
94% of researchers rate our articles as excellent or good
Learn more about the work of our research integrity team to safeguard the quality of each article we publish.
Find out more
ORIGINAL RESEARCH article
Front. Oncol., 06 December 2021
Sec. Hematologic Malignancies
Volume 11 - 2021 | https://doi.org/10.3389/fonc.2021.783703
This article is part of the Research TopicMinimal Residual Disease (MRD) Assessment in Multiple Myeloma PatientsView all 9 articles
 Sandy W. Wong1
Sandy W. Wong1 Nina Shah1
Nina Shah1 Guy Ledergor1
Guy Ledergor1 Thomas Martin1
Thomas Martin1 Jeffrey Wolf1
Jeffrey Wolf1 Amy M. Shui2
Amy M. Shui2 Chiung-Yu Huang2
Chiung-Yu Huang2 Joaquin Martinez-Lopez1,3*
Joaquin Martinez-Lopez1,3*Chimeric antigen receptor T-cell (CAR-T) therapy targeted against B-cell maturation antigen (BCMA) in multiple myeloma (MM) has produced rapid responses but many eventually relapse. In light of this new treatment, novel predictors of progression-free survival (PFS) are needed. We performed a single institution analysis of 54 BCMA-CAR-T patients. We analyzed patient’s overall response rate (ORR) by the IMWG criteria, involved serum-free light chains (iFLC), and minimal residual disease testing by next-generation sequencing (MRD-NGS). Between patients who achieved a ≤SD and those who achieved a ≥PR, PFS differed significantly (p < 0.0001); though there was no difference between patients who achieved a ≥CR vs. VGPR/PR (p = 0.2). In contrast, patients who achieved a nonelevated iFLC at 15 days (p < 0.0001, HR = 6.8; 95% CI, 2.7–17.3) or 30 days (p < 0.001, HR = 16.7; 95% CI, 3.9–71.7) had a prolonged PFS compared with those with an elevated iFLC. Patients achieving MRD-NGS less than the detectable limit at a sensitivity of 10−6 had a better PFS than those with detectable disease at 1 month (p = 0.02) and 3 months (p = 0.02). In conclusion, achieving a nonelevated iFLC and an undetectable MRD-NGS quickly were factors that were strongly associated with improved PFS. Further studies are needed to confirm the role of these markers in MM patients receiving CAR-T therapies.
Chimeric antigen receptor T-cell (CAR-T) therapy against B-cell maturation antigen (BCMA) is a promising new treatment for multiple myeloma (MM) with high and rapid clinical efficacy even in multiply refractory patients. By the International Myeloma Working Group (IMWG) criteria, the overall response rate (ORR) and complete response (CR) have been as high as 80%–90% and 50%, respectively (1–8). Many patients that achieve a CR are minimal residual disease (MRD) negative (1–7). However, despite high and deep responses, most patients eventually relapse (9, 10). Although the PFS is not yet mature for many constructs, bb2121 had a PFS of around 1 year (1). Manipulations of newer CAR-Ts have been focused on improving PFS. Since each novel CAR-T construct requires a long time to generate a PFS evaluation, early clinical predictors of PFS would hasten the assessment of new CAR-T improvements. Here, we report that an early assessment of involved free light chains (iFLC) and minimal residual disease (MRD) after CAR-T is closely correlated with PFS.
We analyzed all consecutive MM patients treated with BCMA-directed CAR-T therapy from five different clinical trials (ClinicalTrials.gov: NCT03430011, NCT03361748, NCT03274219, NCT03601078, and NCT03548207) at the University of California San Francisco from November 1, 2017 to February 26, 2020. This study was approved by the local Institutional Review Board (IRB #15-17721).
All patients had serologically measurable disease prior to clinical trial entry defined as serum M-protein ≥0.5 g/dl or SFLC ≥100 mg/L. Serologic response assessments occurred at 15 days after CAR-T infusion and then monthly. Bone marrow (BM) biopsies were performed at 1, 3, 6, 12, 18, and 24 months. Plasma cell infiltration of the bone marrow core and aspirate were evaluated by conventional methods. BM clonality was defined when the κ/λ ratio is >4:1 or <1:2 for κ and λ patients by immunohistochemistry. Cytogenetics were performed on the bone marrow done before lymphodepleting chemotherapy or at screening if the patient did not receive bridging therapy. Fresh bone marrow samples from patients were sent for MRD assessment by commercially available next-generation sequencing (NGS) of immunoglobulin genes as previously described (Adaptive Biotechnologies, Seattle, WA, USA) with each BM biopsy (11–13). Imaging assessment was performed by PET-CT between 3 and 6 months after infusion. Date of data cutoff was August 27, 2020.
ORR was assessed according to the IMWG uniform response criteria (14). IMWG responses were calculated from laboratory data obtained prior to lymphodepletion chemotherapy. CAR-T toxicities such as CRS were graded using Lee criteria and ICANS was graded by CTCAEv4.03 or ICE depending on the clinical trial (15). The statistical analysis was carried out with SAS version 9.4. Hypothesis tests were two sided, and the significance threshold was set to 0.05. PFS curves were plotted using the Kaplan-Meier method, and log-rank tests were used to test for group differences. Multivariable analysis was performed by using Cox proportional hazards regression models.
Fifty-four myeloma patients were included in our analysis, with all except four having refractory disease prior to enrollment on trial (Supplementary Table S1). At the time of data cutoff, 26 patients relapsed, and 28 patients had ongoing responses to CAR-T treatment. Baseline characteristics were generally similar between the two groups, although the relapsed group had a higher median-involved SFLC. Both groups were heavily pretreated with a median of 6 prior lines of therapy. Overall, the median PFS was 12.7 months, similar to previously studies (1, 7). Median OS was 25.2 months. Median duration of follow-up was 8.8 months (range, 5.6–12.6).
After CAR-T treatment, the involved SFLC dropped more rapidly than the M-protein in patients who responded to treatment. Over time, the depth of the M-protein decline approached that of the iSFLC (Figure 1A). ORR by IMWG criteria defined as ≥PR was 87% with 52% ≥CR, 35% VGPR/PR and 13% <PR. PFS differed significantly between patients who had a ≥PR compared with those who had <PR (p < 0.0001, HR = 63.0; 95% CI, 13.9–285.0) (Figure 1B). However, patients with VGPR/PR did not have a significantly different PFS from those who had a ≥CR (p = 0.20). Likewise, for patients with a detectable serum M-protein, its reduction was not correlated with PFS at 0.5, 1, and 3 months (p = 0.9, p = 0.8, and p = 0.5, respectively).
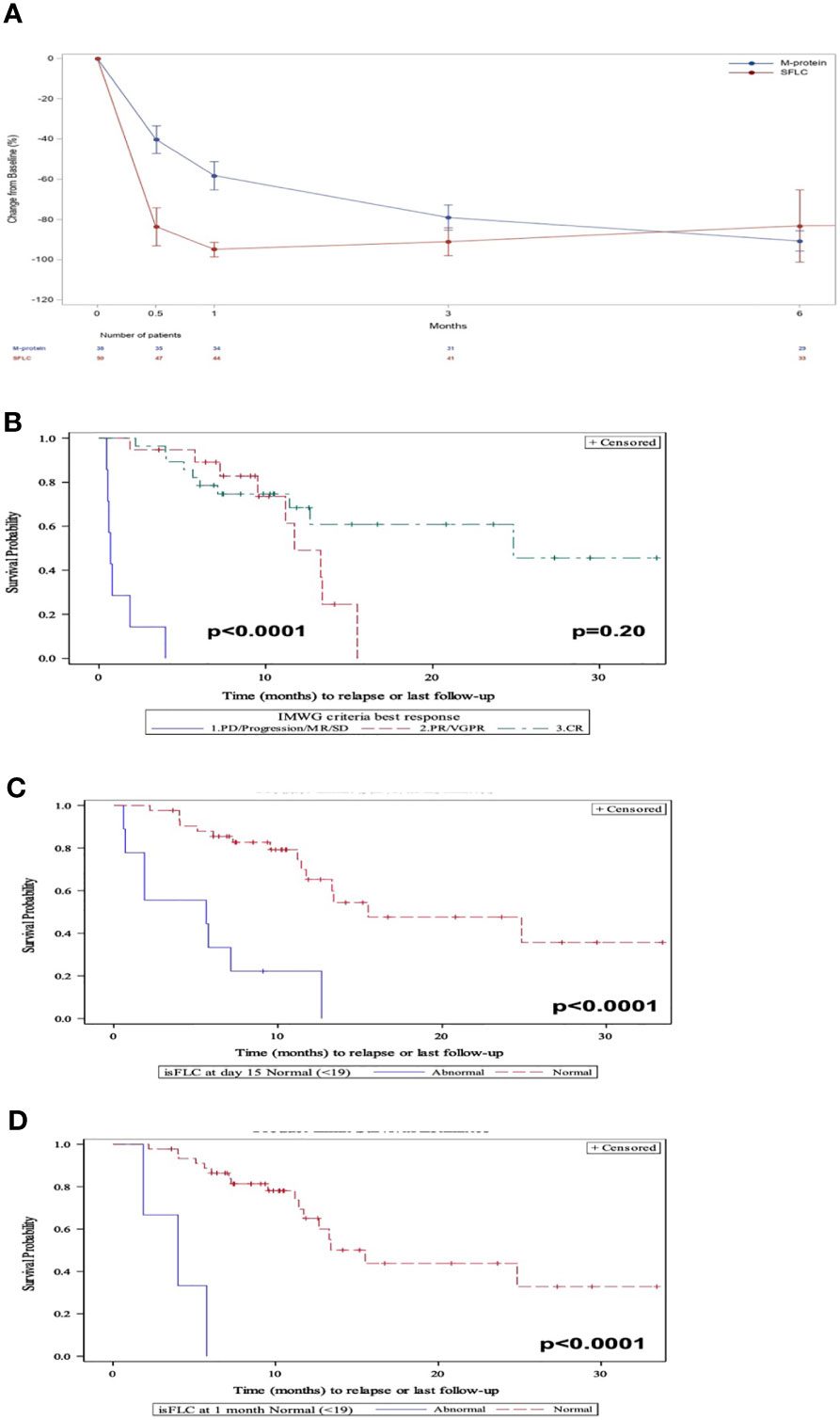
Figure 1 (A) Evolution of the M-protein and involved SFLC after CAR-T therapy in responders. (B) PFS by IMWG response criteria. (C) PFS at 15 days post-CAR-T by elevated iSFLC vs. nonelevated iSFLC. (D) PFS at 30 days post-CAR-T by elevated iSFLC vs. nonelevated iSFLC.
After 15 days of receiving CAR-Ts, 82% of patients had a nonelevated iSFLC which increased to 94% after 1 month. Patients who achieved a nonelevated iFLC at 15 days (Figure 1C) and 1 month (Figure 1D) had a prolonged PFS compared with those with persistently elevated iFLC (p < 0.0001, HR = 6.8; 95% CI, 2.7–17.3 and p = 0.0001, HR = 16.7; 95% CI 3.9–71.7, respectively).
Of patients who responded to CAR-T therapy, 82% achieved MRD-NGS negativity at 10−6 as the best response. Patients achieving MRD-NGS <10−6 at any point tended to have a prolonged PFS compared with those with MRD-NGS ≥10−6 although this did not reach statistical significance (p = 0.08). In contrast, patients achieving MRD-NGS ≤10−6 at 1 month had a prolonged PFS compared with those with MRD-NGS ≥10−6 (p = 0.02) (Figure 2A). Patients achieving MRD-NGS ≤10−6 at 3 months also had a prolonged PFS, but the association did not reach statistical significance (p = 0.06) (Figure 2B). Similar results were seen with MRD-NGS at 10−5. The addition of PET-CT to MRD-NGS at 10−5 allowed for further differentiation of patients who have a better PFS (p = 0.0002) (Figure 2C).
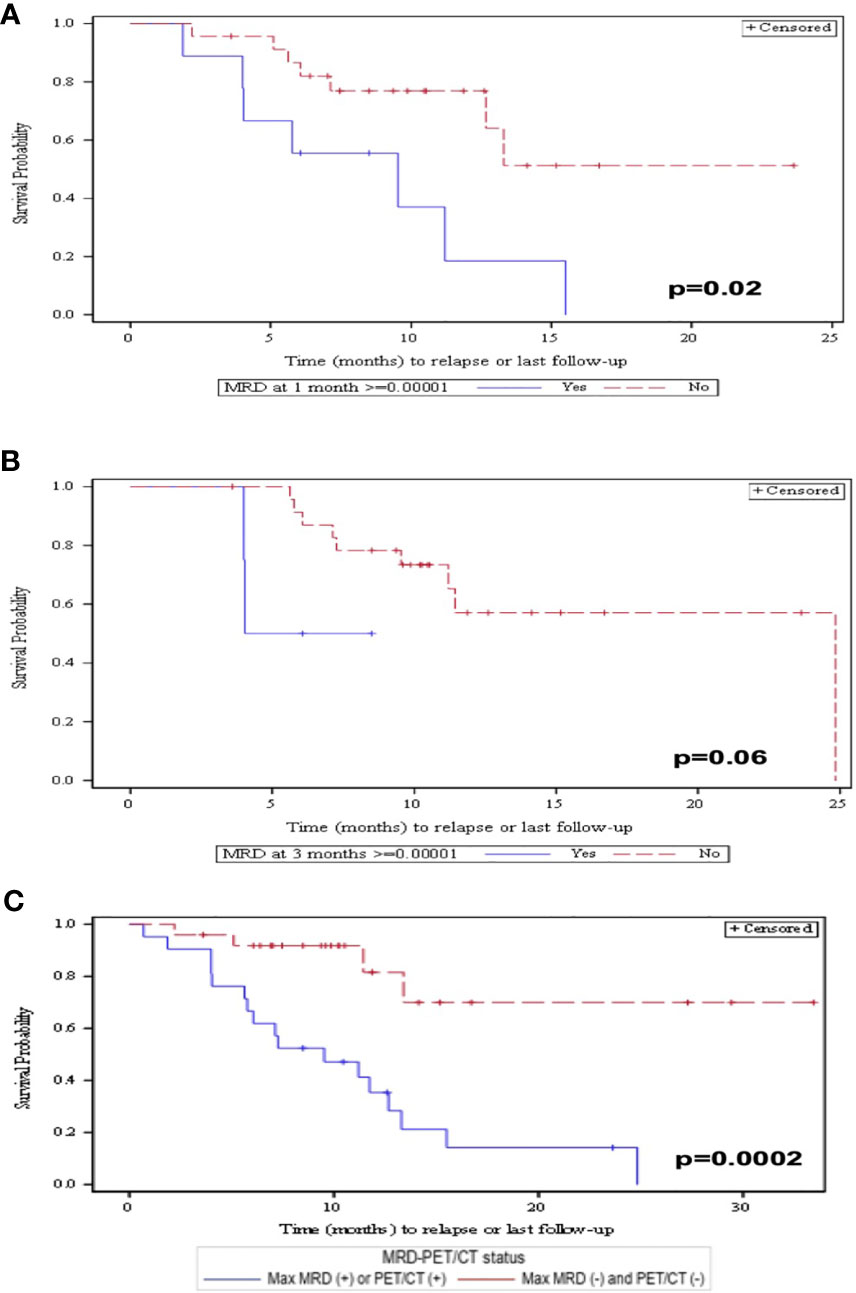
Figure 2 (A) PFS at 1 month post-CAR-T by patients who obtained MRD <10−6 vs. MRD >10−6. (B) PFS at 3 months post-CAR-T by patients who obtained MRD <10−6 vs. MRD >10−6. (C) PFS by patients who achieved MRD <10−5 and PET/CT negativity compared with patients who have either MRD <10−5 or PET/CT positivity.
Lastly, 46 (85%) of the patients had CRS, nine (17%) had neurotoxicity, and 10 (19%) patients had MAS. Toxicities to CAR-T treatment such as cytokine release syndrome (CRS), neurotoxicity and macrophage activation syndrome were not associated with a better PFS (Supplementary Figures S1SA–C).
In our analysis, a ≥PR vs. <PR by IMWG was associated with an improved PFS but CR vs. VGPR/PR was not. Since most patients achieved ≥PR with CAR-T therapy, clinical and radiographic markers which correlate with better PFS are needed. Although the IMWG response criteria are the standard with which responses to myeloma treatment is graded, the criteria rely on changes in M-protein. M-protein reduction by 90% and 50% which represents the cutoff for VGPR and PR respectively, was not associated with PFS in CAR-T treated patients, neither was the M-protein reduction during the first 6 months post-CAR-T. The M-protein has a long half-life of 23 days (16). The slow clearance kinetics may limit its ability to predict PFS in the evaluation of patients whose disease has been rapidly eliminated via CAR-T therapy.
Instead, elevated serum free light chains at 15 days or 1-month post-CAR-T therapy were associated with worse PFS. Unlike the M-protein, SFLC have a half-life of only 2–6 h (17). Thus, the rapid clearance of SFLC may allow it to more closely mirror the myeloma burden in the body. The kinetics of M-protein and SFLC reductions with CAR-T therapy differ from studies with novel agents (18) and traditional chemotherapy (19), likely due to its unique mechanism of action.
Regarding methodology in our study, all the MRD assessments have been performed by NGS. These data should be extrapolated to MFC assessment, always that a 10−5 sensitivity was achieved. In the future, comparing the differences should be of interest to achieve MRD negativity between different CAR-Ts.
Our study is limited by its retrospective nature and its small sample size. Furthermore, patients in this study all had normal to near-normal kidney function, and these findings might not be generalizable to patients with renal impairment in a real-world setting, since kidney function affects light chain clearance.
In conclusion, for MM patients treated with BCMA CAR-T therapy, achieving a nonelevated iFLC as early as 15 days or 1 month, and MRD-NGS negativity with PET/CT negativity were strongly associated with a favorable PFS. Furthermore, larger studies are needed to establish the role of these markers in relation to the conventional IMWG criteria for response assessment in MM patients on CAR-T therapies.
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
The studies involving human participants were reviewed and approved by UCSF. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
SW, JW, and J-ML designed the study, had full access to all of the data in the study, and wrote the paper. NS, GL, TM, and JW did the data collection. AS, SW, JM-L, and C-YH performed the analysis. All authors critically revised the manuscript and gave final approval for the version to be published.
J-ML: speaking bureau of Adaptive and owner of shares of Altum sequencing and HOSEA. JW: consultant for Adaptive. J-ML: Honorarium and speaking bureau from BMS, Janssen, Glaxo, and Novartis. SW: Bristol-Myers Squib (research), Fortis (research), Janssen (research), Genentech (research), GSK (research), Caelum (research), Sanofi (advisory board), Telix (advisory board), Amgen (consultant), Dren (consultant).
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
JM-L was supported by CRIS foundation.
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.783703/full#supplementary-material
1. Raje N, Berdeja J, Lin Y, Siegel D, Jagannath S, Madduri D, et al. Anti-BCMA CAR T-Cell Therapy Bb2121 in Relapsed or Refractory Multiple Myeloma. N Engl J Med (2019) 380(18):1726–37. doi: 10.1056/NEJMoa1817226
2. Yan Z, Cao J, Cheng H, Qiao J, Zhang H, Wang Y, et al. A Combination of Humanised Anti-CD19 and Anti-BCMA CAR T Cells in Patients With Relapsed or Refractory Multiple Myeloma: A Single-Arm, Phase 2 Trial. Lancet Haematology (2019) 6(10):e521–9. doi: 10.1016/S2352-3026(19)30115-2
3. Brudno JN, Maric I, Hartman SD, Rose JJ, Wang M, Lam N, et al. T Cells Genetically Modified to Express an Anti-B-Cell Maturation Antigen Chimeric Antigen Receptor Cause Remissions of Poor-Prognosis Relapsed Multiple Myeloma. J Clin Oncol (2018) 36(22):2267–80. doi: 10.1200/JCO.2018.77.8084
4. Cohen AD, Garfall AL, Stadtmauer EA, Melenhorst JJ, Lacey SF, Lancaster E, et al. B Cell Maturation Antigen-Specific CAR T Cells Are Clinically Active in Multiple Myeloma. J Clin Invest (2019) 129(6):2210–21. doi: 10.1172/JCI126397
5. Garfall AL, Maus MV, Hwang WT, Lacey SF, Mahnke YD, Melenhorst JJ, et al. Chimeric Antigen Receptor T Cells Against CD19 for Multiple Myeloma. N Engl J Med (2015) 373(11):1040–7. doi: 10.1056/NEJMoa1504542
6. Garfall AL, Stadtmauer EA, Hwang WT, Lacey SF, Melenhorst JJ, Krevvata M, et al. Anti-CD19 CAR T Cells With High-Dose Melphalan and Autologous Stem Cell Transplantation for Refractory Multiple Myeloma. JCI Insight (2018) 3(8):e120505. doi: 10.1172/jci.insight.120505
7. Zhao WH, Liu J, Wang BY, Chen YX, Cao XM, Yang Y, et al. A Phase 1, Open-Label Study of LCAR-B38M, a Chimeric Antigen Receptor T Cell Therapy Directed Against B Cell Maturation Antigen, in Patients With Relapsed or Refractory Multiple Myeloma. J Hematol Oncol (2018) 11(1):141. doi: 10.1186/s13045-018-0681-6
8. Roex G, Timmers M, Wouters K, Campillo-Davo D, Flumens D, Schroyens W, et al. Safety and Clinical Efficacy of BCMA CAR-T-Cell Therapy in Multiple Myeloma. J Hematol Oncol (2020) 13(1):164. doi: 10.1186/s13045-020-01001-1
9. Cho SF, Anderson KC, Tai YT. Targeting B Cell Maturation Antigen (BCMA) in Multiple Myeloma: Potential Uses of BCMA-Based Immunotherapy. Front Immunol (2018) 9:1821. doi: 10.3389/fimmu.2018.01821
10. Timmers M, Roex G, Wang Y, Campillo-Davo D, Van Tendeloo VFI, Chu Y, et al. Chimeric Antigen Receptor-Modified T Cell Therapy in Multiple Myeloma: Beyond B Cell Maturation Antigen. Front Immunol (2019) 10:1613. doi: 10.3389/fimmu.2019.01613
11. Wood B, Wu D, Crossley B, Dai Y, Williamson D, Gawad C, et al. Measurable Residual Disease Detection by High-Throughput Sequencing Improves Risk Stratification for Pediatric B-ALL. Blood (2018) 131(12):1350–9. doi: 10.1182/blood-2017-09-806521
12. Martinez-Lopez J, Lahuerta JJ, Pepin F, Gonzalez M, Barrio S, Ayala R, et al. Prognostic Value of Deep Sequencing Method for Minimal Residual Disease Detection in Multiple Myeloma. Blood (2014) 123(20):3073–9. doi: 10.1182/blood-2014-01-550020
13. Martinez-Lopez J, Wong SW, Shah N, Bahri N, Zhou K, Sheng Y, et al. Clinical Value of Measurable Residual Disease Testing for Assessing Depth, Duration, and Direction of Response in Multiple Myeloma. Blood Adv (2020) 4(14):3295–301. doi: 10.1182/bloodadvances.2020002037
14. Kumar S, Paiva B, Anderson KC, Durie B, Landgren O, Moreau P, et al. International Myeloma Working Group Consensus Criteria for Response and Minimal Residual Disease Assessment in Multiple Myeloma. Lancet Oncol (2016) 17(8):e328–46. doi: 10.1016/S1470-2045(16)30206-6
15. Lee DW, Santomasso BD, Locke FL, Ghobadi A, Turtle CJ, Brudno JN, et al. ASTCT Consensus Grading for Cytokine Release Syndrome and Neurologic Toxicity Associated With Immune Effector Cells. Biol Blood Marrow Transplant (2019) 25(4):625–38. doi: 10.1016/j.bbmt.2018.12.758
16. Kendrick F, Evans ND, Arnulf B, Avet-Loiseau H, Decaux O, Dejoie T, et al. Analysis of a Compartmental Model of Endogenous Immunoglobulin G Metabolism With Application to Multiple Myeloma. Front Physiol (2017) 8:149. doi: 10.3389/fphys.2017.00149
17. Mead GP, Carr-Smith HD, Drayson MT, Morgan GJ, Child JA, Bradwell AR. Serum Free Light Chains for Monitoring Multiple Myeloma. Br J Haematol (2004) 126(3):348–54. doi: 10.1111/j.1365-2141.2004.05045.x
18. Yan Y, Mao X, Liu J, Fan H, Du C, Li Z, et al. The Impact of Response Kinetics for Multiple Myeloma in the Era of Novel Agents. Blood Adv (2019) 3(19):2895–904. doi: 10.1182/bloodadvances.2019000432
Keywords: multiple myeloma, CAR-T, minimal residual disease, sFLC, PET-CT
Citation: Wong SW, Shah N, Ledergor G, Martin T, Wolf J, Shui AM, Huang C-Y and Martinez-Lopez J (2021) Early Dynamics and Depth of Response in Multiple Myeloma Patients Treated With BCMA CAR-T Cells. Front. Oncol. 11:783703. doi: 10.3389/fonc.2021.783703
Received: 26 September 2021; Accepted: 02 November 2021;
Published: 06 December 2021.
Edited by:
Angelo Maiolino, Federal University of Rio de Janeiro, BrazilReviewed by:
Carlos Fernandez De Larrea, Hospital Clínic de Barcelona, SpainCopyright © 2021 Wong, Shah, Ledergor, Martin, Wolf, Shui, Huang and Martinez-Lopez. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Joaquin Martinez-Lopez, am1hdGkwMUB1Y20uZXM=
Disclaimer: All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article or claim that may be made by its manufacturer is not guaranteed or endorsed by the publisher.
Research integrity at Frontiers

Learn more about the work of our research integrity team to safeguard the quality of each article we publish.