- 1First Affiliated Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 2Institute of Hematology, Zhejiang University, Hangzhou, China
Donor lymphocyte infusion (DLI) is a key strategy for the treatment of AML relapse after allogeneic hematopoietic cell transplantation (allo-HCT) and has been used for either prophylactic, pre-emptive, or therapeutic purposes. However, the prognosis of these patients remains dismal even after DLI infusion (2-year overall survival, ~25%), and the efficacy is achieved at the cost of toxicities such as graft-versus-host (GVH) disease. Attempts to optimize DLI efficacy and safety, such as dose/timing modification and the use of cytoreduction, before DLI have been performed previously. Recently, a great number of novel targeted and immunomodulatory agents have emerged. Some of them, such as hypomethylating agents, FLT3 and Bcl-2 inhibitors, have been used in combination with DLI, aiming to enhance the graft-versus-leukemia effect. Moreover, manipulation of the DLI graft through cell selection (e.g., donor NK cells) or cell engineering (donor CAR-T cells) has shown potentially superior anti-tumor effects but less GVH effect than conventional DLI in clinical trials. This review summarizes the recent advances on the use of DLI for the prophylaxis/treatment of AML relapse and discusses future strategies which may further improve the treatment efficacy.
Introduction
Allogeneic hematopoietic cell transplantation (allo-HCT) remains the therapy with the highest chance of long-term remission for acute myeloid leukemia (AML), especially for those in first complete remission (CR1) who belong to the European LeukemiaNet (ELN) intermediate or high-risk prognostic groups, those who remain measurable residual disease (MRD)-positive after induction therapy as well as those beyond CR1 (1). Disease relapse has been the main cause of failure for allo-HCT, with a dismal 2-year overall survival (OS) of less than 20% even in the most recent studies (1–4). Although both donor lymphocyte infusion (DLI) (5, 6) and a second allo-HCT (7, 8) have shown definite efficacy in patients deemed for intensive treatments, DLI seems to confer a similar outcome but with a lower incidence of non-relapse mortality (4). However, the survival benefits associated with DLI therapy remains unsatisfactory. In a study recently published, Kharfan-Dabaja et al. observed a 2-year OS of only 25% for patients with AML hematological relapse after allo-HCT who received therapeutic DLI (4). Therefore, there is still a lot of room for progress for DLI therapy in AML relapse after allo-HCT.
DLI is a kind of immunotherapy which can induce durable remission by enhancing the graft-versus-leukemia (GVL) effect (9). It was introduced for the treatment of leukemia relapse in the early 1990s (10) and was shown to be more effective in chronic myeloid leukemia (CML) (11, 12) than in acute leukemias (5, 13). With the application of tyrosine kinase inhibitors, DLI usage for CML relapse treatment has decreased. DLI could be used in either HLA-matched or mismatched allo-HCTs from different donors, including matched related, unrelated, or haploidentical related donors. With the introduction of anti-thymocyte globulin (ATG)-based and post-transplant cyclophosphamide (PT-Cy)-based haplo-HCT systems, the proportion of haploidentical HCT (haplo-HCT) within allo-HCT has increased rapidly (14, 15). Meanwhile, haplo-DLI has emerged as a promising strategy for leukemia relapse post-haplo-HCT since they may provide a stronger GVL effect due to a greater HLA disparity than those from HLA-matched donors (16, 17) and the fact that the availability of haploidentical related donors is superior to unrelated donors (9).
DLI has been used to either treat or prevent AML relapse. The therapeutic use of DLI is limited to T cell-replete allo-HCT protocols. Regarding the claim that DLI alone may not be sufficient to control overt relapse and that high tumor burden prior to DLI is associated with poor therapeutic response (4, 5, 18–20), cytoreduction with chemotherapy prior to therapeutic DLI (chemo+DLI) is frequently used, which may improve the efficacy and exert potential immunomodulatory effects (21, 22). Consistently, chemotherapy plus DLI has been found to be superior to DLI (23) or chemotherapy alone (13) in inducing disease remission after post-HCT AML hematological relapse. Pre-emptive and prophylactic DLIs have also been intensively investigated, which could effectively prevent hematological relapse with lower toxicity compared with therapeutic DLI. Pre-emptive DLI guided by MRD has shown definite efficacy in eliminating residual disease and promote donor chimerism (24, 25). Prophylactic DLI has been used to enhance immune reconstitution and prevent infection/relapse in T cell-depleted allo-HCT (26, 27) and in both ATG-based (28) and PT-Cy-based T cell-replete allo-HCT (29).
In the recent decade, cellular therapy and novel drugs have come into focus for the treatment of hematological malignancies with exciting results. Efforts have been made to “transplant” these modalities to AML relapse after allo-HCT, with the aim to maximize the therapeutic effects while minimizing the toxicity. The recommended strategies to optimize the DLI, including DLI regimen and protocol improvement, additional use of novel drugs, and cell engineering, are schematically shown in Figure 1.
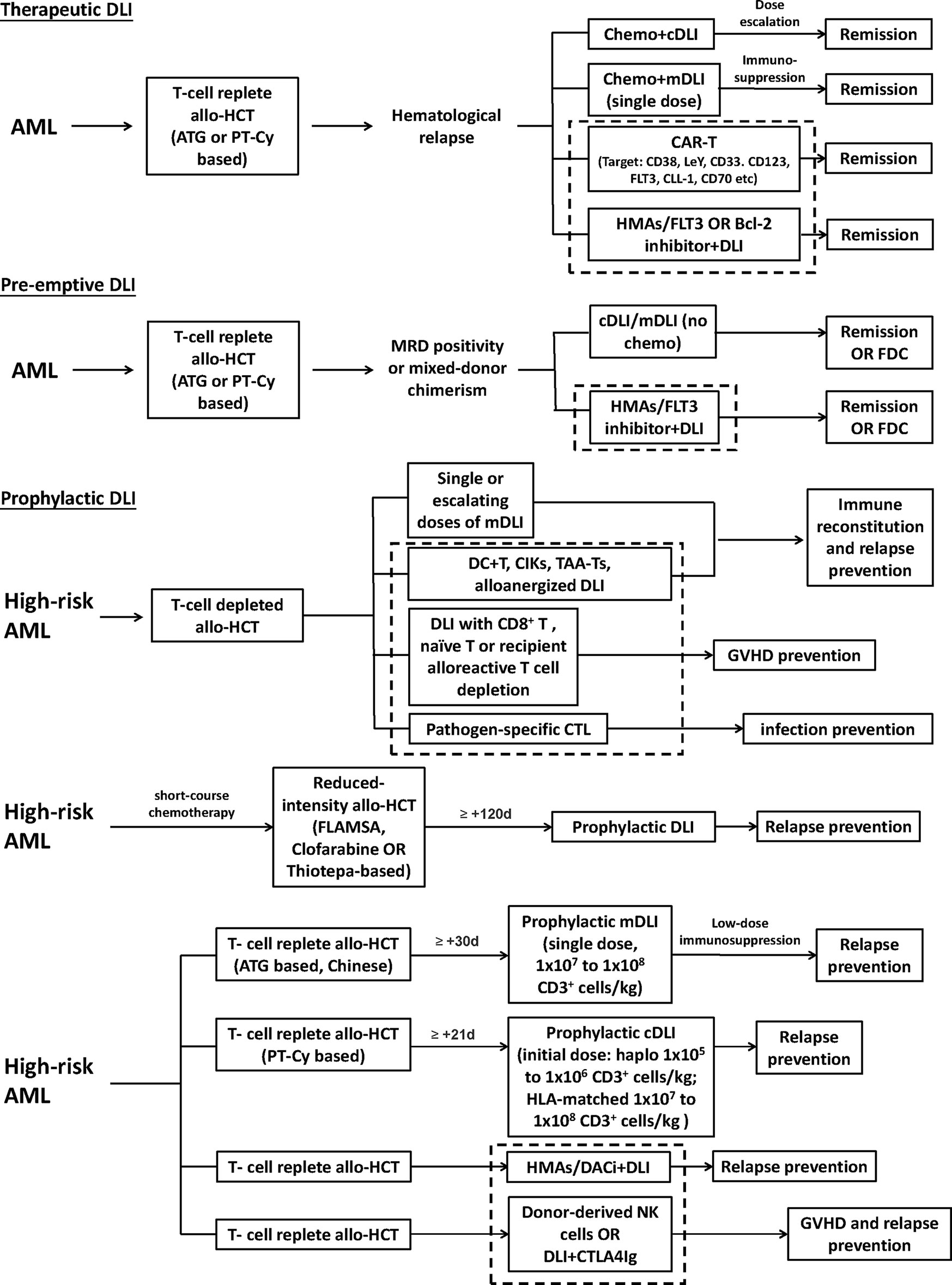
Figure 1 Recommended strategies to optimize the donor lymphocyte infusion (DLI), including DLI regimen and protocol improvement, additional use of novel drugs, and cell engineering. The area within the dotted bordered square indicate strategies, including novel drugs and cell engineering. High-risk AML represents those defined to have a higher risk of relapse in different studies. cDLI, DLI prepared by leukapheresis of unstimulated peripheral blood; mDLI, DLI derived from peripheral blood stem cells mobilized by granulocyte colony-stimulating factor; HMAs, hypomethylating agents; FDC, full donor chimerism; CIK, cytokine-induced killer cells; TAA-T, tumor-antigen-specific T cells; FLAMSA, condition regimen including fludarabine, cytarabine, and amsacrine.
Comparison of cDLI and mDLI
Two major types of DLIs are commonly used: the “conventional DLI” (cDLI in the following chapters) prepared by leukapheresis of unstimulated peripheral blood, and “modified DLI” (mDLI in the following chapters) using granulocyte colony-stimulating factor-mobilized peripheral blood stem cells (PBSCs). Both cDLI (5, 30) and mDLI (6, 31) have shown determined efficacy in the prophylaxis or treatment of AML relapse after allo-HCT. While cDLI is often used in Western countries using the PT-Cy-based allo-HCT protocol, mDLI, developed by Peking University, is regularly used by Chinese groups using the G-CSF/ATG-based protocol. Meanwhile, the cell dose is usually higher for mDLI (1 × 107 to 1 × 108 CD3+ cells/kg in both haplo and HLA-matched settings) (13, 32–34) than cDLI (haplo: 1 × 105 to 1 × 106 CD3+ cells/kg; HLA-matched: 1 × 107 to 1 × 108 CD3+ cells/kg) (35–37). Furthermore, mDLI is often followed by short-term immunosuppression with cyclosporine A (CsA) or methotrexate (38), while cDLI is not used in combination with GVHD prophylaxis (9). Comparative studies between cDLI and mDLI in the PT-Cy- or ATG-based allo-HCT protocols are required.
Therapeutic DLI
Around 1995, inspired by a series of success for therapeutic DLI in CML (39), both cDLI (40) and mDLI (39) were evaluated in relapsed AML after allo-HCT. Collins et al. reported 39 AML patients receiving cDLI without chemotherapy after allo-HCT relapse; the CR rate was only 15.4% (40). With the addition of chemotherapy in prior, the CR rate and survival have increased. Schmid et al. summarized a big cohort of 399 AML patients with hematological relapse after allo-HCT: 75% of patients received chemo+cDLI, and the CR rate for the whole cohort was 34%, bringing an overall aGVHD incidence of 43%. Meanwhile, the 2-year OS was 21% for patients receiving cDLI and only 9% for those not receiving cDLI (5). Chemo+mDLI followed by short-term immunosuppression was developed by the group in Peking University and was majorly used after haplo-HCT (6). In an early report of 20 patients with relapsed hematological malignancies (AML, n = 7) after haplo-HCT, 9 received chemo+mDLI. Moreover, 75% (n = 15) of patients achieved complete remission, and the 2-year leukemia-free survival (LFS) was 40% for the whole cohort (6). Yan et al. evaluated the efficacy of chemo+mDLI in 82 patients (AML, n = 45) who relapsed after haplo-HCT. The CR rate was significantly higher in the chemo+mDLI cohort than in the chemotherapy cohort (64.0 vs. 12.5%) (13). The incidence of grade II–IV aGVHD was 62.7%. In this study, chronic GVHD (cGVHD) and durable MRD negativity after chemo+DLI were found to be associated with a lower relapse rate. Following this observation, a cGVHD- and MRD-guided chemo+DLI consolidation strategy was developed and tested in a prospective trial of 47 patients (AML, n = 25) (32). The 1-year relapse rate (22 vs. 56%) and LFS (71 vs. 35%) were significantly better in the consolidation cohort than in the control. More recently, therapeutic DLI has been investigated in the PT-Cy based haplo-HCT protocol. In a retrospective cohort of 40 patients (AML, n = 16) receiving cDLI-based therapy after relapse from PT-Cy-based haplo-HCT, 30% of patients achieved a CR, with 25% of patients developing aGVHD. Meanwhile, higher rates of CR were achieved among those treated with chemo+cDLI than cDLI alone (41). A study conducted by Ghiso et al. used either cDLI alone or chemo+cDLI for molecular or hematological relapsed leukemia patients after PT-Cy-based haplo-HCT. A response rate of 33% and grades II and III aGVHD incidence of 17% were reported (24). Goldsmith et al. summarized 21 patients with disease relapse (90%) or loss of chimerism (10%) after PT-Cy-based haplo-HCT, in which 76% of patients received chemo+cDLI. Seven patients (33%) finally achieved CR or full donor chimerism, and the grades I–III aGVHD incidence was 23.8% (42). The experience on therapeutic DLI is limited to ATG- or PTCy-based T cell-replete allo-HCT protocols.
Pre-emptive DLI
MRD positivity prior to (43) or after (44) allo-HCT is positively associated with relapse incidence and negatively correlated with disease-free survival for AML patients. Furthermore, early MRD after allo-HCT (within +30 day) predicts the highest risk of relapse (44). Meanwhile, loss of donor chimerism (mixed chimerism, MC) predicts disease relapse and poorer relapse-free survival after allo-HCT in AML (45, 46). Pre-emptive DLI has been used to eradicate MRD and promote donor chimerism after allo-HCT in AML.
Pre-emptive DLI has brought about definite benefits in response rate and survival as compared to therapeutic DLI (24, 25, 47). Ghiso et al. compared pre-emptive cDLI for molecular relapse and therapeutic cDLI for hematological relapse after PT-Cy-based haplo-HCT. A higher response rate in the pre-emptive cohort (45 vs. 33%) was observed (24). Recently, Rettig et al. reported a significantly better survival for post-allo-HCT AML patients receiving pre-emptive than therapeutic mDLI (2-year OS: 64 vs. 26%) (25).
MRD is the most frequently used indicator for pre-emptive DLI, which is detected majorly by either flow cytometry (47), quantitative PCR (qPCR) (48), or, more recently, genomic sequencing (49). Yan et al. compared pre-emptive mDLI and IL-2 therapy on 105 standard-risk acute leukemia patients (AML, n = 61) with persistent MRD after allo-HCT. The 3-year cumulative incidence (CI) of relapse was significantly lower (27.8 vs. 64.4%), and the disease-free survival (55.6 vs. 24.1%) was significantly better in the pre-emptive mDLI cohort (18). The grade II–IV aGVHD and extensive cGVHD incidences after pre-emptive mDLI were 27.9 and 34.2%, respectively. Since patients with late-onset MRD had a lower risk of relapse than patients with early-onset MRD after pre-emptive DLI (50), we further compared the relapse rate and survival between pre-emptive DLI and IL-2 in patients with late-onset MRD (MRD positivity at >100 days after allo-HCT), and similar outcomes for the two strategies were observed (51). Quantitative assessment of WT1 as MRD has been used as a marker for pre-emptive DLI in AML patients after allo-HCT (52), and WT1 copies/104 Abelson cells in marrow cells has been suggested as the cutoff value (48). In order to better prevent disease recurrence, chemo+mDLI as pre-emptive therapy has been evaluated in ATG-based T cell-replete allo-HCT (53). A comparative study between chemo+pre-emptive mDLI and pre-emptive mDLI alone in acute leukemia/MDS patients showed that both strategies led to similar percentages of patients (chemo+DLI: 78.3 vs. DLI: 75%) to turn MRD-negative, thus advocating the use of pre-emptive mDLI alone for MRD+ patients for its lower toxicity. Several studies have also shown that pre-emptive DLI effectively converts MC to full donor chimerism (FDC), while its capacity in preventing disease relapse or prolonging survival remains to be established for lack of comparative studies (54). Caldemeyer et al. reported pre-emptive cDLI use in 29 patients with hematological malignancies who were identified MC without detectable disease after allo-HCT, and 93% of these patients converted to FDC with a cumulative grade II–IV aGVHD and/or extensive cGVHD incidence of 31% (55). Feliu et al. retrospectively analyzed 119 patients (AML, n = 48) receiving pre-emptive cDLI for falling CD3 chimerism, and 60% of patients achieved FDC after treatment (56). Of note is the fact that, in relapsed patients with exclusively recipient chimerism, DLI may not be effective and may lead to severe aplasia (57).
Prophylactic DLI
Prophylactic DLI in T Cell-Depleted Allo-HCT
Allo-BMT or HCT with ex vivo T cell depletion has been carried out extensively from the 1980s in both HLA-identical (58) or haploidentical settings (59). Ex vivo T cell depletion minimizes GVHD but is associated with slow immune recovery and a higher risk of relapse and infections (60). Prophylactic DLI has been used to enhance immune reconstitution so as to reduce the infection and relapse rates (26, 27). Kothari et al. studied, in a prospective trial, 75 patients (AML, n = 37) receiving T cell-depleted-matched related allo-HCT, in which 26 patients received at least one cDLI (median DLI number ≥3). The 2-year PFS was better in the DLI cohort than the entire population (57 vs. 41%), and the 1-year grade II–IV aGVHD rate was 28% for the DLI cohort (61). Gilman et al. studied prophylactic DLI after T cell-depleted haploidentical HCT in a phase I/II trial comprising 35 patients (AML, n = 10) (27). These patients received between day +30 and day +42 a single dose of up to 5◊104 CD3/kg prophylactic mDLI, followed by methotrexate; a 2-year OS of 69% was achieved, with a CI of 11% for grade II–IV aGVHD and 14% for cGVHD (27). Several techniques have been applied to manipulate prophylactic DLI to enhance its anti-leukemic and anti-infectious capacity while sparing the GVH effects. Following the rational that CD8+ T cell depletion reduces the risk of GVHD induced by DLI (62), Dodero et al. reported the results of a phase I/II trial comprising 23 patients with hematological malignancies who received escalating doses of prophylactic CD8+ T-depleted DLI (median DLI number = 2) after T cell-depleted reduced-intensity allo-HCT. A grade II–IV aGVHD incidence of 26% was observed (63). Regulatory T cells (Tregs) could prevent GVHD occurrence by facilitating the immune self-tolerance and homeostasis (64), which have been co-infused to suppress the GVH while maintaining the GVL effects (65). Martelli et al. reported 43 acute leukemia patients (AML, n = 33) receiving T cell-depleted haplo-HCT. These patients received freshly isolated donor Tregs on day -4, followed by a megadose of purified CD34+ cells and Tcons on day 0. Only 15% of the patients developed grade II–IV aGVHD, with a CI of 5% after 46 months of follow-up (66). Recently, the same group has updated the data using this transplant protocol in 50 aged AML patients (age >50) receiving haplo-HCT. Fifteen (30%) patients developed grade II–IV aGVHD, and only 2 patients (4%) relapsed. The OS after a median follow-up of 29 months was 77% (67). Infusion of pathogen-specific T cells may decrease the risk of specific infections after transplantation. Perruccio et al. generated and infused donor alloantigen-deleted Aspergillus and CMV-specific donor T cells after T cell-depleted haplo-HCT. These two adoptive immunotherapy strategies successfully cleared invasive aspergillosis and prevented CMV reactivation, respectively (68). Recently, third-party pathogen-specific cytotoxic T cells have been developed for off-the-shelf use. Olsen et al. reported a trial using third-party BK virus-specific cytotoxic T cell infusions into 59 patients developing BK virus-associated hemorrhagic cystitis after allo-HCT, and a day 14 overall response rate of 67.7% was observed (69). Moreover, several techniques are being investigated for optimal T cell allodepletion with preservation of pathogen-specific responses and Tregs (70). Despite these encouraging results, cell selection remains a time- and labor-consuming technique with high expenses, which has limited its extensive use.
Prophylactic DLI in T Cell-Replete Allo-HCT
Schmid et al. reported an early trial testing of prophylactic cDLI in 75 patients with high-risk AML/MDS defined by progressive/refractory (R/R) disease, second remission after early relapse, or first remission with poor cytogenetics/delayed response to induction chemotherapy. These patients received reduced-intensity T cell-replete HLA-matched allo-HCT. Escalating doses of prophylactic cDLI without immunosuppression were infused from day +120 for patients without immunosuppression or GVHD (matched related donor, escalating from 1◊105 to 2◊106 CD3+ cells/kg; matched unrelated donor, escalating from 2◊105 to 5◊106 CD3+ cells/kg, median DLI number = 2). A 2-year LFS of 40% was achieved (71). Later, Jedlickova et al. compared the long-term results of patients receiving prophylactic cDLI within the prospective trial introduced above with a well-matched control group without DLI. The overall survival at 7 years after transplant was 67%, compared with 31% in the control group (P < 0.001). This study has demonstrated the long-term survival benefit achieved by prophylactic cDLI in AML/MDS (37). Indeed the prophylactic cDLI usage described above is part of a sequential approach combining (1) a short course of chemotherapy followed by a reduced-intensity conditioning regimen (2), reduced-intensity allo-HCT (3), new drugs to prevent relapse, and (4) prophylactic DLI starting at day +120. This sequential regimen aims to reduce the toxicity of allo-HCT and optimize the anti-leukemia effects of DLI. In the two studies described above, the FLAMSA regimen (fludarabine, cytarabine, and amsacrine) was applied. Similarly, other allo-HCT protocols have also been reported as part of this sequential regimen (72). Mohty et al. conducted a phase II study testing a sequential regimen of clofarabine, cytosine arabinoside, and reduced-intensity allo-HCT, followed by delayed prophylactic cDLI, in refractory AML patients (73). The 1-year OS and CI of non-relapse mortality (NRM) were 54 and 8%, respectively. Shelikova et al. reported 22 children with R/R AML who received cytoreduction with fludarabine and cytarabine and subsequent myeloablative conditioning with treosulfan and thiotepa. The following prophylactic mDLI or cDLI comprised of a CD45RA-depleted fraction with or without a hypomethylating agent. The CR rate was 95%, with a 2-year EFS of 49% and TRM of 9% (74).
The Chinese groups evaluated extensively prophylactic mDLI in the ATG-based T cell-replete protocol, which usually included a single mDLI dose (the same for haplo and HLA-matched settings) followed by short-term immunosuppression. Wang et al. retrospectively compared patients receiving prophylactic mDLI and those without in T cell-replete haplo-HCT (median 0.6◊108 CD3+ cells/kg for the entire cohort). The 2-year CI of relapse was lower (36 vs. 55%) and the 3-year OS was better (31 vs. 11%) in the prophylactic DLI group than in the control (28). Later, the same group developed a strategy of prophylactic mDLI followed by MRD- and GVHD-guided multiple DLIs for R/R acute leukemia. In a prospective study comprising 100 patients (AML, n = 59), this protocol achieved a 3-year CI of relapse, LFS, and OS of 32, 50, and 51%, respectively (33). We have recently reported a matched-pair study in ATG-based haplo-HCT between patients (n = 34; AML, n = 28) receiving a single-dose prophylactic mDLI (median: 3.8◊107 CD3+ cells/kg), followed by low-dose CsA, and the same number of patients without. The 5-year LFS was superior (65 vs. 34%), and the CI of relapse (15 vs. 49%) was lower for the mDLI cohort than the control. Meanwhile, the 100-day CI of grade II–IV aGVHD for the mDLI cohort was 26.7% (34). Gao et al. compared haploidentical and matched-sibling mDLI, followed by low-dose CsA (median: 2.3◊107 CD3+ cells/kg for the entire cohort), in high-risk AML defined by progressive disease at transplant, CR1 achievement with ≥3 cycles of chemotherapy, or those carrying TP53, DNMT3a, TET2, or FLT3-ITD gene mutations. They observed a higher 100-day grade II–IV aGVHD CI (60 vs. 31%) and worse 1-year NRM (28 vs. 0%) in the haplo than the matched-sibling cohort, indicating a potentially higher toxicity for haplo than matched-sibling DLI (75).
Prophylactic DLI has also been evaluated in PT-Cy-based T cell-replete allo-HCT. Jaiswal et al. reported a small cohort of 21 AML patients not in remission who underwent PT-Cy-based haplo-HCT and received three consecutive prophylactic mDLIs, with each dose being 1 × 106 CD3+ cells/kg, on days +21, +35, and +60 with concurrent CsA use (71% of the studied patients received all 3 doses), which decreased the progression rate from 66 to 21% (29). Cauchois et al. analyzed 36 patients with high-risk hematological malignancies (AML, n = 21), defined as with a high disease risk index or not in CR before transplant who received escalating doses of prophylactic cDLI (from 1 × 105/kg to 2.5 × 106/kg, median DLI number = 1) after PT-Cy-based haplo-HCT. The CI of relapse at 1 year after prophylactic DLI was 16%, and the PFS was 76%, respectively (76).
Feasibility and Side Effects of DLI
Although DLIs have shown definite efficacy in preventing or controlling disease relapse after allo-HCT, feasibility requires to be examined before each DLI infusion even if it is planned and the donor is willing to donate. Prophylactic or pre-emptive DLI may not be administrated because of early relapse, a history of severe GVHD, active GVHD with continuous need of immunosuppression, severe infections, significant cytopenia, early death or choice of the patients (37). In the trial conducted by Jedlickova et al. testing prophylactic DLI for high-risk AML after allo-HCT, only ~30% of patients received prophylactic DLI as planned in the protocol (37), while in another phase II trial on prophylactic azacytidine + DLI for high-risk AML/MDS after allo-HCT, 56.7% of patients received at least one DLI (36). In the retrospective study conducted by Guillaume et al. evaluating the efficacy of prophylactic/pre-emptive azacytidine + DLI on high-risk AML/MDS after allo-HCT, 79% of patients received at least one DLI (77). For therapeutic DLI, inadequate organ function, active severe acute/chronic GVHD, and severe infections are regarded as contraindications (24). Another crucial barrier for successful DLI usage is HLA loss, which represents an important immune-escape mechanism that allows the leukemia to relapse after allo-HCT (78). Genomic loss of mismatched HLA occurs through a mechanism of copy-neutral loss of heterozygosity, eliminating the incompatible HLA alleles without decreasing the overall level of expression of HLA class I molecules (79). HLA loss accounts for around one-third of relapses after haplo-HCT (78, 80) and also a minority of relapses from matched or mismatched unrelated allo-HCTs (81). Since patients with HLA loss relapse were unlikely to benefit from DLI of the original donor, the 2019 EBMT consensus of DLI after haplo-HCT recommended a second allo-HCT from a related donor with a different mismatched haplotype or a mismatched unrelated donor (9).
GVHD is the main complication of DLI. The morbidity and severity of DLI-related GVHD was closely related to the type of DLI (cDLI or mDLI), the time interval between transplantation and DLI, the transplant protocol, the dose of CD3+ cells, the donor type, and the immunosuppressant. Since G-CSF mobilization alters the cellular composition and cytokine profile of the DLI graft, it was suggested that the mDLI might reduce the GVHD incidence (38), but this hypothesis is not yet confirmed in a head-to-head study. The time interval between transplantation and DLI greatly affects the morbidity and severity of DLI-associated GVHD (34). It is accepted that the shorter the interval, the higher the risk that GVHD will occur. In our study, we initially tested prophylactic mDLI in 5 patients at a median of 71 days post-HCT; 2 patients developed grade IV aGVHD, and 1 had grade I aGVHD, indicating a high incidence of aGVHD for early prophylactic mDLI infusion (31). Su et al. compared the safety of efficacy of prophylactic mDLI on day +60 and day +90 after T cell-replete allo-HCT and observed that prophylactic mDLI on day +90 was associated with a lower extensive cGVHD incidence (10 vs. 28%) and better GVHD-free, relapse-free survival (GRFS) (55 vs. 41%) (82). Meanwhile, pre-emptive/prophylactic DLI infused very early after allo-HCT (within 40 days) may be impaired by the remaining ATG in the human body (83).
In different transplant protocols, the timing of DLI and the related GVHD incidence vary greatly. In an earlier study conducted by Liga et al., 15 patients (AML, n = 8) received HLA-identical allo-HCT with an alemtuzumab-containing conditioning regimen and prophylactic mDLI with dose escalation (dose escalation for unrelated donor: 0.5 to 1 × 106 CD3+ cells/kg, for sibling: 0.75 to 1.5 × 106 CD3+ cells/kg, median DLI number = 3) in the absence of concurrent immunosuppression. The first infusion was given at a median of 162 days post-transplantation, and the DLI-associated GVHD incidence was 47% (84). In the PT-Cy-based haplo-HCT, an earlier study conducted by Legrand et al. included 22 AML patients receiving escalating doses of prophylactic cDLI (median DLI number = 1) after HLA-matched or haploidentical HCT, in which 4 (18%) received PT-Cy as GVHD prophylaxis. The first prophylactic DLI (the dose ranged from 0.1 to 10 × 106 CD3+/kg) was performed in a median time of 130 days post-transplant, and the cumulative incidence of DLI-associated GVHD was 37% (35). Later, Cauchois et al. reported 36 patients receiving prophylactic cDLI with dose escalation after PT-Cy-based haplo-HCT (escalating from 1 × 105/kg to 2.5 × 106 CD3+ cells/kg, median DLI number = 1) at a median time of 109 days between transplant and the first DLI; the cumulative 1-year incidence of cDLI-induced GVHD was 33% (76). Notably, in the study conducted by Jaiswal et al., the first prophylactic mDLI was given as early as +21 day after PT-Cy-based haplo-HCT with concurrent immunosuppression, and the cumulative incidence of aGVHD was only 31% (29). This study indicates that concurrent GVHD prophylaxis might control the GVHD caused by very early DLI infusions.
The CD3+ T cell dose is another important factor affecting DLI-associated GVHD. An early comparative study between escalating cDLI and a single infusion in relapsed CML after allo-HCT showed that the escalating regimen was associated with a lower incidence of GVHD but with similar outcomes in disease control (85). Bar et al. retrospectively analyzed the effects of an initial CD3+ T cell dose within therapeutic cDLI on the GVHD incidence after allo-HCT in 225 patients with hematological malignancies (AML, n = 71). Compared to lower doses, an initial CD3+ T cell dose above 1 × 108/kg in cDLI was found to be associated with a higher 1-year CI of GVHD (55%) but did not decrease the relapse incidence or improve the survival (86). As for donor type, haplo-DLI may bring about a higher incidence of GVHD than HLA-matched DLI due to higher alloreactivity. Gao et al. observed a higher incidence of grade II–IV DLI-associated aGVHD in haploidentical than matched sibling prophylactic mDLI (60 vs. 31%) after allo-HCT in high-risk AML (2◊107 CD3+ cells/kg for both groups) (75). Yu et al. analyzed refractory acute leukemia patients receiving allo-HCT followed by prophylactic mDLI (3◊107 CD3+ cells/kg for both groups). A total of 119 patients (AML, n = 54) received haploidentical and 132 patients (AML, n = 57) received matched-sibling donor allo-HCT. The haploidentical group was associated with a higher incidence of grade II–IV aGVHD (62 vs. 54% p=0.025) but with a similar 3-year incidence of cGVHD (87).
Concurrent immunosuppression has been routinely used with mDLI in the G-CSF/ATG-based allo-HCT protocol to reduce the incidence of DLI-associated GVHD. Immunosuppression with either CsA or methotrexate (MTX) is normally used right after a single dose of mDLI and maintained for 2–6 weeks according to the donor–recipient relationship, the aim of the DLI (prophylactic, pre-emptive, or therapeutic), or at the discretion of the physician. We reported the use of low-dose CsA (1 to 2 mg/kg/day) after DLI for 3 to 4 weeks after prophylactic mDLI (median of 3.8 × 107 CD3+ cells/kg) in the G-CSF/ATG-based allo-HCT protocol, and the CI of grade II–IV DLI-associated aGVHD was 17.6% (34). Yan et al. reported GVHD prophylaxis for 2–4 weeks after HLA-identical related pre-emptive mDLI or 4–6 weeks after an HLA-identical unrelated or HLA-haploidentical mDLI. The grade II–IV DLI-associated aGVHD incidence was 28% (18). When comparing CsA and MTX, MTX might bring about a similar anti-GVHD effect while preserving a stronger GVL effect due to a higher maintenance of absolute lymphocyte count (88). In a retrospective study including 124 patients receiving either prophylactic, pre-emptive, or therapeutic DLI, Yan et al. observed that the duration of GVHD prophylaxis was the only independent factor affecting the development of grade III–IV mDLI-associated aGVHD. The cumulative incidences of grade III–IV acute GVHD in patients with prophylaxis more than 6, 4–6, 2–4, and <2 weeks were 9.3, 14.4, 31.6, and 49.5%, respectively (p = 0.018) (89). In PT-Cy-based myeloablative haplo-HCT, Jaiswal et al. reported CsA use during consecutive prophylactic mDLIs (each dose: 1 × 106 CD3+ cells/kg) until day +60 and tapered over 4 weeks. The cumulative incidence of aGVHD was 31% (29). It is noteworthy that dose escalation strategy is frequently used with cDLI according to the CD3+ cell count for GVHD prevention, while adjuvant immunosuppression has not been reported. Meanwhile, no comparative study has supported differences on the incidences of DLI-associated aGVHD between mDLI plus immunosuppression and cDLI without immunosuppression (9). Finally, the possibility of impaired GVL effect after immunosuppression remains to be excluded.
Aplasia happens in between 18 and 40% of patients after DLI infusion (24, 38). Insufficient donor chimerism and conditions of DLI usage might be important reasons for the development of aplasia (57). Prophylactic (34, 37, 75, 90) and pre-emptive DLIs (56) seem to rarely induce DLI-associated cytopenia, while therapeutic DLIs are associated with a considerable risk of aplasia. Glass et al. reported both neutropenia and thrombocytopenia incidences of 36% among 11 patients (AML, n = 5) with hematological malignancy relapse after allo-HCT who received cytoreductive therapy+mDLI (39). Huang observed incidences of 20% neutropenia and 35% thrombocytopenia among 20 patients (AML, n = 7) with hematological malignancy relapse after allo-HCT and therapeutic DLI (6). The higher incidence of cytopenia during therapeutic DLI is probable due to cytoreductive therapy in prior and lower donor chimerism during infusions. Notably, DLI with adjuvant drugs has also been reported to induce considerable hematological toxicities. Grade III/IV neutropenia and thrombocytopenia occurred during 65 and 63% of treatment cycles in a prospective single-arm multicenter phase II trial, conducted by Schroeder et al., testing azacytidine plus therapeutic cDLI for AML/MDS relapse after allo-HCT (91). Similarly, venetoclax plus therapeutic mDLI led to anemia, neutropenia, and thrombocytopenia in 55, 73, and 64% of patients with relapsed AML after allo-HCT, respectively (92). However, it is important to clarify the reason for cytopenia since it could be due to other reasons than DLI itself, including primary disease, infection, GVHD, etc.
Infection is another non-negligible complication after DLI following DLI-associated GVHD or cytopenia. Viral and fungal infections are main causes of infection-related deaths (71). In T cell-depleted haplo-HCT, Gilman et al. reported that 11% of patients died of fatal viral/fungal infections after prophylactic cDLI (27). In the ATG-based T cell-replete protocol, an earlier study reported a high septicemia incidence of 49% after prophylactic cDLI and an infection-related death incidence of 25% at 1 year after transplant (71). With the advancement of anti-infection strategies, in a recent study conducted within the ATG-based T cell-replete allo-HCT protocol, Su reported an infection-related death rate of 10–15% after prophylactic mDLI (82). In the FLAMSA RIC protocol, Jedlickova reported an incidence of only 4% for infection-related death after prophylactic cDLI after 7 years of follow-up. For therapeutic DLI, Huang et al. previously observed that therapeutic mDLI led to an infection-related death incidence of 20% after T-cell replete ATG. More recently, Rettig et al. reported an infection-related death incidence of only 2% after therapeutic mDLI usage for AML relapse after ATG-based allo-HCT (25). In the PT-Cy-based T cell-replete protocol, Jaiswal documented 5 (24%) CMV infections and 1 (5%) fungal infection among 21 patients receiving PT-Cy-based haplo-HCT and prophylactic mDLI (29). Moreover, Ghiso et al. reported a cumulative infection incidence of 12% after therapeutic or pre-emptive cDLI after PT-Cy-based haplo-HCT (24). Collectively, regardless of the great difference among transplant platforms, advanced infection management strategies may have significantly reduced the incidences of infection-related death after therapeutic DLI.
New Drugs and DLI
Hypomethylating Agents
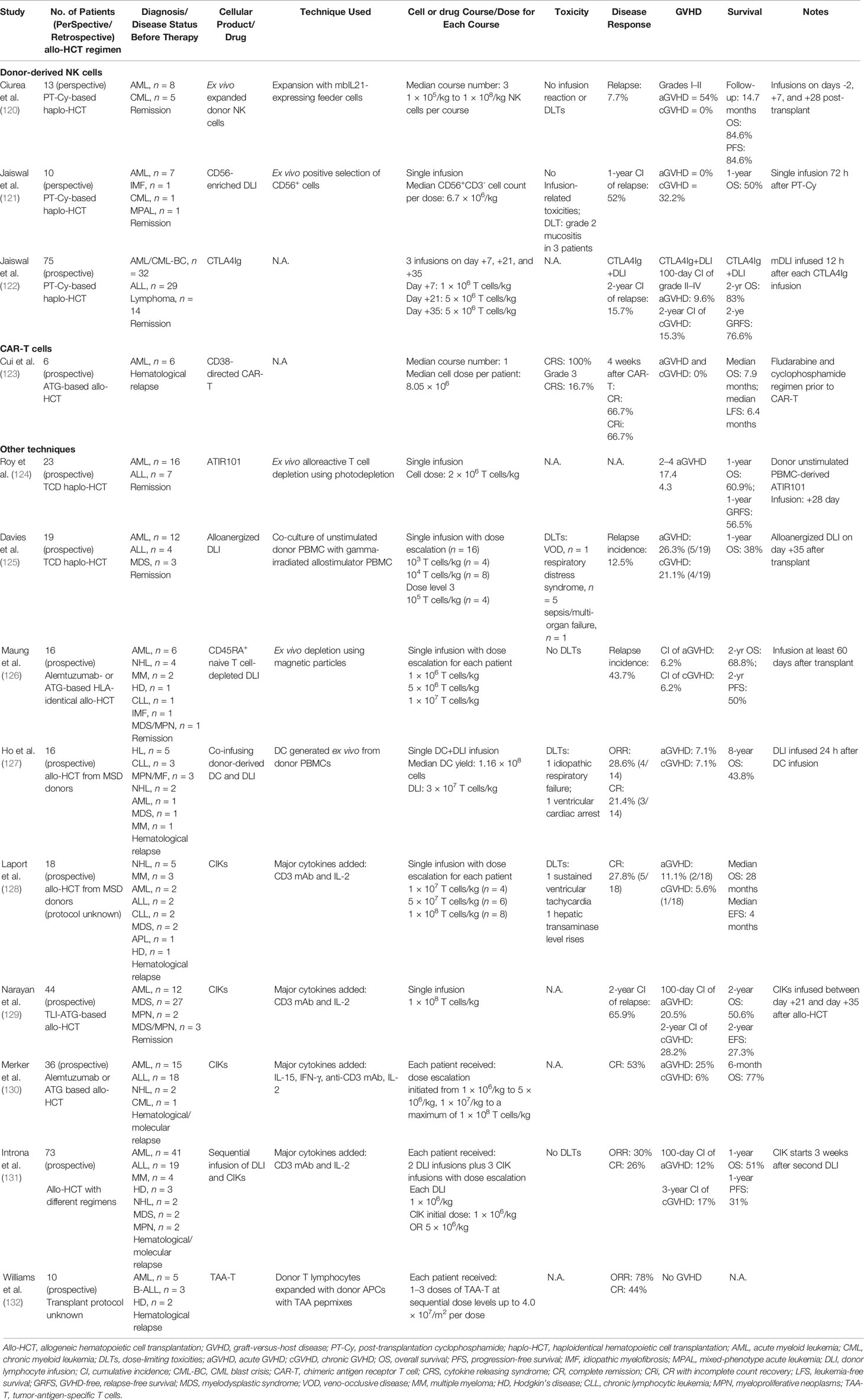
The clinical studies on the combined use of novel drugs and DLI are summarized in Table 1. Hypomethylating agents azacitidine and decitabine are approved drugs with demonstrated efficacy and prolong the survival of MDS and AML patients (102). Early preclinical studies showed that low-dose azacitidine upregulates epigenetically silenced tumor antigens and induces a cytotoxic T cell response (103), and it also mitigates GVHD via Treg induction (104). The hypomethylating agent (HMA)/DLI combination has been used for both salvage therapy and post-transplant maintenance in AML/MDS. In a multi-center retrospective analysis comprising 154 relapsed AML/MDS (AML, n = 124; MDS, n = 28; MPN, n = 2) patients receiving HMA/DLI combination, the overall response rate (ORR) was 33%, and the CR rate was 27% (93). In a bigger retrospective study derived from the EBMT database comprising 181 patients (AML, n = 116; MDS, n = 65) receiving HMA/DLI combination as salvage therapy after hematological relapse, the ORR rate was 29%, and the CR rate was 15% (94). Schroeder et al. reported 30 relapsed AML/MDS (AML, n = 28, MDS, n = 2) patients receiving up to 6 cycles of azacytidine, followed by DLI after every second cycle in a prospective trial. The overall response rate (ORR) was 30%, and the CR rate was 23% (91). Moreover, following the pre-clinical evidence that azacitidine mitigates GVHD, Ghobadi et al. conducted a phase I trial wherein azacitidine was applied on days 4, 6, 8, and 10 post-DLI in 8 patients with post-transplant AML relapse; 6 out of 8 (75%) patients responded with 2 (25%) cytogenetic complete remissions. Of note is the fact that no patient experienced grade III–IV aGVHD, indicating enough safety and GVHD prevention capacity for azacitidine use following DLI (95).
Guillaume et al. conducted a phase II trial evaluating prophylactic azacytidine followed by DLI in 30 high-risk AML/MDS patients. High-risk AML was defined as CR1 with unfavorable cytogenetics, requiring ≥2 cycles of treatment for remission, remission greater than CR2, or progressive disease. High-risk MDS was defined as with an intermediate-2 or higher risk International Prognostic Scoring System (IPSS) score. The DFS was 65.5% at 2 years, and the CI of relapse was 27.6%. Meanwhile, the 2-year CI of grade I–III aGVHD was 31.5% (36). In another retrospective study analyzing 77 high-risk AML/MDS patients based on unfavorable genomic or clinical status at transplantation (AML, n = 54; MDS, n = 23), prophylactic or pre-emptive azacitidine combined with DLI achieved a 2-year CI of relapse of 22%, and the CIs of grade II–IV acute GVHD and cGVHD were 27.4 and 45%, respectively (77).
Like azacitidine, the use of decitabine in combination with DLI has been for both post-transplant salvage and maintenance in AML/MDS. A retrospective multicenter analysis from Germany reported that decitabine plus DLI achieved an ORR of 25% and CR of 17% in 36 patients with relapsed AML (n = 29) or MDS (n = 7). Meanwhile, only 7 patients (19%) experienced aGVHD after treatment. It is however noteworthy that the 2-year OS was only 11% in this cohort (96). In another retrospective analysis, including 26 patients with relapsed hematological malignancies (AML, n = 18, MDS, n = 6, MPN, n = 2), decitabine plus DLI achieved an ORR of 19% and a CR/CRi rate of 15% (97). In a prospective single-arm study, decitabine plus DLI was applied as prophylaxis in 28 patients with high-risk hematological malignancies, defined as with at least one of the unfavorable gene mutations (FLT3- ITD, TP53, ASXL1, DNMT3A, or TET2) (AML, n = 23; MDS, n = 2; ALL, n = 3). A 3-year relapse-free survival of 48.2% and OS of 48.9% were observed, together with a 3-year relapse rate of 26.1% post-DLI. Meanwhile, the incidence of grade II–IV aGVHD at 100 days post-DLI was 25.8%, and the cGVHD incidence at 3 years post-DLI was 21.6% (90). Collectively, the HMA/DLI combination after allo-HCT is safe for both consolidation and salvage therapy, while the efficacy remains to be further improved.
FLT3 Inhibitors
FLT3 internal tandem duplications (ITDs) and FLT3-tyrosine kinase domain (TKD) mutations occur in ~25 and <10% of AML patients, respectively (105). FLT3 mutations, especially FLT3-ITD, indicate an adverse prognosis (106). Sorafenib is currently the only FLT3 inhibitor intensively investigated in the post-transplant setting. Single-agent sorafenib was shown to significantly reduce the relapse rate as maintenance therapy for FLT3-ITD mutant AML after allo-HCT (107, 108). Battipaglia et al. reported 27 patients with FLT3-positive AML who received sorafenib maintenance after allo-HCT, which led to a 1-year PFS of 92% (107). We observed that maintenance sorafenib was superior to prophylactic DLI in FLT3-ITD AML patients, with a lower relapse rate (4.2 vs. 25%) and a lower incidence of grade II–IV aGVHD (8.7 vs. 46.3%) (109).
The effects of post-transplant sorafenib is multifaceted, including direct FLT mutation inhibition, GVL effect augmentation through IL-15 production in FLT3-ITD mutant leukemia cells, and also synergic effects with alloreactive T cells (110). A synergic effect of sorafenib and DLI is therefore anticipated. Xuan et al. analyzed 83 FLT3-ITD mutant AML patients with overt relapse after allo-HCT who received salvage therapies (98). A superior survival was observed in sorafenib-containing regimen to conventional therapy (OS: 46.8 vs. 20%). In a subgroup analysis, the best survival was achieved for patients receiving sorafenib-containing chemotherapy followed by DLI, which is superior to other therapeutic regimens, including sorafenib combined with chemotherapy, chemotherapy followed by DLI, and monochemotherapy. No significant differences were observed concerning GVHD incidences among these therapies. In addition, Bruzzese et al. reported, in a small cohort of four patients, that the pre-emptive use of sorafenib followed by DLI in MRD-positive FLT3-ITD AML patients achieved MRD negativity in 3 patients (75%) (99). Interestingly, in this report, the remaining patient discontinued sorafenib because of toxicity and changed to gilteritinib, and long-term remission was achieved. Therefore, the use of other FLT3 inhibitors before and after allo-HCT as well as the combined use with DLI warrants further investigations.
Bcl-2 Inhibitors
Aberrant Bcl-2 overexpression is identified in patients with AML, rendering survival advantage for the leukemia cells. The Bcl-2 inhibitor venetoclax combined with HMAs has achieved ~70% CR or CRi rate in untreated elderly AML patients unfit for conventional intensive chemotherapy (111, 112). In the post-transplant setting, Schuler et al. retrospectively analyzed 32 AML patients who relapsed after allo-HCT and reported an ORR of 47% after venetoclax/HMA combined therapy. However, 72% of patients experienced severe infections, and 78% of patients died after a median follow-up of 8.4 months (113). We have recently reported 44 patients (AML, n = 34) with post-allo-HCT relapse who received salvage venetoclax/HMA, which achieved a CR/CRi incidence of 34.1% (114).
The reports have shown that venetoclax enhances T cell-mediated cytotoxicity against AML (115) and does not impair activated T cell proliferation (116). Therefore, venetoclax plus DLI has been tried in the post-transplant setting. Amit et al. summarized 22 AML patients with post-transplant relapse who received the venetoclax/DLI combination; a total of 11 patients (50%) responded, and CR/CRi was achieved in 5 patients (23%). Meanwhile, microbiology-documented infections occurred in 8 patients (36%) and aGVHD in 4 patients (18%) (92).
Other Agents
The deacetylase inhibitors (DACi) may enhance leukemia-specific cytotoxicity and mitigate GVHD but, conversely, could impair T and NK cell function (100). DACi panobinostat was evaluated in a phase I/II trial as maintenance therapy for high-risk AML/MDS in hematological complete remission after allo-HCT. High-risk AML was defined as with adverse risk cytogenetics, R/R disease, or secondary AML. In this study, 18 patients received the panobinostat/DLI combination, which showed a good safety profile but whose efficacy was unevaluable (100). Another phase I/II study evaluated the panobinostat/decitabine combination as maintenance in 110 patients with poor-risk AML/MDS after allo-HCT, in which 60 patients received DLI afterwards. The CI of relapse for the whole cohort was 35%, and the 2-year progression-free survival was 49%. Grades 3 and 4 adverse events related to panobinostat and decitabine were observed in 26% of evaluated patients. This study revealed the feasibility of the DACi/HMA/DLI combination as maintenance for AML after allo-HCT with enough safety and efficacy (101). Recently, IDH1/IDH2 inhibitors have been the other hotspot in the field of AML treatment. Instead of toxicity, IDH1/IDH2 inhibitors mainly exert therapeutic effects through the differentiation and maturation of malignant cells (117). In two pilot phase I studies, IDH1 inhibitor ivosidenib (118) achieved an ORR of 41.6%, and IDH2 inhibitor enasidenib (119) obtained an ORR of 40.3% in R/R AML patients with relevant mutations. Of note is that the continuous daily use of these two drugs led to a low frequency of treatment-related adverse events. The combined use of IDH1/IDH2 inhibitors and DLI requires further investigation for post-transplant AML relapse harboring these specific mutations.
DLI Composition Manipulation and Engineering
Donor-Derived NK Cells
The clinical studies on DLI composition manipulation and engineering are summarized in Table 2. Donor-derived natural killer (NK) cells may eliminate recipient malignant cells in the setting of mismatched or haploidentical transplant through alloreactivity (133) and attenuate GVHD via recipient dendritic cell elimination (134) and direct lysis or regulation of alloreactive T cells (135, 136). In a phase I trial, mbIL21 ex vivo-expanded donor NK cells were infused on days -2, +7, and +28 posttransplant for 13 patients with high-risk myeloid malignancies (AML, n = 8) who received PT-Cy-based haplo-HCT (120). In this study, high-risk AML was defined as a refractory disease or with unfavorable cytogenetics/molecular mutations, and high-risk MDS was that with an intermediate- or high-risk IPSS score. The safety of donor NK cell infusion was demonstrated, and it was observed that donor NK cell infusion might be related to lower viral infections and relapse rate. The other phase I trial tested prophylactic IL-2 activated donor-derived NK cell infusions 60–120 days after matched sibling allo-HCT in 16 patients with hematological diseases (AML, n = 6). The safety of this strategy was demonstrated, and promising efficacy was indicated (137). Meanwhile, Jaiswal et al. observed that a well-designed CD56-enriched DLI infusion after PT-Cy-based haplo-HCT prompted the good reconstitution of mature NK cells with a reduced incidence of aGVHD (121). More recently, CTLA4Ig has emerged as a novel and simpler approach in dissociating GVL and GVHD effects due to the rational that CTLA4Ig attenuates T cell activation but is resistant to NK cells. CTLA4Ig has been applied in combination with early sequential mDLI in patients with advanced hematological malignancies who received PT-Cy-based haplo-HCT. Compared to DLI alone, the CTLA4Ig-DLI decreased the acute GVHD (aGVHD) incidence from 18.8 to 9.6% and the cGVHD incidence from 36.5 to 15.3% (122). Interestingly, the disease progression rate was also lower in the CTLA4Ig-DLI group than in the DLI group (15.7 vs. 31.1%), which is probably due to an early expansion of functionally competent adaptive NK cells after the CTLA4Ig treatment (138, 139). Several strategies have been applied to enhance the anti-leukemic capacity of donor-derived NK cells, including the combined use with an immunomodulatory drug (eg., lenalidomide) (140), cytokine pre-activation (141), blocking of the inhibitory receptors [eg., PD1/PD-L1 (142) and iKIR (143)], selection of HLA-mismatched single KIR NK cells (144), or genetically modified NK cells (e.g., CAR-NK cells will be introduced in the next chapter). These techniques are expected to be used soon to potentiate NK cell therapy for AML relapse after allo-HCT.
CAR-T and CAR-NK Cells
CAR-T infusions post-allo-HCT have been intensively investigated, especially in B-ALL. Both allogenic and autologous CAR-T cells targeting CD19 (CART19) have been applied in clinical trials for B-ALL relapse after allo-HCT, with a CR rate of more than 80% (145) (146, 147). Apart from the satisfactory remission rate, CAR-T seems to confer a lower aGVHD incidence compared to DLI. Smith et al. summarized 132 patients from 9 studies who received posttransplant CART19 and observed a total aGVHD rate of 14% for donor-derived CAR-T and only 2% for recipient-derived CAR-T (148).
CAR-T cell therapy in AML has been much more challenging compared to B cell malignancies, majorly due to the lack of AML-specific target antigens and clonal heterogeneity, leading to unwanted on-target off-leukemia toxicity and risk of relapse from minor clones (149). Despite the difficulties, major efforts have been made to develop CAR-T cells for AML. Cui et al. reported a pioneering study using CD38-directed CAR-T cells in 6 patients with relapsed CD38+ AML after allo-HCT, and 4 (66.7%) achieved CR or CR with incomplete count recovery (CRi) (123). Notably, several key CAR-T products have shown promising efficacy in R/R AML and are tested in clinical trials [summarized by Daver et al. (150)]. An earlier study showed that LeY CAR-T achieved 1 CR in 4 adult R/R AML patients (151). The CD33 antibody–drug conjugate gemtuzumab ozogamicin was the only approved antibody-targeted therapy for AML, and the CD33/CD3-bispecific BiTE antibody construct has shown potent pre-clinical anti-AML activity (152). Although an earlier case report showed unsatisfactory results in one refractory AML patient receiving CD33 CAR-T infusion (153), several clinical trials are ongoing to prove its efficacy. CD123 CAR-T has shown potent antileukemic efficacy in a preclinical human AML xenograft model, while severe hematologic toxicities accompany the conventional second-generation CD123 CAR-T (154). More recently, a switchable universal CAR-T platform (UniCAR) has been tested in clinical trials (155). In a preliminary report on 3 R/R AML patients receiving CD123 UniCAR-T, the cell infusion was well tolerated, and CRi was achieved in 2 patients and PR in 1 patient (156). In addition, NKG2D CAR-T cells have been evaluated in a phase I clinical trial on AML/MDS and multiple myeloma patients. Among the 7 reported R/R AML patients, the CR/CRi rate was 42% (157). Moreover, CAR-T cells redirected to several novel targets [FLT3 (158, 159), CLL-1 (160), CD70 (161), IL-10R (162), mesothelin (163), Siglec-6 (164), etc.] have shown potent efficacy in pre-clinical studies, and some of them (eg., CLL-1) are currently being assessed in clinical trials (150). Furthermore, to overcome AML heterogeneity, dual CAR-T cells have also been investigated in AML. In a phase I study, Liu et al. evaluated compound CAR-T cells targeting both CD33 and CLL-1 in R/R AML; 2 patients who had blast counts >20% before cCAR T cell infusion achieved MRD-negative remission and were able to proceed to allo-HSCT (165). Other dual CAR-T, such as CD123/CLL-1 CAR-T cells, and further engineered CAR-T cells, such as CD19 CAR-T cells engineered to secrete a biparatopic anti-CLEC12A bridging protein, have been developed and evaluated (166).
Despite its potency, CAR-T cell therapy owns well-defined limitations, including cytokine release syndrome (CRS) and GVHD. With the potential to dissociate the GVL and GVHD effects, CAR-NK cells potentially confer better disease control with lower toxicity than CAR-T cells in both pre- and post-transplant settings (167). Due to difficulties in manufacturing (168), CAR-NK has been forwarded to clinical trials [summarized by Lu et al. (169)] more lately than CAR-T cells. Following the success of CD19 CAR-NK cells on B cell malignancies (170), few but encouraging results have been published on AML. Tang et al. reported three patients with R/R AML who received CD33-CAR NK-92 cells in a phase I trial. One patient experienced a high fever and two moderate fevers, and grade I CRS was observed in 2 patients, showing enough safety for the CAR-NK cell infusions in patients with high tumor burden (171). Moreover, CAR-NK redirected to several other AML antigens [e.g., NKG2D ligands (172), CD38 (173), CD123 (174), NPM1c (175), CD7 (176), etc.] have shown preclinical efficacy in hematological malignancies and are being investigated in clinical trials for AML.
Other Techniques of DLI Composition Manipulation and Engineering
Efforts have been made to modify the composition of DLI to reduce the GVHD rate but maintain the GVL effects. Infusion of ATIR101, the donor-derived T cell-enriched product selectively depleted of recipient-alloreactive T cells, in T cell-depleted (TCD) haplo-HCT has decreased the NRM rate and improved the GRFS versus TCD haplo-HCT alone (124). In addition, infusion of alloanergized DLI generated ex vivo on day +35 was shown to promote immune reconstitution and expand regulatory T cells after CD34-selected haplo-HCT (125). Inspired by the preclinical studies which indicated that naïve T cells are major drivers of GVHD, a phase I dose escalating study of CD45RA+ naïve T cell-depleted DLI was conducted in allo-HCT patients. Among the 28 patients evaluated, no patient developed grade III/IV acute GVHD or severe cGVHD, indicating the preventive capacity of this technique against GVHD (126). Moreover, since dendritic cells may bolster T cell responses through antigen presentation, a phase I trial has evaluated the co-infusion of DC followed by DLI in 16 relapsed patients with hematological malignancies after allo-HCT. Among the 14 evaluable patients, 4 (29%) achieved long-term remission, and only one developed grade II aGVHD (127).
Cytokine-induced killer cells (CIK cells), which are ex vivo-activated cytotoxic T cells containing predominantly CD3+CD8+NKG2D+ cells along with significantly expanded CD3+CD56+ cells, have shown anti-leukemic activity without causing severe aGVHD in relapse treatment (128) or consolidation (129) after allo-HCT. Merker et al. observed superior disease control but lower incidence of aGVHD for CIK in a comparative study between CIK and DLI in patients with overt hematologic relapse after allo-HCT (130). The sequential infusion of DLI and CIK has also been tested in a phase II trial for hematological relapse post-allo-HCT, but the response rate was unsatisfactory (response rate, 30%) (131). Notably, CAR-engineered CIK cells have shown potent anti-leukemic efficacy in pre-clinical studies in vitro and in vivo (177). Another attractive modality is multiple tumor antigen-specific T cells (TAA-T). In a prospective study conducted by Williams et al., expanded lymphocytes reactive to TAAs, including WT1, PRAME, and surviving, achieved 60% (3/5) CR rate in 5 AML patients relapsed after allo-HCT without causing GVHD (132). Moreover, T cells engineered with specific TCR (TCR-T) have shown potent anti-leukemic efficacy in vitro and in xenograft mouse models of lymphoid malignancies with low risk of aGVHD (178, 179). Finally, adoptive transfer of suicide gene-modified DLI has allowed efficient GVHD control via inducible cell elimination (180, 181).
Conclusions and Perspectives
The aim of DLI optimization is to maximize its GVL while minimizing the GVH effects. Many factors, including transplantation type, donor origin, disease burden, DLI dosage/timing, and immunosuppression, all affect greatly its efficacy/toxicity. It is recommended to use prophylactic/pre-emptive DLIs especially in high-risk AML after allo-HCT since therapeutic DLI for overt relapse is related to a lower chance of disease control. MRD-guided pre-emptive DLI awaits further improvement with the introduction of unbiased techniques with higher specificity, such as whole-genome sequencing. Additional use of novel drugs and composition manipulation are two promising directions which may revolutionize the DLI. Many novel targeted/immunomodulatory drugs are in the pipeline for clinical use in AML, while their efficacy/toxicity as well as their influence on the GVL/GVH effects must be clarified before clinical use after allo-HCT. Finally, cell engineering may help to realize the final aim of “perfect DLI” with full disease control and no GVHD, which awaits breakthrough in cellular immunotherapy for myeloid diseases. Integration of novel techniques (eg., CAR and TCR combined use) and personalized cell engineering techniques may further enhance the efficacy of cellular immunotherapy.
Author Contributions
YL and YY designed this study. YY and LY wrote the manuscript. YL and HH revised each section and gave expert comments. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Science Foundation of China (grant # 82000180 and grant # 82170205).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Bazarbachi A, Schmid C, Labopin M, Beelen D, Blau IW, Potter V, et al. Evaluation of Trends and Prognosis Over Time in Patients With AML Relapsing After Allogeneic Hematopoietic Cell Transplant Reveals Improved Survival for Young Patients in Recent Years. Clin Cancer Res (2020) 26:6475–82. doi: 10.1158/1078-0432.CCR-20-3134
2. Horowitz M, Schreiber H, Elder A, Heidenreich O, Vormoor J, Toffalori C, et al. Epidemiology and Biology of Relapse After Stem Cell Transplantation. Bone Marrow Transplant (2018) 53:1379–89. doi: 10.1038/s41409-018-0171-z
3. Tsirigotis P, Byrne M, Schmid C, Baron F, Ciceri F, Esteve J, et al. Relapse of AML After Hematopoietic Stem Cell Transplantation: Methods of Monitoring and Preventive Strategies. A Review From the ALWP of the EBMT. Bone Marrow Transplant (2016) 51:1431–8. doi: 10.1038/bmt.2016.167
4. Kharfan-Dabaja MA, Labopin M, Polge E, Nishihori T, Bazarbachi A, Finke J, et al. Association of Second Allogeneic Hematopoietic Cell Transplant vs Donor Lymphocyte Infusion With Overall Survival in Patients With Acute Myeloid Leukemia Relapse. JAMA Oncol (2018) 4:1245–53. doi: 10.1001/jamaoncol.2018.2091
5. Schmid C, Labopin M, Nagler A, Bornhäuser M, Finke J, Fassas A, et al. Donor Lymphocyte Infusion in the Treatment of First Hematological Relapse After Allogeneic Stem-Cell Transplantation in Adults With Acute Myeloid Leukemia: A Retrospective Risk Factors Analysis and Comparison With Other Strategies by the EBMT Acute Leukem. J Clin Oncol (2007) 25:4938–45. doi: 10.1200/JCO.2007.11.6053
6. Huang XJ, Liu DH, Liu KY, Xu LP, Chen H, Han W. Donor Lymphocyte Infusion for the Treatment of Leukemia Relapse After HLA-Mismatched/Haploidentical T-Cell-Replete Hematopoietic Stem Cell Transplantation. Haematologica (2007) 92:414–7. doi: 10.3324/haematol.10570
7. Christopeit M, Kuss O, Finke J, Bacher U, Beelen DW, Bornhäuser M, et al. Second Allograft for Hematologic Relapse of Acute Leukemia After First Allogeneic Stem-Cell Transplantation From Related and Unrelated Donors: The Role of Donor Change. J Clin Oncol (2013) 31:3259–71. doi: 10.1200/JCO.2012.44.7961
8. Shimoni A, Labopin M, Finke J, Ciceri F, Deconinck E, Kröger N, et al. Donor Selection for a Second Allogeneic Stem Cell Transplantation in AML Patients Relapsing After a First Transplant: A Study of the Acute Leukemia Working Party of EBMT. Blood Cancer J (2019) 9(12):88. doi: 10.1038/s41408-019-0251-3
9. Dholaria B, Savani BN, Labopin M, Luznik L, Ruggeri A, Mielke S, et al. Clinical Applications of Donor Lymphocyte Infusion From an HLA-Haploidentical Donor: Consensus Recommendations From the Acute Leukemia Working Party of the EBMT. Haematologica (2020) 105:47–58. doi: 10.3324/haematol.2019.219790
10. Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, et al. Donor Leukocyte Transfusions for Treatment of Recurrent Chronic Myelogenous Leukemia in Marrow Transplant Patients. Blood (1990) 76:2462–5. doi: 10.1182/blood.v76.12.2462.2462
11. Chalandon Y, Passweg JR, Schmid C, Olavarria E, Dazzi F, Simula MP, et al. Outcome of Patients Developing GVHD After DLI Given to Treat CML Relapse: A Study by the Chronic Leukemia Working Party of the EBMT. Bone Marrow Transplant (2010) 45:558–64. doi: 10.1038/bmt.2009.177
12. Radujkovic A, Guglielmi C, Bergantini S, Iacobelli S, van Biezen A, Milojkovic D, et al. Donor Lymphocyte Infusions for Chronic Myeloid Leukemia Relapsing After Allogeneic Stem Cell Transplantation: May We Predict Graft-Versus-Leukemia Without Graft-Versus-Host Disease? Biol Blood Marrow Transplant (2015) 21:1230–6. doi: 10.1016/j.bbmt.2015.03.012
13. Yan CH, Wang JZ, Liu DH, Xu LP, Chen H, Liu KY, et al. Chemotherapy Followed by Modified Donor Lymphocyte Infusion as a Treatment for Relapsed Acute Leukemia After Haploidentical Hematopoietic Stem Cell Transplantation Without In Vitro T-Cell Depletion: Superior Outcomes Compared With Chemotherapy Alone and a. Eur J Haematol (2013) 91:304–14. doi: 10.1111/ejh.12168
14. D’Souza A, Fretham C, Lee SJ, Arora M, Brunner J, Chhabra S, et al. Current Use of and Trends in Hematopoietic Cell Transplantation in the United States. Biol Blood Marrow Transplant (2020) 26:e177–82. doi: 10.1016/j.bbmt.2020.04.013
15. Passweg JR, Baldomero H, Chabannon C, Basak GW, Corbacioglu S, Duarte R, et al. The EBMT Activity Survey on Hematopoietic-Cell Transplantation and Cellular Therapy 2018: CAR-T’s Come Into Focus. Bone Marrow Transplant (2020) 55:1604–13. doi: 10.1038/s41409-020-0826-4
16. Wang Y, Liu DH, Xu LP, Liu KY, Chen H, Chen YH, et al. Superior Graft-Versus-Leukemia Effect Associated With Transplantation of Haploidentical Compared With HLA-Identical Sibling Donor Grafts for High-Risk Acute Leukemia: An Historic Comparison. Biol Blood Marrow Transplant (2011) 17:821–30. doi: 10.1016/j.bbmt.2010.08.023
17. Luo Y, Xiao H, Lai X, Shi J, Tan Y, He J, et al. T-Cell-Replete Haploidentical HSCT With Low-Dose Anti-T-Lymphocyte Globulin Compared With Matched Sibling HSCT and Unrelated HSCT. Blood (2014) 124:2735–43. doi: 10.1182/blood-2014-04-571570
18. Yan CH, Liu DH, Liu KY, Xu LP, Liu YR, Chen H, et al. Risk Stratification-Directed Donor Lymphocyte Infusion Could Reduce Relapse of Standard-Risk Acute Leukemia Patients After Allogeneic Hematopoietic Stem Cell Transplantation. Blood (2012) 119:3256–62. doi: 10.1182/blood-2011-09-380386
19. Takami A, Yano S, Yokoyama H, Kuwatsuka Y, Yamaguchi T, Kanda Y, et al. Donor Lymphocyte Infusion for the Treatment of Relapsed Acute Myeloid Leukemia After Allogeneic Hematopoietic Stem Cell Transplantation: A Retrospective Analysis by the Adult Acute Myeloid Leukemia Working Group of the Japan Society for Hematopoietic Cell. Biol Blood Marrow Transplant (2014) 20:1785–90. doi: 10.1016/j.bbmt.2014.07.010
20. Sun W, Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, et al. Chemotherapy Plus DLI for Relapse After Haploidentical HSCT: The Biological Characteristics of Relapse Influences Clinical Outcomes of Acute Leukemia Patients. Bone Marrow Transplant (2019) 54:1198–207. doi: 10.1038/s41409-018-0406-z
21. Schmid C, Labopin M, Nagler A, Niederwieser D, Castagna L, Tabrizi R, et al. Treatment, Risk Factors, and Outcome of Adults With Relapsed AML After Reduced Intensity Conditioning for Allogeneic Stem Cell Transplantation. Blood (2012) 119:1599–606. doi: 10.1182/blood-2011-08-375840
22. Guillaume T, Gaugler B, Chevallier P, Delaunay J, Ayari S, Clavert A, et al. Escalated Lymphodepletion Followed by Donor Lymphocyte Infusion can Induce a Graft-Versus-Host Response Without Overwhelming Toxicity. Bone Marrow Transplant (2012) 47:1112–7. doi: 10.1038/bmt.2011.231
23. Levine JE, Braun T, Penza SL, Beatty P, Cornetta K, Martino R, et al. Prospective Trial of Chemotherapy and Donor Leukocyte Infusions for Relapse of Advanced Myeloid Malignancies After Allogeneic Stem-Cell Transplantation. J Clin Oncol (2002) 20:405–12. doi: 10.1200/JCO.20.2.405
24. Ghiso A, Raiola AM, Gualandi F, Dominietto A, Varaldo R, Van Lint MT, et al. DLI After Haploidentical BMT With Post-Transplant CY. Bone Marrow Transplant (2015) 50:56–61. doi: 10.1038/bmt.2014.217
25. Rettig AR, Ihorst G, Bertz H, Lübbert M, Marks R, Waterhouse M, et al. Donor Lymphocyte Infusions After First Allogeneic Hematopoietic Stem-Cell Transplantation in Adults With Acute Myeloid Leukemia: A Single-Center Landmark Analysis. Ann Hematol (2021) 100:2339–50. doi: 10.1007/s00277-021-04494-z
26. Lewalle P, Triffet A, Delforge A, Crombez P, Selleslag D, De Muynck H, et al. Donor Lymphocyte Infusions in Adult Haploidentical Transplant: A Dose Finding Study. Bone Marrow Transplant (2003) 31:39–44. doi: 10.1038/sj.bmt.1703779
27. Gilman AL, Leung W, Cowan MJ, Cannon M, Epstein S, Barnhart C, et al. Donor Lymphocyte Infusion and Methotrexate for Immune Recovery After T-Cell Depleted Haploidentical Transplantation. Am J Hematol (2018) 93:169–78. doi: 10.1002/ajh.24949
28. Wang Y, Liu DH, Xu LP, Liu KY, Chen H, Zhang XH, et al. Prevention of Relapse Using Granulocyte CSF-Primed PBPCs Following HLA-Mismatched/Haploidentical, T-Cell-Replete Hematopoietic SCT in Patients With Advanced-Stage Acute Leukemia: A Retrospective Risk-Factor Analysis. Bone Marrow Transplant (2012) 47:1099–104. doi: 10.1038/bmt.2011.213
29. Jaiswal SR, Zaman S, Chakrabarti A, Sen S, Mukherjee S, Bhargava S, et al. Improved Outcome of Refractory/Relapsed Acute Myeloid Leukemia After Post-Transplantation Cyclophosphamide-Based Haploidentical Transplantation With Myeloablative Conditioning and Early Prophylactic Granulocyte Colony-Stimulating Factor–Mobilized Donor Ly. Biol Blood Marrow Transplant (2016) 22:1867–73. doi: 10.1016/j.bbmt.2016.07.016
30. Pierini A, Ruggeri L, Mancusi A, Carotti A, Falzetti F, Terenzi A, et al. T Cell Depletion and No Post Transplant Immune Suppression Allow Separation of Graft Versus Leukemia From Graft Versus Host Disease. Bone Marrow Transplant (2019) 54:775–9. doi: 10.1038/s41409-019-0597-y
31. Yang L, Tan Y, Shi J, Zhao Y, Yu J, Hu Y, et al. Prophylactic Modified Donor Lymphocyte Infusion After Low-Dose ATG-F-Based Haploidentical HSCT With Myeloablative Conditioning in High-Risk Acute Leukemia: A Matched-Pair Analysis. Bone Marrow Transplant (2020) 56(3):664–72. doi: 10.1038/s41409-020-01088-7
32. Yan CH, Wang Y, Wang JZ, Chen YH, Chen Y, Wang FR, et al. Minimal Residual Disease- and Graft-vs.-Host Disease-Guided Multiple Consolidation Chemotherapy and Donor Lymphocyte Infusion Prevent Second Acute Leukemia Relapse After Allotransplant. J Hematol Oncol (2016) 9:1–11. doi: 10.1186/s13045-016-0319-5
33. Yan CH, Liu QF, Wu DP, Zhang X, Xu LP, Zhang XH, et al. Prophylactic Donor Lymphocyte Infusion (DLI) Followed by Minimal Residual Disease and Graft-Versus-Host Disease–Guided Multiple DLIs Could Improve Outcomes After Allogeneic Hematopoietic Stem Cell Transplantation in Patients With Refractory/Relapsed Acute. Biol Blood Marrow Transplant (2017) 23:1311–9. doi: 10.1016/j.bbmt.2017.04.028
34. Yang L, Tan Y, Shi J, Zhao Y, Yu J, Hu Y, et al. Prophylactic Modified Donor Lymphocyte Infusion After Low-Dose ATG-F-Based Haploidentical HSCT With Myeloablative Conditioning in High-Risk Acute Leukemia: A Matched-Pair Analysis. Bone Marrow Transplant (2021) 56:664–72. doi: 10.1038/s41409-020-01088-7
35. Legrand F, Le Floch AC, Granata A, Fürst S, Faucher C, Lemarie C, et al. Prophylactic Donor Lymphocyte Infusion After Allogeneic Stem Cell Transplantation for High-Risk AML. Bone Marrow Transplant (2017) 52:620–1. doi: 10.1038/bmt.2016.326
36. Guillaume T, Malard F, Magro L, Labopin M, Tabrizi R, Borel C, et al. Prospective Phase II Study of Prophylactic Low-Dose Azacitidine and Donor Lymphocyte Infusions Following Allogeneic Hematopoietic Stem Cell Transplantation for High-Risk Acute Myeloid Leukemia and Myelodysplastic Syndrome. Bone Marrow Transplant (2019) 54:1815–26. doi: 10.1038/s41409-019-0536-y
37. Jedlickova Z, Schmid C, Koenecke C, Hertenstein B, Baurmann H, Schwerdtfeger R, et al. Long-Term Results of Adjuvant Donor Lymphocyte Transfusion in AML After Allogeneic Stem Cell Transplantation. Bone Marrow Transplant (2016) 51:663–7. doi: 10.1038/bmt.2015.234
38. Wang Y, Xu L, Yan C, Huang X. Modification of Donor Lymphocyte Infusion: How to Improve the Outcome? Sci China Life Sci (2019) 62:1253–6. doi: 10.1007/s11427-019-9597-3
39. Glass MB, Dreger P, Seime R, Santoro A, Vasta S, Sutlorp M, et al. Allogeneic Peripheral Blood Progenitor Cells for Treatment of Relapse After Bone Marrow Transplantation. Bone Marrow Transplant (1997) 20:533–41. doi: 10.1038/sj.bmt.1700934
40. Collins RH, Shpilberg O, Drobyski WR, Porter DL, Giralt S, Champlin R, et al. Donor Leukocyte Infusions in 140 Patients With Relapsed Malignancy After Allogeneic Bone Marrow Transplantation. J Clin Oncol (1997) 15:433–44. doi: 10.1200/JCO.1997.15.2.433
41. Zeidan AM, Forde PM, Symons H, Chen A, Smith BD, Pratz K, et al. HLA-Haploidentical Donor Lymphocyte Infusions for Patients With Relapsed Hematologic Malignancies After Related HLA-Haploidentical Bone Marrow Transplantation. Biol Blood Marrow Transplant (2014) 20:314–8. doi: 10.1016/j.bbmt.2013.11.020
42. Goldsmith SR, Slade M, Dipersio JF, Westervelt P, Schroeder MA, Gao F, et al. Donor-Lymphocyte Infusion Following Haploidentical Hematopoietic Cell Transplantation With Peripheral Blood Stem Cell Grafts and PTCy. Bone Marrow Transplant (2017) 52:1623–8. doi: 10.1038/bmt.2017.193
43. Gilleece MH, Labopin M, Yakoub-Agha I, Volin L, Socié G, Ljungman P, et al. Measurable Residual Disease, Conditioning Regimen Intensity, and Age Predict Outcome of Allogeneic Hematopoietic Cell Transplantation for Acute Myeloid Leukemia in First Remission: A Registry Analysis of 2292 Patients by the Acute Leukemia Working Party E. Am J Hematol (2018) 93:1142–52. doi: 10.1002/ajh.25211
44. Shah MV, Jorgensen JL, Saliba RM, Wang SA, Alousi AM, Andersson BS, et al. Early Post-Transplant Minimal Residual Disease Assessment Improves Risk Stratification in Acute Myeloid Leukemia. Biol Blood Marrow Transplant (2018) 24:1514–20. doi: 10.1016/j.bbmt.2018.02.003
45. Lee HC, Saliba RM, Rondon G, Chen J, Charafeddine Y, . Medeiros LJ, et al. Mixed T-Lymphocyte Chimerism After Allogeneic Hematopoietic Transplantation Is Predictive for Relapse of AML/MDS. Biol Blood Marrow Transplant (2016) 176:1948–54. doi: 10.1016/j.bbmt.2015.07.005.Mixed
46. Reshef R, Hexner EO, Loren AW, Frey NV, Stadtmauer EA, Luger SM, et al. Early Donor Chimerism Levels Predict Relapse and Survival After Allogeneic Stem Cell Transplantation With Reduced-Intensity Conditioning. Biol Blood Marrow Transplant (2014) 20:1758–66. doi: 10.1016/j.bbmt.2014.07.003
47. Tan Y, Du K, Luo Y, Shi J, Cao L, Zheng Y, et al. Superiority of Preemptive Donor Lymphocyte Infusion Based on Minimal Residual Disease in Acute Leukemia Patients After Allogeneic Hematopoietic Stem Cell Transplantation. Transfusion (2014) 54:1493–500. doi: 10.1111/trf.12524
48. Di Grazia C, Pozzi S, Geroldi S, Grasso R, Miglino M, Colombo N, et al. Wilms Tumor 1 Expression and Pre-Emptive Immunotherapy in Patients With Acute Myeloid Leukemia Undergoing an Allogeneic Hemopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2016) 22:1242–6. doi: 10.1016/j.bbmt.2016.03.005
49. Aitken MJL, Ravandi F, Patel KP, Short NJ. Prognostic and Therapeutic Implications of Measurable Residual Disease in Acute Myeloid Leukemia. J Hematol Oncol (2021) 14:1–15. doi: 10.1186/s13045-021-01148-5
50. Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. Comparison of Outcomes After Donor Lymphocyte Infusion With or Without Prior Chemotherapy for Minimal Residual Disease in Acute Leukemia/Myelodysplastic Syndrome After Allogeneic Hematopoietic Stem Cell Transplantation. Ann Hematol (2017) 96:829–38. doi: 10.1007/s00277-017-2960-7
51. Yuan XL, Tan YM, Shi JM, Zhao YM, Yu J, Lai XY, et al. Preemptive Low-Dose Interleukin-2 or DLI for Late-Onset Minimal Residual Disease in Acute Leukemia or Myelodysplastic Syndrome After Allogeneic Hematopoietic Stem Cell Transplantation. Ann Hematol (2021) 100:517–27. doi: 10.1007/s00277-020-04326-6
52. Pozzi S, Geroldi S, Tedone E, Luchetti S, Grasso R, Colombo N, et al. Leukaemia Relapse After Allogeneic Transplants for Acute Myeloid Leukaemia: Predictive Role of WT1 Expression. Br J Haematol (2013) 160:503–9. doi: 10.1111/bjh.12181
53. Mo XD, Zhang XH, Xu LP, Wang Y, Yan CH, Chen H, et al. Salvage Chemotherapy Followed by Granulocyte Colony-Stimulating Factor-Primed Donor Leukocyte Infusion With Graft-vs-Host Disease Control for Minimal Residual Disease in Acute Leukemia/Myelodysplastic Syndrome After Allogeneic Hematopoietic Stem Cell Tran. Eur J Haematol (2016) 96:297–308. doi: 10.1111/ejh.12591
54. Solomon SR, Sizemore CA, Zhang X, Brown S, Holland HK, Morris LE, et al. Preemptive DLI Without Withdrawal of Immunosuppression to Promote Complete Donor T-Cell Chimerism Results in Favorable Outcomes for High-Risk Older Recipients of Alemtuzumab-Containing Reduced-Intensity Unrelated Donor Allogeneic Transplant: A Prospective. Bone Marrow Transplant (2014) 49:616–21. doi: 10.1038/bmt.2014.2
55. Caldemeyer LE, Akard LP, Edwards JR, Tandra A, Wagenknecht DR, Dugan MJ. Donor Lymphocyte Infusions Used to Treat Mixed-Chimeric and High-Risk Patient Populations in the Relapsed and Nonrelapsed Settings After Allogeneic Transplantation for Hematologic Malignancies Are Associated With High Five-Year Survival If Persistent Full. Biol Blood Marrow Transplant (2017) 23:1989–97. doi: 10.1016/j.bbmt.2017.07.007
56. Feliu J, Potter V, Grimaldi F, Clay J, Floro L, Saha C, et al. Full Donor Chimerism Without Graft-Versus-Host Disease: The Key Factor for Maximum Benefit of Pre-Emptive Donor Lymphocyte Infusions (pDLI). Bone Marrow Transplant (2020) 55:562–9. doi: 10.1038/s41409-019-0695-x
57. Keil F, Haas OA, Fritsch G, Kalhs P, Lechner K, Mannhalter C, et al. Donor Leukocyte Infusion for Leukemic Relapse After Allogeneic Marrow Transplantation: Lack of Residual Donor Hematopoiesis Predicts Aplasia. Blood (1997) 89:3113–7. doi: 10.1182/blood.v89.9.3113
58. Lowdell MW, Craston R, Ray N, Koh M, Galatowicz G, Prentice HG. The Effect of T Cell Depletion With Campath-1M on Immune Reconstitution After Chemotherapy and Allogeneic Bone Marrow Transplant as Treatment for Leukaemia. Bone Marrow Transplant (1998) 21:679–86. doi: 10.1038/sj.bmt.1701153
59. Aversa F, Terenzi A, Tabilio A, Falzetti F, Carotti A, Ballanti S, et al. Full Haplotype-Mismatched Hematopoietic Stem-Cell Transplantation: A Phase II Study in Patients With Acute Leukemia at High Risk of Relapse. J Clin Oncol (2005) 23:3447–54. doi: 10.1200/JCO.2005.09.117
60. Or-Geva N, Reisner Y. The Evolution of T-Cell Depletion in Haploidentical Stem-Cell Transplantation. Br J Haematol (2016) 172:667–84. doi: 10.1111/bjh.13868
61. Kothari S, Artz AS, Lee SM, Fulton N, Park JH, Stock W, et al. Dose Escalation Prophylactic Donor Lymphocyte Infusion After T-Cell Depleted Matched Related Donor Allogeneic Hematopoietic Cell Transplantation Is Feasible and Results in Higher Donor Chimerism, Faster Immune Re-Constitution, and Prolonged Progression-Fr. Bone Marrow Transplant (2020) 55:1161–8. doi: 10.1038/s41409-020-0798-4
62. Meyer RG, Britten CM, Wehler D, Bender K, Hess G, Konur A, et al. Prophylactic Transfer of CD8-Depleted Donor Lymphocytes After T-Cell-Depleted Reduced-Intensity Transplantation. Blood (2007) 109:374–82. doi: 10.1182/blood-2006-03-005769
63. Dodero A, Carniti C, Raganato A, Vendramin A, Farina L, Spina F, et al. Haploidentical Stem Cell Transplantation After a Reduced-Intensity Conditioning Regimen for the Treatment of Advanced Hematologic Malignancies: Posttransplantation CD8-Depleted Donor Lymphocyte Infusions Contribute to Improve T-Cell Recovery. Blood (2009) 113:4771–9. doi: 10.1182/blood-2008-10-183723
64. Di Ianni M, Falzetti F, Carotti A, Terenzi A, Castellino F, Bonifacio E, et al. Tregs Prevent GVHD and Promote Immune Reconstitution in HLA-Haploidentical Transplantation. Blood (2011) 117:3921–8. doi: 10.1182/blood-2010-10-311894
65. Edinger M, Hoffmann P, Ermann J, Drago K, Garrison Fathman C, Strober S, et al. CD4+CD25+ Regulatory T Cells Preserve Graft-Versus-Tumor Activity While Inhibiting Graft-Versus-Host Disease After Bone Marrow Transplantation. Nat Med (2003) 9:1144–50. doi: 10.1038/nm915
66. Martelli MF, Di Ianni M, Ruggeri L, Falzetti F, Carotti A, Terenzi A, et al. HLA-Haploidentical Transplantation With Regulatory and Conventional T-Cell Adoptive Immunotherapy Prevents Acute Leukemia Relapse. Blood (2014) 124:638–44. doi: 10.1182/blood-2014-03-564401
67. Pierini A, Ruggeri L, Carotti A, Falzetti F, Saldi S, Terenzi A, et al. Haploidentical Age-Adapted Myeloablative Transplant and Regulatory and Effector T Cells for Acute Myeloid Leukemia. Blood Adv (2021) 5:1199–208. doi: 10.1182/bloodadvances.2020003739
68. Perruccio K, Tosti A, Burchielli E, Topini F, Ruggeri L, Carotti A, et al. Transferring Functional Immune Responses to Pathogens After Haploidentical Hematopoietic Transplantation. Blood (2005) 106:4397–406. doi: 10.1182/blood-2005-05-1775
69. Olson A, Lin R, Marin D, Rafei H, Bdaiwi MH, Thall PF, et al. Third-Party BK Virus-Specific Cytotoxic T Lymphocyte Therapy for Hemorrhagic Cystitis Following Allotransplantation. J Clin Oncol (2021) 39:2710–9. doi: 10.1200/JCO.20.02608
70. Perruccio K, Topini F, Tosti A, Carotti A, Burchielli E, Ruggeri L, et al. Optimizing a Photoallodepletion Protocol for Adoptive Immunotherapy After Haploidentical SCT. Bone Marrow Transplant (2012) 47:1196–200. doi: 10.1038/bmt.2011.237
71. Schmid C, Schleuning M, Ledderose G, Tischer J, Kolb HJ. Sequential Regimen of Chemotherapy, Reduced-Intensity Conditioning for Allogeneic Stem-Cell Transplantation, and Prophylactic Donor Lymphocyte Transfusion in High-Risk Acute Myeloid Leukemia and Myelodysplastic Syndrome. J Clin Oncol (2005) 23:5675–87. doi: 10.1200/JCO.2005.07.061
72. Chevallier P, Labopin M, Socié G, Tabrizi R, Furst S, Lioure B, et al. Results From a Clofarabine-Busulfan-Containing, Reduced-Toxicity Conditioning Regimen Prior to Allogeneic Stem Cell Transplantation: The Phase 2 Prospective CLORIC Trial. Haematologica (2014) 99:1486–91. doi: 10.3324/haematol.2014.108563
73. Mohty M, Malard F, Blaise D, Milpied N, Socié G, Huynh A, et al. Sequential Regimen of Clofarabine, Cytosine Arabinoside and Reduced-Intensity Conditioned Transplantation for Primar Y Refractor Y Acute Myeloid Leukemia. Haematologica (2017) 102:184–91. doi: 10.3324/haematol.2016.150326
74. Shelikhova L, Ilushina M, Shekhovtsova Z, Shasheleva D, Khismatullina R, Kurnikova E, et al. αβ T Cell-Depleted Haploidentical Hematopoietic Stem Cell Transplantation Without Antithymocyte Globulin in Children With Chemorefractory Acute Myelogenous Leukemia. Biol Blood Marrow Transplant (2019) 25:e179–82. doi: 10.1016/j.bbmt.2019.01.023
75. Gao XN, Lin J, Wang LJ, Li F, Li HH, Wang SH, et al. Comparison of the Safety and Efficacy of Prophylactic Donor Lymphocyte Infusion After Haploidentical Versus Matched-Sibling PBSCT in Very High-Risk Acute Myeloid Leukemia. Ann Hematol (2019) 98:1267–77. doi: 10.1007/s00277-019-03636-8
76. Cauchois R, Castagna L, Pagliardini T, Harbi S, Calmels B, Bramanti S, et al. Prophylactic Donor Lymphocyte Infusions After Haploidentical Haematopoietic Stem Cell Transplantation for High Risk Haematological Malignancies: A Retrospective Bicentric Analysis of Serial Infusions of Increasing Doses of CD3 + Cells. Br J Haematol (2019) 185:570–3. doi: 10.1111/bjh.15544
77. Guillaume T, Thépot S, Peterlin P, Ceballos P, Le Bourgeois A, Garnier A, et al. Prophylactic or Preemptive Low-Dose Azacitidine and Donor Lymphocyte Infusion to Prevent Disease Relapse Following Allogeneic Transplantation in Patients With High-Risk Acute Myelogenous Leukemia or Myelodysplastic Syndrome. Transplant Cell Ther (2021) 000:1–6. doi: 10.1016/j.jtct.2021.06.029
78. Vago L, Perna SK, Zanussi M, Mazzi B, Barlassina C, Stanghellini MTL, et al. Loss of Mismatched HLA in Leukemia After Stem-Cell Transplantation. N Engl J Med (2009) 361:478–88. doi: 10.1056/nejmoa0811036
79. Zeiser R, Vago L. Mechanisms of Immune Escape After Allogeneic Hematopoietic Cell Transplantation. Blood (2019) 133:1290–7. doi: 10.1182/blood-2018-10-846824
80. Crucitti L, Crocchiolo R, Toffalori C, Mazzi B, Greco R, Signori A, et al. Incidence, Risk Factors and Clinical Outcome of Leukemia Relapses With Loss of the Mismatched HLA After Partially Incompatible Hematopoietic Stem Cell Transplantation. Leukemia (2015) 29:1143–52. doi: 10.1038/leu.2014.314
81. Jan M, Leventhal MJ, Morgan EA, Wengrod JC, Nag A, Drinan SD, et al. Recurrent Genetic HLA Loss in AML Relapsed After Matched Unrelated Allogeneic Hematopoietic Cell Transplantation. Blood Adv (2019) 3:2199–204. doi: 10.1182/bloodadvances.2019000445
82. Su Q, Fan Z, Huang F, Xu N, Nie D, Lin D, et al. Comparison of Two Strategies for Prophylactic Donor Lymphocyte Infusion in Patients With Refractory/Relapsed Acute Leukemia. Front Oncol (2021) 11:554503. doi: 10.3389/fonc.2021.554503
83. Call SK, Kasow KA, Barfield R, Madden R, Leung W, Horwitz E, et al. Total and Active Rabbit Antithymocyte Globulin (rATG;Thymoglobulin®) Pharmacokinetics in Pediatric Patients Undergoing Unrelated Donor Bone Marrow Transplantation. Biol Blood Marrow Transplant (2009) 15:274–8. doi: 10.1016/j.bbmt.2008.11.027
84. Liga M, Triantafyllou E, Tiniakou M, Lambropoulou P, Karakantza M, Zoumbos NC, et al. High Alloreactivity of Low-Dose Prophylactic Donor Lymphocyte Infusion in Patients With Acute Leukemia Undergoing Allogeneic Hematopoietic Cell Transplantation With an Alemtuzumab-Containing Conditioning Regimen. Biol Blood Marrow Transplant (2013) 19:75–81. doi: 10.1016/j.bbmt.2012.07.021
85. Dazzi F, Szydlo RM, Cross NCP, Craddock C, Kaeda J, Kanfer E, et al. Durability of Responses Following Donor Lymphocyte Infusions for Patients Who Relapse After Allogeneic Stem Cell Transplantation for Chronic Myeloid Leukemia. Blood (2000) 96:2712–6. doi: 10.1182/blood.v96.8.2712
86. Bar M, Sandmaier BM, Inamoto Y, Bruno B, Hari P, Chauncey T, et al. Donor Lymphocyte Infusion for Relapsed Hematological Malignancies After Allogeneic Hematopoietic Cell Transplantation: Prognostic Relevance of the Initial CD3+ T Cell Dose. Biol Blood Marrow Transplant (2013) 19:949–57. doi: 10.1016/j.bbmt.2013.03.001
87. Yu S, Huang F, Fan Z, Xuan L, Nie D, Xu Y, et al. Haploidentical Versus HLA-Matched Sibling Transplantation for Refractory Acute Leukemia Undergoing Sequential Intensified Conditioning Followed by DLI: An Analysis From Two Prospective Data. J Hematol Oncol (2020) 13:1–11. doi: 10.1186/s13045-020-00859-5
88. Yan CH, Xu LP, Liu DH, Chen H, Wang Y, Wang JZ, et al. Low-Dose Methotrexate may Preserve a Stronger Antileukemic Effect Than That of Cyclosporine After Modified Donor Lymphocyte Infusion in Unmanipulated Haploidentical HSCT. Clin Transplant (2015) 29:594–605. doi: 10.1111/ctr.12561
89. Yan CH, Liu DH, Xu LP, Liu KY, Zhao T, Wang Y, et al. Modified Donor Lymphocyte Infusion-Associated Acute Graft-Versus-Host Disease After Haploidentical T-Cell-Replete Hematopoietic Stem Cell Transplantation: Incidence and Risk Factors. Clin Transplant (2012) 26:868–76. doi: 10.1111/j.1399-0012.2012.01618.x
90. Zhang R, Wang L, Chen P, Gao X, Wang S, Li F, et al. Haematologic Malignancies With Unfavourable Gene Mutations Benefit From Donor Lymphocyte Infusion With/Without Decitabine for Prophylaxis of Relapse After Allogeneic HSCT: A Pilot Study. Cancer Med (2021) 10:3165–76. doi: 10.1002/cam4.3763
91. Schroeder T, Czibere A, Platzbecker U, Bug G, Uharek L, Luft T, et al. Azacitidine and Donor Lymphocyte Infusions as First Salvage Therapy for Relapse of AML or MDS After Allogeneic Stem Cell Transplantation. Leukemia (2013) 27:1229–35. doi: 10.1038/leu.2013.7
92. Amit O, On YB, Perez G, Shargian-Alon L, Yeshurun M, Ram R. Venetoclax and Donor Lymphocyte Infusion for Early Relapsed Acute Myeloid Leukemia After Allogeneic Hematopoietic Cell Transplantation. A Retrospective Multicenter Trial. Ann Hematol (2021) 100:817–24. doi: 10.1007/s00277-021-04398-y
93. Schroeder T, Rachlis E, Bug G, Stelljes M, Klein S, Steckel NK, et al. Treatment of Acute Myeloid Leukemia or Myelodysplastic Syndrome Relapse After Allogeneic Stem Cell Transplantation With Azacitidine and Donor Lymphocyte Infusions-A Retrospective Multicenter Analysis From the German Cooperative Transplant Study Group. Biol Blood Marrow Transplant (2015) 21:653–60. doi: 10.1016/j.bbmt.2014.12.016
94. Craddock C, Labopin M, Robin M, Finke J, Chevallier P, Yakoub-Agha I, et al. Clinical Activity of Azacitidine in Patients Who Relapse After Allogeneic Stem Cell Transplantation for Acute Myeloid Leukemia. Haematologica (2016) 101:879–83. doi: 10.3324/haematol.2015.140996
95. Ghobadi A, Choi J, Fiala MA, Fletcher T, Liu J, Eissenberg LG, et al. Phase I Study of Azacitidine Following Donor Lymphocyte Infusion for Relapsed Acute Myeloid Leukemia Post Allogeneic Stem Cell Transplantation. Leuk Res (2016) 49:1–6. doi: 10.1016/j.leukres.2016.07.010
96. Schroeder T, Rautenberg C, Krüger W, Platzbecker U, Bug G, Steinmann J, et al. Treatment of Relapsed AML and MDS After Allogeneic Stem Cell Transplantation With Decitabine and DLI—A Retrospective Multicenter Analysis on Behalf of the German Cooperative Transplant Study Group. Ann Hematol (2018) 97:335–42. doi: 10.1007/s00277-017-3185-5
97. Sommer S, Cruijsen M, Claus R, Bertz H, Wäsch R, Marks R, et al. Decitabine in Combination With Donor Lymphocyte Infusions Can Induce Remissions in Relapsed Myeloid Malignancies With Higher Leukemic Burden After Allogeneic Hematopoietic Cell Transplantation. Leuk Res (2018) 72:20–6. doi: 10.1016/j.leukres.2018.07.005
98. Xuan L, Wang Y, Chen J, Jiang E, Gao L, Wu B, et al. Sorafenib Therapy Is Associated With Improved Outcomes for FMS-Like Tyrosine Kinase 3 Internal Tandem Duplication Acute Myeloid Leukemia Relapsing After Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2019) 25:1674–81. doi: 10.1016/j.bbmt.2019.04.018
99. Bruzzese A, Assanto GM, Diverio D, Quattrocchi L, Carmini D, La Rocca U, et al. Pre-Emptive Use of Sorafenib Combined With DLI Post HSCT in AML FLT3+: A Single Center Experience. Bone Marrow Transplant (2021) 56:1455–7. doi: 10.1038/s41409-020-01174-w
100. Bug G, Burchert A, Wagner EM, Kröger N, Berg T, Güller S, et al. Phase I/II Study of the Deacetylase Inhibitor Panobinostat After Allogeneic Stem Cell Transplantation in Patients With High-Risk MDS or AML (PANOBEST Trial). Leukemia (2017) 31:2523–5. doi: 10.1038/leu.2017.242
101. Kalin B, van Norden Y, van Gelder M, Breems D, Maertens J, Jongen-Lavrencic M, et al. Panobinostat and Decitabine Prior to Donor Lymphocyte Infusion in Allogeneic Stem Cell Transplantation. Blood Adv (2020) 4:4430–7. doi: 10.1182/BLOODADVANCES.2020002074
102. Patel SA, Litzow MR, Cerny J. Targeted and Cytotoxic Therapies as Maintenance Treatment for Non-Transplant Eligible Patients With Acute Myeloid Leukemia. Blood Rev (2021) 50:100863. doi: 10.1016/j.blre.2021.100863
103. Goodyear O, Agathanggelou A, Novitzky-Basso I, Siddique S, McSkeane T, Ryan G, et al. Induction of a CD8+ T-Cell Response to the MAGE Cancer Testis Antigen by Combined Treatment With Azacitidine and Sodium Valproate in Patients With Acute Myeloid Leukemia and Myelodysplasia. Blood (2010) 116:1908–18. doi: 10.1182/blood-2009-11-249474
104. Choi J, Ritchey J, Prior JL, Holt M, Shannon WD, Deych E, et al. In Vivo Administration of Hypomethylating Agents Mitigate Graft-Versus-Host Disease Without Sacrificing Graft-Versus-Leukemia. Blood (2010) 116:129–39. doi: 10.1182/blood-2009-12-257253
105. Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, Roberts ND, et al. Genomic Classification and Prognosis in Acute Myeloid Leukemia. N Engl J Med (2016) 374:2209–21. doi: 10.1056/nejmoa1516192
106. Kantarjian H, Kadia T, DiNardo C, Daver N, Borthakur G, Jabbour E, et al. Acute Myeloid Leukemia: Current Progress and Future Directions. Blood Cancer J (2021) 11(2):41 doi: 10.1038/s41408-021-00425-3
107. Battipaglia G, Ruggeri A, Massoud R, El Cheikh J, Jestin M, Antar A, et al. Efficacy and Feasibility of Sorafenib as a Maintenance Agent After Allogeneic Hematopoietic Stem Cell Transplantation for Fms-Like Tyrosine Kinase 3-Mutated Acute Myeloid Leukemia. Cancer (2017) 123:2867–74. doi: 10.1002/cncr.30680
108. Xuan L, Wang Y, Huang F, Fan Z, Xu Y, Sun J, et al. Sorafenib Maintenance in Patients With FLT3-ITD Acute Myeloid Leukaemia Undergoing Allogeneic Haematopoietic Stem-Cell Transplantation: An Open-Label, Multicentre, Randomised Phase 3 Trial. Lancet Oncol (2020) 21:1201–12. doi: 10.1016/S1470-2045(20)30455-1
109. Shi J, Cao L, Luo Y, Zhao Y, Tan Y, Yu J, et al. Maintenance Sorafenib Is Superior to Prophylactic Donor Lymphocyte Infusion at Improving the Prognosis of Acute Myeloid Leukemia With FMS-Like Tyrosine Kinase 3 Internal Tandem Duplication After Allogeneic Hematopoietic Stem Cell Transplantation. Bone Marrow Transplant (2021) 56:293–6. doi: 10.1038/s41409-020-01015-w
110. Mathew NR, Baumgartner F, Braun L, O’Sullivan D, Thomas S, Waterhouse M, et al. Sorafenib Promotes Graft-Versus-Leukemia Activity in Mice and Humans Through IL-15 Production in FLT3-ITD-Mutant Leukemia Cells. Nat Med (2018) 24:282–91. doi: 10.1038/nm.4484
111. DiNardo CD, Pratz K, Pullarkat V, Jonas BA, Arellano M, Becker PS, et al. Venetoclax Combined With Decitabine or Azacitidine in Treatment-Naive, Elderly Patients With Acute Myeloid Leukemia. Blood (2019) 133:7–17. doi: 10.1182/blood-2018-08-868752
112. DiNardo CD, Jonas BA, Pullarkat V, Thirman MJ, Garcia JS, Wei AH, et al. Azacitidine and Venetoclax in Previously Untreated Acute Myeloid Leukemia. N Engl J Med (2020) 383:617–29. doi: 10.1056/nejmoa2012971
113. Schuler E, Wagner-Drouet EM, Ajib S, Bug G, Crysandt M, Dressler S, et al. Treatment of Myeloid Malignancies Relapsing After Allogeneic Hematopoietic Stem Cell Transplantation With Venetoclax and Hypomethylating Agents—A Retrospective Multicenter Analysis on Behalf of the German Cooperative Transplant Study Group. Ann Hematol (2021) 100:959–68. doi: 10.1007/s00277-020-04321-x
114. Gao F, Gao Y, Luo Y, Yu J, Fu H, Lai X, et al. Venetoclax Plus Hypomethylating Agent for the Salvage Treatment of Relapsing Myeloid Malignancies After Hematopoietic Stem Cell Transplantation: A Multicenter Retrospective Study on Behalf of the Zhejiang Cooperative Group for Blood and Marrow Transplanta. Am J Hematol (2021) 97(2):E44–7. doi: 10.1002/ajh.26405
115. Lee JB, Khan DH, Hurren R, Xu M, Na Y, Kang H, et al. Venetoclax Enhances T Cell–Mediated Antileukemic Activity by Increasing ROS Production. Blood (2021) 138:234–45. doi: 10.1182/blood.2020009081
116. Siblany L, Gaugler B, Stocker N, Ricard L, Ye Y, Mohty M, et al. Venetoclax Does Not Impair Activated T-Cell Proliferation. Bone Marrow Transplant (2021) 56:1740–2. doi: 10.1038/s41409-021-01245-6
117. Amatangelo MD, Quek L, Shih A, Stein EM, Roshal M, David MD, et al. Enasidenib Induces Acute Myeloid Leukemia Cell Differentiation to Promote Clinical Response. Blood (2017) 130:732–41. doi: 10.1182/blood-2017-04-779447
118. DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, Mims AS, et al. Durable Remissions With Ivosidenib in IDH1 -Mutated Relapsed or Refractory AML. N Engl J Med (2018) 378:2386–98. doi: 10.1056/nejmoa1716984
119. Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, Altman JK, et al. Enasidenib in Mutant IDH2 Relapsed or Refractory Acute Myeloid Leukemia. Blood (2017) 130:722–31. doi: 10.1182/blood-2017-04-779405
120. Ciurea SO, Schafer JR, Bassett R, Denman CJ, Cao K, Willis D, et al. Phase 1 Clinical Trial Using Mbil21 Ex Vivo-Expanded Donor-Derived NK Cells After Haploidentical Transplantation. Blood (2017) 130:1857–68. doi: 10.1182/blood-2017-05-785659
121. Jaiswal SR, Zaman S, Nedunchezhian M, Chakrabarti A, Bhakuni P, Ahmed M, et al. CD56-Enriched Donor Cell Infusion After Post-Transplantation Cyclophosphamide for Haploidentical Transplantation of Advanced Myeloid Malignancies Is Associated With Prompt Reconstitution of Mature Natural Killer Cells and Regulatory T Cells With Reduced I. Cytotherapy (2017) 19:531–42. doi: 10.1016/j.jcyt.2016.12.006
122. Jaiswal SR, Bhakuni P, Bhagawati G, Aiyer HM, Soni M, Sharma N, et al. CTLA4Ig-Primed Donor Lymphocyte Infusions Following Haploidentical Transplantation Improve Outcome With a Distinct Pattern of Early Immune Reconstitution as Compared to Conventional Donor Lymphocyte Infusions in Advanced Hematological Malignancies. Bone Marrow Transplant (2021) 56:185–94. doi: 10.1038/s41409-020-01002-1
123. Cui Q, Qian C, Xu N, Kang L, Dai H, Cui W, et al. CD38-Directed CAR-T Cell Therapy: A Novel Immunotherapy Strategy for Relapsed Acute Myeloid Leukemia After Allogeneic Hematopoietic Stem Cell Transplantation. J Hematol Oncol (2021) 14:4–8. doi: 10.1186/s13045-021-01092-4
124. Roy DC, Walker I, Maertens J, Lewalle P, Olavarria E, Selleslag D, et al. ATIR101 Administered After T-Cell-Depleted Haploidentical HSCT Reduces NRM and Improves Overall Survival in Acute Leukemia. Leukemia (2020) 34:1907–23. doi: 10.1038/s41375-020-0733-0
125. Davies JK, Brennan LL, Wingard JR, Cogle CR, Kapoor N, Shah AJ, et al. Infusion of Alloanergized Donor Lymphocytes After CD34-Selected Haploidentical Myeloablative Hematopoietic Stem Cell Transplantation. Clin Cancer Res (2018) 24:4098–109. doi: 10.1158/1078-0432.CCR-18-0449
126. Maung KK, Chen BJ, Barak I, Li Z, Rizzieri DA, Gasparetto C, et al. Phase I Dose Escalation Study of Naive T-Cell Depleted Donor Lymphocyte Infusion Following Allogeneic Stem Cell Transplantation. Bone Marrow Transplant (2021) 56:137–43. doi: 10.1038/s41409-020-0991-5
127. Ho VT, Kim HT, Kao G, Cutler C, Levine J, Rosenblatt J, et al. Sequential Infusion of Donor-Derived Dendritic Cells With Donor Lymphocyte Infusion for Relapsed Hematologic Cancers After Allogeneic Hematopoietic Stem Cell Transplantation. Am J Hematol (2014) 89:1092–6. doi: 10.1002/ajh.23825
128. Laport GG, Sheehan K, Baker J, Armstrong R, Wong RM, Lowsky R, et al. Adoptive Immunotherapy With Cytokine-Induced Killer Cells for Patients With Relapsed Hematologic Malignancies After Allogeneic Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant (2011) 17:1679–87. doi: 10.1016/j.bbmt.2011.05.012
129. Narayan R, Benjamin JE, Shah O, Tian L, Tate K, Armstrong R, et al. Donor-Derived Cytokine-Induced Killer Cell Infusion as Consolidation After Nonmyeloablative Allogeneic Transplantation for Myeloid Neoplasms. Biol Blood Marrow Transplant (2019) 25:1293–303. doi: 10.1016/j.bbmt.2019.03.027
130. Merker M, Salzmann-Manrique E, Katzki V, Huenecke S, Bremm M, Bakhtiar S, et al. Clearance of Hematologic Malignancies by Allogeneic Cytokine-Induced Killer Cell or Donor Lymphocyte Infusions. Biol Blood Marrow Transplant (2019) 25:1281–92. doi: 10.1016/j.bbmt.2019.03.004
131. Introna M, Lussana F, Algarotti A, Gotti E, Valgardsdottir R, Micò C, et al. Phase II Study of Sequential Infusion of Donor Lymphocyte Infusion and Cytokine-Induced Killer Cells for Patients Relapsed After Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant (2017) 23:2070–8. doi: 10.1016/j.bbmt.2017.07.005
132. Williams KM, Grant M, Ismail M, Hoq F, Martin-Manso M, Hoover J, et al. Complete Remissions Post Infusion of Multiple Tumor Antigen Specific T Cells for the Treatment of High Risk Leukemia and Lymphoma Patients After HCT. Cytotherapy (2017) 19:e3. doi: 10.1016/j.jcyt.2017.03.013
133. Gao F, Ye Y, Gao Y, Huang H, Zhao Y. Influence of KIR and NK Cell Reconstitution in the Outcomes of Hematopoietic Stem Cell Transplantation. Front Immunol (2020) 11:2022. doi: 10.3389/fimmu.2020.02022
134. Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, et al. Effectiveness of Donor Natural Killer Cell Aloreactivity in Mismatched Hematopoietic Transplants. Science (80-) (2002) 295:2097–100. doi: 10.1126/science.1068440
135. Pallmer K, Oxenius A. Recognition and Regulation of T Cells by NK Cells. Front Immunol (2016) 7:251. doi: 10.3389/fimmu.2016.00251
136. Sheng L, Mu Q, Wu X, Yang S, Zhu H, Wang J, et al. Cytotoxicity of Donor Natural Killer Cells to Allo-Reactive T Cells Are Related With Acute Graft-Vs.-Host-Disease Following Allogeneic Stem Cell Transplantation. Front Immunol (2020) 11:1534. doi: 10.3389/fimmu.2020.01534
137. Devillier R, Calmels B, Guia S, Taha M, Fauriat C, Mfarrej B, et al. Phase I Trial of Prophylactic Donor-Derived Il-2-Activated Nk Cell Infusion After Allogeneic Hematopoietic Stem Cell Transplantation From a Matched Sibling Donor. Cancers (Basel) (2021) 13(11):2673. doi: 10.3390/cancers13112673
138. Cichocki F, Cooley S, Davis Z, DeFor TE, Schlums H, Zhang B, et al. CD56 Dim CD57+ NKG2C+ NK Cell Expansion Is Associated With Reduced Leukemia Relapse After Reduced Intensity HCT. Leukemia (2016) 30:456–63. doi: 10.1038/leu.2015.260
139. Jaiswal SR, Chakraborty S, Lakhchaura R, Shashi P, Mehta A, Soni M, et al. Early and Sustained Expansion of Adaptive Natural Killer Cells Following Haploidentical Transplantation and CTLA4Ig-Primed Donor Lymphocyte Infusions Dissociate Graft-Versus-Leukemia and Graft-Versus-Host Effects. Transplant Cell Ther (2021) 27:144–51. doi: 10.1016/j.jtct.2020.10.005
140. Hideshima T, Ogiya D, Liu J, Harada T, Kurata K, Bae J, et al. Immunomodulatory Drugs Activate NK Cells via Both Zap-70 and Cereblon-Dependent Pathways. Leukemia (2021) 35:177–88. doi: 10.1038/s41375-020-0809-x
141. Romee R, Rosario M, Berrien-Elliott MM, Wagner JA, Jewell BA, Schappe T, et al. Cytokine-Induced Memory-Like Natural Killer Cells Exhibit Enhanced Responses Against Myeloid Leukemia. Sci Transl Med (2016) 8(357):357ra123. doi: 10.1126/scitranslmed.aaf2341
142. Pesce S, Greppi M, Grossi F, Del Zotto G, Moretta L, Sivori S, et al. PD/1-PD-Ls Checkpoint: Insight on the Potential Role of NK Cells. Front Immunol (2019) 10:1242. doi: 10.3389/fimmu.2019.01242
143. Benson DM, Cohen AD, Jagannath S, Munshi NC, Spitzer G, Hofmeister CC, et al. A Phase I Trial of the Anti-KIR Antibody IPH2101 and Lenalidomide in Patients With Relapsed/Refractory Multiple Myeloma. Clin Cancer Res (2015) 21:4055–61. doi: 10.1158/1078-0432.CCR-15-0304
144. Langenkamp U, Siegler U, Jörger S, Diermayr S, Gratwohl A, Kalberer CP, et al. Human Acute Myeloid Leukemia CD34+CD38- Stem Cells Are Susceptible to Allorecognition and Lysis by Single KIR-Expressing Natural Killer Cells. Haematologica (2009) 94:1590–4. doi: 10.3324/haematol.2009.005967
145. Hu Y, Wang J, Wei G, Yu J, Luo Y, Shi J, et al. A Retrospective Comparison of Allogenic and Autologous Chimeric Antigen Receptor T Cell Therapy Targeting CD19 in Patients With Relapsed/Refractory Acute Lymphoblastic Leukemia. Bone Marrow Transplant (2019) 54:1208–17. doi: 10.1038/s41409-018-0403-2
146. Zhang C, Wang XQ, Zhang RL, Liu F, Wang Y, Yan ZL, et al. Donor-Derived CD19 CAR-T Cell Therapy of Relapse of CD19-Positive B-ALL Post Allotransplant. Leukemia (2021) 35:1563–70. doi: 10.1038/s41375-020-01056-6
147. Ding L, Wang Y, Hong R, Zhao H, Zhou L, Wei G, et al. Efficacy and Safety of Chimeric Antigen Receptor T Cells in Acute Lymphoblastic Leukemia With Post-Transplant Relapse. Front Oncol (2021) 11:750218. doi: 10.3389/fonc.2021.750218
148. Smith M, Zakrzewski J, James S, Sadelain M. Strategies to Improve Graft-Versus-Leukemia Effects Posttransplant Chimeric Antigen Receptor Therapy. Blood (2018) 131:1045–52. doi: 10.1182/blood-2017-08-752121
149. Haubner S, Perna F, Köhnke T, Schmidt C, Berman S, Augsberger C, et al. Coexpression Profile of Leukemic Stem Cell Markers for Combinatorial Targeted Therapy in AML. Leukemia (2019) 33:64–74. doi: 10.1038/s41375-018-0180-3
150. Daver N, Alotaibi AS, Bücklein V, Subklewe M. T-Cell-Based Immunotherapy of Acute Myeloid Leukemia: Current Concepts and Future Developments. Leukemia (2021) 35:1843–63. doi: 10.1038/s41375-021-01253-x
151. Ritchie DS, Neeson PJ, Khot A, Peinert S, Tai T, Tainton K, et al. Persistence and Efficacy of Second Generation CAR T Cell Against the LeY Antigen in Acute Myeloid Leukemia. Mol Ther (2013) 21:2122–9. doi: 10.1038/mt.2013.154
152. Herrmann M, Krupka C, Deiser K, Brauchle B, Marcinek A, Wagner AO, et al. Bifunctional PD-1 3 Acd3 3 Acd33 Fusion Protein Reverses Adaptive Immune Escape in Acute Myeloid Leukemia. Blood (2018) 132:2484–94. doi: 10.1182/blood-2018-05-849802
153. Wang QS, Wang Y, Lv HY, Han QW, Fan H, Guo B, et al. Treatment of CD33-Directed Chimeric Antigen Receptor-Modified T Cells in One Patient With Relapsed and Refractory Acute Myeloid Leukemia. Mol Ther (2015) 23:184–91. doi: 10.1038/mt.2014.164
154. Tasian SK, Kenderian SS, Shen F, Ruella M, Shestova O, Kozlowski M, et al. Optimized Depletion of Chimeric Antigen Receptor T Cells in Murine Xenograft Models of Human Acute Myeloid Leukemia. Blood (2017) 129:2395–407. doi: 10.1182/blood-2016-08-736041
155. Meyer JE, Loff S, Dietrich J, Spehr J, Jurado Jiménez G, von Bonin M, et al. Evaluation of Switch-Mediated Costimulation in Trans on Universal CAR-T Cells (UniCAR) Targeting CD123-Positive AML. Oncoimmunology (2021) 10(1):1945804. doi: 10.1080/2162402X.2021.1945804
156. Wermke M, Kraus S, Ehninger A, Bargou RC, Goebeler ME, Middeke JM, et al. Proof of Concept for a Rapidly Switchable Universal CAR-T Platform With UniCAR-T-CD123 in Relapsed/Refractory AML. Blood (2021) 137:3145–8. doi: 10.1182/blood.2020009759
157. Baumeister SH, Murad J, Werner L, Daley H, Trebeden-Negre H, Gicobi JK, et al. Phase I Trial of Autologous CAR T Cells Targeting NKG2D Ligands in Patients With AML/MDS and Multiple Myeloma. Cancer Immunol Res (2019) 7:100–12. doi: 10.1158/2326-6066.CIR-18-0307
158. Wang Y, Xu Y, Li S, Liu J, Xing Y, Xing H, et al. Targeting FLT3 in Acute Myeloid Leukemia Using Ligand-Based Chimeric Antigen Receptor-Engineered T Cells. J Hematol Oncol (2018) 11:1–12. doi: 10.1186/s13045-018-0603-7
159. Sommer C, Cheng HY, Nguyen D, Dettling D, Yeung YA, Sutton J, et al. Allogeneic FLT3 CAR T Cells With an Off-Switch Exhibit Potent Activity Against AML and Can Be Depleted to Expedite Bone Marrow Recovery. Mol Ther (2020) 28:2237–51. doi: 10.1016/j.ymthe.2020.06.022
160. Wang J, Chen S, Xiao W, Li W, Wang L, Yang S, et al. CAR-T Cells Targeting CLL-1 as an Approach to Treat Acute Myeloid Leukemia. J Hematol Oncol (2018) 11:1–13. doi: 10.1186/s13045-017-0553-5
161. Sauer T, Parikh K, Sharma S, Omer B, Sedloev D, Chen Q, et al. CD70-Specific CAR T Cells Have Potent Activity Against Acute Myeloid Leukemia Without HSC Toxicity. Blood (2021) 138:318–30. doi: 10.1182/blood.2020008221
162. Chen N, Xu Y, Mou J, Rao Q, Xing H, Tian Z, et al. Targeting of IL-10R on Acute Myeloid Leukemia Blasts With Chimeric Antigen Receptor-Expressing T Cells. Blood Cancer J (2021) 11:1–11. doi: 10.1038/s41408-021-00536-x
163. Le Q, Castro S, Tang T, Loeb AM, Hylkema T, McKay C, et al. Therapeutic Targeting of Mesothelin With Chimeric Antigen Receptor T Cells in Acute Myeloid Leukemia. Clin Cancer Res (2021) 27(20):5718–30. doi: 10.1158/1078-0432.ccr-21-1546
164. Jetani H, Navarro-Bailón A, Maucher M, Frenz S, Verbruggen CM, Yeguas A, et al. Siglec-6 Is a Novel Target for CAR T-Cell Therapy in Acute Myeloid Leukemia (AML). Blood (2021) 138(19):1830–42. doi: 10.1182/blood.2020009192
165. Liu F, Cao Y, Pinz K, Ma Y, Wada M, Chen K, et al. First-In-Human CLL1-CD33 Compound CAR T Cell Therapy Induces Complete Remission in Patients With Refractory Acute Myeloid Leukemia: Update on Phase 1 Clinical Trial. Blood (2018) 132:901–1. doi: 10.1182/blood-2018-99-110579
166. Rennert PD, Dufort FJ, Su L, Sanford T, Birt A, Wu L, et al. Anti-CD19 CAR T Cells That Secrete a Biparatopic Anti-CLEC12A Bridging Protein Have Potent Activity Against Aggressive Acute Myeloid Leukemia In Vitro and In Vivo. Mol Cancer Ther (2021) (10):2071–81. doi: 10.1158/1535-7163.mct-20-1030
167. Gurney M, O’dwyer M. Realizing Innate Potential: Car-Nk Cell Therapies for Acute Myeloid Leukemia. Cancers (Basel) (2021) 13:1–26. doi: 10.3390/cancers13071568
168. Klöß S, Oberschmidt O, Morgan M, Dahlke J, Arseniev L, Huppert V, et al. Optimization of Human NK Cell Manufacturing: Fully Automated Separation, Improved Ex Vivo Expansion Using IL-21 With Autologous Feeder Cells, and Generation of Anti-CD123-CAR-Expressing Effector Cells. Hum Gene Ther (2017) 28:897–913. doi: 10.1089/hum.2017.157
169. Lu H, Zhao X, Li Z, Hu Y, Wang H. From CAR-T Cells to CAR-NK Cells: A Developing Immunotherapy Method for Hematological Malignancies. Front Oncol (2021) 11:720501. doi: 10.3389/fonc.2021.720501
170. Liu E, Marin D, Banerjee P, Macapinlac HA, Thompson P, Basar R, et al. Use of CAR-Transduced Natural Killer Cells in CD19-Positive Lymphoid Tumors. N Engl J Med (2020) 382:545–53. doi: 10.1056/nejmoa1910607
171. Tang X, Yang L, Li Z, Nalin AP, Dai H, Xu T, et al. Erratum: First-In-Man Clinical Trial of CAR NK-92 Cells: Safety Test of CD33-CAR NK-92 Cells in Patients With Relapsed and Refractory Acute Myeloid Leukemia. Am J Cancer Res (2018) 8:1899.
172. Leivas A, Valeri A, Córdoba L, García-Ortiz A, Ortiz A, Sánchez-Vega L, et al. NKG2D-CAR-Transduced Natural Killer Cells Efficiently Target Multiple Myeloma. Blood Cancer J (2021) 11:1–11. doi: 10.1038/s41408-021-00537-w
173. Gurney M, Stikvoort A, Nolan E, Kirkham-McCarthy L, Khoruzhenko S, Shivakumar R, et al. CD38 Knockout Natural Killer Cells Expressing an Affinity Optimized CD38 Chimeric Antigen Receptor Successfully Target Acute Myeloid Leukemia With Reduced Effector Cell Fratricide. Haematologica (2020). doi: 10.3324/haematol.2020.271908
174. Morgan MA, Kloos A, Lenz D, Kattre N, Nowak J, Bentele M, et al. Improved Activity Against Acute Myeloid Leukemia With Chimeric Antigen Receptor (CAR)-NK-92 Cells Designed to Target Cd123. Viruses (2021) 13:1365. doi: 10.3390/v13071365
175. Dong H, Xie G, Liang Y, Dongjoo Ham J, Vergara J, Chen J, et al. Engineered Memory-Like NK Cars Targeting a Neoepitope Derived From Intracellular NPM1c Exhibit Potent Activity and Specificity Against Acute Myeloid Leukemia. Blood (2020) 136:3–4. doi: 10.1182/blood-2020-134148
176. You F, Wang Y, Jiang L, Zhu X, Chen D, Yuan L, et al. A Novel CD7 Chimeric Antigen Receptor-Modified NK-92MI Cell Line Targeting T-Cell Acute Lymphoblastic Leukemia. Am J Cancer Res (2019) 9:64–78.
177. Oelsner S, Wagner J, Friede ME, Pfirrmann V, Genßler S, Rettinger E, et al. Chimeric Antigen Receptor-Engineered Cytokine-Induced Killer Cells Overcome Treatment Resistance of Pre-B-Cell Acute Lymphoblastic Leukemia and Enhance Survival. Int J Cancer (2016) 139:1799–809. doi: 10.1002/ijc.30217
178. Jahn L, Hombrink P, Hagedoorn RS, Kester MGD, van der Steen DM, Rodriguez T, et al. TCR-Based Therapy for Multiple Myeloma and Other B-Cell Malignancies Targeting Intracellular Transcription Factor BOB1. Blood (2017) 129:1284–95. doi: 10.1182/blood-2016-09-737536
179. Kawamura K, Tanaka Y, Nakasone H, Ishihara Y, Kako S, Kobayashi S, et al. Development of a Unique T Cell Receptor Gene-Transferred Tax-Redirected T Cell Immunotherapy for Adult T Cell Leukemia. Biol Blood Marrow Transplant (2020) 26:1377–85. doi: 10.1016/j.bbmt.2020.04.006
180. Di Stasi A, Tey S-K, Dotti G, Fujita Y, Kennedy-Nasser A, Martinez C, et al. Inducible Apoptosis as a Safety Switch for Adoptive Cell Therapy. N Engl J Med (2011) 365:1673–83. doi: 10.1056/nejmoa1106152
181. Stabile H, Nisti P, Fionda C, Pagliara D, Gaspari S, Locatelli F, et al. NK Cell Reconstitution in Paediatric Leukemic Patients After T-Cell-Depleted HLA-Haploidentical Haematopoietic Stem Cell Transplantation Followed by the Reinfusion of Icasp9-Modified Donor T Cells. J Clin Med (2019) 8:1904. doi: 10.3390/jcm8111904
Keywords: donor lymphocyte infusion (DLI), AML—acute myeloid leukemia, allogeneic hematopoietic cell transplantation, new drug, cell engineering
Citation: Ye Y, Yang L, Yuan X, Huang H and Luo Y (2022) Optimization of Donor Lymphocyte Infusion for AML Relapse After Allo-HCT in the Era of New Drugs and Cell Engineering. Front. Oncol. 11:790299. doi: 10.3389/fonc.2021.790299
Received: 06 October 2021; Accepted: 28 December 2021;
Published: 27 January 2022.
Edited by:
Giorgia Battipaglia, Federico II University of Naples, ItalyCopyright © 2022 Ye, Yang, Yuan, Huang and Luo. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Luo, bHVveWlqckB6anUuZWR1LmNu
 Yishan Ye
Yishan Ye Luxin Yang1,2
Luxin Yang1,2 He Huang
He Huang Yi Luo
Yi Luo