
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 10 January 2022
Sec. Thoracic Oncology
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.795528
This article is part of the Research Topic Epidemiology, Screening and Diagnosis of Lung Cancer View all 24 articles
Objective: Lung cancer screening has been widely conducted in Western countries. However, population-based lung cancer screening programs in Hebei in China are sparse. Our study aimed to assess the participation rate and detection rate of positive nodules and lung cancer in Hebei province.
Method: In total, 228 891 eligible participants aged 40–74 years were enrolled in the Cancer Screening Program in Hebei from 2013 to 2019. A total of 54 846 participants were evaluated as the lung cancer high-risk population by a risk score system which basically followed the Harvard Risk Index and was adjusted for the characteristics of the Chinese population. Then this high-risk population was recommended for low-dose computed tomography (LDCT) screening. And all participants attended annual passive follow-up, and the active follow-up interval was based on radiologist’s suggestion. All participants were followed-up until December 31, 2020. The overall, group-specific participation rates were calculated, and its associated factors were analyzed by a multivariable logistic regression model. Participation rates and detection of positive nodules and lung cancer were reported.
Results: The overall participation rate was 52.69%, where 28 899 participants undertook LDCT screening as recommended. The multivariable logistic regression model demonstrated that a high level of education, having disease history, and occupational exposure were found to be associated with the participation in LDCT screening. The median follow-up time was 3.56 person-years. Overall, the positive identification of lung nodules and suspected lung cancer were 12.73% and 1.46% through LDCT screening. After the native and passive follow-up, 257 lung cancer cases were diagnosed by lung cancer screening, and the detection rate of lung cancer was 0.89% in the screening group. And its incidence density was 298.72 per 100,000. Positive lung nodule rate and detection rate were increased with age.
Conclusion: Our study identified personal and epidemiological factors that could affect the participation rate. Our findings could provide the guideline for precise prevention and control of lung cancer in the future.
Lung cancer is the second most diagnosed cancer, and it is also the leading cause of cancer death in the world. According to GLOBOCAN 2020, there were approximately 2 206 771 newly diagnosed lung cancer cases and 1 796 144 cancer deaths in 2020, accounting for 11.4% and 18.0% of all new cases from cancer, respectively (1). As reported by the Chinese National Cancer Center (CNCC), with a 36.05/100,000 age-standardized incidence rate and a 28.06/100,000 age-standardized mortality rate, lung cancer was the most common cancer and the leading cause of cancer death in 2016 in China. It also showed an increasing trend in China (2). While the five-year survival rate of lung cancer was only 19.7% (3).
A series of randomized controlled trials, cohort studies, and case-control studies have demonstrated that low-dose computed tomography (LDCT) screening in a high-risk population could reduce mortality due to lung cancer (4–7). By now, lung cancer screening programs have been organized by many countries, such as the national lung cancer screening trial (NLST), National Cancer Institute Prostate, Lung, Colorectal & Ovarian Cancer Screening Trial (PLCO), and others (4, 8, 9). These trails were mainly carried out in Western countries. However, the effectiveness evaluation of lung cancer screening programs in China, in which the lifestyle is different from Western countries, is still rare.
The population-based Cancer Screening Program in Urban China (CanSPUC) was conducted in 2012. It included five type common kinds of cancer: lung cancer, female breast cancer, liver cancer, colorectal cancer, and upper digestive tract cancer (esophagus cancer and gastric cancer). Participants were invited to take a cancer risk assessment using an established clinical cancer risk score system, and those who were evaluated to be at high risk for specific types of cancer were recommended to take the appropriate screening intervention by the study design. Individuals who were found to be at high risk of lung cancer were recommended to undergo LDCT at tertiary-level hospitals.
Combined with follow-up, we aimed to assess the participation rate, screening effectiveness, and results of lung cancer screening in a high-risk population in Hebei province, China. It could provide reliable and effective data support for lung cancer prevention and control.
The study was conducted in Shijiazhuang and Tangshan City which are located in Hebei province (North China), and screenings took place in six tertiary-level hospitals (the Fourth Hospital of Hebei Medical University, the First Hospital of Hebei Medical University, the first Hospital of Shijiazhuang, Hebei Cheat Hospital, Tangshan People’s Hospital, and Kailuan Hospital). The participants who met the following conditions became the screening objects: (1) the residents of the program’s city; (2) residents’ age is 40-74 years old. The program used a cluster sampling method to select the screening participants. And selecting the screening participants was based on the community. The staff of the community mobilized eligible residents of the area under their jurisdiction to participate in the program. Eligible residents took part in face-to-face interviews in the selected communities. After obtaining signed informed consent, all the eligible participants were interviewed by trained staff to complete an epidemiological questionnaire and to assess their cancer risk using an established risk score system. In this study, to maximize the use of limited health resources and increase the detection rate of lung cancer, participants who were put into the high-risk groups of lung cancer were recommended free LDCT examinations in those tertiary hospitals. The present study was approved by the Ethics Board of the Fourth Hospital of Hebei Medical University. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) reporting guideline. A flow diagram showing the recruitment of the study population is shown in Figure 1.
The rationale of the cancer risk score system was based on the Harvard Risk Index (10). According to the Chinese characteristics, the risk score system included risk factors, relative risks, and exposure rates of risk factors that were adjusted. Each risk factor was allocated a score by the expert panel based on the magnitude of its association with lung cancer. The cumulative risk scores were calculated and were then divided by the average risk score in the general population to get the final individual relative risks (11). People who smoke more than one cigarette a day for more than 6 months were defined as smokers. Second-hand smoking exposure was identified in participants living with a smoker on a regular basis in the workplace or at home. The database was established by professional trained community doctors with double-entry and high-quality control to ensure consistency. The questionnaires completed every day required a random sample of 2% for re-examination, and the compliance rate of each item after the re-examination could not be less than 90%.
All participants undertaking the LDCT screening used the 64-section CT machine. The parameters were set as follows: (1) Scan parameters: 120 kVp and ≤30 mAs; scanning thickness: 5 mm and scanning spacing: 5 mm; the reconstructed layer thickness was 1.0-1.25 mm continuous (layer interval is 0); (2) the scanning range was from the lung tip to the costophrenic angle (including all lungs); (3) nodule measurement: Using an electronic measuring ruler to measure the maximum of the nodule length and wide diameter; (4) positive nodule: The mean diameter of solid or partly nodular nodules ≥ 5 mm, or non-solid nodules ≥ 8 mm in average diameter, or endobronchial nodules; and (5) suspicious lung cancer: A suspicious lung cancer case was identified when cases were diagnosed as suspected lung cancer or malignant lesions by senior thoracic radiologists.
All participants were followed-up by active and passive methods until December 31, 2020. An annual passive and regular active follow-up mechanism for the entire cohort population was established and carried out in our program based on the cancer registration system. Through telephone, home visits, and retrieval of medical record information from medical institutions, positive cases were actively followed-up to obtain the final diagnosis and outcome. For people with positive results, regular active follow-up was conducted by radiologist’s suggestion after the LDCT screening.
For passive follow-up, all participants who completed the questionnaire survey were matched by a personal identification number with the local cancer registration database and the all-cause mortality database in 2013-2020. The information of cancer incidence, subsite, topography, and morphology were obtained from these databases. Newly diagnosed cases of lung cancer were classified by sites according to International Statistical Classification of Diseases and Related Health Problems, Tenth Revision (codes C33 and C34).
The overall and group-specific participation rates by different characteristics were calculated and compared by χ2 test. Categorical variables were presented as numbers and percentages. The relationship of variables with participation rate of lung cancer screening were quantified by a multivariable logistics regression model with odds ratios (ORs) and their 95% confidence intervals (CIs). All statistical analyses were performed using R, version 3.4. Statistical significance was established at P ≤ 0.05 on two-sided probabilities.
In the lung cancer screening program, 228 891 participants were recruited and had completed a risk assessment questionnaire in 2013-2019. There were 54 846 high-risk participants for lung cancer accounting for 23.96% of the total population. More women took part in the screening program, while the high-risk rate in women (43.73%) was less than that in men (56.27%). The majority of participants were between 50 and 64 years old. Most participants had junior school education level or below. In the high-risk population group, half (54.86%) had first degree relatives who had history of lung cancer, and three-quarters were smokers (Table 1).
In the 54 846 participants in the lung cancer high-risk population, 28 899 undertook LDCT screening. The total participation rate was 52.69%. The screening program in Shijiazhuang (64.97%) had a higher participation rate than that in Tangshan (48.20%). Although there was a higher high-risk rate in men, the participation rate in men (42.44%) was less than that in women (65.88%). Participants aged 45-49 had the higher participation rate (56.57%), and the participation rates decreased along with the increasing age. It was found that participants with higher educational level, who worked as technical staff, had occupational exposure, never smoked, had second-hand smoke exposure, a history of lung diseases, and family history of lung cancer had relatively higher participant rates (Table 1).
In multivariable analysis, we found that participants who had occupational exposure had 45% higher odds of undertaking screening than other participants (OR: 1.45; 95%CI: 1.39-1.51). Smokers and former smokers were less willing to accept the screening, in which the ORs were 0.87 (95%CI: 0.81-0.92) and 0.83 (95%CI: 0.74-0.93), respectively. After adjusting for year of recruitment, study areas, married condition, Body Mass Index (BMI), drinking consumption, heating methods, and cooking fuels, we found that age, sex, educational level, occupation, occupational exposure, smoke condition, second-hand smoking exposure, history of lung diseases, and family history of lung cancer were associated with participation rate (Table 2).
In the screening program, 3 679 positive nodules and 421 suspected lung cancer cases were detected, yielding rates of 12.73% and 1.46%, respectively. Comparing the results in different genders, the positive nodules rate in men (1757, 13.41%) was higher than that in women (1922, 12.16%). With increasing age, the positive rates gradually increased. The highest positive nodule rate was reached at 70-74 years old in both genders, which was 21.79% in men and 18.35% in women. In the positive nodules rates in ages 40-44 and 65-69, the rates in men were higher than the respective rates in women at the same age range. Along with an increasing age, the suspected lung cancer rates had an increasing trend. At 70-74 years old in both men and women, the rates reached the top which were 5.38% and 3.10%, respectively (Figures 2 and 3).
The characteristics of the nodules are shown in Table 3. The mean diameter of the nodule demonstrated the significant difference in benign nodule and lung cancer groups in which the median sizes were 6.00 mm and 12.25 mm, respectively. The majority of nodules were solid (83.30% in the benign nodule group and 36.63% in the lung cancer group). Non-solid and part-solid nodules accounted for 5.94% and 10.76% in the benign nodule group and 27.91% and 35.47% in the lung cancer group, respectively. A larger number of cancers were observed in the left upper (23.30%) and right upper lobes (38.07%) than in the other lobe (Table 1). In the lung cancer group, the proportion of nodules with stretched pleura and spiculation was higher than those in the benign nodule group.
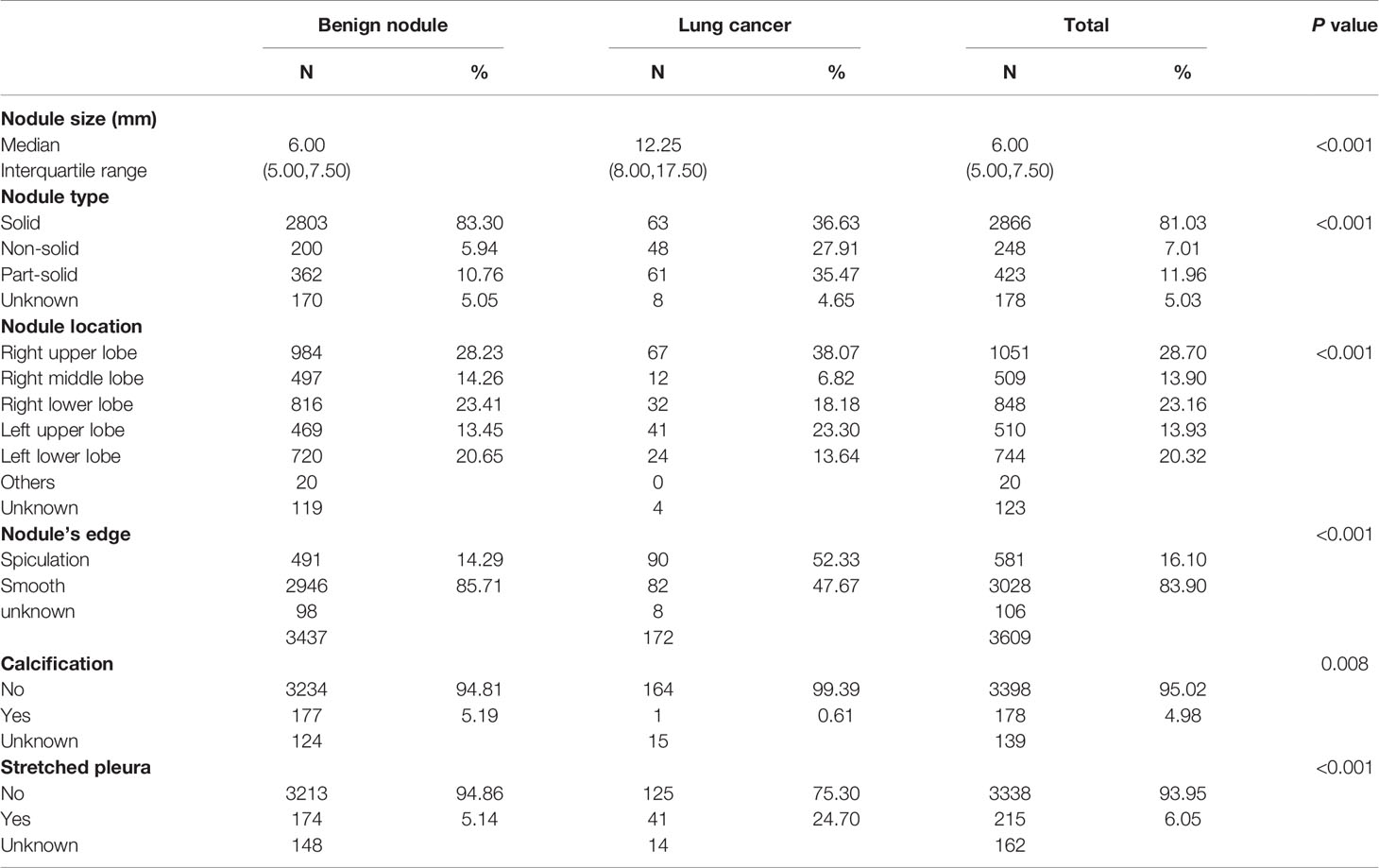
Table 3 Distribution of nodule characteristics in lung cancer screening in Hebei province, 2013-2019.
From 2013 to 2020, the median follow-up time was 3.56 years and the total follow-up time was 828 252.5 person-year. By follow-up, 257 lung cancer cases were screened in the screening group, in which the detection rate in the screening group was 0.89% and incidence density was 298.72/100,000. In the screening group, the participants with positive results (positive nodules and suspicious lung cancer) had the higher detection rate of lung cancer than participants with negative results (4.73% vs. 0.31%). In the high risk of lung cancer population, the detection rate of the screening group (0.89%) was significantly higher than those in the non-screening group (0.44%). Figure 4 shows that the detection rates from lung cancer increased with age and those were higher in men than in women. In the screening and non-screening groups, the most common subsite of lung cancer was the upper lobe. And adenocarcinoma was the main histologic type, followed by squamous cell carcinoma and small-cell carcinoma (Table S1).
This study reported the 228 891 participants undertaking LDCT screening among a large-scale population-based screening program. This is the first study in Hebei province in China that combined epidemiological investigation, risk assessment stratification, and LDCT for participants. Although great efforts have been made by previous studies to develop effective screening, the majority of studies aimed to optimization risk scores and few were truly implemented in large-scale lung cancer screening, especially in Hebei province. The overall participation rate was 52.69% in LDCT screening among the lung cancer high-risk population. The detection rate of lung cancer in the screening group was 0.89%. And we found that the population of nodules with a relatively large mean diameter (6.00 mm vs. 12.25 mm in the benign nodule group vs. lung cancer group), non-solid, spiculation, non-calcification, and stretched pleura would more likely to develop into lung cancer. This study could provide a reliable, reasonable, and precise management strategy for lung cancer prevention and control in Hebei.
The overall participation rate was 52.69% in Hebei province. The participation rate of lung cancer screening in the high-risk population varies in different programs. It might relate to the local management and personal factors. Smoking is one of the most important factors for lung cancer and smokers were more likely to develop lung cancer (5, 12). While we found that the participation rates of lung cancer screening in smokers and former smokers were lower than that in non-smokers, in which the adjusted OR was 0.87 (95%CI: 0.81-0.92) and 0.83 (95%CI: 0.74-0.93), respectively. In smokers, the aversion to encountering adverse screening results might prevent test uptake (13–16). We also found that the population with higher educational level, who were technicians or employees, had second-hand smoking exposure, history of respiratory disease and family history of lung cancer had a higher participation rate. Previous studies demonstrate that the level of education was significantly positively correlated with the level of compliance with screening (17, 18). Our study was consistent with that of Henan province where people with undergraduate degrees or more had higher compliance (OR = 1.34, 95%CI: 1.24-1.44). It might be that the participants with a higher level of education, disease history, and occupational exposure have better understanding, self-health awareness, and pay more attention to self-care. In NLST and the European Dutch-Belgian Randomized Lung Cancer Screening Trial (NELSON), the compliance rate of screening reached 90% (4, 19). And some studies showed that the rates of participation were more than 50% (9, 20). While the overall participation rate was 34.86% in LDCT screening in three provinces (Zhejiang province, Anhui province, and Liaoning province) in China (21). And in the same program in Henan province in China, the overall participation rate was 40.16% which was lower than that in our studies. Different regional compliance was not at the same level, and other factors included the publicity and mobilization of the communities and hospitals involved in the program, the organization and mobilization process, and the health awareness of residents. Another survey conducted among family physicians in South Carolina in 2015 showed that most people had a knowledge gap and there were limited referrals of patients eligible for LDCT screening (16, 22). We conducted multiple training sessions for community physicians to educate them on the necessity and importance of lung cancer screening. As community physicians could influence screening uptake, issues related to penetration and educational outreach around LDCT screening to physicians should be examined. These studies confirmed that community physicians can help improve the compliance to a screening program, especially for people with low educational level and high age. Strengthening health education in the community system and improving the awareness rate of residents’ cancer knowledge will have a positive influence on the compliance of lung cancer screening.
In our study, the positive nodule rate was 12.73% in 2013-2019. After active and passive follow-up, the lung cancer detection rate was 0.89% in the screening group. The study of lung cancer screening in 2013-2017 in China showed that the positive rate of nodules detected by LDCT screening in high-risk groups of lung cancer was 11.36% (23). The detection rate of lung positive nodules reported in various provinces in China showed that Zhejiang province was the highest at 21.61%, followed by Beijing with 10.99%, Chongqing City, Yunnan province, Hunan province, and Henan province with 12.91%, 6.90%, 5.92%, and 5.87%, respectively (24–29). The one reason for the different levels in positive nodules rates is the different skill level of diagnosis of cancer in the early stage. During the implementation of the program, our province conducted multiple clinical diagnosis training sessions and unified the diagnostic standards to ensure the homogeneity of the data. Some of our findings with respect to the initial low-dose CT screening are not fully consistent with previous studies. The prevalence of lung cancer (0.89%) was at the middle of the reported range in some prior large studies [NLST, Early Lung Cancer Action Project (ELCAP) (30), International Early Lung Cancer Action Program (I-ELCAP) (31), NELSON (32), Rural China Screening Programme (RuraCSP) (33), Sone (34)[, which ranged from 0.4% to 2.7%. But it was close to the rate of 1.0% in the NELSON trial and 1.1% in NLST. This relatively low rate may be due to some combination of the following factors: participants in the program were healthier than the general population, and were younger in our study than in other studies. For example, our study included participants aged 40-74 and the NLST criteria included 55-74-year-old and heavy smoker participants. The other reason is that the definitions of a high-risk population were different. Following the NLST age entry criteria, the detection rate of our study in ages 55-74 was 1.28%. If the population only includes smokers, the detection rate will increase. It means that the risk assessment system of our study could concentrate on the high-risk lung cancer population and it could increase the screening effects.
Lung nodules can be effectively detected by LDCT. But discrimination between benign and malignant nodules, and which type of nodule had the greater probability of developing lung cancer are the medical concern (7). Among the positive nodules, 4.85% were malignant in our study, and this corresponded with other studies. In the Pan-Canadian Early Detection of Lung Cancer Study (PanCan) and British Columbia Cancer Agency (BCCA), the rates of cancer in nodules in the two datasets were 5.5% and 3.7% (35). We confirmed that the right upper lobes were the most common sub-site in lung cancer; they accounted for 38.07% of all diagnosed lung cancer cases. Among the screen-detected lung cancers, about three-quarters were adenocarcinomas. And the screening methods for small cell lung cancer and squamous cell carcinoma need to be improved. Lung adenocarcinomas are more likely to be located at the periphery of the lung. And the cancer in the lung periphery had a greater probability of being measured than central lung cancer (36). Lung cancer is most likely to occur in the upper lobe. It is a known phenomenon in non–small cell lung cancer cases and can be explained as the maximum airflow when breathing begins, mainly towards the upper right lobe bronchus. So, tobacco smoke and its carcinogenic toxins accumulates the most in the right upper lobe (37–39). Through our study, we confirmed that nodules with the following characteristics should be paid more attention to in future clinical treatment and diagnosis: larger nodule size, location of the nodule in the upper lobe, non-solid and part-solid nodule type, spiculation, non-calcification, and stretched pleura nodules (35). These nodules were more likely to develop into lung cancer.
This study has strength and limitations. The strengths were as follows: this study was population-based, and it involved a large-scale sample size. Detailed epidemiological questionnaire information was collected in a standardized manner by trained study staff to ensure the quality of the data. A sound annual passive and active follow-up mechanism for the entire cohort population was established and carried out in our program based on the cancer registration system. We obtained information regarding each participant’s cancer incidence in the study. This study has the limitation that some variables, such as smoking status and other variables, were self-reported and it might lead to misclassification. Another limitation is that follow-up work for patients diagnosed with lung cancer is still under way, therefore clinical disease information was not fully obtained. And the study population was a pre-selected high-risk population ascertained by the risk assessment system which might not represent the general population of Hebei province, and selection bias cannot be ruled out.
In summary, in this large-scale lung cancer screening in Hebei, we found that some variables, which were age, sex, educational level, job, smoker, secondhand smoking exposure, history of respiratory, and family history of lung cancer contributed to the participation rate. And the detection rate in the screening group was higher than that in other groups. Our finding may provide data support for lung cancer prevention and it is useful for optimizing screening strategies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by the Ethics Board of the Fourth Hospital of Hebei Medical University (No. 2012KY102). The patients/participants provided their written informed consent to participate in this study.
DiL and YH wrote the main text and conducted data analysis. YH designed the study. JS, DaL, SW, and JJ collected the data. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as potential conflicts of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We gratefully thank the cooperation partners of all cancer screening hospitals and communities in Hebei province. We are grateful to the participants for taking part in this study. The authors assume full responsibility for the analyses and interpretations of the data.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.795528/full#supplementary-material
1. Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin (2021) 71(3):209–49. doi: 10.3322/caac.21660
2. He J, Wei WQ. 2019 China Cancer Registry Annual Report. Beijing, China: People's Medical Publishing House (2021).
3. Zeng H, Chen W, Zheng R, Zhang S, Ji JS, Zou X, et al. Changing Cancer Survival in China During 2003-15: A Pooled Analysis of 17 Population-Based Cancer Registries. Lancet Global Health (2018) 6(5):e555–67. doi: 10.1016/S2214-109X(18)30127-X
4. National Lung Screening Trial Research T, Aberle DR, Adams AM, Berg CD, Black WC, Clapp JD, et al. Reduced Lung-Cancer Mortality With Low-Dose Computed Tomographic Screening. N Engl J Med (2011) 365(5):395–409. doi: 10.1056/NEJMoa1102873
5. Moyer VA. USPSTF. Screening for Lung Cancer: U.S. Preventive Services Task Force Recommendation Statement. Ann Intern Med (2014) 160(5):330–8. doi: 10.7326/m13-2771
6. Yousafkhan U, Carlijn VDA, De Jong PA, Heuvelmans M, Scholten E, Walter J, et al. Risk Stratification Based on Screening History: The NELSON Lung Cancer Screening Study. Thorax (2017) 72(9):819–24. doi: 10.1136/thoraxjnl-2016-209892
7. Jett J. Screening for Lung Cancer: Who Should be Screened? Arch Pathol Lab Med (2012) 136(12):1511–4. doi: 10.5858/arpa.2012-0259-RA
8. Tammemagi CM, Pinsky PF, Caporaso NE, Kvale PA, Hocking WG, Church TR, et al. Lung Cancer Risk Prediction: Prostate, Lung, Colorectal And Ovarian Cancer Screening Trial Models and Validation. J Natl Cancer Inst (2011) 103(13):1058–68. doi: 10.1093/jnci/djr173
9. Quaife SL, Ruparel M, Dickson JL, Beeken RJ, McEwen A, Baldwin DR, et al. Lung Screen Uptake Trial (LSUT): Randomized Controlled Clinical Trial Testing Targeted Invitation Materials. Am J Respir Crit Care Med (2020) 201(8):965–75. doi: 10.1164/rccm.201905-0946OC
10. Colditz GA, Atwood KA, Emmons K, Monson RR, Willett WC, Trichopoulos D, et al. Harvard Report on Cancer Prevention Volume 4: Harvard Cancer Risk Index. Risk Index Working Group, Harvard Center for Cancer Prevention. Cancer Causes Control (2000) 11(6):477–88. doi: 10.1023/a:1008984432272
11. Chen H, Li N, Ren J, Feng X, Lyu Z, Wei L, et al. Participation and Yield of a Population-Based Colorectal Cancer Screening Programme in China. Gut (2019) 68(8):1450–7. doi: 10.1136/gutjnl-2018-317124
12. Jaklitsch MT, Jacobson FL, Austin JHM, Field JK, Jett JR, Keshavjee S, et al. The American Association for Thoracic Surgery Guidelines for Lung Cancer Screening Using Low-Dose Computed Tomography Scans for Lung Cancer Survivors and Other High-Risk Groups. J Thorac Cardiov Sur (2012) 144(1):33–8. doi: 10.1016/j.jtcvs.2012.05.060
13. Carter-Harris L, DuyKhanh PC, Hanna N, Rawl SM. Lung Cancer Screening: What do Long-Term Smokers Know and Believe? Health Expect (2017) 20(1):59–68. doi: 10.1111/hex.12433
14. Raju S, Khawaja A, Han X, Wang X, Mazzone PJ. Lung Cancer Screening: Characteristics of Nonparticipants and Potential Screening Barriers. Clin Lung Cancer (2020) 21(5):e329–36. doi: 10.1016/j.cllc.2019.11.016
15. Sung JJ, Choi SY, Chan FK, Ching JY, Lau JT, Griffiths S. Obstacles to Colorectal Cancer Screening in Chinese: A Study Based on the Health Belief Model. Am J Gastroenterol (2008) 103(4):974–81. doi: 10.1111/j.1572-0241.2007.01649.x
16. Wong MCS. Health Behavioral Models to Find Reasons for Low Rates of Lung Cancer Screening by Low-Dose Computed Tomography. JAMA Oncol (2018) 4(3):425. doi: 10.1001/jamaoncol.2017.0493
17. Dong P, Shi JF, Qiu WQ, Liu CC, Wang K, Huang HY, et al. Analysis on the Health Literacy of the Cancer Prevention and Treatment and Its Related Factors Among Urban Residents in China From 2015 to 2017. Chin J Prev Med (2020) 54(1):76–83. doi: 10.3760/cma.j.issn.0253-9624.2020.01.015
18. Dong P, Qiu WQ, Shi JF, Mao AY, Huang HY, Sun ZX, et al. Cancer Screening Service Utilization Amd Willingness-to-Pay of Urban Populations in China: A Cross-Sectional Survey From Potential Service Demander’s Perspective. Zhonghua Liu Xing Bing Xue Za Zhi (2018) 39(2):165–72. doi: 10.3760/cma.j.issn.0254-6450.2018.02.006
19. de Koning HJ, van der Aalst CM, de Jong PA, Scholten ET, Oudkerk M. Reduced Lung-Cancer Mortality With Volume CT Screening in a Randomized Trial. N Engl J Med (2020) 382(6):503–13. doi: 10.1056/NEJMoa1911793
20. Kinsinger LS, Anderson C, Kim J, Larson M, Chan SH, King HA, et al. Implementation of Lung Cancer Screening in the Veterans Health Administration. JAMA Intern Med (2017) 177(3):399–406. doi: 10.1001/jamainternmed.2016.9022
21. Wen Y, Yu L, Du LB, Wei DH, Liu YY, Yang ZY, et al. Analysis of Low−Dose Computed Tomography Compliance and Related Factors Among High−Risk Population of Lung Cancer in Three Provinces Participating in the Cancer Screening Program in Urban China. Chin J Prev Med (2021) 55(5):633–9. doi: 10.3760/cma.j.cn112150-20201015-01286
22. Ersek JL EJ, McDonnell KK. Knowledge of Attitudes Toward and Use of Low-Dose Computed Tomography for Lung Cancer Screening Among Family Physicians. Cancer (2016) 122(15):2324–31. doi: 10.1002/cncr.29944
23. Chen WQ, Cao MM. Strengthening Cancer Early Diagnosis and Treatment,Implementing the Strategy of Healthy China. China Cancer (2019) 28(9):643. doi: 10.11735/j.issn.1004-0242.2019.09.A001
24. Du J, He M, Qiu H. Results of Lung Cancer Screening Among Urban Residents in Chongqing, 2012~2017. China Cancer (2018) 27(5):328–32. doi: 10.11735/j.issn.1004-0242.2018.05.A002
25. Guo LW, Liu SZ, Zhang SK, Yang FN, Wu Y, Zheng LY, et al. Analysis of the Efficacy of Lung Cancer Screening in Urban Areas of Henan Province by Low-Dose Computed Tomography From 2013 to 2017. Chin J Oncol (2020) 2:155–9. doi: 10.3760/cma.j.issn.0253-3766.2020.02.013
26. Yang L, Zhang X, Liu S, Li HC. J F Ji. Lung Cancer Screening in Urban Beijing From 2014 to 2019. Chin J Prev Med (2021) 3:339–45. doi: 10.3760/cma.j.cn112150-20200817-01126
27. Xiao HF, Yan SP, Xu KK, Zou YH, Shi ZH, Zhu SL, et al. Analysis of Cancer Screening Program in Changsha Urban Area From 2012 to 2018. China Cancer (2019) 11:807–15. doi: 10.11735/j.issn.1004-0242.2019.11.A001
28. Zhang Q, Hhuang YC, Shen LD, Zhao YH, Jiang GX, Zhou H, et al. Analysis of Cancer Risk Assessment and Screening Results Among Urban Residents in Kunming City. China Cancer (2018) 9:641–6. doi: 10.11735/j.issn.1004-0242.2018.09.A001
29. Zhang LH, Du LB, Sun XH, Gao YM, Lei LV, Wang XH, et al. An Analysis on the Result of Early Detection and Treatment of Cancer in Zhejiang Urban Population. Zhejiang Prev Med (2018) 27(12):1189–93. doi: CNKI:SUN:ZYFX.0.2015-12-001
30. Henschke DIM CI, Yankelevitz DF. Early Lung Cancer Action Project: Overall Design and Findings From Baseline Screening. Lancet (1999) 354(9173):99–105. doi: 10.1016/S0140-6736(99)06093-6
31. International Early Lung Cancer Action Program I, Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, et al. Survival of Patients With Stage I Lung Cancer Detected on CT Screening. N Engl J Med (2006) 355(17):1763–71. doi: 10.1056/NEJMoa060476
32. Rob J, van Klaveren MO, Prokop M. Management of Lung Nodules Detected by Volume CT Scanning. N Engl J Med (2009) 361(23):2221–9. doi: 10.1056/NEJMoa0906085
33. Zhou QH, Fan YG, Wu N, Huang YC, Wang Y, Li L, et al. Demonstration Program of Population-Based Lung Cancer Screening in China: Rationale and Study Design. Thorac Cancer (2014) 5(3):197–203. doi: 10.1111/1759-7714.12078
34. Sone S, Takashima S, Li F, Yang Z, Honda T. Y Maruyama,Maruyama YMass Screening for Lung Cancer With Mobile Spiral Computed Tomography Scanner. Lancet (1998) 351(9111):1242–5. doi: 10.1016/S0140-6736(97)08229-9
35. McWilliams A, Tammemagi MC, Mayo JR, Roberts H, Liu G, Soghrati K, et al. Probability of Cancer in Pulmonary Nodules Detected on First Screening CT. N Engl J Med (2013) 369(10):910–9. doi: 10.1056/NEJMoa1214726
36. Horeweg N, van der Aalst CM, Thunnissen E, Nackaerts K, Weenink C, Groen HJ, et al. Characteristics of Lung Cancers Detected by Computer Tomography Screening in the Randomized NELSON Trial. Am J Respir Crit Care Med (2013) 187(8):848–54. doi: 10.1164/rccm.201209-1651OC
37. Subramaniam RP, Asgharian B, Freijer JI, Miller FJ, Anjilvel S. Analysis of Lobar Differences in Particle Deposition in the Human Lung. Inhal Toxicol (2003) 15(1):1–21. doi: 10.1080/08958370304451
38. Lince L, Lulu DJ. Carcinoma of the Lung. A Comparative Series of 687 Cases. Arch Surg (1971) 102(2):103–7. doi: 10.1001/archsurg.1971.01350020013004
Keywords: lung cancer, screening, Hebei province, participation rate, detection rate
Citation: Liang D, Shi J, Li D, Wu S, Jing J and He Y (2022) Participation and Yield of a Lung Cancer Screening Program in Hebei, China. Front. Oncol. 11:795528. doi: 10.3389/fonc.2021.795528
Received: 15 October 2021; Accepted: 09 December 2021;
Published: 10 January 2022.
Edited by:
Lizza E. L. Hendriks, Maastricht University Medical Centre, NetherlandsReviewed by:
Shaokai Zhang, Henan Provincial Cancer Hospital, ChinaCopyright © 2022 Liang, Shi, Li, Wu, Jin and He. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yutong He, aHl0b25nNjlAeWFob28uY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.