- 1Department of Microbiology, Prof. F. Łukaszczyk Oncology Centre, Bydgoszcz, Poland
- 2Department of Urological Oncology, Prof. F. Łukaszczyk Oncology Centre, Bydgoszcz, Poland
- 3Department of Medical Biology, Medical University of Warsaw, Warsaw, Poland
- 4Department of Surgical Oncology, Nicolaus Copernicus University Ludwik Rydygier’s Collegium Medicum, Bydgoszcz, Poland
- 5Department of Clinical Breast Cancer and Reconstructive Surgery, Prof. F. Łukaszczyk Oncology Centre, Bydgoszcz, Poland
Invasive Candida glabrata infections are not common complications after radical cystoprostatectomy. Furthermore, resistance to echinocandins arising during the course of a patient’s treatment is rarely recognised. We described a case of development of echinocandin resistance in a patient with muscle-invasive bladder cancer (pT2b N0 M0, high grade) diagnosis, subjected to radical cystoprostatectomy and exposed to echinocandins. A male patient with a previous surgical history after a traffic accident, who was operated on due to bladder cancer, underwent an episode of candidemia and mixed postoperative wound and urinary tract infection caused by C. glabrata and extended spectrum β-lactamase (ESBL)-producing Escherichia coli during hospital treatment. The patient was started on caspofungin. Repeat blood cultures showed clearance of the bloodstream infection; however, infection persisted at the surgical site. Resistance to echinocandins developed within 2 months from the day of initiation of therapy with caspofungin in the C. glabrata strain obtained from the surgical site. The isolates sequentially obtained during the patient’s treatment demonstrated resistance to echinocandins due to the mutation in hotspot 1 FKS2. Although resistance to echinocandins is relatively rare, it should be considered in oncological patients with increased complexity of treatment and intestinal surgery.
Introduction
The changing distribution of Candida species responsible for infections, with the predomination of Candida glabrata, significantly limits therapeutic options in oncological patients (1, 2). The first-line treatment of invasive infections includes echinocandins, such as C. glabrata, which present a low susceptibility to azoles (3). However, resistance to echinocandins has also been rising over the past decade. In some studied populations, especially in the USA, the rate of resistant isolates among this species exceeded 12.0% (4). In Europe, the prevalence of echinocandin-resistant C. glabrata is lower and reported to be 1.5% (5). One-third of echinocandin-resistant isolates may be multidrug resistant (6). As a consequence, infections due to C. glabrata pose a threat to patients, are difficult to treat, and are associated with a high mortality. Resistance to echinocandins due to a mutation in one or two “hot spot” regions of the FKS1 or FKS2 genes, encoding a subunit of the 1,3-β-D glucan synthase protein (the potential target enzyme echinocandins), is a serious clinical problem, resulting in echinocandin treatment failure and poor clinical outcomes (7). FKS genes are necessary for production of 1,3-β-D-glucan, a component of the Candida cell wall and the target of echinocandins, such as micafungin, anidulafungin, and caspofungin. Unlike amphotericin or azoles, these drugs are the safest for patients and most active on a broad spectrum for pathogenic fungal strains. The acquisition of resistance is often favoured by intra-abdominal candidiasis, repeat operations of the gastrointestinal tract, and poor drug penetration (3, 8). Infection can also be complicated by multidrug-resistant bacterial coinfection, which makes treatment even more difficult (9). We describe a case of rapid development of echinocandin resistance in a patient with an episode of candidemia and mixed postoperative wound and urinary tract infection caused by C. glabrata and extended spectrum β-lactamase (ESBL)-producing Escherichia coli.
Description of Patient
This work describes the case of a 66-year-old male patient who underwent surgery due to a muscle-invasive bladder cancer (pT2b N0 M0, high grade) and was subjected to radical cystoprostatectomy with a permanent, incontinent diversion of urine to the abdominal skin using a separated piece of small intestine as a stoma (ileal conduit). The course of the operation was difficult due to multiorgan traffic injury of the abdomen demanding urgent surgical treatment a few years prior. During the radical cystoprostatectomy, there was a need to dissolve many adhesions, remove ischaemic tissues, and partially resect the small bowel. Surgery was performed under antibiotic prophylaxis: ceftriaxone, 1 g every 12 h for 3 days and metronidazole, 0.5 g every 8 h for 3 days. On the eighth day of treatment, eventration and ileus occurred, requiring urgent surgery (to dissolve adhesions of the small intestine). An ileus recurred on day 11, and during the surgery, necrosis of the stoma was evident. The ischaemic tissues were removed, the ureters recatheterised, and bilateral percutaneous nephrostomies were performed. Wound healing was complicated by skin necrosis, which required several surgical treatments. A small intestine content appeared in the wound on day 20. Severe haemorrhage from the iliac artery occurred on day 57 and after urgent surgery, lower limb ischaemia appeared. The failure of treatment required a limb amputation at the thigh level. Ischaemic changes of the postoperative wounds and lower limb amputation stump progressed in the further course of hospitalisation. Dialysis was performed due to renal failure. On day 125, a stroke occurred. The patient died on day 186 after the primary surgery of excision of the urinary bladder as a result of gradually developing multiorgan failure (Figure 1).

Figure 1 Timeline of the clinical procedures performed in patients with muscle-invasive bladder cancer during Candida glabrata infection. AND, anidulafungin; MICA, micafungin; FLU, fluconazole.
Diagnostic Assessment and Results
Microbiological Monitoring
The patient was monitored microbiologically throughout the hospital stay. Blood, material from the postoperative wound, abscesses, urine, and peritoneal fluid were cultured. Three fungal isolates collected during the course of treatment were analysed. All examined strains were isolated during routine diagnostic procedures and then characterised by phenotypic and genotypic methods (Supplementary Material Data). The clinical cultures were performed on Sabouraud glucose agar. Species identification was performed using VITEK 2 YST ID card and multiplex polymerase chain reaction using a blood culture identification in the FilmArray system. The microorganism was identified as C. glabrata. All clinical isolates were reidentified by MALDI-TOF mass spectrometry.
On day 67, after the primary surgery, positive blood culture for C. glabrata was reported. All blood cultures drawn after 10 days from a positive result remained negative for Candida until the patient’s death. Ten days after the positive blood result, during caspofungin therapy, other fungal infections occurred. We detected C. glabrata in the postoperative wound (on day 126) and urine (nephrostomy, on day 147) with different susceptibility patterns as the isolate originated from the blood. Echinocandin-resistant isolate was detected in a wound sample at day 49 from the first positive result from this infection site, and on day 59 after initiation of therapy with caspofungin. At that time, we also isolated a phenotypically similar strain from urine. Both the postoperative wound and urine isolates were recovered from the same surgical site. The strain isolated from urine at day 80, after starting antifungal treatment with caspofungin, was subjected to molecular testing.
Additionally, the microorganisms inhabiting the digestive tract, specifically E. coli, Enterococcus faecium, Enterococcus faecalis, Bacteroides thetaiotaomicron, and Pseudomonas aeruginosa were isolated. E. coli recovered from the postoperative wound infections was identified as ESBL-E. coli, which is multidrug resistant and described in our previous work as belonging to the B2 phylogenetic group and carrying blaCTX-M-15 gene with a unique pulsed field gel electrophoresis pattern (10). As a result, a number of antibiotics, including those that are broad-spectrum, were ordered.
Antifungal Susceptibility
Antifungal susceptibility was determined by using Micronaut-AM EUCAST AFST 2-Test Plate, which was based on the broth microdilution procedure, as recommended by the manufacturer. Drug susceptibility was assessed according to The EUCAST antifungal clinical breakpoint table (11, 12).
The isolate from the blood sample was susceptible to all examined antifungal echinocandins (micafungin, anidulafungin) and amphotericin B; susceptibility to fluconazole was intermediate/susceptible with increased exposure. Except for resistance to echinocandins, the isolates recovered from the postoperative wound and urine samples were intermediate/increased exposure susceptible to fluconazole (minimal inhibitory concentration (MIC), 8 mg/L) and susceptible to amphotericin B (MIC, 0.5 mg/L) (Table 1). According to the epidemiological cutoff values (EVCs), isolates susceptible to anidulafungin with micafungin (MIC of 0.03 mg/L) did not harbour a FKS mutation conferring resistance to echinocandins. In addition, the susceptibility to fluconazole in the strain obtained from urine was assessed using gradient strips impregnated with fluconazole with E-test system. The interpretation criteria used is outlined above. Using this method, the C. glabrata strain showed fluconazole resistance (MIC, 64 mg/L).
DNA Manipulations and FKS Sequencing
Identification of the Candida isolates was confirmed by PCR using specific ITS1 and ITS4 primers directed against the panfungal internal transcribed spacer ITS1-5.8S-ITS4 region and further were digested with restriction enzymes (13, 14). A multiplex mPCR-ID assay identified the clinical isolates as C. glabrata sensu stricto and analysed by random amplified polymorphic DNA (Supplementary Figure S1) (15, 16).
The main goal of genotyping analysis was to detect FKS1 and FKS2 (β-D-1,3-glucan synthase complex) genes, and sequencing to evaluate the prevalence of hotspot-1 and hotspot-2 mutations. In this study, a search for mutations was performed by the PCR method (Supplementary Materials Data) (17–22). Nucleotide and deduced amino acid sequences obtained for beta-1,3-glucan synthase catalytic subunit Fks1 and Fks2 with hotspot-1 and hotspot-2 regions were compared with corresponding sequences of the C. glabrata ATCC90030 echinocandin-susceptible wild-type reference strain (GeneBank accession number HM366440 and HM3664442 for FKS1 and FKS2, respectively).
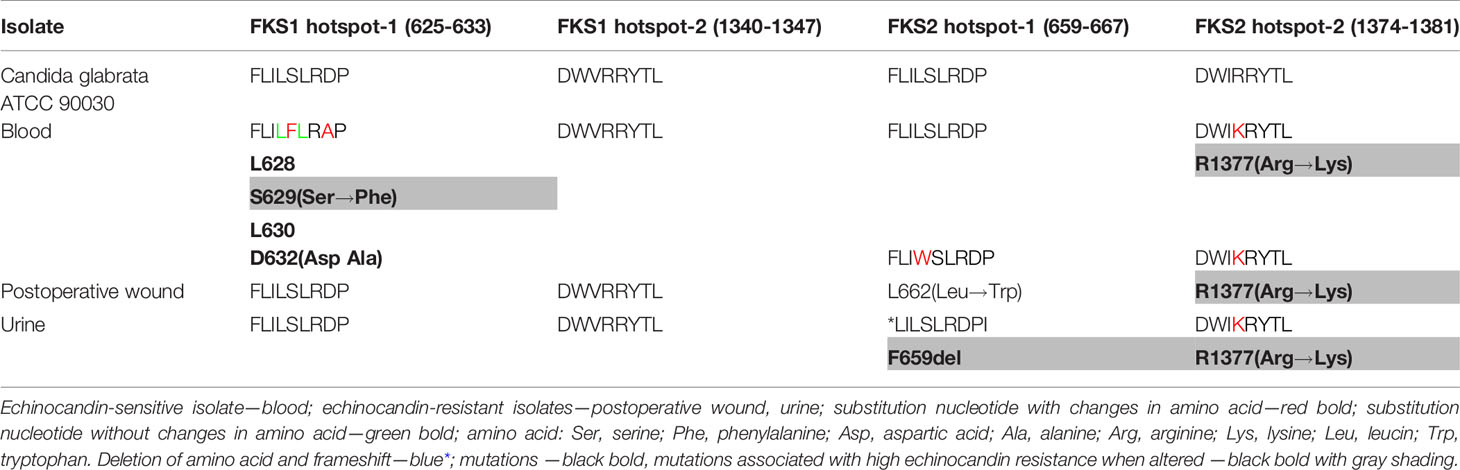
Only one strain, the C. glabrata isolate from blood, in which the phenotypic studies turned out to be sensitive against echinocandins, displayed Fks1 substitutions. The substitutions were in positions L628, S629, L630, and D632, of which S629 and D632 mutations generated amino acid substitutions, from serine to phenylalanine and from aspartic acid to alanine, respectively (Table 2). The mutations detected in the FKS1 hotspot-1 may be spontaneous and related to clinical echinocandin exposure (7, 23, 24). None of the analysed C. glabrata clinical isolates displayed FKS1 hotspot-2 region mutations. In all the clinical strains isolated from the postoperative wound and urine samples that were echinocandin-resistant, mutations in FKS2 hotspot-1 and FKS2 hotspot-2 were identified (Table 2). A single nucleotide changes in the FKS2 hotspot-1 of the wound isolate resulting in a leucin to tryptophan substitution at position 662 was identified. In the same region of the urine isolate, the deletion of three nucleotides leading to the removal of phenylalanine from Fks2 protein was observed. This mutation may result in the altered structure of the Fks2 protein and/or lack of its activity, which explains the negative response to the patient’s further treatment. In addition, one additional mutation in a FKS2 hotspot-2 region, namely R1377, leading to substitution of arginine to lysine, was detected for all clinical strains. Mutations in FKS2 could characterise echinocandin-resistant C. glabrata strains, especially in the hotspot-1 fragment. According to literature data, the mutations detected in the FKS1 hotspot-1 positions S629, FKS2 hotspot-1 positions F659del, and FKS2 hotspot-2 position R1377 may play an important role in rise of echinocandin-resistant mutants (18–22, 25).
Additionally, the epidemiology of fluconazole resistance and other azole resistance (indirectly) was performed with the detection of ERG11 and PDR1 genes (pleiotropic drug resistance). The product of the ERG11 gene leads to inhibition of demetylase needed for ergosterol biosynthesis (22, 26). The PDR1 gene acts as a regulator of the ATP-binding cassette (ABC), relevant in the drug efflux process and mitochondrial dysfunction by Candida species in the susceptibility to azoles (27, 28). The PCR reaction for EGR11 gene was present in the genomes of all tested clinical strains; however, with the second pair of ERG2 primers (Supplementary Table S1), no product was achieved from the urine samples (Supplementary Figure S1). This, in turn, may indicate a fragment deletion in the ERG11 gene from the C. glabrata genome isolated from the urine sample. This observation requires further studies, but undoubtedly, previous exposure to fluconazole could cause mutations in the ERG11 gene, especially the deletion observed in other studies (14, 22, 27). An additional E-test assay performed as an alternative method for the urine isolate resulted in a MIC value of 64 mg/ml. This value may indicate resistance to fluconazole, and thus confirm the deletion in the ERG11 gene in this isolate. In addition, other authors have reported inconsistencies in the results of broth microdilution and the E-test method for echinocandin-resistant C. glabrata strains that were not observed for susceptible strains (22, 29). The PDR1 gene was identified in all clinical isolates by PCR assay, which may result in a reduced susceptibility to fluconazole (14, 27).
Antifungal Treatment
The patient was prophylactically treated with fluconazole from March 10 to 25, 2019 and from April 22 to 27, 2019, receiving a single intravenous (IV) dose of 200 mg once daily. On April 28, 2019 (day 67), the patient was switched from fluconazole to caspofungin, which was maintained until May 9 at a dose of 50 mg daily (70 mg loading dose on the first day). Anidulafungin was then administered from May 10 to 28, 2019 (79th–97th day of stay), a single IV loading dose of anidulafungin 200 mg on day 1, followed by 100 mg once daily and caspofungin from June 01 to 26, 2019 (101st–126th day of stay), as described above. On July 17, 2019 (day 147), the patient was started on liposomal amphotericin B (L-AmB) at a dose of 5 mg/kg of body weight daily and continued until August 06, 2019 (day 167).
Discussion
Growing resistance to antifungal drugs is becoming a serious challenge, especially among oncological patients. The phenomenon of resistance build-up during the treatment of Candida infections is of particular concern. We reported a case of a patient with muscle-invasive bladder cancer and candidemia episode, caused by C. glabrata susceptible to echinocandins and later C. glabrata infection of the postoperative wound and urinary tract complicated by infection with multidrug-resistant E. coli. We have demonstrated that isolates sequentially obtained from the analysed patient during the patient’s treatment developed resistance to echinocandins due to a FKS2 hotspot-1 mutation.
The combination of multiple risk factors in our patient with a solid tumour of the bladder presented a high risk for Candida infections. The main risk factors were the complexity of surgery, reoperations, exposure to broad-spectrum antibiotics in the course of multidrug-resistant bacteria infection treatment (ESBL-E. coli), multiple blood transfusions, and older age. Moreover, a stay in the intensive care unit and a prolonged hospital stay also could favour Candida infection, as previously reported (30, 31). Most significant, however, was the patient’s previous surgical treatment history, which resulted in a large extent of ischaemic intestinal lesions after radical cystoprostatectomy.
In accordance with the adopted strategy of managing infections in our hospital, the patient was monitored microbiologically and the antifungal therapy was selected according to the etiological agents of infection and susceptibility patterns. Moreover, in the case of candidemia, the use of a genetic method-multiplex PCR for the detection of microorganisms in the blood allowed for the implementation of antifungal treatments appropriate to species identification before the assessment of drug susceptibility. The proceedings were consistent with the current literature data, as the delay in administration of antifungals in candidemia is associated with a higher risk of therapeutic failure among patients with candidemia (32, 33).
Candidemia treatment, first with caspofungin and then with anidulafungin, resulted in a positive response without any new positive episode of candidemia during the period of hospitalisation. However, during therapy, other fungal infections were recognised at the surgical site. For that reason, prolonged and repeated antifungal treatment with caspofungin was initiated and continued for 26 days. First, an echinocandin-resistant isolate was detected in the wound sample within 2 months of initiation of caspofungin therapy. At that time, a phenotypically similar strain from the urine was also isolated. Both the postoperative wound and urine isolates were recovered from the same surgical site. Clinical trials show that >5 days of antifungal therapy (especially high-dose caspofungin, >100 mg) was directed to the occurrence of FKS mutations, especially in isolates from the urine tract (22, 34, 35).
It is extremely important to determine the EVCs for echinocandins that may be a phenotypic indicator for the occurrence of Fks mutations. Some authors suggest an EVC of 0.25 mg/L for caspofungin, 0.12 mg/ml for anidulafungin and 0.03 mg/ml for micafungin (17, 20, 35). Similarly, in our study, a MIC of 0.125 mg/ml for anidulafungin and 0.06 mg/ml for micafungin resulted in mutations in FKS2. Micafungin and anidulafungin could play important roles as markers of FKS mutations (21, 22, 35).
In our case report, we demonstrated that the isolates obtained from the surgical site showed increased MIC values of echinocandins that rendered them echinocandin resistant (anidulafungin and micafungin were examined in this study), consistent with the EUCAST antifungal clinical breakpoint table v. 9.0 and table v. 10. According to the last EUCAST recommendation (table v.10), isolates with micafungin MIC >0.03 mg/L are considered to be resistant due to harbouring a FKS mutation and Fks sequencing is recommended. In our case, molecular testing confirmed resistance to echinocandins by hotspot-1 FKS2 mutation. The isolate originating from urine demonstrated amino acid deletion F659del, which is most common in C. glabrata and is associated with resistance and poor prognosis (21, 36, 37). This isolate showed the highest MIC value for echinocandins among the examined isolates. The isolate recovered from the postoperative wound possessed mutation L662 and was demonstrated at low-level resistance for echinocandins (MIC for anidulafungin, 0.125 mg/L; for micafungin, 0.06 mg/L). In the blood isolate, no effect of the S629 mutation in the hotspot-1 region of the FKS1 gene regarding reduced sensitivity to echinocandins was observed in this analysed case. This result was confirmed in many studies that reported Candida strains susceptible to echinocandins with mutations S629 in the FKS1 hotspot-1 (21, 29, 35). Approximately 80% of susceptible strains could present changes in the genotype (21). These results were reported by Aldejohann et al. (29), who tested these same echinocandin-susceptible strains with a few mutations independently in eight different labs in Germany. In contrast, in the study by Castanheira et al. (37), the isolates that displayed this mutation presented resistance to echinocandins.
Prior exposure to antifungal agents, i.e., prophylaxis with fluconazole before candidemia (for a total of 22 days), probably influenced the selection of C. glabrata with naturally reduced azole susceptibility. In addition, prior treatment with caspofungin (for a total of 37 days) influenced the selection pressure for C. glabrata, with increasing MICs for echinocandins and subsequent resistance to this group of antifungals. Echinocandin-susceptible isolates without mutation in hotspot-1 FKS2 gene in the study patient was detected before the isolation of resistant isolates and may indicate the acquisition of resistance during treatment, as reported in other studies (4). Mutations in the FKS1 hotspot-1 in amino acid positions 625 or 632 and in the FKS2 hotspot-1 in positions 659, 662, or 663 were found in C. glabrata resistant to new antifungal drugs (for example, ibrexafungerp), which was investigated by Arendrup et al. (38), and has evolved into a clinical problem for next decades.
It seems that the most likely source of the candidemia episode in our patient was the gastrointestinal tract due to the possible imbalance of the intestinal microbiome, a large extent of intestinal necrosis, damaged mucosal tissue, and intestinal fistulas and intestinal leakage, as previously observed by other authors (34, 39). As a consequence, the local condition of the abdominal cavity likely reduced drug penetration. In turn, poor drug penetration and the persistence of prolonged subinhibitory echinocandin levels could influence selection pressure and the emergence of resistance (3, 9). Coexistence in surgical site infection of C. glabrata and multidrug-resistant bacteria (ESBL-producing E. coli) points to a significant problem among patients with solid tumours and emphasises the importance of the gastrointestinal tract as a reservoir of multidrug-resistant microorganisms in cancer patients. Finally, antifungal treatment with liposomal amphotericin B eventually led to microbiological success. Unfortunately, the ESBL-positive infection persisted, despite targeted treatment with carbapenems. Ultimately, further deterioration of the patient’s clinical condition led to death.
In conclusion, resistance to echinocandins in C. glabrata developed in a patient presenting with muscle-invasive bladder cancer and surgical history due to previous multiorgan traffic injury of the abdomen. Complexity of treatment and intestinal surgery should compel the treating physician to consider resistant Candida infection, although resistance to echinocandins in European countries is still rare.
Strength of the study:
1. The rare case report presenting Candida glabrata FKS mutants in patients with solid tumours.
2. In this case, apart from known mutations (S629; F659del), we described rare mutations in FKS2 hotspot-1 (L662: leucin to tryptophan); in hotspot-2 (R1377: arginine to lysine); and in FKS1 hotspot-1 (D632: aspartic acid to alanine), which led to changes in amino acids and protein properties, echinocandin resistance, and higher MICs values.
3. Correlation of echinocandin MIC values with the results of previous scientific research mapping Fks mutations within the hotspot conserved regions seems to be a good screening method in detecting clinical resistance of C. glabrata to echinocandins. This monitoring could be in a procedure algorithm in relation to all patients with intestinal surgery exposed to endogenous C. glabrata infections.
4. The presented results, especially estimation of the MICs and epidemiological cutoff values (EVCs) for all echinocandins that may be indicative of the presence of a mutation leading to clinical failure, could help in quickly detecting resistance and administering the appropriate doses of antifungal drugs.
Limitation of the study:
1. The substitutions of the nucleotides leading to changes in amino acid in the genes encoding glucan synthase (Fks) constitute only one mechanism that contributes to the lack of echinocandin interaction with the C. glabrata cell wall. There are many possible mechanisms for echinocandin resistance of C. glabrata, for example, exposure to echinocandins (also prophylaxis in risk groups); changes in cell wall composition (increase of chitin/mannan) related to the activation of RHO1 gene and MAPK cascade dependent on calconeurine or regulation in the cell cycle; deletion of yapsin-like proteins in the cell wall; and changes in membrane sphingolipids. For this reason, whole genome or exome sequencing and searching for new resistance mechanisms should be the subject of future research on an appropriate statistically significant number of strains.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material. Further inquiries can be directed to the corresponding author.
Ethics Statement
The studies involving human participants were reviewed and approved by the Bioethical Commission at the Collegium Medicum of Nicolaus Copernicus University in Toruń (KB approval number 453/2021). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
MS was responsible for the conception and design of the study. MS, TN, and KK organised the database and resources. SJ and AK-B performed the genetic examinations and analysis. GO contributed to the interpretation of molecular results. MS, SJ, and AK-B wrote original draft preparation. MS and SJ wrote review and editing. MS provided supervision and project administration. All authors were involved in the analysis of findings, proved the manuscript, and contributed to writing. All authors read and approved the final version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.794235/full#supplementary-material
References
1. Farmakiotis D, Tarrand JJ, Kontoyiannis DP. Drug-Resistant Candida Glabrata Infection in Cancer Patients. Emerg Infect Dis (2014) 20(11):1833–40. doi: 10.3201/eid2011.140685
2. Szymankiewicz M, Nowikiewicz T. Etiology of Candidemia in Patients With Solid Tumors - 7 Years of Experience of One Oncology Center. Neoplasma (2020) 67(6):1391–9. doi: 10.4149/neo_2020_200204N105
3. Perlin DS, Rautemaa-Richardson R, Alastruey-Izquierdo A. The Global Problem of Antifungal Resistance: Prevalence, Mechanisms, and Management. Lancet Infect Dis (2017) 17(12):e383–92. doi: 10.1016/S1473-3099(17)30316-X
4. Alexander BD, Johnson MD, Pfeiffer CD, Jimenez-Ortigosa C, Catania J, Booker R, et al. Increasing Echinocandin Resistance in Candida Glabrata: Clinical Failure Correlates With Presence of FKS Mutations and Elevated Minimum Inhibitory Concentrations. Clin Infect Dis (2013) 56(12):1724–32. doi: 10.1093/cid/cit136
5. Klotz U, Schmidt D, Willinger B, Steinmann E, Buer J, Rath PM, et al. Echinocandin Resistance and Population Structure of Invasive Candida Glabrata Isolates From Two University Hospitals in Germany and Austria. Mycoses (2016) 59(5):312–8. doi: 10.1111/myc.12472
6. Pham CD, Iqbal N, Bolden CB, Kuykendall RJ, Harrison LH, Farley MM, et al. Role of FKS Mutations in Candida Glabrata: MIC Values, Echinocandin Resistance, and Multidrug Resistance. Antimicrob Agents Chemother (2014) 58(8):4690–6. doi: 10.1128/AAC.03255-14
7. Shields RK, Kline EG, Healey KR, Kordalewska M, Perlin DS, Nguyen MH, et al. Spontaneous Mutational Frequency and FKS Mutation Rates Vary by Echinocandin Agent Against Candida Glabrata. Antimicrob Agents Chemother (2019) 63(1):e01692–18. doi: 10.1128/AAC.01692-18
8. Lewis JS 2nd, Wiederhold NP, Wickes BL, Patterson TF, Jorgensen JH. Rapid Emergence of Echinocandin Resistance in Candida Glabrata Resulting in Clinical and Microbiologic Failure. Antimicrob Agents Chemother (2013) 57(9):4559–61. doi: 10.1128/AAC.01144-13
9. Shields RK, Nguyen MH, Press EG, Clancy CJ. Abdominal Candidiasis is a Hidden Reservoir of Echinocandin Resistance. Antimicrob Agents Chemother (2014) 58(12):7601–5. doi: 10.1128/AAC.04134-14
10. Szymankiewicz M, Stefaniuk E, Baraniak A, Nowikiewicz T. Clinical and Molecular Findings of Infections Caused by Extended-Spectrum Beta-Lactamase-Producing Enterobacterales in Patients With Solid Tumors: A Single-Center Study. Microb Drug Resist (2021) 27(11):1470–81. doi: 10.1089/mdr.2020.0530
11. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs for Antifungal Agents, Version 9.0 (2018). Stokholm, Sweden: EUCAST. Available at: http://www.eucast.org/astoffungi/clinicalbreakpointsforantifungals/ (Accessed June 2021 2021).
12. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs for Antifungal Agents, Version 10.0 (2020). Stokholm, Sweden: EUCAST. Available at: http://www.eucast.org/astoffungi/clinicalbreakpointsforantifungals/ (Accessed June 2021 2021).
13. Asadzadeh M, Alanazi AF, Ahmad S, Al-Sweih N, Khan Z. Lack of Detection of Candida Nivariensis and Candida Bracarensis Among 440 Clinical Candida Glabrata Sensu Lato Isolates in Kuwait. PloS One (2019) 14(10):e0223920. doi: 10.1371/journal.pone.0223920
14. Taei M, Chadeganipour M, Mohammadi R. An Alarming Rise of non-Albicans Candida Species and Uncommon Yeasts in the Clinical Samples; a Combination of Various Molecular Techniques for Identification of Etiologic Agents. BMC Res Notes (2019) 12(1):779. doi: 10.1186/s13104-019-4811-1
15. Ergon MC, Gulay Z. Molecular Epidemiology of Candida Species Isolated From Urine at an Intensive Care Unit. Mycoses (2005) 48(2):126–31. doi: 10.1111/j.1439-0507.2004.01086.x
16. Olchawa A, Krawczyk B, Brillowska-Dabrowska A. New PCR Test for Detection of Candida Glabrata Based on the Molecular Target Chosen by the RAPD Technique. Pol J Microbiol (2013) 62(1):81–4. doi: 10.33073/pjm-2013-011
17. Zimbeck AJ, Iqbal N, Ahlquist AM, Farley MM, Harrison LH, Chiller T, et al. FKS Mutations and Elevated Echinocandin MIC Values Among Candida Glabrata Isolates From U.S. Population-Based Surveillance. Antimicrob Agents Chemother (2010) 54(12):5042–7. doi: 10.1128/AAC.00836-10
18. Dudiuk C, Gamarra S, Leonardeli F, Jimenez-Ortigosa C, Vitale RG, Afeltra J, et al. Set of Classical PCRs for Detection of Mutations in Candida Glabrata FKS Genes Linked With Echinocandin Resistance. J Clin Microbiol (2014) 52(7):2609–14. doi: 10.1128/JCM.01038-14
19. Khan Z, Ahmad S, Mokaddas E, Meis JF, Joseph L, Abdullah A, et al. Development of Echinocandin Resistance in Candida Tropicalis Following Short-Term Exposure to Caspofungin for Empiric Therapy. Antimicrob Agents Chemother (2018) 62(4):e01926–17. doi: 10.1128/AAC.01926-17
20. Kritikos A, Neofytos D, Khanna N, Schreiber PW, Boggian K, Bille J, et al. Accuracy of Sensititre YeastOne Echinocandins Epidemiological Cut-Off Values for Identification of FKS Mutant Candida Albicans and Candida Glabrata: A Ten Year National Survey of the Fungal Infection Network of Switzerland (FUNGINOS). Clin Microbiol Infect (2018) 24(11):1214.e1211–1214.e1214. doi: 10.1016/j.cmi.2018.05.012
21. Rivero-Menendez O, Navarro-Rodriguez P, Bernal-Martinez L, Martin-Cano G, Lopez-Perez L, Sanchez-Romero I, et al. Clinical and Laboratory Development of Echinocandin Resistance in Candida Glabrata: Molecular Characterization. Front Microbiol (2019) 10:1585. doi: 10.3389/fmicb.2019.01585
22. Al-Baqsami ZF, Ahmad S, Khan Z. Antifungal Drug Susceptibility, Molecular Basis of Resistance to Echinocandins and Molecular Epidemiology of Fluconazole Resistance Among Clinical Candida Glabrata Isolates in Kuwait. Sci Rep (2020) 10(1):6238. doi: 10.1038/s41598-020-63240-z
23. Pfaller MA, Messer SA, Rhomberg PR, Borroto-Esoda K, Castanheira M. Differential Activity of the Oral Glucan Synthase Inhibitor SCY-078 Against Wild-Type and Echinocandin-Resistant Strains of Candida Species. Antimicrob Agents Chemother (2017) 61(8):e00161–17. doi: 10.1128/AAC.00161-17
24. Spettel K, Galazka S, Kriz R, Camp I, Willinger B. Do Candida Albicans Isolates With Borderline Resistant Micafungin MICs Always Harbor FKS1 Hot Spot Mutations? J Fungi (Basel) (2021) 7(2):93. doi: 10.3390/jof7020093
25. Morio F, Jensen RH, Le Pape P, Arendrup MC. Molecular Basis of Antifungal Drug Resistance in Yeasts. Int J Antimicrob Agents (2017) 50(5):599–606. doi: 10.1016/j.ijantimicag.2017.05.012
26. Xu Y, Chen L, Li C. Susceptibility of Clinical Isolates of Candida Species to Fluconazole and Detection of Candida Albicans ERG11 Mutations. J Antimicrob Chemother (2008) 61(4):798–804. doi: 10.1093/jac/dkn015
27. Tsai HF, Krol AA, Sarti KE, Bennett JE. Candida Glabrata PDR1, a Transcriptional Regulator of a Pleiotropic Drug Resistance Network, Mediates Azole Resistance in Clinical Isolates and Petite Mutants. Antimicrob Agents Chemother (2006) 50(4):1384–92. doi: 10.1128/AAC.50.4.1384-1392.2006
28. Simonicova L, Moye-Rowley WS. Functional Information From Clinically-Derived Drug Resistant Forms of the Candida Glabrata Pdr1 Transcription Factor. PloS Genet (2020) 16(8):e1009005. doi: 10.1371/journal.pgen.1009005
29. Aldejohann AM, Herz M, Martin R, Walther G, Kurzai O. Emergence of Resistant Candida Glabrata in Germany. JAC Antimicrob Resist (2021) 3(3):dlab122. doi: 10.1093/jacamr/dlab122
30. Lortholary O, Renaudat C, Sitbon K, Desnos-Ollivier M, Bretagne S, Dromer F, et al. The Risk and Clinical Outcome of Candidemia Depending on Underlying Malignancy. Intensive Care Med (2017) 43(5):652–62. doi: 10.1007/s00134-017-4743-y
31. De Rosa FG, Busca A, Capparella MR, Yan JL, Aram JA. Invasive Candidiasis in Patients With Solid Tumors Treated With Anidulafungin: A Post Hoc Analysis of Efficacy and Safety of Six Pooled Studies. Clin Drug Investig (2021) 41(6):539–48. doi: 10.1007/s40261-021-01024-7
32. Puig-Asensio M, Padilla B, Garnacho-Montero J, Zaragoza O, Aguado JM, Zaragoza R, et al. Epidemiology and Predictive Factors for Early and Late Mortality in Candida Bloodstream Infections: A Population-Based Surveillance in Spain. Clin Microbiol Infect (2014) 20(4):O245–54. doi: 10.1111/1469-0691.12380
33. Tang HJ, Liu WL, Lin HL, Lai CC. Epidemiology and Prognostic Factors of Candidemia in Cancer Patients. PloS One (2014) 9(6):e99103. doi: 10.1371/journal.pone.0099103
34. Healey KR, Nagasaki Y, Zimmerman M, Kordalewska M, Park S, Zhao Y, et al. The Gastrointestinal Tract Is a Major Source of Echinocandin Drug Resistance in a Murine Model of Candida Glabrata Colonization and Systemic Dissemination. Antimicrob Agents Chemother (2017) 61(12):e01412–17. doi: 10.1128/AAC.01412-17
35. Arastehfar A, Daneshnia F, Salehi M, Yasar M, Hosbul T, Ilkit M, et al. Low Level of Antifungal Resistance of Candida Glabrata Blood Isolates in Turkey: Fluconazole Minimum Inhibitory Concentration and FKS Mutations can Predict Therapeutic Failure. Mycoses (2020) 63(9):911–20. doi: 10.1111/myc.13104
36. Shields RK, Nguyen MH, Press EG, Updike CL, Clancy CJ. Caspofungin MICs Correlate With Treatment Outcomes Among Patients With Candida Glabrata Invasive Candidiasis and Prior Echinocandin Exposure. Antimicrob Agents Chemother (2013) 57(8):3528–35. doi: 10.1128/AAC.00136-13
37. Castanheira M, Woosley LN, Messer SA, Diekema DJ, Jones RN, Pfaller MA. Frequency of Fks Mutations Among Candida Glabrata Isolates From a 10-Year Global Collection of Bloodstream Infection Isolates. Antimicrob Agents Chemother (2014) 58(1):577–80. doi: 10.1128/AAC.01674-13
38. Arendrup MC, Jorgensen KM, Hare RK, Chowdhary A. In Vitro Activity of Ibrexafungerp (SCY-078) Against Candida Auris Isolates as Determined by EUCAST Methodology and Comparison With Activity Against C. Albicans and C. Glabrata and With the Activities of Six Comparator Agents. Antimicrob Agents Chemother (2020) 64(3):e02136–19. doi: 10.1128/AAC.02136-19
Keywords: Candida glabarta, echinocandins, antifungal resistance, FKS mutation, bladder cancer cystoprostatectomy
Citation: Szymankiewicz M, Kamecki K, Jarzynka S, Koryszewska-Bagińska A, Olędzka G and Nowikiewicz T (2021) Case Report: Echinocandin-Resistance Candida glabrata FKS Mutants From Patient Following Radical Cystoprostatectomy Due to Muscle-Invasive Bladder Cancer. Front. Oncol. 11:794235. doi: 10.3389/fonc.2021.794235
Received: 13 October 2021; Accepted: 19 November 2021;
Published: 15 December 2021.
Edited by:
Antonio Augusto Ornellas, National Cancer Institute (INCA), BrazilReviewed by:
Maria Helena Ornellas, Universidade Estadual do Rio de Janeiro, BrazilAyse Banu Demir, University of Economics, Turkey
Copyright © 2021 Szymankiewicz, Kamecki, Jarzynka, Koryszewska-Bagińska, Olędzka and Nowikiewicz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Maria Szymankiewicz, c3p5bWFua2lld2ljem1AY28uYnlkZ29zemN6LnBs
 Maria Szymankiewicz
Maria Szymankiewicz Krzysztof Kamecki2
Krzysztof Kamecki2 Sylwia Jarzynka
Sylwia Jarzynka Gabriela Olędzka
Gabriela Olędzka