- 1Department of Urology, Beijing Tongren Hospital, Capital Medical University, Beijing, China
- 2Beijing Advanced Innovation Center for Big Data-Based Precision Medicine, Beihang University & Capital Medical University, Beijing Tongren Hospital, Beijing, China
- 3Department of Epidemiology and Biostatistics, School of Public Health, Peking University, Beijing, China
Purpose: The purpose of this study was to summarize the existing evidence and develop a comprehensive systematic review of the impact of androgen suppression therapy (AST) on the incidence or clinical outcomes of bladder cancer.
Methods: We systematically searched the PubMed and Embase databases from inception to June 20, 2021 to identify all observational studies examining the incidence or clinical outcomes of bladder cancer in patients who received AST. AST is defined as the use of 5-alpha reductase inhibitors (5-ARIs) or androgen deprivation therapy (ADT).
Results: A total of 18 observational studies were included. Our results showed that AST was not significantly associated with a reduced risk of BCa incidence (OR: 0.92, 95% CI: 0.68–1.24) compared with the lack of AST. The subgroup analysis revealed that finasteride use was significantly associated with a reduction in the risk of BCa incidence (OR: 0.75, 95% CI: 0.64–0.88). Recurrence-free survival (RFS) was improved among AST users compared with nonusers (HR: 0.68, 95% CI: 0.48–0.95), while no significant difference between AST users versus nonusers was identified for cancer-specific survival (CSS), overall survival (OS) or progression-free survival (PFS).
Conclusion: Current evidence indicates that therapy with finasteride may represent a potential strategy aimed at reducing BCa incidence. Moreover, AST has a beneficial effect on the recurrence of bladder cancer. Further well-designed randomized trials or cohort studies with better characterized study populations are needed to validate our preliminary findings.
Systematic Review Registration: International Prospective Register of Systematic Reviews database [https://www.crd.york.ac.uk/PROSPERO/], identifier CRD42021261685.
Introduction
Bladder cancer (BCa), predominantly urothelial carcinoma, is a common malignant genitourinary tumor (1, 2). Men are 3 to 4 times more frequently diagnosed with bladder cancer than women; however, women tend to be diagnosed with more advanced disease at presentation and have less favorable outcomes after treatment (1, 3–5). Female patients with urothelial carcinoma of the bladder have been shown to have worse cancer specific survival, overall survival and recurrence-free survival (5, 6). Recent studies question why there are differences, and the effects of sex hormones and its receptors, especially androgens, have become widely researched (1, 5–7).
Sex hormones and corresponding receptors are relevant modulators of cancer onset and progression in nonreproductive organs, particularly the lung, colorectal, bladder, stomach, kidney, pancreas, and thyroid gland (8). The excessive or reduced expression of these receptors, and the changes in their upstream or downstream pathways are closely related to the outcomes of BCa (8, 9). Numerous studies have focused on the role of androgen receptor (AR) and androgens in the development of bladder cancer. In vitro and vivo evidence highlights a crucial role for AR in BCa development, progression, recurrence and resistance to standard therapies such as chemotherapy, radiotherapy, and Bacillus Calmette Guerin (BCG) (2, 4, 8, 10–14). Emerging clinical evidence also suggests that the manipulation of androgen signaling may affect BCa behavior. Previous meta-analyses included limited clinical literature and some unreported relative risks in studies, suggesting that androgen suppression therapy (AST) consisting of 5-alpha reductase inhibitors (5-ARIs) or androgen deprivation therapy (ADT) can reduce BCa incidence, recurrence and specific mortality (15, 16). However, various results regarding the impact of AST on the incidence and recurrence of bladder cancer have been widely reported recently, and there are disputes among them. Therefore, with the increase in original research on this topic, an updated summary needs to be presented.
Herein, the aim of our study is to summarize the available evidence and develop a comprehensive systematic review of the effect of AST on the incidence of bladder cancer and the clinical outcomes of patients with bladder cancer.
Methods
The protocol of this study has been registered in the International Prospective Register of Systematic Reviews database (CRD42021261685).
Search Strategy and Eligibility Criteria
The PubMed and Embase databases were searched from inception to June 20, 2021. The following search terms were used: “bladder cancer,” “urothelial carcinoma,” or “bladder neoplasms”; one of “androgen suppression therapy” or “5 alpha reductase inhibitor” or “5α-reductase” or “5ARI” or “finasteride” or “dutasteride “ or “androgen deprivation therapy” or “anti-androgen” or “bicalutamide” or “enzalutamide” or “abiraterone” or “GnRH agonist” or “GnRH antagonist” or “castration” or “nilutamide” or “flutamide” or “apalutamide” or “darolutamide”. The titles and abstracts of articles were screened initially to identify relevant studies. Then, the full texts of potentially relevant studies were carefully read to determine those that met the eligibility criteria. Retrospective and prospective studies evaluating the effect of AST (5-ARI or ADT) on BCa incidence, recurrence, or survival were included in the analysis. Articles that did not report AST in patients with BCa were excluded. Reviews, letters, editorials, replies from authors, case reports, conferences and articles not published in English were excluded. Two authors screened the search results and any disagreements were resolved.
Data Extraction and Quality Assessment
Data extracted from the eligible studies included study characteristics (e.g., study type, data source, study period, sample size,median of follow-up), patient characteristics (e.g., patient age, AST type), outcomes (e.g., BCa incidence, BCa recurrence), adjusted risk estimates with 95% confidence interval (CI) for outcomes, and potentially confounding factor adjustments (e.g., age, race, smoking, comorbidities tumor stage and grade, intravesical therapy). The main outcomes were ① incidence of BCa when AST was initiated before diagnosing BCa and ② recurrence-free survival (RFS), progression-free survival (PFS), overall survival (OS) or cancer-specific survival (CSS) when AST was initiated after diagnosing BCa. We used the Risk of Bias in Nonrandomized Studies of Interventions (ROBINS-I) tool to assess methodological quality and summarized the results in Supplementary Table 1.
Statistical Analysis
The meta-analysis was performed by the Review Manager Version 5.3 software. Due to the observational nature of the included studies, we extracted adjusted hazard ratios (HRs) and odds ratios (ORs) with 95% CIs from the multivariate logistic regression analysis to calculate the cumulative effect size (17). Moreover, HRs and incidence density ratios can be regarded as relative risks (RRs) directly (18, 19). Additionally, ORs are close to RRs because of the low incidence of outcome (<10%) (20). The Cochrane Q test and I2 were used to determine the level of heterogeneity among studies. In the case of heterogeneity (p < 0.10 or I2 > 50%), the random effects model was used; otherwise, a fixed effects model was used. A p value < 0.05 was considered statistically significant. In addition, according to the type of AST, we performed a subgroup analysis of the effect of AST on BC incidence. Finally, publication bias was assessed by using a funnel plot when there were more than 10 studies that reported a specific outcome.
Results
Characteristics of Included Studies and Patients
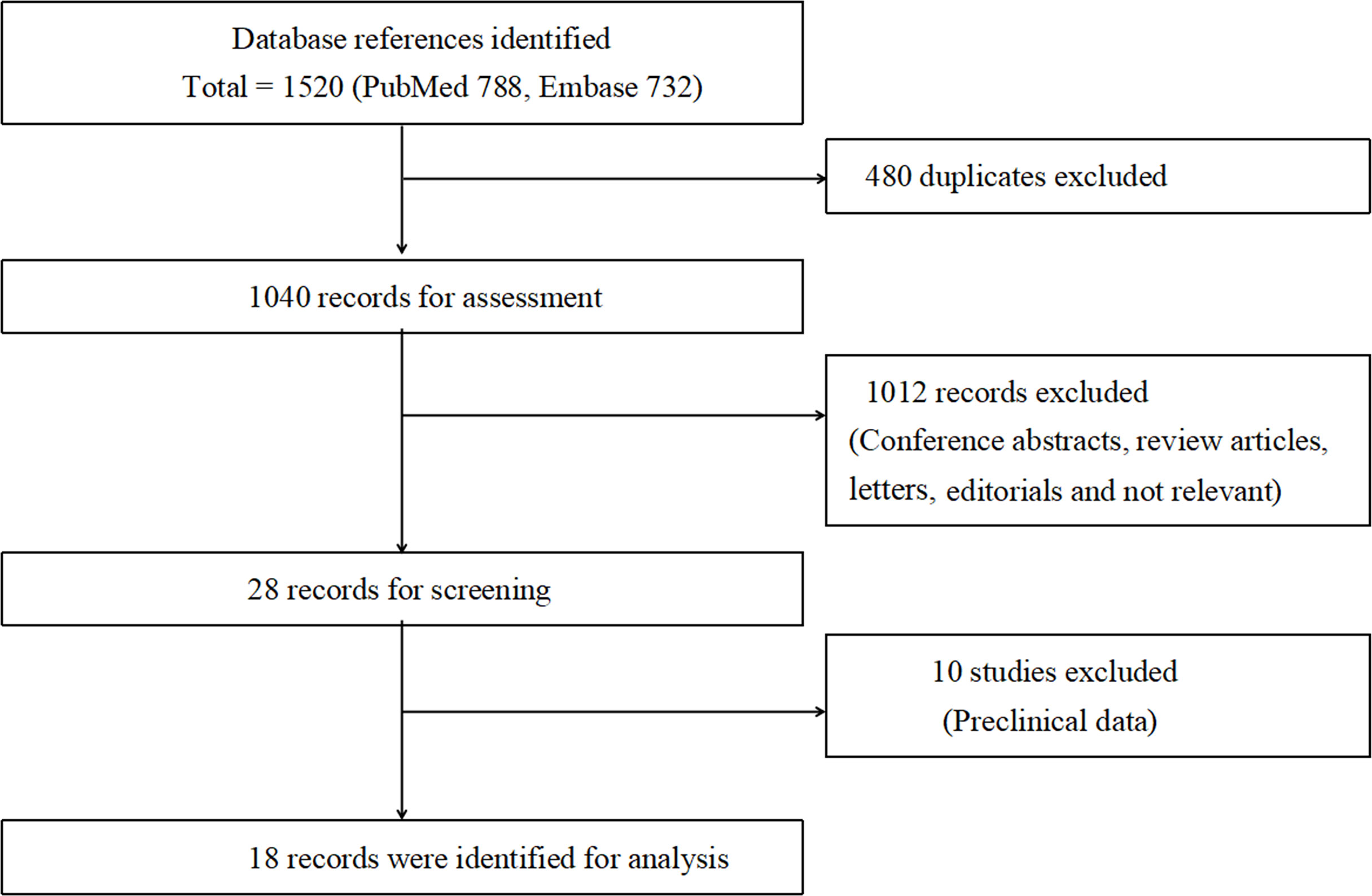
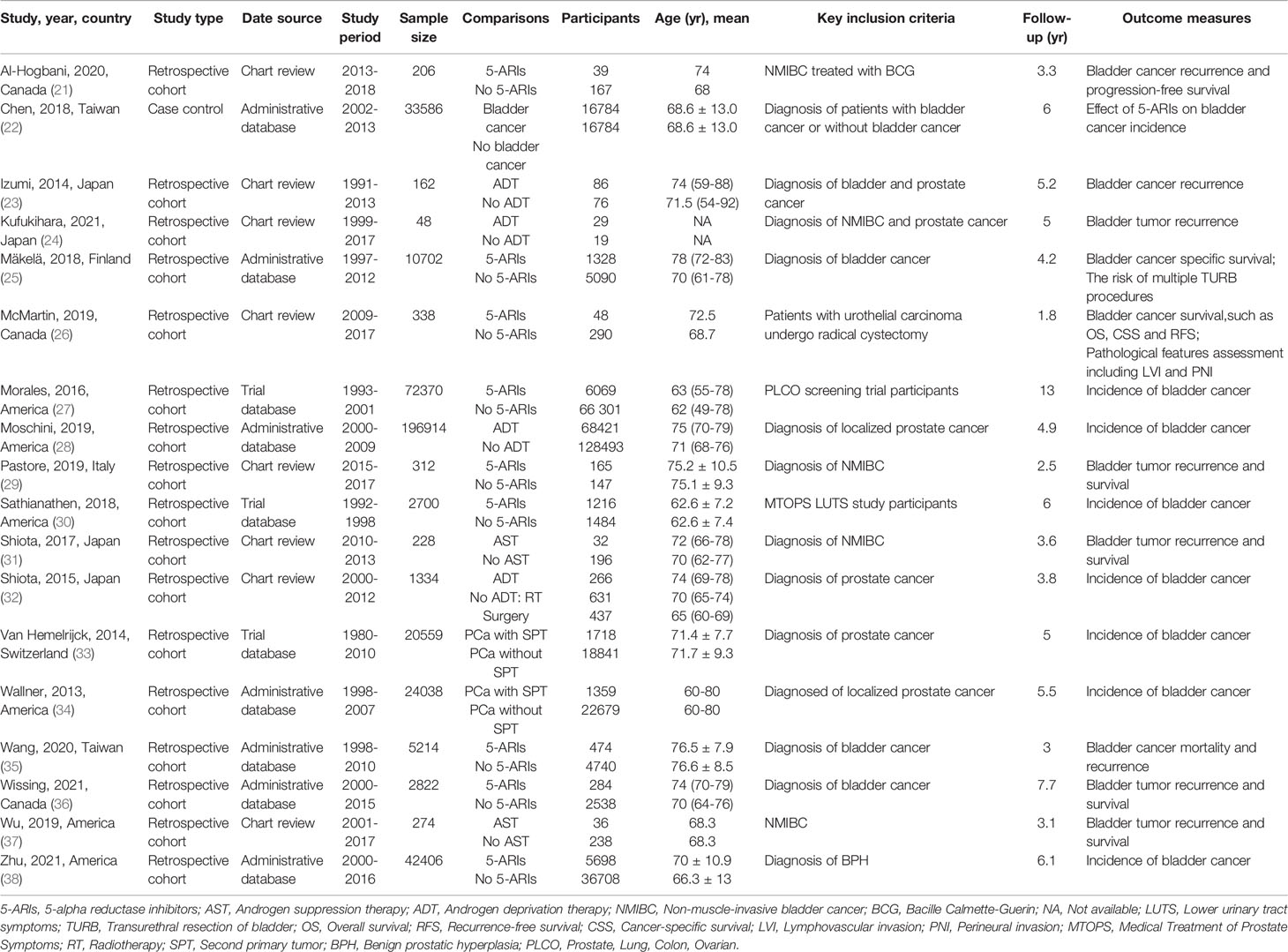
Overall, according to the screening criteria, the systematic review and meta-analysis included 18 studies with a total of 414 007 male patients (21–38) (Figure 1). Eight studies evaluated the effect of AST on bladder cancer incidence (22, 27, 28, 30, 32–34, 38). Ten studies examined the effect of AST on bladder cancer recurrence, progression and survival (21, 23–26, 29, 31, 35–37). The characteristics of the selected studies were summarized in Table 1. The search strategy was presented in Supplementary Table 2.
Effect of AST on Bladder Cancer Incidence
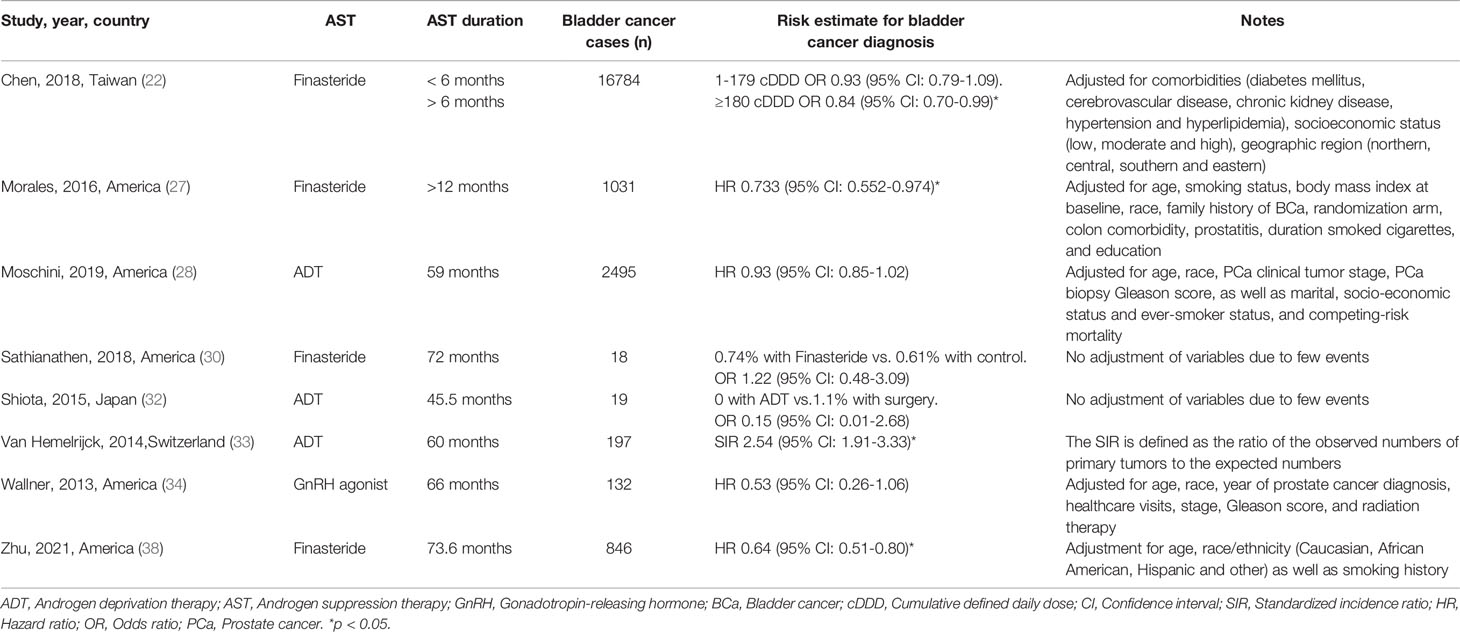
Eight studies with 393 907 participants evaluated whether AST reduced the incidence of bladder cancer diagnosis. The results from these studies are summarized in Table 2. Three studies reported a protective effect of AST on bladder cancer incidence, four reported no association, and one reported an increased risk. The meta-analysis of studies revealed a nonsignificant reduction in BCa incidence (OR: 0.92, 95% CI: 0.68–1.24) (Figure 2). Evidence of statistically significant heterogeneity was found in selected studies (I2 = 90%, p < 0.001). When stratified by the type of AST, we found a statistically lower incidence of bladder cancer among men with finasteride (OR: 0.75, 95% CI: 0.64–0.88), while no statistically significant effect was seen with ADT (OR: 1.00, 95% CI: 0.46–2.15) vs. nonusers. In particular, Chen et al. (22) showed that only patients who received finasteride > 6 months had a lower risk of BCa. In the study of Morales et al. (27), the risk reduction was only observed in well-differentiated and moderately differentiated tumors, while the diagnosis of poorly differentiated or undifferentiated tumors was not reduced. Zhu et al. (38) indicated that the use of finasteride was associated with significant reductions in the risk of high-grade BCa and non-muscle invasive BCa. In addition, a decrease in the risk of BCa was shown only in Caucasians and Hispanics but not among African Americans.
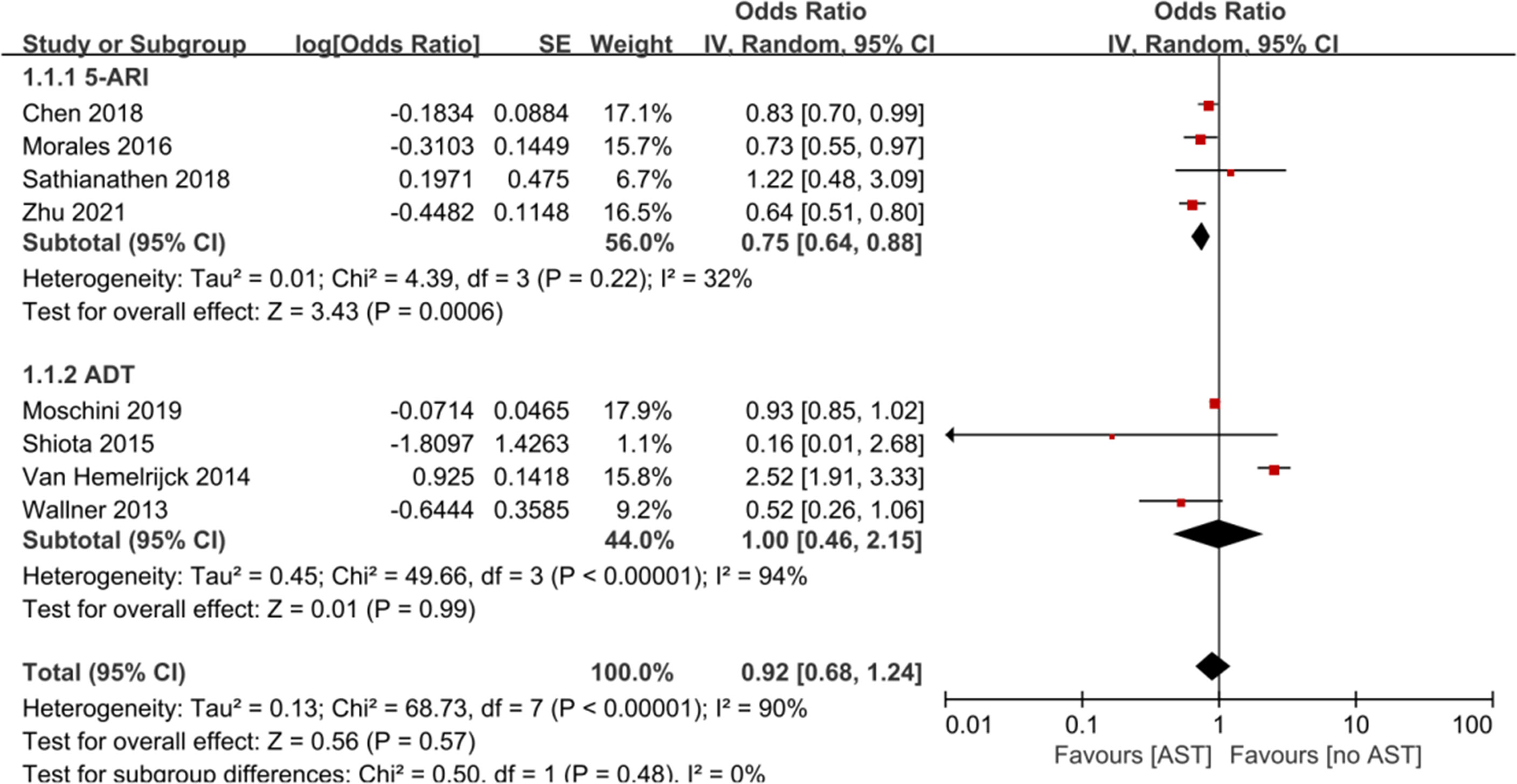
Figure 2 Forest plots showing the effect of AST on bladder cancer incidence. AST, Androgen suppression therapy; 5-ARI, 5-alpha reductase inhibitor; ADT, Androgen deprivation therapy.
Effect of AST on Bladder Cancer Recurrence and Progression
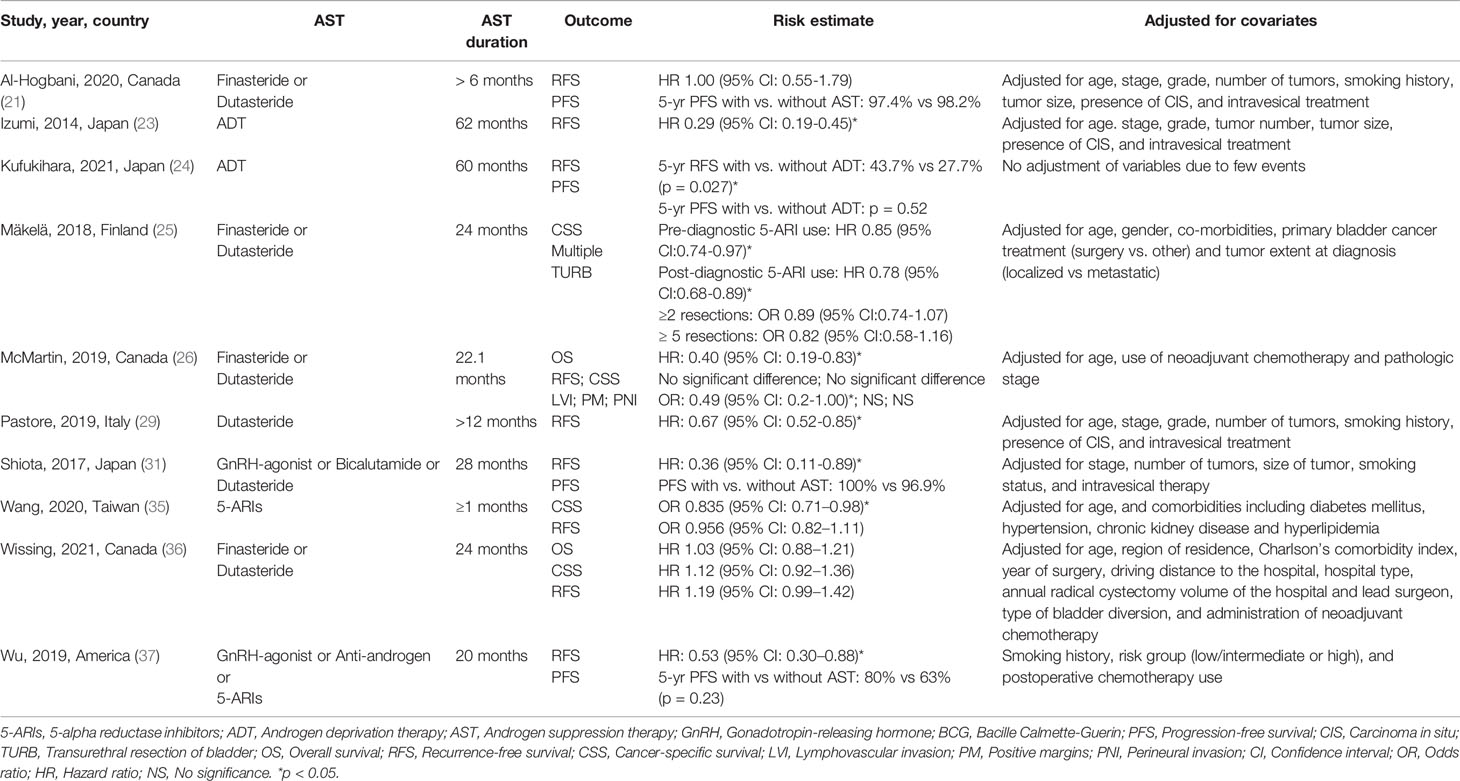
Ten studies including 20 100 participants reported the impact of AST on patients diagnosed with bladder cancer (Table 3). Five studies evaluated patients with non-muscle-invasive bladder cancer (NMIBC), and five included all patients with bladder cancer. The manipulation of the androgen signaling pathway involved the use of 5-ARIs in 4263 patients and ADT in 233 patients. For the analysis of RFS, a meta-analysis of seven studies with corresponding HRs was conducted. Compared with nonusers, AST users had significantly improved RFS (HR: 0.68, 95% CI: 0.48–0.95) (Figure 3A). The pooled analysis for RFS detected significant heterogeneity (I2 = 90%, p < 0.001). Similarly, Kufukihara et al. (24) revealed that the rate of bladder tumor recurrence was significantly lower in the ADT group than in the counterpart (p = 0.027). However, McMartin et al. (26) failed to find a significant difference in RFS between patients undergoing therapy with 5-ARIs and controls.

Figure 3 (A) Forest plots showing the effect of AST on RFS in bladder cancer. (B) Forest plots showing the effect of AST on CSS in bladder cancer. (C) Forest plots showing the effect of AST on OS in bladder cancer. AST, Androgen suppression therapy; RFS, Recurrence-free survival; CSS, Cancer-specific survival; OS, Overall survival.
For the analysis of CSS, a meta-analysis of three studies was conducted. Pooled data for CSS confirmed a nonsignificant difference in patients undergoing therapy with 5-ARIs compared to controls (HR: 0.89, 95% CI: 0.73–1.09) (Figure 3B). The pooled analysis found significant heterogeneity (I2 = 78%, p = 0.01). Similarly, in the study by McMartin et al. (26), there was no significant difference in CSS between patients undergoing therapy with 5-ARIs and controls. Two studies reported OS in patients with 5-ARIs treatment after diagnosing BCa; there was no significant reduction in OS in these patients (HR: 0.69, 95% CI: 0.27–1.73) (Figure 3C). High heterogeneity for OS was observed (I2 = 84%, p = 0.01). Owing to a paucity of HR data from PFS, we performed a descriptive analysis. PFS was investigated in 4 studies, and no differences between AST users and nonusers were found (21, 24, 31, 37).
Discussion
In this comprehensive meta-analysis, we did not find evidence to support the previous hypothesis that the AST is associated with a lower incidence of bladder cancer. Interestingly, subgroup analysis in patients receiving finasteride showed a decreased risk of BCa incidence (OR: 0.75, 95% CI: 0.64–0.88), while ADT had no effect on reducing BCa incidence. In addition, AST significantly reduced RFS in patients with bladder cancer but had no significant effect on CSS, OS or PFS.
Contrary to results of a previous meta-analysis (16), the clinical evidence in this review shows that there is no significant difference in the incidence of BCa between patients with AST and patients without AST. The earlier systematic review and meta-analysis included only three retrospective cohort studies to evaluate the impact of AST on bladder cancer incidence. Obviously, earlier conclusions are easily influenced by the results of newly published studies. In the subgroup analysis based on the type of AST, 5-ARIs exposure was significantly correlated with a decreased risk of subsequent BCa; however, ADT was not significantly correlated with the risk of subsequent BCa. Thus, it seems that suppressing the AR axis more effectively will not yield greater benefits. Nonetheless, we believe that more studies are needed to better evaluate the true benefits of ADT, as this treatment may only affect patients whose BCa does express AR (28). Notably, only a study from Van Hemelrijck et al. (33) indicated an increased risk of bladder cancer associated with ADT use. The authors calculated standardized incidence rates comparing the incidence of second primary malignancies (including bladder cancer) in the prostate cancer (PCa) patient group vs. the general male population in Zurich. However, compared with the general male population, patients with PCa may be monitored more carefully and contact doctors more frequently, thereby increasing the detection rate of bladder cancer and leading to detection bias (39). In our review, bicalutamide was not fully investigated due to limited use of included studies.
The pooled data for RFS in this review show that the risk of BCa recurrence is significantly reduced in patients undergoing hormonal manipulation with AST, which is consistent with the results of previous meta-analyses (15, 16). Creta et al. (15) and Kourbanhoussen et al. (2) indicated that low-grade and low-risk NMIBC may benefit more from the use of AST. This benefit was well reflected in studies including only NMIBC or studies with a high proportion of NMIBC and low-grade patients (23, 29, 31, 37). However, the pooled data for CSS, OS and PFS do not support a protective role of ADT and/or 5-ARIs in terms of BCa progression and survival in the subjects evaluated. Interestingly, Wu et al. (37) indicated that therapy with ADT or 5-ARIs may be associated with lower progression rates in patients with low/intermediate-risk BCa but not in their high-risk counterparts. The negative finding for PFS may be due to the small sample size and the number of events during follow-up, rather than a true lack of correlation. Moreover, the included studies contained many high-risk NMIBC and MIBC patients. It has been reported that these tumor types can reduce dependence on the AR signaling pathway, which may partly explain the lack of association between AST and BCa progression (37, 40–42). As expected, given the prevalence of urinary symptoms in older men, most studies have investigated the role of 5-ARIs in BCa, and only a few studies have investigated the role of ADT in BCa. Because the number of individual studies is insufficient for comparison, it is not clear whether more effective inhibition of the AR axis will yield greater benefits.
Zhu et al. (38) first demonstrated that after using finasteride, although Hispanic men have a similar reduced risk of bladder cancer compared with Caucasian men, African-American men do not. A possible biological explanation for this observation might be the structural variations in the AR protein across different races. African Americans are more likely to contain polymorphisms in AR, which causes their AR to become active and independent of DHT binding (38). The role of AST is not limited to the androgen axis. Clinically, 5-ARIs can increase serum estrogen levels (43, 44). With more effective anti-androgens, the reflex of estrogen increases even higher (2, 45, 46). Estrogens play important roles in BCa development and progression by exerting both stimulatory and inhibitory actions via estrogen receptor α (ERα) and ERβ (2, 8). Overall, it appears that estrogens may protect against or inhibit BCa development, but later—at more advanced stages—they might support tumor progression (8). The estrogen-signaling pathway during AST may still partially explain the observed effects in AST.
The number of included studies for meta-analysis was too small to fully assess the publication bias of the effects of AST on incidence or recurrence. Some of heterogeneities were too high. We speculate that several observational studies included in the present meta-analysis did not adjust for potential confounders such as age, race/ethnicity and smoking history, which may bias the pooled effect estimate and may affect heterogeneity. Moreover, differences in AST type, AST exposure time, follow-up duration and demographic characteristics of the included studies are also important reasons for the heterogeneity of results. The proportion of participants receiving 5-ARIs in the currently included studies is obviously higher than that of participants receiving ADT, making the overall effect of outcomes of AST more inclined to 5-ARIs. In most of the included studies, the average age of participants in the AST group was slightly higher than that in the non-AST group. The AST exposure time and follow-up duration of the included literature vary, although it was mostly longer than 2 years. Compared with a duration of less than 6 months, it seems that the use of 5ARIs for greater than 6 months can lead to more significant benefits (22), but the benefits have not been accumulated with years of 5-ARIs use (25). Most of the original studies did not mention the detailed characteristics of BCa, including grade, stage, and cancer cell type, which further limited the pooled analysis. We still do not know very clearly which types of BCa have a lower incidence and which types of BCa have a low recurrence rate after AST. Although the available preclinical evidence demonstrates that AST can interfere with the sensitivity of BCa to BCG or other therapies, its benefit is not observable when given in clinical studies (2, 15, 21). Further research is needed to better evaluate the role of androgen suppression in specific subgroups of BCa patients, to compare the effects of 5-ARIs and ADT and to better clarify androgen manipulation strategies for patients with BCa undergoing BCG, radiation or chemotherapy.
Conclusion
Our systematic review and meta-analysis identified 18 studies that evaluated androgen suppression on clinical outcomes in BCa patients. AST was not associated with a lower risk of BCa incidence, but a subgroup analysis showed that patients receiving 5-ARIs had a reduced risk of BCa incidence. In addition, AST has a beneficial effect on the recurrence rates of bladder cancer. We did not observe any significant differences in AST on CSS, OS or PFS when compared with the control. Further well-designed prospective studies adjusted for the major and common confounding factors are needed to validate our findings.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author Contributions
Conceptualization: PX, HP, and ZD. Data curation: YH, DG, DL, and PX. Formal analysis: WY, MW, PX, ZD, and YL. Project administration: HP. Resources: HP and ZD. Software: PX and YH. Supervision: HP. Validation: PX and HP. Visualization: PX. Writing - original draft: PX. Writing - review & editing: all authors. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81772698 and 82072833 to HP) and the Open Research Fund from Beijing Advanced Innovation Center for Big Data-Based Precision Medicine, Beijing Tongren Hospital, Beihang University & Capital Medical University (Grant No. BHTR-KFJJ-202005 to HP).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors are very grateful to the urology department of Beijing Tongren Hospital for their selfless help in this work.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.784627/full#supplementary-material
References
1. Lenis AT, Lec PM, Chamie K, Mshs MD. Bladder Cancer: A Review. JAMA (2020) 324(19):1980–91. doi: 10.1001/jama.2020.17598
2. Kourbanhoussen K, McMartin C, Lodde M, Zlotta A, Bryan RT, Toren P. Switching Cancers: A Systematic Review Assessing the Role of Androgen Suppressive Therapy in Bladder Cancer. Eur Urol Focus (2020) 7(5):1044–51. doi: 10.1016/j.euf.2020.10.002
3. Dobruch J, Daneshmand S, Fisch M, Lotan Y, Noon AP, Resnick MJ, et al. Gender and Bladder Cancer: A Collaborative Review of Etiology, Biology, and Outcomes. Eur Urol (2016) 69(2):300–10. doi: 10.1016/j.eururo.2015.08.037
4. Ide H, Miyamoto H. Sex Hormone Receptor Signaling in Bladder Cancer: A Potential Target for Enhancing the Efficacy of Conventional Non-Surgical Therapy. Cells (2021) 10(5):1169. doi: 10.3390/cells10051169
5. Mori K, Mostafaei H, Enikeev DV, Lysenko I, Quhal F, Kimura S, et al. Differential Effect of Sex on Outcomes After Radical Surgery for Upper Tract and Bladder Urothelial Carcinoma: A Systematic Review and Meta-Analysis. J Urol (2020) 204(1):58–62. doi: 10.1097/JU.0000000000000788
6. Uhlig A, Strauss A, Seif Amir Hosseini A, Lotz J, Trojan L, Schmid M, et al. Gender-Specific Differences in Recurrence of Non-Muscle-Invasive Bladder Cancer: A Systematic Review and Meta-Analysis. Eur Urol Focus (2018) 4(6):924–36. doi: 10.1016/j.euf.2017.08.007
7. Martinez-Rojo E, Berumen LC, Garcia-Alcocer G, Escobar-Cabrera J. The Role of Androgens and Androgen Receptor in Human Bladder Cancer. Biomolecules (2021) 11(4):594. doi: 10.3390/biom11040594
8. Costa AR, Lanca de Oliveira M, Cruz I, Goncalves I, Cascalheira JF, Santos CRA. The Sex Bias of Cancer. Trends Endocrinol Metab (2020) 31(10):785–99. doi: 10.1016/j.tem.2020.07.002
9. Moorthy HK, Prabhu GGL, Venugopal P. Clinical and Therapeutic Implications of Sex Steroid Hormone Receptor Status in Urothelial Bladder Cancer. Indian J Urol (2020) 36(3):171–8. doi: 10.4103/iju.IJU_320_19
10. Koti M, Ingersoll MA, Gupta S, Lam CM, Li X, Kamat AM, et al. Sex Differences in Bladder Cancer Immunobiology and Outcomes: A Collaborative Review With Implications for Treatment. Eur Urol Oncol (2020) 3(5):622–30. doi: 10.1016/j.euo.2020.08.013
11. Luna-Velez MV, Dijkstra JJ, Heuschkel MA, Smit FP, van de Zande G, Smeets D, et al. Androgen Receptor Signalling Confers Clonogenic and Migratory Advantages in Urothelial Cell Carcinoma of the Bladder. Mol Oncol (2021) 15(7):1882–900. doi: 10.1002/1878-0261.12957
12. Deng G, Wang R, Sun Y, Huang CP, Yeh S, You B, et al. Targeting Androgen Receptor (AR) With Antiandrogen Enzalutamide Increases Prostate Cancer Cell Invasion Yet Decreases Bladder Cancer Cell Invasion via Differentially Altering the AR/CircRNA-ARC1/MiR-125b-2-3p or Mir-4736/Ppargamma/MMP-9 Signals. Cell Death Differ (2021) 28(7):2145–59. doi: 10.1038/s41418-021-00743-w
13. Ide H, Inoue S, Mizushima T, Jiang G, Chuang KH, Oya M, et al. Androgen Receptor Signaling Reduces Radiosensitivity in Bladder Cancer. Mol Cancer Ther (2018) 17(7):1566–74. doi: 10.1158/1535-7163.Mct-17-1061
14. Tripathi A, Gupta S. Androgen Receptor in Bladder Cancer: A Promising Therapeutic Target. Asian J Urol (2020) 7(3):284–90. doi: 10.1016/j.ajur.2020.05.011
15. Creta M, Celentano G, Napolitano L, La Rocca R, Capece M, Califano G, et al. Inhibition of Androgen Signalling Improves the Outcomes of Therapies for Bladder Cancer: Results From a Systematic Review of Preclinical and Clinical Evidence and Meta-Analysis of Clinical Studies. Diagn (Basel) (2021) 11(2):351. doi: 10.3390/diagnostics11020351
16. Kim A, Kim MS, Ahn JH, Choi WS, Park HK, Kim HG, et al. Clinical Significance of 5-Alpha Reductase Inhibitor and Androgen Deprivation Therapy in Bladder Cancer Incidence, Recurrence, and Survival: A Meta-Analysis and Systemic Review. Aging Male (2020) 23(5):971–8. doi: 10.1080/13685538.2019.1646238
17. Tellini R, Mari A, Muto G, Cacciamani GE, Ferro M, Stangl-Kremser J, et al. Impact of Smoking Habit on Perioperative Morbidity in Patients Treated With Radical Cystectomy for Urothelial Bladder Cancer: A Systematic Review and Meta-Analysis. Eur Urol Oncol (2020) 4(4):580–93. doi: 10.1016/j.euo.2020.10.006
18. Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of Alcohol Consumption With Selected Cardiovascular Disease Outcomes: A Systematic Review and Meta-Analysis. BMJ (2011) 342:d671. doi: 10.1136/bmj.d671
19. Siristatidis C, Sergentanis TN, Kanavidis P, Trivella M, Sotiraki M, Mavromatis I, et al. Controlled Ovarian Hyperstimulation for IVF: Impact on Ovarian, Endometrial and Cervical Cancer–a Systematic Review and Meta-Analysis. Hum Reprod Update (2013) 19(2):105–23. doi: 10.1093/humupd/dms051
20. Zhang J, Yu KF. What’s the Relative Risk? A Method of Correcting the Odds Ratio in Cohort Studies of Common Outcomes. JAMA (1998) 280(19):1690–1. doi: 10.1001/jama.280.19.1690
21. Al-Hogbani M, Gilbert S, Lodde M, Fradet Y, Toren P. Does 5-Alpha Reductase Inhibitor Use Improve the Efficacy of Intravesical Bacille Calmette-Guérin (BCG) for Non-Muscle Invasive Bladder Cancer? Bladder Cancer (2020) 6(1):63–9. doi: 10.3233/blc-190262
22. Chen CC, Huang CP, Tsai YT, Hseih TF, Shyr CR. The Genomic Alterations of 5alpha-Reductases and Their Inhibitor Finasteride’s Effect in Bladder Cancer. Anticancer Res (2017) 37(12):6893–8. doi: 10.21873/anticanres.12152
23. Izumi K, Taguri M, Miyamoto H, Hara Y, Kishida T, Chiba K, et al. Androgen Deprivation Therapy Prevents Bladder Cancer Recurrence. Oncotarget (2014) 5(24):12665–74. doi: 10.18632/oncotarget.2851
24. Kufukihara R, Kikuchi E, Ogihara K, Shigeta K, Yanai Y, Takamatsu K, et al. Role of Previous Malignancy History in Clinical Outcomes in Patients With Initially Diagnosed Non-Muscle Invasive Bladder Cancer. Ann Surg Oncol (2021) 28(9):5349–59. doi: 10.1245/s10434-021-09750-0
25. Makela VJ, Kotsar A, Tammela TLJ, Murtola TJ. Bladder Cancer Survival of Men Receiving 5alpha-Reductase Inhibitors. J Urol (2018) 200(4):743–8. doi: 10.1016/j.juro.2018.04.082
26. McMartin C, Lacombe L, Fradet V, Fradet Y, Lodde M, Toren P. Receipt of 5-Alpha Reductase Inhibitors Before Radical Cystectomy: Do They Render High-Grade Bladder Tumors Less Aggressive? Clin Genitourin Cancer (2019) 17(6):e1122–8. doi: 10.1016/j.clgc.2019.07.016
27. Morales EE, Grill S, Svatek RS, Kaushik D, Thompson IM Jr., Ankerst DP, et al. Finasteride Reduces Risk of Bladder Cancer in a Large Prospective Screening Study. Eur Urol (2016) 69(3):407–10. doi: 10.1016/j.eururo.2015.08.029
28. Moschini M, Zaffuto E, Karakiewicz P, Mattei A, Gandaglia G, Fossati N, et al. The Effect of Androgen Deprivation Treatment on Subsequent Risk of Bladder Cancer Diagnosis in Male Patients Treated for Prostate Cancer. World J Urol (2019) 37(6):1127–35. doi: 10.1007/s00345-018-2504-3
29. Pastore AL, Fuschi A, De Nunzio C, Balzarro M, Al Salhi Y, Velotti G, et al. Possible Role of 5-Alpha Reductase Inhibitors in Non-Invasive Bladder Urothelial Neoplasm: Multicentre Study. Minerva Urol Nefrol (2019). doi: 10.23736/S0393-2249.19.03563-X
30. Sathianathen NJ, Fan Y, Jarosek SL, Lawrentschuk NL, Konety BR. Finasteride Does Not Prevent Bladder Cancer: A Secondary Analysis of the Medical Therapy for Prostatic Symptoms Study. Urol Oncol (2018) 36(7):338.e13–338.e17. doi: 10.1016/j.urolonc.2018.03.020
31. Shiota M, Kiyoshima K, Yokomizo A, Takeuchi A, Kashiwagi E, Dejima T, et al. Suppressed Recurrent Bladder Cancer After Androgen Suppression With Androgen Deprivation Therapy or 5alpha-Reductase Inhibitor. J Urol (2017) 197(2):308–13. doi: 10.1016/j.juro.2016.08.006
32. Shiota M, Yokomizo A, Takeuchi A, Imada K, Kiyoshima K, Inokuchi J, et al. Secondary Bladder Cancer After Anticancer Therapy for Prostate Cancer: Reduced Comorbidity After Androgen-Deprivation Therapy. Oncotarget (2015) 6(16):14710–9. doi: 10.18632/oncotarget.3817
33. Van Hemelrijck M, Feller A, Garmo H, Valeri F, Korol D, Dehler S, et al. Incidence of Second Malignancies for Prostate Cancer. PLoS One (2014) 9(7):e102596. doi: 10.1371/journal.pone.0102596
34. Wallner LP, Wang R, Jacobsen SJ, Haque R. Androgen Deprivation Therapy for Treatment of Localized Prostate Cancer and Risk of Second Primary Malignancies. Cancer Epidemiol Biomarkers Prev (2013) 22(2):313–6. doi: 10.1158/1055-9965.EPI-12-1137
35. Wang CS, Li CC, Juan YS, Wu WJ, Lee HY. 5alpha-Reductase Inhibitors Impact Prognosis of Urothelial Carcinoma. BMC Cancer (2020) 20(1):872. doi: 10.1186/s12885-020-07373-4
36. Wissing MD, O’Flaherty A, Dragomir A, Tanguay S, Kassouf W, Aprikian AG. The Use of 5-Alpha Reductase Inhibitors and Alpha-1 Blockers Does Not Improve Clinical Outcome in Male Patients Undergoing Radical Cystectomy for Bladder Cancer in Quebec, Canada. Clin Genitourin Cancer (2021) 19(4):371–371.e9. doi: 10.1016/j.clgc.2021.01.007
37. Wu SC, Kwon D, Jue JS, Chen FV, Velasquez Escobar MC, Punnen S, et al. Androgen Suppression Therapy Is Associated With Lower Recurrence of Non-Muscle-Invasive Bladder Cancer. Eur Urol Focus (2021) 7(1):142–7. doi: 10.1016/j.euf.2019.04.021
38. Zhu D, Srivastava A, Agalliu I, Fram E, Kovac EZ, Aboumohamed A, et al. Finasteride Use and Risk of Bladder Cancer in a Multiethnic Population. J Urol (2021) 206(1):15–21. doi: 10.1097/JU.0000000000001694
39. Santella C, Rouette J, Brundage MD, Filion KB, Azoulay L. Androgen Deprivation Therapy for Prostate Cancer and the Risk of Bladder Cancer: A Systematic Review of Observational Studies. Urol Oncol (2020) 38(11):816–25. doi: 10.1016/j.urolonc.2020.04.028
40. Miyamoto H, Yao JL, Chaux A, Zheng Y, Hsu I, Izumi K, et al. Expression of Androgen and Oestrogen Receptors and Its Prognostic Significance in Urothelial Neoplasm of the Urinary Bladder. BJU Int (2012) 109(11):1716–26. doi: 10.1111/j.1464-410X.2011.10706.x
41. Sikic D, Breyer J, Hartmann A, Burger M, Erben P, Denzinger S, et al. High Androgen Receptor mRNA Expression Is Independently Associated With Prolonged Cancer-Specific and Recurrence-Free Survival in Stage T1 Bladder Cancer. Transl Oncol (2017) 10(3):340–5. doi: 10.1016/j.tranon.2017.01.013
42. Boorjian S, Ugras S, Mongan NP, Gudas LJ, You X, Tickoo SK, et al. Androgen Receptor Expression Is Inversely Correlated With Pathologic Tumor Stage in Bladder Cancer. Urology (2004) 64(2):383–8. doi: 10.1016/j.urology.2004.03.025
43. Lee YR, Im E, Kim H, Lew BL, Sim WY, Lee J, et al. Untargeted Metabolomics and Steroid Signatures in Urine of Male Pattern Baldness Patients After Finasteride Treatment for a Year. Metabolites (2020) 10(4):131. doi: 10.3390/metabo10040131
44. Kristal AR, Till C, Tangen CM, Goodman PJ, Neuhouser ML, Stanczyk FZ, et al. Associations of Serum Sex Steroid Hormone and 5alpha-Androstane-3alpha,17beta-Diol Glucuronide Concentrations With Prostate Cancer Risk Among Men Treated With Finasteride. Cancer Epidemiol Biomarkers Prev (2012) 21(10):1823–32. doi: 10.1158/1055-9965.EPI-12-0695
45. Wadhwa VK, Weston R, Parr NJ. Bicalutamide Monotherapy Preserves Bone Mineral Density, Muscle Strength and has Significant Health-Related Quality of Life Benefits for Osteoporotic Men With Prostate Cancer. BJU Int (2011) 107(12):1923–9. doi: 10.1111/j.1464-410X.2010.09726.x
46. Liang Z, Cao J, Tian L, Shen Y, Yang X, Lin Q, et al. Aromatase-Induced Endogenous Estrogen Promotes Tumour Metastasis Through Estrogen Receptor-Alpha/Matrix Metalloproteinase 12 Axis Activation in Castration-Resistant Prostate Cancer. Cancer Lett (2019) 467:72–84. doi: 10.1016/j.canlet.2019.09.001
Keywords: androgen suppression therapy, bladder cancer, incidence, recurrence, meta-analysis
Citation: Xiang P, Du Z, Hao Y, Guan D, Liu D, Yan W, Wang M, Liu Y and Ping H (2021) Impact of Androgen Suppression Therapy on the Risk and Prognosis of Bladder Cancer: A Systematic Review and Meta-Analysis. Front. Oncol. 11:784627. doi: 10.3389/fonc.2021.784627
Received: 28 September 2021; Accepted: 24 November 2021;
Published: 14 December 2021.
Edited by:
Scott T Tagawa, Cornell University, United StatesReviewed by:
Andrea Benedetto Galosi, Marche Polytechnic University, ItalyNing Li, Fourth Affiliated Hospital of China Medical University, China
Copyright © 2021 Xiang, Du, Hao, Guan, Liu, Yan, Wang, Liu and Ping. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hao Ping, aGFvcGluZ2N5aEAxNjMuY29t; orcid.org/0000-0002-0321-7921
†These authors have contributed equally to this work
 Peng Xiang1,2†
Peng Xiang1,2† Hao Ping
Hao Ping