
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
REVIEW article
Front. Oncol. , 03 January 2022
Sec. Hematologic Malignancies
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.793274
This article is part of the Research Topic The Elephant in the Room: AML Relapse After Allo-HCT View all 7 articles
Relapsed acute myeloid leukemia (AML) following allogeneic hematopoietic cell transplantation (allo-HCT) is an unfavorable event associated with a poor prognosis, particularly for patients with early relapses. It usually arises from resistant leukemic blasts that escaped both preparative chemotherapy regimen and the graft-versus-leukemia (GVL) effect. Independent from the choice of salvage treatment, only minority of patients can achieve durable remissions. In recent years, better understanding of the disease relapse biology post allo-HCT allowed the application of newer strategies that could induce higher rates of remission, and potential longer survival. Those strategies aim at optimizing drugs that have a direct anti-leukemia activity by targeting different oncogenic mutations, metabolism pathways or surface antigens, and concurrently enhancing the immune microenvironment to promote GVL effect. This review discusses the current treatment landscape of AML relapse post allo-HCT.
Allogeneic hematopoietic cell transplantation (allo-HCT) continues to serve as a potentially curative step in the management of intermediate- and high-risk acute myeloid leukemia (AML) (1). Nowadays, virtually all patients can benefit from allo-HCT owing to the advances in the field from the use of reduced-intensity conditioning (RIC) for older and/or unfit patients, to the availability of alternative donors, and the improvement in supportive care and graft-versus-host disease (GVHD) prophylaxis. Over the past 40 years, the non-relapse mortality (NRM) at one year has declined significantly from 24% in the 1990s to less than 10% from 2013 through 2016 (2). However, disease relapse remains the main treatment failure following allo-HCT, and is associated with dismal outcomes and a median overall survival (OS) of few months (3–5). The cumulative incidence of relapse is largely dependent on the disease risk, the intensity of conditioning regimen, and the type of donor used, varying between 15-20% after a myeloablative conditioning, 30-50% after RIC regimen, and up to 58% when RIC is used for haploidentical transplants (6–9). Majority of the relapses occur within the first year after allo-HCT.
AML relapse post allo-HCT poses a serious challenge to clinicians, as despite increasing survival rates of younger patients relapsing post allo-HCT, their 2-year OS didn’t exceed 26% (3, 10, 11). A minority of patients can be rescued with a second allo-HCT which also provides a long-term survival to no more than one-third of the patients (10). In front of an elephant in the room, traditional therapies have proven to be ineffective in attaining long-term remissions without toxicity and newer therapeutic approaches have become a necessity.
Understanding the factors that led to disease relapse will help in developing and exploring rationally designed combination therapies. First, relapsing leukemia cells showed resistance to preparative chemotherapy either during induction/consolidation or within the conditioning regimen, but most importantly, they had definitely an immune escape mechanism to evade the donor-derived T cells. With the advent of new sequencing technologies, many oncogenic targets have been discovered and served as an additional tool in the armamentarium of AML management. The main goal of treatment is first to eradicate leukemia cells using effective anti-leukemia therapies, and then activate the donor alloreactive T cells toward a graft-versus-leukemia (GVL) direction. This review will discuss current therapeutic options including those directed against leukemia cells, in addition to other strategies aiming at enhancing the GVL effect of immune cells (Figure 1).
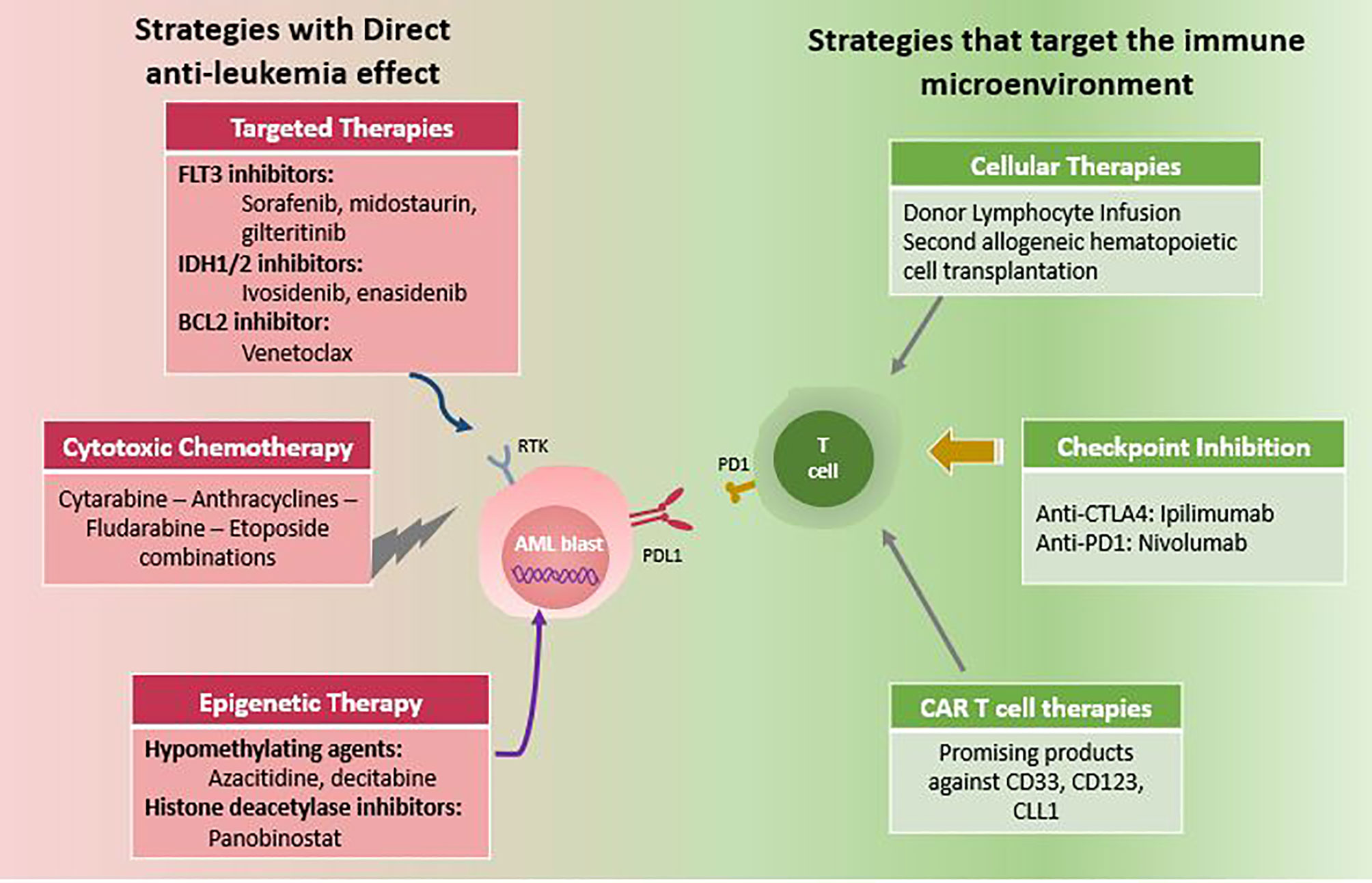
Figure 1 Therapeutic options for post-transplant AML relapse. Note: Combination of two or more options are increasingly used.
Discussing the different escape mechanisms by which leukemia usually relapse post allo-HCT is not within the scope of this review, however our understanding of the biology of disease relapse will help develop strategies to prevent and/or treat AML in the post allo-HCT setting. Multiple patient-, disease-, and transplant related factors are implicated in an increased risk of relapse post allo-HCT, including older age, high-risk cytogenetics, the presence of FLT3 mutation, the absence of NPM1 mutation, the time from achieving remission to allo-HCT, the absence of GVHD and the in-vivo T-cell depletion (12). These factors contribute differently in early and late relapses (12). Early relapses are usually driven by a highly proliferative disease that outstrip the rate of GVL development. The genetic biology of AML plays an important role in determining subgroups of patients with chemoresistance (13). For example, FLT3-ITD mutation is known to be associated with a high risk of relapse and worse survival post allo-HCT (14). Mutations in WT1 and TP53 mutations are also associated with higher risk of relapse post allo-HCT and worse survival (15). Moreover, AML consists usually of multiple genetically distinct subclones that are variable in their phenotype and frequency. These subclones contain different numbers of leukemia initiating cells with self-renewal potential (16). The anti-leukemia therapy exerts different pressures on these subclones, leading to clonal evolution and emergence of therapy-resistant population that cause AML relapse.
In the post allo-HCT setting, the mechanism of disease relapse becomes more complex, involving the immune microenvironment. Multiple mechanisms have been described including the dysregulation in pathways involved in adaptive and innate immunity, such as the downregulation of major histocompatibility complexes (MHC) Class II genes (HLA-DPA1, HLA-DPB1, HLA-DQB1, and HLA-DRB1), the downregulation of NK cell targets, the loss of expression of an HLA haplotype, and the increased expression of inhibitory checkpoint ligands (17–20).
The initial goal in treating AML relapse post allo-HCT is to decrease the leukemia burden and ultimately achieve a deep complete remission (CR) that is sustainable. Among therapies with direct anti-leukemia effect, is the use of intensive cytotoxic chemotherapy. Despite the rapid debulking effect, a minority of patients can tolerate such treatment especially in the early post-transplant phase. In addition, the use of chemotherapy alone is generally not sufficient with a 2-year OS not exceeding 7% (7). The CR rates post intensive chemotherapy rarely exceed 30% to 40% with an increased risk of treatment-related mortality to 20%. Patients with a post-transplant remission duration longer than 6 months seem to better tolerate the treatment, and have a higher likelihood of response. Those who successfully respond to intensive chemotherapy, and were subsequently bridged to a second allo-HCT will have a 2-year OS of 40% (21, 22).
The best results to date have been achieved after donor cell therapies either with donor lymphocyte infusion (DLI) or a second allo-HCT with 2-year OS rate between 20 and 40% (21, 23). Better outcomes are usually encountered in patients who achieved morphologic CR or low burden of disease at time of cellular therapy (3). Thus, other effective anti-leukemia strategies capable of achieving remissions are extremely needed. These can involve targeting tumor-specific surface antigens, cellular metabolism, and activating oncogenic mutations.
Hypomethylating agents (HMAs) such as azacitidine and decitabine are effective anti-leukemia drugs with a proven efficacy in the frontline treatment of AML in older patients. Their use as single agents resulted in improvements in clinical outcomes of older AML patients with a median OS of 8-10 months (24, 25). Due to its efficacy and tolerability, HMA therapy was largely used by many clinicians in the treatment of AML relapse post allo-HCT (26, 27). Initially, it was thought that HMAs are capable of activating silenced tumor suppressor genes through hypomethylation, however many other mechanistic actions were discovered throughout the years, contributing to their effectiveness in the post-transplant setting. By increasing the expression of epigenetically silent tumor antigens, azacitidine can enhance the GVL effect by activating CD8+ T cells directed toward many tumor antigens like melanoma-associated Ag 1, B melanoma antigen 1, and Wilm tumor Ag 1 (28). Concurrently, azacitidine can expand regulatory T cells, thus decreasing the risk of GVHD (29).
Despite the excitement regarding the use of HMAs in the post-transplant relapse, limited number of patients responded, and long-term survival was not possible. Craddock et al. reported the outcomes of patients with AML or high-risk myelodysplastic syndrome (MDS) treated with azacitidine +/- DLI for post-transplant relapse (27). The CR rate was only 15% after a median of 3.6 months. The 2-year OS for all patients was 12.4%, but 48% for those who responded to azacitidine. Interestingly, the addition of DLI to the regimen did not impact the response rate nor the survival (27). Many other retrospective trials investigated the use of DLI in addition to azacitidine or decitabine and resulted in an overall response rate (ORR) of 25-33%, and a 2-year OS between 11% and 29% (30–32).
With the purpose of enhancing the effect of azacitidine, multiple combination therapies were assessed. The combination of azacitidine with lenalidomide might have an additive anti-leukemic effect, while azacitidine decreases the risk of acute GVHD from lenalidomide (33). In the VIOLA trial, 29 patients were treated with standard dose azacitidine (75 mg/m2 for 7 days) with escalated doses of lenalidomide (5-25 mg daily), 15 patients received at least 3 cycles of treatment with modest toxicity, and no increased rates of GVHD. Among evaluable patients, the ORR and CR rates were 47% and 20% respectively, considered to be slightly higher than azacitidine alone when compared to previous reports. Those who responded had a better median OS of 27 months compared to 10 months in non-responders (p=0.004) (33).
The treatment of AML with novel agents is fast evolving, with multiple targeted agents already FDA approved in different settings. Currently, many new targeted agents are effective in AML, and can be used in the post-transplant relapse setting (34). HMAs can also be the backbone treatment for any combination treatment with targeted agents such as BCL2-inhibitors, FLT3 inhibitors and IDH1/2 inhibitors.
Venetoclax is an orally available selective BCL2 inhibitor, that competitively combines to BH3 domain and induces apoptosis of leukemia stem cells. It is currently approved by the Food and Drug Administration (FDA) in combination with azacitidine or low dose cytarabine in newly diagnosed AML patients older than 75 years or unfit for intensive chemotherapy (35, 36). HMA and venetoclax have significantly improved the outcomes of newly diagnosed AML patients with a reported median OS of 14 months and an acceptable toxicity profile (35). The combination regimen has been also used in the relapsed and/or refractory (R/R) setting with an ORR of 21% and short survival (37). In the post-transplant relapse setting, azacitidine and venetoclax were used in a limited number of patients, with a reported ORR of 38-46% (38, 39). More recently, a prospective clinical trial combining FLAG-Ida regimen with venetoclax in the R/R setting reported a composite CR rate of 57% among patients with prior allo-HCT, with a 75% rate of second allo-HCT among responders (40).
Further ways to overcome venetoclax resistance and enhance its efficacy is by targeting MCL1, an anti-apoptotic protein. A recently reported study combined actinomycin D, with azacitidine and venetoclax for the treatment of AML relapse post allo-HCT. The rationale of adding actinomycin D was based on preclinical data suggesting a synergistic anti-leukemic effect through MCL1 degradation, in addition to mitochondrial activity yielding to senescence through PML-nuclear body biogenesis (41–43). Twenty patients were treated with the triplet combination with an impressive ORR of 75% and a median OS of 13.1 months (41). venetoclax- combination therapies have become a new standard of care even in the post-transplant setting.
Somatic mutations in isocitrate dehydrogenases (IDH) 1 and 2 occur in up to 20% of AML patients (44). Targeted agents have been approved in the treatment of R/R AML harboring IDH1 or IDH2 mutations. Enasidenib monotherapy yielded an ORR of 40% with a median OS of 9.3 months, reaching 19.7 months in patients attaining CR in the R/R setting (45, 46). In this trial, 36 patients with IDH2 mutant AML had prior allo-HCT when treated with enasidenib (45). Similarly, 258 patients with R/R IDH1 mutant AML including 79 patients receiving prior allo-HCT were treated with ivosidenib monotherapy (47). The reported ORR was 41.9% with durable responses. Both drugs are very well tolerated. Side effects of special interest include indirect hyperbilirubinemia and IDH-inhibitor related differentiation syndrome. In the setting of R/R AML post allo-HCT, both enasidenib and ivosidenib can be used in combination with HMAs and venetoclax.
Patients with AML who relapse post allo-HCT may harbor a FLT3 internal tandem duplication (ITD) mutation that is usually associated with poor outcomes. Sorafenib is a first generation tyrosine kinase inhibitor that targets FLT3-ITD mutation. In addition to its anti-FLT3 activity, sorafenib has been shown to induce interleukin-15 production by FLT3-positive leukemia cells, thereby activating the donor CD8+ T cells and promoting the GVL effect (48). In the post-transplant relapse setting, sorafenib as monotherapy resulted in durable remissions indicating a clinical synergy with the allogeneic immune effects (49, 50). In a retrospective analysis from the European Bone Marrow Transplantation (EBMT) registry, 30 patients were treated with sorafenib as single agent for relapse post allo-HCT; the CR rate was 39% among evaluable patients. When compared to matched controls, the 2-year OS was 38% compared to 9% for controls (p=0.0001) (51). Gilteritinib is a second generation, more potent FLT3 inhibitor that targets both FLT3-ITD and FLT3-TKD mutations. In the ADMIRAL phase 3 trial, 371 patients with R/R FLT3 mutant AML were randomly assigned to receive either gilteritinib at 120 mg daily or salvage chemotherapy (52). Gilteritinib resulted in a higher CR/CR with incomplete hematologic response rate (34% versus 15%, respectively), and a longer median OS (9.3 months versus 5.6 months, p<0.001). In the trial, 20% of patients were relapsing post allo-HCT. Among those patients, the CR rate achieved with gilteritinib was 35.4% versus 11.5% for those who received salvage chemotherapy. The median OS was also prolonged with gilteritinib compared to chemotherapy (8.3 months versus 4 months, Hazard Ratio: 0.48 (95% Confidence Interval:0.27-0.84) (52).
Although this review focuses on the current treatment landscape of AML relapse post allo-HCT, it is worth noting that several novel therapeutic options are ongoing investigation. Many of these agents are already FDA approved, such as glasdegib, an oral small molecule inhibitor of the Smoothened (SMO) receptor, inhibiting the Hedgedog signaling pathway. Glasdegib was tested in combination with low dose cytarabine in the frontline treatment of AML in patients who are ineligibile for intensive chemotherapy (53). It was used in the R/R setting with modest efficacy (54). Other novel agents include MCL1 inhibitors, MDM2 inhibitors especially in combination with venetoclax (55, 56).
DLI is an adoptive immunotherapy treatment defined as transfusion of non-stimulated lymphocyte concentrate from the original stem cell donor. It was first introduced in the early 1990s and showed high efficacy in patients with relapsed chronic myeloid leukemia after allo-HCT (57, 58). The understanding of the potent GVL effect of DLI has led to its widespread adoption in the management of relapsed hematological malignancies after allo-HCT. However, complications such as acute GVHD remain the main concern. In a retrospective study evaluating the role of DLI in the treatment of relapsed AML after allo-HCT from the EBMT, the estimated survival at 2 years was significantly higher in patients receiving DLI compared to those not receiving DLI (21% vs 9%, respectively). Lower tumor burden, favorable cytogenetics and remission at time of DLI were associated with better survival in DLI recipients. The overall incidence of acute GVHD was 43% at day 100 after DLI (59). This limited efficacy of DLI in the treatment of AML relapse after allo-SCT was confirmed by another retrospective data from the AML working group of the Japan society of hematopoietic cell transplantation. Out of 143 patients treated with DLI at first relapse, OS rates were 32% at 1 year, 17% at 2 years and 7% at 5 years. Long-term survival was almost exclusively associated with CR before DLI (2-year OS of 100%), confirming the necessity of achieving a clinical response before DLI. Acute GHVD was reported in 18% of patients (60). The efficacy of therapeutic DLI was shown to be inferior in the context of hematological relapse compared to mixed-chimerism. In a retrospective study from the Italian group including 180 patients with relapsed AML, the 3-year OS was significantly higher in patients who has received DLI for mixed chimerism compared to those with acute leukemia relapse (55% vs 32% respectively, p=0.002). Although published studies have suggested that outcomes of haplo-identical and HLA-matched DLI are comparable in terms of outcomes with similar incidence of DLI-induced acute GVHD, it is suggested that haplo-DLI might be more beneficial in promoting GVL effect (61).
Patients with relapsed AML after allo-HCT who are eligible for intensive treatment are usually offered a second allo-HCT. However, there is currently no standard of care regarding the donor selection and the type of conditioning regimen used in this setting. In a registry-based study of 418 patients with AML comparing survival at first relapse after allo-HCT, there was no significant difference in OS between the two groups regardless of the disease status at time of treatment. The 2- year and 5-year OS was 25% and 15% respectively in the DLI group compared to 26% and 19% in the allo-HCT group. However, the incidence of NRM was significantly higher in patients who received a second allo-HCT compared to those who received DLI including all patients and those who received treatment with active disease (2-year NRM 9% compared to 26% and 5-year NRM 10% compared to 29% in the DLI group compared to allo-HCT group, respectively). NRM was not significantly different in patients who were in CR at the time of intervention. The rate of grade 2 to 4 acute GVHD was significantly higher in the allo-HCT group (37%) compared to DLI group (20%). Interestingly, there was no difference in the 2-year OS and NRM in patients who received a second allo-HCT by donor type (62). The impact of donor selection on outcome of second allo-HCT was addressed by a study from the Acute Leukemia Working Party of EBMT. The retrospective analysis included 556 patients divided into 3 groups based on allo-HCT donor: same donor, different matched related, and haplo-donor. The 2-year leukemia-free survival (LFS) was comparable between the three groups (LFS 23% for same donor, 23.7% for different matched donor and 21.8% for haplo-donor, p=0.3). Similarly, there was no difference in the 2-year OS between the groups (36.4%, 28.7% and 23.3%, respectively. p=0.21). However, the cumulative incidence of grade 2 to 4 acute GVHD was significantly higher in the same donor group (63). Although it is thought that a haplo-identical donor after a matched related donor might offer an advantage in terms of GVL effect, the outcome of a second allo-HCT is equivalent and the use of a haploidentical donor is not usually associated with better survival. In the setting of allo-HCT for relapsed AML post allo-HSCT, the choice of the donor is mainly limited by the availability as there is no current data to support donor selection based on outcomes and complications of transplant.
Other immune strategies to enhance the T cell activity in AML, especially in the post-transplant setting involve the use of immune checkpoint inhibitors. Although these compounds are not yet approved in the management of AML, however they are gaining more clinical interest especially after a phase 1/1b clinical trial reporting a promising anti-leukemia efficacy of high dose ipilimumab, an anti-CTLA4 antibody, in a subset of AML patients relapsing post allo-HCT (64). In fact, it has been proven that AML cells are capable of evading T-cell responses through the overexpression of PD-L1, the ligand of PD-1 (65). This overexpression was significantly higher in patients with relapsed AML than in untreated patients (65). Furthermore, it appears that HMAs can induce an upregulation of the inhibitory immune checkpoint molecule expression as part of resistance mechanism (66). Thus the rationale for combining azacitidine with nivolumab in relapsed and/or refractory AML (67). In a phase 2 trial, 70 patients with R/R AML were treated, and achieved an ORR of 33% with an encouraging median OS of 10.5 months in patients with first salvage (67). Despite the possibility of durable responses with immune checkpoint inhibitors post allo-HCT relapse, immune mediated adverse effects and exacerbation of severe fatal GVHD remain the main concerns of such strategy.
The field of cellular therapy is fast evolving in the management of hematologic malignancies, especially with the approval of CAR T cells for B-cell acute lymphoblastic lymphoma and non-Hodgkin’s lymphoma. In AML, the development of CAR T cells remain problematic, mainly because many myeloid surface antigens on AML cells, are also expressed on normal hematopoietic or progenitors cells, causing severe bone marrow suppression. Currently, many CAR T cells products are being investigated in AML targeting CD33, CD123, and CLL1 that are predominately expressed on AML blasts (68, 69).
Despite all advances in the field of allo-HCT in AML, disease relapse still represents a major challenge with very limited treatment options. Many factors contribute to disease relapse including clonal evolution with acquisition of new oncogenic mutations, immune escape mechanisms such as downregulation of MHC genes, loss of expression of HLA haplotype, downregulation of NK cells targets, and increased expression of immune inhibitory checkpoint ligands. This progress in our understanding of the biology of disease relapse post allo-HCT is being translated in new therapeutic strategies. Epigenetic regulation using HMAs showed to be effective either alone or with DLI. Immune checkpoint blockade by ipilimumab was also feasible post allo-HCT relapse, particularly in patients with extramedullary involvement (64). More targeted agents are also promising in the post-transplant relapse setting, including FLT3 inhibitors, IDH1 and IDH2 inhibitors, as well as the BCL2 inhibitor, venetoclax. Definitely, there is still potential for improvements in the choice of treatment in the post-transplant relapse setting toward a more personalized approach. Pre-emptive and maintenance strategies post allo-HCT to prevent the relapse to occur is also imperative (70). Enhancing the GVL effect using HMAs is a promising approach, as well as the use of maintenance tyrosine kinase inhibitors such as FLT3 inhibitors post allo-HCT (71–76). Moreover, progress in accurate quantification and monitoring of measurable residual disease post allo-HCT is helpful in predicting impending morphologic relapse that prompts treatment initiation (77, 78).
IA, AA, and AB reviewed the literature and wrote the manuscript. All authors contributed to the article and approved the submitted version.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Döhner H, Estey E, Grimwade D, Amadori S, Appelbaum FR, Büchner T, et al. Diagnosis and Management of AML in Adults: 2017 ELN Recommendations From an International Expert Panel. Blood (2017) 129(4):424–47. doi: 10.1182/blood-2016-08-733196
2. Penack O, Peczynski C, Mohty M, Yakoub-Agha I, Styczynski J, Montoto S, et al. How Much has Allogeneic Stem Cell Transplant-Related Mortality Improved Since the 1980s? A Retrospective Analysis From the EBMT. Blood Adv (2020) 4(24):6283–90. doi: 10.1182/bloodadvances.2020003418
3. Schmid C, de Wreede LC, van Biezen A, Finke J, Ehninger G, Ganser A, et al. Outcome After Relapse of Myelodysplastic Syndrome and Secondary Acute Myeloid Leukemia Following Allogeneic Stem Cell Transplantation: A Retrospective Registry Analysis on 698 Patients by the Chronic Malignancies Working Party of the European Society of Blood and Marrow Transplantation. Haematologica (2018) 103(2):237–45. doi: 10.3324/haematol.2017.168716
4. Horowitz M, Schreiber H, Elder A, Heidenreich O, Vormoor J, Toffalori C, et al. Epidemiology and Biology of Relapse After Stem Cell Transplantation. Bone Marrow Transplant (2018) 53(11):1379–89. doi: 10.1038/s41409-018-0171-z
5. Devillier R, Crocchiolo R, Etienne A, Prebet T, Charbonnier A, Fürst S, et al. Outcome of Relapse After Allogeneic Stem Cell Transplant in Patients With Acute Myeloid Leukemia. Leukemia Lymphoma (2013) 54(6):1228–34. doi: 10.3109/10428194.2012.741230
6. Scott BL, Pasquini MC, Logan BR, Wu J, Devine SM, Porter DL, et al. Myeloablative Versus Reduced-Intensity Hematopoietic Cell Transplantation for Acute Myeloid Leukemia and Myelodysplastic Syndromes. J Clin Oncol (2017) 35(11):1154–61. doi: 10.1200/jco.2016.70.7091
7. Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R, et al. Treatment, Risk Factors, and Outcome of Adults With Relapsed AML After Reduced Intensity Conditioning for Allogeneic Stem Cell Transplantation. Blood J Am Soc Hematol (2012) 119(6):1599–606.
8. Piemontese S, Boumendil A, Labopin M, Schmid C, Ciceri F, Arcese W, et al. Leukemia Relapse Following Unmanipulated Haploidentical Transplantation: A Risk Factor Analysis on Behalf of the ALWP of the EBMT. J Hematol Oncol (2019) 12(1):68. doi: 10.1186/s13045-019-0751-4
9. Ciurea SO, Zhang MJ, Bacigalupo AA, Bashey A, Appelbaum FR, Aljitawi OS, et al. Haploidentical Transplant With Posttransplant Cyclophosphamide vs Matched Unrelated Donor Transplant for Acute Myeloid Leukemia. Blood (2015) 126(8):1033–40. doi: 10.1182/blood-2015-04-639831
10. Bazarbachi A, Schmid C, Labopin M, Beelen D, Wolfgang Blau I, Potter V, et al. Evaluation of Trends and Prognosis Over Time in Patients With AML Relapsing After Allogeneic Hematopoietic Cell Transplant Reveals Improved Survival for Young Patients in Recent Years. Clin Cancer Res an Off J Am Assoc Cancer Res (2020) 26(24):6475–82. doi: 10.1158/1078-0432.ccr-20-3134
11. Bejanyan N, Weisdorf DJ, Logan BR, Wang HL, Devine SM, de Lima M, et al. Survival of Patients With Acute Myeloid Leukemia Relapsing After Allogeneic Hematopoietic Cell Transplantation: A Center for International Blood and Marrow Transplant Research Study. Biol Blood marrow Transplant J Am Soc Blood Marrow Transplant (2015) 21(3):454–9. doi: 10.1016/j.bbmt.2014.11.007
12. Craddock C, Versluis J, Labopin M, Socie G, Huynh A, Deconinck E, et al. Distinct Factors Determine the Kinetics of Disease Relapse in Adults Transplanted for Acute Myeloid Leukaemia. J Internal Med (2018) 283(4):371–9. doi: 10.1111/joim.12720
13. Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N Engl J Med (2016) 374(23):2209–21. doi: 10.1056/NEJMoa1516192
14. Brunet S, Labopin M, Esteve J, Cornelissen J, Socié G, Iori AP, et al. Impact of FLT3 Internal Tandem Duplication on the Outcome of Related and Unrelated Hematopoietic Transplantation for Adult Acute Myeloid Leukemia in First Remission: A Retrospective Analysis. J Clin Oncol (2012) 30(7):735–41. doi: 10.1200/jco.2011.36.9868
15. Luskin MR, Carroll M, Lieberman D, Morrissette JJD, Zhao J, Crisalli L, et al. Clinical Utility of Next-Generation Sequencing for Oncogenic Mutations in Patients With Acute Myeloid Leukemia Undergoing Allogeneic Stem Cell Transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant (2016) 22(11):1961–7. doi: 10.1016/j.bbmt.2016.07.018
16. Goardon N, Marchi E, Atzberger A, Quek L, Schuh A, Soneji S, et al. Coexistence of LMPP-Like and GMP-Like Leukemia Stem Cells in Acute Myeloid Leukemia. Cancer Cell (2011) 19(1):138–52. doi: 10.1016/j.ccr.2010.12.012
17. Zeiser R, Vago L. Mechanisms of Immune Escape After Allogeneic Hematopoietic Cell Transplantation. Blood (2019) 133(12):1290–7. doi: 10.1182/blood-2018-10-846824
18. Christopher MJ, Petti AA, Rettig MP, Miller CA, Chendamarai E, Duncavage EJ, et al. Immune Escape of Relapsed AML Cells After Allogeneic Transplantation. N Engl J Med (2018) 379(24):2330–41. doi: 10.1056/NEJMoa1808777
19. Jan M, Leventhal MJ, Morgan EA, Wengrod JC, Nag A, Drinan SD, et al. Recurrent Genetic HLA Loss in AML Relapsed After Matched Unrelated Allogeneic Hematopoietic Cell Transplantation. Blood Adv (2019) 3(14):2199–204. doi: 10.1182/bloodadvances.2019000445
20. Toffalori C, Zito L, Gambacorta V, Riba M, Oliveira G, Bucci G, et al. Immune Signature Drives Leukemia Escape and Relapse After Hematopoietic Cell Transplantation. Nat Med (2019) 25(4):603–11. doi: 10.1038/s41591-019-0400-z
21. Levine JE, Braun T, Penza SL, Beatty P, Cornetta K, Martino R, et al. Prospective Trial of Chemotherapy and Donor Leukocyte Infusions for Relapse of Advanced Myeloid Malignancies After Allogeneic Stem-Cell Transplantation. J Clin Oncol (2002) 20(2):405–12. doi: 10.1200/jco.2002.20.2.405
22. Motabi IH, Ghobadi A, Liu J, Schroeder M, Abboud CN, Cashen AF, et al. Chemotherapy Versus Hypomethylating Agents for the Treatment of Relapsed Acute Myeloid Leukemia and Myelodysplastic Syndrome After Allogeneic Stem Cell Transplant. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant (2016) 22(7):1324–9. doi: 10.1016/j.bbmt.2016.03.023
23. Christopeit M, Kuss O, Finke J, Bacher U, Beelen DW, Bornhäuser M, et al. Second Allograft for Hematologic Relapse of Acute Leukemia After First Allogeneic Stem-Cell Transplantation From Related and Unrelated Donors: The Role of Donor Change. J Clin Oncol (2013) 31(26):3259–71. doi: 10.1200/jco.2012.44.7961
24. Dombret H, Seymour JF, Butrym A, Wierzbowska A, Selleslag D, Jang JH, et al. International Phase 3 Study of Azacitidine vs Conventional Care Regimens in Older Patients With Newly Diagnosed AML With >30% Blasts. Blood (2015) 126(3):291–9. doi: 10.1182/blood-2015-01-621664
25. Kantarjian HM, Thomas XG, Dmoszynska A, Wierzbowska A, Mazur G, Mayer J, et al. Multicenter, Randomized, Open-Label, Phase III Trial of Decitabine Versus Patient Choice, With Physician Advice, of Either Supportive Care or Low-Dose Cytarabine for the Treatment of Older Patients With Newly Diagnosed Acute Myeloid Leukemia. J Clin Oncol (2012) 30(21):2670–7. doi: 10.1200/jco.2011.38.9429
26. Graef T, Kuendgen A, Fenk R, Zohren F, Haas R, Kobbe G. Successful Treatment of Relapsed AML After Allogeneic Stem Cell Transplantation With Azacitidine. Leukemia Res (2007) 31(2):257–9. doi: 10.1016/j.leukres.2006.03.003
27. Craddock C, Labopin M, Robin M, Finke J, Chevallier P, Yakoub-Agha I, et al. Clinical Activity of Azacitidine in Patients Who Relapse After Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia. Haematologica (2016) 101(7):879–83. doi: 10.3324/haematol.2015.140996
28. Goodyear O, Agathanggelou A, Novitzky-Basso I, Siddique S, McSkeane T, Ryan G, et al. Induction of a CD8+ T-Cell Response to the MAGE Cancer Testis Antigen by Combined Treatment With Azacitidine and Sodium Valproate in Patients With Acute Myeloid Leukemia and Myelodysplasia. Blood (2010) 116(11):1908–18. doi: 10.1182/blood-2009-11-249474
29. Goodyear OC, Dennis M, Jilani NY, Loke J, Siddique S, Ryan G, et al. Azacitidine Augments Expansion of Regulatory T Cells After Allogeneic Stem Cell Transplantation in Patients With Acute Myeloid Leukemia (AML). Blood (2012) 119(14):3361–9. doi: 10.1182/blood-2011-09-377044
30. Schroeder T, Rachlis E, Bug G, Stelljes M, Klein S, Steckel NK, et al. Treatment of Acute Myeloid Leukemia or Myelodysplastic Syndrome Relapse After Allogeneic Stem Cell Transplantation With Azacitidine and Donor Lymphocyte Infusions–a Retrospective Multicenter Analysis From the German Cooperative Transplant Study Group. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant (2015) 21(4):653–60. doi: 10.1016/j.bbmt.2014.12.016
31. Schroeder T, Czibere A, Platzbecker U, Bug G, Uharek L, Luft T, et al. Azacitidine and Donor Lymphocyte Infusions as First Salvage Therapy for Relapse of AML or MDS After Allogeneic Stem Cell Transplantation. Leukemia (2013) 27(6):1229–35. doi: 10.1038/leu.2013.7
32. Schroeder T, Rautenberg C, Krüger W, Platzbecker U, Bug G, Steinmann J, et al. Treatment of Relapsed AML and MDS After Allogeneic Stem Cell Transplantation With Decitabine and DLI-a Retrospective Multicenter Analysis on Behalf of the German Cooperative Transplant Study Group. Ann Hematol (2018) 97(2):335–42. doi: 10.1007/s00277-017-3185-5
33. Craddock C, Slade D, De Santo C, Wheat R, Ferguson P, Hodgkinson A, et al. Combination Lenalidomide and Azacitidine: A Novel Salvage Therapy in Patients Who Relapse After Allogeneic Stem-Cell Transplantation for Acute Myeloid Leukemia. J Clin Oncol (2019) 37(7):580–8. doi: 10.1200/jco.18.00889
34. DiNardo CD, Wei AH. How I Treat Acute Myeloid Leukemia in the Era of New Drugs. Blood (2020) 135(2):85–96. doi: 10.1182/blood.2019001239
35. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med (2020) 383(7):617–29. doi: 10.1056/NEJMoa2012971
36. Wei AH, Montesinos P, Ivanov V, DiNardo CD, Novak J, Laribi K, et al. Venetoclax Plus LDAC for Newly Diagnosed AML Ineligible for Intensive Chemotherapy: A Phase 3 Randomized Placebo-Controlled Trial. Blood (2020) 135(24):2137–45. doi: 10.1182/blood.2020004856
37. DiNardo CD, Rausch CR, Benton C, Kadia T, Jain N, Pemmaraju N, et al. Clinical Experience With the BCL2-Inhibitor Venetoclax in Combination Therapy for Relapsed and Refractory Acute Myeloid Leukemia and Related Myeloid Malignancies. Am J Hematol (2018) 93(3):401–7. doi: 10.1002/ajh.25000
38. Joshi M, Cook J, McCullough K, Nanaa A, Gangat N, Foran JM, et al. Salvage Use of Venetoclax-Based Therapy for Relapsed AML Post Allogeneic Hematopoietic Cell Transplantation. Blood Cancer J (2021) 11(3):49. doi: 10.1038/s41408-021-00437-z
39. Aldoss I, Yang D, Aribi A, Ali H, Sandhu K, Al Malki MM, et al. Efficacy of the Combination of Venetoclax and Hypomethylating Agents in Relapsed/Refractory Acute Myeloid Leukemia. Haematologica (2018) 103(9):e404–7. doi: 10.3324/haematol.2018.188094
40. DiNardo CD, Lachowiez CA, Takahashi K, Loghavi S, Xiao L, Kadia T, et al. Venetoclax Combined With FLAG-IDA Induction and Consolidation in Newly Diagnosed and Relapsed or Refractory Acute Myeloid Leukemia. J Clin Oncol (2021) 39:25. doi: 10.1200/jco.20.03736
41. Zucenka A VV, Maneikis K, Davainis L, Pileckyte R, Trociukas I, et al. Venetoclax Based Salvage Therapy Followed by Venetoclax and DLI Maintenance vs FLAG-Ida for Relapsed or Refractory Acute Myeloid Leukemia After Allogeneic Stem Cell Transplantation. Bone Marrow Transplant (2021) 56(11):2804–12.
42. Wu HC, Rerolle D, Berthier C, Hleihel R, Sakamoto T, Quentin S, et al. Actinomycin D Targets NPM1c-Primed Mitochondria to Restore PML-Driven Senescence in AML Therapy. Cancer Discov (2021) 11:3198–213. doi: 10.1158/2159-8290.cd-21-0177
43. Abou Dalle I, Bazarbachi A. Successful Venetoclax and Actinomycin D-Based Treatment for Relapsed Acute Myeloid Leukemia Post Allogeneic Hematopoietic Cell Transplantation. Bone Marrow Transplant (2021) 56(11):2626–7. doi: 10.1038/s41409-021-01434-3
44. DiNardo CD, Ravandi F, Agresta S, Konopleva M, Takahashi K, Kadia T, et al. Characteristics, Clinical Outcome, and Prognostic Significance of IDH Mutations in AML. Am J Hematol (2015) 90(8):732–6. doi: 10.1002/ajh.24072
45. Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, et al. Enasidenib in Mutant IDH2 Relapsed or Refractory Acute Myeloid Leukemia. Blood (2017) 130(6):722–31. doi: 10.1182/blood-2017-04-779405
46. Abou Dalle I, DiNardo CD. The Role of Enasidenib in the Treatment of Mutant IDH2 Acute Myeloid Leukemia. Ther Adv Hematol (2018) 9(7):163–73. doi: 10.1177/2040620718777467
47. DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable Remissions With Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N Engl J Med (2018) 378(25):2386–98. doi: 10.1056/NEJMoa1716984
48. Mathew NR, Baumgartner F, Braun L, O'Sullivan D, Thomas S, Waterhouse M, et al. Sorafenib Promotes Graft-Versus-Leukemia Activity in Mice and Humans Through IL-15 Production in FLT3-ITD-Mutant Leukemia Cells. Nat Med (2018) 24(3):282–91. doi: 10.1038/nm.4484
49. Metzelder SK, Schroeder T, Finck A, Scholl S, Fey M, Götze K, et al. High Activity of Sorafenib in FLT3-ITD-Positive Acute Myeloid Leukemia Synergizes With Allo-Immune Effects to Induce Sustained Responses. Leukemia (2012) 26(11):2353–9. doi: 10.1038/leu.2012.105
50. Metzelder SK, Schroeder T, Lübbert M, Ditschkowski M, Götze K, Scholl S, et al. Long-Term Survival of Sorafenib-Treated FLT3-ITD-Positive Acute Myeloid Leukaemia Patients Relapsing After Allogeneic Stem Cell Transplantation. Eur J Cancer (Oxford Engl 1990) (2017) 86:233–9. doi: 10.1016/j.ejca.2017.09.016
51. Bazarbachi A, Labopin M, Battipaglia G, Djabali A, Passweg J, Socié G, et al. Sorafenib Improves Survival of FLT3-Mutated Acute Myeloid Leukemia in Relapse After Allogeneic Stem Cell Transplantation: A Report of the EBMT Acute Leukemia Working Party. Haematologica (2019) 104(9):e398–401. doi: 10.3324/haematol.2018.211615
52. Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, Paolini S, et al. Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N Engl J Med (2019) 381(18):1728–40. doi: 10.1056/NEJMoa1902688
53. Cortes JE, Heidel FH, Hellmann A, Fiedler W, Smith BD, Robak T, et al. Randomized Comparison of Low Dose Cytarabine With or Without Glasdegib in Patients With Newly Diagnosed Acute Myeloid Leukemia or High-Risk Myelodysplastic Syndrome. Leukemia (2019) 33(2):379–89. doi: 10.1038/s41375-018-0312-9
54. Zucenka A, Maneikis K, Pugaciute B, Ringeleviciute U, Dapkeviciute A, Davainis L, et al. Glasdegib in Combination With Low-Dose Cytarabine for the Outpatient Treatment of Relapsed or Refractory Acute Myeloid Leukemia in Unfit Patients. Ann Hematol (2021) 100(5):1195–202. doi: 10.1007/s00277-021-04471-6
55. Ramsey HE, Fischer MA, Lee T, Gorska AE, Arrate MP, Fuller L, et al. a Novel MCL1 Inhibitor Combined With Venetoclax Rescues Venetoclax-Resistant Acute Myelogenous Leukemia. Cancer Discov (2018) 8(12):1566–81. doi: 10.1158/2159-8290.cd-18-0140
56. Daver NG, Garcia JS, Jonas BA, Kelly KR, Assouline S, Brandwein JM, et al. Updated Results From the Venetoclax (Ven) in Combination With Idasanutlin (Idasa) Arm of a Phase 1b Trial in Elderly Patients (Pts) With Relapsed or Refractory (R/R) AML Ineligible for Cytotoxic Chemotherapy. Blood (2019). doi: 10.1182/blood-2019-123711
57. Kolb HJ, Mittermüller J, Clemm C, Holler E, Ledderose G, Brehm G, et al. Donor Leukocyte Transfusions for Treatment of Recurrent Chronic Myelogenous Leukemia in Marrow Transplant Patients. Blood (1990) 76(12):2462–5.
58. Dazzi F, Szydlo RM, Cross NC, Craddock C, Kaeda J, Kanfer E, et al. Durability of Responses Following Donor Lymphocyte Infusions for Patients Who Relapse After Allogeneic Stem Cell Transplantation for Chronic Myeloid Leukemia. Blood (2000) 96(8):2712–6.
59. Schmid C, Labopin M, Nagler A, Bornhäuser M, Finke J, Fassas A, et al. Donor Lymphocyte Infusion in the Treatment of First Hematological Relapse After Allogeneic Stem-Cell Transplantation in Adults With Acute Myeloid Leukemia: A Retrospective Risk Factors Analysis and Comparison With Other Strategies by the EBMT Acute Leukemia Working Party. J Clin Oncol (2007) 25(31):4938–45.
60. Takami A, Yano S, Yokoyama H, Kuwatsuka Y, Yamaguchi T, Kanda Y, et al. Donor Lymphocyte Infusion for the Treatment of Relapsed Acute Myeloid Leukemia After Allogeneic Hematopoietic Stem Cell Transplantation: A Retrospective Analysis by the Adult Acute Myeloid Leukemia Working Group of the Japan Society for Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant (2014) 20(11):1785–90. doi: 10.1016/j.bbmt.2014.07.010
61. Zeidan AM, Forde PM, Symons H, Chen A, Smith BD, Pratz K, et al. HLA-Haploidentical Donor Lymphocyte Infusions for Patients With Relapsed Hematologic Malignancies After Related HLA-Haploidentical Bone Marrow Transplantation. Biol Blood Marrow Transplant J Am Soc Blood Marrow Transplant (2014) 20(3):314–8. doi: 10.1016/j.bbmt.2013.11.020
62. Kharfan-Dabaja MA, Labopin M, Polge E, Nishihori T, Bazarbachi A, Finke J, et al. Association of Second Allogeneic Hematopoietic Cell Transplant vs Donor Lymphocyte Infusion With Overall Survival in Patients With Acute Myeloid Leukemia Relapse. JAMA Oncol (2018) 4(9):1245–53. doi: 10.1001/jamaoncol.2018.2091
63. Shimoni A, Labopin M, Finke J, Ciceri F, Deconinck E, Kröger N, et al. Donor Selection for a Second Allogeneic Stem Cell Transplantation in AML Patients Relapsing After a First Transplant: A Study of the Acute Leukemia Working Party of EBMT. Blood Cancer J (2019) 9(12):88. doi: 10.1038/s41408-019-0251-3
64. Davids MS, Kim HT, Bachireddy P, Costello C, Liguori R, Savell A, et al. Ipilimumab for Patients With Relapse After Allogeneic Transplantation. N Engl J Med (2016) 375(2):143–53. doi: 10.1056/NEJMoa1601202
65. Chen X, Liu S, Wang L, Zhang W, Ji Y, Ma X. Clinical Significance of B7-H1 (PD-L1) Expression in Human Acute Leukemia. Cancer Biol Ther (2008) 7(5):622–7. doi: 10.4161/cbt.7.5.5689
66. Daver N, Boddu P, Garcia-Manero G, Yadav SS, Sharma P, Allison J, et al. Hypomethylating Agents in Combination With Immune Checkpoint Inhibitors in Acute Myeloid Leukemia and Myelodysplastic Syndromes. Leukemia (2018) 32(5):1094–105. doi: 10.1038/s41375-018-0070-8
67. Daver N, Garcia-Manero G, Basu S, Boddu PC, Alfayez M, Cortes JE, et al. Efficacy, Safety, and Biomarkers of Response to Azacitidine and Nivolumab in Relapsed/Refractory Acute Myeloid Leukemia: A Nonrandomized, Open-Label, Phase II Study. Cancer Discov (2019) 9(3):370–83. doi: 10.1158/2159-8290.cd-18-0774
68. Michelozzi IM, Kirtsios E, Giustacchini A. Driving CAR T Stem Cell Targeting in Acute Myeloid Leukemia: The Roads to Success. Cancers (Basel) (2021) 13(11):2816. doi: 10.3390/cancers13112816
69. Wang J, Chen S, Xiao W, Li W, Wang L, Yang S, et al. CAR-T Cells Targeting CLL-1 as an Approach to Treat Acute Myeloid Leukemia. J Hematol Oncol (2018) 11(1):7. doi: 10.1186/s13045-017-0553-5
70. Lee CJ, Savani BN, Mohty M, Gorin NC, Labopin M, Ruggeri A, et al. Post-Remission Strategies for the Prevention of Relapse Following Allogeneic Hematopoietic Cell Transplantation for High-Risk Acute Myeloid Leukemia: Expert Review From the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Bone Marrow Transplant (2019) 54(4):519–30. doi: 10.1038/s41409-018-0286-2
71. de Lima M, Giralt S, Thall PF, de Padua Silva L, Jones RB, Komanduri K, et al. Maintenance Therapy With Low-Dose Azacitidine After Allogeneic Hematopoietic Stem Cell Transplantation for Recurrent Acute Myelogenous Leukemia or Myelodysplastic Syndrome: A Dose and Schedule Finding Study. Cancer (2010) 116(23):5420–31. doi: 10.1002/cncr.25500
72. Battipaglia G, Ruggeri A, Massoud R, El Cheikh J, Jestin M, Antar A, et al. Efficacy and Feasibility of Sorafenib as a Maintenance Agent After Allogeneic Hematopoietic Stem Cell Transplantation for Fms-Like Tyrosine Kinase 3-Mutated Acute Myeloid Leukemia. Cancer (2017) 123(15):2867–74. doi: 10.1002/cncr.30680
73. Bazarbachi A, Bug G, Baron F, Brissot E, Ciceri F, Dalle IA, et al. Clinical Practice Recommendation on Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia Patients With FLT3-Internal Tandem Duplication: A Position Statement From the Acute Leukemia Working Party of the European Society for Blood and Marrow Transplantation. Haematologica (2020) 105(6):1507–16. doi: 10.3324/haematol.2019.243410
74. Burchert A, Bug G, Fritz LV, Finke J, Stelljes M, Röllig C, et al. Sorafenib Maintenance After Allogeneic Hematopoietic Stem Cell Transplantation for Acute Myeloid Leukemia With FLT3-Internal Tandem Duplication Mutation (SORMAIN). J Clin Oncol (2020) 38(26):2993–3002. doi: 10.1200/jco.19.03345
75. Antar AI, Otrock ZK, Abou Dalle I, El-Cheikh J, Bazarbachi A. Pharmacologic Therapies to Prevent Relapse of Acute Myeloid Leukemia After Allogeneic Hematopoietic Stem Cell Transplantation. Front Oncol (2020) 10:596134. doi: 10.3389/fonc.2020.596134
76. Bazarbachi A, Labopin M, Battipaglia G, Djabali A, Forcade E, Arcese W, et al. Allogeneic Stem Cell Transplantation for FLT3-Mutated Acute Myeloid Leukemia: In Vivo T-Cell Depletion and Posttransplant Sorafenib Maintenance Improve Survival. A Retrospective Acute Leukemia Working Party-European Society for Blood and Marrow Transplant Study. Clin Hematol Int (2019) 1(1):58–74. doi: 10.2991/chi.d.190310.001
77. Wood H, Sanchez K, Potter V, Kulasekararaj A, de Lavallade H, Yallop D, et al. Post-Transplant Flow Cytometry MRD Predicts Relapse in a Real World AML Cohort. Blood (2019) 134(Supplement_1):4566–6. doi: 10.1182/blood-2019-131711
78. Bastos-Oreiro M, Perez-Corral A, Martínez-Laperche C, Bento L, Pascual C, Kwon M, et al. Prognostic Impact of Minimal Residual Disease Analysis by Flow Cytometry in Patients With Acute Myeloid Leukemia Before and After Allogeneic Hemopoietic Stem Cell Transplantation. Eur J Haematol (2014) 93(3):239–46. doi: 10.1111/ejh.12336
Keywords: AML, allotransplant, relapse, immunotherapy, Graft versus leukaemia (GVL)
Citation: Abou Dalle I, Atoui A and Bazarbachi A (2022) The Elephant in The Room: AML Relapse Post Allogeneic Hematopoietic Cell Transplantation. Front. Oncol. 11:793274. doi: 10.3389/fonc.2021.793274
Received: 11 October 2021; Accepted: 09 December 2021;
Published: 03 January 2022.
Edited by:
Francesco Saraceni, Azienda Ospedaliero Universitaria Ospedali Riuniti, ItalyReviewed by:
Maria Teresa Lupo Stanghellini, San Raffaele Hospital (IRCCS), ItalyCopyright © 2022 Abou Dalle, Atoui and Bazarbachi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Ali Bazarbachi, YmF6YXJiYWNAYXViLmVkdS5sYg==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.