcome and Complications in 29 Patients. Diagn Interv Radiol (2018) 24(3):158–65. doi: 10.5152/dir.2018.17265
34. Zheng L, Chen Z, Sun M, Zeng H, Zuo D, Hua Y, et al. A Preliminary Study of the Safety and Efficacy of Radiofrequency Ablation With Percutaneous Kyphoplasty for Thoracolumbar Vertebral Metastatic Tumor Treatment. Med Sci Monit (2014) 20:556–63. doi: 10.12659/MSM.889742
35. Anchala PR, Irving WD, Hillen TJ, Friedman MV, Georgy BA, Coldwell DM, et al. Treatment of Metastatic Spinal Lesions With a Navigational Bipolar Radiofrequency Ablation Device: A Multicenter Retrospective Study. Pain Physician (2014) 17(4):317–27.
36. Wallace AN, Greenwood TJ, Jennings JW. Radiofrequency Ablation and Vertebral Augmentation for Palliation of Painful Spinal Metastases. J Neurooncol (2015) 124(1):111–8. doi: 10.1007/s11060-015-1813-2
37. Madaelil TP, Wallace AN, Jennings JW. Radiofrequency Ablation Alone or in Combination With Cementoplasty for Local Control and Pain Palliation of Sacral Metastases: Preliminary Results in 11 Patients. Skeletal Radiol (2016) 45(9):1213–9. doi: 10.1007/s00256-016-2404-9
38. Reyes M, Georgy M, Brook L, Ortiz O, Brook A, Agarwal V, et al. Multicenter Clinical and Imaging Evaluation of Targeted Radiofrequency Ablation (T-RFA) and Cement Augmentation of Neoplastic Vertebral Lesions. J Neurointerv Surg (2018) 10(2):176–82. doi: 10.1136/neurintsurg-2016-012908
39. Sutcliffe P, Connock M, Shyangdan D, Court R, Kandala NB, Clarke A. A Systematic Review of Evidence on Malignant Spinal Metastases: Natural History and Technologies for Identifying Patients at High Risk of Vertebral Fracture and Spinal Cord Compression. Health Technol Assess (2013) 17(42):1–274. doi: 10.3310/hta17420
40. Halpin RJ, Bendok BR, Sato KT, Liu JC, Patel JD, Rosen ST. Combination Treatment of Vertebral Metastases Using Image-Guided Percutaneous Radiofrequency Ablation and Vertebroplasty: A Case Report. Surg Neurol (2005) 63(5):469–74; discussion 474-5. doi: 10.1016/j.surneu.2004.04.025
41. Scibilia A, Terranova C, Rizzo V, Raffa G, Morelli A, Esposito F, et al. Intraoperative Neurophysiological Mapping and Monitoring in Spinal Tumor Surgery: Sirens or Indispensable Tools? Neurosurg Focus (2016) 41(2):E18. doi: 10.3171/2016.5.FOCUS16141
42. Leung OC, Poon WL, Nyaw SF, Luk SH. Percutaneous Cementoplasty of Osteolytic Metastases Induces Immediate and Long-Lasting Pain Relief in Oncological Patients. Hong Kong Med J (2013) 19(4):317–22. doi: 10.12809/hkmj133743
43. Scibilia A, Raffa G, Rizzo V, Quartarone A, Visocchi M, Germanò A, et al. Intraoperative Neurophysiological Monitoring in Spine Surgery: A Significant Tool for Neuronal Protection and Functional Restoration. Acta Neurochir Suppl (2017) 124:263–70. doi: 10.1007/978-3-319-39546-3_38
44. Schaefer O, Lohrmann C, Herling M, Uhrmeister P, Langer M. Combined Radiofrequency Thermal Ablation and Percutaneous Cementoplasty Treatment of a Pathologic Fracture. J Vasc Interv Radiol (2002) 13(10):1047–50. doi: 10.1016/s1051-0443(07)61872-7
45. Thanos L, Mylona S, Galani P, Tzavoulis D, Kalioras V, Tanteles S, et al. Radiofrequency Ablation of Osseous Metastases for the Palliation of Pain. Skeletal Radiol (2008) 37(3):189–94. doi: 10.1007/s00256-007-0404-5
46. Maugeri R, Basile L, Gulì C, Banco A, Giordano G, Giugno A, et al. Percutaneous Pedicle-Lengthening Osteotomy in Minimal Invasive Spinal Surgery to Treat Degenerative Lumbar Spinal Stenosis: A Single-Center Preliminary Experience. J Neurol Surg A Cent Eur Neurosurg (2018) 79(5):365–71. doi: 10.1055/s-0038-1641148
47. Cianfoni A, Distefano D, Isalberti M, Reinert M, Scarone P, Kuhlen D, et al. Stent-Screw-Assisted Internal Fixation: The SAIF Technique to Augment Severe Osteoporotic and Neoplastic Vertebral Body Fractures. J Neurointerv Surg (2019) 11(6):603–9. doi: 10.1136/neurintsurg-2018-014481
48. San Millán D. Letter to the Editor. Stent Screw-Assisted Internal Fixation and Combined Radiofrequency Ablation and Vertebroplasty for Stabilization and Local Tumor Control. J Neurosurg Spine (2020) 10:1–3. doi: 10.3171/2020.1.SPINE2053
Keywords: RFA, PMMA, spinal metastases, spinal fixation, vertebral reinforcement
Citation: Giammalva GR, Costanzo R, Paolini F, Benigno UE, Porzio M, Brunasso L, Basile L, Gulì C, Pino MA, Gerardi RM, Messina D, Umana GE, Palmisciano P, Scalia G, Graziano F, Visocchi M, Iacopino DG and Maugeri R (2022) Management of Spinal Bone Metastases With Radiofrequency Ablation, Vertebral Reinforcement and Transpedicular Fixation: A Retrospective Single-Center Case Series. Front. Oncol. 11:818760. doi: 10.3389/fonc.2021.818760
Received: 19 November 2021; Accepted: 28 December 2021;
Published: 21 January 2022.
Edited by:
Alfredo Conti, University of Bologna, ItalyReviewed by:
Fei-Long Wei, Fourth Military Medical University (Air Force Medical University), ChinaAthanasios G. Zafeirakis, Army Share Fund Hospital (NIMTS), Greece
Copyright © 2022 Giammalva, Costanzo, Paolini, Benigno, Porzio, Brunasso, Basile, Gulì, Pino, Gerardi, Messina, Umana, Palmisciano, Scalia, Graziano, Visocchi, Iacopino and Maugeri. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rosario Maugeri, cm9zYXJpby5tYXVnZXJpMTk3N0BnbWFpbC5jb20=
†These authors have contributed equally to this work
 Giuseppe Roberto Giammalva
Giuseppe Roberto Giammalva Roberta Costanzo
Roberta Costanzo Federica Paolini
Federica Paolini Umberto Emanuele Benigno1
Umberto Emanuele Benigno1 Lara Brunasso
Lara Brunasso Giuseppe Emmanuele Umana
Giuseppe Emmanuele Umana Paolo Palmisciano
Paolo Palmisciano Gianluca Scalia
Gianluca Scalia Massimiliano Visocchi
Massimiliano Visocchi Domenico Gerardo Iacopino
Domenico Gerardo Iacopino Rosario Maugeri
Rosario Maugeri