
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
CLINICAL TRIAL article
Front. Oncol. , 06 December 2021
Sec. Hematologic Malignancies
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.789891
This article is part of the Research Topic Insights in Hematologic Malignancies: 2021 View all 28 articles
 Laura Ballotta1,2
Laura Ballotta1,2 Pier Luigi Zinzani3,4
Pier Luigi Zinzani3,4 Stefano Pileri5
Stefano Pileri5 Riccardo Bruna6
Riccardo Bruna6 Monica Tani7
Monica Tani7 Beatrice Casadei3,4
Beatrice Casadei3,4 Valentina Tabanelli5
Valentina Tabanelli5 Stefano Volpetti8
Stefano Volpetti8 Stefano Luminari9,10
Stefano Luminari9,10 Paolo Corradini11
Paolo Corradini11 Elisa Lucchini2
Elisa Lucchini2 Maria Chiara Tisi12
Maria Chiara Tisi12 Michele Merli13
Michele Merli13 Alessandro Re14
Alessandro Re14 Marzia Varettoni15
Marzia Varettoni15 Emanuela Anna Pesce16
Emanuela Anna Pesce16 Francesco Zaja1,2*
Francesco Zaja1,2*Patients with relapsed/refractory (R/R) peripheral T-cell lymphoma (PTCL) have a poor prognosis, with an expected survival of less than 1 year using standard salvage therapies. Recent advances in our understanding of the biology of PTCL have led to identifying B-Cell Lymphoma 2 (BCL2) protein as a potential therapeutic target. BLC2 inhibitor venetoclax was investigated in a prospective phase II trial in patients with BCL2-positive R/R PTCL after at least one previous standard line of treatment (NCT03552692). Venetoclax given alone at a dosage of 800 mg/day resulted in one complete response (CR) and two stable diseases (SDs) among 17 enrolled patients. The majority of patients (88.2%) interrupted the treatment due to disease progression. No relationship with BCL2 expression was documented. At a median follow-up of 8 months, two patients are currently still on treatment (one CR and one SD). No case of tumor lysis syndrome was registered. Therefore, venetoclax monotherapy shows activity in a minority of patients whose biological characteristics have not yet been identified.
Clinical Trial Registration: www.clinicaltrials.gov (NCT03552692, EudraCT number 2017-004630-29).
Peripheral T-cell lymphomas (PTCLs) are a rare and heterogeneous group of T-cell neoplasms, accounting for 5%–10% of all non-Hodgkin lymphomas in Western countries. PTCL arises from mature post-thymic lymphocytes, and the most represented subtypes are PTCL not otherwise specified (PTCL-NOS), angioimmunoblastic T-cell lymphoma (AITL) and other nodal T-cell lymphomas of T-follicular helper origin (TFH), and anaplastic large cell lymphoma (ALCL) ALK-positive (ALCL-ALK+) and ALK-negative (ALCL-ALK-) (1). With few exceptions, PTCLs share an aggressive clinical behavior and poor prognosis; the response to induction chemotherapy is often inadequate and/or short-term (2).
The standard of care for PTCL is based on anthracycline-containing regimens [cyclophosphamide, doxorubicin, vincristine, prednisolone ± etoposide (CHOP/CHOEP)], resulting in a 5-year overall survival (OS) of only 30%–40% (3). High-dose chemotherapy followed by autologous stem cell transplantation (ASCT) is recommended in younger chemosensitive patients, but many of them do not receive the transplant because of ineffective disease control or, eventually, relapse soon after (4).
Following the promising results of a phase 3 clinical trial, the US Food and Drug Administration (FDA) recently approved the use of brentuximab vedotin (BV) in combination with cyclophosphamide, doxorubicin, and prednisolone (CHP) as first-line treatment for CD30-positive PTCL (5). However, only a minority of patients in this study had PTCL-NOS, AITL, or TFH, and the benefit of the addition of BV was not demonstrated in this small population.
Relapsed or refractory (R/R) PTCLs are characterized by a dismal prognosis, and treatment in this setting is an unmet medical need. There is no standard of care in R/R patients; gemcitabine-based regimens are frequently used in this setting, ensuring an overall response rate (ORR) of 30%–70% and median progression-free survival (PFS) of 4–11 months (6).
Romidepsin, belinostat, and pralatrexate received FDA approval for the treatment of R/R PTCLs, but the ORR with these agents is 30%, with a median PFS of a few months (6–8). More promising results were reported with BV, which showed remarkable activity in ALCLs (9), but no similar activity was registered in other PTCLs (10). Other compounds are under investigation in clinical trials alone or in combination (e.g., lenalidomide, copanlisib, duvelisib, 5-azacitidine) (11).
Recent advances in understanding the biology of PTCLs led to identifying BCL2, Cluster of differentiation 38 (CD38), and Programmed death-1 (PD-1) as potential therapeutic targets. Regarding BCL2, an immunohistochemical analysis conducted within the Fondazione Italiana Linfomi (FIL) indicated that BCL2, an antiapoptotic protein, is overexpressed in 88% of patients with AITL, 80% of patients with PTCL-NOS, 58% of patients with ALK- ALCL, and 31% of patients with ALK+ ALCL (12). Moreover, an inverse correlation between BCL2 expression and the apoptotic rate has been demonstrated in PTCL (13), and BCL2 overexpression in PTCLs seems to correlate with disease progression through interactions with the p53-dependent pathway (14). Thus, BCL2 may represent a potential therapeutic target.
Venetoclax is an anti-BCL2 agent approved in Europe for the treatment of chronic lymphocytic leukemia (CLL) that has shown therapeutic activity in mantle cell lymphoma (MCL) and other hematological malignancies (15–17). We report the results of a study from FIL that evaluated the activity of venetoclax monotherapy in BCL2-positive R/R PTCL.
FIL_VERT is an open-label, multicenter, single-arm phase II trial with a two-stage Simon design that aimed to evaluate the activity and safety of venetoclax as a single agent in patients with BCL2-positive PTCL-NOS, AITL, or TFH who were R/R after at least one previous standard line of treatment.
Twenty-one Italian centers belonging to FIL were involved in the present study. Patients aged ≥18 years with a diagnosis of R/R PTCL-NOS, AITL, or TFH and an Eastern Cooperative Oncology Group (ECOG) performance status ≤2 were enrolled. Relapse biopsy, if available, or otherwise the initial biopsy was centrally revised (Division of Haematopathology, European Institute of Oncology, Milan, Italy) according to the revised fourth edition of the WHO classification of hematopoietic and lymphoid tumors (1). The percentage of BCL2-positive tumor cells was scored as follows, according to Bossard et al. (18): 4, >75%; 3, 50%–75%; 2, 25%–49%; 1, 5%–24%; 0, <5%. For central review and confirmation of the diagnosis, fresh sections were cut from the paraffin block(s) and used for immunohistochemistry to assess the expression of BCL2. Only patients with ≥25% BCL2-positive tumor cells in the relapse or initial biopsy were included in the study. For all inclusion and exclusion criteria, see Supplement 1.
All patients provided written informed consent, and the study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the ethical committee of each participating site. The trial was registered at www.clinicaltrials.gov (NCT03552692) and given EudraCT number 2017-004630-29.
The primary objective of the study was to evaluate the efficacy of venetoclax in terms of ORR [complete response (CR) and partial response (PR)] using the Revised Response Criteria for Malignant Lymphoma (Lugano Classification) (19), which was evaluated after three treatment cycles. Secondary objectives included assessment of the CR rate, PR rate, stable disease (SD) rate, overall survival (OS), time to response (TTR), PFS, duration of treatment, and safety. Finally, the explorative objective was to assess the possible relationship between response and BLC2 expression.
Thirty-five patients were planned to be enrolled. Given the two-stage design of the study, a stop in recruitment was planned after the enrollment of 18 patients to perform interim efficacy analysis. To proceed to the second stage, the minimum number of patients with an ORR had to be 3/18. Treatment consisted of oral venetoclax (800 mg/day for 28 days/cycle) administered continuously as a single agent until disease progression, unacceptable toxicity, withdrawal of consent, and/or the investigator determined that further therapy is not in the patient’s best interest. To reduce the risk of tumor lysis syndrome (TLS), the dosage of venetoclax was gradually increased in an initial ramp-up phase (Supplement 2). Patients were also hospitalized for the first 72 h of treatment, and intravenous hydration and antihyperuricemic drugs were administered.
OS and PFS were estimated using the Kaplan–Meier product-limit-method. Adverse events (AEs) were encoded according to National Cancer Institute (NCI) Common Terminology Criteria for Adverse Events v. 4.03.
Between May 2018 and November 2019, 22 patients were enrolled and 17 were determined to be eligible; the diagnosis was not centrally confirmed in one patient, BCL2 was <25% in two patients, one patient withdrew consent, and one patient died before starting the treatment. Patient characteristics are summarized in Table 1. Two of the 17 patients had received ASCT before venetoclax monotherapy. BCL2 positivity in lymphoma cells was 25%–50% in nine patients (53%), 51%–75% in one patient (5.8%), and >75% in seven patients (41.2%).
Overall therapeutic activity was observed in 3/17 patients (18%). These included one 81-year-old patient in PR after three cycles of treatment with venetoclax (Figure 1) that converted to CR at cycle 9 and was maintained 24 months from the beginning of treatment; this patient had PTCL-NOS histology with 50% BCL2 lymphoma cell positivity. He was previously treated with six cycles of age-adjusted CHOP, achieving CR that was maintained for 12 months. Two additional patients had evidence of improvement even without achieving the criteria for PR and for this reason, according to the Lugano classification, were defined as SD. These included a 58-year-old female with relapsed AITL (BCL2 positivity 30%) who received venetoclax for 4 months and experienced disease progression and a 77-year-old male with refractory PTCL-NOS (BCL2 positivity 40%) who is still receiving venetoclax 15 months since the beginning of treatment.
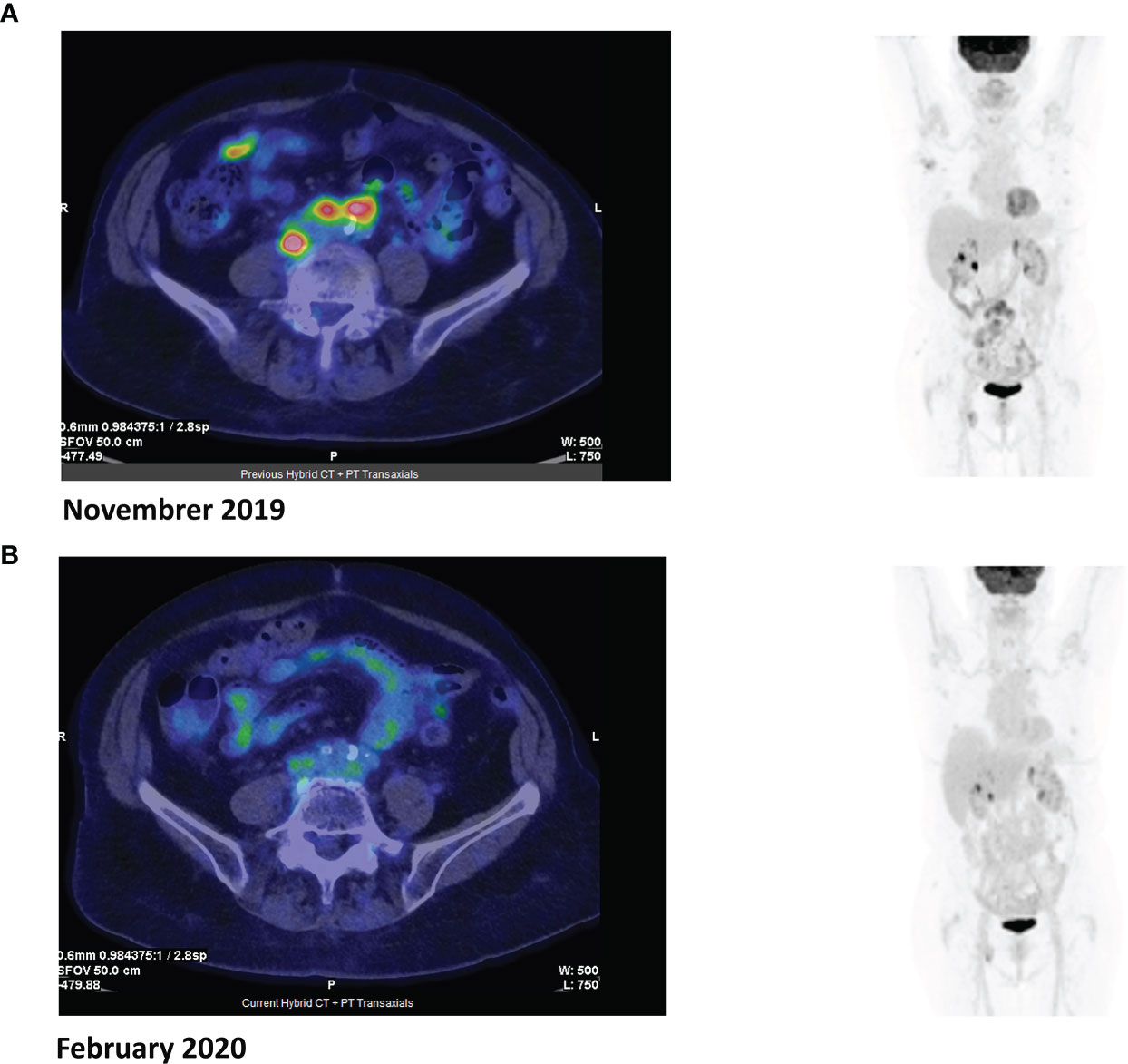
Figure 1 Positron emission tomography (PET) images before (A) and after (B) three cycles of venetoclax.
At a median follow-up of 8 months (range, 1–24 months), two patients are currently still receiving treatment (one CR and one SD). Fifteen patients (88.2%) interrupted the treatment due to disease progression, all but one within five cycles of therapy. Twelve patients (70.6%) died: eight (66.7%) due to Progression of disease (PD) and four (40%) due to worsening of clinical condition and infectious complications. Median OS and PFS were 9 months (95% CI, 7.1–14.2) and 3.8 months (95% CI 2.0–4.8), respectively (Figure 2).
A total of 12 patients experienced grade 3–4 hematological toxicities: neutropenia (42%), thrombocytopenia (25%), and anemia (25%). Nine patients (52.9%) experienced grade 3 extrahematological toxicities (two metabolism and nutrition disorders, particularly hyponatremia and hypocalcemia, three cases of pneumonia, one asthenia, two general disorders, and one acute renal failure), whereas no cases of TLS were registered.
Because of the ORR rate <30%, enrollment was stopped at the 17 patients.
FIL_VERT was designed as a proof-of-concept study to evaluate the activity of venetoclax monotherapy in the setting of R/R BCL2-positive PTCLs with the aim of performing a subsequent trial with venetoclax in combination with other agents in case of positive results. Considering that this was the first therapeutic experience with the use of venetoclax in PTCL, the study was designed in two stages according to Simon.
The characteristics of the 17 patients enrolled in the study indicated high risk/poor prognosis. Unfortunately, despite the biologic rationale and efficacy demonstrated by BCL2 inhibition in other hematological malignancies, our preliminary experience showed that venetoclax monotherapy had therapeutic activity in only 18% of patients, with one case of CR. For this reason, the study was stopped after enrollment of the first 17 patients. It is important to point out that two patients (one CR and one SD) experienced a durable therapeutic effect and are still receiving venetoclax 17 and 18 months after beginning treatment, respectively. No apparent relationship could be found, in our small survey, between the level of BCL2 expression and response. The safety profile was in line with previous studies of other hematological malignancies, and no TLS was observed.
The lack of observed response could be due to cellular mechanisms of resistance that have already been observed in other hematological and solid neoplasms. The major determinants of resistance to venetoclax in MCL and CLL patients have been identified as the overexpression and de novo synthesis of BCL-XL and MCL-1, antiapoptotic proteins belonging to the BCL2 family (20). Other mechanisms of resistance observed in CLL patients treated with venetoclax included early outgrowth of clones with complex karyotype, mutations in BTG1, aberrations of CDKN2A/B (21), and BCL2 mutation Gly101Val alone or associated with other additional acquired BCL2 resistance mutations, as recently reported (22, 23).
A possible strategy for circumventing resistance could be the association with other agents (chemotherapy and/or monoclonal antibodies or biological agents), as reported below.
In R/R follicular lymphoma (FL), despite a high level of BCL2 expression, venetoclax monotherapy has shown limited efficacy, with an ORR of 38% and only 14% CR rate (17). Several trials are ongoing that combine venetoclax with other molecules and/or chemotherapy in both previously untreated patients and in the setting of R/R FL (e.g., NCT04722601, NCT03980171, NCT02956382).
Increased BCL2 levels are also reported in acute myeloid leukemia (AML) patients, and a majority of AML blasts depend on BCL2 for survival. Furthermore, high expression of BCL2 is associated with poor prognosis with an inferior response to chemotherapy (24). Though single-agent venetoclax has had modest activity in AML, the association with azacitidine in previously untreated patients has shown a synergistic effect, with an incidence of a CR and a CR with incomplete hematological recovery of 71% and a median response duration of 21.1 months (25).
Similarly, in the 15%–20% of R/R multiple myeloma patients who carry chromosomal translocation t(11;14), venetoclax has demonstrated promising activity, with an ORR of 40% and 27%, achieving at least a very good partial response (VGPR) (26). To increase the rate of response, several trials of venetoclax in combination with other agents are ongoing and some preliminary results are available (e.g., venetoclax in association with dexamethasone plus/minus bortezomib) (27).
In conclusion, our data suggest that a small number of R/R patients with PTCL may benefit from venetoclax monotherapy. Similarly to what has been observed in other hematological malignancies, it is possible that the combination of venetoclax plus other cytotoxic or biological agents may translate into a synergistic activity and a higher response rate. BCL2 expression alone seems to not be predictive of the response. Further studies should attempt to identify the mechanisms of response and resistance in this setting and define the predictive markers of venetoclax efficacy.
The raw data supporting the conclusions of this article will be made available by the authors without undue reservation.
The study protocol was approved by the ethics committee of each participating site. The trial was registered at www.clinicaltrials.gov, NCT03552692, and given the EudraCT number 2017-004630-29. The patients/participants provided their written informed consent to participate in this study.
FZ designed the study. FZ and LB wrote the article. All other authors contributed to data collection and data analysis, critically revised the article, and approved the final version of the paper.
The study was supported by Abbvie. The funder was not involved in the study design, collection, analysis, interpretation of data, the writing of this article or the decision to submit it for publication.
PZ has received advisory board fees from Secura Bio, Celltrion, Gilead, Janssen-Cilag, BMS, Servier, Sandoz, MSD, TG Therapeutics, Takeda, Roche, Eusapharma, Kyowa Kirin, Novartis, ADC Therapeutics, Incyte, and Beigene; received consultant fees from MSD, Eusapharma, and Novartis; and received speaker’s bureau fees from Celltrion, Gilead, Janssen-Cilag, BMS, Servier, MSD, TG Therapeutics, Takeda, Roche, Eusapharma, Kyowa Kirin, Novartis, Incyte, and Beigene. SL has received advisory board fees from Roche, BMS/Celgene, Gilead/kite, Jannsen, Genmab, and Regeneron. MV has received advisory board fees from Janssen, Beigene, and Roche and travel expenses from Abbvie. FZ has received advisory board fees from Roche, Celgene, Janssen, Sandoz, Gilead, Novartis, Abbvie, Amgen, Sobi, Argenx, and Grifols and received honoraria for giving lectures to medical meetings from Celgene, Janssen, Gilead, Novartis, Roche, Amgen, Abbvie, and Grifols.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
We thank Gianni Ciccone and Luigi Marcheselli for statistical analysis and reporting.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.789891/full#supplementary-material.
1. Swerdlow SH, Campo E, Harris NL, Jaffe ES, Pileri SA, Stein H. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. 4th ed. Lyon, France: IARC (2008). Available at: http://apps.who.int/bookorders/anglais/detart1.jsp?sesslan=1&codlan=1&codcol=70&codcch=4002.
2. Foss FM, Zinzani PL, Vose JM, Gascoyne RD, Rosen ST, Tobinai K. Peripheral T-Cell Lymphoma. Blood (2011) 117(25):6756–67. doi: 10.1182/blood-2010-05-231548
3. Abouyabis AN, Shenoy PJ, Sinha R, Flowers CR, Lechowicz MJ. A Systematic Review and Meta-Analysis of Front-Line Anthracycline-Based Chemotherapy Regimens for Peripheral T-Cell Lymphoma. ISRN Hematol (2011) 2011:623924. doi: 10.5402/2011/623924
4. D’Amore F, Relander T, Lauritzsen GF, Jantunen E, Hagberg H, Anderson H, et al. Up-Front Autologous Stem-Cell Transplantation in Peripheral T-Cell Lymphoma: NLG-T-01. J Clin Oncol (2012) 30(25):3093–9. doi: 10.1200/JCO.2011.40.2719
5. Horwitz S, O’Connor OA, Pro B, Illidge T, Fanale M, Advani R, et al. ECHELON-2 Study Group. Brentuximab Vedotin With Chemotherapy for CD30-Positive Peripheral T-Cell Lymphoma (ECHELON-2): A Global, Double-Blind, Randomised, Phase 3 Trial. Lancet (2019) 393(10168):229–40. doi: 10.1016/S0140-6736(18)32984-2
6. Zinzani PL, Venturini F, Stefoni V, Fina M, Pellegrini C, Derenzini E, et al. Gemcitabine as Single Agent in Pretreated T-Cell Lymphoma Patients: Evaluation of the Long-Term Outcome. Ann Oncol (2010) 21(4):860–3. doi: 10.1093/annonc/mdp508
7. Coiffier B, Pro B, Prince HM, Foss F, Sokol L, Greenwood M, et al. Romidepsin for the Treatment of Relapsed/Refractory Peripheral T-Cell Lymphoma: Pivotal Study Update Demonstrates Durable Responses. J Hematol Oncol (2014) 7:11. doi: 10.1186/1756-8722-7-11
8. O’Connor OA, Pro B, Pinter-Brown L, Bartlett N, Popplewell L, Coiffier B, et al. Pralatrexate in Patients With Relapsed or Refractory Peripheral T-Cell Lymphoma: Results From the Pivotal PROPEL Study. J Clin Oncol (2011) 29(9):1182–9. doi: 10.1200/JCO.2010.29.9024
9. Pro B, Advani R, Brice P, Bartlett NL, Rosenblatt JD, Illidge T, et al. Brentuximab Vedotin (SGN-35) in Patients With Relapsed or Refractory Systemic Anaplastic Large-Cell Lymphoma: Results of a Phase II Study. J Clin Oncol (2012) 30(18):2190–6. doi: 10.1200/JCO.2011.38.0402
10. Horwitz SM, Advani RH, Bartlett NL, Jacobsen ED, Sharman JP, O’Connor OA, et al. Objective Responses in Relapsed T-Cell Lymphomas With Single-Agent Brentuximab Vedotin. Blood (2014) 123(20):3095–100. doi: 10.1182/blood-2013-12-542142
11. Broccoli A, Argnani L, Zinzani PL. Peripheral T-Cell Lymphomas: Focusing on Novel Agents in Relapsed and Refractory Disease. Cancer Treat Rev (2017) 60:120–9. doi: 10.1016/j.ctrv.2017.09.002
12. Zaja F, Tabanelli V, Agostinelli C, Calleri A, Chiappella A, Varettoni M, et al. CD38, BCL-2, PD-1, and PD-1L Expression in Nodal Peripheral T-Cell Lymphoma: Possible Biomarkers for Novel Targeted Therapies? Am J Hematol (2017) 92(1):E1–2. doi: 10.1002/ajh.24571
13. Rassidakis GZ, Jones D, Lai R, Ramalingam P, Sarris AH, McDonnell TJ, et al. BCL-2 Family Proteins in Peripheral T-Cell Lymphomas: Correlation With Tumour Apoptosis and Proliferation. J Pathol (2003) 200(2):240–8. doi: 10.1002/path.1346
14. Jung JT, Kim DH, Kwak EK, Kim JG, Park TI, Sohn SK, et al. Clinical Role of Bcl-2, Bax, or P53 Overexpression in Peripheral T-Cell Lymphomas. Ann Hematol (2006) 85(9):575–81. doi: 10.1007/s00277-006-0127-z
15. Roberts AW, Davids MS, Pagel JM, Kahl BS, Puvvada SD, Gerecitano JF, et al. Targeting BCL2 With Venetoclax in Relapsed Chronic Lymphocytic Leukemia. N Engl J Med (2016) 374(4):311–22. doi: 10.1056/NEJMoa1513257
16. Fischer K, Al-Sawaf O, Fink AM, Dixon M, Bahlo J, Warburton S, et al. Venetoclax and Obinutuzumab in Chronic Lymphocytic Leukemia. Blood (2017) 129(19):2702–5. doi: 10.1182/blood-2017-01-761973
17. Davids MS, Roberts AW, Seymour JF, Pagel JM, Kahl BS, Wierda WG, et al. Phase I First-In-Human Study of Venetoclax in Patients With Relapsed or Refractory Non-Hodgkin Lymphoma. J Clin Oncol (2017) 35(8):826–33. doi: 10.1200/JCO.2016.70.4320
18. Bossard C, Dobay MP, Parrens M, Lamant L, Missiaglia E, Haioun C, et al. Immunohistochemistry as a Valuable Tool to Assess CD30 Expression in Peripheral T-Cell Lymphomas: High Correlation With mRNA Levels. Blood (2014) 124(19):2983–6. doi: 10.1182/blood-2014-07-584953
19. Cheson BD, Fisher RI, Barrington SF, Cavalli F, Schwartz LH, Zucca E, et al. Alliance, Australasian Leukaemia and Lymphoma Group; Eastern Cooperative Oncology Group; European Mantle Cell Lymphoma Consortium; Italian Lymphoma Foundation; European Organisation for Research; Treatment of Cancer/Dutch Hemato-Oncology Group; Grupo Español De Médula Ósea; German High-Grade Lymphoma Study Group; German Hodgkin’s Study Group; Japanese Lymphorra Study Group; Lymphoma Study Association; NCIC Clinical Trials Group; Nordic Lymphoma Study Group; Southwest Oncology Group; United Kingdom National Cancer Research Institute. Recommendations for Initial Evaluation, Staging, and Response Assessment of Hodgkin and Non-Hodgkin Lymphoma: The Lugano Classification. J Clin Oncol (2014) 32(27):3059–68. doi: 10.1200/JCO.2013.54.8800
20. Bose P, Gandhi V, Konopleva M. Pathways and Mechanisms of Venetoclax Resistance. Leuk Lymphoma (2017) 58(9):1–17. doi: 10.1080/10428194.2017.1283032
21. Herling CD, Abedpour N, Weiss J, Schmitt A, Jachimowicz RD, Merkel O, et al. Clonal Dynamics Towards the Development of Venetoclax Resistance in Chronic Lymphocytic Leukemia. Nat Commun (2018) 9(1):727. doi: 10.1038/s41467-018-03170-7
22. Blombery P, Anderson MA, Gong JN, Thijssen R, Birkinshaw RW, Thompson ER, et al. Acquisition of the Recurrent Gly101Val Mutation in BCL2 Confers Resistance to Venetoclax in Patients With Progressive Chronic Lymphocytic Leukemia. Cancer Discovery (2019) 9(3):342–53. doi: 10.1158/2159-8290.CD-18-1119
23. Blombery P, Thompson ER, Nguyen T, Birkinshaw RW, Gong JN, Chen X, et al. Multiple BCL2 Mutations Cooccurring With Gly101Val Emerge in Chronic Lymphocytic Leukemia Progression on Venetoclax. Blood (2020) 135(10):773–7. doi: 10.1182/blood.2019004205
24. Campos L, Rouault JP, Sabido O, Oriol P, Roubi N, Vasselon C, et al. High Expression of Bcl-2 Protein in Acute Myeloid Leukemia Cells Is Associated With Poor Response to Chemotherapy. Blood (1993) 81(11):3091–6. doi: 10.1182/blood.V81.11.3091.3091
25. DiNardo CD, Pratz KW, Letai A, Jonas BA, Wei AH, Thirman M, et al. Safety and Preliminary Efficacy of Venetoclax With Decitabine or Azacitidine in Elderly Patients With Previously Untreated Acute Myeloid Leukaemia: A non-Randomised, Open-Label, Phase 1b Study. Lancet Oncol (2018) 19(2):216–28. doi: 10.1016/S1470-2045(18)30010-X
26. Kumar S, Kaufman JL, Gasparetto C, Mikhael J, Vij R, Pegourie B, et al. Efficacy of Venetoclax as Targeted Therapy for Relapsed/Refractory T(11;14) Multiple Myeloma. Blood (2017) 130(22):2401–9. doi: 10.1182/blood-2017-06-788786
27. Kumar SK, Harrison SJ, Cavo M, de la Rubia J, Popat R, Gasparetto C, et al. Venetoclax or Placebo in Combination With Bortezomib and Dexamethasone in Patients With Relapsed or Refractory Multiple Myeloma (BELLINI): A Randomised, Double-Blind, Multicentre, Phase 3 Trial. Lancet Oncol (2020) 21(12):1630–42. doi: 10.1016/S1470-2045(20)30525-8
Keywords: peripheral T-cell lymphoma, BCL2 protein, venetoclax, BCL2 inhibition, relapsed/refractory
Citation: Ballotta L, Zinzani PL, Pileri S, Bruna R, Tani M, Casadei B, Tabanelli V, Volpetti S, Luminari S, Corradini P, Lucchini E, Tisi MC, Merli M, Re A, Varettoni M, Pesce EA and Zaja F (2021) Venetoclax Shows Low Therapeutic Activity in BCL2-Positive Relapsed/Refractory Peripheral T-Cell Lymphoma: A Phase 2 Study of the Fondazione Italiana Linfomi. Front. Oncol. 11:789891. doi: 10.3389/fonc.2021.789891
Received: 05 October 2021; Accepted: 15 November 2021;
Published: 06 December 2021.
Edited by:
Varsha Gandhi, University of Texas MD Anderson Cancer Center, United StatesReviewed by:
Constantine Tam, Peter MacCallum Cancer Centre, AustraliaCopyright © 2021 Ballotta, Zinzani, Pileri, Bruna, Tani, Casadei, Tabanelli, Volpetti, Luminari, Corradini, Lucchini, Tisi, Merli, Re, Varettoni, Pesce and Zaja. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Francesco Zaja, ZnJhbmNlc2NvLnphamFAYXN1Z2kuc2FuaXRhLmZ2Zy5pdA==
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.