
95% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol. , 24 January 2022
Sec. Head and Neck Cancer
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.786258
This article is part of the Research Topic Challenges and their Implications for the Clinical Practice of Head and Neck Cancer View all 23 articles
Objectives: To compare the oncologic outcomes in patients with cT1N0 tongue squamous cell carcinoma (SCC) who underwent different neck management strategies stratified by sonographic depth of invasion (DOI).
Methods: The included patients were retrospectively enrolled, and divided into two groups: observation (OBS) and elective neck dissection (END). The regional control (RC) and disease-specific survival (DSS) rates were compared and stratified by sonographic DOI.
Results: The mean sonographic and pathologic DOIs were 3.8 and 3.7 mm, respectively; the two DOIs were significantly correlated (Spearman correlation coefficient 0.974. p <0.001). In patients with sonographic DOI <4.0 mm, the 5-year RC rates were 73 and 89% in the OBS and END groups, respectively, and were not significantly different. However, in patients with sonographic DOI ≥4.0 mm, the 5-year RC rate was significantly different between the OBS (57%) and END (80%) groups (p = 0.031). In patients with sonographic DOI <4.0 mm, the 5-year DSS rates were 79 and 89% in OBS and END groups, respectively, and were not significantly different. However, in patients with sonographic DOI ≥4.0 mm, the 5-year DSS rate was significantly different between the OBS (67%) and END (86%) groups (p = 0.033).
Conclusions: Sonographic DOI was notably correlated with pathologic DOI. Moreover, there was a significant survival difference between the OBS and END groups in cT1N0 tongue SCC patients with sonographic DOI ≥4.0 mm but not in those with sonographic DOI <4.0 mm. Our study provides a useful method to aid decision-making in the clinical setting for this patient group.
Surgical excision is the preferred method for managing squamous cell carcinoma (SCC) of the tongue, which is the most common oral malignancy (1). Neck dissection is usually included in the initial treatment of cT3–T4 disease; however, the optimal neck management in cases of cT1N0 tongue SCC is still controversial owing to the wide range of the occult metastasis rate (2). Observation (OBS) and elective neck dissection (END) are two potential approaches for management. Vandenbrouck et al. (3), Fakih et al. (4), and Yuen et al. (5) reported that a comparison of oncologic outcomes between patients undergoing OBS and those indicated for END revealed a similar disease-specific survival (DSS) in both groups. However, some high-quality studies also showed that END could reduce the frequency of regional nodal recurrence and improve DSS in patients with cT1-2N0 oral SCC (6–8). To achieve successful outcomes in such cases, reliable predictors indicating cervical lymph node metastasis, which can be assessed preoperatively, must be identified.
Factors contributing to lymph node metastasis include tumor size, tumor differentiation grade, perineural invasion (PNI), and lymphovascular invasion (LVI) (9–11). Caponio et al. (12) reported that PNI occurred in 40.5% of 200 patients with tongue SCC, and PNI was associated with a higher tendency of lymph node metastasis and a worse disease prognosis. However, the depth of invasion (DOI) is considered the best predictor of occult lymph node metastasis (13). Studies have suggested that neck dissection should be performed if the DOI exceeds 4 mm (14–16). However, in such studies, the DOI was measured postoperatively based on hematoxylin and eosin staining results; this is known as pathologic DOI, which provides little benefit in preoperative decision-making.
Intraoral ultrasound, CT, and MRI are used to evaluate clinical DOI (17, 18). Takamura et al. (17) reported that compared to pathologic DOI, clinical DOI derived by ultrasound was overestimated by an average of 0.2 mm, while CT and MRI-based radiological DOIs were overestimated by an average of 2–3 mm. These findings, combined with the reports of Klein et al. (19) and Marchi et al. (20), highlight the accuracy of ultrasound in determining the clinical DOI. However, to our knowledge, no study has analyzed whether sonographic DOI can be used to guide neck management in cT1N0 tongue SCC. Therefore, this study aimed to compare the oncologic outcomes in patients that underwent different neck management strategies stratified by sonographic DOI.
This study was approved by the Institutional Research Committee of our hospital, and all the participants provided informed consent. All procedures involving human participants were conducted according to the ethical standards of the Institutional and National Research Committees and the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
We retrospectively reviewed the medical records of patients that underwent surgical treatment for primary tongue SCC between January 2015 and January 2021. The following were the criteria for study enrollment: a disease stage of cT1N0 according to the 8th AJCC classification system and the availability of follow-up data. Patients with a history of any other malignancy were excluded. Information on demography, treatment, pathology, and follow-up was extracted and analyzed.
A cT1 tumor was defined as a tumor with a maximum diameter of 2 cm and a maximum clinical DOI of 5 mm based on imaging examination. A cN0 neck referred to a neck with no clinically enlarged lymph nodes on palpation and imaging. PNI was considered present if tumor cells were identified within the perineural space and/or nerve bundle. LVI was considered present if tumor cells were noted within the lymphovascular channels (21, 22).
Sonographic DOI was defined as the vertical distance between the deepest part of the tumor and the virtual line connecting the normal mucosal basal portion adjacent to the tumor (17). Before evaluation, all patients were required to rinse the mouth. Stationary B-mode ultrasound was performed with a 10–12 MHz intracavitary probe (SonoScape, Shenzhen, China) using degassed water as the coupling agent. The tongues of the patients were lightly held with gauze, and the intraoral probes were positioned according to the longitudinal axis of the maximum diameter of the tumor. Scanning was performed with the probe in contact with the lesion, but without compression, to avoid distortion and alteration of the DOI (Figure 1).
Sonographic DOI was frequently assessed for tongue SCC patients from January 2015 in our department. Resection of the primary tumor was performed with a margin of at least 1 cm. The neck management consisted of two strategies: OBS and END. END consisted of suprahyoid neck dissection (SOND) and modified radical neck dissection (MRND). SOND is referred to as a dissection of level I to III, whereas MRND is referred to as a dissection of level I to IV/V. The final neck treatment was based on the preference of the surgeon and the condition of the patient. Postoperative radiotherapy was suggested in cases with cervical nodal disease, positive margin, PNI, LVI, and extracapsular extension. Patients were followed up every three months for the first two years, every six months for the third to fourth year, and once yearly thereafter.
The ROC curve was used to analyze the optimal cutoff value of sonographic DOI in predicting occult metastasis. Bland–Altman and Spearman rank correlation analyses were used to compare sonographic and pathologic DOIs. The chi-square test was used to compare the clinicopathologic variables between the two DOI groups. The main study endpoints were regional control (RC) and DSS. RC time was calculated from the date of surgery to the date of the first neck cancer recurrence or the last follow-up. DSS was calculated from the date of surgery to the date of cancer-related death or the last follow-up. The Kaplan–Meier method (univariate analysis) was used to analyze the RC and DSS rates. Factors which were significant in univariate analyses were then analyzed in Cox model to find out the independent predictor for the survival. All statistical analyses were performed using SPSS 20.0, and p <0.05 was considered significant.
A total of 178 patients (135 men, 42 women) were included in the analysis; the median age was 53 (range: 28–78) years. Smokers and drinkers comprised 100 (56.2%) and 50 (28.1%) patients, respectively. Sixty-five (36.5%) patients underwent OBS for neck treatment, and 113 (63.5%) patients underwent END, with 70 (39.3%) undergoing SOND and 43 (24.2%) undergoing MRND. The mean sonographic DOI was 3.8 (range: 0.4–5.0) mm.
Postoperatively, all patients were pathologic stage T1, and clear margins were noted on histopathologic examination. Pathologic neck lymph node metastasis occurred in 12 patients (10.6%, 12/113), of whom six received SOND and six received MRND. Level I, II, III, and IV metastases were noted in 10 (5.6%), three (2.7%), three (2.7%), and one (0.9%) patient, respectively. PNI and LVI were present in 17 (9.6%) and 13 (7.3%) patients, respectively. The tumors showed good differentiation in 72 (40.4%), intermediate differentiation in 84 (47.2%), and poor differentiation in 22 (12.4%) patients. The two groups had similar distributions regarding clinical and pathologic variables (all p >0.05, Table 1).
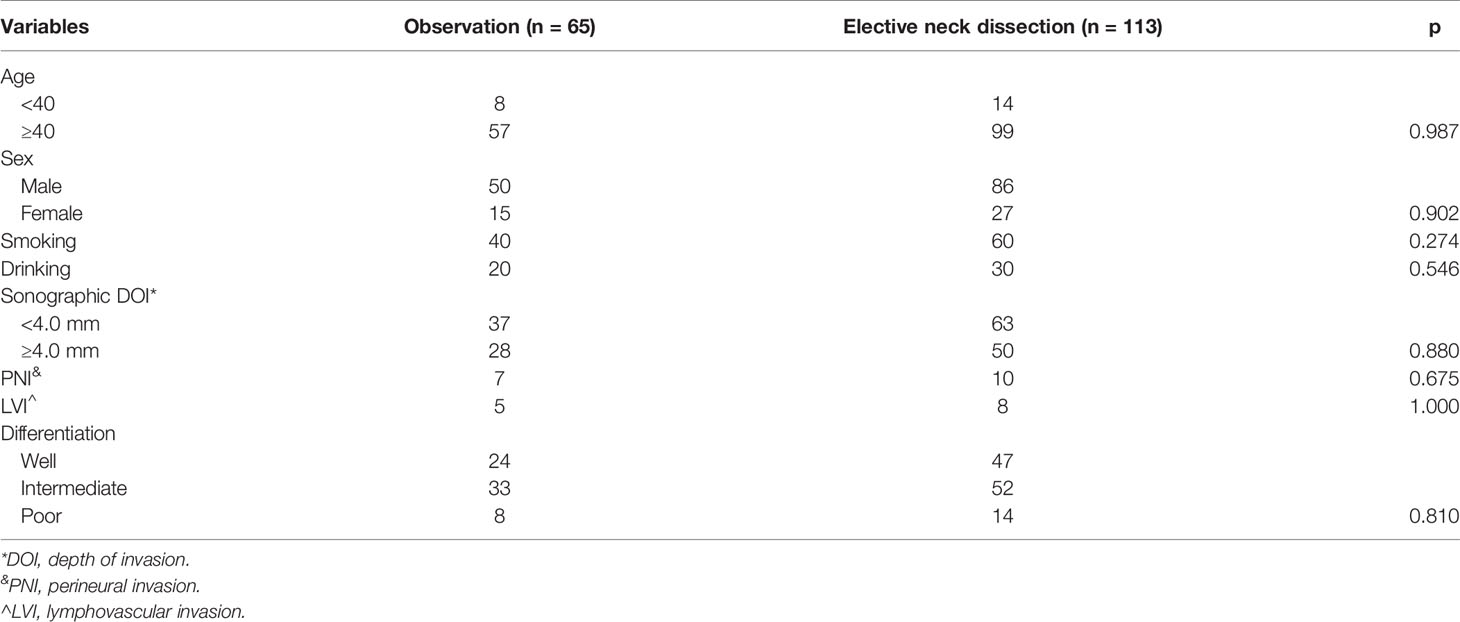
Table 1 Comparison of clinical and pathologic variables between the observation and elective neck dissection groups.
Radiotherapy was performed for 30 patients, of whom six underwent radiation for the primary site, 12 underwent radiation for the primary site and ipsilateral upper neck area, and 12 underwent radiation for the primary site and ipsilateral neck area.
In the END group, the mean sonographic DOI was 3.8 (range: 0.5–5.0) mm. ROC analysis indicated that the best cutoff value for sonographic DOI in predicting occult metastasis was 4.0 mm, with an AUC of 0.759 (Figure 2), sensitivity of 75%, and specificity of 59.4%. Eighteen percent of the 50 tumors with sonographic DOI ≥4.0 mm had occult metastases, which was significantly higher than the 4.8% of the 63 tumors with sonographic DOI <4.0 mm (p = 0.031).
The mean pathologic DOI was 3.7 (range: 0.3–4.8) mm. Spearman analysis of the relationship between sonographic and pathologic DOI yielded a correlation coefficient of 0.974 (p <0.001). Bland–Altman analysis indicated that the sonographic DOI corresponded to the pathologic DOI (Figure 3).
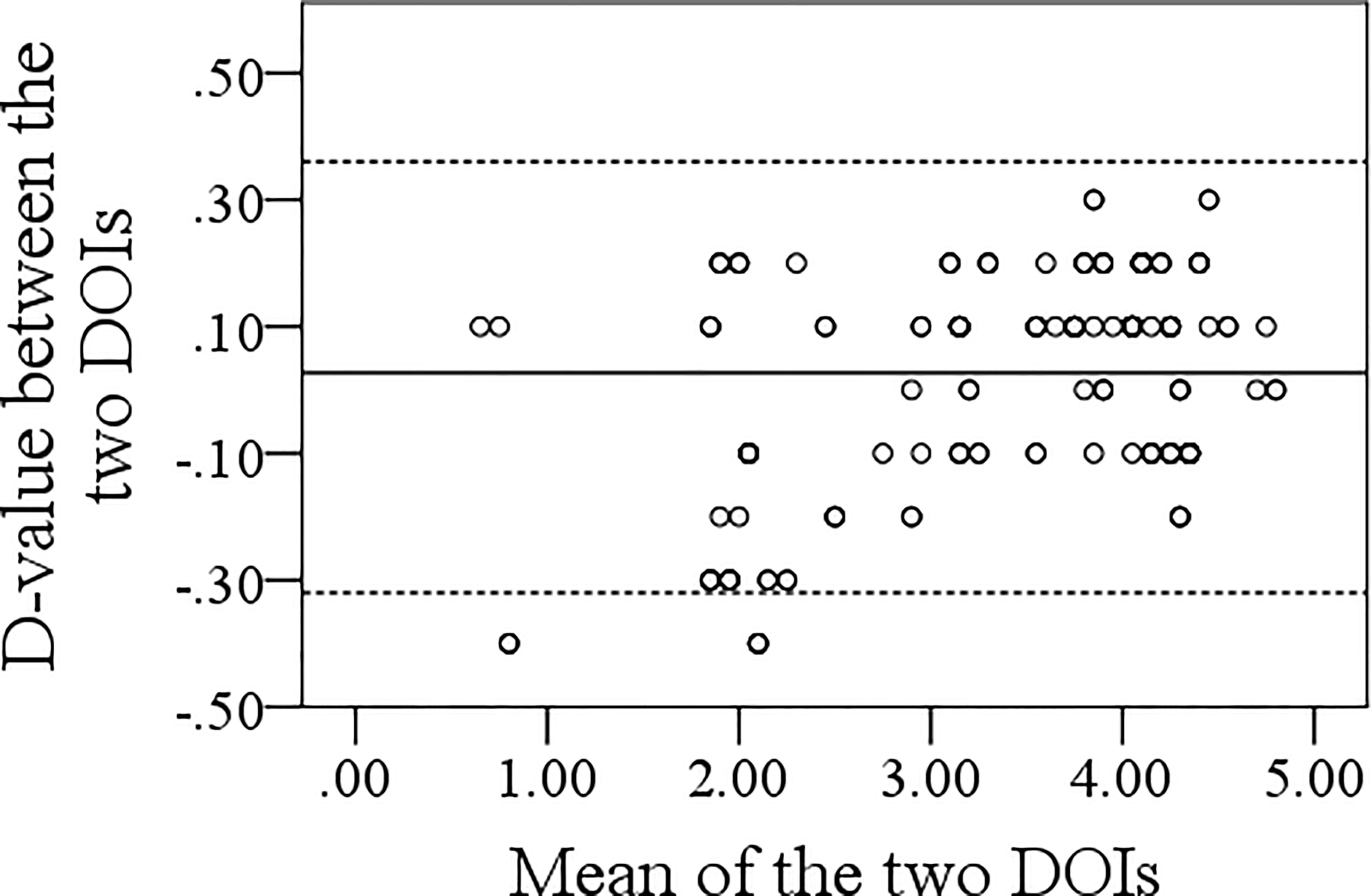
Figure 3 Bland–Altman analysis of the association between sonographic and pathologic depth of invasion.
In tumors with sonographic DOI <4.0 mm, neck recurrence occurred in six and five patients in the OBS and END groups, respectively. In the OBS group, the most common recurrent site was level I; contralateral level II and III recurrence occurred in one patient each. In the END group, the most common recurrent site was level I, while contralateral level II recurrence occurred in one patient. The two groups had a similar recurrence pattern (Table 2).
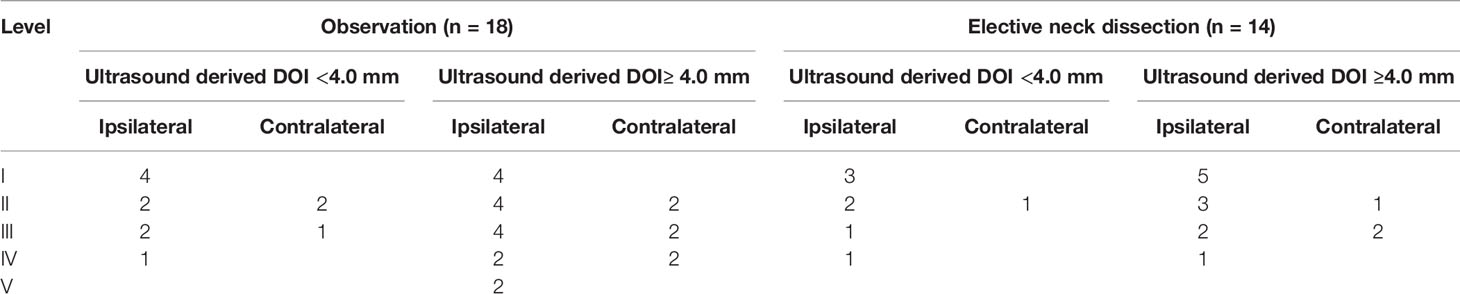
Table 2 Neck recurrence pattern in the observation and elective neck dissection groups stratified by different ultrasound derived depth of invasion (DOI).
In tumors with sonographic DOI ≥4.0 mm, neck recurrence occurred in 12 and nine patients in the OBS and END groups, respectively. In the OBS group, level V recurrence occurred in two patients, while level I, II, and III recurrence occurred in one patient each. In the END group, the most common recurrent site was level I, while contralateral level II and III recurrence occurred in one and two patients, respectively. The recurrence pattern in the OBS group was more complex (Table 2).
After a median follow-up of 2.8 (range: 0.3–6.3) years, in patients with sonographic DOI <4.0 mm, the 5-year RC rates were 73 and 89% in the OBS and END groups, respectively; the difference was not significant (Figure 4A, p = 0.139). In patients with sonographic DOI ≥4.0 mm, the 5-year RC rates were 57 and 80% in the OBS and END groups, respectively, and the difference was significant (Figure 4B, p = 0.031). Further, Cox model analysis confirmed that neck dissection was an independent factor for improving RC (Table 3).
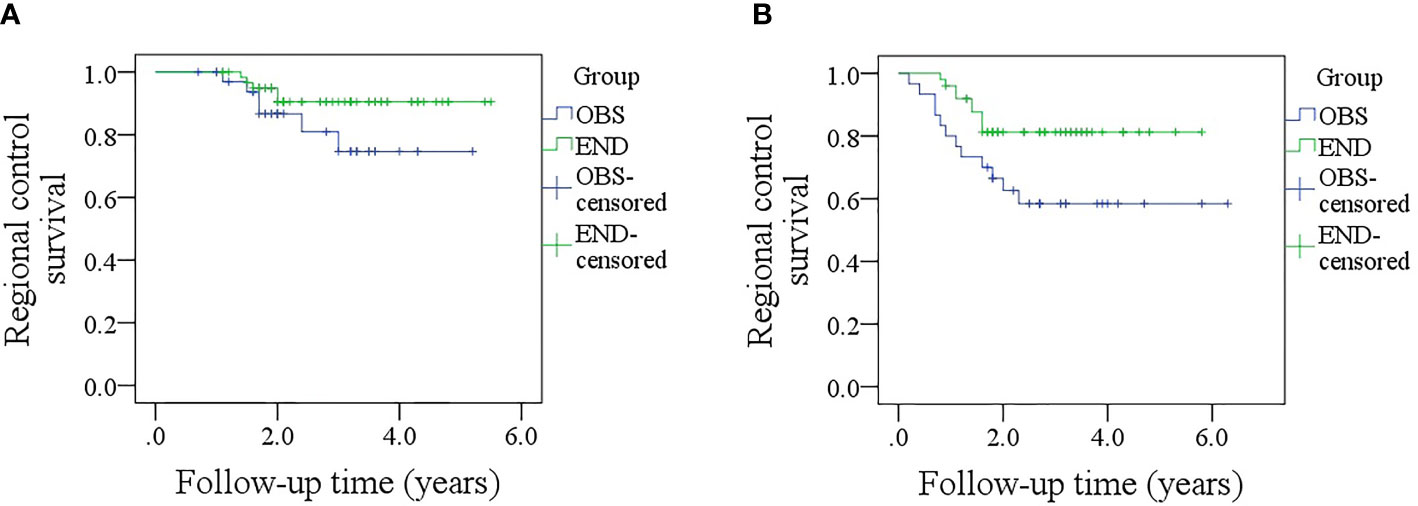
Figure 4 (A) Comparison of regional control rates between the elective neck dissection and observation groups in patients with a sonographic depth of invasion <4.0 mm (p = 0.139); (B) Comparison of regional control survival between the elective neck dissection and observation groups in patients with a sonographic depth of invasion ≥4.0 mm (p = 0.031).
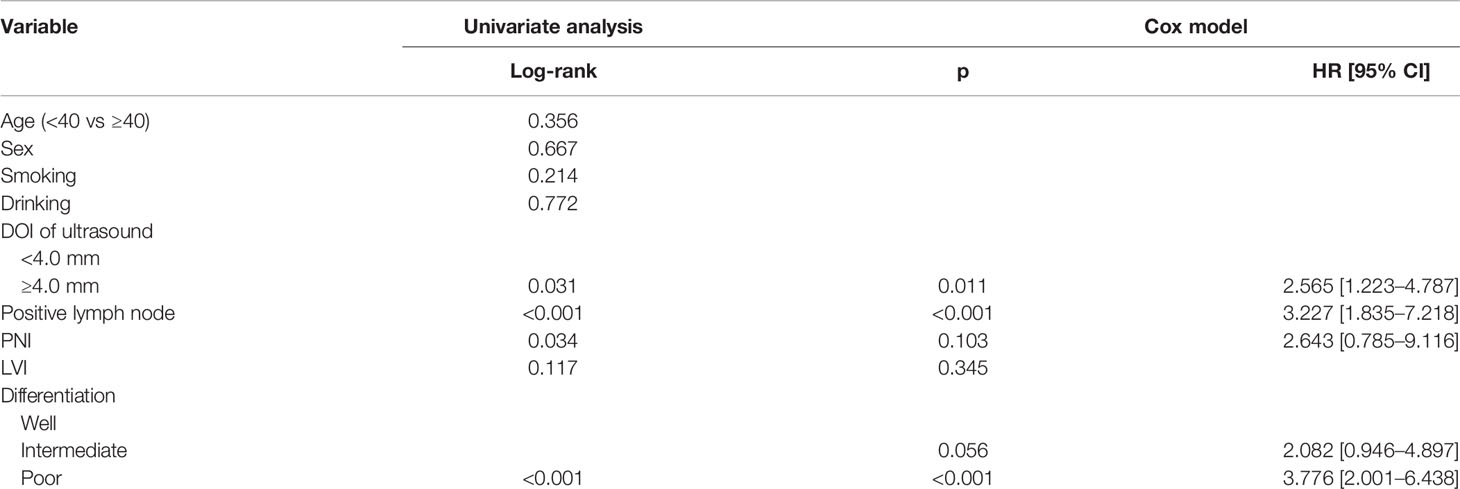
Table 3 Univariate and cox model analyses of regional control survival in patients with ultrasound derived DOI ≥4.0 mm.
In patients with sonographic DOI <4.0 mm, the 5-year DSS rates were 79 and 89% in the OBS and END groups, respectively, and the difference was not significant (Figure 5A, p = 0.381). In patients with sonographic DOI ≥4.0 mm, the 5-year DSS rates were 67 and 86% in the OBS and END groups, respectively, and the difference was significant (Figure 5B, p = 0.033). Further, Cox model analysis confirmed that neck dissection was an independent factor for improving DSS (Table 4).
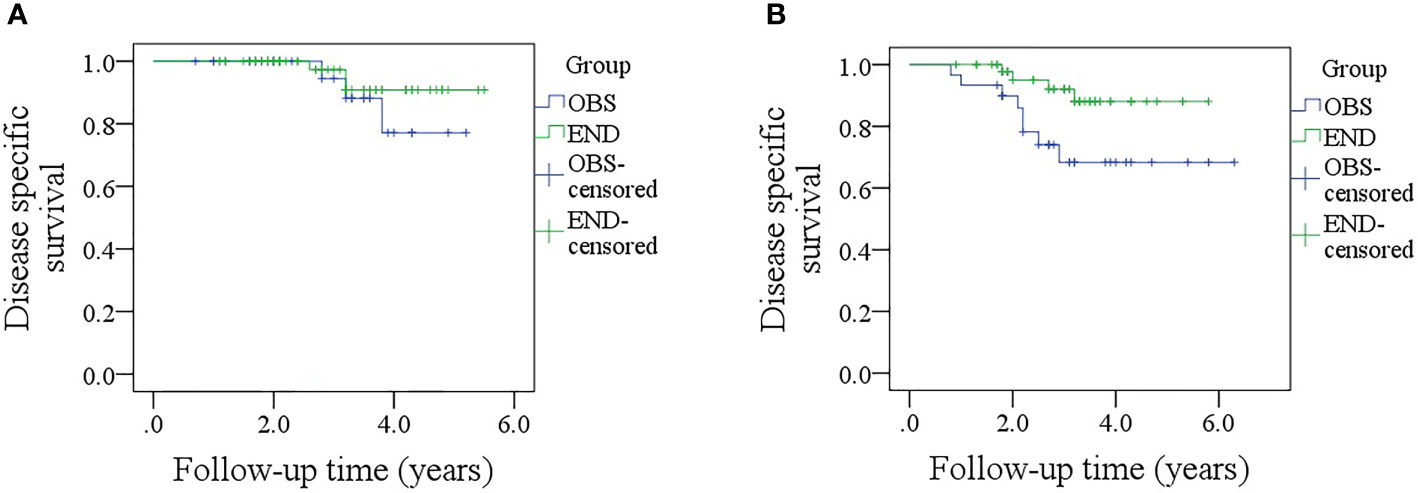
Figure 5 (A) Comparison of disease-specific survival between the elective neck dissection and observation groups in patients with a sonographic depth of invasion <4.0 mm (p = 0.381); (B) Comparison of disease-specific between the elective neck dissection and observation groups in patients with a sonographic depth of invasion ≥4.0 mm (p = 0.033).
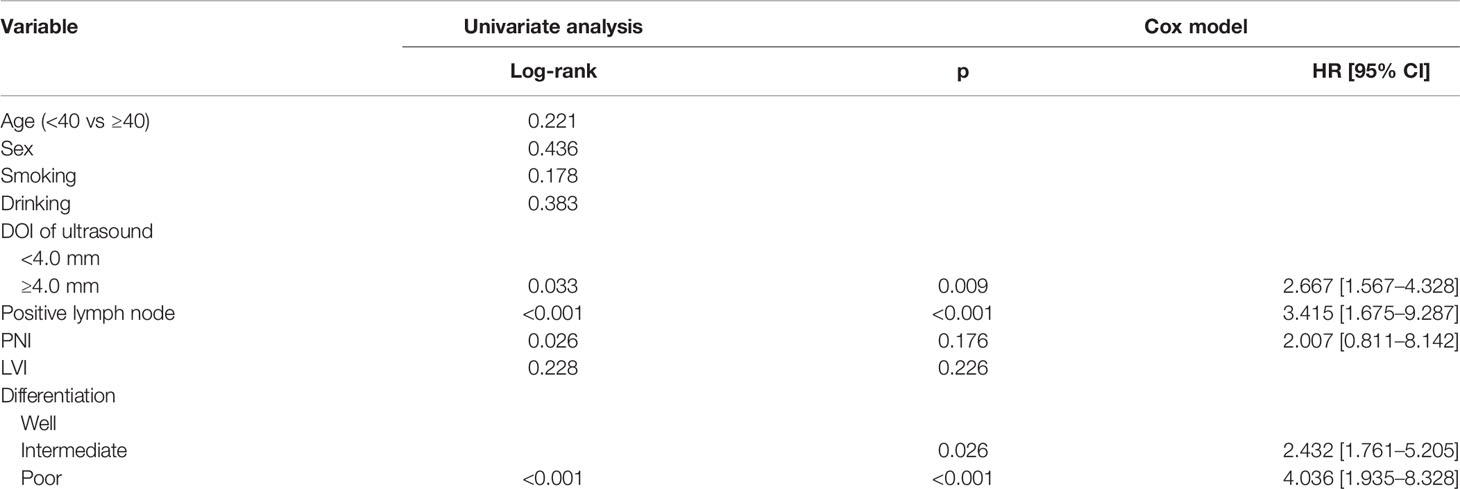
Table 4 Univariate and cox model analyses of disease specific survival in patients with ultrasound derived DOI ≥4.0 mm.
The most important finding in this study was that the sonographic DOI corresponded with pathologic DOI. There was a significant survival difference between the OBS and END groups in patients with cT1N0 tongue SCC with sonographic DOI ≥4.0 mm but not in patients with sonographic DOI <4.0 mm. This finding provides a clear indicator for neck management; hence, END was suggested if there was a presence of sonographic DOI ≥4.0 mm.
Neck lymph node metastasis is an important feature of tongue SCC, and its prevalence differs with tumor stage; END is usually recommended when the estimated risk of lymph node metastasis exceeds 20% (23). However, current evidence suggests that the incidence of occult metastasis in cT1N0 tongue SCC varies from 5 to 10% (6), contributing to debates regarding neck management in patients. A recent high-quality study by D’Cruz et al. (8) showed that, in the results of the first 500 patients with early-stage oral SCC, END resulted in higher overall survival and DSS rates than OBS. However, de Bree et al. (24) discussed the importance of a clear definition of cN0. Questioning the reliability of investigations for this diagnosis, they argued that cN0 was not clearly defined in the Tata Memorial Centre prospective randomized trial; further, to examine the role of ultrasound, some patients with suspicious findings were included, and more importantly, the ultrasound scoring criteria were not described. It was clear that the incidence of delayed metastases and neck recurrence would have been higher if the neck status was staged only by palpation compared with staging using advanced diagnostic techniques. Similar studies reported conflicting results on the benefits of OBS vs END (3–7); thus, there is a need for a reliable preoperative predictor of lymph node metastasis.
DOI was considered for tumor staging in the newest version of the AJCC classification, and it was confirmed as the strongest predictor of lymph node metastasis (11–16), according to the NCCN guidelines (13), END was suggested if pathologic DOI >4.0 mm existed. Pathologic DOI was calculated from the basement membrane to the deepest of invasion, although it was impossible to take the same measurement method, it was important to draft an alternative preoperative indicator of pathologic DOI to create a balance between overtreatment and necessity of lymphadenectomy.
Intraoral ultrasound has gained interest since its introduction by Iro et al. for assessing the tongue and the floor of the mouth (25), and a number of researchers have analyzed the accuracy of intraoral ultrasound in evaluating the DOI of oral SCC patients. Iida et al. (26) found in 56 cases of tongue tumor that the median ultrasound DOI was 3.6 (range: 0.7–9.2) mm, and the median histologic DOI was 3.5 (range: 0–12.0 mm). Compared to histologic DOI, there was an overestimation by only 0.1 mm for ultrasound DOI, with a coefficient of 0.867. If only superficial tumors were analyzed, the compatibility between the two DOIs improved. In another study by Yoon et al. (27) consisting of 22 patients, the mean sonographic DOI and histologic DOI were 6.6 ± 3.4 and 6.4 ± 4.4 mm, respectively, and there was excellent correlation between sonographic and histologic measurement for DOI, with a Pearson correlation coefficient of 0.95 (95%CI: 0.87–0.98). Filauro et al. (28) also noted that the mean difference between sonographic DOI and histologic DOI was only 0.3 mm after analyzing the outcome of 49 patients with cT1-3 tongue SCC, and the two DOIs were significantly related. Together with our results, these findings indicate the high reliability and accuracy of DOI evaluation by ultrasound even in cT1 tumors.
The association between the necessity of END and DOI has been frequently analyzed. Nguyen et al. (29) included 70 patients with cT1N0 oral SCC, of whom 27 underwent END and 43 were observed. Regional relapse occurred in 16.3% of patients who were observed and in 3.7% patients who underwent surgery. Risk factor analysis reported that DOI ≥3.0 mm was related to a poor prognosis, and it was concluded that END should be recommended if DOI ≥3.0 mm. However, the sample size of this study was notably small, and more importantly, it analyzed all regions of the oral cavity together. It is well known that tongue SCC has a significantly different biologic behavior compared to SCC of other oral regions. Kuan et al. (30) recently conduct a review to determine the optimal cutoff DOI value for predicting regional disease for early-stage tongue SCC, and noted that patients with cT1-2N0 oral/tongue SCC with known DOI >3.0 mm should be counseled on the possible survival benefits of END with primary tumor resection. However, the review only focused on the association between regional metastasis and DOI without considering the oncologic outcome. However, compared to T2 disease, a T1 tumor has a lower possibility of occult metastasis, which necessitates a search for a corresponding DOI for each disease stage. To the best of our knowledge, this is the first study to analyze how clinical sonography affects oncologic outcomes in patients undergoing different neck management strategies. Our study indicated that END improved patient prognosis for sonographic DOI ≥4.0 mm, but there was no apparent survival benefit associated with END for sonographic DOI <4.0 mm. This finding provides a useful method to aid decision-making in clinics.
Other studies have compared END and OBS in early-stage oral SCC. In a previous study, we enrolled 175 patients with cT1N0 buccal SCC, and the 5-year locoregional control rates in the END and OBS groups were 92 and 90%, respectively, and the difference was not significant. Moreover, the two groups had comparable 5-year DSS rates. Therefore, we concluded that END did not provide any survival benefit compared to a wait-and-watch policy and could not be suggested for patients with cT1N0 buccal SCC (31). A similar viewpoint was offered by Vandenbrouck et al. (3), Fakih et al. (4), and Yuen et al. (5). However, Huang et al. (32) analyzed the outcome of 380 patients with cT1-2N0 tongue SCC and reported the 5-year overall survival and neck control rates were significantly better in the END group than in the OBS group. Their conclusion was also supported by Abu-Ghanem et al. (6), Ren et al. (7), D’Cruz et al. (8), and de Bree et al. (24). However, these studies did not present the results stratified by the clinical DOI. As DOI is the strongest predictor of occult metastasis, the significance of our study is well highlighted.
The limitations in current study must be acknowledged. First, the study was retrospective with the attendant bias. Second, our sample size was not sufficiently large, we could not analyze the effect of the END extend on the outcome; hence, future studies with a larger sample size need to be conducted.
In conclusion, sonographic DOI corresponded well with pathologic DOI, and there was a significant survival difference between the OBS and END groups in patients with cT1N0 tongue SCC with sonographic DOI ≥4.0 mm but not in patients with sonographic DOI <4.0 mm. Our findings provide a useful method to aid decision-making in the clinic setting for this patient group.
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
This study was approved by the Institutional Research Committee of our hospital, and all the participants signed an informed consent. The patients/participants provided their written informed consent to participate in this study.
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
1. Fang Q, Li P, Qi J, Luo R, Chen D, Zhang X. Value of Lingual Lymph Node Metastasis in Patients With Squamous Cell Carcinoma of the Tongue. Laryngoscope (2019) 129:2527–30. doi: 10.1002/lary.27927
2. Bruschini R, Maffini F, Chiesa F, Lepanto D, De Berardinis R, Chu F, et al. Oral Cancer: Changing the Aim of the Biopsy in the Age of Precision Medicine. A Review. Acta Otorhinolaryngol Ital (2021) 41:108–19. doi: 10.14639/0392-100X-N1056
3. Vandenbrouck C, Sancho-Garnier H, Chassagne D, Saravane D, Cachin Y, Micheau C. Elective Versus Therapeutic Radical Neck Dissection in Epidermoid Carcinoma of the Oral Cavity: Results of a Randomized Clinical Trial. Cancer (1980) 46:386–90. doi: 10.1002/1097-0142(19800715)46:2<386::AID-CNCR2820460229>3.0.CO;2-9
4. Fakih AR, Rao RS, Borges AM, Patel AR. Elective Versus Therapeutic Neck Dissection in Early Carcinoma of the Oral Tongue. Am J Surg (1989) 158:309–13. doi: 10.1016/0002-9610(89)90122-0
5. Yuen AP, Ho CM, Chow TL, Tang LC, Cheung WY, Ng RW, et al. Prospective Randomized Study of Selective Neck Dissection Versus Observation for N0 Neck of Early Tongue Carcinoma. Head Neck (2009) 31:765–72. doi: 10.1002/hed.21033
6. Abu-Ghanem S, Yehuda M, Carmel NN, Leshno M, Abergel A, Gutfeld O, et al. Elective Neck Dissection vs Observation in Early-Stage Squamous Cell Carcinoma of the Oral Tongue With No Clinically Apparent Lymph Node Metastasis in the Neck: A Systematic Review and Meta-Analysis. JAMA Otolaryngol Head Neck Surg (2016) 142:857–65. doi: 10.1001/jamaoto.2016.1281
7. Ren ZH, Xu JL, Li B, Fan TF, Ji T, Zhang CP. Elective Versus Therapeutic Neck Dissection in Node-Negative Oral Cancer: Evidence From Five Randomized Controlled Trials. Oral Oncol (2015) 51:976–81. doi: 10.1016/j.oraloncology.2015.08.009
8. D’Cruz AK, Vaish R, Kapre N, Dandekar M, Gupta S, Hawaldar R, et al. Elective Versus Therapeutic Neck Dissection in Node-Negative Oral Cancer. N Engl J Med (2015) 373:521–9. doi: 10.1056/NEJMoa1506007
9. Jin W, Zhu M, Zheng Y, Wu Y, Ding X, Wu H, et al. Perineural Invasion, Lactate Dehydrogenase, Globulin, and Serum Sodium Predicting Occult Metastasis in Oral Cancer. Oral Dis (2020) 28(1):132–41. doi: 10.1111/odi.13750
10. Vassiliou LV, Acero J, Gulati A, Hölzle F, Hutchison IL, Prabhu S, et al. Management of the Clinically N0 Neck in Early-Stage Oral Squamous Cell Carcinoma (OSCC). An EACMFS Position Paper. J Craniomaxillofac Surg (2020) 48:711–8. doi: 10.1016/j.jcms.2020.06.004
11. Jiang Q, Tang A, Long S, Qi Q, Song C, Xin Y, et al. Development and Validation of a Nomogram to Predict the Risk of Occult Cervical Lymph Node Metastases in Cn0 Squamous Cell Carcinoma of the Tongue. Br J Oral Maxillofac Surg (2019) 57:1092–7. doi: 10.1016/j.bjoms.2019.09.024
12. Caponio VCA, Troiano G, Togni L, Zhurakivska K, Santarelli A, Laino L, et al. Pattern and Localization of Perineural Invasion Predict Poor Survival in Oral Tongue Carcinoma. Oral Dis (2021). doi: 10.1111/odi.13900
13. National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) Head and Neck. Version 1. (2016). p. 2016.
14. van Lanschot CGF, Klazen YP, de Ridder MAJ, Mast H, Ten Hove I, Hardillo JA, et al. Depth of Invasion in Early Stage Oral Cavity Squamous Cell Carcinoma: The Optimal Cut-Off Value for Elective Neck Dissection. Oral Oncol (2020) 111:104940. doi: 10.1016/j.oraloncology.2020.104940
15. Tam S, Amit M, Zafereo M, Bell D, Weber RS. Depth of Invasion as a Predictor of Nodal Disease and Survival in Patients With Oral Tongue Squamous Cell Carcinoma. Head Neck (2019) 41:177–84. doi: 10.1002/hed.25506
16. Faisal M, Abu Bakar M, Sarwar A, Adeel M, Batool F, Malik KI, et al. Depth of Invasion (DOI) as a Predictor of Cervical Nodal Metastasis and Local Recurrence in Early Stage Squamous Cell Carcinoma of Oral Tongue (ESSCOT). PloS One (2018) 13:e0202632. doi: 10.1371/journal.pone.0202632
17. Takamura M, Kobayashi T, Nikkuni Y, Katsura K, Yamazaki M, Maruyama S, et al. A Comparative Study Between CT, MRI, and Intraoral US for the Evaluation of the Depth of Invasion in Early Stage (T1/T2) Tongue Squamous Cell Carcinoma. Oral Radiol (2021) 38(1):114–25. doi: 10.1007/s11282-021-00533-7
18. Xu C, Yuan J, Kang L, Zhang X, Wang L, Chen X, et al. Significance of Depth of Invasion Determined by MRI in Ct1n0 Tongue Squamous Cell Carcinoma. Sci Rep.:4695 Sci Rep (2020) 10:4695. doi: 10.1038/s41598-020-61474-5
19. Klein Nulent TJW, Noorlag R, Van Cann EM, Pameijer FA, Willems SM, Yesuratnam A, et al. Intraoral Ultrasonography to Measure Tumor Thickness of Oral Cancer: A Systematic Review and Meta-Analysis. Oral Oncol (2018) 77:29–36. doi: 10.1016/j.oraloncology.2017.12.007
20. Marchi F, Filauro M, Iandelli A, Carobbio ALC, Mazzola F, Santori G, et al. Magnetic Resonance vs. Intraoral Ultrasonography in the Preoperative Assessment of Oral Squamous Cell Carcinoma: A Systematic Review and Meta-Analysis. Front Oncol (2019) 9:1571. doi: 10.3389/fonc.2019.01571
21. Du W, Fang Q, Liu S, Chen D, Luo R, Zhang X. Feasibility of Submandibular Gland Preservation in Ct1-2N0 Squamous Cell Carcinoma in the Floor of the Mouth. Front Oncol (2020) 10:579. doi: 10.3389/fonc.2020.00579
22. Gu B, Fang Q, Wu Y, Du W, Zhang X, Chen D. Impact of Submandibular Gland Preservation in Neck Management of Early-Stage Buccal Squamous Cell Carcinoma on Locoregional Control and Disease-Specific Survival. BMC Cancer (2020) 20:1034. doi: 10.1186/s12885-020-07534-5
23. Weiss MH, Harrison LB, Isaacs RS. Use of Decision Analysis in Planning a Management Strategy for the Stage N0 Neck. Arch Otolaryngol Head Neck Surg (1994) 120:699–702. doi: 10.1001/archotol.1994.01880310005001
24. de Bree R, van den Brekel MWM. Elective Neck Dissection Versus Observation in the Clinically Node Negative Neck in Early Oral Cancer: Do We Have the Answer Yet? Oral Oncol (2015) 51:963–5. doi: 10.1016/j.oraloncology.2015.08.013
25. Iro H, Nitsche N. Intra-Oral Sonography in Neoplasms of the Mouth and Base of the Tongue. HNO (1989) 37:329–32.
26. Iida Y, Kamijo T, Kusafuka K, Omae K, Nishiya Y, Hamaguchi N, et al. Depth of Invasion in Superficial Oral Tongue Carcinoma Quantified Using Intraoral Ultrasonography. Laryngoscope (2018) 128:2778–82. doi: 10.1002/lary.27305
27. Yoon BC, Bulbul MD, Sadow PM, Faquin WC, Curtin HD, Varvares MA, et al. Comparison of Intraoperative Sonography and Histopathologic Evaluation of Tumor Thickness and Depth of Invasion in Oral Tongue Cancer: A Pilot Study. AJNR Am J Neuroradiol (2020) 41:1245–50. doi: 10.3174/ajnr.A6625
28. Filauro M, Missale F, Marchi F, Iandelli A, Carobbio ALC, Mazzola F, et al. Intraoral Ultrasonography in the Assessment of DOI in Oral Cavity Squamous Cell Carcinoma: A Comparison With Magnetic Resonance and Histopathology. Eur Arch Otorhinolaryngol (2021) 278:2943–52. doi: 10.1007/s00405-020-06421-w
29. Nguyen E, McKenzie J, Clarke R, Lou S, Singh T. The Indications for Elective Neck Dissection in T1N0M0 Oral Cavity Squamous Cell Carcinoma. J Oral Maxillofac Surg (2021) 79:1779–93. doi: 10.1016/j.joms.2021.01.042
30. Kuan EC, Mallen-St Clair J, Badran KW, St John MA. How Does Depth of Invasion Influence the Decision to do a Neck Dissection in Clinically N0 Oral Cavity Cancer? Laryngoscope (2016) 126:547–8. doi: 10.1002/lary.25707
31. Fang Q, Gao H, Gao Q, Sun J, Li P, Cui M, et al. Elective Neck Dissection Versus Wait-and-See Policy in Ct1n0 Buccal Squamous Cell Carcinoma. BMC Cancer (2020) 20:537. doi: 10.1186/s12885-020-07006-w
Keywords: depth of invasion, tongue squamous cell carcinoma, head and neck squamous cell carcinoma, observation, elective neck dissection
Citation: Wu Y, Zhang X, Dai L, Fang Q and Du W (2022) Neck Management in cT1N0 Tongue Squamous Cell Carcinoma as Determined by Sonographic Depth of Invasion. Front. Oncol. 11:786258. doi: 10.3389/fonc.2021.786258
Received: 30 September 2021; Accepted: 28 December 2021;
Published: 24 January 2022.
Edited by:
Steffi Ulrike Pigorsch, Technical University of Munich, GermanyReviewed by:
Aviram Mizrachi, Rabin Medical Center, IsraelCopyright © 2022 Wu, Zhang, Dai, Fang and Du. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Wei Du, ZHV3ZWl0akAxMjYuY29t
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.