- 1Department of Surgery, Oncology and Gastroenterology, Section of Oncology and Immunology, University of Padova, Padova, Italy
- 2Immunology and Diagnostic Molecular Oncology Unit, Veneto Institute of Oncology (IOV), Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Padova, Italy
- 3Department of Neurosciences, Section of Otolaryngology, University of Padova, Treviso, Italy
- 4Department of Medical, Surgical and Health Sciences, Section of Otolaryngology, University of Trieste, Trieste, Italy
- 5Department of Medicine (DIMED), Section of Pathology, University of Padova, Padova, Italy
- 6Department of Neurosciences, Section of Otolaryngology, University of Padova, Padova, Italy
- 7Unit of Oral and Maxillofacial Surgery, Treviso Regional Hospital, Treviso, Italy
- 8Department of Medical, Surgical and Health Sciences, Section of Pathology, University of Trieste, Trieste, Italy
- 9Department of Medicine (DIMED), Section of Pathology, University of Padova, Treviso, Italy
- 10Department of ENT/Head and Neck Surgery, Queen Elizabeth University Hospital Birmingham, Birmingham, United Kingdom
- 11Unit of Cancer Epidemiology, Centro di Riferimento Oncologico di Aviano (CRO) Istituto di Ricovero e Cura a Carattere Scientifico (IRCCS), Aviano, Italy
Objective: To date, no useful prognostic biomarker exists for patients with oral squamous cell carcinoma (OCSCC), a tumour with uncertain biological behaviour and subsequent unpredictable clinical course. We aim to investigate the prognostic significance of two recurrent somatic mutations (-124 C>T and -146 C>T) within the promoter of telomerase reverse transcriptase (TERT) gene and the impact of TERT single nucleotide polymorphism (SNP) rs2853669 in patients surgically treated for OCSCC.
Methods: The genetic frequencies of rs2853669, -124 C>T and -146 C>T as well as the telomere length were investigated in 144 tumours and 57 normal adjacent mucosal (AM) specimens from OCSCC patients.
Results: Forty-five tumours harboured TERT promoter mutations (31.3%), with -124 C>T and -146 C>T accounting for 64.4% and 35.6% of the alterations respectively. Patients with -124 C>T TERT promoter mutated tumours had the shortest telomeres in the AM (p=0.016) and showed higher risk of local recurrence (hazard ratio [HR]:2.75, p=0.0143), death (HR:2.71, p=0.0079) and disease progression (HR:2.71, p=0.0024) with the effect being potentiated by the co-occurrence of T/T genotype of rs2853669.
Conclusion: -124 C>T TERT promoter mutation as well as the T/T genotype of the rs2853669 SNP are attractive independent prognostic biomarkers in patients surgically treated for OCSCC, with the coexistence of these genetic variants showing a synergistic impact on the aggressiveness of the disease.
Introduction
With a worldwide estimated age-standardized incidence rate of 4.0 per 100,000 and an estimated number of new cases in 2018 of 354,864, oral cavity squamous cell carcinoma (OCSCC) is the most common carcinoma developing from the epithelial lining of the upper aero-digestive tract (UADT), thus representing an important burden on health care (1).
Based on the histopathological stage, OCSCC can exhibit an unpredictable behaviour with a fraction of patients with early-stage cancer suffering from poor prognosis (2). Patients curatively treated for OCSCC have indeed a high propensity to develop both recurrences and second field tumours (3). Thus, despite recent improvements in the management strategies of OCSCC, improvements in outcomes have been modest (4).
High-risk human papillomaviruses (HPVs), responsible for more than 50% of oropharyngeal squamous cell carcinomas (SCCs) and robust prognostic biomarkers in risk-stratifying in individuals with these malignancies (5), play only a marginal role in OCSCC (6). Thus, since not all OCSCCs are attributable to tobacco and alcohol exposure, the aetiopathogenesis of these neoplasms remains unknown in several cases and no reliable biomarker capable of stratifying the prognosis of OCSCC exists. It is, therefore, of paramount importance to identify biomarkers and molecular signatures predicting cancer relapse that may guide surveillance follow-up strategies and adjuvant treatments.
The infinite proliferation of malignant cells is a hallmark of oncogenesis and telomere/telomerase interplay dictates cell replicative capacity. Telomerase is indeed usually repressed in normal somatic cells, but it is detectable in the vast majority of tumours (7, 8). By synthesizing the telomere sequences and thus preventing cell senescence and apoptosis, the inappropriate activation of the catalytic component of the telomerase, telomerase reverse transcriptase (TERT), appears crucial for maintaining cellular replicative capacity and allowing tumour formation (9). Furthermore, through its non-canonical extra-telomeric functions, the re-activation of telomerase in cancer cells may affect cancer progression and metastasis (10, 11). These properties make TERT a potentially attractive biomarker in cancer.
Among the different mechanisms leading to the inappropriate reactivation of TERT in cancer, mutually exclusive recurrent C-to-T transitions at nucleotides 1,295,228 (-124 C>T) and 1,295,250 (-146 C>T) within the core promoter of TERT creating de novo binding sites for E-twenty-six (ETS) transcription factors and leading to increased TERT gene expression are particularly interesting: first, their prognostic role was consistently observed in several cancers (12), second, among SCCs of the UADT, TERT promoter mutations were observed to be topographically restricted to OCSCC (13), and third, unlike assessing TERT mRNA levels, TERT promoter mutations can be more easily analysed in formalin-fixed paraffin-embedded (FFPE) specimens from routinely collected biopsies.
Although a previous investigation conducted in a population of subject with OCSCC from Taiwan found that those harbouring the -124 C>T TERT promoter mutation had a worse prognosis, this was not statistically significant. However, current evidence suggests that the effect of TERT promoter mutations may be affected by a common single nucleotide polymorphism (SNP), rs2853669, within the TERT core promoter close to the hotspot mutation sites (14). The minor C-variant allele of the SNP disrupts a pre-existing ETS2 binding site at -245 bp in the TERT promoter region resulting in decreased TERT expression (15) and thus, counteracts the transactivation activity of the TERT promoter hotspots (14). A meta-analysis reports that among cancer patients with TERT promoter mutations, the rs2853669 T/T genotype confers a worse prognosis (16), but the modifying role of this SNP in the prognostic value of TERT promoter mutations is still controversial (12, 17–20). To date, the prognostic value of rs2853669 in OCSCC remains to be elucidated.
Thus, the main aims of this study were to investigate the prevalence and the clinical significance of TERT promoter mutations and the impact of the TERT rs2853669 SNP in a larger series of patients surgically treated for OCSCC.
Materials and Methods
Patients and Tissue Samples
This is a multi-centre retrospective observational study conducted with the approval of the ethics committee of Treviso/Belluno provinces (Ethic vote: 346/AULSS9) and was performed in a cohort of 144 consecutive patients diagnosed with OCSCC from February 1, 2010 to September 30, 2018, who underwent up-front surgery with/without adjuvant (chemo)radiotherapy, whose samples were available for analysis. All patients gave their informed consent. The study network included three University Hospitals in Northeast Italy, located in Padova, Treviso, and Trieste.
Patients were routinely followed-up [median follow-up time: 43 months; interquartile range (IQR), 28-75 months] according to consensus guidelines with endoscopic examination of the upper aero-digestive tract every 1–3 months for the first year, 3–4 months during the second year, 4–6 months during the 3rd year, and every 6 months thereafter. A dedicated CT scan of the chest was performed annually. Additional dedicated head and neck imaging was arranged based on clinical features and local protocol.
Data for 27 OCSCC samples (tumour tissue, adjacent mucosa and patient characteristics) were available from our previous study (13). One hundred and seventeen specimens were FFPE. Estimations of tumour cell content on FFPE OCSCC sections were made by a trained pathologist. When macrodissection was necessary for enrichment in neoplastic cells, the pathologist marked tumour areas on haematoxylin and eosin-stained tissue slides; the corresponding areas were scraped from four to five serial FFPE sections of 10 μm thickness. Adjacent mucosa from 30 of 117 FFPE specimens was analysed in samples from tumours with negative/clear margins, and the stroma immediately adjacent to the neoplastic epithelium was left as a border zone. DNA from FFPE specimens was extracted using the QIAmp DNA mini kit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions.
TERT Promoter Analysis and Telomere Length Measurement
Genomic DNA amplification for TERT promoter region (260 bp) containing -124 C>T and -146 C>T mutation sites, as well as the SNP rs2853669 (-245 T>C), was performed exactly as previously described (21). The amplified products were purified with the Illustra ExoProStar (GE Healthcare, Buckinghamshire, UK) and sequenced on a 3730xl DNA analyzer (Applied Biosystems, Foster City, CA, USA). All samples were analysed in forward and reverse directions.
Telomere length was determined by multiplex PCR assay as previously described (22). Relative telomere length (RTL) values were calculated as telomere/single-copy gene ratio, as previously described (23).
Statistical Analysis
Differences in socio-demographic and clinical characteristics according to TERT promoter were tested through Fisher’s exact test. For each patient, person-time at risk was computed from the date of diagnosis to the event date or the date of last follow-up, whichever came first. Events were defined as death for overall survival (OS), death or recurrence at any site for progression-free survival (PFS), local recurrence for mucosal control, and lymph node recurrence for regional failure. Analyses were truncated at 5 years. The association between TERT promoter and oncological outcomes was evaluated using the Kaplan-Meier method, and difference in survival probabilities was evaluated using the log-rank test (24). To account for competing risks, mucosal and regional control were evaluated using cumulative incidence, and differences according to strata were tested using Gray’s test (25). The risk of unfavourable oncological outcome was evaluated using the Cox proportional hazards model (24); multivariable hazard ratios (HR), and corresponding 95% confidence intervals (CI), were calculated adjusting for gender, age, pathological lymph node status (pN), grading, surgical margins, and extracapsular invasion. For mucosal and regional control, HR were adjusted for competing risk according to Fine-Gray model (25).
Results
Demographic and Clinical Characteristics of Patients
The clinical characteristics of the patients are summarized in Table 1. Globally, the study group had a median age of 65 years (IQR, 54-74 years) at presentation and included 81 (56.2%) male and 63 (43.8%) female patients (Table 1). The majority of patients were ever smoking (61.8%) and never drinking (58.3%). Tumour sub-sites within the oral cavity were as follows: 54.2% (78/144) in the tongue, 15.3% (22/144) in the floor of the mouth, 10.4% (15/144) in both the gingiva and the buccal mucosa, and 9.7% (14/144) in other sub-sites including the lip, the hard palate and the retromolar trigone. Pathological stage was T1-T2 in 99 cases (68.7%) and T3-T4 in 45 (31.3%); 47 (32.6%) of the cases had clinically positive regional lymph nodes and 97 cases (67.4%) were N0; collectively, 69 (47.9%) had advanced disease at diagnosis. Nearly 75% of tumours (104/139) showed G1-G2 grading and 25.2% (35/139) were G3. Close/positive surgical margins and positive extra-capsular spread were present in 21 (14.6%) and 16 (11.1%) cases, respectively (Table 1).
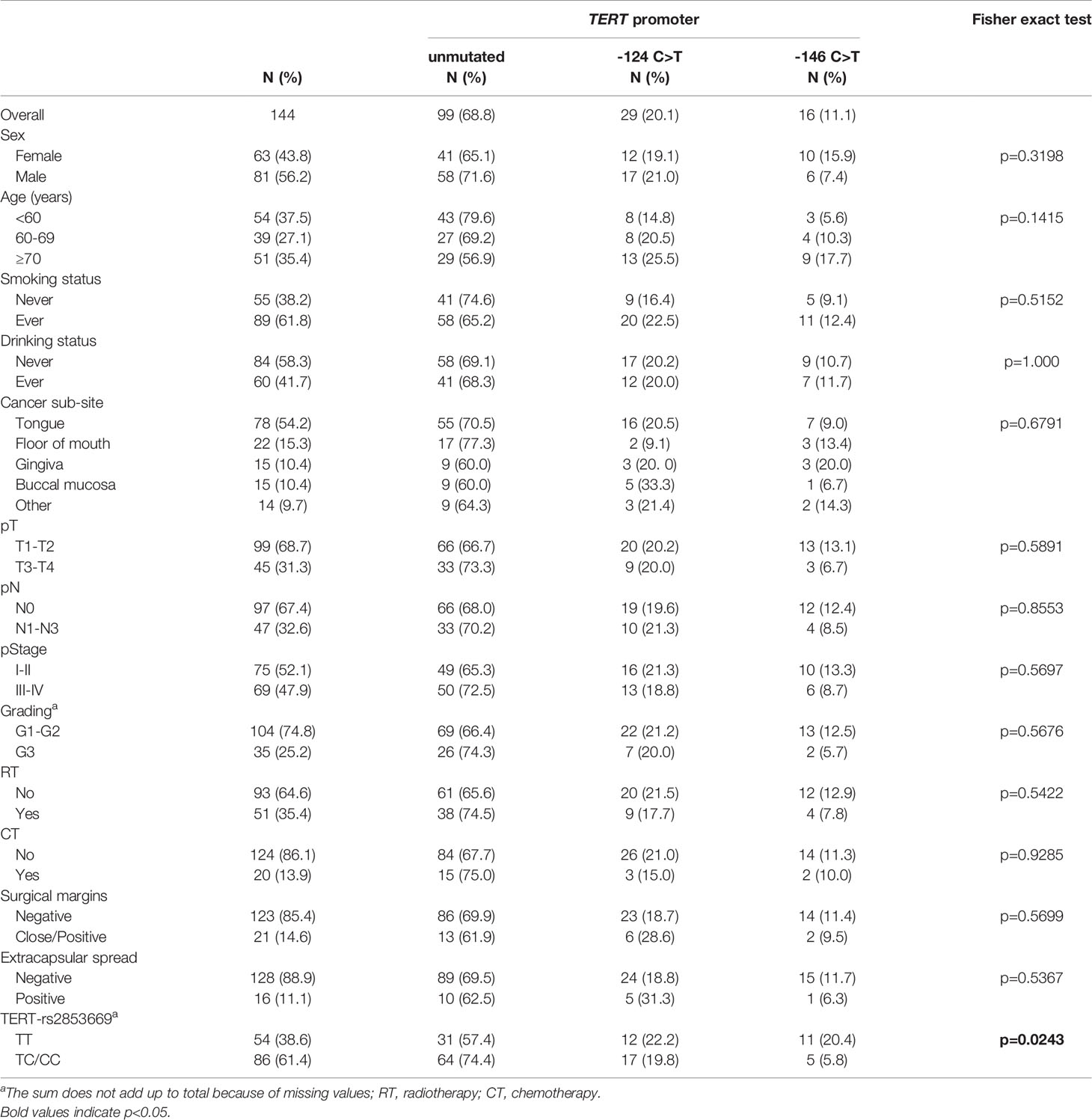
Table 1 Distribution of 144 patients with oral cavity squamous cell carcinoma (OCSCC) according to socio-demographic and clinical characteristics, by TERT promoter mutational status.
TERT Promoter Status
The distribution of TERT promoter mutations according to socio-demographic and clinical characteristics of patients are shown in Table 1. In the overall cohort, the promoter of TERT harboured mutations in 45/144 cases (31.3%). The TERT -124 C>T mutation was more common (29/144, 20.1%) than -146 C>T (16/144, 11.1%). These two mutations occurred in a mutually exclusive manner and with a heterozygous genotype. No mutations were observed in any of 57 adjacent available analysed mucosal specimens, 16 of which were surrounding mutated tumours. There was no statistically significant difference among analysed parameters with regard to TERT promoter mutation rate. We also genotyped 140 of 144 patients of our cohort for the rs2853669 SNP at -245 bp. A total of 86 patients (61.4%) carried the minor C-variant allele, for which 16 patients were homozygous and 70 were heterozygous. Fifty-four patients (38.6%) had the T/T genotype. Notably, patients with TERT promoter mutated tumours had a higher prevalence of the T/T genotype than patients with unmutated TERT promoter (p=0.0243) (Table 1).
Telomere Length
Measurement of RTL was obtained from 132 tumour tissues and 57 surrounding mucosal specimens. Values ranged between 0.42 and 4.42 (median 1.29) in tumours and between 0.62 and 2.93 (median 1.18) in surrounding mucosa; neither correlated with the age (data not shown). Telomere length in tumour cells and surrounding mucosa were not significantly associated with any of the measured demographic or clinical characteristics (Supplementary Table 1). In keeping with our previous findings (13), we found that the mucosa adjacent to tumours harbouring TERT promoter mutations had significantly shorter telomeres than those in adjacent mucosa of cancers with unmutated TERT promoter (p=0.017) (Figure 1A). In particular, the surrounding mucosa adjacent to tumours with -124 C>T mutated TERT promoter showed the shortest telomeres (p=0.016; Figure 1B), despite these patients being younger [median (IQR), 64(58–70) years] than those with unmutated tumours [median (IQR), 67(48–74) years] or with tumours harbouring -146 C>T mutations [median (IQR), 75(71–80) years] (p for age=0.065; data not shown). Conversely, telomere length in tumour tissue did not significantly differ according to the mutational status of TERT promoter (p=0.1182) (Figures 1A, B).
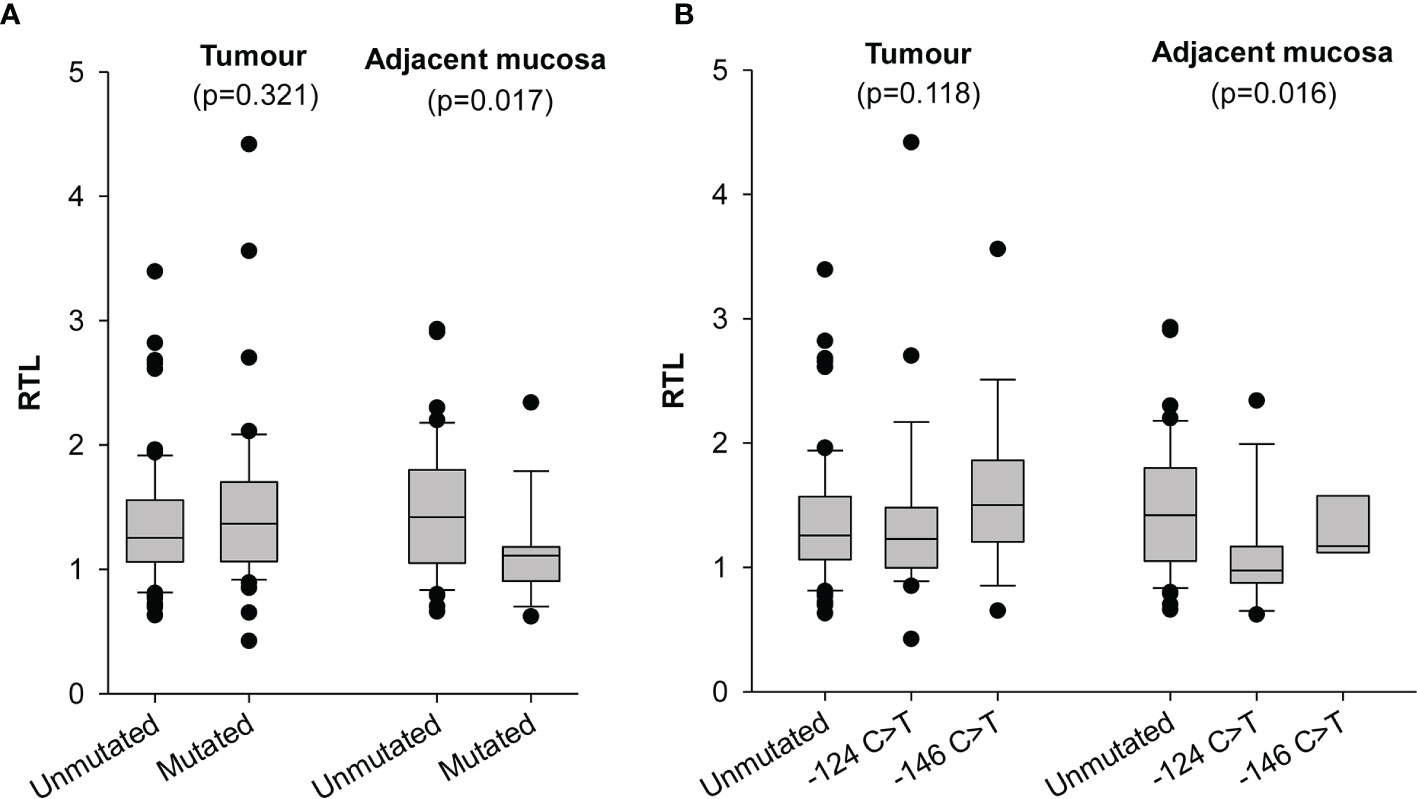
Figure 1 Distribution of relative telomere length (RTL) in tumour and adjacent mucosa according to TERT promoter status. (A) samples were stratified according to absence (Unmutated) and presence of -124 C>T or -146 C>T mutations (Mutated) in the TERT promoter region. (B) samples were stratified according to TERT promoter status in absence (Unmutated), presence of -124 C>T and presence of -146 C>T mutations in the TERT promoter region.
Time-To-Event Analysis
The associations between socio-demographic and clinical characteristics of patients with clinical outcome are summarized in Supplementary Table 2. In a multivariate analysis adjusted for clinical variables (gender, age, pN, grading, surgical margins, and extracapsular invasion), it emerged that buccal mucosa sub-site, pathological lymph nodes, and G3 grading were significantly associated with increased risk of death (HR: 5.96, 95% CI: 1.16-30.73; p=0.0328; HR: 2.30, 95% CI: 1.12-4.75; p=0.0237; HR: 2.28, 95% CI: 1.14-4.56; p=0.0195; respectively).
In order to identify the potential impact of TERT promoter mutations on oncological outcome, we first investigated the association between TERT promoter status with PFS. Kaplan-Meier survival curve showed that the 5-year PFS for patients harbouring the -124 C>T mutation was 42.4% as opposed to 64.3% for patients without mutations, 68.2% for those harbouring the -146 C>T mutation (p=0.0069; Figure 2B). This association was confirmed by multivariate analysis (Table 2) after adjustment for clinical variables with a HR for progression of 2.71 (95% CI: 1.42-5.17; p=0.0024).
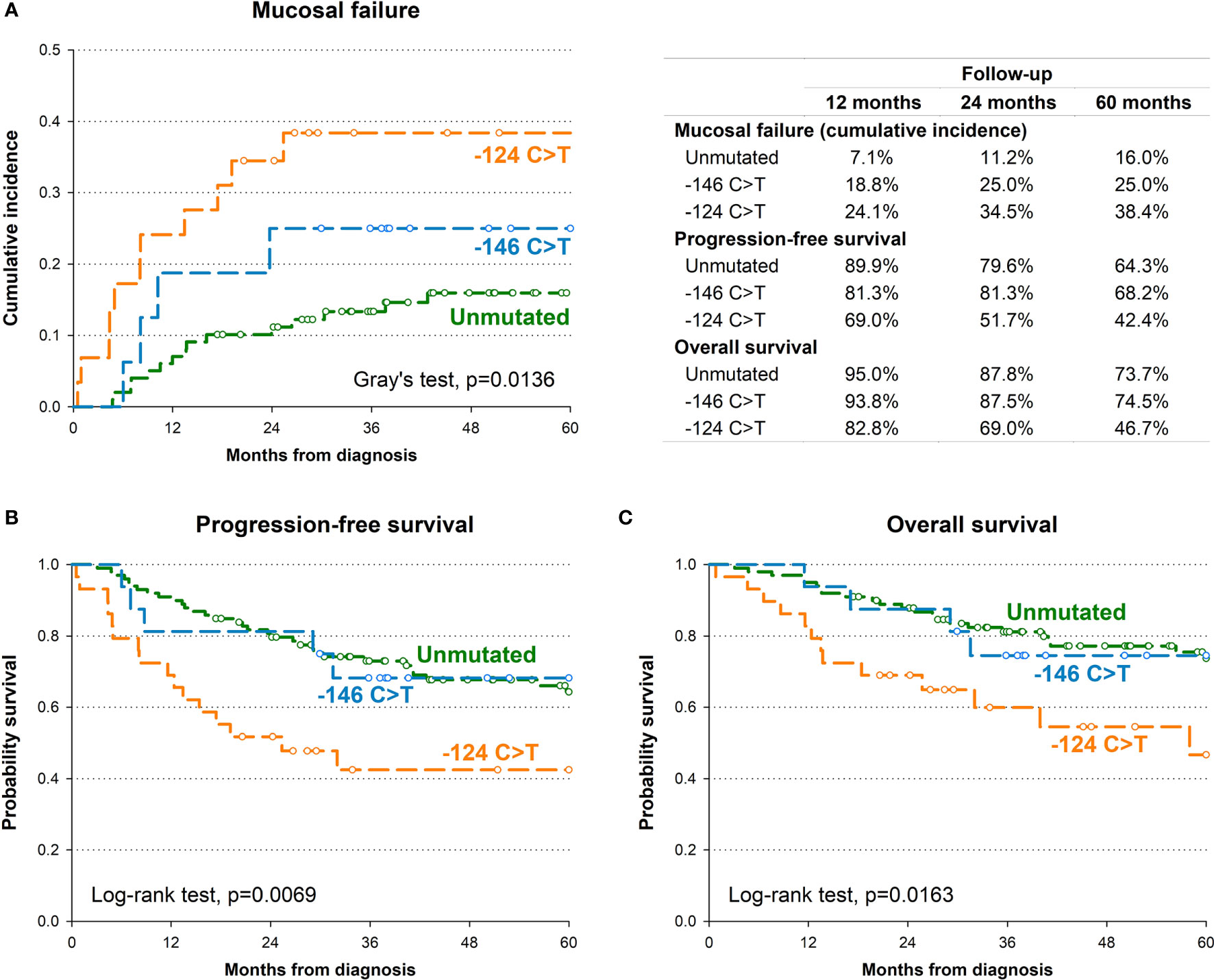
Figure 2 Kaplan-Meier estimates of cumulative incidence of mucosal recurrence (A), progression-free survival (B) and overall survival (C) by TERT promoter.
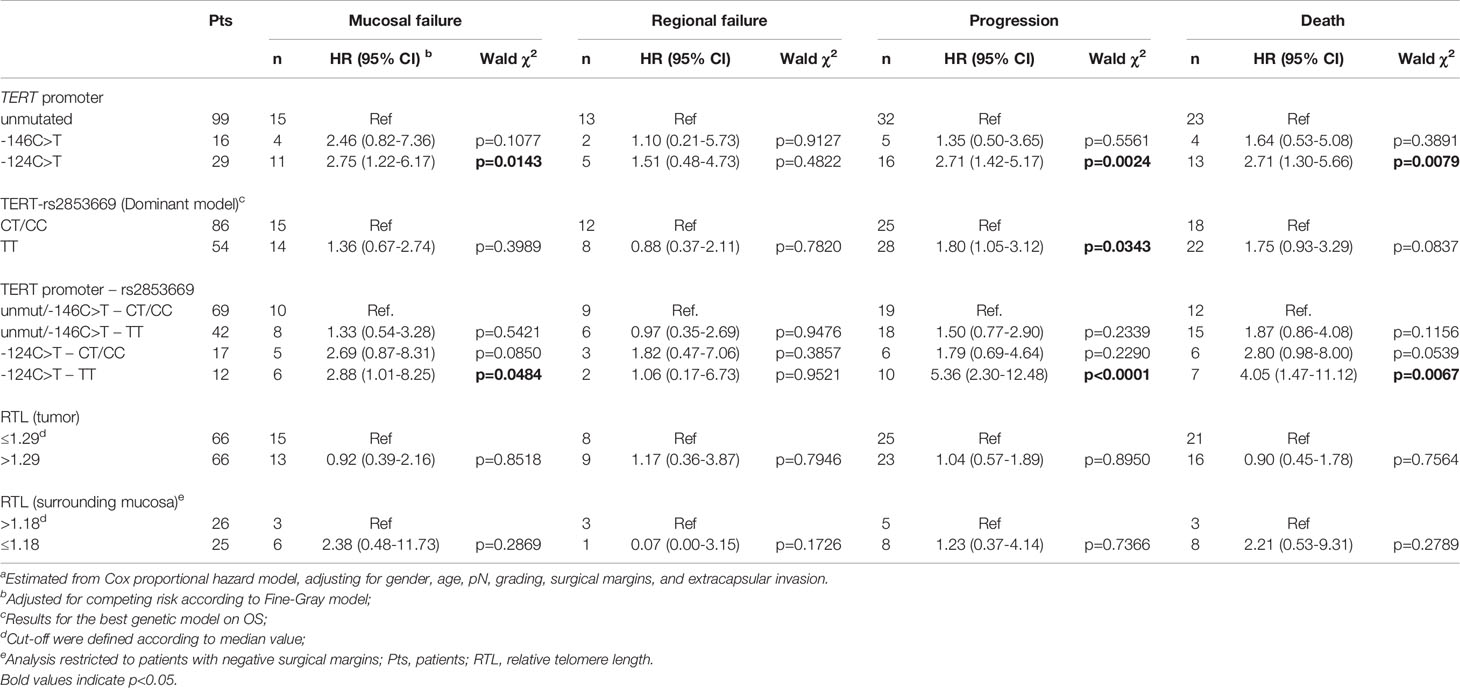
Table 2 Hazard ratio (HR) and corresponding 95% confidence interval (CI)a for mucosal failure, regional failure, progression, and death according to strata of TERT promoter status, rs2853669 genotype and telomere length.
The presence of the -124 C>T mutation was also consistently associated with shorter OS, with 46.7% of patients alive after 5 years, in comparison to 73.7% and 74.5% of patients without mutations or harbouring the -146 C>T mutation, respectively (p=0.0163; Figure 2C). Multivariate analyses confirmed the negative effect of the -124 C>T mutation on prognosis, with a HR of death of 2.71 (95% CI: 1.30-5.66; p=0.0079) (Table 2). The negative impact of the -124 C>T mutation on clinical outcome was likely due to poorer mucosal control; indeed, based upon cumulative incidence estimates, patients with tumours harbouring this mutation suffered a 5-year mucosal failure rate of 38.4% in comparison to 16% and 25% in patients without mutations or harbouring the -146 C>T mutation, respectively (p=0.0136; Figure 2A). This association remained statistically significant in the multivariate analysis (HR: 2.75, 95% CI: 1.22-6.17; p=0.0143) (Table 2). These results suggest that the -124 C>T point mutation may be a risk factor for the aggressiveness of OCSCC compared to the -146 C>T mutation and unmutated TERT promoters which appear to be associated with a more favourable clinical outcome. Notably, the surrounding mucosa adjacent to tumours with -124 C>T mutated TERT promoter had the shortest telomeres (p=0.016; Figure 1B), and, in line with our previous studies (26, 27), adjacent mucosa with shorter telomeres (below the median value) showed a high, albeit not significant risk of tumour relapse (Table 2).
Kaplan-Meier survival analysis revealed that carriers of the T/T rs2853669 genotype showed significantly worse PFS (p=0.008) and OS (p=0.021) compared with C carriers (T/C+C/C genotypes) (Supplementary Figure 1B, C). The negative impact of the T/T genotype was confirmed in the multivariate analysis for progression (HR: 1.80, 95% CI: 1.05-3.12; p=0.0343) but not for death (HR: 1.75, 95% CI: 0.93-3.29; p=0.0837) (Table 2).
To evaluate if the SNP rs2853669 genotype can modulate the effect of TERT promoter mutations on oncological outcome, the potential role of the -124 C>T TERT promoter mutation as a prognostic parameter in OCSCC patients was assessed according to their rs2853669 background. Multivariate analysis revealed that the risk of mucosal failure (HR: 2.88, 95% CI: 1.01-8.25; p=0.0484), progression (HR: 5.36, 95% CI: 2.30-12.48; p<0.0001) and death (HR: 4.05, 95% CI: 1.47-11.12; p=0.0067) were significantly increased in patients with -124 C>T mutated tumours carrying the T/T genotype of the rs2853669 (Table 2) compared to patients without this mutation, and C carriers of the SNP.
Discussion
In the present investigation, we observed that approximately one-third of OCSCC samples harboured TERT promoter mutations with the -124 C>T mutation having a significant adverse impact on the outcome; particularly, when coexisting with the T/T genotype of rs2853669, -124 C>T mutation increased the risk of death by 4 times.
In the literature, the frequency of TERT promoter mutations in OCSCC varies significantly among studies ranging from 30.4 to 75% (13, 28–34). This variability could be attributable to different patient population characteristics or methodological approaches. In our cohort, we found 31.3% (45 of 144) of OCSCC samples harboured TERT promoter mutations, which was in line with other studies (13, 28, 30, 33, 34). In agreement with other studies on OCSCC (20, 28–32), the two mutations have different frequency, with a higher prevalence of -124 C>T (29 of 144) compared to -146 C>T (16 of 144).
With respect to oncological outcomes, an important finding emerging from this study is that the two somatic TERT promoter mutations displayed different behaviour. Indeed, while patients with the -124 C>T TERT promoter mutation had a higher risk of mucosal failure and poorer DFS and OS, patients with tumours harbouring the -146 C>T mutation had an improved clinical outcome, similar to those with unmutated TERT promoter. The recruitment of the transcription factor GABPA, a member of ETS family, specifically to mutant TERT promoters mediates long-range chromatin interaction and enrichment of active histone marks, and hence drives TERT transcription (35). Although both the -124 C>T and -146 C>T mutations generate identical sequences, enable binding of GABPA transcription factors, and are equally efficient in increasing TERT transcription in vitro (36), previous reports demonstrated that these mutations are not functionally identical. Indeed, a peculiar pathway of activation by non-canonical NF-ĸB signalling was only described for the -146 C>T mutation (37, 38). In addition, in vivo, the -124 C>T mutation was associated with higher TERT expression/telomerase activity compared to -146 C>T (39, 40). A significant body of evidence has demonstrated that high levels of tumour TERT expression and/or telomerase activity are significantly associated with aggressiveness of disease, advanced clinical stage, and poor OS and/or DFS in several types of tumours, including UADT SCC (13, 26, 27, 41). The mechanism(s) by which high TERT expression ultimately facilitates cancer progression and constitutes a prognostic factor are not completely elucidated, and seems not be attributable only to TERT’s ability to maintain telomere length. Indeed, accumulating evidence suggests that TERT may also contribute to carcinogenesis via telomere length-independent mechanisms, including enhancement of proliferation, resistance to apoptosis, inflammation, invasion and metastasis altogether contributing towards a more aggressive phenotype of cancer cells (10, 11, 42–50). Therefore, it is conceivable that the -124 C>T TERT promoter mutation, inducing higher expression of TERT in the tumour, results in the increased severity of disease as we observed in our cohort of OCSCC patients. Corroborating our results, Arantes et al. (33) found that the -124 C>T TERT promoter mutation was associated with increased risk of tumour relapse and death in a cohort of 88 Brazilian patients with SCC of the UADT. However, other studies in different tumour types have reported contradicting clinical effects of TERT promoter mutations, ranging from poorer survival associated with the -146 C>T TERT promoter mutation to unchanged clinical outcome (28, 29, 32, 51–54). Given that the two mutations create an identical sequence corresponding to a de novo binding site for ETS transcription factors, these alternative results may depend on the genetic context, including the SNP background in which TERT mutations arise.
For the common polymorphism rs2853669 T>C, which disrupts a pre-existing ETS2 binding site within the TERT core promoter, controversial clinical impacts have been reported (12, 17–20). Our study demonstrates for the first time that the rs2853669 T/T genotype influences the clinical outcome of OCSCC patients, being significantly associated with increased risk of disease progression. Importantly, the coexistence of the T/T genotype of rs2853669 and the -124 C>T TERT promoter mutation is associated with a significantly poorer prognosis including mucosal failure, disease progression and death. The effect of the rs2853669 SNP may be related to higher telomerase activity and TERT expression conferred by the T/T genotype (15) that can also additionally intensify the transactivation activity of TERT promoter mutations (14). Thus, we can speculate that high TERT levels conferred by the -124 C>T TERT promoter mutation and/or rs2853669 T/T genotype may promote tumour progression, probably as a consequence of the extra-telomeric non-canonical functions of telomerase. Unfortunately, we did not have enough tumour material to contemporaneously analyse TERT promoter status and TERT expression/activity, and further studies should be undertaken to extend and validate these findings.
A secondary finding of our study was the absent of TERT promoter mutations in the matched adjacent mucosa. This partly differs from a previous study by Chang et al. (29), and may be due to the reduced number of adjacent normal mucosal specimens available in our cohort. Nonetheless, the finding that metastatic and recurrent head and neck squamous cell carcinomas have more TERT promoter mutations compared to primary tumours (31) suggests that the acquisition of these mutations is a late event in carcinogenesis, and may explain the lack of TERT promoter mutations in the tumour’s adjacent mucosa.
Interestingly, we confirmed that telomeres in mucosa adjacent to TERT promoter mutated tumours were significantly shorter than those adjacent to tumours retaining unmutated TERT promoter (13), and additionally we found that the mucosa adjacent to -124 C>T mutated tumours had the shortest telomeres. As critically short telomeres are a hallmark of genomic instability associated to carcinogenesis and may be considered a marker of field cancerization (26, 27), is not surprising that patients harbouring the -124 C>T TERT promoter mutation showed a significantly increased risk of tumour relapse. These data, for the first time, support a prognostic role for tumour relapse of the -124 C>T TERT promoter mutation in patients with OCSCC likely related to the very short telomeres in the mucosa surrounding the tumour in which the mutation arises.
In conclusion, we found that the -124 C>T TERT promoter mutation, as well as the T/T genotype of the rs2853669 SNP, may be a risk factor for the aggressiveness of OCSCC, and the coexistence of these genetic variations might represent a greater risk of adverse outcome. Supported by the fact that the clinical significance of this mutation is consistent with the biological properties of TERT, that TERT promoter mutations were found to stratify the prognosis in several other cancers, are easy to identify using tissue from routinely collected biopsies and address the unmet clinical need of having a validated prognostic marker for OCSCC, our observations raise the possibility that the -124 C>T TERT promoter mutation in combination with the SNP rs2853669 T/T genotype may serve as a valuable prognostic marker in this cancer, with the ability to guide therapeutic and follow-up strategies.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by the Treviso/Belluno provinces (protocol code 346/AULSS9, March 10, 2015), Italy. The patients/participants provided their written informed consent to participate in this study.
Author Contributions
Conceptualization: ADR, PBR. and SG. Methodology: SG. Statistical analysis: JP. Investigation: SG, PBR, ER, GT, LA, RDC, MR, PN, AM, VC, MT, LB, GS, EE, MMa, MS, RB, APDT, MG, MMo, and GE. Resources: ADR. Writing-original draft preparation: SG and PBR. Writing-review and editing: SG, PBR, JF, and ADR. Supervision: ADR. Project administration: PBR and SG. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2021.782658/full#supplementary-material
References
1. Cancer Today. Available at: http://gco.iarc.fr/today/home (Accessed December 10, 2020).
2. Arora A, Husain N, Bansal A, Neyaz A, Jaiswal R, Jain K, et al. Development of a New Outcome Prediction Model in Early-Stage Squamous Cell Carcinoma of the Oral Cavity Based on Histopathologic Parameters With Multivariate Analysis: The Aditi-Nuzhat Lymph-Node Prediction Score (ANLPS) System. Am J Surg Pathol (2017) 41:950–60. doi: 10.1097/PAS.0000000000000843
3. Brands MT, Smeekens EAJ, Takes RP, Kaanders JHAM, Verbeek ALM, Merkx MAW, et al. Time Patterns of Recurrence and Second Primary Tumors in a Large Cohort of Patients Treated for Oral Cavity Cancer. Cancer Med (2019) 8:5810–19. doi: 10.1002/cam4.2124
4. van Dijk BA, Brands MT, Geurts SM, Merkx MA, Roodenburg JL. Trends in Oral Cavity Cancer Incidence, Mortality, Survival and Treatment in the Netherlands. Int J Cancer (2016) 139:574–83. doi: 10.1002/ijc.30107
5. Paderno A, Morello R, Piazza C. Tongue Carcinoma in Young Adults: A Review of the Literature. Acta Otorhinolaryngol Ital (2018) 38:175–80. doi: 10.14639/0392-100X-1932
6. Castellsagué X, Alemany L, Quer M, Halec G, Quirós B, Tous S, et al. HPV Involvement in Head and Neck Cancers: Comprehensive Assessment of Biomarkers in 3680 Patients. J Natl Cancer Inst (2016) 108:djv403. doi: 10.1093/jnci/djv403
7. Blackburn EH, Greider CW, Szostak JW. Telomeres and Telomerase: The Path From Maize, Tetrahymena and Yeast to Human Cancer and Aging. Nat Med (2006) 12:1133–8. doi: 10.1038/nm1006-1133
8. Shay JW, Wright WE. Role of Telomeres and Telomerase in Cancer. Semin Cancer Biol (2011) 21:349–53. doi: 10.1016/j.semcancer.2011.10.001
9. Hanahan D, Weinberg RA. Hallmarks of Cancer: The Next Generation. Cell (2011) 144:646–74. doi: 10.1016/j.cell.2011.02.013
10. Ségal-Bendirdjian E, Geli V. Non-Canonical Roles of Telomerase: Unraveling the Imbroglio. Front Cell Dev Biol (2019) 7:332. doi: 10.3389/fcell.2019.00332
11. Martínez P, Blasco MA. Telomeric and Extra-Telomeric Roles for Telomerase and the Telomere-Binding Proteins. Nat Rev Cancer (2011) 11:161–76. doi: 10.1038/nrc3025
12. Hafezi F, Perez Bercoff D. The Solo Play of TERT Promoter Mutations. Cells (2020) 9:749. doi: 10.3390/cells9030749
13. Boscolo-Rizzo P, Giunco S, Rampazzo E, Brutti M, Spinato G, Menegaldo A, et al. TERT Promoter Hotspot Mutations and Their Relationship With TERT Levels and Telomere Erosion in Patients With Head and Neck Squamous Cell Carcinoma. J Cancer Res Clin Oncol (2020) 146:381–9. doi: 10.1007/s00432-020-03130-z
14. Park CK, Lee SH, Kim JY, Kim JE, Kim TM, Lee ST, et al. Expression Level of Htert is Regulated by Somatic Mutation and Common Single Nucleotide Polymorphism at Promoter Region in Glioblastoma. Oncotarget (2014) 5:3399–407. doi: 10.18632/oncotarget.1975
15. Hsu CP, Hsu NY, Lee LW, Ko JL. Ets2 Binding Site Single Nucleotide Polymorphism at the Htert Gene Promoter–Effect on Telomerase Expression and Telomere Length Maintenance in Non-Small Cell Lung Cancer. Eur J Cancer (2006) 42:1466–74. doi: 10.1016/j.ejca.2006.02.014
16. Shen N, Lu Y, Wang X, Peng J, Zhu Y, Cheng L. Association Between Rs2853669 in TERT Gene and the Risk and Prognosis of Human Cancer: A Systematic Review and Meta-Analysis. Oncotarget (2017) 8:50864–72. doi: 10.18632/oncotarget.15140
17. Rachakonda P, Hosen I, de Verdier PJ, Fallah M, Heidenreich B, Ryk C, et al. TERT Promoter Mutations in Bladder Cancer Affect Patient Survival and Disease Recurrence Through Modification by a Common Polymorphism. Proc Natl Acad Sci USA (2013) 110:17426–31. doi: 10.1073/pnas.1310522110
18. Ko E, Seo HW, Jung ES, Kim BH, Jung G. The TERT Promoter SNP Rs2853669 Decreases E2F1 Transcription Factor Binding and Increases Mortality and Recurrence Risks in Liver Cancer. Oncotarget (2016) 7:684–99. doi: 10.18632/oncotarget.6331
19. Simon M, Hosen I, Gousias K, Rachakonda S, Heidenreich B, Gessi M, et al. TERT Promoter Mutations: A Novel Independent Prognostic Factor in Primary Glioblastomas. Neuro Oncol (2015) 17:45–52. doi: 10.1093/neuonc/nou158
20. Batista R, Cruvinel-Carloni A, Vinagre J, Peixoto J, Catarino TA, Campanella NC, et al. The Prognostic Impact of TERT Promoter Mutations in Glioblastomas Is Modified by the Rs2853669 Single Nucleotide Polymorphism. Int J Cancer (2016) 139:414–23. doi: 10.1002/ijc.30057
21. Indraccolo S, Lombardi G, Fassan M, Pasqualini L, Giunco S, Marcato R, et al. Genetic, Epigenetic, and Immunologic Profiling of MMR-Deficient Relapsed Glioblastoma. Clin Cancer Res (2019) 25:1828–37. doi: 10.1158/1078-0432.CCR-18-1892
22. Gianesin K, Noguera-Julian A, Zanchetta M, Del Bianco P, Petrara MR, Freguja R, et al. Premature Aging and Immune Senescence in HIV-Infected Children. AIDS (2016) 30:1363–73. doi: 10.1097/QAD.0000000000001093
23. Rampazzo E, Bertorelle R, Serra L, Terrin L, Candiotto C, Pucciarelli S, et al. Relationship Between Telomere Shortening, Genetic Instability, and Site of Tumour Origin in Colorectal Cancers. Br J Cancer (2010) 102:1300–5. doi: 10.1038/sj.bjc.6605644
24. Kalbfleisch JD, Prentice RL. The Statistical Analysis of Failure Time Data. New York: Wiley (2002) 95–147. doi: 10.1002/9781118032985
25. Fine JP, Gray RJ. A Proportional Hazard Model for the Subdistribution of a Competing Risk. J Am Stat Ass (1999) 94:496–509. doi: 10.2307/2670170
26. Boscolo-Rizzo P, Rampazzo E, Polesel J, Giunco S, Menegaldo A, Mantovani M, et al. Predictive and Prognostic Significance of Telomerase Levels/Telomere Length in Tissues and Peripheral Blood in Head and Neck Squamous Cell Carcinoma. Sci Rep (2019) 9:17572. doi: 10.1038/s41598-019-54028-x
27. Boscolo-Rizzo P, Rampazzo E, Perissinotto E, Piano MA, Giunco S, Baboci L, et al. Telomere shortening in mucosa surrounding the tumor: biosensor of field cancerization and prognostic marker of mucosal failure in head and neck squamous cell carcinoma. Oral Oncol (2015) 51:500–7. doi: 10.1016/j.oraloncology.2015.02.100
28. Vinothkumar V, Arunkumar G, Revathidevi S, Arun K, Manikandan M, Rao AK, et al. TERT Promoter Hot Spot Mutations are Frequent in Indian Cervical and Oral Squamous Cell Carcinomas. Tumour Biol (2016) 37:7907–13. doi: 10.1007/s13277-015-4694-2
29. Chang K, Wang CI, Pickering CR, Huang Y, Tsai CN, Tsang NM, et al. Prevalence of Promoter Mutations in the TERT Gene in Oral Cavity Squamous Cell Carcinoma. Head Neck (2017) 39:1131–7. doi: 10.1002/hed.24728
30. Annunziata C, Pezzuto F, Greggi S, Ionna F, Losito S, Botti G, et al. Distinct Profiles of TERT Promoter Mutations and Telomerase Expression in Head and Neck Cancer and Cervical Carcinoma. Int J Cancer (2018) 143:1153–61. doi: 10.1002/ijc.31412
31. Morris LGT, Chandramohan R, West L, Zehir A, Chakravarty D, Pfister DG, et al. The Molecular Landscape of Recurrent and Metastatic Head and Neck Cancers: Insights From a Precision Oncology Sequencing Platform. JAMA Oncol (2017) 3:244–55. doi: 10.1001/jamaoncol.2016.1790
32. Yilmaz I, Erkul BE, Ozturk Sari S, Issin G, Tural E, Terzi Kaya Terzi N, et al. Promoter Region Mutations of the Telomerase Reverse Transcriptase (TERT) Gene in Head and Neck Squamous Cell Carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol (2020) 130:63–70. doi: 10.1016/j.oooo.2020.02.015
33. Arantes LMRB, Cruvinel-Carloni A, de Carvalho AC, Sorroche B, Carvalho AL, Scapulatempo-Neto C, et al. TERT Promoter Mutation C228T Increases Risk for Tumor Recurrence and Death in Head and Neck Cancer Patients. Front Oncol (2020) 10:1275. doi: 10.3389/fonc.2020.01275
34. Mundi N, Prokopec SD, Ghasemi F, Warner A, Patel K, MacNeil D, et al. Genomic and Human Papillomavirus Profiling of an Oral Cancer Cohort Identifies TP53 as a Predictor of Overall Survival. Cancers Head Neck (2019) 4:5. doi: 10.1186/s41199-019-0045-0
35. Akıncılar SC, Khattar E, Boon PL, Unal B, Fullwood MJ, Tergaonkar V. Long-Range Chromatin Interactions Drive Mutant TERT Promoter Activation. Cancer Discov (2016) 6:1276–91. doi: 10.1158/2159-8290.CD-16-0177
36. Bell RJ, Rube HT, Kreig A, Mancini A, Fouse SD, Nagarajan RP, et al. Cancer. The Transcription Factor GABP Selectively Binds and Activates the Mutant TERT Promoter in Cancer. Science (2015) 348:1036–9. doi: 10.1126/science.aab0015
37. Li Y, Zhou QL, Sun W, Chandrasekharan P, Cheng HS, Ying Z, et al. Non-Canonical NF-κb Signalling and ETS1/2 Cooperatively Drive C250T Mutant TERT Promoter Activation. Nat Cell Biol (2015) 17:1327–38. doi: 10.1038/ncb3240
38. Xu X, Li Y, Bharath SR, Ozturk MB, Bowler MW, Loo BZL, et al. Structural Basis for Reactivating the Mutant TERT Promoter by Cooperative Binding of P52 and ETS1. Nat Commun (2018) 9:3183. doi: 10.1038/s41467-018-05644-0
39. Huang DS, Wang Z, He XJ, Diplas BH, Yang R, Killela PJ, et al. Recurrent TERT Promoter Mutations Identified in a Large-Scale Study of Multiple Tumour Types Are Associated With Increased TERT Expression and Telomerase Activation. Eur J Cancer (2015) 51:969–76. doi: 10.1016/j.ejca.2015.03.010
40. Heidenreich B, Rachakonda P, Hosen I, Volz F, Hemminki K, Weyerbrock A, et al. TERT Promoter Mutations and Telomere Length in Adult Malignant Gliomas and Recurrences. Oncotarget (2015) 6:10617–33. doi: 10.18632/oncotarget.3329
41. Giunco S, Rampazzo E, Celeghin A, Petrara MR, De Rossi A. Telomere and Telomerase in Carcinogenesis: Their Role as Prognostic Biomarkers. Curr Pathobiol Rep (2015) 3:315–28. doi: 10.1007/s40139-015-0087-x
42. Mukherjee S, Firpo EJ, Wang Y, Roberts JM. Separation of Telomerase Functions by Reverse Genetics. Proc Natl Acad Sci U.S.A. (2011) 108:E1363–71. doi: 10.1073/pnas.1112414108
43. Park JI, Venteicher AS, Hong JY, Choi J, Jun S, Shkreli M, et al. Telomerase Modulates Wnt Signalling by Association With Target Gene Chromatin. Nature (2009) 460:66–72. doi: 10.1038/nature08137
44. Haendeler J, Hoffmann J, Rahman S, Zeiher AM, Dimmeler S. Regulation of Telomerase Activity and Anti-Apoptotic Function by Protein-Protein Interaction and Phosphorylation. FEBS Lett (2003) 536:180–6. doi: 10.1016/s0014-5793(03)00058-9
45. Dudognon C, Pendino F, Hillion J, Saumet A, Lanotte M, Ségal-Bendirdjian E. Death Receptor Signaling Regulatory Function for Telomerase: hTERT Abolishes TRAIL-Induced Apoptosis, Independently of Telomere Maintenance. Oncogene (2004) 23:7469–74. doi: 10.1038/sj.onc.1208029
46. Celeghin A, Giunco S, Freguja R, Zangrossi M, Nalio S, Dolcetti R, et al. Short-Term Inhibition of TERT Induces Telomere Length-Independent Cell Cycle Arrest and Apoptotic Response in EBV-Immortalized and Transformed B Cells. Cell Death Dis (2016) 7:e2562. doi: 10.1038/cddis.2016.425
47. Giunco S, Zangrossi M, Dal Pozzolo F, Celeghin A, Ballin G, Petrara MR, et al. Anti-Proliferative and Pro-Apoptotic Effects of Short-Term Inhibition of Telomerase In Vivo and in Human Malignant B Cells Xenografted in Zebrafish. Cancers (Basel) (2020) 12:2052. doi: 10.3390/cancers12082052
48. Ghosh A, Saginc G, Leow SC, Khattar E, Shin EM, Yan TD, et al. Telomerase Directly Regulates NF-κb-Dependent Transcription. Nat Cell Biol (2012) 14:1270–81. doi: 10.1038/ncb2621
49. Liu H, Liu Q, Ge Y, Zhao Q, Zheng X, Zhao Y. hTERT Promotes Cell Adhesion and Migration Independent of Telomerase Activity. Sci Rep (2016) 6:22886. doi: 10.1038/srep22886
50. Boscolo-Rizzo P, Da Mosto MC, Rampazzo E, Giunco S, Del Mistro A, Menegaldo A, et al. Telomeres and Telomerase in Head and Neck Squamous Cell Carcinoma: From Pathogenesis to Clinical Implications. Cancer Metastasis Rev (2016) 35:457–74. doi: 10.1007/s10555-016-9633-1
51. Qu Y, Dang S, Wu K, Shao Y, Yang Q, Ji M, et al. TERT Promoter Mutations Predict Worse Survival in Laryngeal Cancer Patients. Int J Cancer (2014) 135:1008–10. doi: 10.1002/ijc.28728
52. Geng J, Liu Y, Guo Y, Wang H, Tai J, Jin Y, et al. Correlation Between TERT C228T and Clinic-Pathological Features in Pediatric Papillary Thyroid Carcinoma. Sci China Life Sci (2019) 62:1563–71. doi: 10.1007/s11427-018-9546-5
53. You H, Wu Y, Chang K, Shi X, Chen XD, Yan W, et al. Paradoxical Prognostic Impact of TERT Promoter Mutations in Gliomas Depends on Different Histological and Genetic Backgrounds. CNS Neurosci Ther (2017) 23:790–7. doi: 10.1111/cns.12724
Keywords: oral cavity squamous cell carcinoma (OCSCC), telomerase, TERT promoter mutations, SNP rs2853669, telomere, prognostic biomarkers, survival
Citation: Giunco S, Boscolo-Rizzo P, Rampazzo E, Tirelli G, Alessandrini L, Di Carlo R, Rossi M, Nicolai P, Menegaldo A, Carraro V, Tofanelli M, Bandolin L, Spinato G, Emanuelli E, Mantovani M, Stellin M, Bussani R, Dei Tos AP, Guido M, Morello M, Fussey J, Esposito G, Polesel J and De Rossi A (2021) TERT Promoter Mutations and rs2853669 Polymorphism: Useful Markers for Clinical Outcome Stratification of Patients With Oral Cavity Squamous Cell Carcinoma. Front. Oncol. 11:782658. doi: 10.3389/fonc.2021.782658
Received: 24 September 2021; Accepted: 25 October 2021;
Published: 10 November 2021.
Edited by:
Panagiota Economopoulou, University General Hospital Attikon, GreeceReviewed by:
Maria Lina Tornesello, Istituto Nazionale Tumori Fondazione G. Pascale (IRCCS), ItalyVinay Tergaonkar, Institute of Molecular and Cell Biology (A*STAR), Singapore
Copyright © 2021 Giunco, Boscolo-Rizzo, Rampazzo, Tirelli, Alessandrini, Di Carlo, Rossi, Nicolai, Menegaldo, Carraro, Tofanelli, Bandolin, Spinato, Emanuelli, Mantovani, Stellin, Bussani, Dei Tos, Guido, Morello, Fussey, Esposito, Polesel and De Rossi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Silvia Giunco, c2lsdmlhLmdpdW5jb0B1bmlwZC5pdA==
†These authors have contributed equally to this work and share first authorship
 Silvia Giunco
Silvia Giunco Paolo Boscolo-Rizzo
Paolo Boscolo-Rizzo Enrica Rampazzo1
Enrica Rampazzo1 Roberto Di Carlo
Roberto Di Carlo Piero Nicolai
Piero Nicolai Giacomo Spinato
Giacomo Spinato Angelo Paolo Dei Tos
Angelo Paolo Dei Tos Marzia Morello
Marzia Morello Jonathan Fussey
Jonathan Fussey Giovanni Esposito
Giovanni Esposito Jerry Polesel
Jerry Polesel Anita De Rossi
Anita De Rossi