- 1Department of Radiation Oncology, The Third Hospital of Nanchang, Nanchang, China
- 2Department of Breast surgery, The Second Affiliated Hospital of Nanchang University, Nanchang, China
- 3Jiangxi Medical College, Nanchang University, Nanchang, China
- 4Department of Oncology, The Second Affiliated Hospital of Nanchang University, Nanchang, China
- 5Department of Cardiovascular Medicine, The Second Affiliated Hospital of Nanchang University, Nanchang, China
- 6Department of Thoracic Surgery, The Second Affiliated Hospital of Nanchang University, Nanchang, China
Background: It is still controversial whether immune checkpoint inhibitors (ICIs) can improve the curative effect when added to original standard chemotherapy treatment for triple-negative breast cancer (TNBC). We compared their antitumor efficacy and adverse effects (AEs) to make a better clinical decision.
Methods: Seven databases were searched for eligible articles. Progression-free survival (PFS), overall survival (OS), and AEs were measured as the primary outcomes.
Results: Nine randomized controlled trials (RCTs) involving 4,501 patients were included. ICI+chemotherapy treatment achieved better PFS (hazard ratio [HR]: 0.78, [0.70–0.86], p < 0.00001), OS (HR: 0.86, [0.74–0.99], p = 0.04), and complete response (584/1,106 vs. 341/825, risk ratio [RR]: 1.38, [1.01–1.89], p = 0.04). With the prolongation of survival, the survival advantage of ICI+chemotherapy increased compared with chemotherapy. Subgroup analysis suggested that the addition of ICIs might not have a better effect in Asian patients, patients with locally advanced disease, or patients with brain metastases. In the toxicity analysis, more Grade 3–5 AEs and serious AEs were found in the ICI+chemotherapy group. For Grade 3–5 AEs, more cases of diarrhea, severe skin reactions, pneumonitis, hepatitis, and adrenal insufficiency were related to the ICI+chemotherapy group.
Conclusions: ICI+chemotherapy appears to be better than chemotherapy alone for TNBC treatment, with better OS and PFS. However, its high rates of serious AEs need to be taken seriously.
Systematic Review Registration: PROSPERO Registration: CRD42021276394.
Introduction
In recent years, breast cancer has been the most common malignancy in women (1). As one of the major subtypes (15–20%), triple-negative breast cancer (TNBC) has the worst prognosis (2). In clinical practice, chemotherapy remains the standard of care (not only in neoadjuvant therapy but also in radical drug therapy) for patients with TNBC (3). However, its poor survival efficacy is not satisfactory for patients and doctors. In recent years, immune checkpoint inhibitors (ICIs) have been incorporated into cancer treatment, and their efficacy has been proven in lung cancer, hepatocellular carcinoma, and gastric cancer (4–6). However, whether ICIs can improve the curative effect when added to original standard chemotherapy treatment for TNBC is still controversial.
In the updated guidelines, ICIs+chemotherapy has been recognized as one of the treatment options for TNBC (7, 8). The KEYNOTE-522 and IMpassion130 trials compared ICIs+chemotherapy with chemotherapy in 2,076 patients with TNBC and suggested that combination therapy prolonged progression-free survival (PFS) and increased the rates of pathological complete response (PCR) (9, 10). Similar results were confirmed by 4 other randomized controlled trials (RCTs) (11–15). However, Bachelot et al.’s, Brufsky et al.’s, and Tolaney et al.’s studies reported that ICIs+chemotherapy could not improve the survival of patients but will cause more adverse effects (AEs) and reduce the quality of life of patients (16–18).
Hence, this meta-analysis of RCTs aimed to compare the efficacy and safety between ICIs+chemotherapy and chemotherapy for TNBC.
Materials and Methods
We conducted this study according to the Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines (PRISMA) (Table S1) (19). (PROSPERO Registration: CRD42021276394)?.
Search Strategy
Studies were retrieved from the following databases: The Cochrane Library, PubMed, Web of Science, Scopus, EMBASE, ScienceDirect, and Ovid MEDLINE. Studies were retrieval time from inception to May 5, 2021. The MeSH terms and keywords were “Breast cancer”, “Immune checkpoint inhibitors”, and “Chemotherapy”. The search details are included in Table S2.
Selection Criteria
The inclusion criteria were as follows: (1) RCTs published in English comparing ICIs+chemotherapy with chemotherapy alone; (2) studies that enrolled patients with TNBC; and (3) the outcomes included survival indicators (OS and PFS), drug responses, and AEs.
The exclusion criteria were as follows: (1) animal studies; (2) meta-analyses and reviews; (3) conference articles; (4) case reports; and (5) studies that did not only enroll patients with TNBC.
Data Extraction
Two investigators extracted the following information independently: the publication year, first author, participant characteristics (quantity, age, etc.), tumor characteristics (histopathology, stage, etc.), antitumor efficacy (OS, PFS, etc.), and number of AEs. All disagreements were resolved by a third investigator.
Outcome Assessments
PFS and OS were the primary outcomes. The subgroup analysis of OS was performed according to age, race, Eastern Cooperative Oncology Group (ECOG) performance status, baseline disease status, metastatic sites, PD-L1 status, neoadjuvant therapy, homologous recombination deficiency (HRD), metastases (brain, bone, liver, or lung), lymph node-only disease, and previous treatment (chemotherapy, taxane, or anthracycline).
Quality Assessment
We assessed the quality of the included RCTs by using the Cochrane Risk of Bias Tool (CRBT) (20) and 5-point Jadad scale (21). We assessed the quality of the results by using the Grading of Recommendations Assessment, Development and Evaluation (GRADE) system (22).
Statistical Analysis
When analyzing survival outcomes (PFS, OS, etc.), we used hazard ratios (HRs). When analyzing dichotomous variables (PFSR, OSR, complete response [CR], AEs, etc.), we used risk ratios (RRs). Heterogeneity was evaluated by the I2 statistic and χ2 test. A random-effects model was used when heterogeneity was significant (I2 < 50% or p > 0.1); otherwise, a fixed-effects model was used. Publication bias was evaluated through visual inspection of the funnel plots. A P < 0.05 was identified as statistically significant. All analyses were performed using Review Manager 5.3 and SPSS 18.0.
Results
Search Results
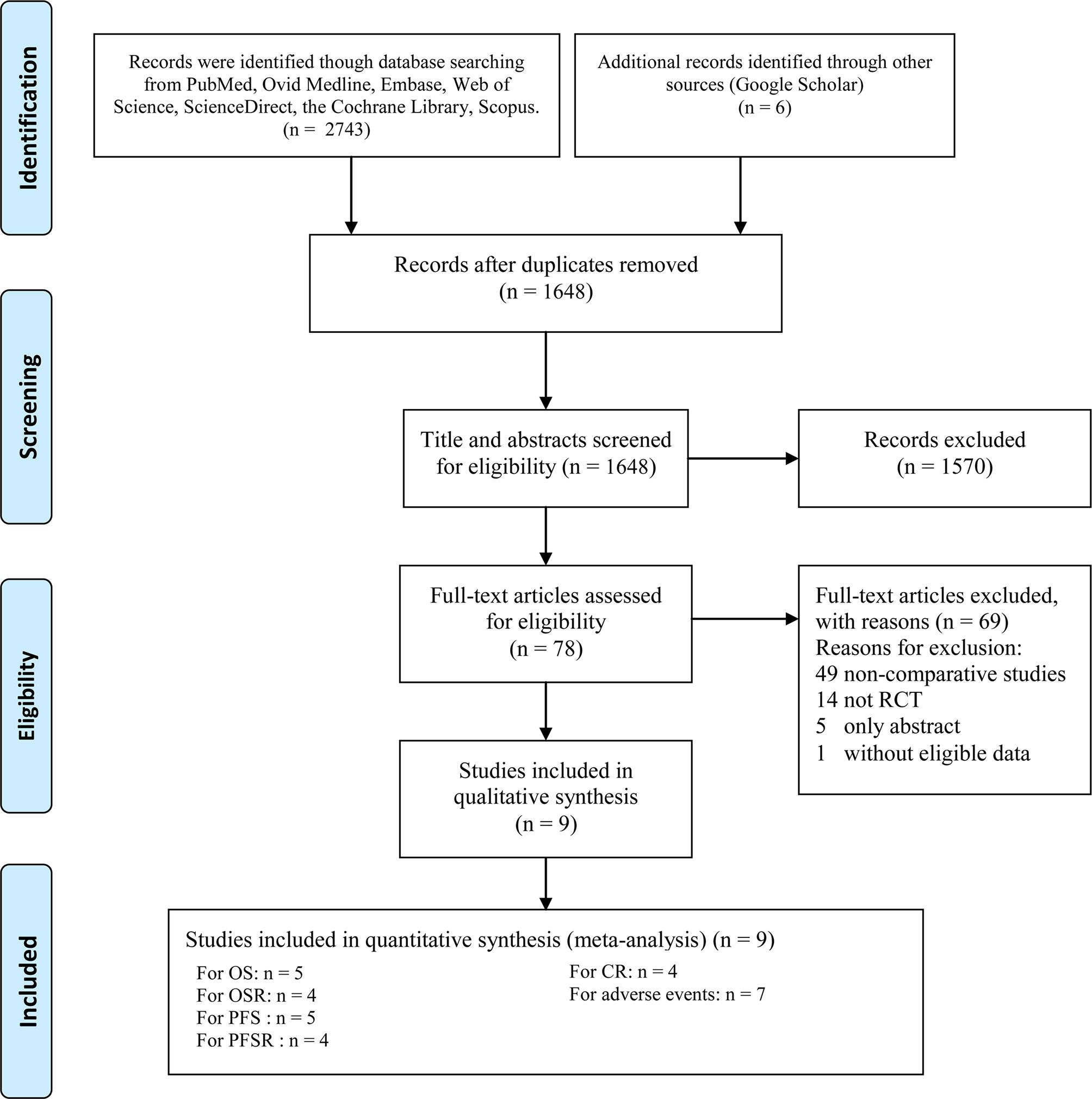
Nine RCTs involving 4,501 patients (2,645 patients in the ICI+chemotherapy group and 1,856 patients in the chemotherapy group) were included (9–16, 18) (Figure 1). Two studies (14, 16) were conducted in Europe, one (18) was conducted in the USA, and the other five studies were global studies (9–13). The essential information of the included studies is summarized in Table 1. According to the Jadad scale (Table S3) and CRBT (Figure S1), all eight RCTs were of high quality. According to the GRADE system, the evidence grades of all the results were medium-high.
Antitumor Efficacy
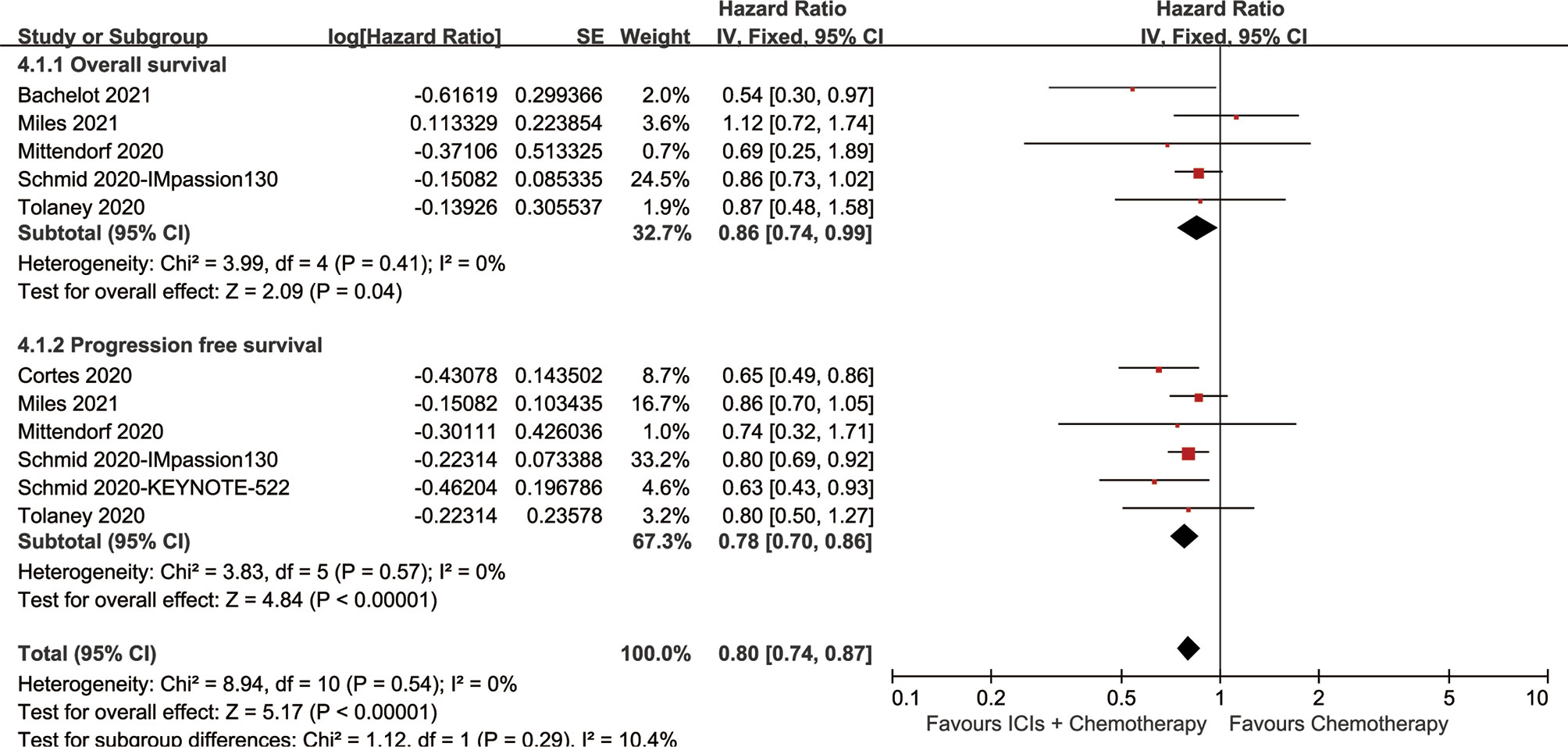
The ICI+chemotherapy group achieved better OS than the chemotherapy group (HR: 0.86, [0.74–0.99], p = 0.04; Figure 2). At all points in time, the overall survival rate (OSR) tended to favor the ICI+chemotherapy group (OSR-6 m, RR: 1.17, [1.13–1.21], p < 0.00001; OSR-12 m, RR: 1.08, [1.00–1.17], p = 0.05; OSR-18 m, RR: 1.11, [0.99–1.24], p = 0.07; OSR-24 m, RR: 1.12, [0.97–1.30], p = 0.13; OSR-30 m, RR: 1.20, [1.00–1.44], p = 0.04; OSR-36 m, RR: 1.33, [1.06–1.67], p = 0.01, Figure S2). With prolonged survival time, ICI+chemotherapy had an increasing advantage for OS (Figures 3A, B).
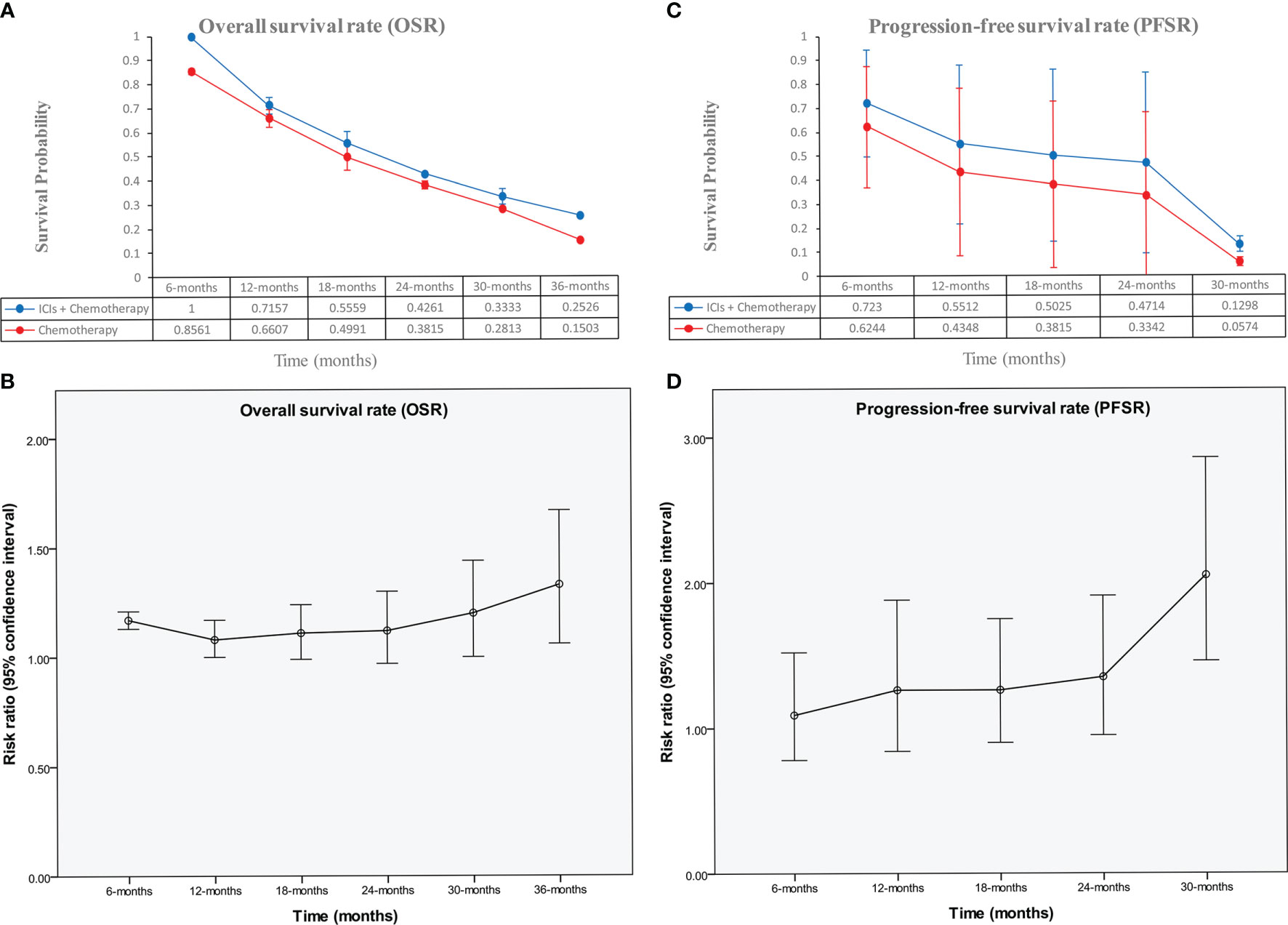
Figure 3 Comparisons of OSR (6–36 months, A, B), and PFSR (6–30 months, C, D) associated with ICIs+Chemotherapy versus Chemotherapy according to survival time.
The ICI+chemotherapy group achieved better PFS than the chemotherapy group (HR: 0.78, [0.70–0.86], p < 0.00001; Figure 2). At all points in time, the progression-free survival rate (PFSR) significantly favored the ICIs+Chemotherapy group (PFSR-6 m, RR: 1.09, [0.78–0.1.52], p = 62; PFSR-12 m, RR: 1.26, [0.84–1.88], p = 0.27; PFSR-18 m, RR: 1.26, [0.90–1.75], p = 0.18; PFSR-24 m, RR: 1.35, [0.95–1.91], p = 0.10; PFSR-30 m, RR: 0.2.05, [1.46–2.86], p < 0.0001, Figure S3). With prolonged survival time, ICI+chemotherapy had an increasing advantage for PFS (Figures 3C, D).
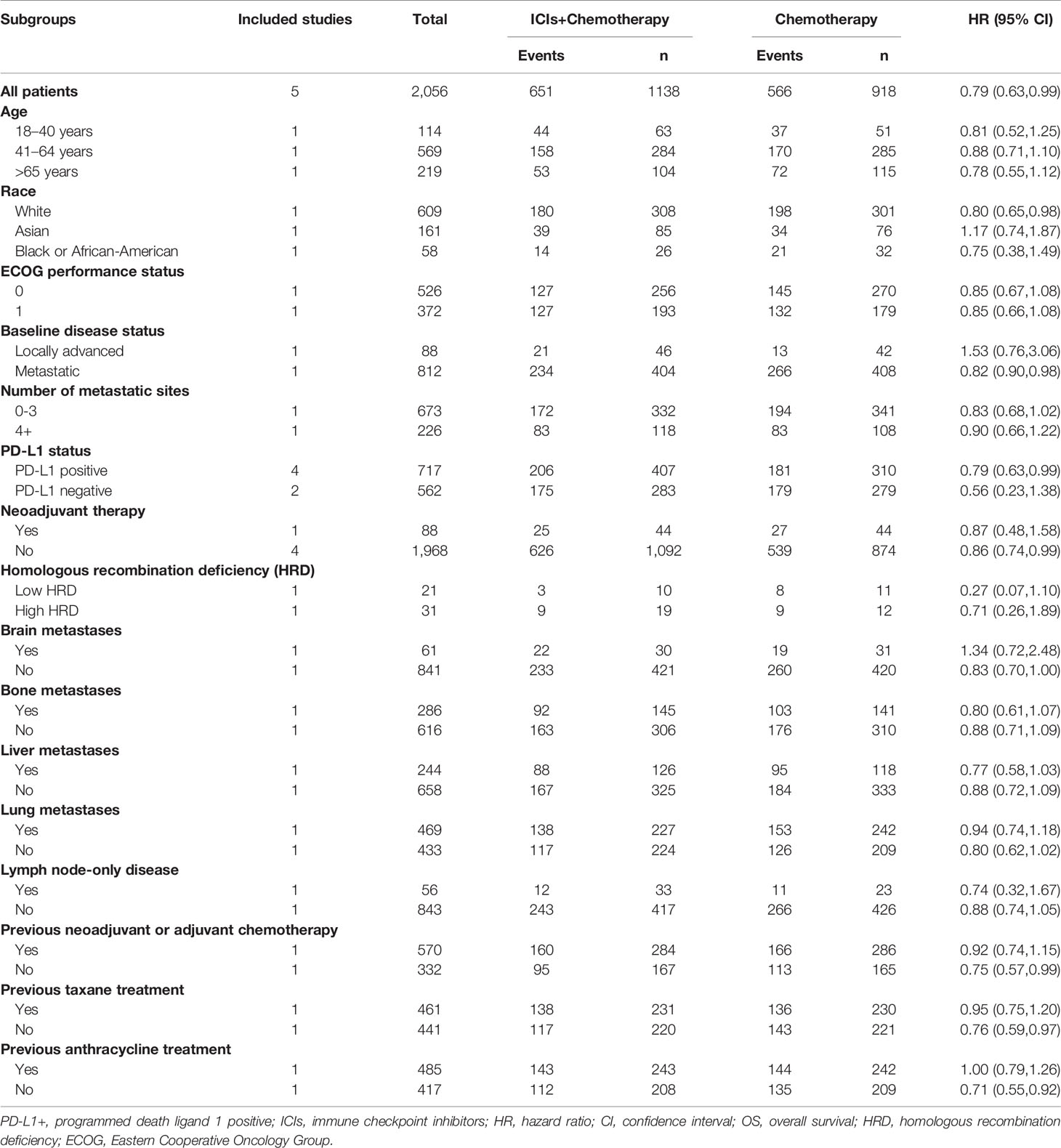
In the subgroup analysis, the favorable tendency of OS did not show significant changes according to age, ECOG performance status, number of metastatic sites, PD-L1 status, neoadjuvant therapy, lymph node-only disease, bone metastases, liver metastases, lung metastases, or previous chemotherapy (chemotherapy, taxane, or anthracycline). The addition of ICIs might have the opposite effect in the subgroups by race (Asian), baseline disease status (locally advanced), and brain metastases (yes) (Table 2).
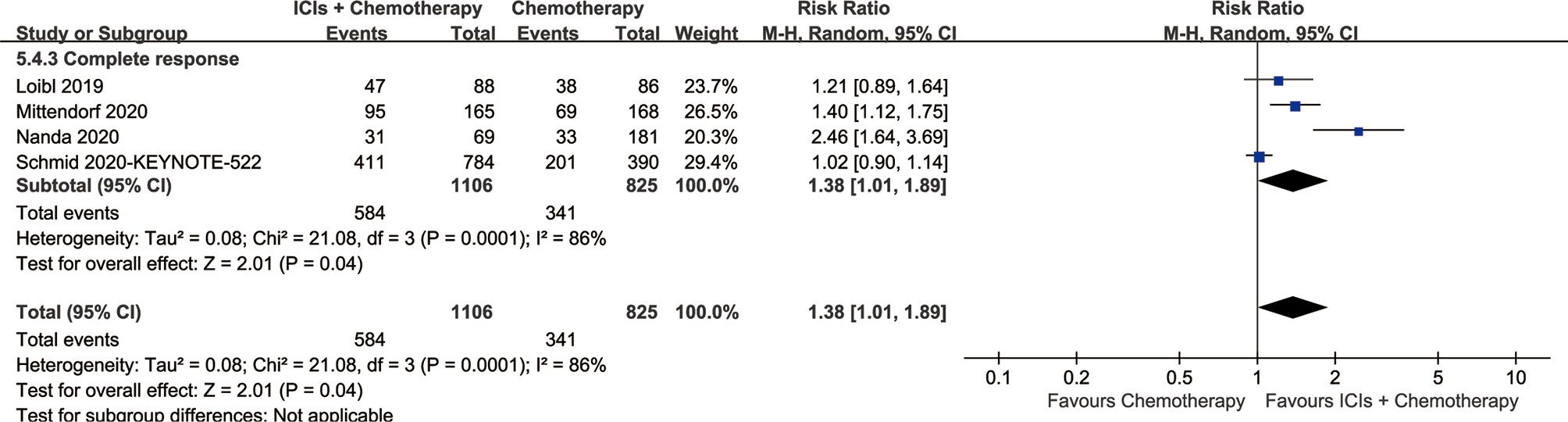
The CR of the ICI+chemotherapy group was higher than that of the chemotherapy group (584/1,106 vs. 341/825, RR: 1.38, [1.01–1.89], p = 0.04; Figure 4).
Toxicity
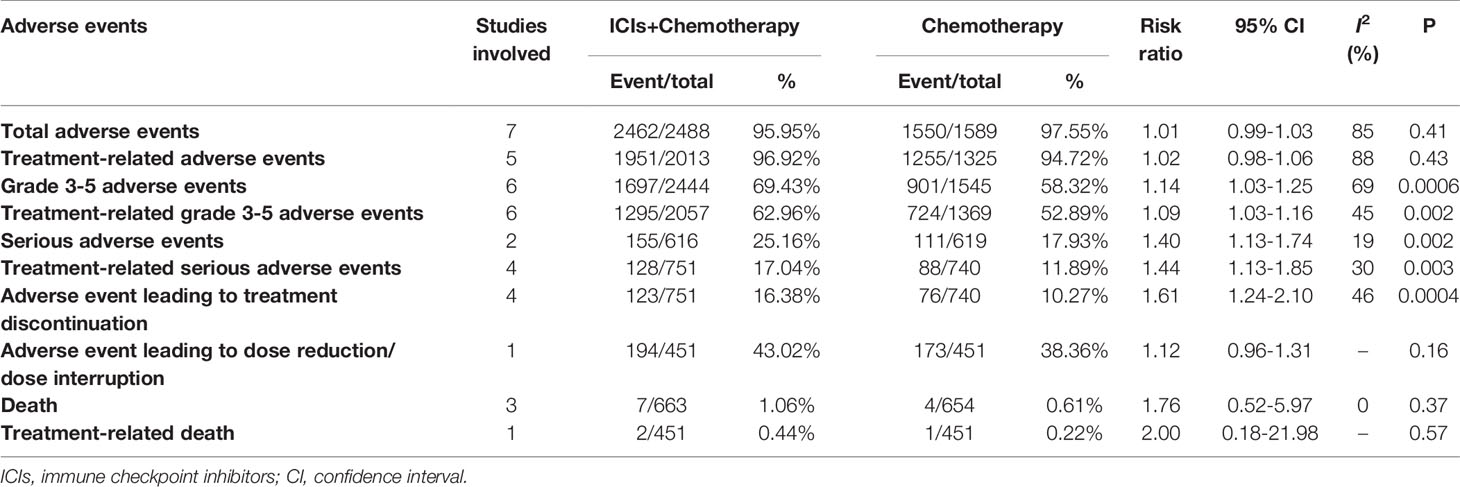
In summary, ICI+chemotherapy treatment was related to more Grade 3–5 AEs, treatment-related Grade 3–5 AEs, serious AEs, treatment-related serious AEs, and AEs leading to treatment discontinuation. Total AEs, treatment-related AEs, death, treatment-related death, and AEs leading to dose reduction/dose interruption were comparable between the two groups (Table 3).
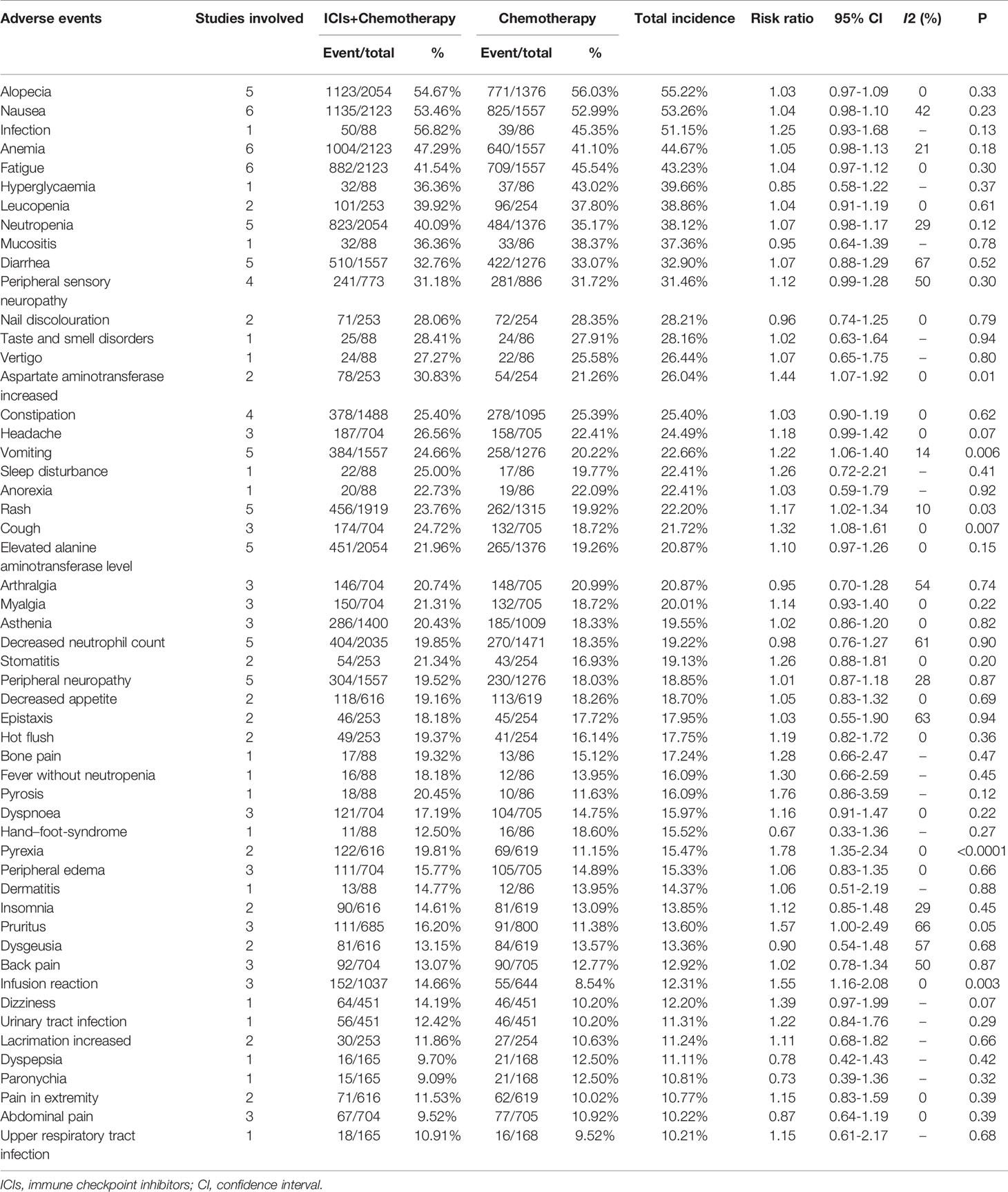
For total AEs, increases in aspartate aminotransferase (AST) levels, vomiting, cough, rash, pyrexia, pruritus, infusion reaction, hypothyroidism, nail disorders, hypokalemia, hyperthyroidism, pneumonitis, hepatitis, and adrenal insufficiencies were related to the ICI+chemotherapy group. Total AEs greater than 10% are summarized in Table 4.
For Grade 3–5 AEs, more cases of diarrhea, severe skin reactions, pneumonitis, hepatitis, and adrenal insufficiencies were related to the ICI+chemotherapy group. Grade 3–5 AEs greater than 1% are summarized in Table 5.
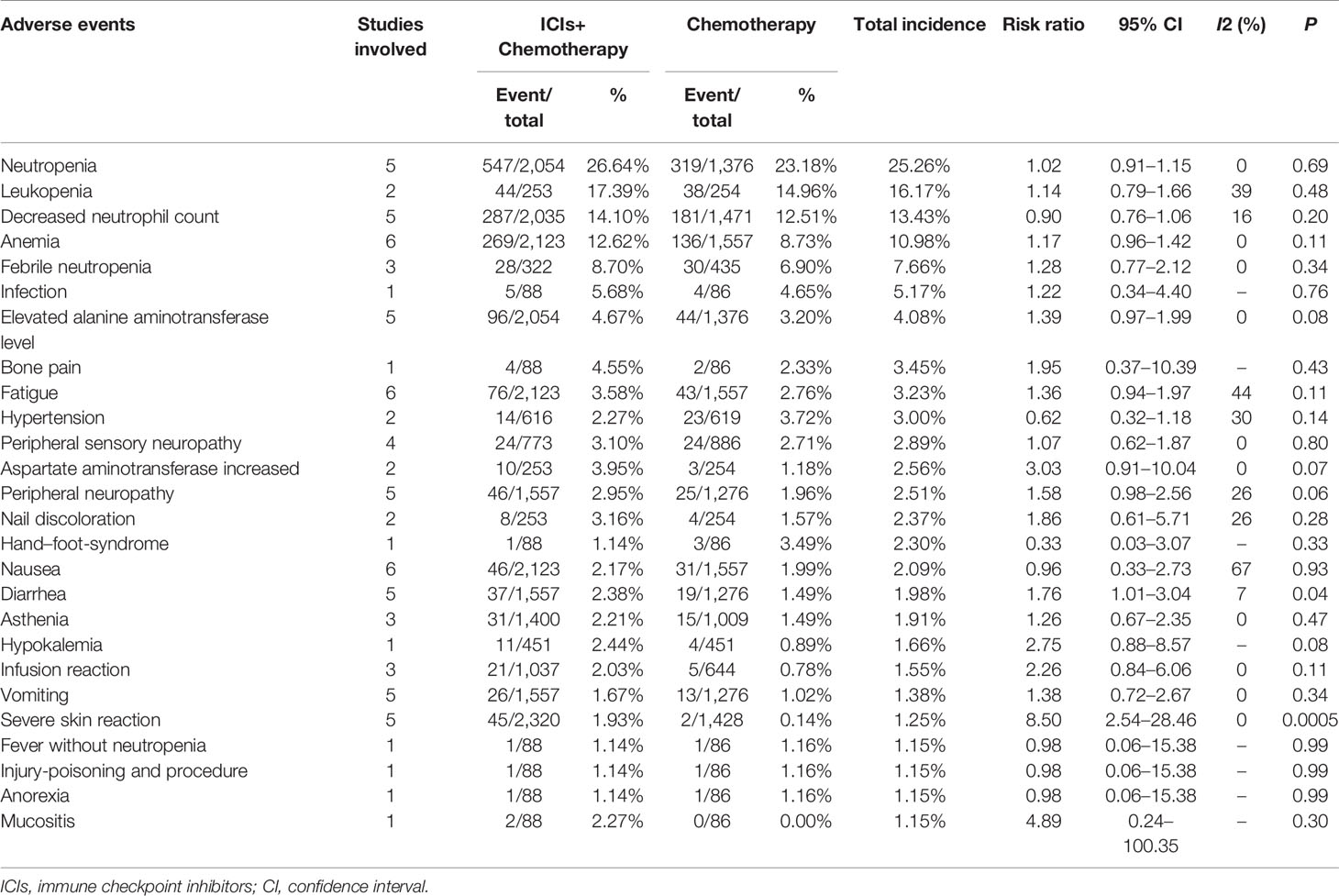
Table 5 Grade 3–5 adverse events an incidence of more than 1% according to combination of two groups.
Sensitivity Analysis
In the analysis of complete response, PFSR, and AEs, the I2 statistic was >50%, which suggests significant heterogeneity. By removing each study, the sensitivity analysis suggested that the results were stable and reliable (Figure S4).
Publication Bias
No significant publication bias was found based on the funnel plots of survival (Figure S5A) and safety (Figure S5B).
Discussion
Due to the lack of targets for therapeutic intervention, the treatment of TNBC is challenging (23). Whether immunotherapy can improve the curative effect when added to original standard chemotherapy treatment is still controversial (7, 8). This meta-analysis first compared ICI+chemotherapy with chemotherapy for TNBC treatment. The results suggest that ICI+chemotherapy treatment showed better efficacy in OS, PFS, and complete response. With the prolongation of survival, the survival advantage of ICI+chemotherapy increased compared with that of chemotherapy. In the toxicity analysis, more Grade 3–5 AEs and serious AEs were found in the ICI+chemotherapy group.
Better survival rates were the main benefit for the ICIs+Chemotherapy group. With the prolongation of survival, the advantage of OS and PFS in the ICIs+Chemotherapy group increased compared with the chemotherapy group. Similar results were confirmed by three large sample RCTs (KEYNOTE-522, IMpassion130 and KEYNOTE-355) (9, 10, 12). The I-SPY2 study and KEYNOTE-522 study suggested that significantly higher rates of CR were achieved in the ICIs+Chemotherapy groups (9, 13). Two reasons may explain the benefit of ICIs+Chemotherapy: (1) ICIs kill tumor cells by activating tumor immunity, which is different from chemotherapy and plays a synergistic role, especially in PD-L1-positive TNBC (9, 24). The antitumor effect may be more significant in early breast cancer than metastatic disease, because the tumor immune microenvironment is more robust (25); and (2) higher CR rates (584/1,106 vs. 341/825, RR: 1.38, [1.01–1.89]) were found in the ICIs+Chemotherapy groups, which is very important for the long-term survival of breast cancer patients after surgery (11, 13). Cortazar et al.’s pooled analysis also confirmed the strong association of PCR (no tumor in either breast or the lymph nodes) after neoadjuvant chemotherapy with an improved long-term benefit with respect to OS and DFS, especially in patients with TNBC (26). However, subgroup analysis suggested that addition of ICIs might not have a better effect in Asian patients, patients with locally advanced disease, or patients with brain metastases. Therefore, we suggested that ICIs+Chemotherapy is better than chemotherapy alone with longer survival, especially for patients with PD-L1-positive TNBC.
Higher rate of AEs, especially Grade 3–5/serious AEs, is the main restrictive factor to add immunotherapy to chemotherapy (9, 10). Twenty-one Grade 3–5 AEs greater >2% were reported in the ICIs+Chemotherapy group (neutropenia, leukopenia, decreased neutrophil count, anemia, febrile neutropenia, infection, elevated alanine aminotransferase [ALT] levels, bone pain, increased AST levels, fatigue, nail discoloration, peripheral sensory neuropathy, peripheral neuropathy, hypokalemia, diarrhea, mucositis, hypertension, severe skin reactions, asthenia, nausea, and infusion reactions) compared with twelve in the chemotherapy group (neutropenia, leukopenia, decreased neutrophil count, anemia, febrile neutropenia, infection, hypertension, hand–foot-syndrome, elevated ALT levels, fatigue, peripheral sensory neuropathy, and bone pain). The frequency of AEs was similar as previously reported by Schmid et al. in the updated report of the IMpassion130 trial (23). Hypothyroidism, hyperthyroidism, pneumonitis, hepatitis, and adrenal insufficiency were five AEs of special interest, which were all significantly increased after the addition of ICIs (27). High levels of AEs leading to treatment discontinuation was found in the ICIs+Chemotherapy group (16.38 vs. 10.27%), which might decrease antitumor efficacy (10). In the subgroup analysis according to the organs, the addition of ICIs might have a greater impact on the gastrointestinal system, hepatobiliary system, respiratory system, and the thyroid. Therefore, we suggested that although ICIs+Chemotherapy has better survival efficacy, the increase in serious complications deserves attention to improve the lifelong treatment of patients during survival.
However, this meta-analysis had some limitations described as follows: (1) The treatments used in the ICIs+Chemotherapy group and chemotherapy group were different between the groups, which might also increase heterogeneity. (2) Four out of the eight included studies (9, 11, 13, 14) focused on neoadjuvant therapy for early breast cancers, and the other 4/8 studies (10, 12, 16, 18) focused on medical therapy for metastatic breast cancers, and the combined analysis might increase heterogeneity. (3) Only RCTs published in English were included, which might introduce language bias; and (4) significant heterogeneity was found in some analyses (CR, PFSR, etc.), which might decrease the credibility of these results.
ICIs+Chemotherapy appears to be better than chemotherapy alone for TNBC with better OS and PFS. With the prolonged survival time, ICIs+Chemotherapy had an increased advantage for survival. However, the high rates of Grade 3–5/serious AEs, especially immunotherapy-related AEs, need to be taken seriously. However, due to the limitations described above, the results must be confirmed by more large-sample and high-quality RCTs.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
JH had full access to all of the data in the manuscript and takes responsibility for the integrity of the data and the accuracy of the data analysis. All authors contributed to the article and approved the submitted version. Concept and design: All authors. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: QJ, JD, MH, and NL. Critical revision of the manuscript for important intellectual content: QJ, JH, and WZ. Statistical analysis: QJ, JH, and WZ. Supervision: QJ and JH.
Funding
This study was supported by Science and Technology Planning Project of Health Commission of Jiangxi Province (Grant number: 20204261) and National Natural Science Foundation of China (NSFC, number of grants: 81560345). Role of the Funding: The funding had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank professor Jichun Liu, MD (Department of Cardio-Thoracic Surgery, The Second Affiliated Hospital of Nanchang University) for his advice and professor Xiaoshu Cheng, MD, PhD (Department of Cardiology, The Second Affiliated Hospital of Nanchang University) for his data collection.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.795650/full#supplementary-material
Supplementary Table 1 | PRISMA 2009 checklist.
Supplementary Table 2 | Search strategies.
Supplementary Table 3 | Quality assessment of the included studies according to Jadad scale.
Supplementary Table 4 | GRADE quality assessment for the outcomes of survival, responses, summary of adverse events, total adverse events, and grade 3–5 adverse events.
Supplementary Figure 1 | Cochrane risk assessment associated with ICIs+Chemotherapy versus Chemotherapy.
Supplementary Figure 2 | Forest plots of OSR (6–36 months) associated with ICIs+Chemotherapy versus Chemotherapy according to survival time.
Supplementary Figure 3 | Forest plots of PFSR (6–30 months) associated with ICIs+Chemotherapy versus Chemotherapy according to survival time.
Supplementary Figure 4 | Sensitivity analysis of complete response (A), PFSR (B), and total adverse events (C) associated with ICIs+Chemotherapy versus Chemotherapy.
Supplementary Figure 5 | Funnel plots of survival summary (A) and safety summary (B) associated with crizotinib versus alectinib.
Abbreviations
AEs, adverse effects; ALT, alanine aminotransferase; AST, aspartate aminotransferase; CR, complete response; CRBT, Cochrane Risk of Bias Tool; ECOG, Eastern Cooperative Oncology Group; GRADE, Grading of Recommendations Assessment, Development and Evaluation; HR, Hazard ratio; HRD, homologous recombination deficiency; ICIs, immune checkpoint inhibitors; OS, overall survival; OSR, overall survival rate; PCR, pathological complete response; PFS, progression-free survival; PFSR, progression-free survival rate; PRISMA, Preferred Reporting Items for Systematic Review and Meta-Analysis guidelines; RCTs, randomized controlled trials; RR, risk ratio; TNBC, triple-negative breast cancer.
References
1. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin (2021) 71(1):7–33. doi: 10.3322/caac.21654
2. DeSantis CE, Ma J, Gaudet MM, Newman LA, Miller KD, Goding Sauer A, et al. Breast Cancer Statistics, 2019. CA Cancer J Clin (2019) 69(6):438–51. doi: 10.3322/caac.21583
3. Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Breast Cancer. Lancet (2021) 397(10286):1750–69. doi: 10.1016/S0140-6736(20)32381-3
4. Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, et al. The Effect of Advances in Lung-Cancer Treatment on Population Mortality. N Engl J Med (2020) 383(7):640–9. doi: 10.1056/NEJMoa1916623
5. Yang JD, Heimbach JK. New Advances in the Diagnosis and Management of Hepatocellular Carcinoma. BMJ (2020) 371:m3544. doi: 10.1136/bmj.m3544
6. Janjigian YY, Shitara K, Moehler M, Garrido M, Salman P, Shen L, et al. First-Line Nivolumab Plus Chemotherapy Versus Chemotherapy Alone for Advanced Gastric, Gastro-Oesophageal Junction, and Oesophageal Adenocarcinoma (CheckMate 649): A Randomised, Open-Label, Phase 3 Trial. Lancet (2021) 398(10294):27–40. doi: 10.1016/S0140-6736(21)00797-2
7. Gradishar WJ, Moran MS, Abraham J, Aft R, Agnese D, Allison KH, et al. NCCN Guidelines Insights: Breast Cancer, Version 4.2021. J Natl Compr Canc Netw (2021) 19(5):484–93. doi: 10.6004/jnccn.2021.0023
8. Denduluri N, Somerfield MR, Chavez-MacGregor M, Comander AH, Dayao Z, Eisen A, et al. Selection of Optimal Adjuvant Chemotherapy and Targeted Therapy for Early Breast Cancer: ASCO Guideline Update. J Clin Oncol (2021) 39(6):685–93. doi: 10.1200/JCO.20.02510
9. Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for Early Triple-Negative Breast Cancer. N Engl J Med (2020) 382(9):810–21. doi: 10.1056/NEJMoa1910549
10. Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N Engl J Med (2018) 379(22):2108–21. doi: 10.1056/NEJMoa1809615
11. Mittendorf EA, Zhang H, Barrios CH, Saji S, Hae Jung K, Hegg R, et al. Neoadjuvant Atezolizumab in Combination With Sequential Nab-Paclitaxel and Anthracycline-Based Chemotherapy Versus Placebo and Chemotherapy in Patients With Early-Stage Triple-Negative Breast Cancer (IMpassion031): A Randomised, Double-Blind, Phase 3 Trial. Lancet (2020) 396(10257):1090–100. doi: 10.1016/S0140-6736(20)31953-X
12. Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im S-A, Yusof M, et al. Pembrolizumab Plus Chemotherapy Versus Placebo Plus Chemotherapy for Previously Untreated Locally Recurrent Inoperable or Metastatic Triple-Negative Breast Cancer (KEYNOTE-355): A Randomised, Placebo-Controlled, Double-Blind, Phase 3 Clinical Trial. Lancet (2020) 396(10265):1817–28. doi: 10.1016/S0140-6736(20)32531-9
13. Nanda R, Liu MC, Yau C, Shatsky R, Pusztai L, Wallace A, et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women With Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol (2020) 6(5):676–84. doi: 10.1001/jamaoncol.2019.6650
14. Loibl S, Untch M, Burchardi N, Huober J, Sinn BV, Blohmer J-U, et al. A Randomised Phase II Study Investigating Durvalumab in Addition to an Anthracycline Taxane-Based Neoadjuvant Therapy in Early Triple-Negative Breast Cancer: Clinical Results and Biomarker Analysis of GeparNuevo Study. Ann Oncol (2019) 30(8):1279–88. doi: 10.1093/annonc/mdz158
15. Miles D, Gligorov J, André F, Cameron D, Schneeweiss A, Barrios C, et al. Primary Results From IMpassion131, a Double-Blind, Placebo-Controlled, Randomised Phase III Trial of First-Line Paclitaxel With or Without Atezolizumab for Unresectable Locally Advanced/Metastatic Triple-Negative Breast Cancer. Ann Oncol (2021) 32(8):994–1004. doi: 10.1016/j.annonc.2021.05.801
16. Bachelot T, Filleron T, Bieche I, Arnedos M, Campone M, Dalenc F, et al. Durvalumab Compared to Maintenance Chemotherapy in Metastatic Breast Cancer: The Randomized Phase II SAFIR02-BREAST IMMUNO Trial. Nat Med (2021) 27(2):250–5. doi: 10.1038/s41591-020-01189-2
17. Brufsky A, Kim SB, Zvirbule Ž, Eniu A, Mebis J, Sohn JH, et al. A Phase II Randomized Trial of Cobimetinib Plus Chemotherapy, With or Without Atezolizumab, as First-Line Treatment for Patients With Locally Advanced or Metastatic Triple-Negative Breast Cancer (COLET): Primary Analysis. Ann Oncol (2021) 32(5):652–60. doi: 10.1016/j.annonc.2021.01.065
18. Tolaney SM, Barroso-Sousa R, Keenan T, Li T, Trippa L, Vaz-Luis I, et al. Effect of Eribulin With or Without Pembrolizumab on Progression-Free Survival for Patients With Hormone Receptor-Positive, ERBB2-Negative Metastatic Breast Cancer: A Randomized Clinical Trial. JAMA Oncol (2020) 6(10):1598–605. doi: 10.1001/jamaoncol.2020.3524
19. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred Reporting Items for Systematic Review and Meta-Analysis Protocols (PRISMA-P) 2015: Elaboration and Explanation. BMJ (2015) 350:g7647. doi: 10.1136/bmj.g7647
20. Guyatt GH, Oxman AD, Schünemann HJ, Tugwell P, Knottnerus A. GRADE Guidelines: A New Series of Articles in the Journal of Clinical Epidemiology. J Clin Epidemiol (2011) 64(4):380–2. doi: 10.1016/j.jclinepi.2010.09.011
21. Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ, et al. Assessing the Quality of Reports of Randomized Clinical Trials: Is Blinding Necessary? Control Clin Trials (1996) 17(1):1–12. doi: 10.1016/0197-2456(95)00134-4
22. Higgins JP, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The Cochrane Collaboration's Tool for Assessing Risk of Bias in Randomised Trials. BMJ (2011) 343:d5928. doi: 10.1136/bmj.d5928
23. Franzoi MA, Romano E, Piccart M. Immunotherapy for Early Breast Cancer: Too Soon, Too Superficial, or Just Right? Ann Oncol (2021) 32(3):323–36. doi: 10.1016/j.annonc.2020.11.022
24. Li S, Liu M, Do MH, Chou C, Stamatiades EG, Nixon BG, et al. Cancer Immunotherapy via Targeted TGF-Beta Signalling Blockade in T(H) Cells. Nature (2020) 587(7832):121–5. doi: 10.1038/s41586-020-2850-3
25. Hutchinson KE, Yost SE, Chang C-W, Johnson RM, Carr AR, McAdam PR, et al. Comprehensive Profiling of Poor-Risk Paired Primary and Recurrent Triple-Negative Breast Cancers Reveals Immune Phenotype Shifts. Clin Cancer Res (2020) 26(3):657–68. doi: 10.1158/1078-0432.CCR-19-1773
26. Cortazar P, Zhang L, Untch M, Mehta K, Costantino JP, Wolmark N, et al. Pathological Complete Response and Long-Term Clinical Benefit in Breast Cancer: The CTNeoBC Pooled Analysis. Lancet (2014) 384(9938):164–72. doi: 10.1016/S0140-6736(13)62422-8
27. Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab Plus Nab-Paclitaxel as First-Line Treatment for Unresectable, Locally Advanced or Metastatic Triple-Negative Breast Cancer (IMpassion130): Updated Efficacy Results From a Randomised, Double-Blind, Placebo-Controlled, Phase 3 Trial. Lancet Oncol (2020) 21(1):44–59. doi: 10.1016/S1470-2045(19)30689-8
Keywords: chemotherapy, triple-negative breast cancer, meta-analysis, immune checkpoint inhibitors, systematic review
Citation: Ji Q, Ding J, Hao M, Luo N, Huang J and Zhang W (2021) Immune Checkpoint Inhibitors Combined With Chemotherapy Compared With Chemotherapy Alone for Triple-Negative Breast Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 11:795650. doi: 10.3389/fonc.2021.795650
Received: 15 October 2021; Accepted: 22 November 2021;
Published: 16 December 2021.
Edited by:
Tomoharu Sugie, Kansai Medical University Hospital, JapanReviewed by:
Lironne Wein, Peter MacCallum Cancer Centre, AustraliaElham Sajjadi, University of Milan, Italy
Copyright © 2021 Ji, Ding, Hao, Luo, Huang and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jiabing Huang, YXdlc29tZWhAMTI2LmNvbQ==
 Qiao Ji1
Qiao Ji1 Meiqi Hao
Meiqi Hao Nachuan Luo
Nachuan Luo Jiabing Huang
Jiabing Huang Wenxiong Zhang
Wenxiong Zhang